Abstract
• Background and Aims Drought is a major environmental constraint affecting growth and production of Coffea canephora. Selection of C. canephora clones has been largely empirical as little is known about how clones respond physiologically to drought. Using clones previously shown to differ in drought tolerance, this study aimed to identify the extent of variation of water use and the mechanisms responsible, particularly those associated morphological traits.
• Methods Clones (14 and 120, drought-tolerant; 46 and 109A, drought-sensitive, based on their abilities to yield under drought) were grown in 120-L pots until they were 12-months old, when an irrigation and a drought treatment were applied; plants were droughted until the pressure potential (ψx) before dawn (pre-dawn) reached −3·0 MPa. Throughout the drought period, ψx and stomatal conductance (gs) were measured. At the end of the experiment, carbon isotope ratio and parameters from pressure–volume curves were estimated. Morphological traits were also assessed.
• Key Results and Conclusions With irrigation, plant hydraulic conductance (KL), midday ψx and total biomass were all greater in clones 109A and 120 than in the other clones. Root mass to leaf area ratio was larger in clone 109A than in the others, whereas rooting depth was greater in drought-tolerant than in drought-sensitive clones. Predawn ψx of −3·0 MPa was reached fastest by 109A, followed progressively by clones 46, 120 and 14. Decreases in gs with declining ψx, or increasing evaporative demand, were similar for clones 14, 46, and 120, but lower in 109A. Carbon isotope ratio increased under drought; however, it was lower in 109A than in other clones. For all clones, ψx, gs and KL recovered rapidly following re-watering. Differences in root depth, KL and stomatal control of water use, but not osmotic or elastic adjustments, largely explained the differences in relative tolerance to drought stress of clones 14 and 120 compared with clones 46 and 109A.
Key words: Carbon isotope ratio, elastic and osmotic adjustments, robusta coffee, vapour pressure deficit, water potential, water relations, water-use efficiency
INTRODUCTION
Drought is an environmental factor that produces water deficit or water stress in plants. Internal water deficit is initiated when low water potential develops and cell turgor begins to fall below its maximum value (Kozlowski and Pallardy, 1997). There has not been a great deal of attention given to separating productivity under drought, which is important for cultivated plants, from survival mechanisms, particularly for woody species. Species or cultivars more tolerant to drought generally differ morphologically and/or physiologically, with mechanisms allowing greater production under limited water supply. These mechanisms involve maximization of water uptake by deep, dense root systems and/or minimization of water loss by stomatal closure and reduction of leaf area (Kramer and Boyer, 1995). These improve plant water status and particularly turgor maintenance, which may be achieved through osmotic adjustment and/or changes in cell wall elasticity, and is essential for maintaining physiological activity for extended periods of drought (Kramer and Boyer, 1995; Turner, 1997).
Coffee (Coffea arabica and C. canephora), a tropical tree crop, is the most important commodity in international agricultural trade, generating over US$90 billion each year and involving about 500 million people in its management, from cultivation to final product for consumption. Currently, robusta coffee (C. canephora) produces about 38 % of coffee consumed (Rezende and Rosado, 2004). It is indigenous to African regions characterized by abundantly distributed rainfall and atmospheric humidity frequently approaching saturation (Willson, 1999). For this reason, robusta coffee probably evolved as a ‘water-spender’ species (DaMatta and Rena, 2001). However, in Brazil, a major area of production, it has been largely cultivated in regions where water availability constitutes the major environmental constraint affecting crop production. Even short periods of drought can substantially decrease coffee yields, and consequently irrigation is indispensable for production. Older progenies of robusta coffee differed little in response to drought, however, plant breeders have recently selected some promising clones with relatively high, and low-year-to-year variation of, bean production under rain-fed conditions. The selection has been largely empirical as relatively little is known about how clones of robusta respond physiologically to drying soil. Many mechanisms have been suggested to be important. Lima et al. (2002), from a study of two clones rapidly drought stressed, proposed that drought tolerance might, at least in part, be associated with enhanced activity of antioxidant enzymes. In contrast, Pinheiro et al. (2004) did not find a general link between protection against oxidative stress and drought tolerance when four clones of robusta were subjected to long-term drought, and so did not corroborate that suggestion. DaMatta et al. (2003) found that the better crop yield of a drought-tolerant clone, compared with a drought-sensitive one, was associated with maintenance of leaf area and higher tissue water potentials, as a consequence of smaller stomatal conductance (gs), which would result in less carbon isotope discrimination. Despite these efforts, the causes of the differences in clonal tolerance to drought in robusta coffee still remain largely unknown. For instance, as yet there is no consistent information about the stomatal control of water use in response to both soil and atmospheric drought stress.
In this work, clones 14, 120, 46 and 109A of robusta coffee were compared. These clones all produce a good crop when grown under irrigation; under limited soil water, however, survival and productivity (Ferrão et al., 2000a, b) as well as maintenance of tissue water status (DaMatta et al., 2000) are impaired to a greater extent in 46 and 109A, which are therefore classified as drought-sensitive, than in 14 and 120, classified as drought-tolerant. One group of plants was continuously irrigated while water was withheld from a second group to promote a drought response. Clones were grown in large containers in an attempt to develop internal water deficits slowly, thus allowing adaptation (acclimation) to occur (DaMatta, 2003). Leaf water relations were examined at a similar internal water status, permitting more reliable comparisons among clones to be made.
The overall aim of this work was to expand our earlier studies quoted above (which explored drought tolerance mostly at a biochemical level) to improved understanding of the physiological and morphological basis of drought tolerance in robusta coffee. This would provide greater opportunities for intensifying selection of promising clones for drought-prone regions. Specific objectives were: (1) to identify the extent and mechanisms of intra-specific variation of water use by examining how stomatal behaviour and leaf water relations adjusted to changes in soil water supply and evaporative demand; and (2) to assess whether differences in drought tolerance are associated with morphological characteristics such as root depth and leaf area. For these purposes, morphological traits, leaf xylem pressure potential (ψx), gs, plant hydraulic conductance (KL), stable carbon isotope ratio, δ13C (to estimate long-term water-use efficiency; Farquhar et al., 1989), and water relations parameters derived from pressure–volume curves were evaluated.
MATERIALS AND METHODS
Experimental design
The experiment was conducted in Viçosa (20°45′S, 650 m a.s.l.), south-eastern Brazil. Plants were grown under shade (about 45 % of natural light) in a screen house with walls of coarse mesh screen, which allowed air exchange with the external environment. The experiment was a completely randomized design, with eight treatment combinations, forming a 4 × 2 factorial (four clones and two watering regimes) with five plants in individual pots per treatment combination as replication. The experimental plot was one plant per container. Clones (14 and 120, drought-tolerant; 46 and 109A, drought-sensitive) of C. canephora ‘Kouillou’ (known in Brazil as ‘Conilon’) raised as rooted stem cuttings were obtained from the Institute for Research and Rural Assistance of the Espírito Santo State (INCAPER), Brazil. Forty plants were grown in plastic, cylindrical pots (0·8 m high, 0·44 m internal diameter) containing 120 L of a mixture of soil, sand and manure (3 : 1 : 1, v/v/v) with a gravel layer at the bottom. Plants received an average midday photosynthetic photon flux of about 900 µmol m−2 s−1. When 12-months old, plants of each clone were separated into two groups: one continued to receive regular irrigation (control plants), and in the other water was withheld (drought-stressed plants) until ψx at pre-dawn (ψpd) reached about −3·0 MPa. During the course of drying, ψx and gs were measured on five occasions on the third or fourth pair of leaves from the apex of plagiotropic branches. Once the desired ψpd was reached, expanding leaves (approximately half of the final size of those from control plants) were collected and their δ13C was determined. Expanding leaves were selected to ensure that most of the carbon analysed was incorporated into tissue during the drought treatment. Fully expanded leaves were also collected for measuring pressure–volume relationships. All the plants were then irrigated (at about 1800 h) and ψx, gs and KL were determined on the following two consecutive days (ψpd measured at 12 h and 36 h after irrigation).
Biometric measurements
At the end of the experiment, well-watered plants were harvested and separated into above-ground parts and roots. Leaf area was measured with an area meter (Area Measurement System, Delta-T Devices, Cambridge, UK). Roots were washed thoroughly with tap water above a 0·5 mm screen sieve. Plant tissues were then oven-dried at 72 °C for 72 h, after which dry matter was determined. Shoot height and root depth (after washing) were also measured.
Water relations
Xylem pressure potential was measured before dawn (0430–0530 h), between 0700–0900 h and at midday (ψmd) using a Scholander-type pressure chamber. About 12 h after irrigating both control and drought-stressed plants (ψx typically above −0·10 MPa), fully expanded leaves were detached by cutting their petioles under deionized water and brought to the laboratory to produce pressure–volume curves. Fresh weight and ψx were measured at intervals during dehydration (free transpiration technique; Hinckley et al., 1980) until a ψx of about −3·5 MPa was reached. Turgid weight was estimated from the linear relationship between fresh weight and ψx in the positive turgor range, by extrapolating to ψx = 0. The inverse of ψx was plotted as a function of relative water content (RWC). From the pressure–volume curves, the osmotic potential at full (ψπ(100)) and zero (ψπ(0)) turgor, RWC at zero turgor (RWC(0)) and the bulk modulus of elasticity (ε; Melkonian et al., 1982) were estimated. Further details are given by DaMatta et al. (1993).
Stomatal and hydraulic conductance
Stomatal conductance to water vapour was measured with a portable, open-system infrared gas analyser (LCA-4, ADC, Hoddesdon, UK), as described in DaMatta et al. (1997). Measurements were made between 0700 and 0900 h (25 ± 2 °C air temperature, 90 ± 2 % relative humidity) and between 1100 and 1300 h (30 ± 2 °C air temperature, 80 ± 2 % relative humidity).
Plant hydraulic conductance [KL = (gs × Δw)/(ψpd − ψmd)] was calculated using ψpd to approximate soil water potential, and gs and Δw (leaf-to-air vapour pressure deficit, estimated according to Landsberg, 1986) were measured at the same time as ψmd (Hubbard et al., 1999; Donovan et al., 2000).
Carbon isotope ratio
Leaf δ13C was measured relative to the international PDB standard using a mass spectrometer (Delta-S, Finnigan MAT, Bremen, Germany), as previously described (DaMatta et al., 2002). Differences in δ13C from duplicates for each sample were below 0·2 ‰.
Statistics
Significant differences between treatment means were tested by the Newman–Keuls and F-tests, at P ≤ 0·05. Regression analyses were used to examine relationships between physiological and/or environmental variables. Equality of the regression models was tested using the indicator variable technique (Neter and Wasserman, 1974), at P ≤ 0·05. Separate regression models for clones 14, 46 and 120 did not differ statistically. Therefore, data for these clones were pooled and single regressions were fitted to the combined data.
RESULTS
It should be noted that a ψpd of −3·0 MPa was reached at different times in different clones (see below) upon discontinuing irrigation and, thus, changes in growth traits are not fully comparable between drought-stressed clones. In addition, because treatments were applied over a relatively short time, drought effects on growth were small, and so not significant (not shown). Therefore, only growth data for control plants are presented. The clones could be grouped into two types of contrasting canopy morphology, with 109A and 120 taller (Table 1) with less dense crowns than 14 and 46. There was no significant difference in total leaf area or specific leaf area between clones, but total dry matter was greater in clones 109A and 120 than in 14 and 46, whereas root mass to leaf area ratio was larger in 109A than in the other clones (Table 1). Drought-tolerant clones had a considerably deeper (Table 1) and more regularly distributed root system down the profile than drought-sensitive clones (Fig. 1).
Table 1.
Morphological characteristics of 1-year-old clones of robusta coffee (Coffea canephora) under full irrigation
| Drought-tolerant clones |
Drought-sensitive clones |
|||||
|---|---|---|---|---|---|---|
| Parameters |
Clone 14 |
Clone 120 |
Clone 46 |
Clone 109A |
||
| Shoot height, m | 0·78 ± 0·05a | 0·94 ± 0·03b | 0·73 ± 0·07a | 0·92 ± 0·02b | ||
| Leaf area, m2 | 1·89 ± 0·11a | 2·51 ± 0·13a | 1·91 ± 0·08a | 2·36 ± 0·10a | ||
| Specific leaf area, m2 kg−1 | 11·18 ± 0·39a | 10·39 ± 0·96a | 12·15 ± 1·09a | 9·32 ± 0·64a | ||
| Root mass to leaf area ratio, g m−2 | 93·5 ± 2·63a | 107·2 ± 8·91a | 113·0 ± 7·36a | 120·8 ± 22·8a | ||
| Total biomass, g | 434 ± 21b | 645 ± 31a | 455 ± 59b | 744 ± 58a | ||
| Root depth, m | 0·76 ± 0·03a | 0·75 ± 0·04a | 0·48 ± 0·04b | 0·53 ± 0·03b | ||
Different letters denote significant differences between clonal means (P ≤ 0·05; Newman–Keuls test). Each value represents the mean ± s.e. of five replicates.
Fig. 1.

Typical root systems of four clones of robusta coffee grown under full irrigation.
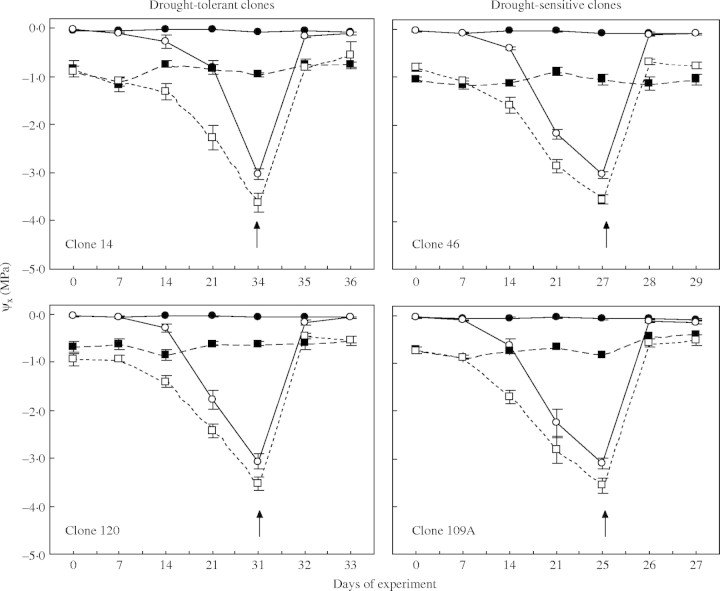
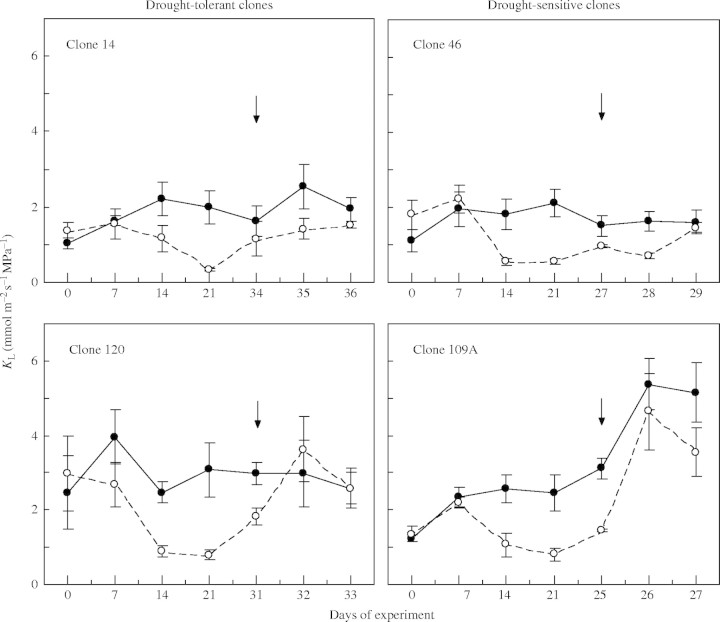
For control plants, ψpd was always above −0·08 MPa, but the average ψmd tended to be lower in clones 14 and 46 (−0·89 and −1·06 MPa, respectively) than in 109A and 120 (both −0·65 MPa; Fig. 2). On average, KL tended to be higher in 109A and 120 (about 3·0 mmol m−2 s−1 MPa−1) than in 14 and 46 (about 1·7 mmol m−2 s−1 MPa−1).
Fig. 2.
Time-course of leaf xylem pressure potential (ψx), both pre-dawn (circles) and at midday (squares), of four clones of robusta coffee either fully irrigated (solid lines) or droughted (dotted lines). Arrows indicate when predawn ψx reached −3·0 MPa, when the drought-stressed plants were re-watered (at 1800 h); measurements were then made for a further 2 d. Note differences in scale on horizontal axes. Each point represents the mean ± s.e. of five replicates.
Plant water stress developed faster in drought-sensitive clones. After withholding irrigation for 14 d, ψpd was significantly lower in clone 109A than in the other clones; 7 d latter, ψpd dropped to about −2·3 MPa in clones 46 and 109A, compared with − 0·8 MPa in clone 14 and −1·6 MPa in clone 120. A similar trend, but clearly shifted to lower values, was found for ψmd (Fig. 2). As expected from the above, clone 109A attained a ψpd of −3·0 MPa earlier than the other clones, followed in order by 46, 120, and then 14 (Fig. 2). Under drought stress, average KL tended to be greater in clones 109A and 120 than in the other clones (Fig. 3). ψx and KL recovered within 2 d of re-watering for all clones (Figs 2, 3).
Fig. 3.
Time-course of hydraulic conductance from soil to leaf (KL) of four clones of robusta coffee subjected to full irrigation (solid circles) and drought conditions (open circles). See Fig. 1 for details.
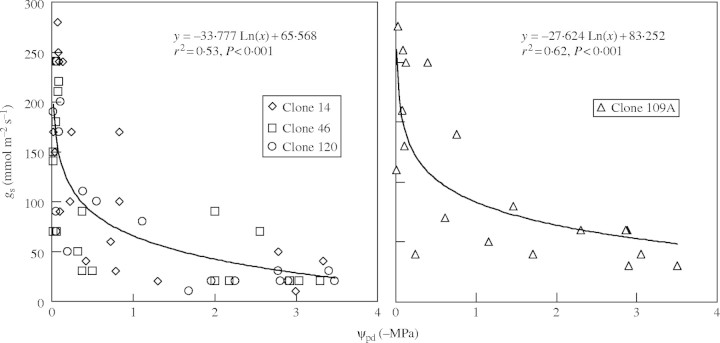
Curvilinear decreases in gs as ψpd declined were similar for clones 14, 46 and 120, but smaller in clone 109A (Fig. 4). For example, as ψpd decreased from −0·5 to −3·0 MPa, gs decreased, on average, from 89 to 28 mmol m−2 s−1 in clones 14, 46 and 120, and from 102 to 52 mmol m−2 s−1 in clone 109A (estimated from the equations in Fig. 4). Similar changes were found when gs was associated with ψx, both variables being measured on the same leaf at 0700–0900 h (data not shown).
Fig. 4.
Stomatal conductance (gs) in relation to pre-dawn leaf xylem pressure potential (ψpd) in four clones of robusta coffee. The gs was measured between 0700–0900 h and represents the entire data set from plants during dehydration after selecting a narrow range of leaf-to-air vapour pressure deficits (1·5 kPa at most).
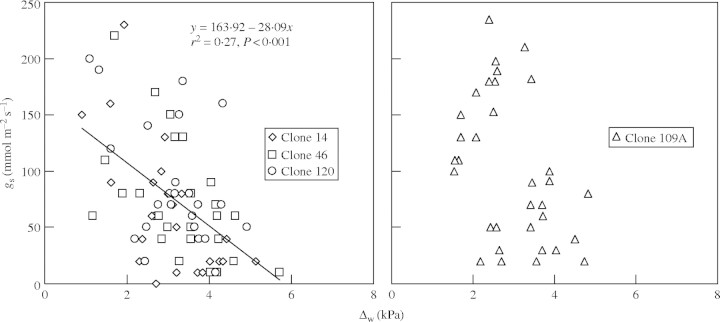
As Δw increased, gs decreased linearly in a similar way in clones 14, 46 and 120. By contrast, there was no relationship between gs and Δw in clone 109A (Fig. 5). In spite of Δw and leaf temperature being strongly associated to each other (r2 = 0·920, P < 0·001), co-variance analysis revealed no direct effect of leaf temperature in the response of gs to Δw (P = 0·173).
Fig. 5.
Stomatal conductance (gs) in relation to leaf-to-air vapour pressure deficit (Δw) in four clones of robusta coffee under irrigated conditions, measured over several days under naturally fluctuating Δw. Data were collected between 1100–1300 h in plants grown under full irrigation to ensure comparable internal water status (xylem pressure potential = −0·8 ± 0·2 MPa). For clone 109A the relationship between gs and Δw was not significant.
Clonal differences in drought tolerance were not associated with osmotic or elastic adjustments, since parameters from pressure–volume curves, irrespective of treatments, were similar (ψπ(100) = −1·83 ± 0·08 MPa; ψπ(0) = −2·26 ± 0·09 MPa; ε = 18·4 ± 0·6 MPa; RWC(0) = 89·8 ± 0·5 %) and constant for all clones (data not shown). The only exception was observed in clone 109A in which drought resulted in a slight, but significant decrease (0·19 MPa) in ψπ(0).
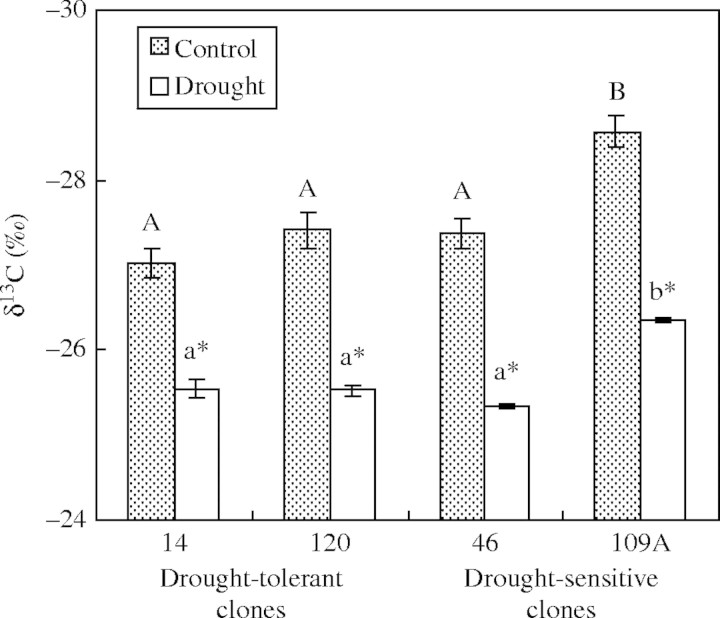
After imposing water deficit, δ13C increased significantly (1·48 to 2·22 ‰; Fig. 6) in all clones, suggesting increased long-term water-use efficiency (WUE). However, absolute values of δ13C were lower in 109A than in the other clones irrespective of the irrigation treatments. There was no difference in δ13C between clones 14, 46 and 120 (Fig. 6). Overall, gs decreased more than net carbon assimilation rate, which did not differ among clones (data not shown) and, thus, changes in δ13C would have been predominantly from changes in gs.
Fig. 6.
Effects of drought on leaf carbon isotope ratio (δ13C) of four clones of robusta coffee. Different capital letters denote significant differences between means for irrigated clones, and different lower case letters represent significant differences between means for drought-stressed clones (Newman–Keuls test at P ≤ 0·05; clone effect). Means for drought-stressed plants marked with an asterisk differ from those for control plants (F-test at P ≤ 0·05; treatment effect). Data are means ± s.e. of four replicates.
DISCUSSION
Plant water stress developed more slowly in the drought-tolerant than in the drought-sensitive clones. Morphological traits such as leaf area and root mass to leaf area ratio were not associated with that response. Instead, the much deeper root system of the tolerant clones enabled them to gain greater access to water towards the bottom of the pots and, therefore, to maintain a more favourable internal water status longer than in drought-sensitive clones. Differences between drought-tolerant and drought-sensitive clones in postponing tissue dehydration are even more evident in the field (DaMatta et al., 2000, 2003), where the development of the root system is much less restricted.
Hydraulic conductance is positively associated with rates of water use, as has been found in genotypes of C. arabica (Tausend et al., 2000). Thus the larger KL, as observed in clones 109A and 120 under full irrigation might at least partially explain their smaller variations in ψx (as indicated by higher ψmd values) than in clones 14 and 46, which may help to avoid limitations to photosynthesis. As a consequence, clones 109A and 120 might have achieved a greater carbon gain, which would to some extent explain their greater biomass accumulation under well-watered conditions. This would be advantageous with non-limiting soil water or with brief periods of water deficit, but disadvantageous with long-term drought since a high KL may hasten the development of severe internal water deficit. This could be partially offset by a deeper root system, as is the case of clone 120. However, because KL was estimated using data from instantaneous gas-exchange measurements, rather than transpiration integrated over the morning, the above considerations should be interpreted cautiously.
Stomatal conductance decreased sharply with decreasing ψx, with no apparent threshold value of ψpd at which stomatal closure was observed. The positive relationship between gs and ψx is expected when soil moisture changes and indirectly affects stomata through a hydraulic feedback (Jones, 1998). The rapid increase of ψx after re-watering, which was accompanied by increased gs, emphasizes the role of leaf water status on stomatal control, as suggested by Fuchs and Livingston (1996). In addition, stomatal sensitivity to evaporative demand, as observed in clones 14, 46 and 120, might indicate a feedforward response that would avoid large internal water deficits. Such sensitivity appears to be weaker in C. canephora than in C. arabica since, in the latter, gs decreases curvilinearly with increasing Δw (Gutiérrez et al., 1994; Kanechi et al., 1995). When considered together, these responses largely explain why C. canephora responds strongly, and better than C. arabica, to irrigation (DaMatta, 2004a).
Less negative δ13C can arise because of low gs or high carbon assimilation, both leading to a high WUE (Farquhar et al., 1989). The observed increases in δ13C in all drought-stressed clones should therefore reflect an increase in long-term WUE. However, in clone 109A stomata closed less in response to both soil and atmospheric drought, probably resulting in a more prodigal use of water and also in a relatively more negative δ13C (and thus lower long-term WUE) than in the other clones regardless of the watering regime. These observations are partially in line with those of Meinzer et al. (1990a), who showed that genotypes of C. arabica with higher carbon isotope discrimination (more negative δ13C) under full irrigation resulted from higher gs rather than lower carbon assimilation, depleted soil water more rapidly, and experienced symptoms of physiological stress earlier when water was withheld. One must be cautious, however, since this present study was too small to demonstrate conclusively the usefulness of δ13C as an index for ranking clones of robusta coffee in terms of drought tolerance.
Osmotic adjustment has been associated with maintenance of gas exchange under drought conditions (Turner, 1997). In our work, however, its amplitude was small and limited to clone 109A and could hardly explain the low stomatal sensitivity to drought in this clone. It should be noted that leaf water deficits may develop faster upon discontinuing irrigation in coffee genotypes having greater amplitude of osmotic adjustment (DaMatta, 2004b). Therefore, osmotic adjustment seems of limited importance (Munns, 1988) in determining drought tolerance in robusta coffee; this has also been reported for several other woody species (Fan et al., 1994). Where it occurs, osmotic adjustment either may not persist for long under drought, or functions over a limited range of ψx values (Blake et al., 1991).
The clones we evaluated lost turgor at values of ψx between −2·1 and −2·4 MPa. They showed relatively high values of ε (i.e. greater tissue rigidity), which resulted in high RWC(0), as reported for robusta coffee in both pot (DaMatta et al., 1993, 2002) and field (DaMatta et al., 2003) studies. These traits were consistent with the strong stomatal sensitivity to soil water deficit mediated by rapid loss of turgor as a consequence of inelastic leaf tissues (White et al., 2000). One must be cautious, however, because changes in relative symplast volume rather than changes in leaf turgor per se have been associated with stomatal aperture, as shown by Meinzer et al. (1990b) working with cultivars of C. arabica subjected to drought. In any case, maintenance of high RWC at low ψx appears to be a means of the coffee tree avoiding, instead of tolerating, dehydration.
In summary, clonal ability to postpone dehydration was more important than dehydration tolerance, and cell water relations were largely unable to adjust to drought stress in any clone. Clone 109A, although possessing a greater root mass to leaf area ratio, is shallow-rooted and showed relatively poor stomatal control of transpiration; these features could explain why it experienced symptoms of drought stress earlier than other clones after irrigation was suspended. Clone 46 is also shallow-rooted, but its stomata closed more with both soil and atmospheric drought than in clone 109A; hence clone 46 dehydrated more slowly than 109A. Similarly to clone 46, stomatal sensitivity to drought was well developed in clones 14 and 120, but these clones showed substantially deeper root systems than the drought-sensitive clones, which could explain their better avoidance of drought. In any case, the larger KL in clone 120 than in clone 14 might be involved in the faster decrease in ψpd in the former. The direct response of stomata to changes in ψx and Δw should have important consequences for clonal ability to support relatively long periods of soil drought associated with high atmospheric evaporative demand. Such behaviour would be advantageous, allowing for maximization of WUE and survival as soil water availability decreases. In this case, stomatal sensitivity to soil drying should be negatively associated with the stability of crop yield under rainfed conditions. However, stomata of mature field-grown trees may not respond to soil water limitations as readily and dramatically as those of the young plants in this study with less expanded root systems (Gucci et al., 1996). If so, a deeper root system compensating for water loss during the day would be of paramount importance. Of course, this should be possible if the plant maintains a sufficient KL, as appears to be the case with clone 120. In this clone, the combination of deep roots with relatively large KL (this study), better protection against oxidative stress (Pinheiro et al., 2004), and maintenance of capacity for sucrose synthesis—and possibly export, allowing extra root growth (Praxedes et al., 2005)—under drought, should contribute to dampen variations in its productivity, as has been observed in test-trials under rainfed conditions (Ferrão et al., 2000a). In any case, clones with better yield stability under drought (120), or better able to survive drought episodes through a more conservative use of water (14), may be of greater value than clones selected for improved environments (46 and 109A), particularly under low-input conditions typical of many farming systems of drought-prone regions (DaMatta, 2004b).
Acknowledgments
The authors gratefully acknowledge the Institute for Research and Rural Assistance of the State of Espírito Santo (INCAPER)—Brazil, for providing the coffee seedlings. This work was supported by the Brazilian Consortium for Coffee Research and Development. A research fellowship (F.M. DaMatta) and scholarships (A.R.M. Chaves and H.A. Pinheiro) granted by the Brazilian Council for Scientific and Technological Development are also acknowledged.
LITERATURE CITED
- Blake TJ, Bevilacqua E, Zwiazek JJ. 1991. Effects of repeated stress on turgor pressure and cell elasticity changes in bleak spruce seedlings. Canadian Journal of Forest Research 21: 1329–1333. [Google Scholar]
- DaMatta FM. 2003. Drought as a multidimensional stress affecting photosynthesis in tropical tree crops. In: Hemantaranjan A, ed. Advances in plant physiology. Vol. 5. Jodhpur: Scientific Publishers, 227–265. [Google Scholar]
- DaMatta FM. 2004. Ecophysiological constraints on the production of shaded and unshaded coffee: a review. Field Crops Research 86: 99–114. [Google Scholar]
- DaMatta FM. 2004. Exploring drought tolerance in coffee: a physiological approach with some insights for plant breeding. Brazilian Journal of Plant Physiology 16: 1–6. [Google Scholar]
- DaMatta FM, Rena AB. 2001. Tolerância do café à seca. In: Zambolim L, ed. Tecnologias de produção de café com qualidade. Viçosa: Universidade Federal de Viçosa, 65–100. [Google Scholar]
- DaMatta FM, Maestri M, Barros RS, Regazzi AJ. 1993. Water relations of coffee leaves (Coffea arabica and C. canephora) in response to drought. Journal of Horticultural Science 68: 741–746. [Google Scholar]
- DaMatta FM, Maestri M, Mosquim PR, Barros RS. 1997. Photosynthesis in coffee (Coffea arabica and C. canephora) as affected by winter and summer conditions. Plant Science 128: 43–50. [Google Scholar]
- DaMatta FM, Silveira JSM, Ducatti C, Loureiro ME. 2000. Eficiência do uso da água e tolerância à seca em Coffea canephora In: I Simpósio de Pesquisa dos Cafés do Brasil. Vol. 2. Brasília: EMBRAPA, 907–910. [Google Scholar]
- DaMatta FM, Loos RA, Silva EA, Loureiro ME, Ducatti C. 2002. Effects of soil water deficit and nitrogen nutrition on water relations and photosynthesis of pot-grown Coffea canephora Pierre. Trees 16: 555–558. [Google Scholar]
- DaMatta FM, Chaves ARM, Pinheiro HA, Ducatti C, Loureiro ME. 2003. Drought tolerance of two field-grown clones of Coffea canephora Plant Science 164: 111–117. [Google Scholar]
- Donovan LA, West JB, McLeod KW. 2000.Quercus species differ in water and nutrient characteristics in a resource-limited fall-line sandhill habitat. Tree Physiology 20: 929–936. [DOI] [PubMed] [Google Scholar]
- Fan S, Blake TJ, Blumwald E. 1994. The relative contribution of elastic and osmotic adjustment to turgor maintenance of woody plants. Physiologia Plantarum 90: 414–419. [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. 1989. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40: 503–537. [Google Scholar]
- Ferrão RG, Fonseca AFA, Ferrão MAG, Santos LP. 2000. Avaliação de clones elites de café Conilon em condição de estresse hídrico no estado do Espírito Santo. In: I Simpósio de Pesquisa dos Cafés do Brasil. Vol. 1. Brasília: EMBRAPA, 402–404. [Google Scholar]
- Ferrão RG, Fonseca AFA, Ferrão MAG, Santos LP. 2000. Comportamento de clones elites de café Conilon em condições de alta tecnologia no estado do Espírito Santo. In: I Simpósio de Pesquisa dos Cafés do Brasil. Vol. 2. Brasília: EMBRAPA, 769–771. [Google Scholar]
- Fuchs EE, Livingston NJ. 1996. Hydraulic control of stomatal conductance in Douglas fir [Pseudotsuga menziesii (Mirb.) Franco] and alder [Alnus rubra (Bong)] seedlings. Plant, Cell and Environment 19: 1091–1098. [Google Scholar]
- Gucci R, Massai R, Xiloyannis C, Flore JA. 1996. The effect of drought and vapour pressure deficit on gas exchange of young kiwifruit (Actinidia deliciosa var. deliciosa) vines. Annals of Botany 77: 605–613. [Google Scholar]
- Gutiérrez MV, Meinzer FC, Grantz DA. 1994. Regulation of transpiration in coffee hedgerows: Covariation of environmental variables and apparent responses of stomata to wind and humidity. Plant, Cell and Environment 17: 1305–1313. [Google Scholar]
- Hinckley TM, Duhme F, Hinckley AR, Richter H. 1980. Water relations of drought hardy shrubs: osmotic potential and stomatal reactivity. Plant, Cell and Environment 3: 131–140. [Google Scholar]
- Hubbard RM, Bond BJ, Ryan MG. 1999. Evidence that hydraulic conductance limits photosynthesis in old Pinus ponderosa trees. Tree Physiology 19: 165–172. [DOI] [PubMed] [Google Scholar]
- Jones HG. 1998. Stomatal control of photosynthesis and transpiration. Journal of Experimental Botany 49: 387–398. [Google Scholar]
- Kanechi M, Uchida NU, Yasuda T, Yamaguchi T. 1995. Water stress effects on leaf transpiration and photosynthesis of Coffea arabica L. under different irradiance conditions. In: Proceedings of the 16th International Scientific Colloquium on Coffee. Paris: Association Scientifique Internationale du Café, 520–527. [Google Scholar]
- Kozlowski TT, Pallardy SG. 1997.Physiology of woody plants. San Diego: Academic Press. [Google Scholar]
- Kramer PJ, Boyer JS. 1995.Water relations of plants and soils. San Diego: Academic Press. [Google Scholar]
- Landsberg JJ. 1986.Physiological ecology of forest production. San Diego: Academic Press. [Google Scholar]
- Lima ALS, DaMatta FM, Pinheiro HA, Totola MR, Loureiro ME. 2002. Photochemical responses and oxidative stress in two clones of Coffea canephora under water deficit conditions. Environmental and Experimental Botany 47: 239–247. [Google Scholar]
- Meinzer FC, Goldstein G, Grantz DA. 1990. Carbon isotope discrimination in coffee genotypes grown under limited water supply. Plant Physiology 92: 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer FC, Grantz DA, Goldstein G, Saliendra NZ. 1990. Leaf water relations and maintenance of gas exchange in coffee cultivars grown in drying soil. Plant Physiology 94: 1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkonian JJ, Wolfe J, Steponkus PL. 1982. Determination of the volumetric modulus of elasticity of wheat leaves by pressure-volume relations and the effects of drought conditioning. Crop Science 22: 116–123. [Google Scholar]
- Munns R. 1988. Why measure osmotic adjustment? Australian Journal of Plant Physiology 5: 207–218. [Google Scholar]
- Neter J, Wasserman W. 1974.Applied linear statistical models. Regression, analysis of variance and experimental designs. Illinois: Richard D. Irwin Inc. [Google Scholar]
- Pinheiro HA, DaMatta FM, Chaves ARM, Fontes EPB, Loureiro ME. 2004. Drought tolerance in relation to protection against oxidative stress in clones of Coffea canephora subjected to long-term drought. Plant Science 167: 1307–1314. [Google Scholar]
- Praxedes SC, DaMatta FM, Loureiro ME, Ferrão MAG, Cordeiro AT. 2005. Effects of long-term soil drought on photosynthesis and carbohydrate metabolism in mature robusta coffee (Coffea canephora Pierre var. kouillou) leaves. Environmental and Experimental Botany, in press. [Google Scholar]
- Rezende AM, Rosado PL. 2004. A informação no mercado de café. In: Zambolim L, ed. Produção integrada de café. Viçosa: Universidade Federal de Viçosa, 1–46. [Google Scholar]
- Tausend PC, Goldstein G, Meinzer FC. 2000. Water utilization, plant hydraulic properties and xylem vulnerability in three contrasting coffee (Coffea arabica) cultivars. Tree Physiology 20: 159–168. [DOI] [PubMed] [Google Scholar]
- Turner NC. 1997. Further progress in crop water relations. Advances in Agronomy 58: 293–338. [Google Scholar]
- White DA, Turner NC, Galbraith JH. 2000. Leaf water relations and stomatal behavior of four allopatric Eucalyptus species planted in Mediterranean southwestern Australia. Tree Physiology 20: 1157–1165. [DOI] [PubMed] [Google Scholar]
- Willson KC. 1999.Coffee, cocoa and tea. Cambridge: CABI Publishing. [Google Scholar]