Abstract
• Background and Aims The ability of partial dehydration and abscisic acid pretreatments to increase desiccation tolerance in the cyanobacterial lichen Peltigera polydactylon was tested.
• Methods Net photosynthesis and respiration were measured using infrared gas analysis during a drying and rehydration cycle. At the same time, the efficiency of photosystem two was measured using chlorophyll fluorescence, and the concentrations of chlorophyll a were spectrophotometrically assayed. Heat production was also measured during a shorter drying and rehydration cycle using differential dark microcalorimetry.
• Key Results Pretreating lichens by dehydrating them to a relative water content of approx. 0·65 for 3 d, followed by storing thalli hydrated for 1 d in the light, significantly improved their ability to recover net photosynthesis during rehydration after desiccation for 15 but not 30 d. Abscisic acid pretreatment could substitute for partial dehydration. The improved rates of photosynthesis during the rehydration of pretreated material were not accompanied by preservation of photosystem two activity or chlorophyll a concentrations compared with untreated lichens. Partial dehydration and ABA pretreatments appeared to have little direct effect on the desiccation tolerance of the mycobiont, because the bursts of respiration and heat production that occurred during rehydration were similar in control and pretreated lichens.
• Conclusions Results indicate that the photobiont of P. polydactylon possesses inducible tolerance mechanisms that reduce desiccation-induced damage to carbon fixation, and will therefore improve the supply of carbohydrates to the whole thallus following stress. In this lichen, ABA is involved in signal transduction pathways that increase tolerance of the photobiont.
Key words: Lichens, desiccation tolerance, abscisic acid, photosynthesis, chlorophyll, PSII activity, respiration, heat production
INTRODUCTION
Most lichens are desiccation tolerant, and can survive in an air-dried state for long or short periods even at relative water contents (RWC) below 10 % (Kershaw, 1985). Work on desiccation-tolerant higher plants and cryptogams suggests that desiccation is harmful for many reasons (Black and Pritchard, 2002). These include damage to the cytoskeleton as a result of the large changes in cell volume that accompany desiccation. Water removal can damage membranes, increase ionic strength, change pH, crystallize solutes and denature proteins. Apparently, desiccation tolerance is achieved not by any one simple adaptive feature, but by a complex interplay of many mechanisms (Black and Pritchard, 2002). Little information is available on desiccation-tolerance mechanisms in lichens, but these include maintaining effective concentrations of enzymic and non-enzymic antioxidants, and in lichens with chlorophycean photobionts, an efficient xanthophyll cycle to prevent reactive oxygen species (ROS) formation in the photosystems (Mayaba and Beckett, 2001; Zorn et al., 2001; Kranner, 2002; Kranner et al., 2003).
Oliver et al. (1998) divided desiccation-tolerant plants into two groups. In the first group, plants will only survive if drying is slow enough to induce mechanisms that will ‘protect’ the plants during desiccation or facilitate recovery during rehydration. Plants in the second group possess constitutive tolerance mechanisms, and can tolerate even rapid drying. It is often assumed that lichens rely mainly on constitutive mechanisms, as they frequently grow in highly stressful environments where desiccation may be sudden and severe. However, the disadvantage of constitutive mechanisms is that they are present even when not needed, and at these times divert energy away from growth and reproduction. It was therefore hypothesized that selection may have favoured inducible tolerance mechanisms in environments that are usually moist, and in which lichens are predictably (and probably slowly) desiccated. The latter conditions typify the habitat occupied by cyanobacterial species of Peltigera growing in the Afromontane forests of South Africa. These forests are moist for much of the year, but experience a regular winter drought. The aim of the work presented here was to test for inducible tolerance in a species from this genus, P. polydactylon, common in this and other similar habitats.
Under field conditions, partial dehydration often precedes desiccation stress, and therefore the effects of partial dehydration on desiccation tolerance were tested in Peltigera polydactylon. Much evidence exists that in poikilohydric plants ABA plays an important role in desiccation tolerance (Hartung et al., 1997), and therefore the effect of an ABA pretreatment on desiccation tolerance in this lichen was tested. Desiccation tolerance was assessed by measuring the effects of desiccation followed by rehydration on photobiont metabolism [net photosynthesis, photosystem two (PSII) activity and the concentrations of chlorophyll a (chla)] and mycobiont metabolism (respiration and heat production). Results presented here show that partial dehydration and ABA pretreatment increase the tolerance of carbon fixation to desiccation stress, which will reduce the effects of stress on the whole lichen thallus.
MATERIALS AND METHODS
Lichen material
Peltigera polydactylon (Necker) Hoffm. was collected from moss-covered boulders under a tree canopy in the Olandweni Valley, Cathedral Peak area, Drakensberg Mountains, KwaZulu-Natal Province, South Africa (28°45′ S, 29°10′ E). Once collected, lichens were cleaned in deionized distilled water then stored for 10 d at 15 °C, and a photosynthetic photon fluence density (PPFD) of 75 µmol photons m−2 s−1 under continuous fluorescent light. PPFDs were measured here and elsewhere over the waveband 400–700 nm using the light meter in the Parkinson leaf chamber of an Analytical Development Corporation (ADC, Hoddeston, UK) Mark III portable infra-red gas analyser. These storage conditions were assumed to remove most of the effects of stress present when lichens were collected, without being long enough to cause the harmful effects that can occur following prolonged moist storage. For each experiment, discs were cut, pooled, and each replicate derived from discs randomly sampled from this pool. Thallus water contents were calculated as RWC, estimated as (fresh mass − dry mass)/(turgid mass − dry mass), and measured as described in Beckett (2002).
Measurement of photosynthesis and respiration
Net photosynthesis and respiration were measured at 25 °C and relative humidity of 50 % using an ADC Mark III portable infrared gas analyser with a barrel-shaped Parkinson leaf chamber, modified with a water-cooled jacket. The flow rate through the leaf chamber was 120 mL min−1 and the PPFD was 350 µmol photons m−2 s−1. Preliminary experiments showed that net photosynthesis saturated at approx. 150 µmol photons m−2 s−1 and no photoinhibition occurred until at least 700 µmol photons m−2 s−1. Optimum rates of net photosynthesis occurred in lightly blotted hydrated material, i.e. no ‘oversaturation’ or suppression of photosynthesis at high RWCs occurred in the material. Equilibrating fully hydrated samples for 10 min gave steady-state rates of gas exchange without causing enough water loss to reduce photosynthesis. Respiration measurements were made in the same way, except lichens were maintained in the dark. Each treatment comprised five replicates of four 1-cm discs (approx. 40 mg fresh mass).
Chlorophyll fluorescence measurements
Chlorophyll fluorescence was measured using an FMS 2 modulated fluorometer (Hansatech Instruments, King's Lynn, UK). Lichens were clamped in standard Hansatech leaf-clips with the probe withdrawn slightly from the clip to allow sampling of a larger than normal thallus area. Each replicate comprised four 1-cm thallus discs. To take a measurement, each replicate was pretreated at a PPFD of 10 µmol photons m−2 s−1 for 10 min as recommended by Campbell et al. (1998), placed in a leaf clip, and Fo and Fm recorded using a 0·8 s flash of light at approx. 1500 µmol photons m−2 s−1. The actinic light at a PPFD of 33 µmol photons m−2 s−1 was then switched on, and F and F′m measured after 5 min. The fluorescence parameters were calculated following Schreiber and Bilger (1993), Schreiber et al. (1995) and Campbell et al. (1998); Fv/Fm = (Fm – Fo)/Fm and ΦPSII=(F′m – F)/F′m). Non-photochemical quenching was estimated as the Stern–Volmer quotient (Fm/F′m − 1). Each treatment comprised five replicates.
Determination of chla
Chla content was determined by grinding 50 mg fresh mass lichen material in liquid nitrogen followed by extraction in 5 mL CaCO3-saturated dimethylsulfoxide. Samples were incubated in a water bath at 60 °C for 40 min, centrifuged at 1500 g for 6 min, and chla measured spectrophotometrically using equations in Palmqvist and Sundberg (2002). Each treatment comprised five replicates.
Heat production measurements
Heat production was measured using a differential dark microcalorimeter (LKB -2277 BioActivity Monitor, Thermometric AB, Jarfalla, Sweden) with a sensitivity of 100 μW in 3·0 cm3 glass vessels. Each treatment comprised three or four replicates of two 6-mm discs. Each point represents an average from 400 digital measurements performed with frequency 0·1 s. Data were analysed using the ‘Spline’ program of Hunt and Parsons (1974).
Pretreatments
Control material comprised lichens stored fully hydrated at a PPFD of 75 µmol photons m−2 s−1 of continuous fluorescent light at 15 °C. The other pretreatments were partial dehydration and treatment with ABA as described by Beckett (1999). Briefly, for the partial dehydration pretreatment, initially fully hydrated lichens were stored at 100 % relative humidity (stored on dry filter paper suspended above distilled water in a desiccator) as above for 3 d. After 3 d of storage this way, lichens reached a RWC of approx. 0·65. They were then fully hydrated by storage on wet filter paper for 1 d at a PPFD of 75 µmol photons m−2 s−1 of continuous fluorescent light at 15 °C. In the ABA treatment, ABA (± cis-trans; Sigma, St Louis, MO, USA) was dissolved in a drop of 1 m NaOH, and the pH of the resulting solution adjusted to 5·6 with HCl. Lichens were pretreated by gently shaking them in 100 μm ABA for 1 h, and then storing them hydrated as above for 3 d.
Desiccation and rehydration cycle
After pretreatment, all lichens were placed in 2 × 5 cm specimen bottles in a desiccator over silica gel at 15 °C and a PPFD of 75 µmol photons m−2 s−1 under continuous fluorescent light. After 1 d the RWC reached approx. 0·02 and did not decline further. After 15 and 30 d the lichens were suddenly rehydrated by addition of 10 mL distilled water. Photosynthesis and respiration were measured in lichens at the start of the experiment, after application of pretreatments, after desiccation, then at intervals during the 8 h following rehydration. Chlorophyll fluorescence parameters and the concentrations chla were measured in control material and material treated with ABA at these same times. Microcalorimetric measurements were made in untreated and ABA-treated material, following desiccation over silica gel for 2·5 h, after which time the RWC of the lichens had declined to approx. 0·05, and during rehydration following desiccation. Fluorescence parameters, chla concentrations and microcalorimetric measurements were not determined in lichens given a partial dehydration pretreatment, because results for net photosynthesis and respiration showed that partial dehydration had almost the same effect as ABA pretreatment. Lichens were normally rehydrated under dim laboratory lighting (aprox. 5 µmol photons m−2 s−1). However, in the experiments that measured respiration and heat production, lichens were rehydrated in complete darkness. For measurement of net photosynthesis, during the 10 min while actual readings of photosynthesis were taking place, lichens were exposed to 350 µmol photons m−2 s−1. During the measurement of chlorophyll fluorescence parameters lichens were exposed to 33 µmol photons m−2 s−1 for 5 min. In the experiments in which photosynthesis, chlorophyll fluorescence and heat production were measured, the same individual replicates of material were used for each measurement throughout the entire experiment. In the experiments in which chla concentrations were measured, different material was assayed at each sampling time, because of the destructive nature of the assay.
Statistical analysis
All data were statistically analysed using a two-way analysis of variance (ANOVA) with pretreatment and time as factors, and the significance of pretreatment tested using Fisher's least significant difference test (Gomez and Gomez, 1983).
Results
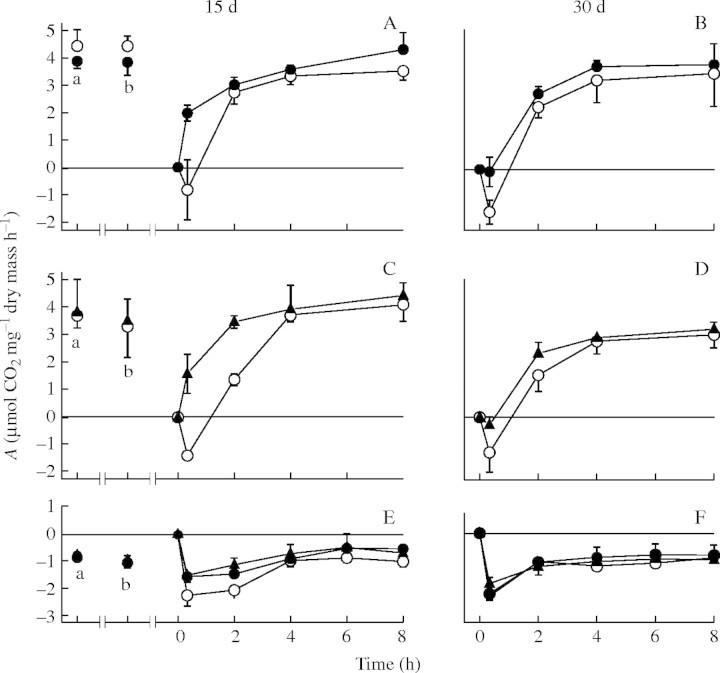
The initial rates of net photosynthesis in P. polydactylon were approx. 4 µmol CO2 mg−1 dry mass h−1, and these rates were not affected by partial dehydration or ABA (Fig. 1A and C). During rehydration after desiccation for 15 d, control material initially (after 20 min) displayed a net release of CO2, but after 2 h regained net CO2 fixation. Photosynthesis had fully recovered after 4 h. By contrast, after the same desiccation time, lichens subjected to a partial dehydration or ABA pretreatment displayed significant net photosynthesis after rehydration for 20 min, and recovered to initial rates faster than untreated material (Fig. 1A and C). ANOVA analyses showed that the effects of these two pretreatments were highly significant (P < 0·01). During rehydration following desiccation for 30 d, control material again initially displayed a net release of CO2, and photosynthesis had fully recovered after 4 h (Fig. 1B and D). The beneficial effects of partial dehydration or ABA were mostly lost after desiccation for 30 d (ANOVA analyses indicating that the effects of pretreatment were not significant; P < 0·05). However, compared with the controls, these lichens tended to display lower net losses of CO2 during the early stages of rehydration. The initial respiration rates of P. polydactylon were slightly less than 1 µmol CO2 mg−1 dry mass h−1, and were not affected by partial dehydration or ABA (Fig. 1E). During rehydration following desiccation for 15 d, respiration in control material initially increased to approx. 2 µmol CO2 mg−1 dry mass h−1, but after 4 h decreased to control rates. Partially dehydrated or ABA-pretreated material tended to display smaller increases in respiration, to approx. 1·5 µmol CO2 mg−1 dry mass h−1 (Fig. 1E), although ANOVA analysis indicated that rates did not differ significantly from control material. After rehydration for 4 h, the respiration rates of these lichens, like those for the controls, returned to their initial values. After desiccation for 30 d, respiration in material from all treatments initially increased to approx. 2 µmol CO2 mg−1 dry mass h−1, and then returned to initial values within 4 h (Fig. 1F).
Fig. 1.
The effect of desiccation for 15 and 30 d followed by rehydration on photosynthesis in Peltigera polydactylon pretreated with partial dehydration (A and B) or 100 μM ABA (C and D), and the effects of these two pretreatments on respiration (E and F). a, After collection from the field; b, following 3-d pretreatment or maintenance moistened with distilled water (‘control’). Time 0 indicates the start of rehydration. In A and C the effects of pretreatment were significant (P < 0·001 and 0·002, respectively), and in the other graphs the effects of pretreatments were not significant. Open circles, control material; closed circles, material pretreated by partial dehydration; closed triangles, ABA-pretreated material; error bars denote the standard deviation, n = 5.
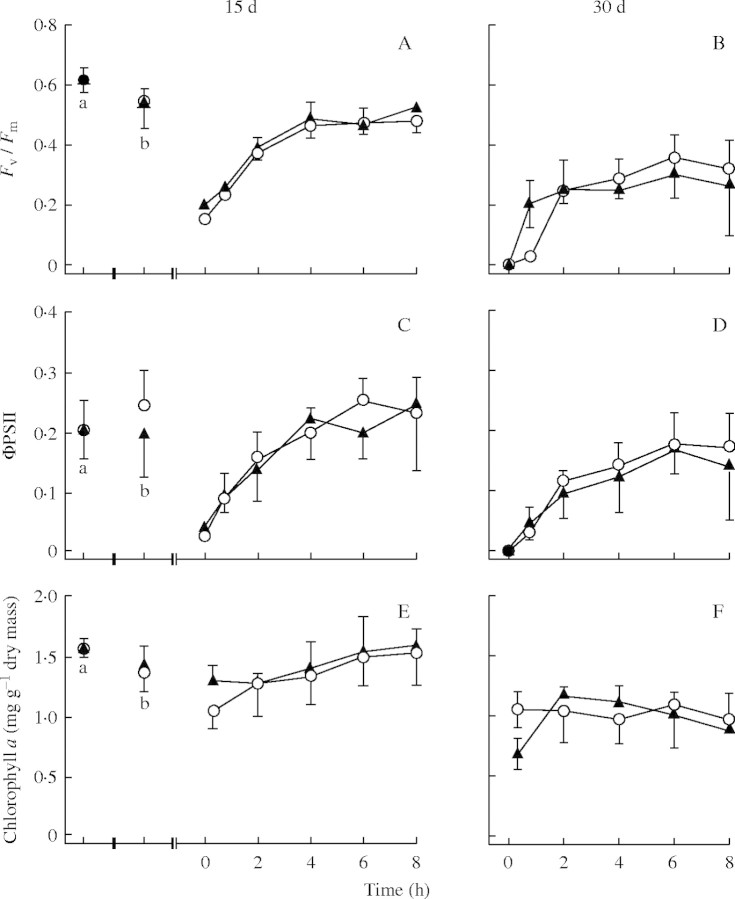
During 3 d of storage following water (control) or ABA pretreatment, the maximum photochemical efficiency of photosystem II (Fv/Fm) declined slightly from approx. 0·6 to approx. 0·55 (compare points a and b in Fig. 2A). Desiccation reduced Fv/Fm to approx. 0·2 after 15 d, and to almost 0 after 30 d (Fig. 2A and B). During rehydration following desiccation for 15 d Fv/Fm recovered to approx. 0·5 after 4 h, but did not increase further by 8 h (Fig. 2A). Control and ABA-pretreated material recovered similarly. Following desiccation for 30 d, even after 8 h the recovery of Fv/Fm was only partial, to approx. 0·3, or half the initial value (Fig. 2B). ABA-pretreated material tended to recover faster than control during the first 20 min of rehydration, but after 2 h no differences existed between these treatments. The actual quantum yield of PSII (ΦPSII) was approx. 0·2 (Fig. 2C). Desiccation reduced ΦPSII to zero. During rehydration following desiccation for 15 d, ΦPSII gradually increased, recovering to initial values after 6 h (Fig. 2C). After desiccation for 30 d, ΦPSII recovered more slowly, and after 8 h had only recovered to approx. 0·15, or about three-quarters of the initial values (Fig. 2D). ANOVA analysis indicated that ABA had no significant effect on the recovery of Fv/Fm or ΦPSII during rehydration following desiccation for 15 or 30 d. NPQ was almost zero in all treatments (data not shown).
Fig. 2.
The effect of ABA on the maximum quantum efficiency (Fv/Fm; A and B), the actual quantum efficiency (ΦPSII; C and D) of PSII, and chla concentration (E and F) in Peltigera polydactylon before and after desiccation for 15 and 30 d followed by rehydration. a, After collection from field; b, following 3-d pretreatment. Time 0 indicates the start of rehydration. Two-way analysis of variance showed that in all graphs the effects of ABA were not significant. Open circles, control material; closed triangles, ABA-pretreated material; error bars denote the standard deviation, n = 5.
Chla concentrations did not change during pretreatment (Fig. 2E). Desiccation for 15 d reduced chla concentrations by about one-quarter; after rehydration for 8 h they had recovered their initial values. Desiccation for 30 d reduced chla concentrations by about one-third, and they did not recover during rehydration (Fig. 2F). ANOVA showed that the changes of chla concentrations in response to desiccation were the same in ABA and water-treated lichens.
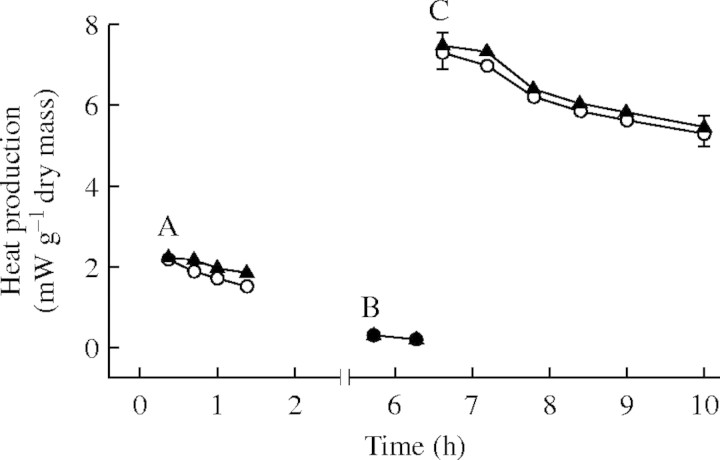
Control and ABA-pretreated lichens produced heat at rates of approx. 2 mW g−1 dry mass. ABA pretreatment induced a small increase in heat production (Fig. 3). After desiccation for approx. 2·5 h, heat production by both control and ABA-pretreated lichens dropped to almost zero. Following rehydration, heat production increased to approx. 8 mW g−1 dry mass and then gradually declined, although production was still approx. 6 mW g−1 dry mass 3 h after rehydration. No significant differences existed in heat production during rehydration between control and ABA-pretreated lichens.
Fig. 3.
The effect of ABA pretreatment on heat production by Peltigera polydactylon before desiccation, while desiccated and during rehydration. (A) Following 3-d pretreatment with ABA or distilled water (control); (B) after desiccation for 3 h to a water content of approx. 0·1; (C) after rehydration. Open circles, control material; closed triangles, ABA-pre treated material. Points represent selected fitted values with 95 % confidence limits, calculated using the ‘Spline’ program of Hunt and Parsons (1974), n = 3 (control) or 4 (ABA-pretreated).
DISCUSSION
Partial dehydration increases the tolerance of carbon fixation to desiccation in Peltigera
Results presented here show that reducing the RWC of the lichen Peltigera polydactylon to approx. 0·65 for a few days increases the tolerance of carbon fixation to desiccation, even if material is stored fully hydrated for 1 d after partial dehydration (Fig. 1). Care is therefore needed when testing the effects of desiccation on lichens, because the hydration state of a sample before measurement can influence tolerance, even if material is maintained fully hydrated before testing. The Peltigera used in this study was from an Afromontane forest, and in such habitats, lichens predictably desiccate during the dry winter months, allowing enough time for the lichens to induce tolerance mechanisms. It is envisaged that lichens from harsher, xeric sites will be found to have largely constitutive desiccation tolerance mechanisms, because if desiccation is rapid and irregular, insufficient time will be available to induce tolerance. However, in P. polydactylon, partial dehydration clearly hardens carbon fixation to the harmful effects of subsequent desiccation stress.
ABA can substitute for partial dehydration
Exogenous application of ABA is as effective at inducing tolerance as partial dehydration (Fig. 1). Presumably, for lichens growing in the field, as for higher plants (Bray, 1997), mild water stress induces ABA biosynthesis, which in turn activates signal transduction pathways that increase tolerance. Only very limited information is available on the role of ABA in lichens, but Dietz and Hartung (1998) showed that ABA occurs in both the mycobiont and the photobiont of a range of lichens, including cyanobacterial species. Dietz and Hartung (1999) later used chlorophyll fluorescence to show that ABA pretreatment does not increase the desiccation tolerance of PSII in Xanthoria parietina, Hypogymnia physodes or Peltigera praetextata. While the present results on PSII activity agree with those of Dietz and Hartung (1999) (Fig. 2C and D), they also show that ABA can protect net photosynthesis (Fig. 1). Apart from measuring different parameters, Dietz and Hartung (1999) desiccated their lichens for 3 months after ABA pretreatment. The present results show that the beneficial effects of ABA pretreatment become less with time, decreasing considerably from 15 to 30 d of desiccation (Fig. 1). The difference in the duration of desiccation may explain why Dietz and Hartung found no beneficial effect of ABA pretreatment, while in the present study ABA hardened lichens to desiccation.
Compared with the little information available on ABA in lichens, more is known about its occurrence and function in free-living cyanobacteria and fungi. In cyanobacteria, ABA application can affect heterocyst frequency, Ca2+ transport and nitrogenase activity (Huddart et al., 1986; Marsálek and Simek, 1992; Pandey et al., 1996). Close and Lammers (1993) showed that while ABA is present in the cyanobacterium Anabaena, dehydration does not promote its synthesis, and exogenous ABA applications do not stimulate the synthesis of dehydrin proteins. Dehydrin proteins are often believed to be involved in desiccation tolerance in seeds and poikilohydric angiosperms (Black and Pritchard, 2002). However, it would be unwise to conclude that ABA has no role in desiccation tolerance in cyanobacteria based on the single report of Close and Lammers (1993) and, moreover, the photobiont of P. polydactylon is Nostoc rather than Anabaena. Some fungi contain ABA (Dörffling et al., 1984), although it appears to be synthesized by a different pathway to higher plants (Hirai et al., 2000). The widespread occurrence of ABA in free-living cyanobacteria and fungi is consistent with the present finding that this hormone may have an important role in desiccation tolerance in Peltigera.
Desiccation tolerance of the photobiont rather than the mycobiont is increased
In P. polydactylon, partial dehydration and ABA treatment probably improve the desiccation tolerance of the photobiont more than that of the mycobiont. While these pretreatments significantly increased the recovery of net photosynthesis during rehydration following desiccation, they had little significant effect on respiration and heat production, parameters that mostly indicate fungal metabolism (Figs 1E and F and 3). By volume, the fungus comprises 90 % of a lichen thallus (Collins and Farrar, 1978), and it seems likely that respiration and heat production largely originate from the mycobiont. The small effect of partial dehydration and ABA pretreatments on respiration also indicates that the improved recovery of net photosynthesis during rehydration is genuine, and cannot be explained simply by a reduction in the respiratory burst. However, recovery of respiration and heat production following desiccation are probably rather insensitive measures of fungal tolerance. In future studies more sensitive assays for fungal metabolism, e.g. rates of eukaryotic protein synthesis, will be used to test whether hardening can increase the desiccation tolerance of the mycobiont.
The mechanisms involved in the improved rates of recovery of net photosynthesis during rehydration following desiccation in the lichens receiving partial dehydration or ABA pretreatments remain unclear. These treatments do not appear to preserve electron transport (Fig. 2A–D) or promote chlorophyll retention during desiccation (Fig. 2E and F). The moderate chlorophyll losses that occur during desiccation were probably the result of photooxidation of chlorophyll, as lichens were desiccated at a PPFD of 75 µmol photons m−2 s−1 (Proctor and Tuba, 2002). It would appear unlikely that hardening treatments could influence the rate of chlorophyll photooxidation. Kranner et al. (2003) also reported some chlorophyll loss in Peltigera during desiccation for several weeks, followed by recovery within hours during rehydation. The ability of Peltigera to relatively rapidly re-synthesize chlorophyll merits further investigation. As discussed in the Introduction, differences in the desiccation tolerance of lichen species from contrasting habitats have been suggested to be related to an ability to maintain effective concentrations of enzymic and non-enzymic antioxidants during stress (Mayaba and Beckett, 2001; Kranner, 2002; Kranner et al., 2003). In future work, it is the intention to test if hardening treatments can maintain the efficiency of antioxidant systems in Peltigera during desiccation and rehdyration cycles.
Significance of the burst of heat production during rehydration
Rehydration following desiccation induced a large, sustained increase in heat production in Peltigera (Fig. 3). During the first few minutes of rehydration, some of the heat produced will be generated by the dissolving of intracellular ions and rehydration of proteins. This heat is not reflected in Fig. 3 because, to rehydrate the lichens, the sample chambers from the microcalorimeter had to be temporarily removed, and measurements could not be resumed for 15 min. However, such physico-chemical heat production will only be short lived, and cannot explain the continued high rate of heat production observed.
Production of heat following stress in fungi and other organisms is generally considered to result from the controlled uncoupling of respiration in mitochondria. Uncoupling can occur, firstly, via the alternative oxidase that dissipates the redox potential, and, secondly, via uncoupling proteins that dissipate the proton motive force (for reviews, see Jarmuszkiewcz, 2001; Hourton-Cabassa et al., 2004). While stress disrupts normal mitochondrial function and increases superoxide production (Møller, 2001), uncoupling electron flow from phosphorylation protects cells by reducing the formation of harmful ROS (Skulachev, 1998). In lichens, possession of efficient alternative oxidase and protein uncoupling systems may be an essential component of desiccation tolerance, because they will reduce mitochondrial superoxide synthesis during rehydration following desiccation. Surprisingly, the data presented here appear to be the first to describe heat production by lichens. While ABA pretreatment does not affect heat production (Fig. 3), microcalorimetry may be a useful tool for future studies on desiccation tolerance.
Conclusions
Although constitutive desiccation tolerance mechanisms are probably essential in lichens growing habitats in which they experience sudden and severe stress, these mechanisms are present even when not needed, and at these times divert energy away from growth and reproduction. In less harsh habitats, in which desiccation is preceded by a period of more moderate stress, selection may have favoured inducible tolerance mechanisms. In P. polydactylon, the physiology of hardened and non-hardened lichens can readily be compared. When studying desiccation tolerance, this may be a more useful approach than comparing the physiology of dissimilar species, differing in tolerance, because it can be difficult to know which metabolic components contribute to tolerance. Future work needs to focus on precisely how partial dehydration and ABA pretreatments protect net photosynthesis from desiccation. Improved net photosynthesis during rehydration will directly benefit the photobiont, but because the mycobiont depends upon the photobiont for a supply of carbohydrates, hardening will benefit the whole lichen thallus.
Supplementary Material
Acknowledgments
This study was funded by the University of KwaZulu-Natal Research Fund, the Russian Foundation for Basic Research (grant number 03-04-48671) and Presidium of RAS, Program of Molecular and Cellular Biology. We gratefully acknowledge the financial support of the South African–Russian bilateral agreement for scientific collaboration for travel for F.V.M., and the National Research Foundation for a bursary for N.M. We thank I. Kranner for valuable discussions.
LITERATURE CITED
- Beckett RP. 1999. Partial dehydration and ABA induce tolerance to desiccation induced ion leakage in the moss Atrichum androgynum South African Journal of Botany 65: 212–217. [Google Scholar]
- Beckett RP. 2002. Determination of the water potential and its components in lichens. In: Kranner I, Beckett RP, Varma AK, eds. Protocols in lichenology. Berlin: Springer-Verlag, 236–255. [Google Scholar]
- Black M, Pritchard HW. 2002.Desiccation and survival in plants: drying without dying. Wallingford, UK: CABI Publishing. [Google Scholar]
- Bray EA. 1997. Plant responses to water deficit. Trends in Plant Science 2: 48–54. [Google Scholar]
- Campbell D, Hurry V, Clarke AK, Gustafsson P, Oquist G. 1998. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiology and Molecular Biology Reviews 62: 667–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close TJ, Lammers PJ. 1993. An osmotic stress protein of cyanobacteria is immunologically related to plant dehydrins. Plant Physiology 101: 773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CR, Farrar JF. 1978. Structural resistances to mass transfer in the lichen Xanthoria parietina New Phytologist 81: 71–83. [Google Scholar]
- Dietz S, Hartung W. 1998. Abscisic acid in lichens: variation, water relations and metabolism. New Phytologist 138: 99–106. [Google Scholar]
- Dietz S, Hartung W. 1999. The effect of abscisic acid on chlorophyll fluorescence in lichens under extreme water regimes. New Phytologist 143: 495–501. [DOI] [PubMed] [Google Scholar]
- Dörffling K, Petersen W, Sprecher E, Ursbasch I, Hanssen HP. 1984. Abscisic acid in phytopathogenic fungi of the genera Botrytis, Ceratocystis, Fusarium and Rhizoctonia Zeitschrift fur Naturforschung 39C: 683–684. [Google Scholar]
- Gomez KA, Gomez AA. 1983.Statistical procedures for agricultural research, 2nd edn. New York: John Wiley. [Google Scholar]
- Hartung W, Schiller P, Dietz KJ. 1997. The physiology of poikilohydric plants. Progress in Botany 59: 299–327. [Google Scholar]
- Hirai N, Yoshida R, Todoroki Y, Ohigashi H. 2000. Biosynthesis of abscisic acid by the non-mevalonate pathway in plants, and by the mevalonate pathway in fungi. Bioscience Biotechnology and Biochemistry 64: 1448–1458. [DOI] [PubMed] [Google Scholar]
- Hourton-Cabassa C, Rita Matos A, Zachowski A, Moreau F. 2004. The plant uncoupling protein homologues: a new family of energy dissipating proteins in plant mitochondria. Plant Physiology and Biochemistry 42: 283–290. [DOI] [PubMed] [Google Scholar]
- Huddart H, Smith RJ, Langton P, Hetherington AM, Mansfield, TA. 1986. Is abscisic acid a universal calcium antagonist? New Phytologist 104: 161–173. [DOI] [PubMed] [Google Scholar]
- Hunt R, Parsons IT. 1974. A computer program for deriving growth functions in plant growth analysis. Journal of Applied Ecology 11: 297–307. [Google Scholar]
- Jarmuszkiewicz W. 2001. Uncoupling proteins in mitochondria of plants and some microorganisms. Acta Biochimica Polonica 48: 145–155. [PubMed] [Google Scholar]
- Kershaw KA. 1985.Physiological ecology of lichens. Cambridge: Cambridge University Press. [Google Scholar]
- Kranner I. 2002. Glutathione status correlates with different degrees of desiccation tolerance in three lichens. New Phytologist 154: 451–460. [DOI] [PubMed] [Google Scholar]
- Kranner I, Zorn M, Turk B, Wornik S, Beckett RP, Batic F. 2003. Biochemical traits of lichens differing in relative desiccation tolerance. New Phytologist 160: 167–176. [DOI] [PubMed] [Google Scholar]
- Marsálek B, Simek M. 1992. Abscisic acid and its synthetic analog in relation to growth and nitrogenase activity of Azotobacter chroococcum and Nostoc muscorum Folia Microbiologica (Praha) 37: 159–160. [DOI] [PubMed] [Google Scholar]
- Mayaba N, Beckett RP. 2001. The effect of desiccation on the activities of antioxidant enzymes in lichens from habitats of contrasting water status. Symbiosis 31: 113–121. [Google Scholar]
- Møller IM. 2001. Plant mitochondria and oxidative stress. Electron transport, NADPH turnover and metabolism of reactive oxygen species. Annual Review of Plant Physiology and Plant Molecular Biology 52: 561–591. [DOI] [PubMed] [Google Scholar]
- Oliver MJ, Wood AJ, O'Mahony P. 1998. ‘To dryness and beyond’: preparation for the dried state and rehydration in vegetative desiccation tolerant plants. Plant Growth Regulation 24: 193–201. [Google Scholar]
- Palmquist K, Sundberg B. 2002. Characterising photosynthesis and respiration in freshly isolated or cultured lichen photobionts. In: Kranner I, Beckett RP, Varma AK, eds. Protocols in lichenology. Berlin: Springer-Verlag, 162–181. [Google Scholar]
- Pandey PK, Singh BB, Mishra R, Bisen PS. 1996. Ca2+ uptake and its regulation in the cyanobacterium Nostoc MAC. Current Microbiology 32: 332–335. [DOI] [PubMed] [Google Scholar]
- Proctor MCF, Tuba M. 2002. Poikilohydry and homoihydry: antithesis or spectrum of possibilities? New Phytologist 156: 327–349. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Bilger W. 1993. Progress in chlorophyll-fluorescence research: major developments during the past years in retrospect. Progress in Botany 54: 151–173. [Google Scholar]
- Schreiber U, Harmann H, Neubauer C, Klughammer C. 1995. Assessment of PSII photochemical quantum yield by chlorophyll fluorescence quenching analysis. Australian Journal of Plant Physiology 22: 209–220. [Google Scholar]
- Skulachev VP. 1998. Uncoupling: new approaches to an old problem of bioenergetics. Biochimica et Biophysica Acta 1363: 100–124. [DOI] [PubMed] [Google Scholar]
- Zorn M, Pfeifhofer HW, Grill D, Kranner I. 2001. Responses of plastid pigments to desiccation and rehydration in the desert lichen Ramalina maciformis Symbiosis 31: 201–211. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.