Abstract
• Background and Aims Selaginella is the largest genus of heterosporous pteridophytes, but karyologically the genus is known only by the occurrence of a dysploid series of n = 7–12, and a low frequency of polyploids. Aiming to contribute to a better understanding of the structural chromosomal variability of this genus, different staining methods were applied in species with different chromosome numbers.
• Methods The chromosome complements of seven species of Selaginella were analysed and, in four of them, the distribution of 45S rDNA sites was determined by fluorescent in situ hybridization. Additionally, CMA/DA/DAPI and silver nitrate staining were performed to investigate the correlation between the 45S rDNA sites, the heterochromatic bands and the number of active rDNA sites.
• Key Results The chromosome numbers observed were 2n = 18, 20 and 24. The species with 2n = 20 exhibited chromosome complement sizes smaller and less variable than those with 2n = 18. The only species with 2n = 24, S. convoluta, had relatively large and asymmetrical chromosomes. The interphase nuclei in all species were of the chromocentric type. CMA/DA/DAPI staining showed only a weak chromosomal differentiation of heterochromatic bands. In S. willdenowii and S. convoluta eight and six CMA+ bands were observed, respectively, but no DAPI+ bands. The CMA+ bands corresponded in number, size and location to the rDNA sites. In general, the number of rDNA sites correlated with the maximum number of nucleoli per nucleus. Ten rDNA sites were found in S. plana (2n = 20), eight in S. willdenowii (2n = 18), six in S. convoluta (2n = 24) and two in S. producta (2n = 20).
• Conclusions The remarkable variation in chromosome size and number and rDNA sites shows that dramatic karyological changes have occurred during the evolution of the genus at the diploid level. These data further suggest that the two putative basic numbers of the genus, x = 9 and x = 10, may have arisen two or more times independently.
Keywords: Pteridophyta, Selaginella, chromosome number, fluorochrome staining, silver nitrate staining, in situ hybridization, 45S rDNA
INTRODUCTION
Selaginella P. Beauv. is a heterosporous genus of the family Selaginellaceae, comprising approx. 750 species distributed throughout the tropical regions, including 250 species in the Americas (Tryon and Tryon, 1982; Kramer and Green, 1990). They are generally found in humid and shady forests, although they can also be present in dry forests, swamps, on damp rocks along riverbanks or near waterfalls, and even in cold regions such as the Alps, or in rocky deserts (Tryon and Tryon, 1982).
Polyploidy and hybridization have occurred numerous times in the evolution of the pteridophytes, resulting in the characteristically high chromosome numbers seen in this group (Walker, 1984). Nonetheless, high chromosome numbers are rare among the heterosporous pteridophytes. Selaginella, the largest heterosporous genus, has only a few polyploid species, although a large dysploid variation has been reported. The chromosome number ranges between 2n = 14 and 2n = 60, and different authors have reported different basic chromosome numbers for the genus: x = 7, 8, 9, 10, 11 and 12 (Kuriachan, 1963; Jermy et al., 1967; Takamiya, 1993).
In spite of several cytotaxonomical studies, the karyological evolution of this group remains poorly understood. Cytological investigations have been based primarily on chromosome counts and, in a few cases, on chromosome morphology. Meanwhile, in angiosperms and gymnosperms the karyological studies in the last two decades have turned to more specific methods such as banding methods and fluorescent in situ hybridization (FISH) (Greilhuber, 1995; Stace and Bailey, 1999). Heterochromatic bands in plants have been analysed mainly by C-banding or by base-specific fluorochrome staining (Guerra, 2000). Fluorochrome banding has the advantage of being a simpler, more reproducible and less destructive method, as compared with C-banding (Guerra, 1993). The fluorochromes chromomycin A (CMA) and 4′,6-diamidino-2-phenylindole (DAPI) exhibit preferential staining for GC and AT-rich DNA sequences, respectively, allowing the identification of different types of heterochromatin. Distamycin A (DA) has also been used to enhance the contrast between CMA and DAPI (Schweizer and Ambros, 1994; Marcon et al., 2003). The 45S rDNA sites are generally positively stained with CMA and negatively stained with DAPI. In many species, the rDNA sites are the only positively stained CMA bands (see, for example, Melo and Guerra, 2003). The variation in number and position of the rDNA sites has been demonstrated to be an additional important karyotype feature to the cytotaxonomic analyses of some angiosperm genera, such as Clivia (Ran et al., 1999) and Sanguisorba (Mishima et al., 2002). Silver nitrate staining is another method that allows the visualization of nucleolus organizer regions (NORs), which are also related to chromosomal secondary constrictions and rDNA sites, but after the silver nitrate staining only those sites activated in an interphase nucleus are stained in the subsequent prophase or metaphase (Moscone et al., 1995). In general, the maximum number of silver-stained sites in prophase or metaphase chromosomes and the maximum number of nucleoli in interphase nuclei correspond to the number of 45S rDNA sites (see, for example, Moscone et al., 1995). This relationship seems to be true also for homosporous pteridophytes (Kawakami et al., 1999; Marcon et al., 2003).
In order to better understand the cytogenetics and evolution of this genus, the chromosome numbers were analysed in seven Brazilian species of Selaginella and in four of them the distribution of 45S rDNA sites was observed. Additionally, the heterochromatic blocks stained with CMA/DA/DAPI and the number of nucleoli per nucleus were investigated, as an indication of the number of NORs. The results are discussed in relation to the chromosomal evolution of the genus.
MATERIALS AND METHODS
The species investigated, with their respective collection sites, chromosome numbers and previous chromosome counts are listed in Table 1. Part of the material collected was prepared for the herbarium, and the vouchers were deposited in the UFP herbarium (Federal University of Pernambuco, Brazil). Another part of the material was maintained at the experimental garden of the Department of Botany, at the Federal University of Pernambuco, for cytogenetic analysis.
Table 1.
Species of Selaginella analysed, with their respective voucher number, provenance, chromosome number and previous counting
| Species |
Voucher number |
Provenance |
Chromosome number (2n) |
Previous countings (2n) |
References |
|---|---|---|---|---|---|
| S. muscosa Spring | 36588 | Bonito | 18 | – | – |
| S. simplex Baker | 36412 | Gravatá | 18 | – | – |
| S. willdenowii (Desv. ex Poiret) Baker | 36589 | Recife | 18 | 18 | Jermy et al. (1967); Fabri (1963); Kuriachan (1963) |
| Selaginella sp. | – | Itamaracá | 18 | – | – |
| S. producta Baker | 3641036411 | PaulistaIgarassú | 2020 | – | – |
| 36591 | Bonito | 20 | – | – | |
| S. plana (Desv. ex Poiret) Hieron | 36409 | Recife | 20 | 20 | Jermy et al. (1967); Fabri (1963); Kuriachan (1963) |
| 36590 | Rio de Janeiro | 20 | 20 | Jermy et al. (1967); Fabri (1963); Kuriachan (1963) | |
| S. convoluta (Arnott) Spring | 36408 | Petrolina | 24 | – | – |
All the samples were collected in the Brazilian state of Pernambuco, except S. plana, which is from the state of Rio de Janeiro.
Leaf buds were collected and pretreated in 0·002 m 8-hydroxyquinoline for 1 h at room temperature, followed by 23 h at 10 °C. Subsequently, they were fixed in Carnoy (ethanol : acetic, acid 3 : 1) for 24 h at room temperature, and stored at −20 °C.
For conventional chromosome staining, the fixed leaf buds were washed twice in distilled water for 5 min, hydrolysed in 5 n HCl for 30 min, and then the meristem was isolated and squashed in 45 % acetic acid (Guerra, 1983). The preparation was stained with Giemsa 5 % and mounted with Entellan. Chromosome measurements were performed on amplified photographs with the aid of a pachymeter.
The triple staining with the fluorochromes CMA, DAPI and DA was performed according to Schweizer and Ambros (1994). Leaf buds were washed twice in distilled water for 5 min, digested in 2 % cellulase/20 % pectinase for 2 h at 37 °C, and squashed in 45 % acetic acid. After coverslip removal the slides were aged for 3 d at room temperature, stained with CMA (0·5 mg mL−1, 1 h), counterstained with distamycin A (0·1 mg mL−1, 30 min), stained with DAPI (2 µg mL−1, 30 min) and mounted in McIlvaine's (pH 7·0) buffer–glycerol (v/v, 1 : 1) containing 2·5 mm MgCl2. CMA/DAPI staining without distamycin A produced a poorer differentiation.
Leaf buds without pretreatment were fixed directly into Carnoy and used for silver nitrate staining. Slides were prepared using the same enzyme treatment employed for fluorochromes, except that they were not aged before staining. A small drop of 50 % silver nitrate diluted in formic acid was placed over the cells, covered with a coverslip and incubated in a moist chamber at 60 °C (Rufas et al., 1987) for approx. 10 min or until adequate staining was attained.
To locate the 45S rDNA sites, probes SK18S and SK25S were used, containing 18S and 25S rDNA of Arabidopsis thaliana (Unfried et al., 1989; Unfried and Gruendler, 1990), kindly provided by Prof. D. Schweizer of the University of Vienna, and labelled by nick translation with biotin-11-dUTP (Sigma) or digoxigenin-11-dUTP (Roche). The probe was detected with mouse anti-biotin monoclonal antibody (Dakopatts no. M743) and visualized with rabbit anti-mouse antibody conjugated to tetramethyl rhodamine isothiocyanate (TRITC) (Dakopatts no. R270) or detected with sheep anti-digoxigenin antibody conjugated to fluorescein isothiocyanate (FITC) (Boehringer Mannheim no.1207741) and FITC-conjugated rabbit anti-sheep (Dakopatts F135, DAKO). The technique was based on Moscone et al. (1996), with some modifications (denaturation at 80 °C and the post-hybridization washes in 0·1 × SSC at 42 °C). The hybridization mixture contained 60 % formamide, 5 % dextran sulfate, 2 × SSC, 0·01 % salmon sperm DNA, and probe at a final concentration of 1·2–3·0 ng µL−1. In most hybridization experiments, a 5S rDNA probe obtained from total genomic DNA of Passiflora edulis Sims (Melo and Guerra, 2003) was added to the mixture but no clear signal was observed. Slides were stained with DAPI (2 µg mL−1) and mounted in Vectashield (Vector).
The best cells were captured with a CCD Cohu camera on a Leica DMLB microscope, or photographed using Kodak Imagelink HQ ASA 25 film for bright field, or Kodak ASA 400 film for fluorescence photography.
RESULTS
The seven species of Selaginella analysed in the present work showed the following chromosome numbers: 2n = 18 [S. muscosa Spring, S. simplex Baker, S. willdenowii (Desv. ex Poiret) Baker and Selaginella sp.], 2n = 20 [S. producta Baker and S. plana (Desv. ex Poiret) Hieron.] and 2n = 24 [(S. convoluta (Arnott) Spring] (Fig. 1A–G).
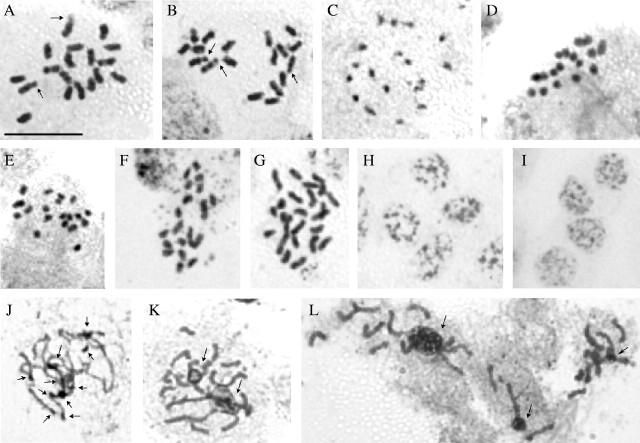
Fig. 1.
Chromosome complement (A–G), interphase nuclei (H and I) and number of NORs and nucleoli (J–L) observed in species of Selaginella: (A) S. willdenowii (2n = 18) with two satellites (arrows); (B) Selaginella sp. (2n = 18) with three satellites (arrows); (C) S. simplex (2n = 18); (D) S. muscosa (2n = 18); (E) S. plana (2n = 20); (F) S. producta (2n = 20); (G) S. convoluta (2n = 24); (H and I) chromocentric interphase nuclei of S. plana and S. willdenowii, respectively; (J) prometaphase cell showing NORs (arrows); (K and L) prometaphase cells showing chromosomes associated with nucleoli (arrows) in S. plana (K) and S. convoluta (L). The bar in A = 5 µm.
Among the species with 2n = 18, S. willdenowii and Selaginella sp. exhibited the largest chromosomes, most of them meta- to submetacentric. The chromosomes in S. willdenowii varied between 1·44 and 2·48 µm (Fig. 1A), and showed up to six satellites: two on the short arms and four on the long arms of submetacentric chromosomes. In Selaginella sp. the chromosome size varied between 1·35 and 2·32 µm and three satellites were observed: two on a metacentric pair, and a third on a submetacentric chromosome (Fig. 1B). On the other hand, S. simplex and S. muscosa had much smaller chromosomes, varying, respectively, between 0·85 and 1·26 µm and 0·80 and 1·62 µm (Fig. 1C and D).
The species with 2n = 20 had smaller chromosomes, ranging from 0·70 to 1·04 µm in S. plana, and 0·78 to 1·43 µm in S. producta (Fig. 1E and F). The position of the centromere could not be observed in any of these four species, mainly due to the small size of their chromosomes. Satellites were also not clearly identified.
In the only species with 2n = 24, S. convoluta, the morphology of the chromosomes seemed to vary from metacentric to acrocentric. Chromosome size varied between 0·70 and 1·77 µm and satellites were not observed (Fig. 1G).
In all species analysed, the interphase nucleus structure was granulated, with small chromocentres, corresponding to the chromocentre type, according to the classification of Tanaka (1971). Some species showed larger and more sharply outlined chromocentres (Fig. 1H and I).
Staining with silver nitrate revealed that the maximum number of nucleoli per nucleus varied among species (Table 2). In S. producta the maximum number of nucleoli per nucleus was two, whereas in the other three species investigated, the maximum number ranged between six and ten, although most nuclei exhibited only three nucleoli. Selaginella willdenowii exhibited a variation of one to ten nucleoli per interphase nucleus and up to ten NORs were observed in prometaphase (Fig. 1J). In S. convoluta and S. plana, it was not possible to identify NORs, but six to ten prophase or prometaphase chromosomes associated with nucleoli were observed (Fig. 1K and L).
Table 2.
Variation in number of chromosomes, rDNA sites and nucleoli per nucleus in four species of Selaginella
| Variation in the number of nucleoli per nucleus |
|||||||
|---|---|---|---|---|---|---|---|
| Species |
2n |
Number of rDNA sites |
No. of cells analysed |
Range |
Most frequent numbers |
||
| S. willdenowii | 18 | 8 | 1015 | 1–10 | 3 (29·5 %) and 4 (21·1 %) | ||
| S. plana | 20 | 10 | 800 | 1–10 | 2 (27·8 %) and 3 (31·7 %) | ||
| S. producta | 20 | 2 | 308 | 1–2 | 1 (94·2 %) | ||
| S. convoluta | 24 | 6 | 528 | 1–6 | 2 (28·0 %) and 3 (35·7 %) | ||
A high number of metaphase cells was obtained from S. willdenowii, S. plana, S. producta and S. convoluta making it possible to analyse the chromosomes with fluorochromes and in situ hybridization. Staining with CMA/DA/DAPI revealed differentiation of bands only in S. willdenowii and S. convoluta. The chromosomes of the other species stained deeply and homogeneously with both CMA and DAPI−, even when counterstained with distamycin A. In prometaphase cells of S. willdenowii, up to eight CMA+/DAPI− terminal bands were observed (Fig. 2A and B). In S. convoluta (2n = 24), six terminal CMA+/DAPI− bands were localized in three chromosome pairs (Fig. 2D and E). No DAPI+ bands were observed in these two species, while the CMA+ bands were often negatively stained with DAPI.
Fig. 2.
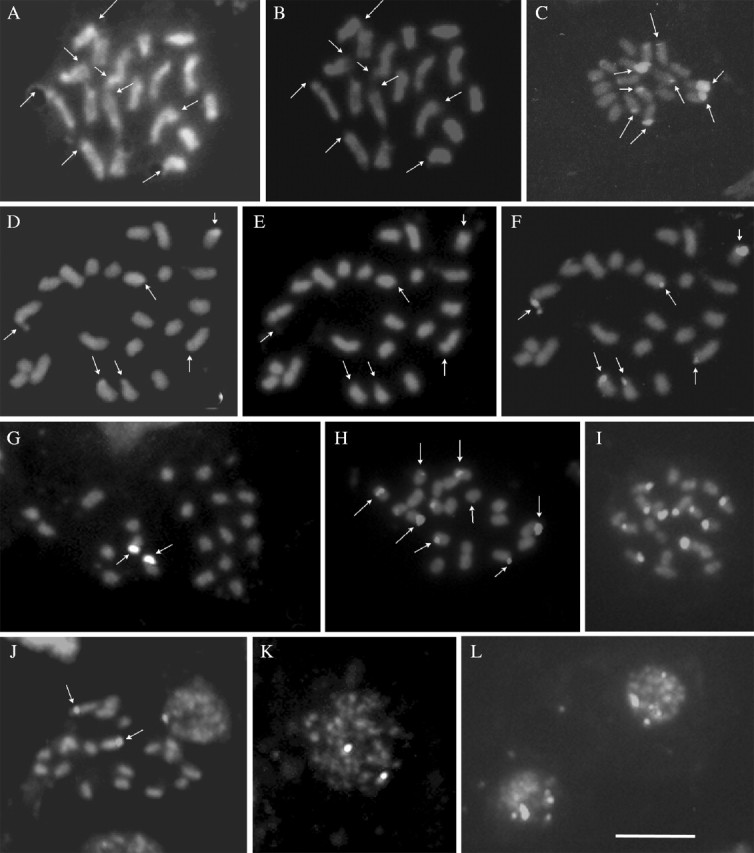
Distribution of the CMA/DAPI bands and 45S rDNA sites in species of Selaginella: S. willdenowii (A and B) and S. convoluta (D and E), stained with CMA (A and D) and DAPI (B and E), respectively. Arrows in A, B, D and E indicate the CMA+/DAPI− bands. 45S rDNA sites (C and F–L) in S. willdenowii (C), S. convoluta (F), in two populations of S. producta (G and J), and in two populations of S. plana (H and I). Interphase nuclei of S. producta (K), showing the two sites of 45S rDNA and of S. plana (L), showing up to ten sites. The bar in L = 5 µm.
In situ hybridization of 45S rDNA in chromosomes of S. willdenowii revealed the presence of terminal sites on the long arms of two chromosome pairs and on the short arms of another two chromosome pairs (Fig. 2C). In two populations of S. plana analysed, five submetacentric pairs were labelled terminally: three pairs on the long arms and two pairs on the short arms (Fig. 2H and I). While in one of the populations it was not possible to observe clearly the smaller site, for this stains weakly, in the another population analysed the ten sites of 45S rDNA were clearly labelled (Fig. 2I). In S. convoluta there were four sites on two apparently acrocentric pairs and two sites on a submetacentric pair (Fig. 2F). In S. producta (Fig. 2G and J) only one chromosome pair was labelled, at the terminal position, in the two populations. These two signals were always remarkably distinct in interphase nuclei (Fig. 2K), whereas up to ten signals were observed in the interphase nuclei of S. plana (Fig. 2L).
DISCUSSION
The chromosome numbers and the structure of interphase nuclei reported in the present work are compatible with the karyological data known for the genus Selaginella (Kuriachan, 1963; Jermy et al., 1967; Ghatak, 1977; Takamiya, 1993). Of the seven species examined, only S. plana and S. willdenowii had been analysed previously. The chromosome numbers observed for these species agreed with the previous counts.
With regard to chromosome size and morphology, there is a strong trend to conserve the karyotype symmetry, with predominance of metacentric and submetacentric chromosomes. The species with 2n = 20 analysed in this sample were more stable in chromosome size than those with 2n = 18. Takamiya (1993) found similar results among species with x = 10 and x = 9. A higher number of meta- and submetacentric chromosomes was observed in the karyotype of all species analysed, and appears to be a common trend in this genus (Takamiya, 1993), and in other heterosporous pteridophytes (Kuriachan, 1979, 1994). On the other hand, acro- and telocentric chromosomes are more frequent among homosporous pteridophytes (Kawakami, 1982; Takamiya et al., 1992; Marcon et al., 2003).
In situ hybridization with 45S rDNA revealed the most prominent structural variation between species. One species with 2n = 20, S. plana, displayed ten sites of 45S rDNA whereas another one with the same chromosome number, S. producta, exhibited only two. On the other hand, S. willdenowii with 2n = 18, showed more rDNA sites (eight) than S. convoluta (six) with 2n = 24. In angiosperms a similar variation has been widely reported. In Passiflora, for instance, the number of 45S rDNA sites varied from one to three pairs among diploid species (Melo and Guerra, 2003). In Paeonia (Zhang and Sang, 1999), five diploid species (2n = 10) exhibited three to ten pairs of rDNA sites. Variation in the number of rDNA sites among angiosperm species of the same ploidy level has been attributed to chromosome rearrangements, transpositional events and gene silencing (Moscone et al., 1999). Likewise, similar mechanisms may be acting in Selaginella species.
In S. plana, S. convoluta and S. producta there was a perfect correlation between the maximum number of nucleoli (10, 6 and 2, respectively) and the number of 45S rDNA sites. The wide variation in the number of nucleoli per nucleus observed in all species examined, with a higher frequency of nuclei with few nucleoli, is probably due to the fusion of nucleoli, as observed in angiosperms (Moscone et al., 1995).
Curiously, S. willdenowii, although having up to ten NORs, displayed only eight 45S rDNA sites and six chromosomes with satellites. This apparent lack of correlation between NORs and rDNA sites may be due to a pair of rDNA sites very reduced in size, not detected by FISH. The number of satellites observed, on the other hand, is often lower than the number of rDNA sites (see, for example, Guerra et al., 1996), and in some cases they are not observed at all, as in S. convoluta, S. plana and S. producta.
The CMA/DA/DAPI staining revealed only CMA+/DAPI− bands in S. willdenowii and S. convoluta. The number of CMA+ bands was positively correlated with the number of rDNA sites and the maximum number of nucleoli observed in these species. Similar results were found in the homosporous genus Acrostichum (Marcon et al., 2003), suggesting that these three features are also positively correlated in pteridophyte chromosomes. No previous report of CMA bands in pteridophytes was found in the literature.
Jermy et al. (1967) proposed that the primary basic number of Selaginella would be x = 10, characteristic of species growing in dense tropical and subtropical forests (see also Kuriachan, 1963). However, later karyological analyses (Takamiya, 1993), as well as morphological, anatomical and paleobotanical data (Mukhopadhyay, 1998) suggest that the basic number of the genus would be x = 9, generating successive dysploids with n = 10, 11 and 12 on one hand and n = 8 and 7 on the other. Takamiya (1993) proposed that dysploidy might have occurred repeatedly in different evolutionary lines within each subgenus. Indeed, the remarkable variation in chromosome number and in size and number of rDNA sites, seems to indicate that structural variation is rather common in the genus and that its karyological evolution may be far more complex, with each basic number arising two or more times.
Acknowledgments
The authors are very greatful to Dr Iván Valdespino from University of Panama, for species identification, and to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) for financial support.
LITERATURE CITED
- Fabri F. 1963. Primo supplemento alle tavole cromosomiche delle Pteridophyta di Alberto Chiarugi. Cariologia 16: 237–335. [Google Scholar]
- Ghatak J. 1977. Biosystematic survey of pteridophytes from Shevaroy Hills, South India. Nucleus 20: 105–108. [Google Scholar]
- Greilhuber J. 1995. Chromosomes of the monocotyledons (general aspects). In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ, eds. Monocotyledons: systematics and evolution. Kew: Royal Botanic Gardens, 379–414. [Google Scholar]
- Guerra M. 1983. O uso de Giemsa na citogenética vegetal—comparação entre a coloração simples e o bandeamento. Ciência e Cultura 35: 191–193. [Google Scholar]
- Guerra M. 1993. Cytogenetics of Rutaceae. V. High chromosomal variability in Citrus species revealed by CMA/DAPI staining. Heredity 71: 234–241. [Google Scholar]
- Guerra M. 2000. Patterns of heterochromatin distribution in plant chromosomes. Genetics and Molecular Biology 23: 1029–1041. [Google Scholar]
- Guerra M, Kenton A, Bennett MD. 1996. rDNA sites in mitotic and polytene chromosomes of Vigna unguiculata (L.) Walp. and Phaseolus coccineus L. revealed by in situ hybridization. Annals of Botany 78: 157–161. [Google Scholar]
- Jermy AC, Jones K, Colden C. 1967. Cytomorphological variation in Selaginella Botanical Journal of the Linnean Society 60: 147–158. [Google Scholar]
- Kawakami S. 1982. Karyomorphological studies on Japanese Pteridaceae. IV. Discussion. Bulletin of Aichi University of Education (Natural Science) 31: 175–186. [Google Scholar]
- Kawakami SM, Kondo K, Kawakami S. 1999. Analysis of nucleolar organizer constitution by fluorescent in situ hybridization (FISH) in diploid and artificially produced haploid sporophytes of the fern Osmunda japonica (Osmundaceae). Plant Systematics and Evolution 216: 325–331. [Google Scholar]
- Kramer KU, Green PS. 1990.The families and genera of vascular plants. Vol. I. Pteridophytes and gymnosperms. Berlin: Springer-Verlag. [Google Scholar]
- Kuriachan PI. 1963. Cytology of the genus Selaginella Cytologia 28: 376–380. [Google Scholar]
- Kuriachan PI. 1979. Interspecific origin of Salvinia molesta Mitchell: evidence from karyotype. Indian Journal of Botany 2: 51–54. [Google Scholar]
- Kuriachan PI. 1994. Karyotype of Regnellidium diphyllum Lindm. Caryologia 47: 311–314. [Google Scholar]
- Marcon AB, Barros ICL, Guerra M. 2003. A karyotype comparison between two closely related Acrostichum L. (Pteridaceae) species. American Fern Journal 93: 116–125. [Google Scholar]
- Melo NF, Guerra M. 2003. Variability of the 5S and 45S rDNA sites in Passiflora L. species with distinct base chromosome numbers. Annals of Botany 92: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima M, Ohmido N, Fukui K, Yahara T. 2002. Trends in site number change of rDNA loci during polyploid evolution in Sanguisorba (Rosaceae). Chromosoma 110: 550–558. [DOI] [PubMed] [Google Scholar]
- Moscone EA, Klein F, Lambrou M, Fuchs J, Schweizer D. 1999. Quantitative karyotyping and dual-color FISH mapping of 5S and 18S-25S rDNA probes in the cultivated Phaseolus species (Leguminosae). Genome 42: 1224–1233. [DOI] [PubMed] [Google Scholar]
- Moscone EA, Loidl J, Ehrendorfer F, Hunziker AT. 1995. Analysis of active nucleolus organizing regions in Capsicum (Solanaceae) by silver staining. American Journal of Botany 82: 276–287. [Google Scholar]
- Moscone EA, Matzke MA, Matzke AJM. 1996. The use of combined FISH/GISH in conjunction with DAPI counterstaining to identify chromosomes containing transgene inserts in amphidiploid tobacco. Chromosoma 105: 231–236. [PubMed] [Google Scholar]
- Mukhopadhyay R. 1998. Cytotaxonomic observations on Selaginella Beauv. Phytomorphology 48: 343–347. [Google Scholar]
- Ran Y, Murray BG, Hammett KRW. 1999. Karyotype analysis of the genus Clivia by Giemsa and fluorochrome banding and in situ hybridization. Euphytica 106: 139–147. [Google Scholar]
- Rufas JS, Giménez-Abián J, Suja JA, Garcia De La Vega C. 1987. Chromosome organization in meiosis revealed by light microscope analysis of silver-staining cores. Genome 29: 706–712. [Google Scholar]
- Schweizer D, Ambros PF. 1994. Chromosome banding. In: Gosden JR, ed. Methods in molecular biology, Vol. 29. Totowa: Humana Press, 97–112. [DOI] [PubMed] [Google Scholar]
- Stace CA, Bailey JP. 1999. The value of genomic in situ hybridization (GISH) in plant taxonomic and evolutionary studies. In: Hollingsworth PM, Bateman RM, Gornall RJ, eds. Molecular systematics and plant evolution. London: Taylor & Francis, 199–210. [Google Scholar]
- Takamiya M. 1993. Comparative karyomorphology and interrelationships of Selaginella in Japan. Journal of Plant Research 106: 149–166. [Google Scholar]
- Takamiya M, Osato K, Ono K. 1992. Karyomorphological studies on Woodwardia sensu lato of Japan. Botanical Magazine 105: 247–263. [Google Scholar]
- Tanaka R. 1971. Types of resting nuclei in Orchidaceae. Botanical Magazine 84: 118–122. [Google Scholar]
- Tryon RM, Tryon AF. 1982.Ferns and allied plants—with special reference to tropical America. New York: Springer-Verlag. [Google Scholar]
- Unfried I, Gruendler P. 1990. Nucleotide sequence of the 5·8S and 25S rRNA genes and of the internal transcribed spacers from Arabidopsis thaliana Nucleic Acids Research 18: 4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unfried I, Stocker U, Gruendler P. 1989. Nucleotide sequence of the 18S rRNA gene from Arabidopsis thaliana Co10. Nucleic Acids Research 17: 7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TG. 1984. Chromosomes and evolution in pteridophytes. In: Sharma AK, Sharma A, eds. Chromosome evolution of eukaryotic groups. Vol. II. Boca Raton, FL: CRC Press, 103–141. [Google Scholar]
- Zhang D, Sang T. 1999. Physical mapping of ribosomal RNA genes in peonies (Paeonia, Paeoniaceae) by fluorescent in situ hybridization: implications for phylogeny and concerted evolution. American Journal of Botany 86: 735–740. [PubMed] [Google Scholar]