Abstract
• Background and Aims The shrub Viburnum tinus is widely distributed in mattoral vegetation of the Mediterranean basin. The purpose of the present study was to classify the seed dormancy type and examine the requirements for embryo growth, root protrusion and shoot emergence.
• Methods Overwintered fruits were collected in western Spain in April 2001 and prepared in three ways: entire pericarp was removed, exocarp and mesocarp were removed or fruits were left intact. Fruits treated in these three ways were subjected to artificial annual temperature cycles or to constant temperature regimes for 1·5 years.
• Key Results Removal of exocarp and mesocarp was necessary for embryo growth and germination. High temperature favoured dormancy alleviation and embryo growth, intermediate to low temperatures favoured root protrusion, and intermediate temperature shoot emergence. There was substantial germination at constant temperature regimes, indicating an overlap between temperature intervals suitable for the different stages of embryo and seedling development. Functionally, V. tinus has the same root and shoot emergence pattern that is described for other Viburnum species considered to have epicotyl dormancy. However, the requirement for high and low temperatures for radicle protrusion and epicotyl emergence, respectively, was missing in V. tinus; these characters are the foundation for the epicotyl dormancy classification.
• Conclusions It is concluded that V. tinus does not have epicotyl dormancy. Instead, there is a combination of a weak morphophysiological dormancy and a slow germination process, where different temperatures during an annual cycle favour different development stages. The present study suggests that the first complete seedlings would emerge in the field 1·5 years after fruit maturation in October, i.e. seed dispersal during winter, embryo growth during the first summer, root protrusion and establishment during the second autumn and winter, and cotyledon emergence during the second spring.
Keywords: Adoxaceae, Caprifoliaceae, epicotyl dormancy, mattoral, morphophysiological dormancy, pericarp, persistent fruits, ruminant endosperm, seedling emergence, shrub, Viburnaceae, Viburnum tinus
INTRODUCTION
Viburnum tinus is a large shrub with a wide distribution in the Mediterranean basin (Dirr, 1998), and it is a characteristic or dominant species in sclerophyllous shrubland (matorral) vegetation (Le Houérou, 1981; Tomaselli, 1981). It has horticultural (Dirr, 1998; Nobre et al., 2000) as well as medicinal properties (Cometa et al., 1998). The fruit is a one-seeded drupe consisting of a fleshy exocarp and mesocarp and a hard endocarp that is united with the seed coat (cf. Ferguson, 1966). The drupes are metallic blue that later turn blue-black to black and ripen in October (Debussche et al., 1987; Dirr, 1998). They are relatively dry (Debussche et al., 1987; Herrera, 1987) with 53 % water content in pulp (Debussche et al., 1987), and are removed from plants mainly by birds during winter (Thebaud and Debussche, 1992; Herrera, 1998). The embryo is surrounded by ruminate endosperm (Dragone-Testi, 1933), a feature also known to occur in other species of the genus (Rehder, 1940). Viburnum tinus is evergreen (Cornelissen et al., 1997), which is unusual within this predominantly deciduous genus. This species is placed in the section Tinus, along with five to six other taxa that occur mostly in China (Rehder, 1940; Krüssmann, 1986).
The genus Viburnum (Caprifoliaceae, Adoxaceae or Viburnaceae) consists of about 150 species of small trees and shrubs distributed primarily in the northern hemisphere but extending south in the mountains of south-east Asia and South America (Krüssmann, 1986; Mabberley, 1997; Donoghue et al., 2004). Seeds of Viburnum species have underdeveloped embryos (Martin, 1946; Gill and Pogge, 1974). Species containing embryos described as underdeveloped are dormant at maturity, and may need warm (≥15 °C) and/or cold (0–10 °C) stratification before embryo growth and germination can take place (Nikolaeva, 1977; Baskin and Baskin, 1998). This class of dormancy is called morphophysiological, and eight types are distinguished (Baskin and Baskin, 2004). In the case of deep simple epicotyl morphophysiological dormancy, the shoot does not immediately emerge after the radicle. Instead, both the radicle and epicotyl are dormant and require different temperature treatments to overcome dormancy; warm temperatures alleviate radicle dormancy, whereupon cold temperatures alleviate epicotyl dormancy in those seeds where the radicle has already emerged (Barton, 1933; Nikolaeva, 1977; Baskin and Baskin, 1998). The inhibitory mechanism of shoot growth appears to be in the cotyledons and not in the epicotyl per se (Knowles and Zalik, 1958).
Epicotyl dormancy has been reported in nine Viburnum taxa growing in northern and central North America [V. acerifolium (Giersbach, 1937; Hidayati et al., in press), V. dentatum, V. dilatatum, V. lentago, V. opulus, V. prunifolium, V. pubescens (Giersbach, 1937), V. alnifolium (Gould, 1966) and V. trilobum (Fedec and Knowles 1973)], one species in the southern United States [V. rufidulum (Giersbach, 1937)] and one species in Russia [V. opulus (Zolobova, 1970; Nikolaeva et al., 1985)]. In contrast, V. nudum and V. scabrellum in southern United States are reported to have morphologically dormant seeds, i.e. roots and shoots readily emerged without any treatments (Giersbach, 1937). Chien et al. (2002) demonstrated that cold stratification alone overcame dormancy in seeds of a Taiwanese species, V. odoratissimum, in the section Solenotinus (Thyrsoma). Based on these results, members of sections Lentago and Odontotinus principally have epicotyl dormancy, with the exception of one species in each section with morphological dormancy; two members in the section Opulus and one in Pseudotinus have epicotyl dormancy. The phylogeny of 42 Viburnum species was investigated recently (Donoghue et al., 2004); in general, the traditional classification in sections was supported, but Lentago was paraphyletic, and Odontotinusnon-monophyletic.
It has been proposed that epicotyl dormancy, in the case of Viburnum, might be an adaptation to temperate climates (Kollmann and Grubb, 2002). One way of determining whether a particular character is an adaptation requires the examination of patterns in nature among relatives. The issue that needs to be resolved is whether the observed trait is an adaptation or merely a phylogenetically conservative one (Westoby, 1999). On the other hand, groups of related species inhabiting similar environments tend to be homogeneous, referred to as phylogenetic niche conservatism, due to stabilizing selection or developmental constraints (Lord et al., 1995; Webb et al., 2002). Species growing in different vegetation types and on different continents offer prospects to explore adaptations, and begin to clarify evolutionary factors, such as constraints or conservatism, that influence traits (Hidayati and Walck, 2002).
When comparing dormancy and germination patterns between species it is important to use a consistent dormancy classification. Otherwise, there is a substantial possibility of grouping species together just because they, for example, germinate the same time of the year, even if different environmental cues alleviated their seed dormancy. In this research, the seed dormancy definitions stated by Vleeshouwers et al. (1995) and Baskin and Baskin (1998), in which dormancy is an innate attribute of the seed and is not related to the particular environment the seed happens to be in, were followed.
The purpose of the present study was to classify the nature of seed dormancy exhibited by V. tinus and, if possible, compare it with the dormancy known to occur in congeneric species primarily found in the temperate deciduous forest of North America. Viburnum tinus grows in a vegetation type and is classified in a section of the genus in which epicotyl dormancy has not been investigated before. Understanding the kind of dormancy found in V. tinus would fill a gap in our knowledge, especially with regards to the adaptive importance of epicotyl dormancy in the temperate region. Specifically, the requirements for embryo growth, root protrusion and shoot emergence, and the influence of the pericarp on dormancy and germination for V. tinus were examined. Using the results of this study, a conceptual model for the timing of germination in the field could be produced.
MATERIALS AND METHODS
Seed collection, pre-treatment and experimental set-up
Fruits were collected on 28 Apr. 2001 at Las Batuecas (in Sierra de Francia), approx. 65 km south-west of Salamanca, in western Spain. The fruits had remained on the plants over the winter and were relatively hard and dry. There was no indication that animals had removed any part of the seed crop. The fruits were kept dry for approx. 1 month at room temperature (approx. 20 °C) before initiation of the studies.
Two thousand fruits were prepared in each of three ways: (1) ‘seed’—the entire pericarp was removed from dry fruits; (2) ‘stone’—the fruits were soaked in water for 3–5 h, the pulp (exocarp and mesocarp) were carefully removed from the stone with the aid of a scalpel, and the stones allowed to dry again (for at least 48 h); and (3) ‘drupe’—the fruits were left intact. Hereafter, the collective of the three different preparations is referred to as ‘fruits’.
Experiments were conducted in three temperature- and light-controlled incubators (Rubarth Apparatebau, Laatzen, Germany) and in a cold room. The incubators were set at alternating 12/12 h (day/night) temperatures of 15/5, 20/10 or 25/15 °C, with a 2-h linear transition between the maximum and minimum temperature. The cold room, equipped with shelving and lighting similar to the incubators, was set at a constant temperature of 5 °C. These four temperature regimes approximate the mean monthly temperatures in Salamanca (Weather Channel, 2004a) and the mean monthly minimum and maximum temperatures in Madrid, approx. 170 km from Salamanca (Weather Channel, 2004b): 5 °C, December–February; 15/5 °C, March and November; 20/10 °C, May, April and October; and 25/15 °C, June–September.
Fruits were exposed to a photoperiod of 12 h per day in both the incubators and room. Warm white fluorescent light tubes were used as the light source in the incubators (two tubes at 18 or 20 W) and in the room (two tubes at 36 W and one at 20 W). The photon flux density (400–700 nm) in the incubators and room was 22–59 µmol m−2 s−1 (photometer: SKP 200, sensor SKP 215, Skye Instruments Ltd, Wales, measure precision 1 µmol m−2 s−1) and the 660/730 nm photon fluence ratio was 9·4–9·5 during the first 53 weeks and 2·6–3·5 during the remaining time (photometer: SKR 100, sensor SKR 110, Skye Instruments Ltd, Wales).
On 1 Jun., 2001, fruits were placed on two filter papers (Munktell 1003) in 9-cm-diameter Petri dishes and moistened with 6 mL of deionized water. The dishes were wrapped with parafilm to reduce water loss during incubation. They were rearranged randomly on the shelves of the incubators and in the room every second week. Water was added to the dishes as needed to keep the filter papers at a near-constant moisture level.
Requirements for root protrusion and shoot emergence
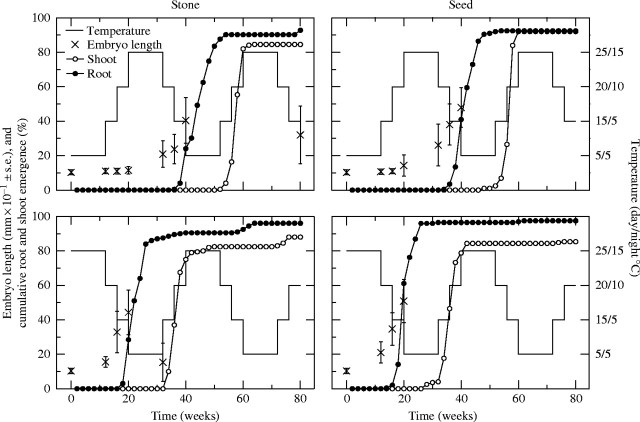
Four or five Petri dishes of 40 fruits per dish were used in each treatment. Fruits were exposed to two types of treatments: continuous temperature regime or annual temperature cycle. The four continuous temperature regimes corresponded approximately to winter (5 °C), early spring/late autumn (15/5 °C), late spring/early autumn (20/10 °C) and summer (25/15 °C). The annual temperature cycles simulated a natural sequence of the seasonal temperatures above, either beginning at summer or at winter regime (Fig. 1). Each artificial year, fruits were subjected to 12 weeks of summer and winter, and 4 weeks of both spring and autumn temperature levels. Experiments with seeds and with stones lasted for 80 weeks (i.e. for two annual temperature cycles), while those with drupes were terminated after 60 weeks.
Fig. 1.
Mean (±s.d.) embryo length on different occasions, and cumulative root protrusion and shoot emergence of Viburnum tinus fruits subjected to annual temperature cycles starting at a winter (5 °C) or summer (25/15 °C) regime. The fruits were treated in two ways before the start of the experiment: the entire pericarp was removed (seeds) or the pulp was removed (stones). In each annual cycle, 200 seeds or stones were used. Embryo lengths were measured in viable fruits remaining in an embryonic stage.
Roots were determined to have protruded when the radicle had split the endosperm at which point it was clearly visible. Shoots were judged to have emerged when at least one cotyledon was completely exposed from the seed coat. At this stage, both the cotyledons and epicotyls (if visible) were green. Fruits with radicles were counted and removed from the dishes every 2 weeks. When a root had protruded, the fruit was removed from the dish and planted in a small pot (65 mm diameter, 70 mm deep), filled with approx. 20 mm moist sand (90 % gneiss and/or granite, 10 % other minerals, 0·2–0·6 mm grain size) and sealed with parafilm on top. Germinated fruits from the same dish were planted in the same pot and a small stake placed beside each one indicated the day of root protrusion. The pots were checked for shoots every 2 weeks. Once a shoot had emerged, it was counted and the plant discarded (during the first 32 weeks, plants at 15/5 and 20/10 °C were retained until the emergence of the first true leaves). Fruits from which radicles did not protrude were checked to determine if the embryos were white and firm, indicating they were viable, or if the embryos were brown and soft, indicating they were nonviable. Percentages of root protrusion and shoot emergence are based on number of viable seeds at the end of the experiment.
Requirements for embryo growth
Lengths of embryos were measured at the beginning of the experiment and at various times. The point in development used to define the end of the embryonic stage was root protrusion. Embryos were excised using a scalpel, and their lengths measured under a dissecting microscope equipped with a micrometer. At the beginning of the experiment, 40 drupes were placed for 24 h on moist filter paper at room temperature before embryos were removed and measured.
One dish of 40 fruits was used on each occasion when measurements were made, using fruits still in the embryonic stage. In each of the continuous temperature regimes, measurements were taken after 60 weeks and for seeds and stones also at 80 weeks. In the annual temperature cycles, embryos were measured each time dishes were moved to a new temperature regime (Fig. 1). At 5 °C and 25/15 °C continuous temperature regimes, embryo lengths were recorded after 12 weeks; the data were obtained from fruits in the annual temperature cycle treatment after the first 12-week period at the winter or summer regimes, respectively. Embryo lengths were measured if ≥8 (20 %) of the fruits in one dish remained in embryonic stage, but on the last occasion (after 60 or 80 weeks) they were measured if in total ≥8 fruits (4–5 %) in the treatment remained in embryonic stage. Only fruits in which embryo and endosperm were white and firm, indicating viability, were used for measurements. Means and standard deviations were calculated each time measurements were obtained.
RESULTS
Effects of pericarp removal
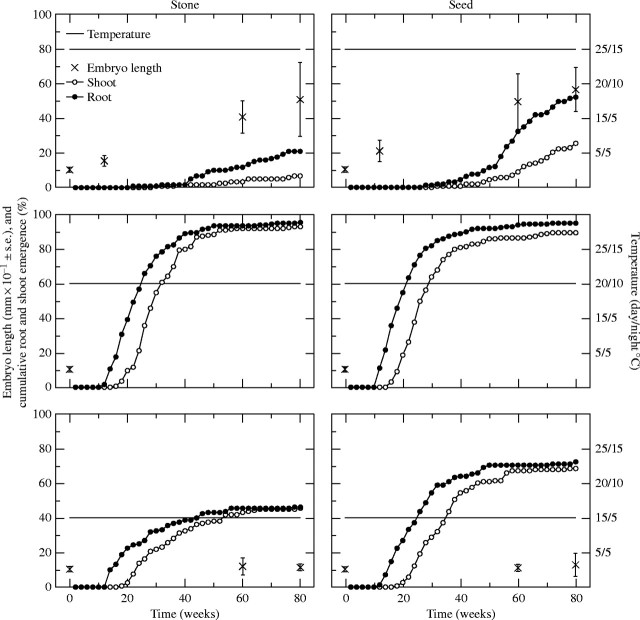
In general, embryos did not grow and germination did not occur in drupes. Of 686 embryos from drupes, only two, both in the annual cycle with summer start, were considerably longer (3·33 and 4·05 mm, respectively) than in fresh drupes (0·76–1·29 mm). Roots protruded from two (one in the annual cycle with summer start, one at continuous 20/10 °C) of 1159 drupes, and no shoot emergence occurred from drupes. In contrast, embryos grew and roots and shoots emerged from fruits in which the pulp was removed (‘stones’) and the entire pericarp was removed (‘seeds’) (Figs 1 and 2). Percentages of root protrusion and shoot emergence were higher for seeds than for stones at continuous 25/15 and 15/5 °C, but were similar at 20/10 °C (Fig. 2).
Fig. 2.
Mean (±s.d.) embryo length on different occasions, and cumulative root protrusion and shoot emergence of Viburnum tinus fruits subjected to continuous temperature regimes (15/5, 20/10 or 25/15 °C day/night, indicated by the line on each graph). The fruits were treated in two ways before the start of the experiment: the entire pericarp was removed (seeds) or the pulp was removed (stones). In each temperature regime, 160–200 seeds or stones were used. Embryo lengths were measured in viable fruits remaining in an embryonic stage.
Requirements for embryo growth
Embryos in fresh (untreated) drupes were 1·03 mm (s.d. 0·14) long, approx. 14 % the length of the seed. In seeds and stones that had been subjected to summer temperature followed by autumn in the annual cycles, lengths of embryos were 8·5–9·1 mm (n = 3) when the radicles were very close to protrusion. In the annual temperature cycles, embryos of seeds and stones started to increase their lengths at 25/15 °C and they continued to do so when transferred to 20/10 and 15/5 °C (Fig. 1). At continuous temperatures, embryos in seeds and stones did not differ (t-test, P > 0·15) from embryos in fresh drupes after 80 weeks of incubation at 5 °C, but growth of embryos occurred in seeds and stones at 15/5 °C and higher temperatures (Fig. 2).
Requirements for root protrusion and shoot emergence
In the annual temperature cycles, roots protruded and shoots emerged from 85–97 % of stones and seeds regardless of whether they were subjected to winter or summer temperatures first (Fig. 1). Only after stones and seeds had been exposed to the summer temperature did roots protrude. Root protrusion occurred during late autumn (15/5 °C) and winter (5 °C) (Fig. 1). Shoots emerged 13·7–15·7 weeks after root protrusion (Table 1). Shoot emergence began at the end of the winter temperature period after root protrusion (Fig. 1).
Table 1.
Time between root protrusion and shoot emergence for Viburnum tinus seeds (pericarp removed from fruits) and stones (pulp removed from fruits) subjected either to annual temperature cycles or to continuous temperature regimes (15/5, 20/10 or 25/15 °C day/night)
| Treatment |
Condition |
Mean time [weeks (s.d.)] |
No. of shoots |
Dead fruits (%) |
Remaining fruits (%) |
|
|---|---|---|---|---|---|---|
| Annual temperature cycles | Starting at a winter regime | Seed | 15·2 (2·6) | 184 | 2·1 | 0·0 |
| Stone | 13·7 (3·2) | 165 | 2·8 | 4·5 | ||
| Starting at a summer regime | Seed | 15·7 (2·3) | 170 | 6·1 | 0·0 | |
| Stone | 14·5 (3·3) | 174 | 7·9 | 0·0 | ||
| Continuous temperature regimes | 15/5 °C | Seed | 8·8 (1·3) | 107 | 2·7 | 1·8 |
| Stone | 9·0 (2·8) | 73 | 0·0 | 0·0 | ||
| 20/10 °C | Seed | 6·5 (2·0) | 178 | 4·8 | 0·0 | |
| Stone | 6·9 (2·4) | 186 | 2·1 | 1·0 | ||
| 25/15 °C | Seed | 6·4 (2·2) | 40 | 45·1 | 6·1 | |
| Stone | 6·3 (1·3) | 8 | 64·0 | 4·0 |
In each treatment 160–200 seeds or stones were used.
‘Dead fruits’ produced roots but died before producing shoots. ‘Remaining fruits’ had roots but not shoots when the experiment was terminated after 80 weeks.
In the continuous temperature regimes, neither roots protruded nor shoots emerged from 160 stones or 160 seeds during 80 weeks of incubation at 5 °C. The highest percentage of root protrusion and shoot emergence for stones and seeds (≥90 %) occurred at 20/10 °C, followed by 15/5 °C (46 % for stones, 69–72 % for seeds) and then 25/15 °C (7–21 % for stones, 25–52 % for seeds) (Fig. 2). The time lag between root protrusion and shoot emergence in the continuous temperature regimes was much shorter than that in the annual temperature cycles, being 6·3–6·9 weeks at 20/10 and 25/15 °C and 8·8–9·0 weeks at 15/5 °C (Table 1). Once cotyledons had emerged, the development of true leaves proceeded without further delay (data not shown).
In general, only a small percentage of the stones and seeds produced roots and then died during the experiment, or produced roots but failed to produce shoots before the experiment was terminated (Table 1). However, the percentage of seeds and stones that produced roots but died before shoots emerged was high (45 and 64 %, respectively) in the 25/15 °C continuous temperature regime (Table 1).
DISCUSSION
Removal of the exocarp and mesocarp was necessary for embryo growth and germination in V. tinus seeds, and removal of the entire pericarp improved germination (Fig. 2). Endocarp removal or exocarp/mesocarp (pulp) removal enhanced germination in V. trilobum (Knowles and Zalik, 1958) and V. dentatum (Meyer and Witmer, 1998). Pulp affects germination by preventing imbibition due to its imperviousness, its high osmotic pressure from dissolved sugars, or by containing germination inhibitors (Mayer and Poljakoff-Mayber, 1989). Meyer and Witmer (1998) found no differences in germination between seeds manually cleaned of pulp, and cleaned bird-passed seeds, regardless if seeds were regurgitated or defecated. Fruits of V. tinus are consumed and dispersed by birds (Thebaud and Debussche, 1992; Herrera, 1998), and the present results suggest that removal of pulp from seeds by frugivores is an important factor for germination.
The present study suggests that the first complete seedlings of V. tinus would start to emerge, in the Mediterranean region, 1·5 years after fruit maturation in October. Seed dispersal would occur during winter, embryo growth during the first summer, root protrusion and establishment during the second autumn and winter, and cotyledon emergence during the second spring. Seedlings would probably emerge at the same occasion regardless of when dispersal occurred during a winter season (autumn to spring).
When collecting the seeds used in the present study, it was noteworthy that the entire seed crop was still on the plants in late spring. The timing of germination has been an aspect evaluated in the past to test hypotheses concerning the adaptive reproduction strategy of plants that produce ‘persistent fruits’ (cf. Jones and Wheelwright, 1987). Jones and Wheelwright (1987) suggested that their germination results for V. opulus supported theoretically expected predictions for fruit retention. They found no difference in the timing or success of germination of seeds eaten in November vs. April, even though the length of time between dispersal and germination differed (9 vs. 4 months). However, seeds dispersed in November and April would have been exposed to equal amounts of warm (summer) temperatures. Seeds of V. opulus, reported to have epicotyl dormancy (Giersbach, 1937; Zolobova, 1970; Nikolaeva et al., 1985), are likely to have similar phenology of emergence as those of V. tinus (Fig. 1). It is predicted that if fruits of V. opulus were available and eaten at a time of the year (e.g. late summer) when they would not have received a sufficient temperature treatment for embryo growth and overcoming dormancy, germination would have been delayed. The timing of dispersal events, particularly when investigating plants with persistent fruits like some Viburnum species, needs to be placed into a framework of knowledge about the phenology of embryo growth, dormancy alleviation and germination.
Seeds and stones of V. tinus treated in annual cycles managed to germinate quickly at 15/5 and also at 5 °C (Fig. 1), while they did not germinate at continuous 5 °C, and at continuous 15/5 °C they germinated more slowly and to a lesser degree than in annual cycles (Fig. 2). Consequently, V. tinus seems to have a physiological mechanism, in addition to the morphological one, that prevents germination when directly subjected to some environmental conditions. However, after exposure to a factor that overcomes dormancy, these conditions are suitable for germination. Hence, it is concluded that V. tinus exhibits morphophysiological dormancy. Considering the dormancy of the 16 Viburnum taxa investigated so far, 14 apparently have morphophysiological dormancy (Adams, 1927; Giersbach, 1937; Gould, 1966; Zolobova, 1970; Fedec and Knowles, 1973; Gill and Pogge, 1974; Nikolaeva et al., 1985; Chien et al., 2002; Hidayati et al., in press), so this classification is not unexpected.
Functionally, V. tinus has the same root protrusion and shoot emergence pattern as previously described for most other Viburnum species (Giersbach, 1937; Gould, 1966; Zolobova, 1970; Fedec and Knowles, 1973; Nikolaeva et al., 1985; Hidayati et al., in press). When subjected to an annual cycle, root protrusion occurs after the first summer (or warm) period and shoot emergence after or during the following winter (or cold) period (Fig. 1). This type of germination pattern for Viburnum has been reported as epicotyl dormancy. Nevertheless, the present results are not completely supportive for inferring epicotyl dormancy in V. tinus.
First, there was no strict requirement of a warm period for germination in V. tinus, but warm summer temperature alleviated a weak physiological dormancy. The fact that substantial germination (45–75 %) was recorded at continuous 15/5 °C (Fig. 2) suggests that high temperatures are not necessary to initiate embryo growth, nor a prerequisite for root protrusion. In contrast, radicles protruded from 72 and 50 % of V. acerifolium seeds held for 48 weeks at 25/15 and 20/10 °C, respectively; no seeds germinated at 15/6 °C (Hidayati et al., in press). At 15/5 °C, the absence of increased mean length of embryos in seeds and stones after 60 and 80 weeks (Fig. 2) can be explained by a possible difference in dormancy level between individuals. In the seeds and stones with the weakest dormancy, embryo growth and subsequent root protrusion occurred, whereas the embryos in seeds and stones with stronger dormancy remained at their initial length. Such a differentiation in responses among seeds in a batch could be expected at suboptimal conditions for the species, and especially so as near-unsuitable conditions are approached. At continuous 25/15 °C embryo growth was considerable during the first 12 weeks but root protrusion was prevented for another 30 weeks (Fig. 2). This indicates that the high summer temperature is not suitable for root protrusion, but that it eventually can happen at such temperatures after embryo growth has occurred.
Secondly, there was no requirement for a cold period for shoot emergence in V. tinus. A substantial time lag occurred between root protrusion and shoot emergence (Table 1), but the development of a shoot continued regardless of continuous temperature regime or changes between temperature regimes in the annual cycles (Figs 1 and 2). It appears that, after planting, roots grew to a substantial size, whereupon shoot development took place. The development was actually quicker at high than at low continuous temperatures (Table 1), which should not have been the case if low temperatures (<10 °C) were essential to weaken dormancy of the epicotyl. During 5 °C in annual cycles, plant development slowed down, but when transferred to 15/5 °C quick and simultaneous shoot emergence occurred (Fig. 1). At continuous 25/15 °C approx. 50 % of germs failed to develop shoots (Table 1), indicating that this temperature was not suitable for shoot emergence.
The interpretation of the present V. tinus results is that dormancy alleviation and germination is a slow process: the first phase (alleviation of dormancy, embryo growth and root protrusion) requires a minimum of 10 weeks (Fig. 2) and the second (epicotyl growth and shoot emergence) a minimum of 6 weeks (Table 1). This slow process is then combined with different temperature regimes suitable for embryo growth (15/5 °C to 25/15 °C), and root protrusion and shoot emergence (5 °C to 20/10 °C) (Figs 1 and 2 and Table 1). The substantial germination at continuous temperatures (Fig. 2) can be explained by the overlap between temperature regimes suitable for the different stages of seedling development. For example, continuous 20/10 °C was suitable for all parts of the germination process; dormancy alleviation, embryo growth, root protrusion and shoot emergence.
Epicotyl dormancy is commonly reported in Viburnum species, in members of several sections of the genus growing in different vegetation types. Standardized laboratory experiments have not been performed in most previous germination studies on members of this genus. In fact, several of the published dormancy classifications involving epicotyl dormancy are based on observations of seedling emergence patterns, rather than on experiments with controlled temperature sequences. It is preferable that seed dormancy is defined as a strict seed characteristic, not affected by ambient environmental conditions (Vleeshouwers et al., 1995; Baskin and Baskin, 1998). The concept of epicotyl dormancy becomes unclear if based on functional properties, partly affected by germination conditions, rather than on innate physiological mechanisms in the seed. Hence, data among the various species of Viburnum are not comparable to the degree necessary for a meaningful phylogenetic analysis. The present results also emphasize the importance of adequate controls in a move-along experiment or exposing seeds to an annual temperature cycle (sensu Baskin and Baskin, 2003). Without seeds kept at the continuous temperature regimes for the entire length of the experiment, the interpretation of data could have been incorrect in the present study.
It is concluded that V. tinus does not have deep simple epicotyl morphophysiological dormancy according to the strict definition (Nikolaeva, 1977; Baskin and Baskin, 1998). Instead, after a weak physiological dormancy is alleviated, continuous development of the seedling occurs, even though the phenological phenomenon in response to annual changing temperature conditions is easily mistaken for epicotyl dormancy.
Supplementary Material
Acknowledgments
We thank Maria Perez-Fernandez for assistance, and CF Lundströms Stiftelse for financial support.
LITERATURE CITED
- Adams J. 1927. The germination of the seeds of some plants with fleshy fruits. American Journal of Botany 14: 415–428. [Google Scholar]
- Barton LV. 1933. Seedling production of tree peony. Contributions from Boyce Thompson Institute 5: 451–460. [Google Scholar]
- Baskin CC, Baskin JM. 1998.Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press. [Google Scholar]
- Baskin CC, Baskin JM. 2003. When breaking seed dormancy is a problem try a move-along experiment. Native Plants Journal 4: 17–21. [Google Scholar]
- Baskin JM, Baskin CC. 2004. A classification system for seed dormancy. Seed Science Research 14: 1–16. [Google Scholar]
- Chien C-T, Chen S-Y, Chang W-L. 2002. Stratification and gibberellin treatments for seeds of four Taiwanese tree species. Taiwan Journal of Forest Science 17: 51–57 [in Chinese with English summary]. [Google Scholar]
- Cometa MF, Mazzanti G, Romassini L. 1998. Sedative and spasmolytic effects of Viburnum tinus L. and its major pure compounds. Phytotherapy Research 12: S89-S91. [Google Scholar]
- Cornelissen JHC, Werger MJA, Castro-Diez P, van Rheenen JWA, Rowland AP. 1997. Foliar nutrients in relation to growth, allocation and leaf traits in seedlings of a wide range of woody plant species and types. Oecologia 111: 460–469. [DOI] [PubMed] [Google Scholar]
- Debussche M, Cortez J, Rimbault I. 1987. Variation in fleshy fruit composition in the Mediterranean region: the importance of ripening season, life-form, fruit type and geographical distribution. Oikos 49: 244–252. [Google Scholar]
- Dirr MA. 1998.Manual of woody landscape plants, 5th edn. Champaign, IL: Stipes Publishing Company. [Google Scholar]
- Donoghue MJ, Baldwin BG, Li J, Winkworth C. 2004.Viburnum phylogeny based on chloroplast trnK intron and nuclear ribosomal ITS DNA sequences. Systematic Botany 29: 188–198. [Google Scholar]
- Dragone-Testi G. 1933. Osservazioni sul significato fisiologico dei lipoidi nel seme di Viburnum tinus L. Annali di Botanica 20: 255–266. [Google Scholar]
- Fedec P, Knowles RH. 1973. After ripening and germination of seeds of American highbush cranberry (Viburnum trilobum). Canadian Journal of Botany 51: 1761–1764. [Google Scholar]
- Ferguson IK. 1966. The genera of Caprifoliaceae in the southeastern United States. Journal of the Arnold Arboretum 47: 33–59. [Google Scholar]
- Giersbach J. 1937. Germination and seedling production of species of Viburnum Contributions from Boyce Thompson Institute 9: 79–90. [Google Scholar]
- Gill JD, Pogge FL. 1974.Viburnum L. In: Schopmeyer CS, technical coordinator. Seeds of woody plants in the United States. USDA Forest Service Agriculture Handbook No. 450, 844–850. [Google Scholar]
- Gould WP. 1966.The ecology of Viburnum alnifolium Marsh. PhD Thesis, Syracuse University, USA. [Google Scholar]
- Herrera CM. 1987. Vertebrate-dispersed plants of the Iberian peninsula: a study of fruit characteristics. Ecological Monographs 57: 305–331. [Google Scholar]
- Herrera CM. 1998. Long-term dynamics of Mediterranean frugivorous birds and fleshy fruits: a 12-year study. Ecological Monographs 68: 511–538. [Google Scholar]
- Hidayati SN, Baskin JM, Baskin CC. Epicotyl dormancy in Viburnum acerifolium (Caprifoliaceae). American Midland Naturalist (in press). [Google Scholar]
- Hidayati SN, Walck JL. 2002. Contrasting seed germination patterns for intracontinental disjuncts of Heuchera (Saxifragaceae) in North America: the eastern temperate H. parviflora and western montane H. cylindrica Canadian Journal of Botany 80: 1185–1192. [Google Scholar]
- Jones E, Wheelwright NT. 1987. Seasonal changes in the fruits of Viburnum opulus, a fleshy-fruited temperate-zone shrub. Canadian Journal of Botany 65: 2291–2296. [Google Scholar]
- Knowles RH, Zalik S. 1958. Effects of temperature treatment and of a native inhibitor on seed dormancy and of cotyledon removal on epicotyl growth in Viburnum trilobum Marsh. Canadian Journal of Botany 36: 561–566. [Google Scholar]
- Kollmann J, Grubb PJ. 2002. Biological flora of the British Isles: Viburnum lantana L. and Viburnum opulus L. (V. lobatum Lam., Opulus vulgaris Borkh.). Journal of Ecology 90: 1044–1070. [Google Scholar]
- Krüssmann G. 1986.Manual of cultivated broad-leaved trees and shrubs. English translation by Epp ME. Portland: Timber Press. [Google Scholar]
- Le Houérou HN. 1981. Impact of man and his animals on Mediterranean vegetation. In: di Castri F, Goodall DW, Specht RL, eds. Ecosystems of the world. Vol. 11. Mediterranean-type shrublands. Amsterdam: Elsevier, 479–521. [Google Scholar]
- Lord J, Westoby M, Leishman M. 1995. Seed size and phylogeny in six temperate floras: constraints, niche conservatism, and adaptation. American Naturalist 146: 349–364. [Google Scholar]
- Mabberley DJ. 1997.The plant-book: a portable dictionary of the vascular plants. Cambridge: Cambridge University Press. [Google Scholar]
- Martin AC. 1946. The comparative internal morphology of seeds. American Midland Naturalist 36: 513–660. [Google Scholar]
- Mayer AM, Poljakoff-Mayber A. 1989.The germination of seeds, 4th edn. Oxford: Pergamon Press. [Google Scholar]
- Meyer GA, Witmer MC. 1998. Influence of seed processing by frugivorous birds on germination success of three North American shrubs. American Midland Naturalist 140: 129–139. [Google Scholar]
- Nikolaeva MG. 1977. Factors controlling the seed dormancy pattern. In: Kahn AA, ed. The physiology and biochemistry of seed dormancy and germination. Amsterdam: North-Holland Publishing Company, 51–74. [Google Scholar]
- Nikolaeva MG, Rasumova MV, Gladkova VN. 1985.Reference book on dormant seed germination. Leningrad: ‘Nauka’ Publishers [in Russian]. [Google Scholar]
- Nobre J, Santos C, Romano A. 2000. Micropropagation of the Mediterranean species Viburnum tinus Plant Cell, Tissue and Organ Culture 60: 75–78. [Google Scholar]
- Rehder A. 1940.Manual of cultivated trees and shrubs, 2nd edn. New York: Macmillan Co. [Google Scholar]
- Thebaud C, Debussche M. 1992. A field-test of the effects of infructescence size on fruit removal by birds in Viburnum tinus Oikos 65: 391–394. [Google Scholar]
- Tomaselli R. 1981. Main physiognomic types and geographic distribution of shrub systems related to Mediterranean climates. In: di Castri F, Goodall DW, Specht RL, eds. Ecosystems of the world. Vol. 11. Mediterranean-type shrublands. Amsterdam: Elsevier, 95–106. [Google Scholar]
- Weather Channel. 2004.Monthly averages for Salamanca, Spain http://www.weather.com/outlook/travel/climatology/monthly/SPXX0196 (10 Jun. 2004). [Google Scholar]
- Weather Channel. 2004.Monthly averages for Madrid, Spain, average temperature: high, low. Minimum period of record: 20 years http://www.weather.com/outlook/travel/climatology/monthly/SPXX0050 (10 Jun. 2004). [Google Scholar]
- Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. 2002. Phylogenies and community ecology. Annual Review of Ecology and Systematics 33: 475–505. [Google Scholar]
- Westoby M. 1999. Generalization in functional plant ecology: the species sampling problem, plant ecology strategy schemes, and phylogeny. In: Pugnaire FI, Valladares F, eds. Handbook of functional plant ecology. New York: Marcel Dekker, 847–872. [Google Scholar]
- Vleeshouwers LM, Bouwmeester HJ, Karssen CM. 1995. Redefining seed dormancy: an attempt to integrate physiology and ecology. Journal of Ecology 83: 1031–1037. [Google Scholar]
- Zolobova ZP. 1970. Some results of studies on the effect of temperatures on the seeds and plumules of Viburnun opulus L. during stratification in order to hasten seedling emergence. In: Bailobok S, Suszka B, eds. Proceedings of the international symposium on seed physiology of woody plants, held at Kórnik, Poland, 3–8 September 1968. Warsaw: Institute of Dendrology and Kórnik Arboretum, Polish Academy of Sciences, 141–146. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.