Abstract
Introduction
Transport of patients undergoing extracorporeal membrane oxygenation is currently available in 5 referral centers in our country.
Methods
Retrospective case series of patients managed by our mobile extracorporeal membrane oxygenation team and transferred to San Gerardo University Hospital from December 2004 to December 2012.
Results
42 patients were transported. The mean age was 42.11 (standard deviation ±18.11) years, with a range between 2 years and 70. 14 patients were females (33%) and 28 males (67%). The average transport distance was 121.69 km (±183.08) with a range between 9 km and 1044 Km. The mission’s mean time was equal to 508 minutes (±185) with range of 120-960 minutes. 29 patients (69%) were transported with extracorporeal membrane oxygenation support, while 13 patients (31%) were transported with conventional ventilation. In 28 patients (97%) a veno-venous bypass was utilized, while in one case (3%) a Veno-Arterial cannulation was performed. 32 patients survived (76%) and have been discharged alive from hospital. No major clinical or technical issues were observed during the transport.
Conclusions
According to our data, we conclude that a dedicated mobile team allowed safe ground transportation of patients with severe acute lung injury to our tertiary care institution.
Keywords: severe acute respiratory syndrome, extracorporeal membrane oxygenation, transportation of patients, mobile emergency unit, ECMO
Introduction
The in- and out-of-hospital transport of the critically ill patient is a virtual risk of adverse clinical events also related to a potential equipment’s failure [1,2,3]. Patient’s safety is dependant on the adherence to transport’s protocols [4, 5]. Patients affected by Adult Respiratory Distress Syndrome (ARDS) present with hypoxia and instability of gases exchange, a situation which may require the use of extracorporeal respiratory support (ECMO - Extra Corporeal Membrane oxygenation) as an emergency life-saving therapy or as a protective strategy to prevent further barotraumas from mechanical ventilation (ALI - acute lung injury) [6,7,8,9,10].
In many cases, patients with ARDS should be referred to an ECMO centre, but the severe and often rapid onset of hypoxia, not manageable with conventional treatments, may not allow for a safe conventional transport [11,12,13,14]. The General Intensive Care of San Gerardo University Hospital-Monza has been working since 1989 as an Italian ECMO referring centre. Up to 2004, patients from different hospitals eligible for ECMO treatment were transported with conventional ventilation techniques by the same hospital’s clinical team, increasing in this way risks related to unsafe transport.
In 2004, inspired by similar programs carried out in other countries, we implemented and put in practice a new program of ECMO positioning at the reporting ICU and consequent inter-hospital transport of these patients [8, 11]. The basic team consist of two intensivists, an ICU nurse and a perfusionist. The aim of this paper is to present our 8-year experience on inter-hospital transport of patients with severe acute respiratory failure managed by our mobile transport team (which also has ECMO capability).
Methods
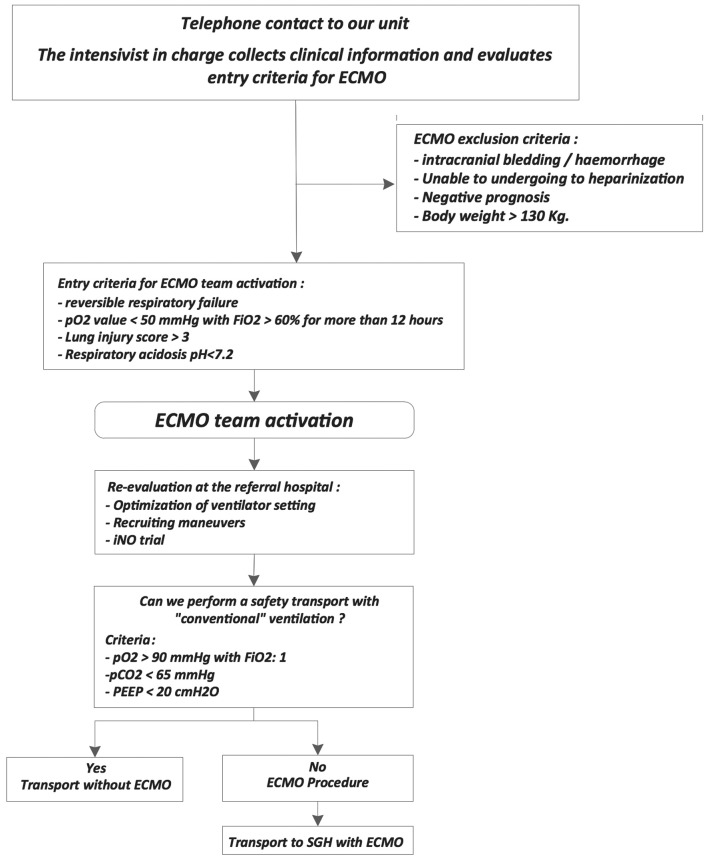
From December 2004 to December 2012 we collected data concerning the activity of our Mobile ECMO team. All consecutive patients transferred by our mobile transport team were included in the study. Clinical records (Innovian Medical Suite© - Drager Medical) and manual data sheets were retrospectively reviewed. Due to the retrospective observational design of the study, informed consent was waived, according to Italian Regulations (AIFA, Directive of March 20, 2008). Data from the first 14 transports have previously been published [13]. All patient transfers were performed from local trust to San Gerardo Hospital (SGH) and subsequently admitted into our 10-bedded General ICU. The National Health insurance covered the expenses of the transport, and the average cost was 10.000,00€ ± 800,00 per transport (ambulance 720,00 € ± 278,00 - staff 2.500,00 € ± 780,00 - medical devices: 6.500,00 € ± 100,00). The alert protocol and procedure for patient eligible for ECMO treatment was based on phone call to our unit for the first evaluation and commence for possible transport activation (Figure 1).
Figure 1.
Management algorithm for the referrals to the SGH ECMO center. ECMO = extracorporeal membrane oxygenation; PEEP = positive end-expiratory pressure.
Criteria for ECMO team activation were: reversible respiratory failure, PaO2 value < 50 mmHg with FiO2 ≥60% for more 12 hours, Lung Injury Score ≥3; or respiratory acidosis pH ≤7.2 [13]. Exclusion criteria were: intracranial bleeding/haemorrhage, unable to undergoing to heparinization, negative prognosis, body weight ≥130 Kg (logistic and equipment restrictions). The internal standard procedure, in case of positive assessment for transport, guarantee an ECMO crew activation time within 60 to 90 minutes after the distress call. The basic team includes two intensivists (MD), one Registered Nurse specialist (RN) and one perfusionist. When possible, a professional on training joined the mission for transport education.
The transportation program began in 2004 based on experience gained by our ICU staff from in-hospital patient transport on extracorporeal support [14]. Since 1994, in hospital transfers were performed by a mobilization system able to house all the biomedical equipment. This tool underwent continuous evaluation to optimize logistics and performance [15].
This device is composed of an x-ray spine board compatible and multi-storey counter top. The pulmonary ventilator, the ECMO pump, syringe pumps and monitoring are placed on shelves.
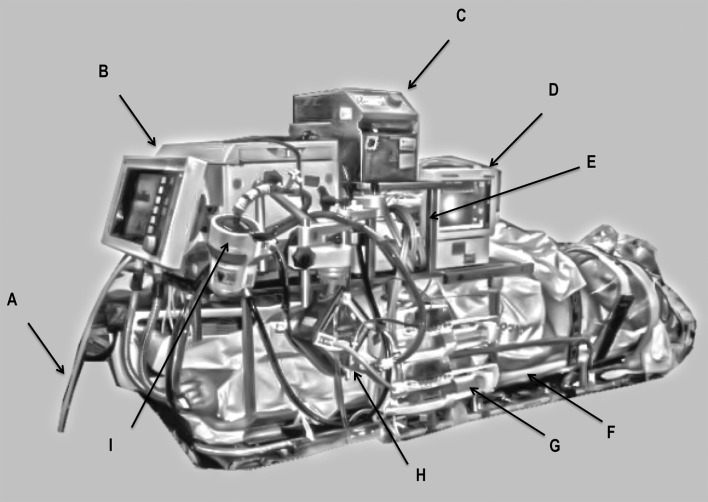
The counter top is equipped with an electrical junction box that provides power to all equipment by a single connection from the main electricity network. Figure 2 shows the system’s structure and Figure 3 shows the possible transport solutions.
Figure 2.
Transport system used.
A: gas inlet, B: intensive care ventilator, C: centrifugal pump - Consoles, D: vital signs monitor, E: water Pump Heater, F: spine board, G: syringe pumps, H: extracorporeal membrane oxygenation (ECMO) pump head, I: head of centrifugal pump.
Figure 3.
Patient seated for transport.
In order to use this system and to perform out-of hospital transports more effectively, a specifically adapted emergency ambulance should be present: it should be able to meet the special needs related to an extra power supply and medical gases storage.
The White Cross of Carugate (Milano) provided us, through a special agreement, all the ambulances used during this study period. The vehicles are based on Renault mechanics (Master model®) and all the tech features are reported in Table 1.
Table 1.
Technical data of ambulances used in inter-hospital transports.
The gas circuit has 4 connections: 2 for oxygen and 2 for compressed air (UNI grafts), controlled by an electronic unit with automatic switching from one cylinder to another. A gas monitoring and alarm system is in situ to check the working pressure and the residual gas availability. In case of electric failure it is possible to switch into manual pneumatic dispensing delivery of the gases.
In order to reduce risks and guarantee the best comfort during transfer from ground to ambulance floor, the vehicle is equipped with a stretcher supported with hydro pneumatic suspension (model: STEM-Piuma®).
In addition to oxygen and compressed air, it is possible to deliver nitric oxide via cylinder 10 L volume/200 atm pressure via a nitric system. An additional vehicle transports the rest of the crew and equipment.
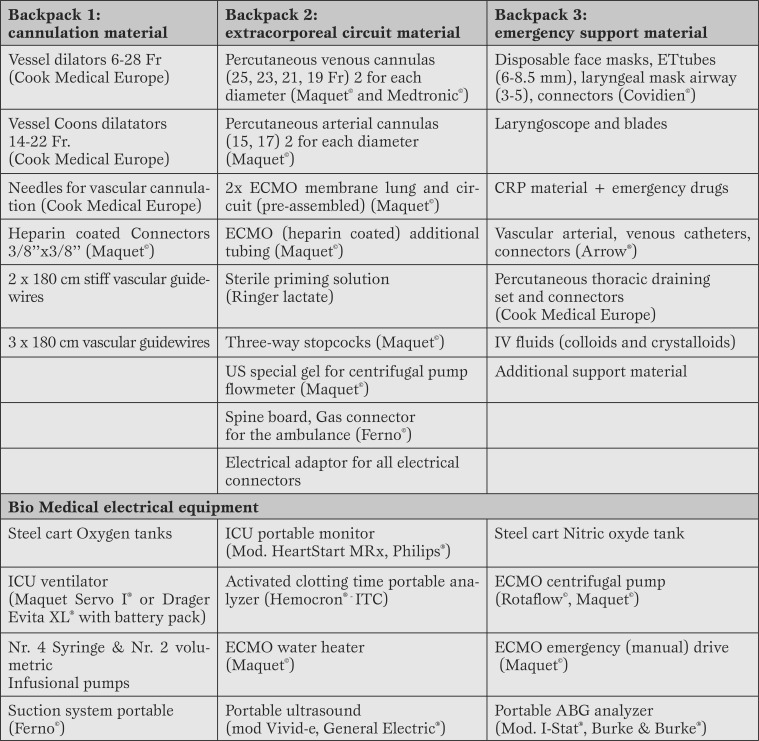
All the equipment required to perform the cannulation and transport has been split into three bags. All the materials (Table 2) are stored, checked and sealed before every transport.
Table 2.
Equipment, devices, and spare materials.
Fr. = French; CPR = cardiopulmonary resuscitation; IV = intra-venous; ICU = intensive care unit; ECMO = extracorporeal membrane oxygenation; ABG = arterial blood gases.
For all eligible ECMO patients, heparinized tubing set (BIOLINE coated®) by MAQUET Holding GmbH & Co. KG-Germany (mod. PLS-BE or HLS-BE) was used. The percutaneous cannulas used are produced by MAQUET (multistage series BEPVL 55 cm) and Medtronic Inc. U.S.A (Mod. CB96605).
As far as the veno-venous support (two-sites approach) is concerned, a drainage percutaneous cannula-multistage type was usually positioned in the left femoral vein and a percutaneous no multistage-cannula type was used for the reinfused blood, to avoid blood recirculation.
Two types of centrifugal pump have been used: Rotaflow® and Cardiohelp® System. The first choice was the two-site veno-venous approach to allow for quick weaning from sedative drugs and an early mobilization of the patient [9]. All the cannulations were performed via percutaneous approach, using real-time ultrasound and a dilatation modified technique to reduce the enlargement stages [16].
At the time of arrival of the ECMO team, the following procedures were carried out: patient assessment and optimization of the ventilator setting (recruitment manoeuvre, lung protective ventilation with tidal volumes <6 mL/Kg, trial of inhaled Nitric Oxide, apparatus dead space reduction by removal of mount connector or HME (heat and moisture exchange), close suctioning system and continuous EtCO2 monitoring) [17,18,19], to evaluate the safety and feasibility of patient transport with “conventional” ventilation (i.e. without ECMO). Criteria for transport with “conventional” ventilation were: pO2>90 mmHg with FiO2 = 1, pCO2 < 65 mmHg and positive end-expiratory pressure (PEEP) < 20 cmH2O.
If these criteria were not met or in case for any reason a transport with “conventional” ventilation was not judged safe, an ECMO support was started on site and the patients were transferred on ECMO.
After the ECMO support has been established, the minute ventilation is gradually reduced through respiratory rate decrease, according to end-tidal CO2 values and later a blood gas sample is performed. PEEP level and inspiratory/expiratory cycling are settled to avoid any unplanned reduction in the mean airways pressure. When the ECMO circuit, ventilation setting and cardiovascular parameters are stabilized, the nurse prepares the patient and all devices for the transport: all the Intra Venous (IV) lines are replaced with needless valve lines, the IV lines extension used are polyurethane 250 cm long. After this procedure, all medical devices are placed in the following order: ECMO pump, membrane lung fixation support plus swivel joint-bracket, stand alone unit (manual drive), heater unit, pulmonary ventilator and monitoring. While these processes were performed, the pulmonary ventilator was supplied with the gas available at patient bed space (inside backpack 2 are present all the air/oxygen joint adapter available in the Italian hospitals). Only when the patient was settled, the gas delivery was switched from main to cylinders delivery.
Connection to main electrical and gases are tested in ambulance prior the departure.
Statistical analysis. Data are presented as mean ± standard deviation or with median and interquartile range (IQR). Recorded data were compared using the two-tailed paired t test date (SPSS version 19. Inc., Chicago, USA). Differences were considered significant when p<0.05.
Results
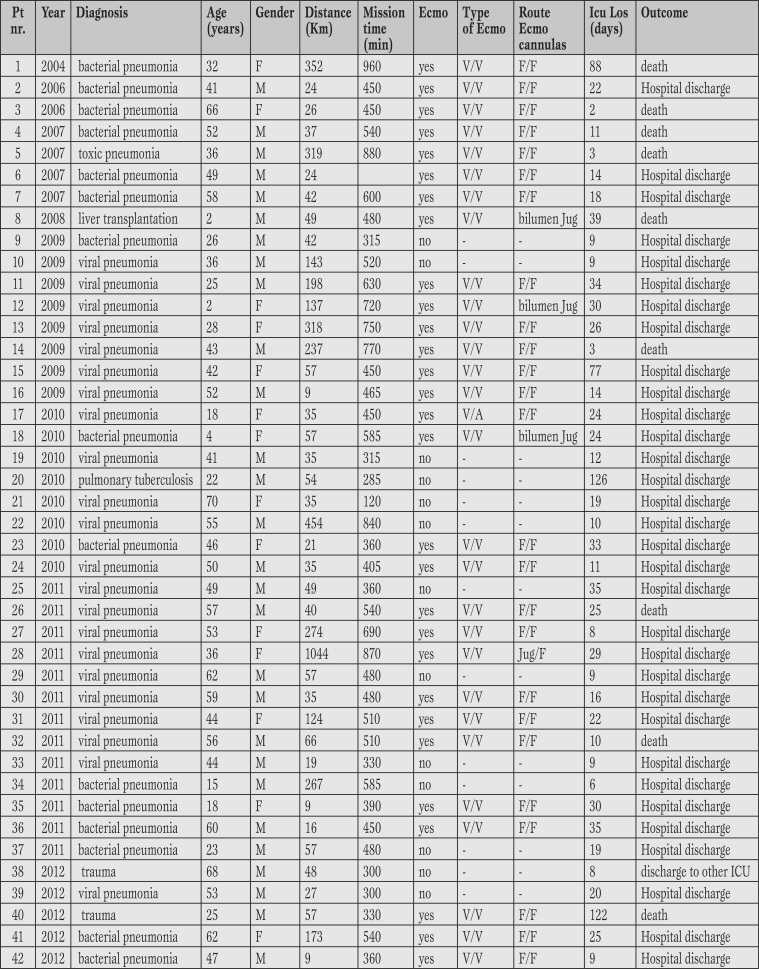
42 patients were transported. The mean age was 42.11 (SD±18.11) years, with a range between 2 years and 70. 14 patients were female (33%) and 28 males (67%). The average transport distance was 121.69 km (SD±183.08) with a range between 9 km and 1044 km. The mission’s mean time (departure from and back to SGH) was equal to 508 minutes (±185 with range of 120-960 minutes). The longest distance of 1044 km was performed by transportation of the ambulance and team on the ‘Hercules C 130’ aircraft provided by the Italian Air Force. No mission was postponed or cancelled due to organizational failure or extreme weather conditions. Table 3 shows the features of patients.
Table 3.
Population characteristics.
V/V = veno-venous Ecmo; V/A = veno-arterial Ecmo; F/F = femo-femoral cannulation; Jug/F= jugular/femoral cannulation; bilumen Jug = double lumen Jugular cannula; Icu Los : intensive care unit lenght of stay.
At the moment of arrival of the ECMO team, the average pO2 was 64.96 mmHg (±18.15), pCO2 was 70.71 mmHg (SD±22.06) and pH was 7.27 (±0.18). After the optimization of ventilation an improvement in oxygenation parameters (average pO2= 91.26 ± 29.85 mmHg, pCO2= 60.53 ± 20.07 mmHg) was registered in 13 patients (31%): these patients were transferred with “conventional” ventilation, without any need for ECMO implementation.
In the remaining 29 patients (69%), optimization of ventilation did not result in an improvement of gas exchanges (pO2= 61.45 ± 15.61 mmHg and pCO2 70.65 ± 23.26 mmHg): in these subjects an ECMO support was instituted on site and they were transferred on ECMO to SGH. In 28 cases (97%) a veno-venous, approach was positioned while in one patient (3%) a Veno-Arterial cannulation was performed for cardiovascular support, due to simultaneous presence of ARDS and cardiogenic shock. The two sites of cannulation were the following: veno-venous femoral/femoral in 24 patients (84%), in one patient only femoral/jugular (3%) and in 3 paediatric patients (10%) a double lumen jugular cannula 15 FR was placed.
For the Veno-Arterial support (3%) femoral vein-artery were cannulated. The average Blood Flow was 2.96 L/min (SD±0.93 - range: 0.6/4.3), and an average Gas-Flow average equal to 3.27 L/min (±1.47 - range: 1-6). No adverse events happened during all the cannulation’s performance.
For 28 (67%) transports, transfer pulmonary ventilator EVITA® (4 or XL - Dräger Medical) were used, while in 13 cases (31%) SERVO I® Maquet ventilator was the choice; and only for the first transport the Siemens SERVO 300® ventilator was used. The median number of syringe pumps used during transport was 4 (IQR 3-4/range 2-6). The median number of inotropic drugs (Dopamine, Dobutamine, Norepinephrine) administered during transport was equal to 1 (IQR 0-1/range 0-1).
We did not report any morbidity or mortality due to the transport or to the ECMO support on transfer.
The following complications were observed: in one patient battery failure of Evita XL® ventilator during transport from the ICU to the ambulance; in another case it was difficult to obtain an acceptable extracorporeal flow due to the patient’s position and it was necessary to stop the ambulance to reposition the patient. No patient required transfusion of blood products during transport.
The average days on ECMO were equal to 18.21 (±22.96 - range 2/116). The average length of stay in ICU was 26.07 days (±27.96 - range 2/126). 33 patients (79%) were discharged alive from SGH, 9 died in the ICU (21%).
All patients who died were transported with ECMO support: no patient died during transport, but they all died while still on ECMO. In the group of patients who required ECMO support, a subgroup analysis was performed to investigate differences between survivors and non-survivors with regard to: pre-ECMO ventilation days, days on ECMO and length of hospital stay.
The only statistically significant difference was observed in the duration of mechanical ventilation before the activation of the ECMO team: in survivors the median of pre-ECMO ventilation days was 2 (IQR:1-4) while in non-survivors was equal to 8 days (IQR:3-13) (p=0.04).
Table 4 collects data of all patients related to pre-transfer, data collected on arrival to SGH and the ones related to patients transferred on ECMO versus no ECMO support.
Table 4.
Clinical characteristics of the enrolled patients.
Baseline = 30 minutes before ECMO; ECMO = at 60 minutes after initiation, SGH ICU admission: parameters at the end of transport when patients were admitted at SGH general ICU.
SD = standard deviation; sABP-dABP-mABP: systolic, diastolic and medium Arterial Blood pressure; ABG = arterial blood gas analysis, ICU LOS = intensive care unit lenght of stay; ECMO = extracorporeal membrane oxygenation; PEEP = positive end-expiratory pressure.
Discussion
The early commence of ECMO support provides an extra source of recovery for patients who develop an acute respiratory failure not responding to standard strategy of care; furthermore for long ventilated patients, the ECMO support let to perform respiratory tidal volume less than 6 mL/kg and low respiratory rate.
Many patients referred for ECMO treatment cannot take benefits from this advanced support due to high risks of death related to an ordinary transfer based on standard respiratory setting.
Implementing the ECMO cannulation standards outside of SGH required several steps. The first transport required two days of preparation to assemble the equipment and suitable staff.
The ambulance choice required a strict collaboration between ICU staff, ambulance crew and the SGH medical physics dept., to get through/to rule out all the technical issues related to the vehicle (electricity power supply and medical gas circuit).
The nursing staff that carried out the transport was trained on fieldwork by tutoring, which was held after the first transport, according to the literature [20, 21]. In order to be qualified for transport, these are the minimum requirements: two years experience in ICU, 2 in hospital cannulation, 2 in intra-hospital transport experience and two out of hospital missions supervised by a qualified nurse.
The nursing staff education (theoretical and practical) was managed by the head nurse and a senior qualified nurse.
To sort out the mission different paperwork (flow charts and checklists) were created in order to verify the equipments [22, 23].
Patients included in this report had an average severe hypoxia (pO2: 64.96 ± 18.15 mmHg). Despite their respiratory instability, the cardiovascular status of patients was stable. The transport described above requireed a great preparation time compared with traditional techniques.
Benefits of this system involve the absence of extra adjustments after this stage, so patient will be ready to be transported straight to ambulance, furthermore after positioning in the back, there is no need to rearrange any equipment (syringe pumps, circuit, ventilator) because they are already settled, reducing the time of transfer and possible complications according to the literature [13, 14].
All the equipment’s packs for transport are dedicated to ECMO transports only [24]. It makes easier to perform the quality checks and battery packs levels. These are relevant facts, identified as the most responsible in the case of adverse events such as battery failure [25, 26].
Finally, it is important to point out our attitude and confidence, as referral centre, in using intensive care ventilators to perform ECMO transport compared with dedicated transport’s ventilator [27, 28].
Our choice is related to an operating principle of veno-venous circuit. With a severe hypoxic patient due to ARDS, the oxygenation is strictly related to the amount of blood volume able to get trough the gas exchange membrane surface (natural and artificial lung).
In case of high cardiac output, not unusual in patients with ARDS, just a portion of the total flow could pass through the artificial membrane lung. The average Blood Flow of the subjects investigated was equal to 2.96 L/min (±0.93).
It means that a certain part of the blood flow should be oxygenated via the natural lung. In order to perform advanced respiratory care with high levels of PEEP despite a very poor compliance, it is essential to use high performance ventilators able to get advanced ventilation settings, allowing to mix them as well (Volume guaranteed steady pressure® - CPPV Autoflow®, Sigh option on control ventilation®) and with high air-operating performance. The average level of PEEP during missions was equal to 16.50 (±3.84) cm H2O.
Our results confirm two important findings reported in the recent literature:
a) transfer to a tertiary-level ICU with ECMO capability has a positive impact on patients’ outcome [30];
b) since the number of ventilation days before ECMO institution is clearly related to survival, it is of paramount importance that all patients deemed eligible for ECMO treatment are referred as early as possible to a specialized ICU. These data is similar to those published by all centers related to Italian “ECMOnet” [12].
In addition, it must be underlined the importance of clinical stabilization of the patients before transport [29].
The number of adverse events observed in our patients during transport were comparable to those reported by other centers or networks that have implemented similar programs aimed at the centralization of ECMO candidates [7, 8, 12, 30].
Limitations
The main limitation of this study is related to retrospective analysis.
Data were collected from medical records and it was not possible to get back all the clinical data for each patient prior to ambulance transfer.
For this reason, we could not compare the different subgroups with regard to severity scores (like SOFA or APACHE) or to the incidence of other comorbidities. However, our goal was only to report our 8-years experience of inter-hospital transportation of critically ill patients and to demonstrate the safety and feasibility of a “Mobile ECMO team” program. We are now performing a prospective study with capillary recording of all relevant clinical variables, in order to perform adequate comparison between different groups of patients; in addition, we are planning to perform a follow-up analysis to investigate the quality of life of surviving patients.
Conclusion
Analysis of risk factors and a plan for actions allow to improve a program for ECMO transport of persons affected by ALI or ARDS to a referring centre.
In order to achieve this goal, four factors seem to be crucial: identification of a vehicle with specific tech features, systematic and precise settings of equipment, a tool-system to sort out equipment and a dedicated training program for nurses involved in these missions.
Acknowledgments
The authors thank Mrs. Samantha Harris-Fox (Ma, Bsc-Hons), RN, Senior ECMO Specialist at Glenfield Hospital, University Hospital of Leicester-NHS Trust (UK), for reviewing the manuscript before submission. The authors thank all the San Gerardo General ICU staff for their enormous, continued efforts in caring for these complex patients. The authors also thank Mr. Angelo Elettrini, Mr. Gianfranco Zappa and the excellent crew of the Milan-Carugate unit of the White Cross (Croce Bianca).
Footnotes
Source of Support Nil.
Disclosures None declared.
Cite as: Lucchini A, De Felippis C, Elli S, Gariboldi R, Vimercati S, Tundo P, Bondi H, Costa MC. Mobile ECMO team for inter-hospital transportation of patients with ARDS: a retrospective case series. Heart, Lung and Vessels. 2014; 6(4): 262-273
References
- Brokalaki H J, Brokalakis J D, Digenis G E, Baltopoulos G, Anthopoulos L, Karvountzis G. Intrahospital transportation: monitoring and risks. Crit Care Nurs. 1996;12:183–186. doi: 10.1016/s0964-3397(96)80554-4. [DOI] [PubMed] [Google Scholar]
- Lovell M A, Mudaliar M Y, Klineberg P L. Intrahospital transport of critically ill patients: complications and difficulties. Anaesth Intensive Care. 2001;29:400–405. doi: 10.1177/0310057X0102900412. [DOI] [PubMed] [Google Scholar]
- Beckmann U, Gillies D M, Berenholtz S M, Wu A W, Pronovost P. Incidents relating to the intra-hospital transfer of critically ill patients. An analysis of the reports submitted to the Australian Incident Monitoring Study in Intensive Care. Intensive Care Med. 2004;30:1579–1585. doi: 10.1007/s00134-004-2177-9. [DOI] [PubMed] [Google Scholar]
- Ehrenwerth J, Sorbo S, Hackel A. Transport of critically ill adults. Crit Care Med. 1986;14:543–547. doi: 10.1097/00003246-198606000-00005. [DOI] [PubMed] [Google Scholar]
- Winter M W. Intra-hospital transfer of critically ill patients; a prospective audit within Flinders Medical Centre. Anaesth Intensive Care. 2010;38:545–549. doi: 10.1177/0310057X1003800321. [DOI] [PubMed] [Google Scholar]
- Mamprin F, Pesenti A, Fumagalli R. Extracorporeal circulation for acute respiratory failure. Intensive Care Med. 2001;27:934–936. doi: 10.1007/s001340100867. [DOI] [PubMed] [Google Scholar]
- Lindén V, Palmér K, Reinhard J, Westman R, Ehrén H, Granholm T. et al. Inter-hospital transportation of patients with severe acute respiratory failure on extracorporeal membrane oxygenation-national and international experience. Intensive Care Med. 2001;27:1643–1648. doi: 10.1007/s001340101060. [DOI] [PubMed] [Google Scholar]
- Zimmermann M, Bein T, Philipp A, Ittner K, Foltan M, Drescher J. et al. Interhospital transportation of patients with severe lung failure on pumpless extracorporeal lung assist. Br J Anaesth. 2006;96:63–66. doi: 10.1093/bja/aei274. [DOI] [PubMed] [Google Scholar]
- Pesenti A, Zanella A, Patroniti N. Extracorporeal gas exchange. Curr Opin Crit Care. 2009;15:52–59. doi: 10.1097/MCC.0b013e3283220e1f. [DOI] [PubMed] [Google Scholar]
- Patroniti N, Bellani G, Pesenti A. Nonconventional support of respiration. Curr Opin Crit Care. 2011;17:527–532. doi: 10.1097/MCC.0b013e32834a4be7. [DOI] [PubMed] [Google Scholar]
- Foley D S, Pranikoff T, Younger J G, Swaniker F, Hemmila M R, Remenapp R A. et al. A review of 100 patients transported on extracorporeal life support. ASAIO J. 2002;48:612–619. doi: 10.1097/00002480-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A. et al. The Italian ECMO network experience during the 2009 influenza A (H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37:1447–1457. doi: 10.1007/s00134-011-2301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgrò S, Patroniti N, Bombino M, Marcolin R, Zanella A, Milan M. et al. Extracorporeal membrane oxygenation for interhospital transfer of severe acute respiratory distress syndrome patients: 5-year experience. Int J Artif Organs. 2011;34:1052–1060. doi: 10.5301/ijao.5000011. [DOI] [PubMed] [Google Scholar]
- Lucchini A, Elli S, Gariboldi R, Tundo P, Doni V, De Felippis C. et al. Standardization of procedures for the transport of critically ill patients in intensive care: observational study of 68 intra-hospital transport. Scenario. 2012;29:15–20. [Google Scholar]
- Lucchini A, Aliprandi L, Foti G, Monti I. Transport of adult patient. Minerva Anestesiol. 2005;71:127–133. [Google Scholar]
- Grasselli G, Pesenti A, Marcolin R, Patroniti N, Isgrò S, Tagliabue P. Percutaneous vascular cannulation for extracorporeal life support (ECLS): a modified technique. Int J Artif Organs. 2010;33:553–557. doi: 10.1177/039139881003300806. [DOI] [PubMed] [Google Scholar]
- Malhotra A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med. 2007;357:1113–1120. doi: 10.1056/NEJMct074213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terragni P P, Del Sorbo L, Mascia L, Urbino R, Martin E L, Birocco A. et al. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111:826–835. doi: 10.1097/ALN.0b013e3181b764d2. [DOI] [PubMed] [Google Scholar]
- Quintel M, Moerer O. Is smaller high enough? Another piece in the puzzle of stress, strain, size, and systems. Crit Care. 2009;13:126–126. doi: 10.1186/cc7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtnyk S. Secondary transportation of critically ill people: implications for nurses and the need for specialist training. Intensive Crit Care Nurs. 1992;8:234–239. doi: 10.1016/0964-3397(92)90055-o. [DOI] [PubMed] [Google Scholar]
- Lee L L Y, Lo W Y L, Yeung K L, Kalinowski E, Tang SYH, Chan J T S. Risk stratification in providing inter-facility transport: experience from a specialized transport team. World J Emerg Med. 2010;1:49–52. [PMC free article] [PubMed] [Google Scholar]
- Jarden R J, Quirke S. Improving safety and documentation in intrahospital transport: development of an intrahospital transport tool for critically ill patients. Intensive Crit Care Nurs. 2010;26:101–107. doi: 10.1016/j.iccn.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Bérubé M, Bernard F, Marion H, Parent J, Thibault M, Williamson D R. et al. Impact of a preventive programme on the occurrence of incidents during the transport of critically ill patients. Intensive Crit Care Nurs. 2013;29:9–19. doi: 10.1016/j.iccn.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Gebremichael M, Borg U, Habashi N M, Cottingham C, Cunsolo L, McCunn M. et al. Interhospital transport of the extremely ill patient: the mobile intensive care. Crit Care Med. 2000;28:79–85. doi: 10.1097/00003246-200001000-00013. [DOI] [PubMed] [Google Scholar]
- Papson J P, Russell K L, Taylor D M. Unexpected events during the intrahospital transport of critically ill patients. Acad Emerg Med. 2007;14:574–577. doi: 10.1197/j.aem.2007.02.034. [DOI] [PubMed] [Google Scholar]
- Coppola C P, Tyree M, Larry K, DiGeronimo R. A 22-year experience in global transport extracorporeal membrane oxygenation. J Pediatr Surg. 2008;43:46–52. doi: 10.1016/j.jpedsurg.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Barton A C, Tuttle-Newhall J E, Szalados J E. Portable power supply for continuous mechanical ventilation during intrahospital transport of critically ill patients with ARDS. Chest. 1997;112:560–563. doi: 10.1378/chest.112.2.560. [DOI] [PubMed] [Google Scholar]
- Blakeman T C, Robinson B R, Branson R D. Battery performance of 4 intensive care ventilator models. Respir Care. 2010;55:317–321. [PubMed] [Google Scholar]
- Warren J, Fromm R E, Orr R A, Rotello L C, Horst H M. American College of Critical Care Medicine. Guidelines for the inter and intrahospital transport of critically ill patients. Crit Care Med. 2004;32:256–262. doi: 10.1097/01.CCM.0000104917.39204.0A. [DOI] [PubMed] [Google Scholar]
- Peek G J, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany M M. et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]