Abstract
• Background and Aims Information on the initial growth characteristics of annuals found in Chinese deserts is very limited. The aim of this study was to investigate seed germination and interactive effects of irrigation and seed burial depth in sand on seedling emergence and seedling survival in three annuals (Agriophyllum squarrosum, Bassia dasyphylla and Aristida adscensionis) commonly growing on sand dunes in these regions.
• Methods Effects of temperature, light and polyethylene glycol-6000 on seed germination were examined by irrigating seeds sown on filter paper in Petri dishes. Seedling emergence was examined for seeds sown on the surface of, or at different depths (5, 10, 20, 30, 40 and 50 mm) in, sand-filled pots, which were irrigated under different regimes. For seeds buried at a depth of 50 mm, seed viability was examined after irrigation of the pots.
• Key Results Seeds of three species germinated at most temperatures recorded between spring and autumn in their native habitats. No seed dormancy was found in any species. For all three species, seedling emergence was most favoured when seeds were buried at a depth of 10 mm. When seeds sown on the sand surface were irrigated, seed germination was considerably suppressed due to water deficiency, but many seeds remained viable. For A. squarrosum and B. dasyphylla, many seeds that were deeply buried and irrigated remained ungerminated but viable, while for A. adscensionis deeply buried seeds germinated, but the seedlings did not emerge due to unfavourable seedling growth in deep sand.
• Conclusions Precipitation is the most crucial factor in determining the seasonal emergence of seedlings of the three tested species in the field. The vertical distribution of seeds in sand determines the proportion of seeds that germinate after precipitation and acts to maintain seed banks over multiple years.
Keywords: Agriophyllum squarrosum, Aristida adscensionis, Bassia dasyphylla, irrigation regimes, mechanical resistance of sand, oxygen deficiency, sand hardening, seed burial depth, seedling survival, temperature effects
INTRODUCTION
In desert environments, plant survival is strongly limited by temporal changes in water availability. Compared with mature plants, seedlings are more likely to suffer from water deficiency (Beatley, 1967; Ackerman, 1979; Jordan and Nobel, 1981) because their short roots are distributed only in dry shallow soil layers. Since seed germination determines when and where seedling growth begins, the success of seedling establishment depends greatly on seed germination responses to the environment. For annual plants, which produce seed only once, seed germination responses to environmental conditions are crucial for recruitment.
Many studies have been reported on seed germination and seedling emergence of annuals growing in deserts in North America (Tevis, 1958; Beatley, 1967; Freas and Kemp, 1983; Baskin et al., 1993; Philippi, 1993a, b), Australia (Mott, 1972, 1974; Mott and McComb, 1974) and Israel (Koller, 1957; Loria and Noy-Meir, 1979/1980; Gutterman and Evenari, 1994); however, information is limited on the initial growth of plants found in deserts of China and neighbouring countries (Kigel, 1995).
Deserts in China are characterized by a colder climate compared with many other deserts in the world—mean winter temperature in Chinese deserts is below 0 °C, and winter is an unfavourable season for seed germination and plant growth. This is in contrast to many other deserts, in which different annuals appear in winter and summer. Precipitation is highest in summer due to a continental monsoon (Fullen and Mitchell, 1994), especially in middle to eastern parts of the desert regions in China. Another characteristic of deserts in China is that a high proportion of the area is composed of sand dunes with different degrees of stability (Fullen and Mitchell, 1994).
Precipitation moistens sand differently depending on sand depth—on the one hand, sand near the surface is moistened even by a light rainfall, but moisture near the sand surface evaporates quickly; on the other hand, sand in deep layers will be moistened only after a heavier rainfall, but moisture in deeper sand is expected to persist longer due to a lower evaporation rate. Thus, seeds located at different depths in sand would be exposed to different moisture conditions after a precipitation event. In addition, seed germination and seedling emergence are expected to be affected by the depth of seed burial in the sand.
Apart from moisture conditions, other depth-dependent environmental factors may affect seed germination and seedling emergence in sand. While seeds of some species will germinate irrespective of how deep they are buried (Watkinson, 1978; Maun and Riach, 1981), seeds of other species when buried deeply do not germinate (Pemadasa and Lovell, 1975; Wang et al., 1998) or dormancy is induced (Maun and Lapierre, 1986; Zhang and Maun, 1990), even if the seeds do not require light for germination. When seeds in sand germinate, the deeper the seeds are buried, the farther the seedlings must elongate to emerge from the sand surface.
This study investigated seed germination and seedling emergence of three annuals commonly found on desert sand dunes in China: Agriophyllum squarrosum (Chenopodiaceae), Bassia dasyphylla (Chenopodiaceae) and Aristida adscensionis (Poaceae). Agriophyllum squarrosum is found on mobile sand dunes, while the other two species are found on semi-stable or stable sand dunes. This study included examining (1) effects of temperature, light and polyethylene glycol (PEG)-6000 on seed germination, and (2) emergence and survival of seedlings when seeds were sown on the surface of, or at different depths in, sand, and irrigated under different regimes. In addition, an attempt was made to determine the fates of seeds that were deeply buried but from which seedlings did not emerge at the sand surface after irrigation.
MATERIALS AND METHODS
Seeds
Seeds of A. squarrosum were collected in October 1995 and of the other two species in October 1997 from desert sand dunes in Shapotou, China (37°26′N, 104°57′E), except the B. dasyphylla seeds used for expt 1 (a) and the A. adscensionis seeds used for expt 1 (b) which were collected in October 1995. Mean seed weights of A. squarrosum, B. dasyphylla and A. adscensionis were 1·8, 1·0 and 1·3 mg, respectively. The seeds collected were initially stored at room temperature until they were transported (within 2–3 months) to Japan. In Japan the seeds were stored in a refrigerator (approx. 0 °C), except for those used for seed longevity tests. During this storage period, the seeds stored in the refrigerator were periodically tested for germination by incubating the seeds with deionized water at 20 °C in darkness. Neither an indication of seed dormancy nor a reduction in the ability of seeds to germinate (germination percentage >85 %) was found. Experiments were carried out from April 1997 to March 2004.
Annual mean precipitation is 188 mm and annual mean temperature is 10·4 °C in Shapotou (1990–1995). Eighty-seven per cent of annual precipitation occurred between April and September and 55 % in July and August (1990–1995). Between October and early June, daily precipitation was usually <8 mm and periods of precipitation were often interrupted by long dry days; however, between late June and September, daily precipitation often exceeded 16 mm and precipitation occurred at shorter intervals. Annual variation in precipitation between April and September in 1990–1995 was rather modest (max. 212 mm in 1995; min. 104 mm in 1991), compared with those reported for other deserts in North America and Israel (Tevis, 1958; Loria and Noy-Meir, 1979/1980).
Expt 1: germination experiments
Replicates of 25 seeds were sown on three layers of filter paper (no. 1) in a 90-mm glass Petri dish. About 15 ml of deionized water or a PEG-6000 solution was added to each dish so that about half the volume of each seed was immersed. The Petri dishes were covered with lids and maintained at a constant or diurnally alternating temperature regime in the dark or in 12 h light/12 h dark in an incubator. When the temperature was alternated, the seeds were exposed to a lower temperature for 12 h in the dark and to a higher temperature for 12 h in the light. In the light, the seeds were illuminated with fluorescent lamps (photon flux density (PFD) at wavelengths 400–700 nm at the surface of the seeds: 80–90 µmol m−2 s−1). During exposure to light, the temperature of the fluid in the Petri dishes was approx. 2 °C higher than that in the incubator. For all treatments, except for (c) below, seeds were observed daily through a magnifying glass with a scale, and seeds with radicles longer than 5 mm were regarded as having germinated and were discarded; about two-thirds of the volume of the fluid in each Petri dish was replaced daily. For dark treatments, daily seed observation was performed under dim light (PFD; approx. 2 µmol m−2 s−1); during each day's observation, seeds were usually illuminated with dim light for 15–60 s. After 10, 14 or 20 d of incubation, the final germination percentage (GF) was determined. Each treatment was replicated four times.
(a) Seed longevity. Agriophyllum squarrosum and B. dasyphylla seeds collected in October 1995 were initially stored at room temperature for 2 months in China, and then in a refrigerator (approx. 0 °C) for 4 months in Japan; thereafter, some of the seeds were stored in the refrigerator and others were stored at room temperature (approx. 23 °C). Seed germination was examined by incubating the seeds at 20 °C in the dark for 14 d for seeds stored at approx. 23 °C for 0·8, 6·3 or 7·1 years (1·3, 6·9 or 7·7 years after seed maturation). Seed germination was evaluated by GF.
(b) Effects of temperature and light on seed germination. To examine the effects of temperature and light on seed germination, seeds were incubated with deionized water in the dark or in 12 h light/12 h dark under a different temperature regime. GF was determined after 10 d of incubation for B. dasyphylla and after 14 d of incubation for the other two species.
(c) Seed germination in complete darkness. Even brief illumination with dim light, such as that used to observe seeds daily, can stimulate seed germination (Dixit and Amritphale, 1996); therefore seed germination in darkness in the above experiment (b) may not be illustrative of the germination behaviour of seeds completely deprived of light. Thus, seed germination was examined when seeds were incubated at 12 h 15 °C/12 h 25 °C in darkness for 14 d without daily observation. Seed germination was evaluated by GF.
(d) Effects of PEG and light on seed germination. Seeds were incubated at 20 °C in either darkness or 12 h light/12 h dark with deionized water or a PEG-6000 solution of known water potential (ΨW) (0 to −1·0 MPa for A. squarrosum and 0 to −1·7 MPa for the other two species). The PEG solution of known ΨW was prepared according to a calibration curve that was determined from isopiestic psychrometer measurements (Boyer and Knipling, 1965) at 20 °C. After incubation for 20 d, GF was determined.
Expt 2: examination of seedling emergence
Replicates with 16 seeds were sown on the surface of or at different depths (5, 10, 20, 30, 40 and 50 mm) in sand in cylindrical plastic pots (inner diameter, 80 mm; sand surface area, approx. 50 cm2) with a height of 50 mm (short pots) or 100 mm (long pots). At the bottom of each pot was a circular drainage hole (diameter, 12 mm) covered with cloth. Sand, collected from a seashore in Japan, was washed repeatedly to remove salt and mud and air-dried before being put into the pots to a depth of 45 mm (short pots) or 90 mm (long pots). The particle-size distribution of the sand (91 %wt was 63–250 µm) was similar to that of the sand dunes in Shapotou (Buckley et al., 1986).
The pots were placed in an incubator and maintained at 12 h 25 °C (light)/12 h 15 °C (dark), which is representative of the temperature conditions in Shapotou from late spring to early summer when seeds are expected to germinate. The pots were illuminated with light in the light period as described in expt 1 (PFD at the surface of the sand, 80–130 µmol m−2 s−1). Relative humidity in the incubator was usually 40–70 % in the 25 °C (light) and 90–95 % in the 15 °C (dark) periods (mean relative humidity in Shapotou was 59 % in July 1996). Irrigation was performed at the beginning of a light period by slowly and evenly dripping water on the sand with a pipette. The pots were observed daily and the number of emerging and surviving seedlings in each pot was counted. A seedling was regarded to have emerged when its height exceeded 3 mm or it extended cotyledons, and a seedling was regarded to have died when it fell over or most of the seedling turned yellowish or brownish. Treatments were replicated five or six times.
(a) Seedling emergence and survival when seeds were sown on the surface of or at a depth of 10 mm in sand and irrigated under different regimes. Replicates of 16 seeds were sown on the sand surface or at a depth of 10 mm in short pots. The pots were either initially irrigated with water of 16 mmP (x mmP denotes irrigation equivalent to x mm precipitation) and subsequently with water of 3 mmP at 1-d intervals for 14 d, or initially irrigated with water of 8 mmP and subsequently with water of 3 mmP at 2-d, 4-d, or 6-d intervals for 14 d.
Where seeds were sown on the surface of the sand and irrigated for 14 d, these seeds which did not develop established seedlings were transferred to a Petri dish and were held in an incubator (12 h 15 °C/12 h 25 °C in the dark) as described in expt 1 (b), and seed germination and seedling growth were observed daily for another 10 d. On the other hand, to examine whether seeds remained viable after an unfavourable irrigation regime (seeds sown at a depth of 10 mm and irrigated at 6-d intervals), pots were irrigated with 8 mmP water on the 14th day and subsequently with 3 mmP water at 1-d intervals for 10 d, and emergence of seedlings was observed daily.
(b) Seedling emergence and survival when seeds were buried at different depths in sand and irrigated under different regimes. Replicates of 16 seeds were sown at different depths (5, 10, 20, 30, 40 and 50 mm) in pots (long pots). The pots were either initially irrigated with deionized water of 8, 16 or 24 mmP and subsequently not irrigated at all for 16 or 20 d ([8+0], [16+0] and [24+0] treatments, respectively), or they were irrigated initially with deionized water of 16 mmP and subsequently with water of 3 mmP at 1-d intervals for 20 or 35 d ([16+3] treatment).
To investigate the fate of seeds that were deeply buried and did not generate emerging seedlings after irrigation, seeds sown at a depth of 50 mm in pots and treated with the [16+3] irrigation for 35 d or with the [16+0] or [24+0] irrigation for 20 d were dug out from the sand, and examined for evidence of germination. Ungerminated seeds and seeds with emerging seedlings <30 mm long were transferred to a Petri dish and were held in an incubator (12 h 15 °C/12 h 25 °C in the dark) as described in expt 1 (b), and seed germination and seedling growth were observed daily for another 10 d.
To evaluate the moisture content of the sand at different depths during this experiment, long pots in which no seeds were sown were irrigated under the same conditions as in each irrigation regime of this experiment. On different days after the beginning of irrigation, sand at each of the different depth ranges in the pot was weighed to determine the moist sand weight, and after the sand was dried at 100 °C for >1 d, the dry sand weight was determined; the weight of water in the sand was determined by subtracting the dry sand weight from the moist sand weight. The moisture content in each sand sample was determined as [(kg water in sand)/(kg dry sand)] × 100 %. For the [16+3] treatment, the moisture content in the sand was measured just before the daily 3-mmP irrigation (1 d after the last 3-mmP irrigation).
Statistical analysis
Student's t-test was used to statistically test the difference between two means. For comparison of multiple means, two-way ANOVA or Tukey's test was used. Percentage values were arcsine transformed prior to statistical analysis. Statistical tests were conducted at P = 0·05.
RESULTS
Seed longevity
GF of seeds 7·0 years after maturation, following storage at approx. 0 °C, was 96 ± 1·6 % for A. squarrosum and 95 ± 1·9 % for B. dasyphylla (mean ± s.e.). GF of B. dasyphylla seeds stored at approx. 23 °C was 85 ± 4·7 % after 0·8 years but fell to 0 ± 0·0 % after 6·3 years. For A. squarrosum, GF of seeds stored at approx. 23 °C was 88 ± 4·5 %, 76 ± 5·6 % and 13 ± 5·2 % after 0·8, 6·3 and 7·1 years, respectively.
Effects of temperature and light on seed germination
Seed germination in both darkness and light/dark was most rapid for B. dasyphylla, followed by A. adscensionis and A. squarrosum at 15 °C/25 °C (Fig. 1) and at many other temperatures. The effect of temperature was significant (P = 0·05; two-way ANOVA, temperature × light) for GF in all three species. No temperature fluctuation requirement for seed germination was found for any species. Seed germination of B. dasyphylla was favoured more at lower temperatures (GF in darkness was 94 % at 5 °C and 15 % at 40 °C) (Fig. 2B), while GF of A. adscensionis seeds was high at higher temperatures (GF in darkness was 33 % at 10 °C and 73 % at 40 °C; Fig. 2C). For A. squarrosum (Fig. 2A), although GF in darkness was low at both high (e.g. 8 % at 30 °C) and low (e.g. 4 % at 10 °C) constant temperature regimes, GF in darkness was high in alternating temperature regimes with the high and low temperatures (e.g. 99 % at 10 °C/30 °C).
Fig. 1.
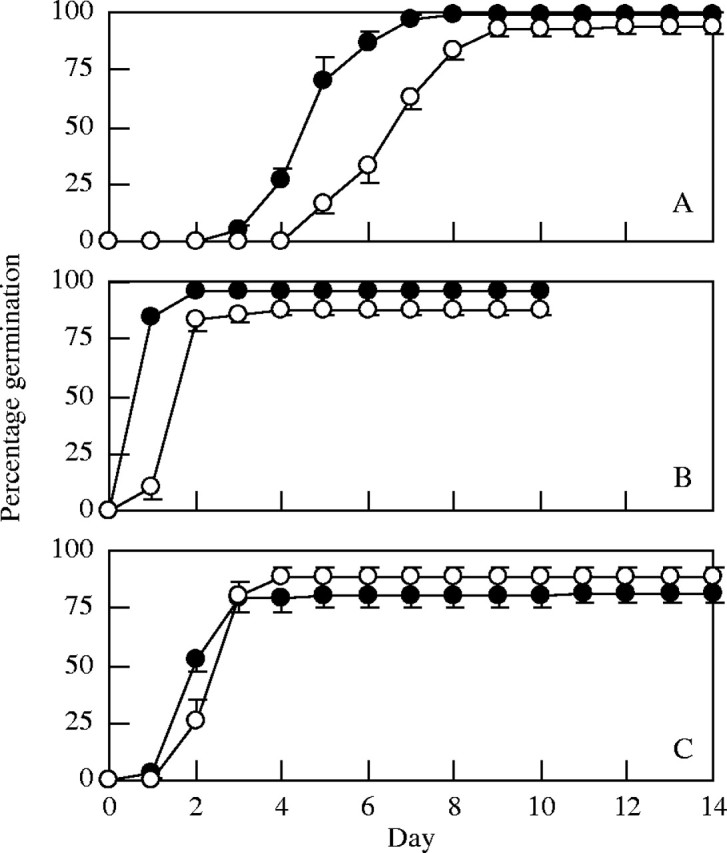
Changes over time in percentage germination of the three tested species (A, A. squarrosum; B, B. dasyphylla; C, A. adscensionis) at 15 °C/25 °C in either dark (filled circles) or light/dark (open circles).Each point represents the mean of four replications; error bars indicating standard error are shown only where it was larger than the point size.
Fig. 2.
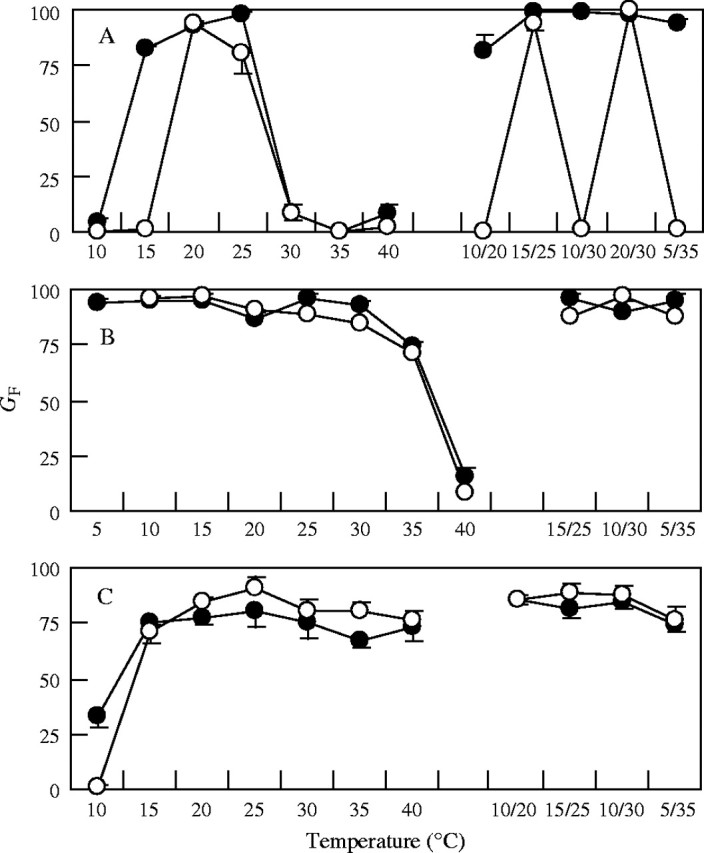
Final percentage germination (GF) of seeds (A, A. squarrosum; B, B. dasyphylla; C, A. adscensionis) at different temperature regimes in dark (filled circles) or light/dark (open circles). Each point represents the mean of four replications; error bars indicating standard error are shown only where it was larger than the point size.
In Shapotou, the means of the daily lowest and highest temperatures in April 1996 were, respectively, 4·2 °C and 17·4 °C, and both the lowest daily mean temperature and highest mean temperature were greatest in July (18·0 °C and 29·0 °C, respectively, in 1996). Comparing the data in the field with the results of the present experiment, it was found that seeds of these three species will germinate, at least in the dark, at most temperatures recorded between spring and autumn in Shapotou and many other desert regions in China.
The effect of light on GF was significant only for A. squarrosum. Seeds of A. squarrosum showed a characteristic interaction of the effects of light and temperature (Fig. 2A)–for temperature regimes at which the temperature of the dark period was 10 °C or lower, and for a constant temperature of 15 °C, GF was almost zero in light/dark but higher than 90 % in darkness; in other temperature regimes, GF was similar between light/dark and dark treatments. The fact that temperature in the dark period was important for seed germination of A. squarrosum suggested that some temperature-dependent germination process proceeds only in the dark period for this species. This was supported by the result of an additional experiment, which indicated that GF was zero at 20 °C in continuous light (data not presented) compared with 94 % in 12 h light/12-h dark at 20 °C (Fig. 2).
Germination experiments in complete darkness (without daily observation in dim light) showed that GF of both B. dasyphylla and A. adscensionis in complete darkness exceeded 90 % and was not significantly different from that of seeds observed daily with dim light. However, for A. squarrosum, GF in complete darkness was high (75 ± 6·7 %; mean ± s.e.) but significantly lower than that of seeds observed daily (99 ± 1·0 %). It is possible that the lower GF of A. squarrosum seeds maintained in complete darkness resulted from water in the dish not being changed daily, because for seeds of some species, leaching of germination-inhibiting chemicals from seeds is necessary for seed germination (Koller, 1957; Barbour, 1968). To examine this possibility, an additional experiment was carried out in which A. squarrosum seeds in dishes were observed daily in dim light, but water in the dishes was not replaced at all; however, GF in this treatment was also high (100 ± 0·0 %), indicating that the lower GF of A. squarrosum seeds maintained in complete darkness is not attributable to the water not being changed daily. The result indicated that, although a proportion of A. squarrosum seeds may require low-fluence light for their germination, seeds of all three species do not substantially require light for germination.
Effects of PEG and light on seed germination
The effect of ΨW on GF was significant in all three species, and the effect of light on GF was significant for A. squarrosum and B. dasyphylla (P = 0·05; two-way ANOVA, ΨW × light). Effects of light on the germination responses to PEG were most pronounced in A. squarrosum (Fig. 3A). Both the ΨW at which GF began to decrease and the ΨW at which GF approached 0 % varied among species. The ΨW at which GF in darkness decreased to 50 % of that at 0 MPa was the lowest (approx. −1·6 MPa) for B. dasyphylla, followed by A. adscensionis (approx. −1·0 MPa), and the highest for A. squarrosum (approx. −0·8 MPa).
Fig. 3.
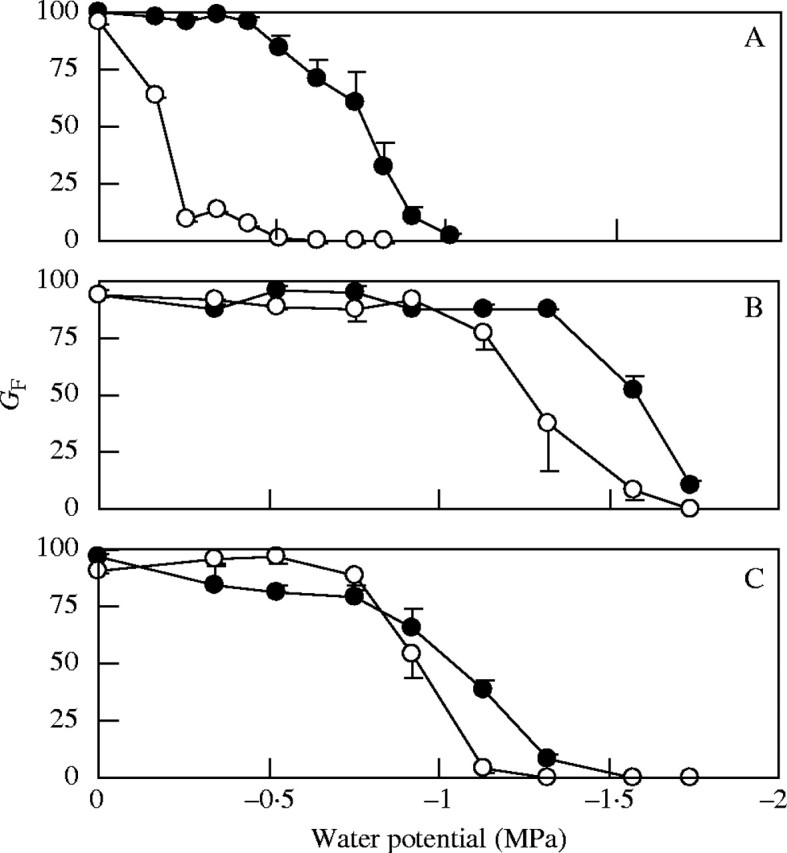
Final percentage germination (GF) of seeds (A, A. squarrosum; B, B. dasyphylla; C, A. adscensionis) when seeds were treated with a PEG solution of a known water potential at 20 °C in dark (filled circles) or light/dark (open circles). Each point represents the mean of four replications; error bars indicating standard error are shown only where it was larger than the point size.
Seedling emergence and survival when seeds were sown on the surface of, or at a depth of 10 mm in, sand and irrigated under different regimes
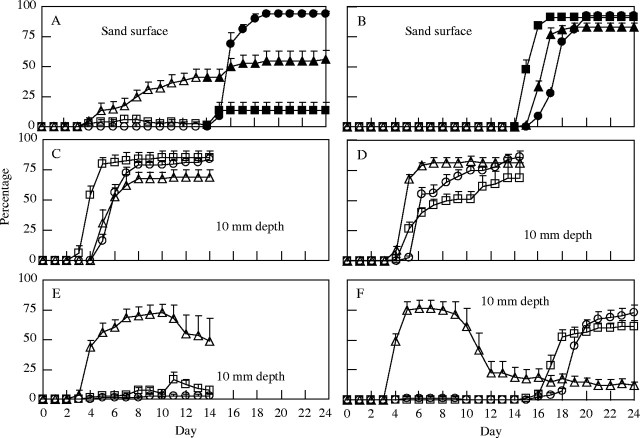
For A. adscensionis seeds sown on the surface of sand and irrigated at 1-d intervals (Fig. 4A), 41 % of seeds generated normal established seedlings (although root bases were exposed by 2–3 mm above the sand). In addition, 19 % were found to have germinated on the sand, but the emerging radicles and plumules desiccated, and the seedlings did not develop into normal seedlings. When A. adscensionis seeds that did not develop into established seedlings were transferred to Petri dishes, only 4 % of them subsequently germinated. For B. dasyphylla seeds sown on the surface of the sand and irrigated at 1-d intervals, only 1 % of seeds became established; and 19 % of seeds were found to have germinated, but their radicles elongated laterally and eventually desiccated. When B. dasyphylla seeds were transferred to Petri dishes, most seeds germinated, but only 13 % of seeds developed normal radicles, and the rest developed abnormal radicles, which were missing tips and elongated upward. Only normally germinated seeds of B. dasyphylla were counted in the germination percentage shown in Fig. 4A. For A. squarrosum, no seeds germinated on the surface of the sand, but after transfer to Petri dishes, 94 % of the seeds germinated normally. When seeds were sown on the surface of the sand and irrigated at 2-d intervals (Fig. 4B), no seeds of any species germinated on the sand surface, and after transfer to Petri dishes, high percentages of seeds germinated normally.
Fig. 4.
Percentage of emerging and surviving seedlings when seeds were sown on the surface (A and B) or at the 10-mm depth (C–F) of sand (open symbols). The pots were irrigated initially with water of 16 mmP (A and C) or 8 mmP (B, D, E and F), and subsequently with 3 mmP at 1-d (A and C), 2-d (B and D), 4-d (E), or 6-d (F) intervals for 14 d. When seeds were sown on the sand surface, seeds were transferred to Petri dishes on day 14 and observed for another 10 d (the germination percentage is shown by filled symbols). When seeds were sown at the 10-mm depth and irrigated at 6-d intervals, seeds were irrigated with 8 mmP on day 14 and subsequently with 3 mmP at 1-d intervals for 10 d (F). Circles, A. squarrosum; squares, B. dasyphylla; triangles, A. adscensionis. Each point represents the mean of six replications; error bars indicating standard error are shown only where it was larger than the point size.
When seeds were sown at a depth of 10 mm, both irrigation at 1-d (Fig. 4C) and 2-d (Fig. 4D) intervals resulted in high percentages of seedling emergence 4–6 d after the beginning of irrigation in all three species, while irrigation at 4-d or 6-d intervals (Fig. 4E and F) resulted in pronounced seedling emergence only in A. adscensionis. For B. dasyphylla, the percentage of emerging seedlings was higher and seedling emergence was more rapid after irrigation at 1-d intervals than at 2-d intervals, while for A. adscensionis, the percentage of seedling emergence was lower after irrigation at 1-d intervals. When irrigated at both 1-d and 2-d intervals, all the emerging seedlings of all three species survived until the end of the experiment (Fig. 4C and D). However, during irrigation at 4-d or 6-d intervals, some or most of the emerging seedlings died before the end of the initial 14-d treatment.
When pots irrigated at 6-d intervals for 14 d were subsequently irrigated at 1-d intervals (Fig. 4F), 83 % (A. squarrosum), 61 % (B. dasyphylla) or 6 % (A. adscensionis) of seedlings newly emerged. All the newly emerging seedlings and many of the A. adscensionis seedlings surviving at the end of the initial 14-d irrigation survived until the end of the experiment. At the end of the later favourable irrigation, percentages of surviving seedlings were considerably lower in A. adscensionis (12 %) than in B. dasyphylla (61 %) and A. squarrosum (72 %).
Seedling emergence and survival when seeds were buried at different depths in sand and irrigated under different regimes
Moisture content in sand at different depths in long pots irrigated under different regimes is shown in Fig. 5. For single initial-day irrigation treatments ([8+0], [16+0] and [24+0]), an increase in the initial day irrigation resulted in higher moisture contents in all sand layers and moistening of sand in deeper sand layers. When the sand appeared to be dry, the sand had a minimum moisture content of approx. 0·6 %, which was regarded to be the moisture content of the sand when water is in equilibrium between the air and the sand. Moisture in deeper sand layers persisted for longer periods, while moisture in shallower sand layers was lost quickly. For example, 8-mmP irrigation caused sand moistening to a depth of 30 mm and most of the moisture in the sand was lost within 5 d, while 24-mmP irrigation moistened all the sand layers in the pot and moisture persisted in deeper sand layers on 25 d after irrigation (moisture content at 60–90 mm, 2·6 %).
Fig. 5.
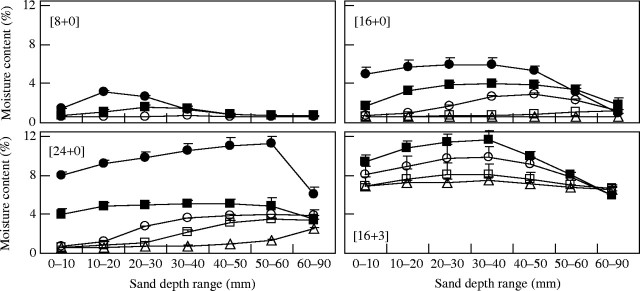
Moisture content in sand at different depth ranges in pots when the pots were irrigated initially with water equivalent to 8 mm, 16 mm or 24 mm of rainfall and subsequently not irrigated at all ([8+0], [16+0] and [24+0], respectively), or irrigated initially with water equivalent to 16 mm of rainfall and subsequently with water equivalent to 3 mm of rainfall at 1-d intervals ([16+3]). Moisture content in sand was measured 3 d (filled circles), 5 d (filled squares), 10 d (open circles), 15 d (open squares) or 25 d (open triangles) after the beginning of irrigation. Moisture contents 15 d and 25 d after irrigation for [8+0] and moisture content 3 d after the beginning of irrigation for [16+3] were not measured. Each point represents the mean of four replications; error bars indicating standard error are shown only where it was larger than the point size.
On the other hand, the [16+3] treatment maintained sufficiently moist conditions without large variations either temporally or vertically in the moisture content of the sand (Fig. 5). Moisture content 25 d after the beginning of irrigation was in the range 6·5–7·5 % in different sand layers. This result indicated that repeated 3-mmP irrigation after 16-mmP irrigation was effective in keeping the sand moist for relatively long periods, while a single 4-mmP irrigation moistened only a 0–10 mm sand layer for <3 d (data not shown).
To investigate seedling emergence and survival when all sand layers are maintained sufficiently moist, pots were treated with [16+3] irrigation (Table 1). In this treatment, percentage seedling emergence of B. dasyphylla and A. adscensionis was considerably lower at a seed burial depth of 20 mm than at 10 mm, and was almost zero at seed burial depths of 30 and 40 mm. For seeds of A. squarrosum, however, percentage seedling emergence decreased less markedly with increasing seed burial depth, although seedling emergence was considerably slower and tended to continuously increase until the end of the experiment when the seed burial depth was 20 mm or more. For all three species, all the emerging seedlings survived until the end of the experiment.
Table 1.
Final percentage of emerging seedlings when seeds sown at different depths in sand in pots were treated with [16+3] irrigation for 20 d, or [16+0] or [8+0] irrigation for 16 d (refer to the caption of Fig. 5 for each treatment)
|
Agriophyllum squarrosum |
Bassia dasyphylla |
Aristida adscensionis |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depth (mm) |
[16+3] |
[16+0] |
[8+0] |
[16+3] |
[16+0] |
[8+0] |
[16+3] |
[16+0] |
[8+0] |
||||||
| 5 | – | 10c,d | 0d | – | 55a | 7b | – | 56a,b,c | 14d,e | ||||||
| 10 | (84a) | 41b | 0d | (85a) | 64a | 19b | (69a,b) | 76a | 69a,b | ||||||
| 20 | 76a | 12c,d | 9c,d | 16b | 19b | 2b | 19d,e | 67a,b | 40b,c,d | ||||||
| 30 | 52a,b | 7c,d | 0d | 0b | 2b | 1b | 4e | 27c,d | 2e | ||||||
| 40 | 30b,c | – | – | 0b | – | – | 2e | – | – | ||||||
For each species, the values with the same letter are not significantly different from each other (P = 0·05; Tukey's test).
Dashes denote that no experiment was carried out under these conditions.
Data from Fig. 4 are indicated in parenthesis.
Data are the means of five replications.
To determine the amount of precipitation that results in seedling emergence and survival, pots were treated with [8+0] or [16+0] irrigation (Table 1). In both treatments, percentage seedling emergence tended to be higher at a seed burial depth of 10 mm than at 5 mm (Table 1). At seed burial depths of 10 mm or more, percentage seedling emergence of all three species decreased with increasing seed burial depth, except that in the [8+0] treatment, seedling of A. squarrosum emerged only from seeds buried at a depth of 20 mm.
In the [16+0] treatment, percentage seedling emergence was lower for A. squarrosum and B. dasyphylla than for A. adscensionis at seed burial depths of 20 and 30 mm. For A. adscensionis, seedlings generally tended to emerge more in the [16+0] treatment than in the [16+3] treatment, indicating that higher sand moisture content did not always favour seedling emergence of this species. On the other hand, for A. squarrosum, percentage seedling emergence was lower in the [16+0] treatment than in the [16+3] treatment. No marked difference in percentage seedling emergence was evident for B. dasyphylla between the [16+3] and the [16+0] treatments. Less than 30 % of emerging seedlings of B. dasyphylla and A. adscensionis died before the end of the experiment. For A. squarrosum, 90 % and 61 % of seedlings emerging from seeds buried at respective depths of 5 and 10 mm died before the end of the experiment, while at seed burial depths of 20 and 30 mm, seedling emergence of this species was considerably slower, and all the emerging seedlings survived until the end of the experiment.
Percentage seedling emergence was lower in the [8+0] treatment than in the [16+0] treatment for all three species (Table 1). Seedling emergence in the [8+0] treatment was the most favourable for A. adscensionis and the least favourable for A. squarrosum. In the [8+0] treatment, seedlings of B. dasyphylla and A. adscensionis began to emerge after 2–6 d from irrigation but all the seedlings died after 4–6 d from emergence. For A. squarrosum, seedlings emerged 6–12 d after the irrigation from 9 % of seeds sown at a depth of 20 mm, and some of them survived until the end of the experiment.
To investigate the fate of deeply buried seeds from which seedlings did not emerge at the sand surface, seed germination and seedling growth were examined when seeds were irrigated ([16+3], [16+0], [24+0]; Table 2) in sand at a depth of 50 mm and subsequently transferred to Petri dishes. Percentage seedling emergence from sand was 1–7 % for A. squarrosum and 0 % for the other two species. When seeds were dug out from the sand, the percentage of seeds that had germinated, but from which seedlings did not emerge at the surface of the sand, were 0–5 % for A. squarrosum and 2–10 % for B. dasyphylla (Table 2); all the radicles or seedlings emerging from A. squarrosum seeds in sand were white and healthy, but all the radicles emerging from B. dasyphylla seeds in sand were short (5–17 mm) and brownish. For A. adscensionis, many (40–49 %) seeds seemed to have germinated in the sand, but all the radicles and plumules (total length 1–12 mm) emerging in the sand were brownish, and some were detached from seeds or decomposed. When seeds dug out from the sand were transferred to Petri dishes, high percentages of A. squarrosum and B. dasyphylla seeds germinated newly 2–3 d (A. squarrosum) or 1–2 d (B. dasyphylla) after transfer. For both species, the germination velocity was similar to that when seeds that had not been pre-irrigated in sand were irrigated in Petri dishes under the same conditions. The percentage of seed germination in Petri dishes was lower after the [16+3] treatment than after any of the other treatments. For seeds that had germinated in sand, the emerging radicles of A. squarrosum continued to elongate in Petri dishes, whereas those of B. dasyphylla did not elongate in Petri dishes. For A. adscensionis, no seeds germinated newly in Petri dishes and no emerging radicles or plumules continued to elongate in Petri dishes.
Table 2.
Fates of seeds sown at a depth of 50 mm and treated with [16+3] irrigation for 35 d, or [16+0] or [24+0] irrigation for 20 d (refer to the caption of Fig. 5 for each treatment)
| Species |
Emerged from sand |
Germinated in sand but not emerged |
Newly germinated in Petri dishes |
Total germination |
||||
|---|---|---|---|---|---|---|---|---|
| [16+3] (35 d after beginning of irrigation) | ||||||||
| A. squarrosum | 7 (4·6) | 1 (1·3) | 59 (8·9) | 68 (9·2) | ||||
| B. dasyphylla | 0 (0) | 11 (2·3) | 62 (9·8) | 73 (9·5) | ||||
| A. adscensionis | 0 (0) | >40 (5·8) | 0 (0) | >40 (5·8) | ||||
| [16+0] (20 d after irrigation) | ||||||||
| A. squarrosum | 1 (1·2) | 0 (0) | 91 (2·5) | 92 (1·2) | ||||
| B. dasyphylla | 0 (0) | 9 (3·5) | 82 (3·7) | 91 (5·0) | ||||
| A. adscensionis | 0 (0) | >49 (4·4) | 0 (0) | >49 (4·4) | ||||
| [24+0] (20 d after irrigation) | ||||||||
| A. squarrosum | 1 (1·3) | 5 (3·2) | 84 (4·1) | 91 (3·1) | ||||
| B. dasyphylla | 0 (0) | 2 (1·5) | 85 (4·6) | 88 (4·0) | ||||
After irrigation in pots, ungerminated seeds in sand were transferred to filter paper in Petri dishes and irrigated with water, and seeds in the Petri dishes were examined for germination for another 10 d.
Data are the means of five replications with standard errors in parenthesis.
In an additional experiment, seeds of these species that had not been pre-irrigated in sand were irrigated on filter paper in Petri dishes at 12 h 15 °C/12 h 25 °C in the dark. For all three species, high percentages of seeds germinated and produced normal seedlings in Petri dishes, the lengths of the seedlings (mean ± s.e.) being 58 ± 7·5 mm (A. squarrosum; 7 d after irrigation; n = 20), 74 ± 5·2 mm (B. dasyphylla; 4 d after irrigation; n = 21) and 69 ± 3·5 mm (A. adscensionis; 5 d after irrigation; n = 19). This result indicated that the inhibition of seedling elongation of B. dasyphylla and A. adscensionis in deep sand was caused by one or more factors in the deep sand environment.
DISCUSSION
Seeds of all three tested species germinated at most temperatures common between spring and autumn in Shapotou and many other desert regions in China, indicating that temperature is not a critical factor limiting seed germination of these species in the field. Moreover, seed dormancy was not found in any of the three species. Additionally, seed longevity of A. squarrosum and B. dasyphylla at room temperature was rather short. These results suggest that seeds of the three species tested germinate readily and tend not to remain ungerminated for long periods.
In some reports on seed bank dynamics of desert plants, persistence in seed banks has been reported to be important for species adaptation to environments with unpredictable precipitation (Philippi, 1993a, b). On the other hand, others reported rapid depletion of seed banks for species distributed in deserts of North America and Australia (Mott, 1972; Kemp, 1989; Kigel, 1995). It seems that characteristics of seed banks (temporary vs. persistent) in desert environments depend on the reliability and abundance of precipitation in the growing season of plants (Freas and Kemp, 1983; Baskin et al., 1993).
The fact that three annuals tested in this study showed rather temporary characteristics in germination responses may partly be attributable to monoseasonality of plant growth in deserts of China, where a dry and cold winter is disadvantageous for seed germination and plant growth. This is in contrast to the conditions in many other desert regions in the world, where dependence of seed germination on temperature (Went, 1948; Mott, 1972; Ackerman, 1979) or seed dormancy (Mott, 1972; Baskin et al., 1993) determines seasonal emergence of different species. In addition, higher predictability of precipitation in deserts in China may be related to the germination characteristics of these species. That is, in Shapotou and many other deserts in China, a greater amount of precipitation is expected annually in the summer; many seeds will germinate in late spring to early summer and the emerging seedlings are provided with sufficient water, resulting in the survival of a large proportion of seedlings.
Photoinhibition of seed germination was found to be very noticeable in A. squarrosum, but was very modest in the other two species. Photoinhibition of seed germination has been reported in a wide range of species including those distributed in deserts (Koller, 1957; Koller et al., 1963; Barbour, 1968) and shore dunes (Thanos et al., 1991, 1994), and is reported to inhibit the germination of seeds located on the surface where emerging seedlings would be likely to die of desiccation. Nevertheless, it is unknown whether photoinhibition substantially affects seed germination of these species in the field, because as will be discussed later, inhibition of the germination of seeds located on the surface in the field may result mostly from limitations of seed water uptake.
Water potentials that reduced seed germination of the three species (−1·0 MPa to −2·0 MPa) were not greatly different from those that reduced seed germination of other species distributed in deserts (e.g. Mott, 1974) or more mesic habitats (e.g. Evans and Etherington, 1990). Among the three species, A. squarrosum had reduced germination at the highest water potential and took the longest time to germinate after irrigation, and required the most favourable irrigation for seedling emergence from sand. This may be related to the fact that the habitat of A. squarrosum is mobile sand dunes in which the water conditions in sand are more favourable than in semi-stable or stable sand dunes (Mo et al., 1997) where the other two species grow. On the other hand, while B. dasyphylla seeds germinated more rapidly and at lower water potentials than A. adscensionis seeds, seedling emergence from sand with limited irrigation was favoured more for A. adscensionis than for B. dasyphylla. Sand does not tend to strongly retain water by matric forces (Bowers, 1982), and seeds in sand tend to be exposed to either favourable or unfavourable water conditions for seed water uptake. In such environments, seed germinability at lower water potentials may not favour seed germination. One possibility for favourable seedling emergence of A. adscensionis is that water absorbed by appendages of A. adscensionis seeds persisted longer and water was supplied to the seeds even after the sand had dried. The longer imbibition time of A. adscensionis seeds may therefore be compensated for by retention of water by seed appendages.
Although the initial 8-mmP irrigation and the subsequent 3-mmP irrigation at 2-d intervals allowed high percentages of seedlings to emerge and survive in all three species, a single 8-mmP irrigation, which moistened sand near the surface only for several days, allowed smaller percentages of seedlings to emerge and was not sufficient to allow emerging seedlings to survive for long in any species. On the other hand, a single 16-mmP irrigation, which caused moistening of sand to deeper sand layers and persistence of moisture for longer periods, resulted in favourable seedling emergence and seedling survival. These results indicate that both the amount and frequency of precipitation are important for seedling emergence and survival.
When these results were compared with precipitation in Shapotou, it was found that emergence or survival of seedlings of the three species tested from early to middle spring is inhibited due to low and infrequent precipitation, and that precipitation from late spring to early autumn results in seed germination, seedling emergence and seedling survival of these species. These results indicate that precipitation is the most important determinant regulating seasonal emergence of seedlings of these species in the field.
The amount and frequency of irrigation required to cause seedling emergence differed among species. This is in agreement with the report of field studies in a desert in Israel by Loria and Noy-Meir (1979/1980). For the species (A. adscensionis) in which the seeds germinated under unfavourable moisture conditions, emerging seedlings were more liable to be killed by desiccation and the seeds to be lost from the seed bank, thus indicating that seed germination under unfavourable moisture conditions is not always advantageous for adaptation to the environment. This indicates that precipitation patterns determine which species are advantageously established. In these experiments, seed density in pots may be higher than that in the field, and competition among seedlings for moisture hastened the death of seedlings that were subjected to unfavourable irrigation regimes. However, general features of the responses of seedlings to moisture conditions in the field are not expected to differ substantially.
Reduced seedling emergence from seeds sown on the sand surface or buried at 5 mm may be due to deficiency of moisture at shallower sand layers (Batanouny and Ziegler, 1971; Maun and Lapierre, 1986). When seeds were sown on the sand surface, seed germination of A. squarrosum was almost completely suppressed even in irrigation at 1-d intervals. This can be attributable to the requirement of the higher water potential and longer imbibition time of this species' seeds for germination, and photoinhibition of seed germination. For the other two species, however, irrigation at 1-d intervals caused germination of considerable percentages of seeds sown on the surface of sand, but germinated seeds did not become established seedlings, except for some of the A. adscensionis seeds. In this irrigation treatment, high percentages of seeds of these two species germinated but many radicles died, because low percentages of seeds germinated normally after transfer to Petri dishes. The radicle death of these two species would have been caused primarily by water deficiency, and some radicles would have died before they became visibly detectable. For B. dasyphylla, very short and nearly invisible radicle tips seemed to have emerged from some seeds but were desiccated and lost, and after transfer of the seeds to Petri dishes, radicles without tips seemed to have emerged from the seeds. Another reason for the failure of the establishment of B. dasyphylla seedlings emerging from seeds sown on the surface of sand seemed to be that the emerging radicles could not penetrate into the sand; the same type of seeds has been reported in other species (Maun and Riach, 1981; Maun and Lapierre, 1986). These results indicate that for most of B. dasyphylla seeds and some of A. adscensionis seeds, seed germination at the surface of sand tends to result in loss of seeds from seed banks. On the other hand, when irrigation was limited to 2-d intervals, the germination of seeds sown on the surface of sand was almost completely suppressed and most seeds remained viable. In these species' habitat, the sand surface frequently may not be moistened sufficiently as in the conditions of the 1-d interval irrigation treatment in this study. In the field, many of these species' seeds located on the surface of sand are expected to remain ungerminated and viable. In addition, photoinhibition may inhibit seed germination on the surface of sand more strongly in the field, where light intensity is much stronger than that in our experimental conditions.
Seedling emergence was suppressed when seeds were buried at 20 mm or deeper for all three species. For A. squarrosum and B. dasyphylla, many seeds buried deeply did not germinate. The results for A. squarrosum in this study are essentially in agreement with those reported by Wang et al. (1998), who examined seed germination of this species when the seeds were buried at different depths in sand and irrigated sufficiently. While seeds of some species were reported to have become dormant when they were deeply buried (Maun and Lapierre, 1986; Zhang and Maun, 1990), this would not be the case for A. squarrosum or B. dasyphylla because their seeds germinated soon after being transferred to Petri dishes. On the other hand, for A. adscensionis, many seeds that were deeply buried seemed to have germinated, but the emerging seedlings seemed to have been prevented from elongating for some reason. A similar response was found in B. dasyphylla seedlings generated from seeds that were deeply buried, while the seedlings of A. squarrosum seemed to elongate favourably in deep sand. Unfavourable growth of seedlings in deep sand has also been reported for other species (Pemadasa and Lovell, 1975; Chen and Maun, 1999).
Two hypotheses can be given to explain the suppression of seed germination of A. squarrosum and B. dasyphylla and inhibition of seedling elongation in A. adscensionis and B. dasyphylla in deep sand. One hypothesis is that deficiency of oxygen in deep sand caused these responses–as water content in sand increases, air content in sand decreases, and the resulting oxygen deficiency may inhibit seed germination and/or seedling elongation. Indeed, a reduction in oxygen availability causes deleterious effects on seed germination and seedling growth in many species (Gill and Miller, 1956; VanderZee and Kennedy, 1981; Crawford, 1992). Based on this hypothesis, more favourable seedling emergence of A. adscensionis with [16+0] treatment than with [16+3] treatment may have resulted from the lower air content in sand with a higher moisture content. However, based on this hypothesis, it is difficult to explain the result of an additional experiment (data not presented) that A. squarrosum seeds buried at a depth of 5 mm in submerged sand were maintained ungerminated but viable, while 90 % of A. squarrosum seeds germinated when they were sown on the surface of submerged sand. Another hypothesis is that higher mechanical resistance of deeper sand impeded seed germination and/or seedling elongation. Mechanical restraint by soil can impede shoot or root growth of seedlings (Gill and Miller, 1956; Barley and Greacen, 1967), or cause adverse effects on seed germination (Collis-George and Williams, 1968; Hadas, 1977). When sand contains water, sand particles coagulate and sand hardens (Hornbaker et al., 1997); hardened sand may suppress seed germination and/or inhibit seedling elongation. At deeper sand layers where the water content is higher, the sand becomes more hardened and the weight of sand in upper layers increases the hardening of the sand, and seedlings need to elongate a greater distance in the hardened sand before they reach the surface. Based on this hypothesis, more favourable seedling emergence of A. adscensionis in sand of lower moisture content can be interpreted to have resulted from the lower hardness of sand with lower moisture content. Germination suppression of A. squarrosum seeds at shallower depths in submerged sand may be attributable to stronger hardening of sand with higher water content. Unfortunately, no clear-cut conclusion can be drawn from the present study on how seed germination was suppressed or seedling elongation was inhibited in deep sand.
The present study indicated that temperature or dormancy is not crucial in determining the seed germination of the three species tested, and suggested that seedling emergence in the field of the three species tested is regulated primarily by precipitation. Many of the A. squarrosum and B. dasyphylla seeds that were located deeply or shallowly in sand and the A. adscensionis seeds that were located shallowly in sand would remain ungerminated but viable even after a heavy rainfall, and these seeds may germinate in future years if they are moved to a depth appropriate for germination. Thus, the vertical distribution of seeds in sand is expected to determine the proportion of seeds that germinate after precipitation and act to maintain seed banks over many years.
LITERATURE CITED
- Ackerman TL. 1979. Germination and survival of perennial plant species in the Mojave Desert. Southwestern Naturalist 24: 399–408. [Google Scholar]
- Barbour MG. 1968. Germination requirements of the desert shrub Larrea divaricata Ecology 49: 915–923. [Google Scholar]
- Barley KP, Greacen EL. 1967. Mechanical resistance as a soil factor influencing the growth of roots and underground shoots. Advances in Agronomy 19: 1–43. [Google Scholar]
- Baskin CC, Chesson PL, Baskin JM. 1993. Annual seed dormancy cycles in two desert winter annuals. Journal of Ecology 81: 551–556. [Google Scholar]
- Batanouny KH, Ziegler H. 1971. Eco-physiological studies on desert plants. II. Germination of Zygophyllum coccineum L. seeds under different conditions. Oecologia 8: 52–63. [DOI] [PubMed] [Google Scholar]
- Beatley JC. 1967. Survival of winter annuals in the northern Mojave Desert. Ecology 48: 745–750. [DOI] [PubMed] [Google Scholar]
- Bowers JE. 1982. The plant ecology of inland dunes in western North America. Journal of Arid Environments 5: 199–220. [Google Scholar]
- Boyer JS, Knipling EB. 1965. Isopiestic technique for measuring leaf water potentials with a thermocouple psychrometer. Proceedings of the National Academy of Sciences of the USA 54: 1044–1051. [PMC free article] [PubMed] [Google Scholar]
- Buckley RC, Chen W, Liu Y, Zhu Z. 1986. Characteristics of Tengger dunefield, north-central China, and comparison with the central Australian dunefields. Journal of Arid Environments 10: 97–101. [Google Scholar]
- Chen H, Maun MA. 1999. Effects of sand burial depth on seed germination and seedling emergence in Cirsium pitcheri Plant Ecology 140: 53–60. [Google Scholar]
- Collis-George N, Williams J. 1968. Comparison of the effects of soil matric potential and isotropic effective stress on the germination of Lactuca sativa Australian Journal of Soil Research 6: 179–192. [Google Scholar]
- Crawford RMM. 1992. Oxygen availability as an ecological limit to plant distribution. In: Begon M, Fitter AH, eds. Advances in biological research, Vol. 23. London: Academic Press, 93–185. [Google Scholar]
- Dixit S, Amritphale D. 1996. Very low fluence response in the induction and inhibition of seed germination in Celosia argentea Seed Science Research 6: 43–48. [Google Scholar]
- Evans C-E, Etherington JR. 1990. The effect of soil water potential on seed germination of some British plants. New Phytologist 115: 539–548. [DOI] [PubMed] [Google Scholar]
- Freas KE, Kemp PR. 1983. Some relationships between environmental reliability and seed dormancy in desert annual plants. Journal of Ecology 71: 211–217. [Google Scholar]
- Fullen MA, Mitchell DJ. 1994. Desertification and reclamation in North-Central China. Ambio 23: 131–135. [Google Scholar]
- Gill WR, Miller RD. 1956. A method for study of the influence of mechanical impedance and aeration on the growth of seedling roots. Soil Science Society of America Proceedings 20: 154–157. [Google Scholar]
- Gutterman Y, Evenari M. 1994. The influences of amounts and distribution of irrigation during the hot and dry season on emergence and survival of some desert winter annual plants in the Negev Desert. Israel Journal of Plant Sciences 42: 1–14. [Google Scholar]
- Hadas A. 1977. Water uptake and germination of leguminous seeds in soils of changing matric and osmotic water potential. Journal of Experimental Botany 28: 977–985. [Google Scholar]
- Hornbaker DJ, Albert R, Albert I, Barabási A-L, Schiffer P. 1997. What keeps sandcastles standing? Nature 387: 765. [Google Scholar]
- Jordan PW, Nobel PS. 1981. Seedling establishment of Ferocactus acanthodes in relation to drought. Ecology 62: 901–906. [Google Scholar]
- Kemp PR. 1989. Seed banks and vegetation processes in deserts. In: Leck MA, Parker VT, Simpson RL, eds. Ecology of soil seed banks. New York: Academic Press, 257–281. [Google Scholar]
- Kigel J. 1995. Seed germination in arid and semiarid regions. In: Kigel J, Galili G, eds. Seed development and germination. New York: Marcel Dekker, 645–699. [Google Scholar]
- Koller D. 1957. Germination-regulating mechanisms in some desert seeds. IV. Atriplex dimorphostegia Kar. et Kir. Ecology 38: 1–13. [Google Scholar]
- Koller D, Poljakoff-Mayber A, Berg A, Diskin T. 1963. Germination-regulating mechanisms in Citrullus colocynthis American Journal of Botany 50: 597–603. [Google Scholar]
- Loria M, Noy-Meir I. 1979. Dynamics of some annual populations in a desert loess plain. Israel Journal of Botany 28: 211–225. [Google Scholar]
- Maun MA, Lapierre J. 1986. Effects of burial by sand on seed germination and seedling emergence of four dune species. American Journal of Botany 73: 450–455. [Google Scholar]
- Maun MA, Riach S. 1981. Morphology of caryopses, seedlings and seedling emergence of the grass Calamovilfa longifolia from various depths in sand. Oecologia 49: 137–142. [DOI] [PubMed] [Google Scholar]
- Mo W, Natori T, Jiang S, Nishimura N, Omasa K. 1997. Responses of photosynthesis and water use to drought in two desert annuals, Agriophyllum squarrosum and Bassia dasyphylla Journal of Arid Land Studies 7: 185–195. [Google Scholar]
- Mott JJ. 1972. Germination studies on some annual species from an arid region of Western Australia. Journal of Ecology 60: 293–304. [Google Scholar]
- Mott JJ. 1974. Factors affecting seed germination in three annual species from an arid region of Western Australia. Journal of Ecology 62: 699–709. [Google Scholar]
- Mott JJ, McComb AJ. 1974. Patterns in annual vegetation and soil microrelief in an arid region of Western Australia. Journal of Ecology 62: 115–126. [Google Scholar]
- Pemadasa MA, Lovell PH. 1975. Factors controlling germination of some dune annuals. Journal of Ecology 63: 41–59. [Google Scholar]
- Philippi T. 1993. Bet-hedging germination of desert annuals: beyond the first year. American Naturalist 142: 474–487. [DOI] [PubMed] [Google Scholar]
- Philippi T. 1993. Bet-hedging germination of desert annuals: variation among populations and maternal effects in Lepidium lasiocarpum American Naturalist 142: 488–507. [DOI] [PubMed] [Google Scholar]
- Tevis L. 1958. Germination and growth of ephemerals induced by sprinkling a sandy desert. Ecology 39: 681–688. [Google Scholar]
- Thanos CA, Georghiou K, Delipetrou P. 1994. Photoinhibition of seed germination in the maritime plant Matthiola tricuspidata Annals of Botany 73: 639–644. [Google Scholar]
- Thanos CA, Georghiou K, Douma DJ, Marangaki CJ. 1991. Photoinhibition of seed germination in Mediterranean maritime plants. Annals of Botany 68: 469–475. [Google Scholar]
- VanderZee D, Kennedy RA. 1981. Germination and seedling growth in Echinochloa crus-galli var. oryzicola under anoxic conditions: structural aspects. American Journal of Botany 68: 1269–1277. [Google Scholar]
- Wang Z-L, Wang G, Liu X-M. 1998. Germination strategy of the temperate sandy desert annual chenopod Agriophyllum squarrosum Journal of Arid Environments 40: 69–76. [Google Scholar]
- Watkinson AR. 1978. The demography of a sand dune annual: Vulpia fasciculata II. The dynamics of seed populations. Journal of Ecology 66: 35–44. [Google Scholar]
- Went FW. 1948. Ecology of desert plants. I. Observations on germination in the Joshua Tree National Monument, California. Ecology 29: 242–253. [Google Scholar]
- Zhang J, Maun MA. 1990. Effects of sand burial on seed germination, seedling emergence, survival, and growth of Agropyron psammophilum Canadian Journal of Botany 68: 304–310. [Google Scholar]