Abstract
• Background The incidence of blossom-end rot (BER) is generally associated with a calcium (Ca) deficiency in the distal portion of tomato fruits. The visible symptom is a necrotic lesion, which is presumed to be a consequence of cell death and the subsequent leakage of solutes into the extracellular space. Environmental factors that affect either fruit cell expansion or Ca delivery to the distal portion of the fruit influence the occurrence of BER. However, since no absolute, critical fruit Ca concentration for the occurrence of BER has been identified, it is now important to define the role of Ca in fruit cell physiology and to seek the cause of BER at the cellular level.
• Hypothesis Here, it is suggested that BER is initiated by a cellular dysfunction in the distal portion of a young fruit during rapid cell expansion. It is proposed that insufficient Ca2+ is available for critical apoplastic and cytoplasmic functions when the cellular Ca demand imposed by vacuolation exceeds the Ca delivery to an expanding cell. A local Ca deficiency, therefore, may result in aberrant intracellular Ca2+ signals, a weakening of cell walls and a loss of cellular integrity. Ultimately it may lead to cell death and the visible symptoms of BER. Several experimental strategies are suggested to confirm the occurrence of aberrant Ca2+ concentrations in cells contributing to BER.
• Perspective Many genetic and genomic resources are becoming available for tomato. Ultimately, these will allow genes affecting the occurrence of BER to be identified. Such knowledge will inform breeding strategies to eliminate BER. In the meanwhile, increasing the apoplastic Ca concentration in susceptible fruit tissue should provide a simple and reliable, practical solution for the prevention of BER in tomatoes. It is suggested that current horticultural practices, such as the manipulation of the mineral composition of the feed or the growth environment, are not completely effective in reducing BER because they affect apoplastic Ca concentration in fruit tissue indirectly. Therefore, spraying Ca directly onto young fruits is recommended for the prevention of BER.
Keywords: Annexin, blossom-end rot (BER), calcium (Ca2+), environmental conditions, fruit, mineral nutrition, phloem, tomato (Lycopersicon esculentum), xylem
INTRODUCTION
The symptoms, occurrence and search for the cause of blossom-end rot (BER) in tomato (Lycopersicon esculentum) have been described frequently in the scientific literature of the last century (see Brooks, 1914; Spurr, 1959; Saure, 2001). The majority of studies have identified a local Ca deficiency in the distal fruit tissue as the primary cause of BER (Lyon et al., 1942; Ward, 1973; Bradfield and Guttridge, 1984; Adams and Ho, 1992). For this reason, BER was considered to be a symptom of a Ca-related physiological disorder (see Shear, 1975; Bangerth, 1979; Kinet and Peet, 1997). However, the induction of BER in modern glasshouse tomato production is rarely caused by insufficient Ca in the feed. More often, BER occurs in plants with an adequate Ca supply when grown under conditions that either (a) reduce the transport of Ca to rapidly growing distal fruit tissue or (b) increase the demand of the distal fruit tissue for Ca by accelerating fruit expansion (Ho, 1998b). In practice, BER can be prevented by increasing Ca transport toward the fruit by reducing canopy transpiration (Li et al., 2001) or by canopy Ca sprays (Geraldson, 1957; Wilcox et al., 1973; Wada et al., 1996; Ho, 1998a; Schmitz-Eiberger et al., 2002). Nevertheless, since BER may occur in plants and fruits with apparently adequate tissue Ca concentrations, predicting and preventing the occurrence of BER in glasshouse tomatoes from measurements of their Ca status has not always been effective. This has led to a recent opinion that Ca nutrition is neither a primary, nor an independent factor in the development of BER (Saure, 2001).
The main objections to a primary role for Ca in the induction of BER raised in recent years are (a) that no universal critical Ca level in the BER fruit tissue has been identified (Nonami et al., 1995); (b) that BER can be induced by changing the concentrations of mineral nutrients other than Ca in the feed (Nukaya et al., 1995); and (c) that there is no conclusive evidence for a role of Ca when BER is induced by various environmental stresses (Saure, 2001). However, these objections appear to be based on a paucity of detailed information in the literature. Here are some examples. (1) The inability to find an universal critical Ca level for the induction of BER in fruit could be because, in general (a) Ca has been measured as total Ca (mainly Ca-oxalate and Ca-pectate) rather than as the fraction of Ca relevant to a particular cell function (Minamide and Ho, 1993) and (b) Ca has been measured in whole fruit, or at most in distal tissue, rather than at the cellular level pertinent to cell function. It is possible that a local Ca (or Ca2+) deficiency for individual cells in the distal tissues might be responsible for BER (Schmitz-Eiberger et al., 2002; Suzuki et al., 2003). (2) The induction of BER by changing the concentration of mineral ions other than Ca in the feed can be interpreted as resulting from their effects on Ca2+ uptake by roots and transport within the plant, or their effects on cellular biochemistry (Geraldson, 1957; Willumsen et al., 1996). (3) The induction of BER by stress factors such as heat, hormones and oxidants does not necessarily exclude the involvement of Ca, since changes in cytosolic Ca2+ ([Ca2+]cyt) are likely to have a role in coordinating the cellular responses to all these stress factors (White and Broadley, 2003). From these counter-arguments, it appears necessary to consider the induction of BER as a cellular phenomenon.
This paper presents a reappraisal of the role of Ca in the induction of BER in tomato fruit. It considers the induction of BER as a cellular event that occurs during rapid cell expansion in a young fruit. How this physiological process might be affected by the responses of the whole plant to the environment and, in particular, how interactions between Ca and other nutritional (i.e. N, K and P), hormonal (i.e. auxin and gibberellins) and stress (i.e. heat, water and oxidative) factors could affect the induction of BER, is discussed.
THE SYMPTOMS AND OCCURRENCE OF BER
Blossom-end rot occurs in tomatoes grown in fields or glasshouses as a horticultural crop. No report on its occurrence in wild species is found. This implies that BER is associated with large fruit growing under conditions favourable for fruit expansion. The induction of BER occurs within 2 weeks of fruit set (Spurr, 1959; Adams and El-Gizawy, 1988), when the Ca concentration in the fruit is at its lowest (Fig. 1). The external symptoms of BER are the collapse of cells in the epidermis and subepidermal parenchyma (Spurr, 1959; Suzuki et al., 2003) and the appearance of a watery, discoloured, necrotic tissue at the blossom-end of the fruit (Fig. 2B and C; Maynard et al., 1957). An internal BER (termed ‘black seeds’) may also occur when the necrotic region develops in the parenchyma tissue surrounding the young seeds and in the distal placenta (Fig. 2A; Estabrooks and Tiessen, 1972; Adams and Ho, 1992). Internal BER is considered to be either a milder form of the disorder or an earlier phase in the development of external BER. Both are presumed to be consequences of cell death, and the subsequent leakage of cell contents into the extracellular space (van Goor, 1968; Suzuki et al., 2003). The incidence of BER is infrequent, but it can cause a substantial financial loss when it occurs. It can occur in any truss, throughout the seasons (Ho et al., 1993), but is only induced in fruit soon after fruit set, when the relative growth rate of young fruit is at its highest (Ho et al., 1987). This suggests that the induction of BER is associated with rapid cell expansion in the distal fruit tissue. It can be induced by a number of growing conditions, such as low Ca (Raleigh and Chucka, 1944; Maynard et al., 1957; Greenleaf and Adams, 1969; Ward, 1973; Adams and El-Gizawy, 1988; Franco et al., 1994; de Kreij, 1996; Paiva et al., 1998; Adams, 2002) or low P supply (Cerda et al., 1979; de Kreij, 1996; Ho, 1998a), high Mg (Raleigh and Chucka, 1944; Geraldson, 1957; Hao and Papadopoulos, 2003, 2004), high N (particularly NH4) (Raleigh and Chucka, 1944; Wilcox et al., 1973; Pill et al., 1978; Pill and Lambeth, 1980; Hartman et al., 1986; Ikeda and Osawa, 1988; Barker and Ready, 1994; Nukaya et al., 1995), high K (Raleigh and Chucka, 1944; Adams, 2002) or high salinity (Adams, 1991; Adams and Ho, 1992; Willumsen et al., 1996; Cuartero and Fernández-Muñoz, 1999; del Amor et al., 2001; Dorais et al., 2001), drought (Shaykewich et al., 1971; van der Boon, 1973) or water logging in the root zone (Tachibana, 1988), and low humidity (Gerard and Hipp, 1968; Bradfield and Guttridge, 1984; de Kreij, 1996) or high light or temperature (Ho et al., 1993) in the shoot environment. Since these conditions can either inhibit or promote plant growth, BER appears to be unrelated to plant growth rate per se. However, BER does appear to be related to fruit growth rate and/or potential fruit size among cultivars (i.e. fruit shape and fruit expansion rate), and there is a clear genetic influence in the susceptibility of different cultivars to BER (Brooks, 1914; Maynard et al., 1957; Greenleaf and Adams, 1969; Adams and Ho, 1992; Ho et al., 1995; Sperry et al., 1996; Cuartero and Fernández-Muñoz, 1999). Plum tomatoes are more susceptible to BER than round tomatoes, and no BER is ever observed in cherry tomatoes (Ho, 1998a). This has led to the suggestion that the susceptibility of cultivars to BER is related to the differential delivery of phloem-borne leaf assimilate and xylem-borne Ca to the distal end of the fruit in response to the growing environment. Indeed, fruit from cultivars susceptible to BER generally have lower Ca concentrations than those from non-susceptible cultivars, especially immediately post-anthesis (Franco et al., 1994; Willumsen et al., 1996). This may be a consequence of the reduced xylem network in fruit of cultivars susceptible to BER (Belda et al., 1996). A higher incidence of BER is also associated with an earlier onset of ethylene production (Barker and Ready, 1994), cessation of growth and premature ripening.
Fig. 1.
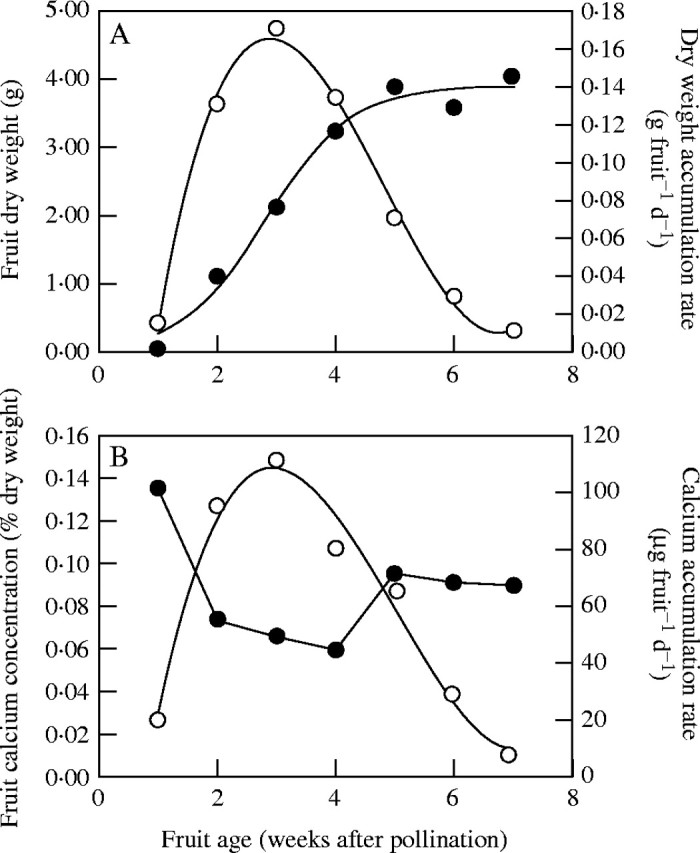
The time-course of accumulation of dry matter and calcium in a tomato fruit during its development. (A) The accumulation of dry matter (closed circles) follows a sigmoidal relationship with time. Fruit growth rate (open circles) is slow immediately after ovule fertilization during the phase of cell division (Phase 1), accelerates during the phase of cell expansion (Phase 2), and slows again as the fruit approaches its final size (Phase 3). The rate of dry matter accumulation is maximal during the phase of cell expansion. (B) Fruit calcium concentration (closed circles) declines as fruit development proceeds. However, although the rate of Ca accumulation (open circles) is maximal during the phase of cell expansion, the rate of Ca accumulation does not increase as much as the rate of accumulation of dry matter during this phase. Thus, fruit calcium concentration reaches a minimal value during the phase of rapid cell expansion. Data were abstracted from Ehret and Ho (1986a, b) and Ho et al. (1987).
Fig. 2.
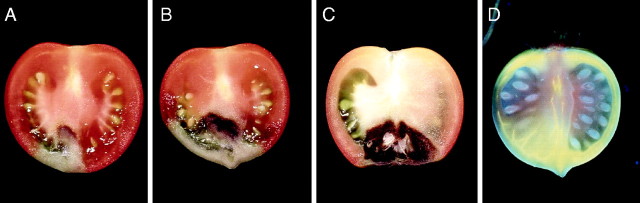
The symptoms of (A) internal BER, (B) and (C) external BER in ripe tomato fruit, and (D) the development of the xylem network in young tomato fruit. To visualize xylem development, the dye Lucifer Yellow CH was used to stain functioning xylem in vivo (Malone and Andrews, 2001). The young tomato fruit appears to possess only two functioning xylem strands in the placental tissue.
CALCIUM AND CELL EXPANSION
Since the susceptibility of tomato fruit to BER appears to occur during the early phase of rapid cell expansion, it is pertinent to examine the many critical roles of Ca in this process and to observe that the Ca concentration in fruit decreases substantially during this period (Fig. 1B; Ehret and Ho, 1986a; Cho et al., 1997). It is also significant that Ca is delivered via the xylem to the expanding fruit cells, that the density of xylem vessels decreases during fruit expansion and that there are far fewer, and narrower, xylem vessels at the blossom-end of the fruit than at the proximal end (Belda and Ho, 1993; Ho et al., 1993; Belda et al., 1996). The xylem : phloem ratio also decreases towards the distal end of the fruit. Furthermore, during the critical phase of rapid fruit expansion, although the xylem network in the pericarp tissue increases, there remain only two single functioning strands in the placental tissue (Fig. 2D). These features are thought to be the anatomical reason why BER is initiated in the distal placental tissues.
Hormones such as auxins and gibberellins trigger fruit cells to expand (Gillaspy et al., 1993). Specific changes in [Ca2+]cyt are initiated by these hormonal signals, which effect appropriate developmental responses (White and Broadley, 2003). These signals are generated by Ca2+ influx to the cytosol from extracellular and/or intracellular (endoplasmic reticulum or vacuolar) compartments (White and Broadley, 2003). Thus, Ca has a role in initiating cell expansion, and a local lack of available Ca2+ may result in aberrant [Ca2+]cyt signals.
Cell expansion then depends upon the generation of hydrostatic (turgor) pressure, through the accumulation of osmotically active solutes, and the yielding of the cell wall (Nobel, 1999). Although cellulose microfibrils form the basic scaffolding of the cell wall, the rigidity of the cell walls is thought to reside predominantly in the crosslinking of non-esterified pectins by Ca2+ (Carpita and McCann, 2000). Cell wall loosening probably proceeds through auxin-induced apoplastic acidification and the activation of endoglycosidases, xyloglucan endotransglycosylases (XETs) and expansins that cleave the load-bearing bonds tethering the wall's cellulose microfibrils to other polysaccharides. As the cell expands, however, pectins in the cell wall become progressively de-esterified through the activity of pectin methylesterases, crosslinked by Ca2+, and branched. This eventually halts cell expansion. In addition, pectins appear to restrict the access of XETs and expansins to their substrates. Thus, Ca has a role in regulating cell expansion, and an insufficient apoplastic Ca2+ concentration ([Ca2+]apoplast) may result in excessive cell enlargement. It is noteworthy, therefore, that the cells in necrotic regions of BER fruit have ill-formed walls (Suzuki et al., 2003) and that BER appears to be increased in fruit lacking Ca by accelerated canopy transpiration (Gerard and Hipp, 1968; Paiva et al., 1998).
As a cell expands, its plasma membrane and cell wall must increase in area. The incorporation of new material into the plasma membrane and cell wall is also a Ca2+-dependent process and an elevated [Ca2+]cyt has been associated with expansion growth in several cell types (White, 1998; White and Broadley, 2003). It is thought that [Ca2+]cyt controls cell expansion by influencing the incorporation of vesicles containing the materials and enzymes required for membrane and wall construction into the plasma membrane. In elongating root cells, Ca2+ influx through hyperpolarization-activated Ca2+ channels (HACCs) appears to be responsible for elevating [Ca2+]cyt (White et al., 2002; White and Broadley, 2003). Similar channels have been observed in many plant cells, including suspension-cultured tomato cells (Blumwald et al., 1998). Since the activity of HACCs is induced by the presence of reactive oxygen species (ROS) (White and Broadley, 2003), it is interesting to note that ROS are most abundant in fruits during the cell expansion phase (Aktas et al., 2003) and that the production of ROS can be induced by both auxin and gibberellins (Mori and Schroeder, 2004). It is thought that HACCs are formed by annexins (White et al., 2002), and several genes encoding annexins are expressed in tomato fruit (Table 1; Harms, 2003). In tomato, as in other fruit, the expression of genes encoding annexins changes during fruit development (Wilkinson et al., 1995; Proust et al., 1996). These observations indicate the importance of [Ca2+]cyt in coordinating cell expansion.
Table 1.
The expression of genes encoding annexins in tomato fruit
| Annexin |
Synonym |
Transcript and clone |
Expressed in fruit |
Reference |
|---|---|---|---|---|
| AnnLe1 | annexin p34 | AF079232 | Yes | Lim et al. (1998) |
| Smallwood et al. (1992) | ||||
| AnnLe2 | annexin p35 | AF079231 | Yes | Lim et al. (1998) |
| AnnLe3A | TC128826 | Yes | TIGR and HRI, unpublished | |
| cLEM6A12 | ||||
| AnnLe3B | TC122423 | – | TIGR | |
| AnnLe4A | TC124457 | Yes | TIGR | |
| AnnLe4B | AW650868 | – | TIGR and HRI, unpublished | |
| cLEI14P1 | ||||
| AnnLe4C | BG642726 | – | TIGR | |
| AnnLe5A | TC127030 | Yes | TIGR and HRI, unpublished | |
| cTOA5H24 | ||||
| AnnLe5B | AI778652 | – | TIGR |
Each unique tomato transcript has been numbered according to the homologue in Arabidopsis thaliana with which it has greatest similarity (White et al., 2002).
When several tomato transcripts were most similar to the same Arabidopsis transcript, these were differentiated by an alphabetical postscript.
Sequence data were obtained for tentative consensus (TC) sequences and cDNA clones from the Institute for Genomic Research, USA (http://www.tigr.org/tdb/lgi/). Subsequent sequencing of cDNA clones was performed by H. C. Bowen and P. J. White (Warwick HRI).
A putative full-length sequence was obtained for all transcripts highlighted in bold.
During the intial phase of cell expansion, cells increase the relative volume of their vacuoles (Gillaspy et al., 1993). This results in an increased intracellular Ca demand, because the Ca concentration in the cytoplasm is generally less than that of the vacuole. It has been observed that when Ca movement to the vacuole exceeds the rate at which Ca is supplied to a cell, Ca deficiency symptoms become apparent. So, for example, leaves of plants overexpressing H+/Ca2+-antiporters that remove Ca2+ from the cytoplasm to the vacuole exhibit Ca deficiency symptoms despite having a ‘sufficient’ Ca concentration (Hirschi, 2001). These symptoms might be a consequence of lowered [Ca2+]cyt or [Ca2+]apoplast.
Finally, both [Ca2+]apoplast and [Ca2+]cyt concentrations impact on solute fluxes across the plasma membrane. The integrity of the plasma membrane is maintained by Ca2+ binding to negatively charged lipids. In the absence of Ca2+, membranes loose their semi-permeability and become ‘leaky’ to charged solutes (Kirkby and Pilbeam, 1984). If this occurs, cell death will ensue. The disruption of cell membranes has been observed as an early symptom of BER (Suzuki et al., 2003) and might occur if the [Ca2+]apoplast around a cell was reduced to a critical level. Hence, it is noteworthy (a) that there is lower Ca2+ in the plasma membrane and walls surrounding cells that collapse during rapid cell expansion in BER fruit, and that there is a strong negative correlation between the incidence of BER and the apoplastic Ca concentration in the distal half of tomato fruits (Schmitz-Eiberger et al., 2002; Suzuki et al., 2003), and (b) that BER can be reduced by spraying young fruit with cations, such as Ca or Sr, that stabilize membrane structure (Bangerth, 1973; Ho, 1998a; Schmitz-Eiberger et al., 2002). In addition, the activity of many transporters are modulated either directly by Ca2+ or by signalling cascades triggered by changes in [Ca2+]cyt. Since controlled accumulation of osmotically active solutes is required for cell expansion, appropriate [Ca2+]apoplast and [Ca2+]cyt must be maintained.
These observations imply that Ca2+ is required for several essential functions during cell expansion and it is possible that reduced Ca2+ concentrations could be responsible for the initiation of BER in tomato fruit. In summary, since changes in [Ca2+]cyt control cell development, Ca deficiency might cause a cell to develop abnormally. Since Ca2+ contributes to cell wall structure by crosslinking pectins, Ca deficiency may lead to cell wall weakness and aberrant cell expansion. Since [Ca2+]cyt coordinates the cellular biochemistry of cell expansion, Ca deficiency might result in morphological defects. Since Ca2+ regulates membrane permeability, Ca deficiency may result in abnormal leakage of solutes from cells and cell death.
It is now necessary to test the hypothesis that aberrant Ca2+ concentrations initiate BER by confirming that (a) local and/or cellular Ca2+ concentrations differ between normal cells and cells that contribute to BER and (b) that perturbation of Ca2+ concentration through experimental manipulations affect the incidence of BER appropriately. This could be achieved using transgenic tomato plants expressing the Ca2+-sensor aequorin either in the cytosol (Moyen et al., 1998; Harms, 2003) or in the apoplast (cf. Gao et al., 2004). For this strategy to succeed, however, a method to recognize the cells in a developing fruit that will subsequently contribute to BER in vivo will be required. This might be realized by identifying characteristic genes that are expressed solely in cells that will contribute to BER. Microarray technology (e.g. Wang et al., 2001, 2002; Moore et al., 2002; Zhang et al., 2004) will facilitate this by allowing the transcriptional profiles in distal cells from young fruit developing on a plant without BER to be compared with those developing on the same plants when BER is induced by a transient environmental perturbation. Appropriate environmental perturbations might include transient elevations of temperature and/or light intensity, transient increases in the NH4+ concentration of the feed or the removal of Ca from the feed. Provided a suitable gene can be identified, the promoter for this gene could be used to drive the expression of apoaequorin and/or a ‘marker’ gene encoding a visible, non-toxic protein, such as the green fluorescent protein (GFP), solely in cells initiating BER. A similar strategy has been used previously, for example, to mark specific cell types for electrophysiological studies (Maathuis et al., 1998; Kiegle et al., 2000) and to develop ‘smart’ plants to monitor their nutritional status (Hammond et al., 2003). This will not only allow the [Ca2+]cyt and [Ca2+]apoplast to be assayed in pre-symtomatic cells that will ultimately contribute to BER, but will also allow the transcriptional changes occurring during the induction of BER to be investigated at the cellular level if combined with single cell sampling and analytical techniques (Tomos and Sharrock, 2001; Brandt et al., 2002). The latter approach could, for example, resolve whether cells undergo uncontrolled (necrotic) or controlled (apoptotic) cell death during the induction of BER by comparing the genes expressed in cells contributing to BER with the characteristic biochemical and genetic events that occur during apoptotic cell death (Gechev et al., 2004). To explore the critical role of [Ca2+]cyt in the initiation of BER, transgenic plants with altered [Ca2+]cyt homeostasis could also be assayed using this approach. Such plants can be genetically engineered by overexpressing Ca2+-ATPases or Ca2+/H+ antiporters under the control of a fruit specific promoter (cf. Hirschi, 2001). Once the involvement of Ca2+ in the initiation of BER can be confirmed, the precise mechanism can then be studied.
ENVIRONMENTAL AND DEVELOPMENTAL FACTORS INFLUENCING THE INCIDENCE OF BER
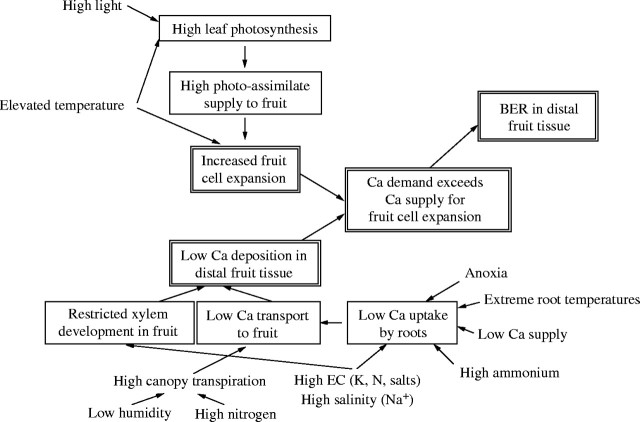
The induction of BER in young fruit is influenced by a number of environmental factors. These are most likely to exert their effects by affecting the transport of Ca to the fruit and/or the rate of cell expansion (Fig. 3). Although each adverse environmental condition might reduce Ca accumulation and/or accelerate cell expansion in a young fruit through a different physiological process, all are able to reduce the Ca concentration in distal fruit tissue to a critical level. This interpretation is corroborated by the observation that different cultivars show a wide range of responses to growing conditions that induce BER (Adams, 1992; Ho et al., 1995) and the susceptibility of different cultivars to BER appears to be related to the development of the xylem network and the rate of cell expansion during early fruit development (Ho et al., 1993; Belda et al., 1996). Environmental factors that influence Ca uptake and/or the partitioning of Ca to the fruit include the composition of the feed, its salinity or osmotic strength, and the humidity of the air. Environmental factors that influence the rate of fruit growth, either directly or indirectly by affecting the concentrations of hormones, include water availability in the root zone, light intensity and ambient temperature.
Fig. 3.
Environmental factors that influence the incidence of blossom-end rot (BER) in tomato fruit (Ho, 1998b; Adams, 2002). It is hypothesized that BER occurs in distal tissues of young, rapidly expanding fruit when their demand for Ca exceeds their Ca supply. Thus, BER is promoted by environmental factors, such as high light and elevated temperatures, that accelerate fruit growth, in addition to environmental factors that reduce the uptake, transport and deposition of Ca in the fruit.
It has been observed that increasing the concentrations in the feed of ions that antagonize Ca uptake promotes BER. In this respect, NH4+ (Pill et al., 1978; Barker and Ready, 1994; Nukaya et al., 1995), Na+ (Adams, 1991; Willumsen et al., 1996; Cuartero and Fernández-Muñoz, 1999; del Amor et al., 2001) and K+ (Adams, 2002) are particularly noteworthy. Similarly, extreme low or high temperatures (Chong and Ito, 1982; Adams, 1988; Petersen and Willumsen, 1992) or anoxia (Tachibana, 1988) in the root zone reduce Ca uptake and promote BER. In addition, since Ca movement to the xylem is proportional to water uptake (Ho et al., 1995; White, 2001), environmental factors that reduce water uptake, such as extreme root temperatures, anoxia, drought and osmotic stress, also reduce Ca fluxes to the shoot and, thereby, promote BER (Guichard et al., 2001). Indeed, uneven watering or saline conditions in the field or high electrical conductivity (EC) in the feed for glasshouse tomato production have been identified as the most common cause of BER in tomatoes (Cuartero and Fernández-Muñoz, 1999; Franco et al., 1999). Environmental factors that reduce Ca fluxes to the developing fruit, either by diverting the xylem stream preferentially to leaves, such as high canopy transpiration rates (Ho, 1989; Adams and Holder, 1992; Li et al., 2001), or by impairing xylem development within the fruit, such as high EC in the feed (Belda and Ho, 1993; Ho et al., 1993; Belda et al., 1996) also promote BER. It has been suggested that one reason why high N in the feed might promote BER is that it leads to the development of a large canopy and, thereby, augments canopy transpiration (Pill and Lambeth, 1980; Ikeda and Osawa, 1988; Ho et al., 1999).
Cultural practices, e.g. thinning trusses, and environmental factors, e.g. high light intensities and elevated temperatures, which accelerate fruit expansion also promote BER (DeKock et al., 1982; Wui and Takano, 1995). The seasonal trend of BER (Gerard and Hipp, 1968; Ho et al., 1993) and the incidental increase in BER to a sudden change from dull to bright weather (Ho and Grimbly, 1988) are good examples of induction of BER by high light intensities and elevated temperatures. During these periods, fruit expansion is accelerated by high temperatures directly, and high light intensities and elevated temperatures increase canopy photosynthesis and the supply of photoassimilate to the fruit (Ho, 1998b). Under these conditions, it is likely that BER is induced in the rapidly expanding distal fruit tissue because its demand for Ca exceeds the immediate xylem supply (Ho et al., 1993).
Finally, Saure (2001) appears to suggest a direct influence of hormones on the induction of BER. However, although it is possible that various stress conditions produce hormonal imbalances, it is likely that hormones exert their effects indirectly by influencing cell expansion and plant development. For example, auxins and gibberellins are thought to trigger fruit cells to expand (Gillaspy et al., 1993). The highest concentration of these hormones in fruit occurs prior to cell expansion (El-Beltagy et al., 1976). It is known that the application of auxins and/or giberellins increases cell division, rapid fruit growth and the incidence of BER (Bangerth, 1973; Castro, 1980). Thus, the acceleration of fruit growth, and the inability of the plant to supply sufficient Ca to rapidly growing fruit, might explain the effects of these hormones on the incidence of BER in the majority of cases. However, blocking polar auxin transport to the fruit can also induce BER. Intriguingly, it has been suggested that this phenomenon might be a consequence of reduced xylogenesis and, therefore, impaired Ca movement to the distal tissue (Battey, 1990). An involvement of ethylene in the induction of BER has also been proposed (Saure, 2001). In addition to its effect on tomato fruit ripening, ethylene has been implicated in initiating responses to wounding and pathogens through [Ca2+]cyt signals (White and Broadley, 2003). The precocious production of ethylene is consistent with the necrotic phenotype of BER and is likely, therefore, to be a consequence not a cause of BER. Nevertheless, it is also possible that ethylene, and other ‘stress’ factors that increase the production of ROS could influence the occurrence of BER through the activation of HACCs and the consequent elevation of [Ca2+]cyt and rapid cell expansion.
In conclusion, most environmental factors that affect the incidence of BER perturb the relationship between the rate of cell expansion and Ca accumulation in developing fruit. Further insight on the physiological processes that promote BER could be obtained using the genetic and genomic resources rapidly becoming available for tomato. Firstly, the relative contribution of genetic and environmental factors to the occurrence of BER might be quantified by screening a ‘foundation set’ of tomato varieties selected to comprise a high proportion of the genetic variation in Lycopersicon esculentum. This could then be complemented by the identification of chromosomal loci impacting on the occurrence of BER through quantitative trait (QTL) analysis using genetic mapping populations. Ideally, the parents of these populations would be tomato varieties that show extreme sensitivities to BER, and the populations should show transgressive segregation for this trait. Nevertheless, several mapping populations are already available (e.g. Doganlar et al., 2002; van der Knaap et al., 2002; Causse et al., 2004; Lecomte et al., 2004; Tanksley, 2004), which might be used in the interim. An alternative approach would be to survey the allelic variation in the entire ‘foundation set’ population and utilize association analysis to correlate phenotypic data with allelic diversity (e.g. Nesbitt and Tanksley, 2002). An appraisal of the genes present in chromosomal loci that influence the occurrence of BER will provide information on the physiological processes contributing to BER and direct further experimentation and breeding programmes. The analysis of chromosomal loci will be facilitated greatly by the sequencing of the tomato genome, which will be completed in the near future (Solanaceae Genomics Network, http://www.sgn.cornell.edu/help/about/tomato_sequencing.html).
A WORKING HYPOTHESIS FOR THE INDUCTION OF BER IN TOMATO
All environmental and genetic factors that influence the occurrence of BER in tomato affect either the rate of cell expansion or the delivery of Ca to the young fruit (Fig. 3). It has been observed that BER occurs solely in distal fruit tissue during the initial phase of rapid cell expansion and vacuolation, before any locular tissue is present. Thus, BER occurs during a period of high cellular Ca demand, when fruit growth is accelerated or Ca delivery to the fruit is limited. Recent evidence suggests that BER is initiated as a cellular event, and it has been argued earlier that more detailed information on cellular Ca dynamics during the development of BER could address any substantive argument that Ca is not a primary factor in the development of BER.
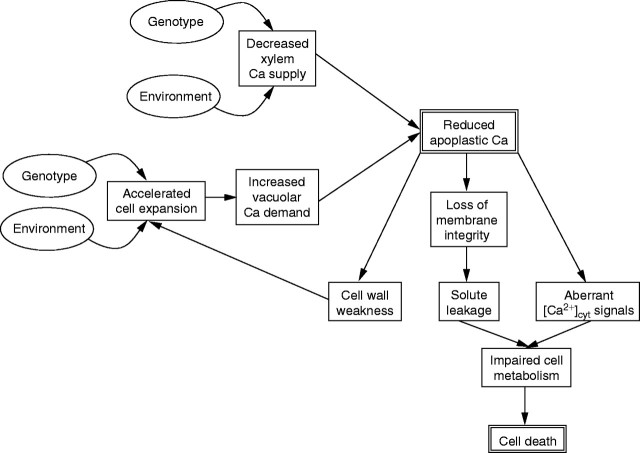
This section presents a hypothesis that BER is initiated by a cellular dysfunction in a fruit cell during expansion upon a local, transient Ca-deficiency (Fig. 4). During cell expansion, there is a considerable demand for Ca2+ (a) as a structural component of new cell walls and membranes, (b) as a cytosolic signal orchestrating the allometry and biochemistry of cell expansion and (c) as a counter-cation in the enlarging vacuole (White and Broadley, 2003). When Ca is in limited supply, any preferential distribution of Ca to a particular process might impair cellular function. Thus, excessive Ca accumulation in the vacuole may lead to both aberrant [Ca2+]cyt signals and a weakening of cell walls. This is illustrated by the observation that leaves of transgenic plants overexpressing vacuolar H+/Ca2+-antiporters, which remove Ca2+ from the cytoplasm to the vacuole, exhibit symptoms of Ca-deficiency (tip-burn) despite having greater tissue Ca concentrations than leaves of wild-type plants (Hirschi, 2001). It was suggested that this phenomenon might be a consequence of altered [Ca2+]cyt homeostasis (Hirschi, 2001), but this has yet to be verified. Likewise, it is possible that Ca sequestration in the enlarging vacuoles of a fruit cell expanding during a period of limited Ca supply could starve the cytoplasm or apoplast of the Ca2+ required for intracellular signalling or cellular integrity. This might be exacerbated by certain mineral stresses that inhibit Ca uptake or promote the synthesis of organic acids that chelate Ca. A reduction in [Ca2+]apoplast could result in impaired cell wall properties, leading to structural weakness and precocious cell expansion, alterations in plasma membrane permeability, leading to solute leakage and unregulated solute fluxes, and aberrant responses to environmental or developmental signals initiated by Ca2+ influx. These would ultimately lead to an uncontrolled cell death. Thus, the symptoms of BER, such as the appearance of a watery, discoloured and necrotic tissue, are consistent with Ca deficiency in the apoplast. Furthermore, it is also consistent with the morphology of individual cells in the BER-affected fruit tissue and the initiation of BER in the unsupported epidermis and subepidermal parenchyma (Spurr, 1959; Suzuki et al., 2003). This hypothesis can explain why the Ca concentration is not necessarily lower in BER-affected tissue than in unaffected fruit tissue, since the vacuolar Ca concentrations in BER-affected tissue could still be high. However, it can be predicted that the Ca2+ concentrations in the apoplast and/or cytoplasm of BER-affected tissue will be lower than those of unaffected fruit tissue and this local Ca2+ deficiency will initiate BER.
Fig. 4.
A hypothesis relating the induction of blossom-end rot (BER) in tomato fruit to effects at the cellular level. Environmental and genetic factors influence the rates of cell expansion and Ca delivery to the rapidly expanding cells in the distal portion of young tomato fruit. An imbalance between Ca supply and cell expansion-led Ca demand leads to a decrease in apoplastic Ca concentration, which impacts on cell wall structure, membrane integrity and cytosolic Ca2+ signals. Adverse effects on these critical cellular properties result in uncontrolled solute fluxes and, eventually, cell death.
In a horticultural context, BER can be considered simply as a symptom of Ca deficiency in the distal fruit tissue during rapid cell expansion. Thus, BER in a tomato crop can be minimized by spraying Ca onto young tomato fruit (Fig. 5; Wilcox et al., 1973; Ho, 1998a; Schmitz-Eiberger et al., 2002). In the glasshouse, this treatment prevents BER more effectively than other current horticultural practices, such as the manipulation of the mineral composition of the feed (e.g. lower N supply) or the growth environment (e.g. lower canopy transpiration), because it increases the Ca concentration of distal fruit tissues directly. However, this treatment can only be effective when regular Ca sprays are targeted to young fruit before any symptom of BER is observed. The occasional failure of Ca sprays to prevent BER previously may be due to an indiscriminate application to the entire canopy, without any appreciation of fruit development. An appreciation of how environmental factors affect both fruit growth rates and Ca delivery to the fruit has also informed other management practices to limit BER (Adams, 2002; Ho, 2002). These practices aim to limit growth spurts, and to optimize Ca accumulation, in young tomato fruit. From the forgoing discussion, it is clear that (a) fruit growth spurts can be avoided by preventing momentary high light intensities and temperatures in the canopy, (b) the uptake of Ca2+ by roots can be maximized by optimizing the mineral composition of the feed, avoiding high salinity (i.e. <5 dS m−1) or excessive NH4+ (i.e. <10 % total N), K+ and Mg2+ concentrations, whilst maintaining adequate Ca2+ concentrations, and/or by preventing extreme root temperatures (i.e. not <14 °C or >30 °C) and drying in the root environment, and (c) Ca delivery to the fruit in the transpiration stream can be maximized by increasing humidity (i.e. by reducing the potential transpiration rate of the crop by one-third), reducing the leaf : fruit ratio, and by avoiding high light intensities and temperatures in the canopy. In addition to informing crop husbandry, an appreciation of fruit Ca physiology at the cellular level can also inform the selection of cultivars for insensitivity to BER. This selection might consider a range of growth characteristics, such as fruit size and shape, fruit : leaf ratio, and the responses of fruit-cell expansion to light and temperature, as well as the efficiency of Ca transport to and within the fruit. It is hoped that, in the future, through the combination of appropriate management practices and the use of BER-resistant cultivars, the occurrence of BER in commercial tomato crops might be eliminated.
Fig. 5.
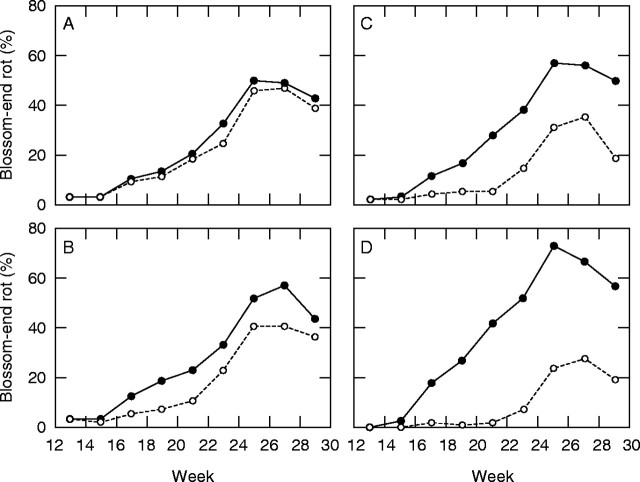
The effects of nutritional and Ca spray treatments on the incidence of blossom-end rot in plum tomatoes (‘Mariella’) grown hydroponically. Comparisons of the incidence of BER, expressed as a percentage of the bi-weekly fruit yield, when (A) N in the feed was decreased from 240 (closed circles) to 120 mg L−1 (open circles), (B) P in the feed was decreased from 30 (open circles) to 5 mg L−1 (closed circles), (C) spraying young fruits weekly with 0·5 % (w/v) CaCl2 (open circles; unsprayed = closed circles), and (D) combining all these treatments, with standard N, lowered P and no Ca spray (closed circles) or with reduced N, standard P plus a Ca spray (open circles) (Ho, 1998a).
Acknowledgments
We thank our colleagues Dr John Andrews and Dr John Hammond for helping with the figures and Helen Bowen for sequencing the tomato annexin cDNA clones. This work was funded by the UK Biotechnology and Biological Sciences Research Council (BBSRC) and the UK Department for Environment, Food and Rural Affairs (Defra).
LITERATURE CITED
- Adams P. 1988. Some effects of root temperature on the growth and calcium status of tomato. Acta Horticulturae 222: 167–172. [Google Scholar]
- Adams P. 1991. Effects of increasing the salinity of the nutrient solution with major nutrients or sodium chloride on the yield, quality and composition of tomatoes grown in rockwool. Journal of Horticultural Science 66: 201–207. [Google Scholar]
- Adams P. 2002. Nutritional control in hydroponics. In: Savvas D, Passam H, eds. Hydroponic production of vegetables and ornamentals. Athens, Greece: Embryo Publications, 211–261. [Google Scholar]
- Adams P, El-Gizawy AM. 1988. Effect of calcium stress on the calcium status of tomatoes grown in NFT. Acta Horticulturae 222: 15–22. [Google Scholar]
- Adams P, Ho LC. 1992. The susceptibility of modern tomato cultivars to blossom-end rot in relation to salinity. Journal of Horticultural Science 67: 827–839. [Google Scholar]
- Adams P, Holder R. 1992. Effects of humidity, Ca and salinity on the accumulation of dry matter and Ca by the leaves and fruit of tomato (Lycopersicon esculentum). Journal of Horticultural Science 67: 137–142. [Google Scholar]
- Aktas H, Karni L, Aloni B, Bar-Tal A. 2003. Physiological and biochemical mechanisms leading to blossom-end rot in greenhouse-grown peppers, irrigated with saline solution. Acta Horticulturae 609: 81–88. [Google Scholar]
- del Amor FM, Martinez V, Cerdá A. 2001. Salt tolerance of tomato plants as affected by stage of plant development. HortScience 36: 1260–1263. [Google Scholar]
- Bangerth F. 1973. Investigations upon Ca related physiological disorders. Phytopathologische Zeitschrift 77: 20–37. [Google Scholar]
- Bangerth F. 1979. Calcium-related physiological disorders of plants. Annual Review of Phytopathology 17: 97–122. [Google Scholar]
- Barker AV, Ready KM. 1994. Ethylene evolution by tomatoes stressed by ammonium nutrition. Journal of the American Society for Horticultural Science 119: 706–710. [Google Scholar]
- Battey NH. 1990. Calcium deficiency disorders of fruits and vegetables. Postharvest News and Information 1: 23–27. [Google Scholar]
- Belda R, Ho LC. 1993. Salinity effects on the network of vascular bundles during tomato fruit development. Journal of Horticultural Science 68: 557–564. [Google Scholar]
- Belda RM, Fenlon JS, Ho LC. 1996. Salinity effects on the xylem vessels in tomato fruit among cultivars with different susceptibilities to blossom-end rot. Journal of Horticultural Science 71: 173–179. [Google Scholar]
- Blumwald E, Aharon GS, Lam BC-H. 1998. Early signal transduction pathways in plant–pathogen interactions. Trends in Plant Science 3: 342–346. [Google Scholar]
- Bradfield EG, Guttridge CG. 1984. Effects of night-time humidity and nutrient solution concentration on the calcium content of tomato fruit. Scientia Horticulturae 22: 207–217. [Google Scholar]
- Brandt S, Kloska S, Altmann T, Kehr J. 2002. Using array hybridization to monitor gene expression at the single cell level. Journal of Experimental Botany 53: 2315–2323. [DOI] [PubMed] [Google Scholar]
- Brooks C. 1914. Blossom-end rot of tomatoes. Phytopathology 4: 345–374. [Google Scholar]
- Carpita N, McCann M. 2000. The cell wall. In: Buchanan BB, Gruissem W, Jones RL, eds. Biochemistry and molecular biology of plants. Rockville, USA: American Society of Plant Physiologists, 52–108. [Google Scholar]
- Castro PRC. 1980. Plant growth regulators in tomato crop production. Acta Horticulturae 100: 99–104. [Google Scholar]
- Causse M, Duffe P, Gomez MC, Buret M, Damidaux R, Zamir D, Gur A, Chevalier C, Lemaire-Chamley M, Rothan C. 2004. A genetic map of candidate genes and QTLs involved in tomato fruit size and composition. Journal of Experimental Botany 55: 1671–1685. [DOI] [PubMed] [Google Scholar]
- Cerda A, Bingham FT, Labanauskas CK. 1979. Blossom-end rot of tomato fruit as influenced by osmotic potential and phosphorous concentrations of nutrient solution media. Journal of the American Society for Horticultural Science 104: 236–239. [Google Scholar]
- Cho I-H, Woo Y-H, Lee E-H, Kim H-J. 1997. Changes in cuticular transpiration and calcium content of tomato fruits and prevention of blossom-end rot through environmental control. Journal of the Korean Society for Horticultural Science 38: 98–102. [Google Scholar]
- Chong PK, Ito T. 1982. Growth, fruit yield and nutrient absoption of tomato plant as influenced by solution temperature in nutrient film technique. Journal of the Japanese Society for Horticultural Science 51: 44–50. [Google Scholar]
- Cuartero J, Fernández-Muñoz R. 1999. Tomato and salinity. Scientia Horticulturae 78: 83–125. [Google Scholar]
- DeKock PC, Inkson RHE, Hall A. 1982. Blossom-end rot of tomato as influenced by truss size. Journal of Plant Nutrition 5: 57–62. [Google Scholar]
- de Kreij C. 1996. Interactive effects of air humidity, calcium and phosphate on blossom-end rot, leaf deformation, production and nutrient contents of tomato. Journal of Plant Nutrition 19: 361–377. [Google Scholar]
- Doganlar S, Frary A, Ku HM, Tanksley SD. 2002. Mapping quantitative trait loci in inbred backcross lines of Lycopersicon pimpinellifolium (LA1589). Genome 45: 1189–1202. [DOI] [PubMed] [Google Scholar]
- Dorais M, Papadopoulos AP, Gosselin A. 2001. Influence of electric conductivity management on greenhouse tomato yield and fruit quality. Agronomie 21: 367–383. [Google Scholar]
- Ehret DL, Ho LC. 1986. Translocation of calcium in relation to tomato fruit growth. Annals of Botany 58: 679–688. [Google Scholar]
- Ehret DL, Ho LC. 1986. The effects of salinity on dry matter partitioning and fruit growth in tomatoes grown in nutrient film culture. Journal of Horticultural Science 61: 361–367. [Google Scholar]
- El-Beltagy AS, Patrick JP, Hewitt EW, Hall MA. 1976. Endogenous plant growth regulator levels in tomato fruits during development. Journal of Horticultural Science 51: 15–30. [Google Scholar]
- Estabrooks DN, Tiessen H. 1972. Blossom-end rot and black seeds of tomatoes. Canadian Journal of Plant Science 52: 1076–1077. [Google Scholar]
- Franco JA, Bañón S, Madrid R. 1994. Effects of a protein hydrolysate applied by fertigation on the effectiveness of calcium as a corrector of blossom-end rot in tomato cultivated under saline conditions. Scientiae Horticulturae 57: 283–292. [Google Scholar]
- Franco JA, Pérez-Saura PJ, Fernández JA, Parra M, García AL. 1999. Effect of two irrigation rates on yield, incidence of blossom-end rot, mineral content and free amino acid levels in tomato cultivated under drip irrigation using saline water. Journal of Horticultural Science and Biotechnology 74: 430–435. [Google Scholar]
- Gao DJ, Knight MR, Trewavas AJ, Sattelmacher B, Plieth C. 2004. Self-reporting arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiology 134: 898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechev TS, Gadjev IZ, Hille J. 2004. An extensive microarray analysis of AAL-toxin-induced cell death in Arabidopsis thaliana brings new insights into the complexity of programmed cell death in plants. Cellular and Molecular Life Sciences 61: 1185–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldson CM. 1957. Factors affecting calcium nutrition of celery, tomato and pepper. Soil Science Society Proceedings 21: 621–625. [Google Scholar]
- Gerard CJ, Hipp BW. 1968. Blossom-end rot of ‘Chico’ and ‘Chico Grande’ tomatoes. Proceedings of the American Society for Horticultural Science 93: 521–531. [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W. 1993. Fruits: a developmental perspective. Plant Cell 5: 1439–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf WH, Adams F. 1969. Genetic control of blossom-end rot disease in tomatoes through calcium metabolism. Journal of the American Society for Horticultural Science 94: 248–250. [Google Scholar]
- Guichard S, Bertin N, Leonard C, Gary C. 2001. Tomato fruit quality in relation to water and carbon fluxes. Agronomie 21: 385–392. [Google Scholar]
- Hammond JP, Bennett MJ, Bowen HC, Broadley MR, Eastwood DC, May ST, Rahn C, Swarup R, Woolaway KE, White PJ. 2003. Changes in gene expression in Arabidopsis shoots during phosphate starvation and the potential for developing smart plants. Plant Physiology 132: 578–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X, Papadopoulos AP. 2003. Effects of calcium and magnesium on growth, fruit yield and quality in a fall greenhouse tomato crop grown on rockwool. Canadian Journal of Plant Science 83: 903–912. [Google Scholar]
- Hao X, Papadopoulos AP. 2004. Effects of calcium and magnesium on plant growth, biomass partitioning, and fruit yield of winter greenhouse tomato. HortScience 39: 512–515. [Google Scholar]
- Harms K. 2003.An investigation into the role of calcium signalling in fruit ripening. M.Phil Thesis, University of Nottingham. [Google Scholar]
- Hartman PL, Mills HA, Jones JB. 1986. The influence of nitrate: ammonium ratios on growth, fruit development, and element concentrations in ‘Floradel’ tomato plants. Journal of the American Society for Horticultural Science 111: 487–490. [Google Scholar]
- Hirschi K. 2001. Vacuolar H+/Ca2+ transport: who's directing the traffic. Trends in Plant Science 6: 100–104. [DOI] [PubMed] [Google Scholar]
- Ho LC. 1989. Environmental effects on the diurnal accumulation of 45Ca by young fruit and leaves of tomato plants. Annals of Botany 63: 281–288. [Google Scholar]
- Ho LC. 1998.To quantify environmental and physiological factors controlling calcium uptake, transport and utilization on yield and quality of tomato and sweet peppers in glasshouses. Final report on MAFF project HH1309SPC. [Google Scholar]
- Ho LC. 1998. Improving tomato fruit quality by cultivation. In: Cockshull KE, Gray D, Seymour GB, Thomas B, eds. Genetic and environmental manipulation of horticultural crops. Wallingford, UK: CAB International, 17–29. [Google Scholar]
- Ho LC. 2002. Manipulation of physiological processes for better yield and quality in crop plants in controlled environment. Acta Horticulturae 578: 273–279. [Google Scholar]
- Ho LC, Adams P, Li XZ, Shen H, Andrews J, Xu ZH. 1995. Responses of Ca-efficient and Ca-inefficient tomato cultivars to salinity in plant growth, calcium accumulation and blossom-end rot. Journal of Horticultural Science 70: 909–918. [Google Scholar]
- Ho LC, Belda R, Brown M, Andrews J, Adams P. 1993. Uptake and transport of calcium and the possible causes of blossom-end rot in tomato. Journal of Experimental Botany 44: 509–518. [Google Scholar]
- Ho LC, Grange RI, Picken AJ. 1987. An analysis of the accumulation of water and dry matter in tomato fruit. Plant, Cell and Environment 10: 157–162. [Google Scholar]
- Ho LC, Grimbly P. 1988. Stemming the rot. Grower 109: 13–16. [Google Scholar]
- Ho LC, Hand DJ, Fussell M. 1999. Improvement of tomato fruit quality by calcium nutrition. Acta Horticulturae 481: 463–468. [Google Scholar]
- Ikeda H, Osawa T. 1988. The effects of NO3/NH4 ratios and temperature of nutrient solution on growth, yield and blossom-end rot incidence in tomato. Journal of the Japanese Society for Horticultural Science 57: 62–69. [Google Scholar]
- Kiegle E, Gilliham M, Haseloff J, Tester M. 2000. Hyperpolarisation-activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots. Plant Journal 21: 225–229. [DOI] [PubMed] [Google Scholar]
- Kinet JM, Peet MM. 1997. Tomato. In: Wien HC, ed. The physiology of vegetable crops. Wallingford, UK : CAB International, 207–258. [Google Scholar]
- Kirkby EA, Pilbeam DJ. 1984. Calcium as a plant nutrient. Plant, Cell and Environment 7: 397–405. [Google Scholar]
- Lecomte L, Saliba-Colombani V, Gautier A, Gomez-Jimenez MC, Duffe P, Buret M, Causse M. 2004. Fine mapping of QTLs of chromosome 2 affecting the fruit architecture and composition of tomato. Molecular Breeding 13: 1–14. [Google Scholar]
- Li YL, Stanghellini C, Challa H. 2001. Effect of electrical conductivity and transpiration on production of greenhouse tomato (Lycopersicon esculentum L.). Scientia Horticulturae 88: 11–29. [Google Scholar]
- Lim EK, Roberts MR, Bowles DJ. 1998. Biochemical characterization of tomato annexin p35. Independence of calcium binding and phosphatase activities. Journal of Biological Chemistry 273: 34920–34925. [DOI] [PubMed] [Google Scholar]
- Lyon CB, Beeson KC, Barrentine M. 1942. Macro-element nutrition of the tomato plant as correlated with fruit fullness and occurrence of blossom-end rot. Botanical Gazette 103: 651–667. [Google Scholar]
- Maathuis FJM, May ST, Graham NS, Bowen HC, Jelitto TC, Trimmer P, Bennett MJ, Sanders D, White PJ. 1998. Cell marking in Arabidopsis thaliana and its application to patch-clamp studies. Plant Journal 15: 843–851. [DOI] [PubMed] [Google Scholar]
- Malone M, Andrews J. 2001. The distribution of xylem hydraulic resistance in the fruiting truss of tomato. Plant, Cell and Environment 24: 565–570. [Google Scholar]
- Maynard DN, Barham WS, McCombs CL. 1957. The effect of calcium nutrition of tomatoes as related to the incidence and severity of blossom-end rot. Proceedings of the American Society for Horticultural Science 69: 318–322. [Google Scholar]
- Minamide RT, Ho LC. 1993. Deposition of calcium compounds in tomato fruit in relation to calcium transport. Journal of Horticultural Science 68: 755–762. [Google Scholar]
- Moore S, Vrebalov J, Payton P, Giovannoni J. 2002. Use of genomics tools to isolate key ripening genes and analyse fruit maturation in tomato. Journal of Experimental Botany 53: 2023–2030. [DOI] [PubMed] [Google Scholar]
- Mori IC, Schroeder JI. 2004. Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiology 135: 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyen C, Hammond-Kosack KE, Jones J, Knight MR, Johannes E. 1998. Systemin triggers an increase of cytosolic calcium in tomato mesophyll cells: Ca2+ mobilisation from intra- and extracellular compartments. Plant, Cell and Environment 21: 1101–1111. [Google Scholar]
- Nesbitt TC, Tanksley SD. 2002. Comparative sequencing in the genus Lycopersicon: implications for the evolution of fruit size in the domestication of cultivated tomatoes. Genetics 162: 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel PS. 1999.Physicochemical and environmental plant physiology, 2nd edn. London: Academic Press. [Google Scholar]
- Nonami H, Fukuyama T, Yamamoto M, Yang L, Hashimoto Y. 1995. Blossom-end rot of tomato plants may not be directly caused by calcium deficiency. Acta Horticulturae 395: 107–114. [Google Scholar]
- Nukaya A, Goto K, Jang H, Kano A, Ohkawa K. 1995. Effect of NH4-N level in the nutrient solution on the incidence of blossom-end rot and gold specks on tomato fruit grown in rockwool. Acta Horticulturae 401: 381–388. [Google Scholar]
- Paiva EAS, Martinez HEP, Casali VWD, Padilha L. 1998. Occurrence of blossom-end rot in tomato as a function of calcium dose in the nutrient solution and air relative humidity. Journal of Plant Nutrition 21: 2663–2670. [Google Scholar]
- Petersen KK, Willumsen J. 1992. Effects of root zone warming and season on blossom-end rot and chemical composition of tomato fruit. Danish Journal of Plant and Soil Science 96: 489–498. [Google Scholar]
- Pill WG, Lambeth VN. 1980. Effect of soil water regime and nitrogen form on blossom-end rot, yield, water relations and elemental composition of tomato. Journal of the American Society for Horticultural Science 105: 730–734. [Google Scholar]
- Pill WG, Lambeth VN, Hinckley TM. 1978. Effects of nitrogen form and level on ion concentrations, water stress, and blossom-end rot incidence in tomato. Journal of the American Society for Horticultural Science 103: 265–268. [Google Scholar]
- Proust J, Houlné G, Schantz M-L, Schantz R. 1996. Characterization and gene expression of an annexin during fruit development in Capsicum annuum FEBS Letters 383: 208–212. [DOI] [PubMed] [Google Scholar]
- Raleigh SM, Chucka JA. 1944. Effect of nutrient ratio and concentration on growth and composition of tomato plants and on the occurrence of blossom-end rot of the fruit. Plant Physiology 19: 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saure MC. 2001. Blossom-end rot of tomato (Lycopersicon esculentum Mill.)—a calcium- or a stress-related disorder? Scientia Horticulturae 90: 193–208. [Google Scholar]
- Schmitz-Eiberger M, Haefs R, Noga G. 2002. Calcium deficiency—influence on the antioxidative defense system in tomato plants. Journal of Plant Physiology 159: 733–742. [Google Scholar]
- Shaykewich CF, Yamaguchi M, Campbell JD. 1971. Nutrition and blossom-end rot of tomatoes as influenced by soil water regime. Canadian Journal of Plant Science 51: 505–511. [Google Scholar]
- Shear CB. 1975. Calcium-related disorders of fruits and vegetables. HortScience 10: 361–365. [Google Scholar]
- Smallwood MF, Gurr SJ, McPherson MJ, Roberts K, Bowles DJ. 1992. The pattern of plant annexin gene expression. Biochemical Journal 281: 501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry WJ, Davis JM, Sanders DC. 1996. Soil moisture and cultivar influence cracking, blossom-end rot, zippers, and yield of staked fresh-market tomatoes. HortTechnology 6: 21–23. [Google Scholar]
- Spurr AR. 1959. Anatomical aspects of blossom-end rot in the tomato with special reference to calcium nutrition. Hilgardia 28: 269–295. [Google Scholar]
- Suzuki K, Shono M, Egawa Y. 2003. Localization of calcium in the pericarp cells of tomato fruits during the development of blossom-end rot. Protoplasma 222: 149–156. [DOI] [PubMed] [Google Scholar]
- Tachibana S. 1988. The influence of withholding oxygen supply to roots by day and night on the blossom-end rot of tomatoes in water culture. Soilless Culture 4: 41–50. [Google Scholar]
- Tanksley SD. 2004. The genetic, developmental, and molecular bases of fruit size and shape variation in tomato. Plant Cell 16: S181–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomos AD, Sharrock RA. 2001. Cell sampling and analysis (SiCSA): metabolites measured at single cell resolution. Journal of Experimental Botany 52: 623–630. [PubMed] [Google Scholar]
- van der Boon J. 1973. Influence of K/Ca ratio and drought on physiological disorders in tomato. Netherlands Journal of Agricultural Science 21: 56–67. [Google Scholar]
- van der Knaap E, Lippman ZB, Tanksley SD. 2002. Extremely elongated tomato fruit controlled by four quantitative trait loci with epistatic interactions. Theoretical and Applied Genetics 104: 241–247. [DOI] [PubMed] [Google Scholar]
- van Goor BJ. 1968. The role of calcium and cell permeability in the disease blossom-end rot of tomatoes. Physiologia Plantarum 21: 1110–1121. [Google Scholar]
- Wada T, Ikeda H, Ikeda M, Furukawa H. 1996. Effects of foliar application of calcium solutions on the incidence of blossom-end rot of tomato fruit. Journal of the Japanese Society for Horticultural Science 65: 553–558. [Google Scholar]
- Wang Y-H, Garvin DF, Kochian LV. 2001. Nitrate-induced genes in tomato roots. Array analysis reveals novel genes that may play a role in nitrogen nutrition. Plant Physiology 127: 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-H, Garvin DF, Kochian LV. 2002. Rapid induction of regulatory and transporter genes in response to phosphorus, potassium, and iron deficiencies in tomato roots. Evidence for cross talk and root/rhizosphere-mediated signals. Plant Physiology 130: 1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward GM. 1973. Causes of blossom-end rot of tomatoes based on tissue analysis. Canadian Journal of Plant Science 53: 169–174. [Google Scholar]
- White PJ. 1998. Calcium channels in the plasma membrane of root cells. Annals of Botany 81: 173–183. [Google Scholar]
- White PJ. 2001. The pathways of calcium movement to the xylem. Journal of Experimental Botany 52: 891–899. [DOI] [PubMed] [Google Scholar]
- White PJ, Bowen HC, Demidchik V, Nichols C, Davies JM. 2002. Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochimica et Biophysica Acta 1564: 299–309. [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR. 2003. Calcium in plants. Annals of Botany 92: 487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox GE, Hoff JE, Jones CM. 1973. Ammonium reduction of calcium and magnesium content of tomato and sweet corn leaf tissue and influence on incidence of blossom end rot of tomato fruit. Journal of the American Society for Horticultural Science 98: 86–89. [Google Scholar]
- Wilkinson JQ, Lanahan MB, Conner TW, Klee HJ. 1995. Identification of mRNAs with enhanced expression in ripening strawberry fruit using polymerase chain reaction differential display. Plant Molecular Biology 27: 1097–1108. [DOI] [PubMed] [Google Scholar]
- Willumsen J, Petersen KK, Kaack K. 1996. Yield and blossom-end rot of tomato as affected by salinity and cation activity ratios in the root zone. Journal of Horticultural Science 71: 81–98. [Google Scholar]
- Wui M, Takano T. 1995. Effect of temperature and concentration of nutrient solution during the stage of the fruit development on the incidence of blossom-end rot in fruits of tomato, Lycopersicon esculentum L. Environmental Control in Biology 33: 7–9. [Google Scholar]
- Zhang X, Fowler SG, Cheng H, Lou Y, Rhee SY, Stockinger EJ, Thomashow MF. 2004. Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis Plant Journal 39: 905–919. [DOI] [PubMed] [Google Scholar]