Abstract
• Background and Aims The anatomy of bamboo culms and the multilayered structure of fibre cell walls are known to be the main determinant factors for its physical and mechanical properties. Studies on the bamboo cell wall have focussed mainly on fully elongated and mature fibres. The main aim of this study was to describe the ultrastructure of primary and secondary cell walls in culm tissues of Dendrocalamus asper at different stages of development.
• Methods The development of fibre and parenchyma tissues was classified into four stages based on light microscopy observations made in tissues from juvenile plants. The stages were used as a basis for transmission electron microscopy study on the ultrastructure of the cell wall during the process of primary and early secondary cell wall formation. Macerations and phloroglucinol–HCl staining were employed to investigate fibre cell elongation and fibre cell wall lignification, respectively.
• Key Results The observations indicated that the primary wall is formed by the deposition of two distinct layers during the elongation of the internode and that secondary wall synthesis may begin before the complete cessation of internode and fibre elongation. Elongation was followed by a maturation phase characterized by the deposition of multiple secondary wall layers, which varied in number according to the cell type, location in the culm tissue and stage of shoot development. Lignification of fibre cell walls started at the period prior to the cessation of internode elongation.
• Conclusions The structure of the primary cell wall was comprised of two layers. The fibre secondary cell wall began to be laid down while the cells were still undergoing some elongation, suggesting that it may act to cause the slow-down and eventual cessation of cell elongation.
Keywords: Bamboo, Dendrocalamus asper, primary cell wall, secondary cell wall, TEM, ultrastructure
INTRODUCTION
The anatomical structure of bamboo is known to determine the physical and mechanical properties of the culm material. Of particular importance is the multilayered structure of the fibre and parenchyma cell walls, which arises from the alternation in the orientation of cellulose microfibrils and results in a structure with high tensile strength (Parameswaran and Liese, 1980). Investigations concerning bamboo cell walls have focussed mainly on the structure of fully elongated fibres and few studies have described the development of bamboo cell walls in detail, especially during the early elongation period of the shoot.
In bamboo, cell divisions and the differentiation of parenchyma and fibre cells are not as clearly organized as in wood. This makes it difficult to follow the process of cell wall formation precisely. Therefore it is necessary of define stages of development within the shoot that can be used as reference points for subsequent studies (Crow, 2000; Crow et al., 2000). Crow (2000) defined three stages of fibre and parenchyma development that occur during internode elongation in the temperate bamboo Phyllostachys viridi-glaucescens. Briefly, the first stage involves cell divisions in both cell types. During the second stage, as the elongation of the internode proceeds, parenchyma cells continue to divide whereas fibres begin to elongate. Finally, in the third stage, both cell types elongate.
In a simplified model of the conifer tracheid cell wall, the primary cell wall is depicted as being composed of a single layer of randomly arranged cellulose microfibrils (e.g. Dinwoodie, 2000). However, the primary wall has been shown to possess two layers with different microfibrillar orientations (Harada and Côté, 1985; Abe et al., 1995). In the model of the mature bamboo fibre cell proposed by Parameswaran and Liese (1976), cell wall microfibrils are shown randomly arranged in the primary wall in a single layer. More recently, Crow (2000) observed that microfibrils of the primary wall in cells of Phyllostachys viridi-glaucescens were initially deposited in an axial orientation, but were then quickly and abruptly superseded by layers of transversely aligned microfibrils, suggesting that in bamboo the primary wall might also be composed of two parts.
The initiation of secondary wall deposition has been considered to commence after the cell has stopped growing (Wardrop, 1964; Brett and Waldron, 1996). However, several reports have demonstrated that secondary wall deposition may start before the cessation of cell expansion (Schubert et al., 1973; Juniper et al., 1981; Abe et al., 1997). This aspect of secondary wall deposition in bamboo cells does not appear to have been reported in the literature.
The aim of present study is to describe the deposition of primary and secondary cell wall layers in detail as seen primarily under the transmission electron microscope, with regard to the different stages of fibre and parenchyma cell differentiation in the tropical bamboo species Dendrocalamus asper.
MATERIALS AND METHODS
Plant material
Dendrocalamus asper (Schultes f.) Backer ex Heyne plantlets were grown in vitro in MS (Murashige and Skoog, 1962) basal liquid medium (MS, Sigma M-5519) supplemented with sucrose and BA (6-benzylamino-purine, Sigma B-3274), at pH 5·0 under a 16 h photoperiod provided by fluorescent lighting, at 22 ± 1 °C. Subculturing was carried out every 3 to 5 weeks. Three- to 5-week-old cultures were rooted by subculturing whole plantlets into the same medium supplemented with α-naphthalene acetic acid (Sigma N-1641) at pH 5·0 for 2–4 weeks. Once roots developed, individual plantlets were transferred into peat compost and grown for 2–4 weeks in a growth room at 25 ± 2 °C, 12 h photoperiod under high humidity, and gradually exposed to air. Rooted plants were transplanted into fresh multipurpose compost and grown under greenhouse conditions with additional fluorescent lighting provided daily during winter months.
Sampling
Samples were taken from ‘juvenile’ plants, which had been grown in the greenhouse as described above. Both ‘immature’ and ‘mature’ shoots were selected. The immature shoots were fully sheathed and had no developed leaves or branches, whereas the mature shoots had several developed branches, many leaves and the culm sheaths were detached. The immature shoots were chosen on the basis of size and presence of both very young developing as well as elongating internodes and at least one maturing internode.
Sample preparation and microscopy
Several internodes were selected from an ‘immature’ shoot measuring about 20 cm in height and from the first internode above ground of a ‘mature’ shoot measuring 60 cm in height that had stopped elongating. The morphological characteristics of the selected internode material are summarized in Table 1. Samples were taken from the middle region of the internode. Cell division occurs mainly at the intercalary meristem at the base of the internode, but also throughout the internode at early stages of internode development.
Table 1.
Characteristics of internode material from juvenile shoots (sampled from a 2-year-old plant) used for light microscopy and TEM
| Shoot |
Internode |
Characteristics |
|---|---|---|
| Immature (20 cm in height) | Apex material (containing apex and un-elongated internodes) | Very soft, light green in colour |
| Internode 4 (1st elongating internode) | Very soft, light green in colour, 1·0 cm long | |
| Internode 3 (elongating) | Soft, green in colour, 3·0 cm long | |
| Internode 1 (maturing) | Hard and brownish in colour, 4·5 cm long | |
| Mature (60 cm in height) | Internode 1 (maturing) | Hard and brownish in colour, 4·0 cm long |
Samples were fixed following the protocol developed at Imperial College London for Phyllostachys viridi-glaucescens (Crow, 2000). In summary, fresh tissues from mid-internode positions were cut and immediately fixed in half-strength Karnovsky's fixative (Karnovsky, 1965) composed of 2 % paraformaldehyde and 2·5 % glutaraldehyde in 0·1 m sodium cacodylate buffer, at pH 8 for 2 h. Primary fixation was completed overnight at 4 °C in the same fixative but at pH 6·8. Following 3 × 20 min rinses in 0·1 m sodium cacodylate buffer with 10 % sucrose, pH 6·8, samples were post-fixed for 2 h with 1 % osmium tetroxide in the same buffer and pH, washed with distilled water (3 × 15 min), and pre-stained in the dark with 0·25 % uranyl acetate overnight at 4 °C. After dehydration in a graded ethanol series and 2 × 20 min changes of propylene oxide, specimens were infiltrated and embedded in hard formulation of Spurr's resin (1969).
Transverse (TS) and longitudinal (LS) 2-mm thick sections were cut with a glass knife using a Reichert ultramicrotome and stained with Richardson's stain (mixture of equal volumes of 1 % methylene blue in 1 % Borax and 1 % Azure II; Richardson et al., 1960) and permanently mounted in DPX medium (Agar, R1340) on glass slides. These sections were used to identify key developmental stages with the light microscope.
Transverse ultra-thin sections of gold to silver interference colour were obtained using a diamond knife, collected onto 200 mm mesh copper grids coated with Formvar, stained with 2 % (aq) uranyl acetate for 30 min at 60 °C and Reynolds (1963) lead citrate for 10 min. Observations were made from areas located two or three bundles away from the outer epidermis. Thin sections were examined and photographed with a Phillips EM301 Transmission Electron Microscope (TEM) and thick sections with a Leitz Diaplan light microscope.
Macerations
Two additional immature (elongating) and mature shoots from a 4-year-old plant grown in the greenhouse were used to measure fibre length at stages 3 and 4. Small matchstick pieces of tissue from the middle of each of four internodes from the immature shoot (2, 3, 4 and 5 counted from the base) and from internode 2 of the mature shoot were macerated to release individual fibres by incubation in a solution of 1 : 4 : 5 hydrogen peroxide (30 %): distilled water: glacial acetic acid at 56 °C for 3 d (Ruzin, 1999). Fibres were pipetted onto slides, dried on a hotplate, stained with 0·03 % Toluidine blue, rinsed, dried again and mounted in DPX. A total of 50 randomly selected fibres were measured using the public domain NIH Image programme (developed at the US National Institutes of Health and available on the internet at http://rsb-info.nih.gov/nih-image). The data were graphically represented and analysed using Microsoft Excel and GraphPad InStat version 3·00 for Windows 95 (GraphPad Software, San Diego California USA, www.graphpad.com).
Test for lignification—Wiesner reaction (Phloroglucinol–HCl)
Sections from the same samples used for the macerations were prepared and stained with Phloroglucinol solution (3 % w/v phloroglucinol in 96 % ethanol) for 30 s, immersed in concentrated HCl for a few seconds, and mounted in glycerol. The sections were observed with a Leitz Diaplan light microscope and photographed immediately. Polarized light micrographs were also taken in order to follow the process of secondary wall formation.
RESULTS
Ultrastructure
Four stages of internode tissue development were identified under the light microscope using thick sections of material prepared for TEM. The first three stages were similar to those described by Crow et al. (2000) for fibre and parenchyma cells. The fourth stage represents the period when cells have ceased to elongate and undergo further maturation. This stage was typical of internode 1 in both the immature and the mature shoots.
Stage 1: cell divisions leading to differentiation of fibre and parenchyma cells. Early stages of vascular bundle formation. Primary cell wall synthesis occurring in both cell types (Fig. 1)
Fig. 1.
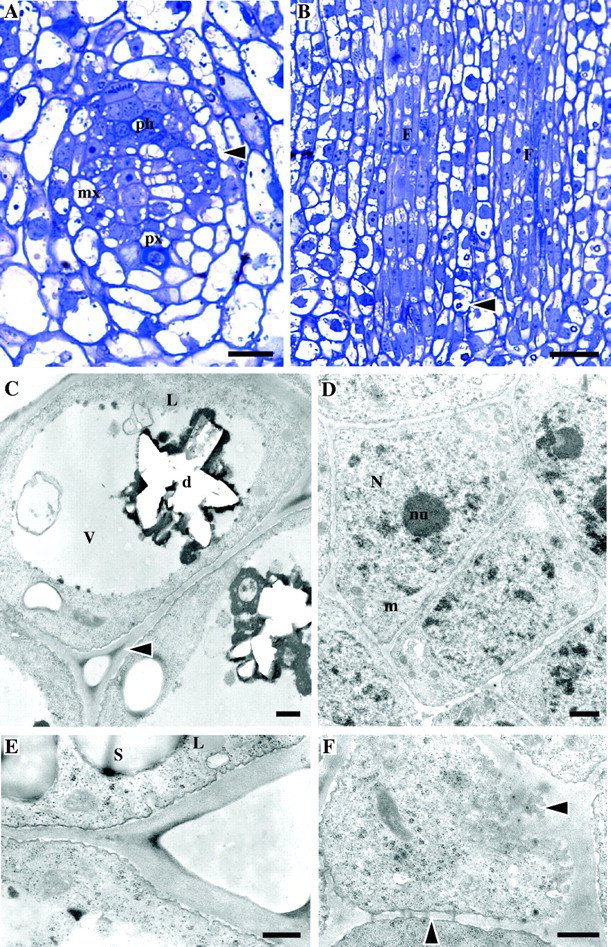
Stage 1 of development (un-elongated internodes close to apex of the immature shoot). Light (A, B) and TEM (C–F) micrographs. (A) TS of a provascular strand of an internode close to the apex showing differentiation of vascular elements. Ph, protophloem; px, protoxylem; mx, metaxylem; arrow, dividing fibre cells. (B) LS of a similar area showing the procambium strands which give rise to fibre cells (labelled ‘F’). The young fibres have a central protoplast and vacuolated ends. Note crystals in parenchyma cells (arrow). (C) Parenchyma cells (TS) with peripheral cytoplasm and large central vacuole (v) containing a hollow structure representing a druse (d). Intercellular spaces are present at cell corners, which are more electron-dense than the rest of the wall (arrow). Lipid globules (L) can be seen in cytoplasm. (D) Meristematic cells of the procambium (TS), which give rise to fibres, showing large nuclei (N) with prominent nucleoli (nu) and dense cytoplasm. Cells are surrounded by a continuous wall. The plasmalemma has an undulating appearance. (E) Detail of parenchyma cell wall showing vesicles fusing with the plasma membrane (TS). Starch grains (s) are present. L = lipid globule. (F) Glancing section of the end wall of a parenchyma cell where plasmodesmata can be seen in cross-section in the plasma membrane and in LS in lateral cell walls (arrows). Scale bars: (A) 50 μm, (B) 25 μm, (C–F) 0·5 μm.
This stage was characteristic of the un-elongated internodes close to the apex in the immature shoot. Vascular elements were at early stages of differentiation. In longitudinal section, periclinal divisions occurred in the procambium strands leading to the differentiation of fibre cells (Fig. 1A, B). Fibre initials were elongated in shape and their protoplasts and nuclei occupied a central location in the cell, whereas small vacuoles concentrated at cell ends. Parenchyma cells were either rectangular or irregularly shaped (Fig. 1B). They usually had a peripherally positioned cytoplasm and a large central vacuole, usually containing a single druse crystal.
Under the TEM it could be seen that the primary wall was at early stages of formation and a single thin continuous matrix surrounded the cells (Fig. 1C, D). The thickness of the combined cell walls between cells was similar in both fibres and parenchyma. For example, between parenchyma cells the wall usually measured approximately 0·15 µm, whereas between fibres it was about 0·12 µm. Unlike parenchyma cells, young fibres were not separated by intercellular spaces (Fig. 1C, D). Large nuclei occupied much of the cytoplasm and some small vacuoles were present at cell ends. Their cell walls were usually lightly stained and, in contrast to parenchyma, the cell corners were stained with the same intensity as the rest of the wall. Parenchyma cells possessed a large central vacuole in which a star-shaped hollow structure indicated that a single a druse crystal had been present and was lost during sectioning (Fig. 1C). Their cytoplasm was confined to the periphery. The parenchyma cell wall was pale-staining, except for the cell corner region of the middle lamella, which was generally more electron-dense (Fig. 1C). Both cell types had dense cytoplasm rich in organelles including ribosomes, mitochondria and rough endoplasmic reticulum (Fig. 1C–E). Lipid globules were present, particularly in parenchyma cells (Fig. 1C). The plasma membrane of both cell types had an undulating appearance, due to the fusion of many vesicles containing material seemingly being transported to the cell wall and to some degree of plasmolysis (Fig. 1C–F). Vesicles containing similar material were also observed within the cytoplasm. Parenchyma cells contained numerous starch grains. Many plasmodesmata were present between adjacent cells, but at this stage no primary pit fields had been formed in the cell walls (Fig. 1F).
Stage 2: cell divisions occurring in most parenchyma cells, most fibres undergoing elongation. Primary cell wall deposition continues in both cell types (Fig. 2)
Fig. 2.
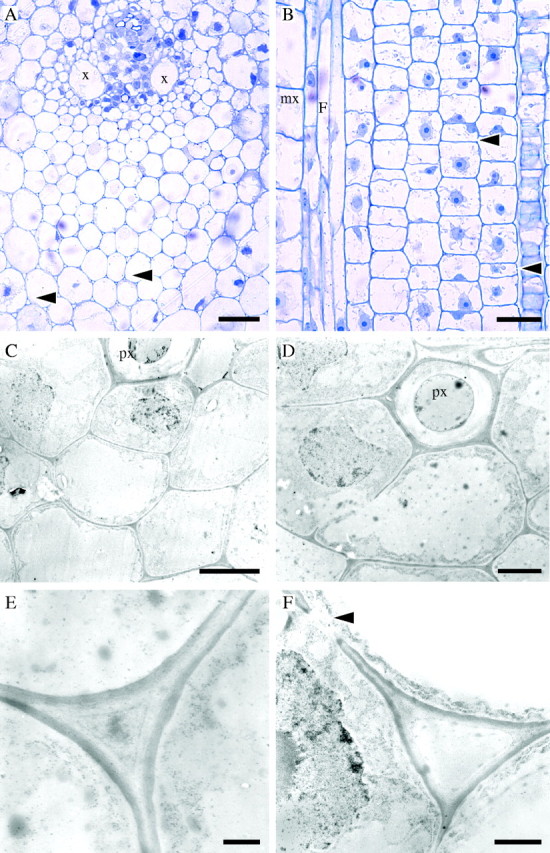
Stage 2 of development (internode 4 of the immature shoot). Light (A, B) and TEM (C–F) micrographs. (A) TS of early vascular bundle development. Some cells adjacent to xylem vessels (x) have prominent protoplasts. Intercellular spaces separate parenchyma cells in ground tissue and fibre cells, especially those in the main portion of the protoxylem fibre sheath (arrows). Parenchyma cells have a round or oval shape whereas fibres tend to be polygonal. (B) LS of a similar area showing elongating fibre cells (F) next to a differentiating metaxylem vessel (mx). Fibres have tapering tips, which grow intrusively at both ends of the cell. Most parenchyma cells continue to divide by periclinal divisions. Elongation of some parenchyma cells gives rise to long and short cells (arrows). (C) Developing fibre cells (TS) at the protoxylem (px) pole. Cells adjacent to the protoxylem element have large nuclei. (D) Detail of fibre cells (TS). (E) Cell wall of fibre cells from the central region of the protoxylem fibre sheath showing a newly deposited electron-dense layer on the cytoplasmic side. An intercellular space is developing, but it appears filled with unidentified material. (F) Cell wall between parenchyma cells showing similar characteristics to those of fibres. A primary pit field is present (arrow). Scale bars: (A, B) 50 μm, (C, D) 5·0 μm, (E, F) 1·0 μm.
At internode 4 of the immature shoot, fibres became distinctly polygonal in shape and some fibres were separated by intercellular spaces, particularly in the main body of the fibre sheath at the protoxylem pole (Fig. 2A). Fibres adjacent or close to protoxylem had prominent protoplasts. In contrast, parenchyma cells had larger diameters and, in transverse section, were characterized by their roundish or oval shape (Fig. 2A). Many parenchyma cells continued to divide in the periclinal plane. Some elongation, possibly leading to the differentiation of long and short parenchyma cells, was apparent (Fig. 2B). Fibres were elongating. Elongation was likely to be occurring at both ends of the cell as indicated by their tapering tips and intrusive growth (Fig. 2B).
The cell walls of both fibre and parenchyma cells were still very thin (Fig. 2C, D). The combined thickness of the cell wall between two cells was approximately 0·3 µm for fibres and 0·2 µm for parenchyma. A conspicuous dark layer approximately 0·1 µm in thickness was deposited on the lighter stained inner side of the cell wall in both cell types (Fig. 2E, F). Primary pit fields were formed in both fibre and parenchyma cell walls (Fig. 2D, F). In most fibres the cytoplasm became restricted to the periphery of the cell and a large central vacuole developed (Fig. 2D). As in light micrographs, some of the fibres adjacent to the protoxylem element had prominent nuclei. Examination of the cytoplasmic content was greatly hindered as the quality of fixation had severely deteriorated by this stage.
Stage 3: cell divisions are complete in both cell types. Elongation is occurring in fibres and parenchyma. Secondary cell wall deposition has started in fibres. Parenchyma cells have only primary walls (Fig. 3)
Fig. 3.
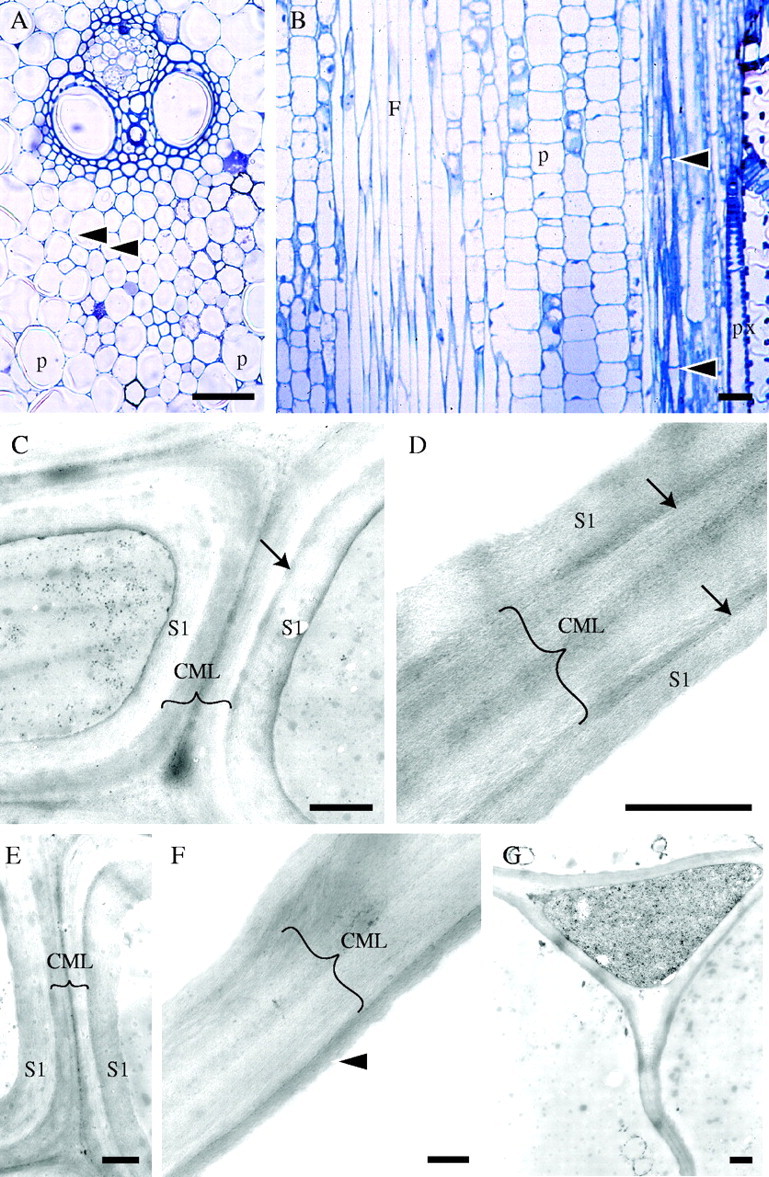
Stage 3 of development (internode 3 of the immature shoot). Light (A, B) and TEM (C–G) micrographs. (A) TS of a vascular bundle. Secondary wall deposition and thickening is occurring mainly in cell walls of fibres, especially in those close to vascular elements as indicated by the darker stain and thicker walls. Intercellular spaces (arrows) are more developed and can be clearly seen separating the fibres. Parenchyma cells (p) remain thin-walled and cell corners are darkly stained. (B) LS of a similar area showing elongating parenchyma (p) and fibre cells (F). The tips of the fibres have become more pointed than in the previous stage. Cross-walls have developed mainly in fibres located in the periphery of the culm or bundle (arrows). Protoxylem vessel (px) can be seen on the right. (C) Cell walls of fibres located two cells away from a metaxylem vessel (TS). At least one secondary wall layer has been deposited (S1). The arrow points to the dark-stained boundary between the primary and the secondary wall (only present in the cell on the right). Brackets indicate the compound middle lamella (CML) composed of the middle lamellae and primary wall. (D) Cell walls of fibres located three cells away from the metaxylem vessel (TS). Wall layers are less well defined. One narrow electron-dense and one broad light-staining layer may be distinguished. Here also a dark layer separates the primary and secondary walls, possibly part of the S1 layer (arrows). (E) Cell walls of fibres two cells away from a metaxylem vessel with one secondary wall layer. The boundary layer is clear in the cell on the right. (F) Cell wall of less developed fibres approximately at the middle of the protoxylem sheath. Distinction of primary and secondary wall layers is less obvious. One dark layer of secondary wall is present (arrowhead). (G) Cell wall of parenchyma cells showing a wavy appearance. The wall is still thin and composed only of middle lamella and primary wall similar to those at stage 2. Scale bars: (A, B) 50 μm, (C–G) 0·5 μm.
At internode 3 of the immature shoot, fibre cell walls were strongly stained and had thickened considerably compared with the previous stage, particularly those cells close to the vascular elements (Fig. 3A). Intercellular spaces became more evident (Fig. 3A) and cross-walls developed, especially in fibres located toward the periphery of the stem (Fig. 3B). Parenchyma cell walls also stained darker, particularly at the cell corners (Fig 3A). The variation in the length of parenchyma cells in longitudinal section suggested that they were still elongating. Short isodiametric parenchyma cells could be distinguished amongst expanding longer cells (Fig. 3B). Fibres became needle-shaped and were still elongating (see results on fibre length below). Their sharply pointed ends indicated additional growth at the tips.
Under the TEM it could be confirmed that at this stage secondary wall deposition was being initiated in most fibres (Fig. 3C–F). In fibres adjacent to or close to the vascular elements it was clear that at least one layer of secondary wall had been laid down. A dark, not clearly defined lamella or ‘boundary’ layer was sometimes present between the primary and the secondary walls (Fig. 3C, D). ‘Less mature’ fibres located towards the centre of the protoxylem sheath exhibited less-developed walls, but there were signs that secondary wall deposition had also started. In these fibres the distinction between primary and secondary wall layers was not always obvious, but at least one layer appeared to be secondary. Unlike fibres, most parenchyma cells still had very thin walls with a similar appearance to those of cells at stage 2, and were frequently wavy, possibly an artefact of specimen preparation procedures (Fig. 3G). As in the previous stage, cytoplasmic contents were almost completely lost due to poor fixation.
Stage 4: cessation of elongation, maturation of fibre and parenchyma cells. Secondary wall deposition is occurring in parenchyma; additional secondary wall layers are deposited in fibres (Figs 4 and 5)
Fig. 4.
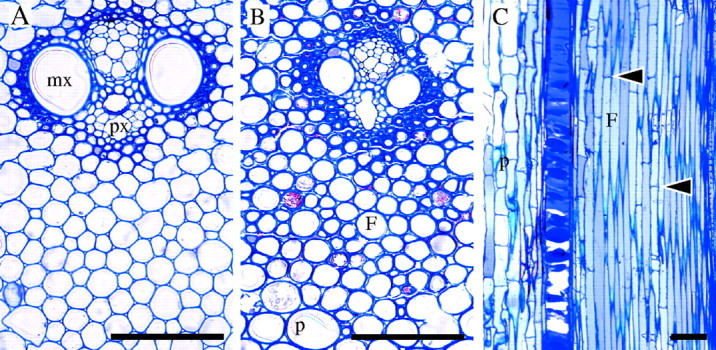
Stage 4 of development (internode 1 of immature and mature shoots). Light micrographs. (A) TS of a vascular bundle from internode 1 of immature shoot showing thickened cell walls in fibres close to metaxylem (mx) and protoxylem (px) vascular elements. Fibres in the main body of the protoxylem sheath have thinner walls and some have become rounded. (B) TS of vascular bundle at internode 1 of mature shoot showing further cell wall thickening in fibres (F) and parenchyma (p). (C) LS of young shoot showing elongated fibres and parenchyma. Many cross-walls have developed in fibres (arrows). Scale bars: 100 μm.
Fig. 5.
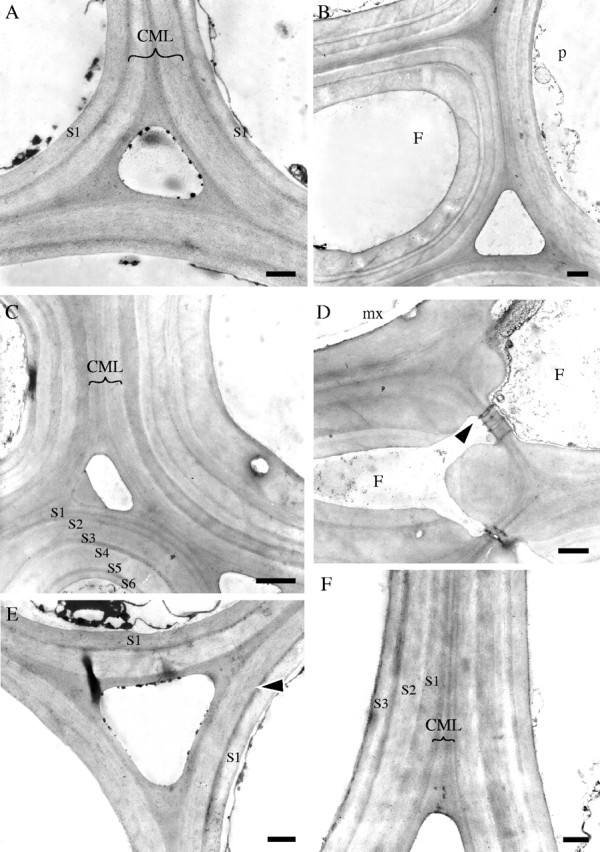
Stage 4 of development (internode 1 of immature and mature shoots). TEM micrographs (all TS). (A) Cell walls of three fibres from the centre of protoxylem sheath at internode 1 of the immature shoot showing compound middle lamella (CML), a thin dark transition layer, and one layer of secondary wall (S1). (B) Fibre (F) from the middle of the protoxylem sheath located next to a parenchyma cell (p) at the periphery of the sheath (internode 1, mature shoot). At least three secondary wall layers can be recognized. Note variation in the thickness of individual layers. (C) Cell walls of fibres three cells away from a metaxylem vessel (mature shoot). At least six secondary wall layers can be distinguished (S1–S6). (D) Cell walls of flattened fibres (F) adjacent to a metaxylem vessel (mx) showing wall layering with less clear boundaries. Plasmodesmata connect pits between two cells (arrow) (mature shoot). (E, F) Parenchyma cell walls. In the immature shoot (E) only a thin dark boundary layer (arrow) and one secondary layer (S1) have been deposited, whereas in the mature shoot (F) further layers (S1–S3) have been deposited. Scale bars: (A, D–F) 0·5 μm, (B, C) 1·0 μm.
At internode 1 of the immature and the mature shoots, both fibre and parenchyma cells had probably attained their full size. Parenchyma cells were thin-walled, especially towards the inner part of the stem in the immature shoot (Fig. 4A). In the mature shoot parenchyma walls were thicker and more strongly stained (Fig. 4B). Similarly, the cell walls of fibres were thicker in the older shoot. Fibre cells were rounder in shape compared to previous stages, especially in the mature shoot (Fig. 4A, B). Many fibre cells developed cross-walls and fibre tips were characterized by further intrusive growth, shown by their sharply pointed tips (Fig. 4C).
TEM observations revealed that additional secondary wall layers had been deposited in fibre cells and there was an increase in the number of layers in the mature shoot (Fig. 5A–D). Cell wall layers were more clearly defined than at stage 3 and some fibres exhibited the typical alternation of broad and narrow layers (Fig. 5B). The cell walls of fibres surrounding the metaxylem vessels were obviously layered, but the boundary between the layers was not so well defined when compared with those of cells located elsewhere (Fig. 5D). In the immature shoot, the cell wall of parenchyma cells usually had one secondary wall layer (Fig. 5E). In contrast, in the mature shoot parenchyma cell walls were composed of at least three secondary wall layers (Fig. 5F).
Fibre elongation, secondary wall deposition and lignification during stages 3 and 4 of tissue development
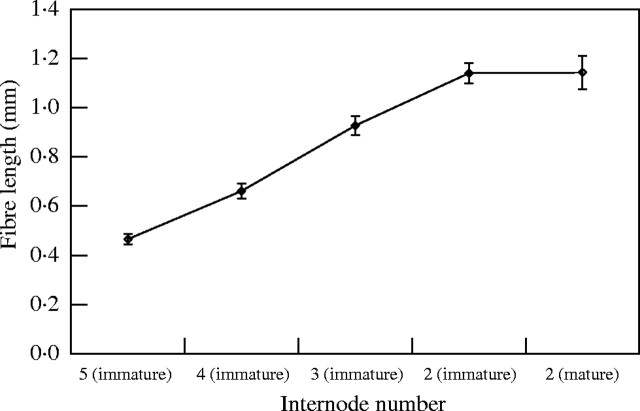
Fibres in tissues at stage 3 (elongation) of tissue development underwent significant elongation until they reached their maximum length, which occurred in internode 2 of the immature shoot (stage 4). Cessation of elongation was confirmed by the observation that fibres at internode 2 of the mature shoot had a similar length to those of the immature shoot at the same internode number (Fig. 6). Statistically significant differences were found between the mean lengths of all fibres, except between internode 2 of the immature shoot and internode 2 of the mature shoot (Table 2).
Fig. 6.
Fibre length during shoot development. The term ‘immature’ refers to the young elongating shoot, whereas ‘mature’ refers to the fully elongated shoot. Each point represents the mean for 50 measurements. The internodes were counted from the base of the shoot upwards. Internodes 5, 4 and 3 of the immature shoot represent stage 3 of tissue development, whereas internode 2 of both shoots is at stage 4 of development. Bars indicate s.e.
Table 2.
Summary of Tukey–Kramer Multiple Comparisons test performed after ANOVA on results on fibre length
| Comparison |
P-value |
|---|---|
| INT 5 vs. INT 4 | **P < 0·01 |
| INT 5 vs. INT 3 | ***P < 0·001 |
| INT 5 vs. INT 2 | ***P < 0·001 |
| INT 5 vs. INT 2 (mature) | ***P < 0·001 |
| INT 4 vs. INT 3 | ***P < 0·001 |
| INT 4 vs. INT 2 | ***P < 0·001 |
| INT 4 vs. INT 2 (mature) | ***P < 0·001 |
| INT 3 vs. INT 2 | ***P < 0·001 |
| INT 3 vs. INT 2 (mature) | ***P < 0·001 |
| INT 2 vs. INT 2 (mature) | ns P > 0·05 |
The only pair showing no significant difference between fibre length means is internode 2 of the immature shoot vs. internode 2 of the mature shoot. INT 2 refers to the immature shoot unless other wise indicated in parentheses.
At internode 5 (immature shoot), birefringence of fibre cell walls was pronounced in cells adjacent to the vascular tissues, indicating that at least those cells had secondary walls (Fig. 7A). Fibres distal to the vascular elements as well as the parenchyma cells in the ground tissue had weakly birefringent cell walls, which may be attributed to a transverse orientation of cellulose microfibrils in the primary cell wall. No positive staining was observed with the Wiesner test, suggesting that cell walls were still unlignified (Fig. 7B). Birefringence of cell walls increased towards the outer parts of the fibre sheaths during fibre elongation and at internode 3 it was clear secondary walls had been deposited in both fibres and parenchyma cells (Fig. 7C, E). Lignification also followed a similar pattern, but it was mainly restricted to fibres close to the vascular elements (Fig. 7D, F). Birefringence and the strength of phloroglucinol–HCl staining continued to increase at internode 2 of the immature and mature shoots, indicating that further secondary wall layers and subsequent lignification occurred after the cessation of elongation (Fig. 7G–J).
Fig. 7.
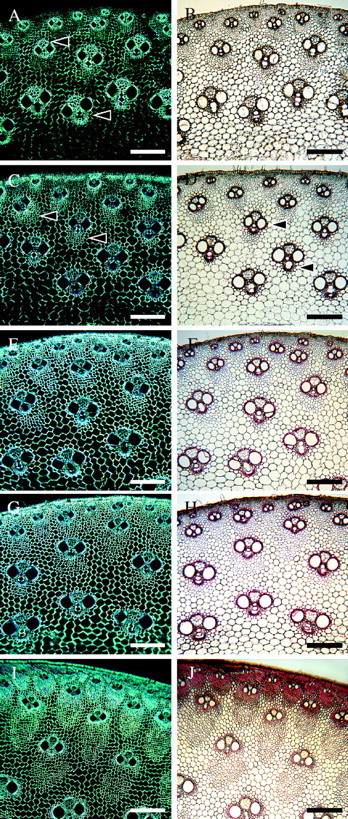
Stages 3 and 4 of tissue development as observed with polarized light (A, C, E, G, I) and with phloroglucinol–HCl staining (B, D, F, H, J). (A, B) Internode 5. Birefringence is pronounced mainly in fibres adjacent to the vascular elements (arrows), indicating presence of secondary cell walls. Cell walls remain unlignified (B). (C, D) Internode 4. Increase in birefringence in fibres not adjacent to vascular elements (arrows). Lignification starts in fibres surrounding the vascular tissues (arrows in D). (E, F) Internode 3. All cell walls become birefringent, including parenchyma. Lignification is still mainly confined to vascular tissues and cells close to their vicinity; however, staining is more intense. (G, H) Internode 2, immature shoot. All cell walls are highly birefringent. Phloroglucinol staining is slightly more pronounced than in the previous internode (H). (I, J) Internode 2, mature shoot. Further secondary wall deposition is evident due to the high level of birefringence. Cell walls continue to lignify in an inward direction. Scale bars: all 200 μm.
DISCUSSION
The observations in the present study showed that the formation of primary and secondary walls occurs at specific stages of tissue development. At the cellular level, as opposed to tissue level, the development of each individual cell type may be divided into three major phases: (1) division and differentiation, (2) elongation, and (3) maturation. These, however, do not occur in both fibre and parenchyma cells simultaneously, a process that is particularly noticeable during early internode growth when parenchyma cell development lags behind that of fibres. This developmental sequence is similar to that observed in Phyllostachys viridi-glaucescens (Crow 2000; Crow et al., 2000).
As pointed out in the Introduction, the primary wall of the conifer tracheid is often depicted as being composed of single layer (e.g. Dinwoodie, 2000). However, it has been shown that it is usually composed of two layers with different microfibrillar orientations (Harada and Côté, 1985; Abe et al., 1995). In cotton (Gossypium hirsutum), for example, the primary wall of fibres has two layers with randomly distributed microfibrils in the outermost layer and transverse microfibrils in the inner layer (Itoh, 1974). In the present study, the primary wall of D. asper was also formed by the deposition of two layers, resulting in a double-layer structure. Primary wall synthesis took place during division and the bulk of cell elongation in both parenchyma and fibre cells. At the earliest stage, the first layer of the primary wall appeared pale and featureless under the TEM. A second layer that was more electron-dense was subsequently deposited in both cell types during stage 2 of tissue development (parenchyma dividing, fibres elongating), and during which primary pit fields were also formed. The orientation of cellulose microfibrils, however, could not be resolved with the TEM. A separate detailed investigation on cellulose microfibril orientation in bamboo cell walls has been carried out and results will be presented in a subsequent paper.
The data from the macerations and the TEM observations indicated that at least one secondary wall layer may be deposited whilst fibre elongation is still taking place (late stage 3 of tissue development). As parenchyma cell elongation lags behind that of fibres, secondary wall deposition begins later; however, we did not investigate whether it starts before cessation of elongation, as observed in fibres. Following the end of elongation, both fibre and parenchyma cells entered a ‘maturation’ phase during which additional secondary wall layers were added to the growing wall and considerable thickening of the cell wall occurred in fibres. Elongation of fibre cells at early stages was both diffuse and by intrusive growth, but as elongation progressed, it is possible that elongation is mainly diffuse given the significant increase in fibre length during internode elongation.
He et al. (2000) proposed four stages of fibre development in the bamboo Phyllostachys pubescens: cell differentiation, cell elongation (primary wall formation), cell wall thickening, and lignification. During the last two stages secondary wall formation takes place. Their results suggested that secondary wall formation occurred only after fibres had stopped elongating, contrary to the present observations that indicate that secondary wall synthesis begins before the complete cessation of elongation. Evidence that secondary wall deposition occurs whilst cells are still elongating has also been shown in cotton (Gossypium hirsutum) fibres (Schubert et al., 1973). The authors postulated that elongation and secondary wall thickening are not separate events and a substantial amount of secondary wall is deposited before elongation stops. Similarly, Abe et al. (1997) reported that in conifer tracheids secondary wall synthesis begins before cells stop radial expansion. They proposed that cell expansion ceases as a result of secondary wall deposition and that the dimensions of the tracheids are probably determined by the initiation of secondary wall formation. Our work shows that an amount of secondary wall is also laid down in bamboo fibres during elongation and that this may act to cause the slow-down and eventual cessation of cell elongation.
Although not studied in detail, it was noted that the septate fibres observed in the present study were associated with older tissues and with the periphery of the stem. Septate fibres are of relatively common occurrence in bamboos. They are often present in mature culms (Liese, 1998) and have been reported to be present in several bamboo species (Parameswaran and Liese, 1977; Murphy and Alvin, 1997). Usually only one septum is formed by cytokinesis of the nucleus in fibres with already multilayered walls. As the fibre matures, the septum also becomes multilayered and lignified, unlike those of dicotyledons. Septate fibres have been reported to be most abundant in the periphery and towards the inner part of the culm, in the middle of the xylem and metaxylem fibre caps, but are rarer in phloem caps (Parameswaran and Liese, 1977).
Finally, it is worth noting that a full examination of protoplast contents was not possible due to poor fixation of specimens. Crow (2000) tested several fixation protocols using Phyllostachys viridi-glaucescens and found that ultrastructural preservation of bamboo tissues tended to be erratic even in neighbouring cells. From our experience with other bamboo species, fixation trials also produced similar results. In this study, only the youngest tissues were reasonably well preserved. Older tissues showed a poor preservation of membranes and, consequently, of organelles. He et al. (2000) considered organelle swelling and plasmolysis, among other features, to be evidence of programmed cell death in bamboo fibres. Their samples, however, were not particularly mature. Furthermore, bamboo fibre cell walls, particularly those of large diameter fibres, are known to continue thickening even in very old culms over 9 years of age, and so they clearly retain protoplasts (Liese and Weiner, 1996; Murphy and Alvin, 1997).
CONCLUSIONS
The primary cell wall of fibre and parenchyma cells in Dendrocalamus asper is constructed by the deposition of two distinct layers resulting in a double-layer structure. The deposition of an amount of secondary wall before the complete cessation of internode elongation indicated that secondary wall deposition may be responsible, wholly or in part, for stopping cell elongation. Although some lignification takes place prior to cessation of elongation, it occurs mainly after the cessation of elongation. Further detailed immunocytochemical work and field emission scanning electron microscopy focussing on the control and the orientation of cellulose microfibrils will be reported in a subsequent paper.
Acknowledgments
We thank the European Commission for funding the INCO-DC project (ERBIC18CT970176) under which this research was undertaken. We are also very grateful to Dr Johan Gielis and Oprins Plant NV (Belgium) for the donation of initial cultures of D. asper, Mr Ian Morris for his invaluable advice on electron microscopy techniques, and Dr Emma Crow for kindly sharing with us her knowledge on bamboo cell development.
LITERATURE CITED
- Abe H, Funada R, Imaizumi H, Ohtani J, Fukazawa K. 1995. Changes in the arrangement of microtubules and microfibrils in conifer tracheids during the expansion of cells. Annals of Botany 75: 305–310. [Google Scholar]
- Abe H, Funada R, Ohtani J, Fukazawa K. 1997. Changes in the arrangement of cellulose microfibrils associated with the cessation of cell expansion in tracheids. Trees Structure and Function 11: 328–332. [Google Scholar]
- Brett CT, Waldron KW. 1996.Physiology and biochemistry of plant cell walls, 2nd edn. Cambridge: Chapman and Hall/University Press. [Google Scholar]
- Crow E. 2000.Development of fibre and parenchyma cells in the bamboo Phyllostachys viridi-glaucescens (Carr.) Riv. & Riv. PhD Thesis Imperial College London, University of London, UK. [Google Scholar]
- Crow E, Murphy RG, Cutler DF. 2000. Development of fibre and parenchyma cells in the bamboo (Phyllostachys viridi-glaucescens (Carr.) Riv. & Riv.) with emphasis on the cytoskeleton during cell wall deposition. In: Savidge RA., Barnett JR, Napier R, eds. Cell and molecular biology of wood formation. Oxford: BIOS Scientific Publishers Ltd., 265–276. [Google Scholar]
- Dinwoodie JM. 2000.Timber, its nature and behaviour, 2nd edn. New York, USA: E & FN Spon, 26. [Google Scholar]
- Harada H, Côté WA Jr. 1985. Structure of wood. In: Higuchi T, ed. Biosynthesis and biodegradation of wood components. Orlando: Academic Press, 1–42. [Google Scholar]
- He XQ, Wang YQ, Hu YX, Lin JX. 2000. Ultrastructural study of secondary wall formation in the stem fibre of Phyllostachys pubescens Acta Botanica Sinica 42: 1003–1008. [Google Scholar]
- Itoh T. 1974. Fine structure and formation of cell wall of developing cotton fiber. Wood Research. Bulletin of the Wood Research Institute, Kyoto University 56: 49–61. [Google Scholar]
- Juniper BE, Lawton JR, Harris PJ. 1981. Cellular organelles and cell-wall formation in fibres from the flowering stem of Lolium temulentum L. New Phytologist 89: 609–619. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ. 1965. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. Journal of Cell Biology 27: 137A. [Google Scholar]
- Liese W. 1998.The anatomy of bamboo culms. Technical Report. Beijing, China: INBAR. [Google Scholar]
- Liese W, Weiner G. 1996. Ageing of bamboo culms. A review. Wood Science and Technology 30: 77–89. [Google Scholar]
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15: 473–497. [Google Scholar]
- Murphy RJ, Alvin KL. 1997. Fibre maturation in the bamboo Gigantochloa scortechinii IAWA Journal 18: 147–156. [Google Scholar]
- Parameswaran N, Liese W. 1976. On the fine structure of bamboo fibres. Wood Science and Technology 10: 231–246. [Google Scholar]
- Parameswaran N, Liese W. 1977. Structure of septate fibres in bamboo. Holzforschung 31: 55–57. [Google Scholar]
- Parameswaran N, Liese W. 1980. Ultrastructural aspects of bamboo cells. Cellulose Chemistry and Technology 14: 587–609. [Google Scholar]
- Reynolds ES. 1963. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. Journal of Cell Biology 17: 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson KC, Jarett L, Finke EH. 1960. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technology 35: 313–323. [DOI] [PubMed] [Google Scholar]
- Ruzin SE. 1999.Plant microtechnique and microscopy. Oxford: Oxford University Press. [Google Scholar]
- Schubert AM, Benedict CR, Berlin JD, Koehel RJ. 1973. Cotton fiber development—kinetics of cell elongation and secondary wall thickening. Crop Science 13: 704–709. [Google Scholar]
- Spurr AR. 1969. A low viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructural Research 26: 31–43. [DOI] [PubMed] [Google Scholar]
- Wardrop AB. 1964. The structure of formation of cell wall in xylem. In: Zimmermann MA, ed. The formation of wood in forest trees. New York: Academic Press, 87–134. [Google Scholar]