Abstract
• Background and Aims The ability to detect and respond to the impending threat of shade can confer significant selective advantage to plants growing in natural communities. This Botanical Briefing highlights (a) the regulation of shade-avoidance responses by endogenous and exogenous factors and (b) current understanding of the molecular components involved in red to far-red ratio signal transduction.
• Scope The Briefing covers: (a) the shade-avoidance syndrome in higher plants; (b) the adaptive significance of shade avoidance in natural light environments; (c) phytochrome regulation of shade-avoidance responses; (d) the role of blue light signals in shade avoidance; (e) gating of rapid shade-avoidance responses by the circadian clock; (f) potential signalling components and future perspectives.
Keywords: Phytochromes, red light, far-red light, red to far-red ratio, blue light, elongation growth, early flowering, circadian clock, ethylene
INTRODUCTION
Natural plant communities, as well as agricultural crops, are frequently resource-limited, and competition between individuals often results in plastic developmental responses to the specific resource shortage. As photoautotrophs, higher plants depend upon the acquisition of light energy for their survival and competition for light is characteristic of plant communities. Being sessile organisms that cannot choose their surroundings, plants must adapt their growth and development to the ambient light environment. Monitoring changes in the quantity, quality and direction of light enables plants to optimize both the timing of germination and their subsequent growth and development for the optimal acquisition of light energy to drive photosynthesis. In addition, through interactions with the central circadian oscillator, light signals enable plants to monitor day length (photoperiod) and adapt their growth and development, especially the timing of the transition from vegetative to reproductive development, in relation to changing seasonal environments. Such developmental plasticity in response to light signals is conferred by specialized information-transducing photoreceptors. In higher plants, three major families of such photoreceptors have been identified and characterized. These are the red (R)/far-red (FR) light-absorbing phytochromes and the blue/UV-A light-absorbing cryptochromes (Cashmore et al., 1999) and phototropins (Briggs and Huala, 1999).
R : FR RATIO AND SHADE AVOIDANCE
Plants have evolved mechanisms that enable them to respond to the presence of neighbours. The spectral energy distribution of daylight is dramatically altered by vegetation. The photosynthetic pigments, chlorophylls and carotenoids, absorb light over most of the visible spectrum, although some green light is reflected or transmitted. Radiation in the FR region is very poorly absorbed and, consequently, the light that is transmitted through, or reflected, from vegetation is depleted in R and significantly enriched in FR wavelengths. A useful parameter to describe the natural light environment is therefore the ratio of photon irradiance in the R, to that in the FR (R : FR ratio). This parameter is often directly defined as follows:
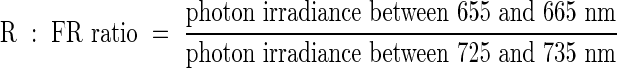 |
The R : FR ratio of daylight is around 1·15 and varies little with weather conditions or time of year (Smith, 1982). Reported R : FR ratios underneath canopies of vegetation are typically in the range 0·05–0·7 (Smith, 1982). A spectral photon distribution of both incident and reflected daylight is shown in Fig. 1.
Fig. 1.

The spectral photon distributions of daylight (blue line) and light reflected from leaves of Fallopia japonica (green line).
Changes in light quality in the red and far-red regions of the spectrum (i.e. R : FR ratio) are detected by the phytochromes. Higher plants contain multiple phytochromes, the apoproteins of which are encoded by a small divergent gene family (Quail, 1994). Three major phytochromes exist in angiosperms, phyA, phyB and phyC, encoded by the genes PHYA, PHYB and PHYC. Two additional phytochromes exist in dicotyledonous plants, phyD and phyE, and likely represent the products of more recent gene duplication events (Mathews and Sharrock, 1997). In the model plant species Arabidopsis thaliana, five apophytochrome-encoding genes (PHYA–PHYE) have been sequenced and characterized (Sharrock and Quail, 1989; Clack et al., 1994). The protein products of the PHYB and PHYD genes share approx. 80 % sequence identity and are slightly more related to PHYE than they are to either PHYA or PHYC. Thus, the PHYB, PHYD and PHYE genes are considered to form a distinct subgroup within the arabidopsis PHY gene family (Goosey et al., 1997).
Phytochromes exist as a homodimer of two independently reversible subunits. Each subunit consists of a polypeptide (approx. 124 kDa) covalently linked to a light-absorbing, linear tetrapyrrole chromophore, phytochromobilin, via a thioether linkage (Furuya and Song, 1994). In the dark, phytochrome is synthesized in the red light-absorbing (Pr) form (absorption maximum approx. 660 nm) and is generally regarded to be biologically inactive. Activity is acquired upon photo-conversion to the far red light-absorbing (Pfr) form (absorption maximum approx. 730 nm). Under almost all irradiation conditions, an equilibrium mixture of the two forms will exist. The relative amounts of R and FR light in incident radiation will be translated by the phytochromes into different relative concentrations of the active Pfr form of phytochrome. A reduced R : FR ratio, leading to relatively low concentrations of Pfr, is considered to be a key signature of light reflected from, or transmitted through, neighbouring vegetation.
In response to low R : FR ratio signals, many plants display a rapid and pronounced increase in the elongation growth rate of stems and petioles, often at the expense of leaf and storage organ development. This is demonstrated in Fig. 2, which shows arabidopsis and Brassica rapa plants grown in prolonged irradiation of both high and low R : FR ratios. In addition to reduced chlorophyll content, a reduction in leaf thickness is often observed in plants receiving low R : FR ratio signals (McClaren and Smith, 1978). These architectural modifications are accompanied by elevated leaf angles (hyponasty) and an increase in apical dominance leading to reduced branching in dicots and reduced tillering in grasses (Casal et al., 1986). Such responses, collectively termed the shade-avoidance syndrome, serve to elevate leaves towards unfiltered daylight and provide an essential survival strategy in rapidly growing populations.
Fig. 2.
The R : FR ratio-mediated shade-avoidance response. The appearance of arabidopsis (A) and Brassica rapa (B) plants grown under high or low R : FR ratio conditions. All plants were grown under white fluorescent light providing equal photosynthetically active radiation (400–700 nm). For each species, the plants on the right received supplementary FR to reduce the R : FR ratio.
Shade-avoidance responses are typically initiated in advance of canopy closure and light becoming limiting. Thus, plants respond predominantly to the reduction in R : FR ratio of light reflected from surrounding vegetation (a proximity signal) and therefore initiate escape responses in anticipation of being shaded (Ballaré et al., 1990). The morphology, size and distribution of leaves are key factors in the generation of these proximity signals (Gilbert and Smith, 2001). If the reduced R : FR ratio signal persists and the plant is unable to overtop competing vegetation, flowering is accelerated, thereby promoting seed set and enhancing the probability of reproductive success (Halliday et al., 1994; Smith and Whitelam, 1997; Dorn et al., 2000).
THE ADAPTIVE SIGNIFICANCE OF SHADE AVOIDANCE
The adaptive significance of shade avoidance has been assessed in a number of ecological investigations. Such studies have suggested that the ability of stems and petioles to elongate in response to proximity-to-vegetation signals can confer high relative fitness in dense stands of plants (Schmitt et al., 2003). Constitutive expression of an oat PHYA gene has previously been used to suppress shade-avoidance responses in transgenic tobacco plants (McCormac et al., 1991, 1992). In these plants, persistence of a normally transient phyA-mediated inhibition of stem elongation resulted in an inability of plants to increase stem elongation in response to low R : FR ratio signals. When grown in dense stands, these transgenic plants displayed decreased fitness compared with wild-type tobacco plants (Schmitt et al., 1995; Robson et al., 1996). A similar finding was obtained from a recent study using transgenic tobacco plants that are insensitive to ethylene (see ‘The role of blue light and other signals in shade avoidance’). Compared with wild types, the transgenic plants display a significant delay in the key shade-avoidance traits—increased leaf hyponasty and stem elongation in response to reduced R : FR ratio. When grown in mixed populations alongside wild-type seedlings, the ethylene-insensitive transgenic plants suffered a severely reduced competitive ability compared with their wild-type neighbours (Pierik et al., 2003). However, when grown in crowded monocultures, biomass accumulation in the ethylene-insensitive transgenic plants was comparable with that of crowded wild-type monocultures. This suggests that when all neighbouring plants suffer the same disruption of shade-avoidance responses, there are no obvious fitness consequences (Pierik et al., 2004a).
Although shade avoidance may have major fitness benefits in crowded communities, in the absence of competition for light, the reallocation of resources towards elongation growth that typifies the shade-avoidance response may reduce the competitive success of an individual as well as leading to an increased risk of lodging and mechanical injury (Casal and Smith, 1989). Experiments in which mutants that are constitutively elongated have been grown at low densities, in either the glasshouse or in the field, support this notion. Under these conditions, the elongated internode (ein) mutant of Brassica rapa displayed decreased dry biomass and a reduction in the number of reproductive structures compared with wild-type plants (Schmitt et al., 1995). Likewise, seedlings of the long hypocotyl (lh) mutant of cucumber were subject to increased stem damage compared with wild-type cucumber seedlings (Casal et al., 1994).
PHYTOCHROME REGULATION OF SHADE AVOIDANCE
The roles of individual phytochromes in mediating responses to low R : FR ratios have been largely inferred from studies using mutants deficient in one or more family members. When grown in white light (i.e. high R : FR ratio conditions), phytochrome B-deficient mutants of a number of plants species characteristically display elongated stems/petioles, reduced leaf size, decreased chlorophyll content and early flowering—responses often described as ‘constitutive shade avoidance’ (Somers et al., 1991; Devlin et al., 1992; López-Juez et al., 1992; Reed et al., 1993). Such studies have confirmed a major role for phyB in transducing the low R : FR ratio signal, although the retention of some, attenuated shade-avoidance responses in phytochrome B-deficient null mutants indicated the involvement of additional phytochromes (Whitelam and Smith, 1991). Daytime reductions in R : FR ratio and end-of-day (EOD) FR treatments, known to mimic the effects of growth in reduced R : FR ratio conditions, were subsequently shown to elicit small, yet significant, shade-avoidance responses in phyB mutants of multiple species (Robson et al., 1993; Halliday et al., 1994; Devlin et al., 1996). The discovery of a naturally occurring phyD mutation in the Wassilewskija (Ws) ecotype of arabidopsis enabled the role of this phytochrome in shade avoidance to be examined. Adult mutants deficient in phyD displayed wild-type responses to both low R : FR ratio and EOD FR treatments (Aukerman et al., 1997; Devlin et al., 1999). Comparison of a phyBphyD mutant with a phyB mutant, however, revealed the double mutant to display greater petiole elongation and earlier flowering, phenotypes reminiscent of the shade-avoidance syndrome (Devlin et al., 1999). These findings suggested a redundancy of function between phyB and phyD in regulating shade avoidance, a proposal supported by their sequence similarity and similar patterns of expression of their genes (Goosey et al., 1997; Mathews and Sharrock, 1997).
A pronounced acceleration of flowering and internode growth between rosette leaves were observed in arabidopsis phyAphyB mutants subject to EOD FR treatments, forming the basis of a screen from which the phyE mutation was isolated (Devlin et al., 1998). These phenotypes were constitutively displayed by the phyAphyBphyE triple mutant, implicating phyE in the regulation of these responses (Devlin et al., 1998). The subsequent creation of mutants null for multiple phytochrome species led to confirmation that the shade-avoidance syndrome is regulated exclusively by phytochromes B, D and E, all acting together in a functionally redundant manner (Franklin et al., 2003a). These phytochromes represent the most recently evolved members of the phytochrome family, forming a distinct subgroup (Mathews and Sharrock, 1997). It can therefore be speculated that competition for light may have provided the selective pressure for their evolution (Devlin et al., 1998).
The elongated appearance and early flowering response displayed by phyB mutants grown in high R : FR ratio, phenotypes not displayed by phyD or phyE monogenic mutants, have implicated phyB as the major regulator of shade-avoidance responses in Arabidopsis. Recent studies have, however, revealed evidence that action of different phytochromes is modified by other environmental signals, particularly ambient growth temperature (Blázquez et al., 2003; Halliday et al., 2003). For instance, a modest reduction in ambient temperature from 22 °C to 16 °C was sufficient to completely abolish the early flowering phenotype of phyB mutants. Accelerated flowering responses in low R : FR were, nevertheless, still observed in wild-type plants grown at 16 °C, suggesting that phytochromes, other than phyB, play a more dominant role in repressing flowering in plants growing at lower temperatures. Studies using mutants null for multiple phytochromes revealed that phyE, and to a lesser extent phyD, do indeed play greater roles in the control of flowering under cooler conditions (Halliday and Whitelam, 2003; Halliday et al., 2003).
In contrast to the other phytochromes, phyA is subject to rapid proteolytic degradation upon photoconversion to Pfr and therefore only accumulates to high levels in etiolated seedlings (Quail, 1994). A major role for phyA, and the basis upon which phyA mutants were identified, is mediating FR high irradiance responses in de-etiolating seedlings (Smith and Whitelam, 1990). Despite being present at reduced levels in light-grown plants, phyA performs an important role in the regulation of hypocotyl elongation in response to changes in R : FR ratio. When grown in continuous low R : FR ratio light, phyA mutant seedlings displayed enhanced hypocotyl elongation compared with wild-type controls (Johnson et al., 1994). These observations led to the suggestion that in wild-type plants, phyA action was antagonizing phyB-mediated shade avoidance by constraining hypocotyl extension. This notion is supported by observations showing that the hypocotyls of phyAphyB double mutants display enhanced elongation growth when compared with monogenic phyB mutants (Johnson et al., 1994). In the field, the action of phyA in constraining shade-avoidance elongation responses has been shown to be of fundamental importance in seedling establishment under dense natural vegetational shade. Under these conditions, phyA mutants display an extreme hypocotyl elongation response, with many seedlings failing to become established and dying prematurely (Yanovsky et al., 1995). The growth-inhibitory action of phyA in low R : FR ratio conditions has been successfully exploited in the production of transgenic tobacco plants which over-express PHYA, thus leading to the elimination of unwanted elongation responses in more densely planted crops (Robson et al., 1996).
A role for phyC in mediating shade-avoidance responses was excluded following observations that phyBphyDphyE triple mutants were blind to the reduced R : FR ratio signal and EOD FR treatments (Franklin et al., 2003a). This proposal was endorsed by the isolation of mutants, null at the PHYC locus, which displayed no aberrancy in R : FR ratio perception, alone, or when present in combination with other phytochrome mutations (Franklin et al., 2003b).
THE ROLE OF BLUE LIGHT AND OTHER SIGNALS IN SHADE AVOIDANCE
In addition to changes in R : FR ratio, reductions in total light quantity can provide information to plants about the proximity of neighbours in closed canopies (Ballaré et al., 1991a; Ballaré, 1999). Indeed, reductions in both photosynthetically active radiation and blue light can induce shade-avoidance responses in cucumber hypocotyls (Ballaré et al., 1991b) and tobacco stems (Casal and Sánchez, 1994). Changes in blue light quantity are sensed by the blue light photoreceptors cryptochromes and phototropins (Briggs and Huala, 1999; Cashmore et al., 1999). Studies using transgenic tobacco plants that are insensitive to ethylene revealed their delayed shade-avoidance responses to the presence of neighbouring vegetation were the result of insensitivity to reduced fluence rates of blue light (Pierik et al., 2004b). The ethylene insensitive plants displayed more or less wild-type responses to reductions in R : FR ratio. Such observations indicate that reductions in incident blue light play an important role in shade avoidance in dense communities and suggest that ethylene is an important regulatory component in these blue light-mediated responses (Pierik et al., 2004b). In addition to reductions in R : FR ratio and blue light irradiance, exposure to low concentrations of ethylene has also been shown to initiate shade-avoidance responses in wild-type tobacco plants (Pierik et al., 2003). This, together with the finding that ethylene concentrations increase in the canopy atmosphere, suggest that ethylene accumulation in crowded stands may provide a further warning to plants that competitors are nearby (Pierik et al., 2004b).
SIGNALLING IN SHADE AVOIDANCE
Proximity perception in natural light environments is achieved through the convergence of multiple signals. Of these, a reduction in R : FR ratio may be considered to be the most effective (Holmes and Smith, 1975; Ballaré et al., 1990; Smith and Whitelam, 1997). Although the individual phytochrome species involved in the perception of R : FR ratio signals have been defined, relatively little is known about the molecular mechanisms involved in transducing the signal into changed patterns of growth and development. Phytochrome B plays a major role in R : FR ratio perception and an understanding of the molecular mechanisms underlying phytochrome B signal transduction in relation to its role in seedling de-etiolation is emerging. The isolation of multiple phytochrome B-interacting factors and observations of light-induced translocation of the Pfr form of phyB (and indeed other phytochromes) to the nucleus suggest that phytochrome signal transduction is a highly complex network of events occurring in multiple cellular compartments (for reviews, see Møller et al., 2002; Schäfer and Bowler, 2002). As yet, no direct experimental evidence exists to link the nuclear translocation of Pfr or the binding of phyB to interacting factors with R : FR ratio signalling. These events are beyond the scope of this review and will not be discussed further here.
The transition to flowering in arabidopsis is controlled by multiple regulatory pathways which converge to regulate the expression of meristem identity genes such as LFY (Simpson and Dean, 2002). This is achieved using a number of floral pathway integrating genes such as FT and SOC1. These are, in turn, regulated by transcriptional regulators such as FLC and CO (Simpson and Dean, 2002). The concept of an independent light quality (i.e. R : FR ratio) pathway was suggested by the discovery that the early flowering phenotype of phyB mutants involved regulation of LFY expression, in a manner that was independent from CO, the floral regulator implicated in the photoperiodic regulation of flowering (Blázquez and Weigel, 1999). More recent studies have revealed this pathway involves regulation of FT (Cerdán and Chory, 2003; Halliday et al., 2003) in a temperature-conditional manner (Halliday et al., 2003). Mutant screening has resulted in the identification of pft1, a recessive mutation which suppresses the early flowering phenotype associated with phyB deficiency (Cerdán and Chory, 2003). The PFT1 gene encodes a nuclear-localized protein with structural similarity to some transcriptional regulators (Cerdán and Chory, 2003). Petiole length was largely unaffected in pft1phyB double mutants, whilst the pft1 mutation led to a significant impairment of the flowering response to EOD FR treatments. Taken together, these data suggest that the major role of PFT1 is to regulate flowering time downstream of phytochromes B, D and E (Cerdán and Chory, 2003).
Reports of genes whose expression is strongly regulated by changes in R : FR ratio are limited. The most cited examples to date include the homeodomain ZIP transcription factors ATHB-2 (also known as HAT4) and ATHB-4. Both these genes show a rapid increase in transcript abundance upon transfer of arabidopsis plants to low R : FR ratio (Carabelli et al., 1993, 1996). Analyses of mutants deficient in multiple phytochrome combinations have revealed the regulation of ATHB-2 transcript abundance to be controlled by phyB and phyE acting in a functionally redundant manner (Franklin et al., 2003a). The possible involvement of ATHB-2 in shade avoidance has been inferred from studies using plants with altered levels of ATHB-2 expression (Schena and Davis, 1992; Steindler et al., 1999). Transgenic plants with elevated levels of ATHB-2 displayed some phenotypes similar to those of wild-type plants grown in reduced R : FR ratio. Plants with decreased levels of ATHB-2 behaved oppositely, suggesting a role for ATHB-2 in the regulation of shade avoidance (Carabelli et al., 1996; Steindler et al., 1999).
More recently, microarray analyses in arabidopsis have revealed two genes, PIL1 (PIF3-LIKE 1) and PIL2 (PIF3-LIKE 2) to show rapid and significant increases in transcript abundance upon transfer of plants to low R : FR ratio (Salter et al., 2003). Both genes encode basic helix–loop–helix transcription factors with high protein sequence similarity to the phytochrome-interacting protein PIF3 (Ni et al., 1998). The increase in PIL1 transcript level is extremely rapid, with quantitative reverse transcription polymerase chain reaction (qRT-PCR) revealing detectable increases in PIL1 transcript after just 8 min of low R : FR ratio treatment (Salter et al., 2003). The kinetics of PIL2 transcript abundance appear slightly different, with longer exposures to low R : FR required for maximum de-repression of its expression (Salter et al., 2003). More detailed expression studies of PIL1 and PIL2 showed de-repression of both genes to be gated by the circadian clock, with maximum increases occurring at subjective dawn (Salter et al., 2003). The role of PIL1 in shade avoidance was established following observations that a pil1 null mutant was impaired in rapid hypocotyl elongation in response to transient reductions in R : FR ratio (Salter et al., 2003).
The gating of PIL1 and PIL2 gene de-repression by the circadian clock led to speculation that physiological responses to low R : FR ratio may be regulated in a similar manner. It was subsequently revealed that a 2-h transient reduction in R : FR ratio was sufficient to induce a 30 % increase in arabidopsis hypocotyl extension within the following 24-h period. This response was also gated by the circadian clock, with maximum elongation occurring at subjective dusk (Salter et al., 2003). A considerably attenuated response was observed in pil1 null mutants, suggesting that the presence of PIL1 is required. Paradoxically, an inhibition of hypocotyl length was observed in seedlings treated with low R : FR ratio at subjective dawn, a time when maximum de-repression of PIL1 transcript occurs (Salter et al., 2003). This response was absent in phyA mutants, confirming the role of this phytochrome family member in antagonizing shade-avoidance elongation growth responses. The growth of arabidopsis hypocotyls is reported to be under circadian control, with a daily arrest at dawn and a period of rapid elongation at dusk (Dowson-Day and Millar, 1999). Hypocotyl extension in rapid shade avoidance therefore coincides with the seedling's natural endogenous rhythm of elongation growth. Observations that pil1 null mutants appear phenotypically similar to wild-type plants in prolonged low R : FR ratios suggest that PIL1 only plays a role in rapid responses to shade (Salter et al., 2003).
FUTURE PERSPECTIVES
Although the photoreceptors involved in mediating shade-avoidance responses are well established, a thorough understanding of this important biological phenomenon requires elucidation of the molecular components involved. The use of microarray technology has already enabled the identification of novel genes whose expression is strongly regulated by R : FR ratio (Salter et al., 2003). The rapid and transient phenotypes of pil1 null mutants suggest redundancy of function to exist in the regulation of elongation responses to shade. Future investigations of multiple physiological responses will undoubtedly reveal further candidates and ultimately the signal transduction processes involved.
LITERATURE CITED
- Aukerman MJ, Hirschfeld M, Wester L, Weaver M, Clack T, Amasino RM, Sharrock RA. 1997. A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell 9: 1317–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré CL. 1999. Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends in Plant Science 4: 97–102. [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL, Sánchez RA. 1990. Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247: 329–332. [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL, Sánchez RA. 1991. Photocontrol of stem elongation in pant neighbourhoods: effects of photon fluence rate under natural conditions of radiation. Plant, Cell and Environment 14: 57–65. [Google Scholar]
- Ballaré CL, Casal JJ, Kendrick RE. 1991. Responses of light-grown wild-type and long-hypocotyl mutant cucumber seedlings to natural and stimulated shade light. Photochemistry and Photobiology 54: 819–826. [Google Scholar]
- Blázquez MA, Weigel D. 1999. Independent regulation of flowering by phytochrome B and gibberellins in Arabidopsis. Plant Physiology 120: 1025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez MA, Ahn JH, Weigel D. 2003. A thermosensory pathway controlling flowering time in Arabidopsis thaliana Nature Genetics 33: 168–171. [DOI] [PubMed] [Google Scholar]
- Briggs WR, Huala E. 1999. Blue-light photoreceptors in higher plants. Annual Review of Cell and Developmental Biology 15: 33–62. [DOI] [PubMed] [Google Scholar]
- Carabelli M, Sessa G, Ruberti I, Morelli G. 1993. The Arabidopsis ATHB-2 and -4 genes are strongly induced by far-red-rich light. The Plant Journal 4: 469–479. [DOI] [PubMed] [Google Scholar]
- Carabelli M, Morelli G, Whitelam GC, Ruberti I. 1996. Twilight-zone and canopy shade induction of the ATHB-2 homeobox gene in green plants. Proceedings of the National Academy of Sciences USA 93: 3530–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ, Sánchez RA. 1994. Impaired stem growth response to blue light irradiance in light-grown transgenic tobacco seedlings overexpressing Avena phytochrome A. Physiologia Plantarum 91: 268–272. [Google Scholar]
- Casal JJ, Smith H. 1989. The function, action and adaptive significance of phytochrome in light-grown plants. Plant, Cell and Environment 12: 855–862. [Google Scholar]
- Casal JJ, Ballaré CL, Tourn M, Sánchez RA. 1994. Anatomy, growth and survival of a long-hypocotyl mutant of Cucumis sativus deficient in phytochrome B. Annals of Botany 73: 569–575. [Google Scholar]
- Casal JJ, Sánchez RA, Deregibus VV. 1986. The effect of plant density on tillering: the involvement of R/FR ratio and the proportion of radiation intercepted per plant. Environmental and Experimental Botany 26: 365–371. [Google Scholar]
- Cashmore AR, Jarillo JA, Wu YJ, Liu D. 1999. Cryptochromes: blue light receptors for plants and animals. Science 284: 760–765. [DOI] [PubMed] [Google Scholar]
- Cerdán PD, Chory J. 2003. Regulation of flowering time by light quality. Nature 423: 881–885. [DOI] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA. 1994. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE Plant Molecular Biology 25: 413–427. [DOI] [PubMed] [Google Scholar]
- Devlin PF, Rood SB, Somers DE, Quail PH, Whitelam GC. 1992. Photophysiology of the elongated internode (ein) mutant of Brassica rapa: ein mutant lacks a detectable phytochrome B-like protein. Plant Physiology 100: 1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Halliday KJ, Harberd NP, Whitelam GC. 1996. The rosette habit of Arabidopsis thaliana is dependent upon phytochrome action: novel phytochromes control internode elongation and flowering time. The Plant Journal 10: 1127–1134. [DOI] [PubMed] [Google Scholar]
- Devlin PF, Patel SR, Whitelam GC. 1998. Phytochrome E influences internode elongation and flowering time in Arabidopsis Plant Cell 10: 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Robson PRH, Patel SR, Goosey L, Sharrock RA, Whitelam GC. 1999. Phytochrome D acts in the shade-avoidance syndrome in Arabidopsis by controlling elongation and flowering time. Plant Physiology 119: 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LA, Hammond Pyle E, Schmitt J. 2000. Plasticity to cues and resources in Arabidopsis thaliana: testing for adaptive value and costs. Evolution 54: 1982–1994. [DOI] [PubMed] [Google Scholar]
- Dowson-Day MJ, Millar AJ. 1999. Circadian dysfunction causes aberrant hypocotyl elongation patterns in Arabidopsis. The Plant Journal 17: 63–71. [DOI] [PubMed] [Google Scholar]
- Franklin KA, Praekelt U, Stoddart WM, Billingham OE, Halliday KJ, Whitelam GC. 2003. Phytochromes B, D and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiology 131: 1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Davis SJ, Stoddart WM, Vierstra RD, Whitelam GC. 2003. Mutant analyses define multiple roles for phytochrome C in Arabidopsis thaliana photomorphogenesis. Plant Cell 15: 1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M, Song P-S. 1994. Assembly and properties of holophytochrome. In: Kendrick RE, Kronenberg GHM, eds. Photomorphogenesis in plants. Dordrecht: Kluwer, 105–140. [Google Scholar]
- Gilbert IR, Jarvis PG, Smith H. 2001. Proximity signal and shade avoidance differences between early and late successional trees. Nature 411: 792–795. [DOI] [PubMed] [Google Scholar]
- Goosey L, Palecanda L, Sharrock RA. 1997. Differential patterns of expression of the Arabidopsis PHYB, PHYD, and PHYE phytochrome genes. Plant Physiology 115: 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday KJ, Whitelam GC. 2003. Changes in photoperiod or temperature alter the functional relationships between phytochromes and reveal roles for phyD and phyE. Plant Physiology 131: 1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday KJ, Koornneef M, Whitelam GC. 1994. Phytochrome B and at least one other phytochrome mediate the accelerated flowering response of Arabidopsis thaliana L to low red/far-red ratio. Plant Physiology 104: 1311–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday KJ, Salter MG, Thingnaes E, Whitelam GC. 2003. Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT. The Plant Journal 33: 875–885. [DOI] [PubMed] [Google Scholar]
- Holmes MG, Smith H. 1975. The function of phytochrome in plants growing in the natural environment. Nature 254: 512–514. [Google Scholar]
- Johnson E, Bradley JM, Harberd NP, Whitelam GC. 1994. Photoresponses of light-grown phyA mutants of Arabidopsis: phytochrome A is required for the perception of daylength extensions. Plant Physiology 105: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Juez E, Nagatani A, Tomizawa K-I, Deak M, Kern R, Kendrick RE, Furuya M. 1992. The cucumber long hypocotyl mutant lacks a light-stable PHYB-like phytochrome. Plant Cell 4: 241–251. [PMC free article] [PubMed] [Google Scholar]
- McCormac AC, Cherry JR, Hershey HP, Vierstra RD, Smith H. 1991. Photoresponses of transgenic tobacco plants expressing an oat phytochrome gene. Planta 185: 162–170. [DOI] [PubMed] [Google Scholar]
- McCormac AC, Whitelam GC, Smith H. 1992. Light grown plants of transgenic tobacco expressing an introduced oat phytochrome A gene under the control of a constitutive viral promoter exhibit persistent growth inhibition by far-red light. Planta 188: 173–181. [DOI] [PubMed] [Google Scholar]
- McLaren JS, Smith H. 1978. The function of phytochrome in the natural environment. VI. Phytochrome control of the growth and development of Rumex obtusifolius under simulated canopy light environments. Plant, Cell and Environment 1: 61–67. [Google Scholar]
- Mathews S, Sharrock RA. 1997. Phytochrome gene diversity. Plant, Cell and Environment 20: 666–671. [Google Scholar]
- Møller SG, Ingles PJ, Whitelam GC. 2002. The cell biology of phytochrome signalling. New Phytologist 154: 553–590. [DOI] [PubMed] [Google Scholar]
- Ni M, Tepperman JM, Quail PH. 1998. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix–loop–helix protein. Cell 95: 657–667. [DOI] [PubMed] [Google Scholar]
- Pierik R, Visser EJW, de Kroon H, Voesenek LACJ. 2003. Ethylene is required in tobacco to successfully complete with proximate neighbours. Plant, Cell and Environment 26: 1229–1234. [Google Scholar]
- Pierik R, Voesenek LACJ, de Kroon H, Visser EJW. 2004. Density-induced plant size reduction and size inequalities in ethylene-sensing and ethylene-insensitive tobacco. Plant Biology 6: 201–205. [DOI] [PubMed] [Google Scholar]
- Pierik R, Whitelam GC, Voesenek LACJ, de Kroon H, Visser EJW. 2004. Canopy studies on ethylene-insensitive tobacco identify ethylene as a novel element in blue light and plant-plant signalling. The Plant Journal 38: 310–319. [DOI] [PubMed] [Google Scholar]
- Quail PH. 1994. Phytochrome genes and their expression. In: Kendrick RE, Kronenberg GHM, eds. Photomorphogenesis in plants, 2nd edn. Dordrecht: Kluwer, 71–104. [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. 1993. Mutations in the gene for red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson PRH, Whitelam GC, Smith H. 1993. Selected components of the shade-avoidance syndrome are displayed in a normal manner in mutants of Arabidopsis thaliana and Brassica rapa deficient in phytochrome B. Plant Physiology 102: 1179–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson PRH, McCormac AC, Irvine AS, Smith H. 1996. Genetic engineering of harvest index in tobacco through overexpression of a phytochrome gene. Nature Biotechnology 14: 995–998. [DOI] [PubMed] [Google Scholar]
- Salter MG, Franklin KA, Whitelam GC. 2003. Gating of the rapid shade-avoidance response by the circadian clock in plants. Nature 11: 680–683. [DOI] [PubMed] [Google Scholar]
- Schena M, Davis RW. 1992. HD-Zip proteins: members of an Arabidopsis homeodomain protein superfamily. Proceedings of the National Academy of Sciences of the USA 89: 3894–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer E, Bowler C. 2002. Phytochrome-mediated photoperception and signal transduction in higher plants. EMBO Reports 3: 1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J, McCormac AC, Smith H. 1995. A test of the adaptive plasticity hypothesis using transgenic and mutant plants disabled in phytochrome-mediated elongation responses to neighbours. American Naturalist 146: 937–953. [Google Scholar]
- Schmitt J, Stinchcombe JR, Heschel MS, Huber H. 2003. The adaptive evolution of plasticity: phytochrome-mediated shade avoidance responses. Integrative and Comparative Biology 43: 459–469. [DOI] [PubMed] [Google Scholar]
- Sharrock RA, Quail PH. 1989. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes and Development 3: 1745–1757. [DOI] [PubMed] [Google Scholar]
- Simpson GG, Dean C. 2002.Arabidopsis, the Rosetta stone of flowering time? Science 296: 285–289. [DOI] [PubMed] [Google Scholar]
- Smith H. 1982. Light quality, photoperception and plant strategy. Annual Review of Plant Physiology 33: 481–518. [Google Scholar]
- Smith H, Whitelam GC. 1990. Phytochrome, a family of photoreceptors with multiple physiological roles. Plant, Cell and Environment 13: 695–707. [Google Scholar]
- Smith H, Whitelam GC. 1997. The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant, Cell and Environment 20: 840–844. [Google Scholar]
- Somers DE, Sharrock RA, Tepperman JM, Quail PH. 1991. The hy3 long hypocotyl mutant of Arabidopsis is deficient in phytochrome B. Plant Cell 3: 1263–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, Aoyama T, Morelli G, Ruberti I. 1999. Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development 126: 4235–4245. [DOI] [PubMed] [Google Scholar]
- Whitelam GC, Smith H. 1991. Retention of phytochrome-mediated shade avoidance responses in phytochrome-deficient mutants of Arabidopsis, cucumber and tomato. Journal of Plant Physiology 39: 119–125. [Google Scholar]
- Yanovsky MJ, Casal JJ, Whitelam GC. 1995. Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: weak de-etiolation of the phyA mutant under dense canopies. Plant, Cell and Environment 18: 788–794. [Google Scholar]



