Abstract
• Background and Aims Water adhesion forces, water absorption capacity and permeability of the pine exine were investigated to consider a possible function of sporopollenin coatings in the control of water transport.
• Methods The experiments were carried out with sporopollenin capsules obtained from pine pollen consisting of an empty central capsule and two sacci. Changes in the concentration of excluded dextran molecules in the medium were analysed to quantify water absorption by purified exine fragments and the osmotic volume flow out of the intact central capsule.
• Key Results The contact angle of sporopollenin to water is higher than the one to ethanol and lower than the one to n-heptane. The water-filled pore space in pine sporopollenin amounts to only 20·6 % of the matrix volume. A monosaccharide was excluded from 15 % and a trisaccharide from about 38 % of this space. Shrinkage of the central capsule induced by permeable osmotica was transient, whereas that induced by sodium polyacrylate (2100 g mol−1) was stable. Values obtained for the hydraulic conductance LP of the exine (0·39–0·48 µm s−1 MPa−1) are comparable in size to those of biomembranes. Sodium sulfate solutions induced a significant osmotic flow through the exine (reflection coefficient at least 0·6). The exine around the central capsule can be ruptured by equilibration of its lumen with a concentrated electrolyte solution and subsequent transfer to water. The denatured protoplast along with the intact intine was ejected when pollen grains were subjected to this osmotic shock treatment.
• Conclusions The pine exine is easily wetted with water and does not represent a significant barrier to water exchange either liquid or gaseous. Through osmotic burst, it can be separated from the intine. The effect of salts and small solute molecules on water fluxes may be functionally significant for rehydration upon pollination.
Keywords: Sporopollenin, exine, intine, wettability, water absorption, hydraulic conductivity, reflection coefficient, osmotic shock, dextran, pollen, Pinus sylvestris, Pinus nigra
INTRODUCTION
Exines and sporodermata consist of sporopollenin, a solvent-resistant polymer composed of highly cross-linked aliphatic alcohols, carbonic acids and aromatic components (Wiermann and Gubatz, 1992; Wilmesmeier et al., 1993; Ahlers et al., 2000). The sporopollenin coatings of spores and pollen grains have been regarded as water transport barriers (Heslop-Harrison, 1973; Raven, 2000). Indeed, a principal function of sporopollenin is probably related to the dispersal of pollen and spores through the atmosphere. Ducker et al. (1978) pointed to the fact that evolution of the submerged-flowering sea grasses resulted in an extreme reduction of the sporopollenin coat of its pollen. On the other hand, only inaperturate exines might be a water transport barrier as such. Porate or colpate exines cannot prevent the loss of water vapour efficiently without a hydrophobic pollen coat. In addition, it is questionable whether retention of water is significant for pollen that is shed in the dry state. At present, there is a lack of experimental data characterizing a sporopollenin envelope with respect to hydraulic conductivity. There is also a need for data on adhesion of polar and non-polar liquids to sporopollenin, water absorption capacity and other parameters of this polymer that might be relevant to the water relationships of the reproductive cells.
This study concentrates on these parameters using purified exines (sporopollenin capsules) prepared from pine pollen, which consist of a central capsule and two lateral sacci (Bohne et al., 2003). The ontogenesis and structure of the inaperturate pine exine has been described recently (Rowley et al., 1999, 2000a, b). Two dense inner sporopollenin layers, the endexine and the foot layer of the ectexine, cover the cell wall (intine) as an inaperturate envelope (nexine). The nexine has a relatively small exclusion limit and a limited permeability for sugars that is strongly size-dependent (Bohne et al., 2003). The two outer porous exine layers (designated as sexine) are partly separated from the nexine to build the air bladders (sacci). The wall of the sacci consists of a thin porous surface layer (tectum) and a rigid supporting sporopollenin frame (columellae). The sacci do not have apertures visible by light microscope, but they are permeable even for microparticles with a radius of 100 nm (Bohne et al., 2003).
MATERIAL AND METHODS
Purified exine fragments
Sporopollenin capsules were prepared as described previously (Bohne et al., 2003). One gram of dry sporopollenin capsules was suspended, together with 4 g crystalline sodium chloride, in 15 mL 96 % ethanol, and treated for 20 min with the sonifier Sonoplus HD200 (Bandelin Electronic GmbH&Co KG, Berlin, Germany) in a cooled 50-mL glass centrifuge vial. The vibrating rod (sonotrode: TT 13, diameter 12·7 mm) was directly applied to the suspension with a power of 120 W in the 50 % cycle mode. Complete rupture of central capsules was microscopically checked. The exine fragments were washed with 50 % ethanol on an S4 glass filter to remove salt and then incubated with a pancreas enzyme preparation and cellulolytic enzymes as described previously (Bohne et al., 2003) to remove residues of the intine and the protoplasts. Absence of cellulose was checked by means of the Calcofluor White method. Having been washed on a centrifuge, the exine fragments were lyophilized by means of the lyophilizer Alpha 1-4 (Christ, Osterrode/Harz, Germany).
Capillary forces
A suspension of intact sporopollenin capsules in the respective liquid (water, ethanol, n-heptane) was used for packing a dense bed (diameter 7 mm, height 25 mm) on a polypropylene filter in a glass column. The column outlet was connected with a suction flask. The bed was washed with 5 mL of the respective liquid using a suction of 50 kPa. During this step, care was taken that the bed remained covered by liquid. Before measurement, the suction flask was opened to allow liquid saturation of the bed at the suction resulting from gravity (<200 Pa). Subsequently, the air pressure within the flask was slowly decreased or the air pressure above the bed was slowly increased to determine the critical pressure difference that induced outflow of the mobile liquid from the interparticle capillary space. For this purpose, a pressure sensor (AktivSensor GmbH, Stahnsdorf, Germany) was applied.
Change in solute concentration by water absorption and solute exclusion
Two hundred milligrams of lyophilized exine fragments were shaken at room temperature with 6 mL of the respective dextran or sugar solutions (each 30 g L−1) for 3 d in sealed polyethylene tubes. To obtain a clear supernatant, small air bubbles had to be separated from a part of the exine fragments. This was carried out by repeated centrifugation (11 400 g) and incubation of the closed vessels in an ultrasonic bath. The dextran or sugar concentration in the original dextran solution and the supernatant was measured at 25 °C with a relative accuracy of at least 0·2 % using the Perkin & Elmer polarimeter 141 M (Ueberlingen, Germany) at a wavelength of 366 nm.
Continuous recording of water efflux from the capsules
Volume changes of the central capsules were determined by recording the change in concentration of excluded dextran molecules (cf. Ehwald et al., 1973; Fleischer and Ehwald, 1995). The device applied in this study (Fig. 1) consists of a tube-shaped thermostated incubation vessel (inner diameter 12 mm, length 91 mm). A piece of a thin porous paper filter was fitted into the incubation vessel in such a way that it completely covered the vertical rifles in the inner tube wall and the horizontal ring channel that directed the filtrate flow to the outlet. The air-lift system was driven by the flow of water-saturated air (10 mL min−1). It agitated the suspension and moved the filtrate through the measuring cell. About 20 s were needed for one full circulation of the filtrate. The total liquid volume was 9–9·5 mL, including the volume of the filtrate within the tubes and the polarimeter cell (2·3 mL). The optical activity was generally measured at 25 °C at a wavelength of 366 nm. Experiments were started by adding one-half of the desired final volume of a concentrated dextran solution (Dextran T70, 60 g L−1) together with approx. 2 mL of water into the air-lift system. Subsequently a defined mass (approx. 0·5 g) of the water-saturated sporopollenin capsules obtained by filtration was suspended in the incubation vessel. The dextran-excluding volume (close to the volume of the central capsules) was determined for each experiment by measuring the angle of rotation before and after this step. Evaporation or condensation effects were suppressed by regulating the temperature of the moistening flask until the dextran concentration in the cell remained constant. Then water was added to the suspension until the theoretical angle of rotation (15·925°) of the standard dextran concentration (30 g L−1) was reached. This way the volume of the medium outside the central capsules was also adjusted to the desired value. Water efflux from the central capsules was induced by addition of a defined volume (0·1–0·5 mL) of a dextran solution (30 g L−1) containing the osmoticum. Care was taken that the angle of rotation of the added solution did not deviate from that recorded actually in the polarimetric system by more than 0·010°. While adding the osmoticum, the flow through the silicon tubing was manually interrupted for 10 s to prevent entrance of the unmixed solute into the filtrate flow, which would have caused measuring errors by striation within the polarimeter cell. After the water efflux curve had been registered, the record was calibrated by addition of 100 μL water to the incubation vessel (see Fig. 4). A freezing point osmometer (Knauer GmbH, Berlin, Germany) was applied for the measurement of the osmotic potential of the medium.
Fig. 1.
Air-lift system for circulation of the dextran medium and polarimetric registration of the dextran concentration. 1, Thermostated and aerated incubation vessel containing the pine exines; 2, cylindrical particle filter covering a rifled drainage area and a ring channel in front of the filtrate outlet; 3, thermostated polarimeter cell (1 mL).
Measuring the ultrafilter coefficient
Moist filtered sporopollenin microcapsules (50 g, water content 47 g) were mixed with 50 mL of a solution containing Dextran T70 (60 g L−1, α0 = 31·850 ± 0·010°). Some of the suspension obtained was centrifuged and the angle of rotation of the supernatant α1 was measured to determine the volume VC (mL) of the central capsules that excludes dextran
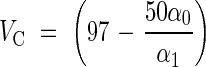 |
and to calculate the water volume which had to be added to reach the standard dextran concentration (30 g L−1) in the experimental stock suspension.
Portions of 4·5–4·9 mL of the stock suspension were shaken in sealed polypropylene tubes. At time zero 0·1–0·5 mL of a solution containing the osmoticum and dextran (the latter exactly at the concentration of 30 g L−1) was added. After the incubation period Δt (usually 10 s), the suspension was vacuum filtered through a dry nylon sieve fixed to a polyethylene tube. The filtrate was centrifuged and the angle of rotation α was measured. To obtain a reference value α′, the dextran solution containing the osmoticum was mixed in the respective relationship with the supernatant of the stock suspension obtained by centrifugation. The osmotic change of the central capsule volume ΔV was obtained from the polarimeter readings
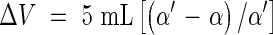 |
and referred to the capsule volume obtained from eqn (1). Mean rates of shrinkage
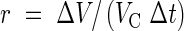 |
were determined for the respective incubation period Δt. The ultrafilter coefficient was calculated from the difference in osmotic pressure (Δπ), the rate (r), and the ratio of volume to surface area of the central capsule (β)
 |
Values of β (5·01 µm for P. sylvestris L., 5·58 µm for P. nigra Arnold) were taken from a previous study (Bohne et al., 2003).
Chemicals used
Methyl-α-d-glucoside (Sigma, St Louis, USA); raffinose (Difco Laboratories, Detroit, MI, USA); Dextran T20 and Dextran T70 (Pharmacia, Uppsala, Sweden); Polyacrylic acid 2100, sodium salt (Fluka Chemie AG, Buchs, Switzerland), Evans blue (Reanal, Budapest, Hungary), Calcofluor® White ST (American Cyanamid Company, Bound Brook, NJ, USA).
RESULTS AND DISCUSSION
Adhesion of liquids to sporopollenin
When the sporopollenin capsules or intact pollen were suspended in emulsions of water and n-heptane or water and butanol, they sedimented in the aqueous bottom phase. The same result was obtained with purified exines prepared from Alnus glutinosa. Figure 2 shows a part of a dispersion containing n-butanol, water and sporopollenin capsules. The aqueous phase is darkened by Indian ink. None of the sporopollenin capsules is enclosed completely by the butanol phase. One of them, which is situated within the unstained butanol area, is surrounded by a thin aqueous film. Capillary rise in packings of the vacuum-dried sporopollenin capsules has been observed with all tested liquids (water, ethanol and n-heptane). The adhesive force of different liquids to the sporopollenin surface was estimated as the critical pressure difference for the entrance of air into a dense, liquid-saturated packing of the capsules. Outflow of ethanol and water from the inter-particle space did not occur unless the air pressure difference reached the critical value of nearly 10 kPa. In contrast, n-heptane was held within the packing by a much smaller adhesive force (Table 1). The critical air pressure difference obtained for the ethanol-saturated packings is equal to that of an ethanol-containing capillary with a radius of 4·4 µm wherein the liquid adheres with a contact angle of 0°. The extra-particle volume in packings of the sporopollenin capsules (smallest diameter approx. 30 µm) is about 40 % of the packing volume. It may be concluded that the equivalent radius of the critical capillary spaces between the sporopollenin capsules cannot be far below 4·4 µm. Hence, the contact angle of ethanol to sporopollenin (γeth) is close to zero. Water was held with a force similar to, or slightly smaller, than the one of ethanol within the extra-particle spaces of the bed. Since the capillary force is proportional to both cos γ and the surface tension σ, cos γH2O is equal to or lower than σeth/σH2O (γH2O ≥ 70°). Likewise, it can be concluded from Table 1, that the contact angle of n-heptane is even higher than the one of water.
Fig. 2.
Partition of sporopollenin capsules within a water/n-butanol emulsion. Water-saturated capsules prepared from Pinus sylvestris pollen (5 mg of a filtered mass) were suspended in 0·5 mL of n-butanol and the suspension mixed with 1 mL of a suspension of Indian ink in water to stain the aqueous phase.
Table 1.
Critical pressure difference for the entrance of air into the inter-particle space of liquid-saturated beds packed with sporopollenin capsules
| Surface tension σ to air at 20 °C (mN m−1) | Critical pressure difference (102 Pa)* |
|||
|---|---|---|---|---|
| Suction |
Compression |
|||
| Deionized water | 72·6 | 80 ± 11 | 93 ± 13 | |
| Ethanol | 22·8 | 82 ± 19 | 139 ± 4 | |
| n-Heptane | 19·7 | 19 ± 3 | 27 ± 8 | |
Beds (height 25 mm, diameter 7 mm) of sporopollenin capsules obtained from Pinus nigra were packed in glass columns and subjected to decreasing air pressure (suction) at the lower end or to increasing air pressure (compression) on the top until the mobile liquid phase was drained out at the critical pressure difference.
Mean values and s.d., n = 5.
In conclusion, sporopollenin surfaces are not hydrophobic but amphiphilic with a strong preference for polar liquids. The authors assume that this is due to a relatively high density of negative charges in the sporopollenin matrix (mainly carboxylic and acid phenolic groups). Titration curves of purified sporopollenin prepared from Pinus sylvestris and Alnus glutinosa resulted in carboxylic group contents of 23 and 98 µmol g−1 d. wt, respectively.
Salter et al. (2002) found that several saccate and non-saccate conifer pollens, in contrast to the fern spores investigated, are easily wetted with water. The wettability of the ectexine layers of the sacci results in cohesive inclusion of air bubbles when pine falls into water. Therefore the particles do float whereas the corpus is submerged. This phenomenon has been suggested to be crucial for the orientation of the pollen grain on the surface of the micropylar droplet (Salter et al., 2002).
A hydrophilic character of sporopollenin might be significant to enable water transfer from the stylar tissue to the pollen grain in plants that need the presence of liquid oils (triglycerides) for pollen hydration or pollen-tube growth on the stylar tissue (Lush et al., 1998; Wolters-Arts et al., 1998, 2002; Zinkl and Preuss, 2000). The essential lipids are produced by a secretory zone of the stigma or they are a constituent of the pollen coat (plants with a dry stigma). Possibly the oils that cover pollen grains on the stigma prevent evaporation, whereas a hydrophilic ectexine and eventually adsorbed hydrophilic solutes enable the development of an aqueous film around the germinating pine pollen (cf. Fig. 2).
Water absorption and size-dependent solute sugar partition
When dry exine fragments were suspended in dextran solutions, the polymer concentration increased due to selective water absorption by the sporopollenin matrix (Table 2). The effect of water absorption on polymer concentration was larger in solutions of Dextran T70 (mean Stokes' radius 4·56 nm) than in those of Dextran T20 (mean Stokes' radius 3·99 nm). As this difference was found to be significant on the confidence level of 95 %, at least Dextran T20 was not completely excluded from the sporopollenin matrix. The exine fragments comprise the endexine and the strata of the ectexine (foot layer, columellae and tectum), which differ in stainability and electron density (Rowley, 1996; Rowley et al., 2001). Hence, the presence of a pore space that is accessible to Dextran T20 does not imply the intact sporopollenin envelope to be permeable to this polymer (cf. Bohne et al., 2003). The water-filled pore space in the sporopollenin matrix which excludes Dextran T70 amounts to 0·180 ml g−1 d. wt. With respect to the density of swollen sporopollenin (approx. 1·14 kg L−1) the volume fraction of this space is about 20·6 %. The value is close to the maximum water absorption capacity, since the osmotic tension by the applied dextran (30 g L−1) solutions is negligible. Compared with lyophilized plant cell wall fragments, which absorb >4 mL water per gram dry weight from dextran solutions (R. Ehwald, unpubl. res.), the water absorption capacity of sporopollenin is low. The relatively high solid content of the sporopollenin matrix is physiologically important. It results in a significant mechanical resistance to deformation at cohesive shrinkage and is certainly correlated with the remarkable ability of pollen grains to dry from the concentrated locular suspension without uncontrolled aggregation (clumping).The mean partition coefficients of sugars in the water space defined by the exclusion of Dextran T70 have been calculated from the respective concentration change. The values obtained are rather high, even for the trisaccharide raffinose (Table 2). The partition coefficients given in Table 2 relate to the exine fragments as a whole, whereas permeability may be controlled by one of the exine layers. The permeability coefficient of the exine for methyl-glucoside was found to be about three times higher than that for raffinose (Bohne et al., 2003). Probably, relatively large pores with high sugar partition coefficients dominate the measured water space but are absent in the thin exine layers (endexine and/or foot layer) that control permeability.
Table 2.
Selective absorption of water by pine exine fragments
| Solute |
Increase of solute concentration (%)* due to equilibration with lyophilized exine fragments (33·3 mg mL−1) |
Solute excluding volume (ml g−1 sporopollenin)† |
s.d. |
Solute partition coefficient in the exclusion volume of Dextran T70 |
|---|---|---|---|---|
| Dextran T70 | 0·69 | 0·180 | 0·023 | 0·00 |
| Dextran T20 | 0·59 | 0·155 | 0·009 | 0·14 |
| Raffinose | 0·26 | 0·069 | 0·012 | 0·62 |
| Methyl-α-d-glucoside | 0·10 | 0·027 | 0·008 | 0·85 |
Mean value, n = 5.
Differences between all given mean values are significantly different as revealed by the 95 % LSD test.
Water fluxes through the sporopollenin coat of the central capsule
When water-saturated pine sporopollenin capsules were transferred into sugar or salt solutions of sufficiently high osmotic pressure, rapid shrinkage of the central capsule could be observed (Fig. 3). However, no shrinkage occurred, when concentrated solutions of sucrose or sodium sulfate were pumped slowly into a stirred suspension of the sporopollenin capsules. This way, the central capsules could be equilibrated with high concentrations of permeable solutes without shrinkage. However, shrinkage could not be prevented when sodium polyacrylate with a mean molecular weight of 2100 g mol−1 was used as an osmoticum, even though the osmotic pressure increased slowly (0·1 kPa min−1).
Fig. 3.
Sporopollenin capsules, prepared from Pinus sylvestris pollen, in a sodium polyacrylate solution. Concentration of polyacrylate 2100 = 50 g L−1 (0·47 MPa). The sacci are brought together by the shrinkage of the central capsule.
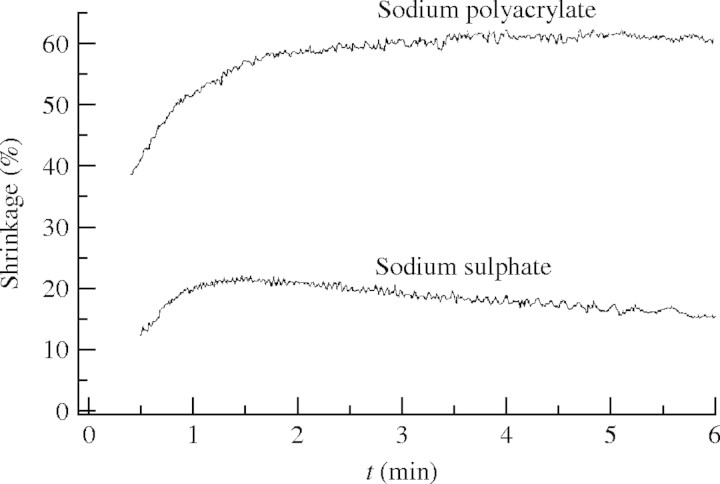
The device shown in Fig. 1 was used to follow the time course of water efflux. Water efflux induced by the addition of sodium polyacrylate was completed after approx. 5 min (Fig. 4). The limited filtrate flow velocity in the measuring system and transient initial recording errors due to striation prevented the registration of an initial water flow rate. However, the final volume reduction and its dependence on sodium polyacrylate concentration could be obtained accurately (Fig. 5). Most of the water can be sucked out of the capsules by a moderate osmotic force. Water efflux recorded after the addition of permeable osmotica like sodium sulfate was followed by a slow subsequent water uptake (Fig. 6). Re-swelling of the shrunken central capsule was much slower than the dissipation of osmotic force due to salt entering (G. Bohne et al., unpubl. res.). Likewise re-swelling was slow, when capsules, shrunken in a sodium polyacrylate solution, were transferred to water. Hence, the shape-restoring forces are weak in the absence of an osmotic pressure difference.
Fig. 4.
Osmotically induced water efflux from the central capsules. (A) Course of the dextran concentration change after the addition of sodium polyacrylate. The polarimetric system contained 902 mg (f. wt) of packed water-saturated sporopollenin capsules (prepared from Pinus sylvestris). Dextran concentration, 30 g L−1; volume outside of the central capsule, 9 mL; volume Vc of the central capsule, 322 μL. At time zero, a volume of 0·5 mL of a dextran/sodium polyacrylate solution was added (final polyacrylate concentration = 24 g L−1, π = 0·225 MPa). The scale of recording was calibrated by addition of water (100 μL) as indicated. (B) Control experiment, osmoticum added to the dextran solution without suspended capsules. The transient change in the recording was caused by schlieren in the polarimeter cell.
Fig. 5.

Dependence of capsule shrinkage on the osmotic potential of sodium polyacrylate 2100 solutions. Final shrinkage was determined from records of the type shown in Fig. 4.
Fig. 6.
Course of the volume changes induced by sodium polyacrylate 2100 and sodium sulfate in isotonic concentration. Final osmotic pressure, 0·225 MPa; final concentrations: sodium sulfate, 0·035 M; sodium polyacrylate, 23·9 g L−1. Sporopollenin capsules were prepared from Pinus sylvestris. Water efflux is given as percentage of the central capsule volume.
Initial rates of the osmotic volume change were measured to determine ultrafilter coefficients. The absolute value of the hydraulic conductivity of a membrane (LP = Jv/ΔP) is equal to the ultrafilter coefficient (LPD = Jv/Δπ) provided that the osmoticum is not permeable and that the flow-mediated concentration gradients of the osmoticum at the membrane surface can be neglected. These conditions apply to the envelope of a central capsule in a vigorously stirred solution of sodium polyacrylate. The hydraulic efficiency of an osmolyte with a given osmotic pressure, in comparison to the hydraulic efficiency of the equivalent hydrostatic pressure, is expressed by the term reflection coefficient σ. It is equal to the quotient between the ultrafilter coefficient LPD and the hydraulic conductivity Lp (σ = –LPD/LP). Since LPD* for an impermeable solute is equal to –LP, σ for for a permeable solute can be obtained from its ultrafilter coefficient LPD (σ = LPD/LPD*). Short-term experiments were carried out with a relatively small osmotic force which induced the loss of only 5–10 % of water within 10 s. The prolongation of the measuring period to 20 s caused an only slight decrease in the absolute value of LPD (Table 3). Ultrafilter coefficients LPD (=–LP) for sodium polyacrylate 2100 (Table 4) were found to be −0·39 µm s−1 MPa−1 (P. sylvestris) and −0·48 µm s−1 MPa−1 (P. nigra). Values are in the range typical of LP of plasma membranes (0·1–10 µm s−1 MPa−1; Lösch, 2001). The ultrafilter coefficient LPD of the exine for sodium sulfate corresponds to a reflection coefficient (σ = −LPD/LP) of approx. 0·6. It might have been slightly underestimated due to salt entrance during the measuring period. The osmotic efficiency of a salt is in accord with the relatively low permeability of the exine to sugars (Bohne et al., 2003).
Table 3.
Ultrafilter coefficients (LPD) of the pine exine covering the central capsule for sodium polyacrylate obtained at different values of the incubation time and the osmotic force
| Osmotic potential [MPa] |
|||||||
|---|---|---|---|---|---|---|---|
| −0·09 |
−0·18 |
||||||
| t (s) | 12 | 20 | 12 | 20 | |||
| Shrinkage (%) | 10·0 | 16·0 | 15·2 | 21·0 | |||
| LPD (µm s−1 MPa−1) | −0·489 | −0·472 | −0·353 | −0·292 | |||
Material, Pinus nigra; osmoticum, sodium polyacrylate (2100 g mol−1).
Table 4.
Ultrafilter coefficients (LPD) of the pine exine covering the central capsule
| Ultrafilter coefficient LPD (µm s−1 MPa−1) |
||||
|---|---|---|---|---|
| Osmoticum |
π (MPa) |
Pinus sylvestris |
Pinus nigra |
|
| Sodium polyacrylate 2100 | 0·090 | −0·387 ± 0·055 (n = 9) | −0·476 ± 0·144 (n = 25) | |
| Sodium sulfate | 0·118 | −0·227* | −0·285 ± 0·093 | |
| −0·208* | (n = 15) | |||
LPD was obtained from initial shrinkage rates (incubation time 10 s).
Individual measurement.
Osmotic burst
When the sporopollenin capsules are loaded with a concentrated electrolyte solution and then transferred to water, a high pressure can be transiently developed due to the relatively high value of the reflection coefficient. It has been investigted whether the capsules can be ruptured by such a procedure. The integrity of the central capsule envelope could be documented by the exclusion of Evans blue, a tetra-valent anionic dye (Stokes' radius 1·3 nm), for which the sporopollenin envelope is an efficient barrier. Most capsules were resistant to the transfer from a saturated sodium sulfate solution (1·3 mol L−1) to water. A large proportion of the capsules was ruptured after their transfer to water, when they had previously been loaded with a saturated sodium dihydrogenphosphate solution (7·1 mol L−1). More than 90 % of the capsules were opened by an osmotic down-shock, which was carried out after equilibration with 85 % phosphoric acid (Fig. 7). Osmotic rupture of the exine was also stated with intact pollen grains that had been preloaded with the concentrated salt or acid (Fig. 8). In the latter case, it was observed in a large number of the pollen grains, that the male gametophyte was ejected along with the intact intine. The permselectivity of an inaperturate exine can thus be utilized to obtain pure sporopollenin structures without harsh hydrolytic treatments and to deliberate the intine from its envelope.
Fig. 7.

Integrity of central capsules before and after osmotic shock. (A) Control preparation. Only a small percentage of the central capsules is stained (due to fissures in the exine). (B) Preparation after loading with phosphoric acid (final concentration, 850 mL L−1) and subsequent osmotic down-shock. Most of the central capsules do not exclude the dye. Sporopollenin capsules prepared from pollen of Pinus sylvestris. Concentration of Evans blue, 10 g L−1.
Fig. 8.

Pine pollen grain (Pinus nigra) after osmotic shock. The denatured gametophyte has been catapulted out of the exine. Cellulose staining with Calcofluor White demonstrates that the ejected protoplast remained covered by the intine. The pollen grain has been slowly equilibrated with phosphoric acid (final concentration 850 mL L−1) and subsequently transferred to water.
Functional aspects with respect to water permeability and osmosis
As shown in Table 2, sporopollenin of pine forms a polymer matrix with a relatively small volume fraction (approx. 18 %) of water-filled pores (Table 2). It is by no means hydrophobic but exerts higher adhesive forces to water than to nonpolar liquids and even to amphiphilic ones (Fig. 2 and Table 1). The inaperturate pine exine forms a significant, but not an absolute diffusion barrier for low-molecular-weight substances (Bohne et al., 2003). As shown in this study, it is highly permeable to liquid water (Table 3). Obviously the exine investigated differs from a typical cuticle in this respect, albeit it is comparable to a cutinized cell wall regarding the low pore size (Schönherr, 2000; Schönherr and Schreiber, 2004). Indeed, a strong barrier to water vapour and liquid water does not seem to be essential for pine pollen grains, since they do not have an enclosed gaseous volume that has to be protected against the entrance of liquid water.
There is no obvious ecophysiological need to prevent rapid evaporation from mature pine pollen. In the course of pollen ripening a negative water balance coincides with the regulated ageing process of the pine strobili. The pollen sacs open by a cohesive mechanism that is triggered by a low internal humidity. Pine pollen like that of many other species is shed in a highly dehydrated state and remains viable for a long period of anhydrobiosis. Even the whole envelope (exine + pollen coat) of maize pollen, which is highly sensitive to the drying stress, is not an efficient barrier against evaporation (Aylor, 2003).
The ultrafilter coefficient for the permeable salt sodium sulfate was significantly smaller than that for the impermeable sodium polyacrylate (Table 4). The derived reflection coefficient (about 0·6) is nevertheless sufficient to induce significant osmotic effects on the volume of the central capsule (Fig. 5). The hydraulic efficiency of small osmolytes may be significant for pollination. Poikilohydric protoplasts can suffer from rehydration stress (Hoekstra et al., 2001). Solutes delivered by the micropyle's exudate (sugars, amino acids and electrolytes) might effect mechanics and kinetics of pollen swelling by their osmotic effect on the exine. Solutes released from the protoplasts might be retained in the space between the plasma membrane and the endexine during the swelling phase and enable the presence of a liquid film between the rigid exine and the soft intine.
Acknowledgments
The study was supported by the German Federal Ministry of Economics (project ‘Chromatographieträger’ No 1279/00).
LITERATURE CITED
- Ahlers F, Bubert H, Steuernagel S, Wiermann R. 2000. The nature of oxygen in sporopollenin from the pollen of Typha angustifolia L. Zeitschrift für Naturforschung 55c: 129–136. [DOI] [PubMed] [Google Scholar]
- Aylor DE. 2003. Rate of dehydration of corn (Zea mays L.) pollen in the air. Journal of Experimental Botany 54: 2307–2312. [DOI] [PubMed] [Google Scholar]
- Bohne G, Richter E, Woehlecke H, Ehwald R. 2003. Diffusion barriers of tripartite sporopollenin microcapsules prepared from pine pollen. Annals of Botany 92: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducker SC, Pettitt JM, Knox RB. 1978. Biology of Australian seagrasses: pollen development and submarine pollination in Amphibolis antarctica and Thalassodendron ciliatum (Cymodoceaceae). Australian Journal of Botany 26: 265–285. [Google Scholar]
- Ehwald R, Sammler P, Göring H. 1973. Different affinities of the α- and β-anomers of D-glucose, D-mannose and D-xylose for the glucose uptake system of Baker's yeast. Folia Microbiologica 18: 102–177. [DOI] [PubMed] [Google Scholar]
- Fleischer A, Ehwald R. 1995. The free space of sugars in plant tissues—external film and apoplastic volume. Journal of Experimental Botany 46: 647–654. [Google Scholar]
- Heslop-Harrison J. 1973. The pollen wall: structure and development. In: J. Heslop-Harrison, ed. Pollen: development and physiology. London: Butterworths, 75–98. [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J. 2001. Mechanisms of plant desiccation tolerance. Trends in Plant Science 6: 431–438. [DOI] [PubMed] [Google Scholar]
- Lösch R. 2001.Wasserhaushalt der Pflanzen, 1 edn, 88. Wiebelsheim: Quelle & Meyer. [Google Scholar]
- Lush WM, Grieser F, Wolters-Art M. 1998. Directional guidance of Nicotiana alata pollen tubes in vitro and on the stigma. Plant Physiology 118: 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA. 2000. Land plant biochemistry. Philosophical Transactions of the Royal Society of London. Series B. Biological Sciences 355: 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JR. 1996. 14D-Exine origin, development and structure in pteridophytes, gymnosperms and angiosperms. In: Jansonius J, McGregor DC eds. Palynology: principles and applications, Vol. 1. American Association of Stratigraphic Palynologists Foundation, 443–462. [Google Scholar]
- Rowley JR, Skvarla JJ, Walles B. 1999. Microsporogenesis in Pinus sylvestris VII. Exine expansion and tapetal development. Taiwania 44: 325–344. [Google Scholar]
- Rowley JR, Skvarla JJ, Walles B. 2000. Microsporogenesis in Pinus sylvestris VI. Exine and tapetal development during the tetrad period. Nordic Journal of Botany 20: 67–87. [Google Scholar]
- Rowley JR, Skvarla JJ, Walles B. 2000. Microsporogenesis in Pinus sylvestris L. VIII. Tapetal and late pollen grain development. Plant Systematics and Evolution 225: 201–224. [Google Scholar]
- Rowley JR, Gabarayeva NI, Skvarla JJ, El-Ghazaly G. 2001. The effect of 4-methylmorpholine N-oxide monohydrate (MMNO·H2O) on pollen and spore exines. Taiwania 46: 246–273. [Google Scholar]
- Salter J, Murray BG, Braggins JE. 2002. Wettable and unsinkable: the hydrodynamics of saccate pollen grains in relation to the pollination mechanism in the two New Zealand species of Prumnopitys Phil. (Podocarpaceae). Annals of Botany 89: 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr J. 2000. Cuticular penetration of calcium salts: effects of humidity, anions, and adjuvants. Journal of Plant Nutrition and Soil Sciences 164: 225–231. [Google Scholar]
- Schönherr J, Schreiber L. 2004. Size selectivity of aqueous pores in astomatous cuticular membranes isolated from Populus canescens (Aiton) Sm. leaves. Planta 219: 405–411. [DOI] [PubMed] [Google Scholar]
- Wiermann R, Gubatz S. 1992. Pollen wall and sporopollenin. International Review of Cytology 140: 35–72. [Google Scholar]
- Wilmesmeier S, Steuernagel S, Wiermann R. 1993. Comparative FTIR and 13C CP/MAS NMR spectroscopic investigations on sporopollenin for different systematic origins. Zeitschrift für Naturforschung 48c: 697–701. [Google Scholar]
- Wolters-Arts M, Lush WM, Mariani C. 1998. Lipids are required for directional pollen-tube growth. Nature 392: 818–821. [DOI] [PubMed] [Google Scholar]
- Wolters-Arts M, van der Weerd L, van Aelst AC, van der Weerd J, van As H, Mariani C. 2002. Water-conducting properties of lipids during pollen hydration. Plant, Cell and Environment 25: 513–519. [Google Scholar]
- Zinkl GM, Preuss D. 2000. Dissecting Arabidopsis pollen–stigma interactions reveals novel mechanisms that confer mating specificity. Annals of Botany 85: Suppl. A, 15–21. [Google Scholar]