Abstract
• Background and Aims Salinity can affect germination of seeds either by creating osmotic potentials that prevent water uptake or by toxic effects of specific ions. Most studies have only used monosaline solutions, although these limit the extent to which one can interpret the results or relate them to field conditions. The aim of this work was to evaluate the germination of Prosopis strombulifera seeds under increasing salinity by using the most abundant salts in central Argentina in monosaline or bisaline iso-osmotic solutions, or in solutions of mannitol and polyethylene glycol.
• Methods Seeds were allowed to germinate under controlled conditions in a germination chamber at 30 ± 1 °C and at 80 % r.h. Salinizing agents were KCl, NaCl, Na2SO4, K2SO4, NaCl + Na2SO4 and KCl + K2SO4 and osmotic agents were polyethylene glycol 6000 and mannitol. Treatments for all osmotica consisted of 0·0, −0·4, −0·8, −1·2, −1·5, −1·9 and −2·2 MPa solutions.
• Key Results The percentage of germination decreased as salinity increased.  in monosaline solutions, with osmotic potentials −1·2 MPa and lower, was more inhibitory than Cl− at iso-osmotic concentrations. This
in monosaline solutions, with osmotic potentials −1·2 MPa and lower, was more inhibitory than Cl− at iso-osmotic concentrations. This  toxicity was alleviated in salt mixtures and was more noticeable in higher concentrations. K+ was more inhibitory than Na+ independently of the accompanying anion.
toxicity was alleviated in salt mixtures and was more noticeable in higher concentrations. K+ was more inhibitory than Na+ independently of the accompanying anion.
• Conclusions Different responses to different compositions of iso-osmotic salt solutions and to both osmotic agents indicate specific ionic effects. This study demonstrates that the germination of P. strombulifera is strongly influenced by the nature of the ions in the salt solutions and their interactions. Comparative studies of Cl− and  effects and the interaction between
effects and the interaction between  and Cl− in salt mixtures indicate that extrapolation of results obtained with monosaline solutions in the laboratory to field conditions can be speculative.
and Cl− in salt mixtures indicate that extrapolation of results obtained with monosaline solutions in the laboratory to field conditions can be speculative.
Keywords: Prosopis strombulifera, seed germination, salinity, osmotic potential, ionic effects
INTRODUCTION
Environmental abiotic stress conditions, especially drought and salinity, are currently the major factors that reduce crop yields worldwide. Salinity, in particular, is an increasing problem affecting 20 % of the world's cultivated land and nearly half of the area under irrigation. Breeding of salt-resistant crop varieties will require a clear understanding of the complex mechanisms of salt-stress tolerance, an understanding which is still lacking despite intensive research during the last decade (Holmberg and Bülow, 1998; Yeo, 1998; Zhu, 2001; Apse and Blumwald, 2002).
While all major crops, as well as most wild species, are glycophytes, i.e. sensitive to relatively low salt concentrations, there are also plants naturally adapted to conditions of high salinity in the soil. These plants, known as halophytes, include a large taxonomic variety and occupy diverse habitats, from extremely dry to temporarily waterlogged sites or salt marshes. Tobe et al. (2000) reported that germination responses of halophytic species to environmental parameters determine their distribution in saline environments. Nevertheless, recent investigations on the germination response of dimorphic seeds of Atriplex prostrata and Salicornia europaea to environmental conditions showed that zonation within the inland marsh is not determined at the germination stage of development (Carter and Ungar, 2003). Similar conflicting results are found in the literature related to the genus Prosopis. Catalán et al. (1994) reported that the effects of salinity on the germination of P. flexuosa seeds from habitats differing in soil salinity levels were similar. This was in agreement with results reported for P. farcta by Dafni and Negbi (1978), who found no correlation between salinity of the habitat where the seed originated and various salt tolerance criteria studied at germination. In contrast, Bazzaz (1973) found differences in the germination behaviour of seeds of the same species which originated in different habitats and suggested that they belonged to different ‘salt ecotypes’. In any case, although germination in a saline substrate does not necessarily correlate with salt tolerance at other growth stages (Noble, 1983) it is a legitimate criterion for selecting for tolerance in saline environments (Blum, 1988).
Prosopis is a genus with many widely studied species because they are an economically important arboreal component of saline zones in America (Burkart, 1976). However, within this genus there are other species which constitute fundamental shrub-like members of native communities such as P. strombulifera, a spiny shrub frequently found in the salinized areas of Córdoba and San Luis (central area of Argentina).
Previous results with P. strombulifera seedlings grown hydroponically under increasing NaCl concentrations showed that 80 % of the treated seedlings developed stem and root growth stimulation up to 450 mm in relation to non-treated plants (Reinoso et al., 2000). This response is different to that obtained in other Prosopis species (Jarrell and Virginia, 1990; Catalán et al., 1994; M. A. Cony, Universidad Nacional de Mendoza, Argentina, pers. comm.) in which growth was reduced in response to increasing salinity. Anatomical and physiological studies have demonstrated that P. strombulifera is a salt-excluder halophytic species that constitutes an interesting model to improve knowledge on salinity tolerance (Reinoso et al., 2004).
It has been proposed that seed germination in salt-affected soils is influenced by the total concentration of dissolved salt (or the osmotic pressure) as well as by the type of salts involved (Ryan et al., 1975). Ungar (1978) reported that inorganic ions were not more inhibitory than mannitol and polyethylene glycol (PEG) in several halophytes, indicating that seeds are mainly affected by osmotic stress rather than specific ion toxicities. The same author stated that the distribution of halophytes in the United States indicates that there are species tolerant of a wide range of combinations of anions such as Cl−,  and
and 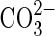 and cations such as Na+, K+ and Mg2+ (Ungar, 1991), suggesting that for these species specific ionic effects would have less influence on seed germination than the soil water potential. More recent results by Egan et al. (1997) on the effect of different sodium and potassium salts on the germination of Atriplex prostrata concluded that inhibition of seed germination and early growth was primarily due to an osmotic effect and not to a specific ion toxicity of either the chloride or sulfate salts.
and cations such as Na+, K+ and Mg2+ (Ungar, 1991), suggesting that for these species specific ionic effects would have less influence on seed germination than the soil water potential. More recent results by Egan et al. (1997) on the effect of different sodium and potassium salts on the germination of Atriplex prostrata concluded that inhibition of seed germination and early growth was primarily due to an osmotic effect and not to a specific ion toxicity of either the chloride or sulfate salts.
The existing data on germination of Prosopis species under salinity have been obtained using only NaCl (López-Villagra and Galera, 1992; Villagra, 1997; Cony and Trione, 1998). The use of NaCl as the sole salinizing agent in salinity studies is due to the fact that generally it is the main component of the soluble salts mixture present in saline soils (Fan et al., 1993). Nevertheless, in the salinized areas of Córdoba and San Luis the NaCl/Na2SO4 proportions are similar, and in some areas Na2SO4 is more abundant (Sosa et al., 2002) in agreement with observations made by Cisneros et al. (1997).
Several studies compared the effects of chloride and sulfate salts on germination and seedling growth to determine if the results obtained for NaCl-affected soils could be appropriately applied to soils salinized with sulfate salts (Romo and Haferkamp, 1978; Bal and Chattopadhyay, 1985; Curtin et al., 1993). Due to conflicting evidence from different experiments, this question remains unanswered.
Few studies have recently focused on the effects of Na2SO4 on germination and plant growth in spite of the fact that their comparison with the NaCl effects is still of high interest (Grattan and Grieve, 1999). In addition, most studies have used monosaline solutions, which limits the extent to which one can interpret the results or relate them to field conditions.
The aims of this work, then, were (a) to obtain information about the germination response of Prosopis strombulifera, a native wild species common in South American salt marshes, (b) to determine the effect of increasing salinity with monosaline iso-osmotic solutions of KCl, NaCl, Na2SO4, K2SO4 and bisaline iso-osmotic solutions of NaCl + Na2SO4 and KCl + K2SO4, on germination, and (c) to differentiate the osmotic from the ion toxicity effects, through comparison of salt solutions with the osmotic agents mannitol and PEG 6000. If salts have only osmotic effects, iso-osmotic salts, PEG and mannitol solutions should affect the seeds in the same manner and to the same extent.
MATERIALS AND METHODS
Seeds of Prosopis strombulifera (Lam.) Benth. were collected from an area in the south-west of the Province of San Luis, Argentina. Geographically, the location is 33°43′S, 66°37′W at 400–500 m a.s.l. with a temperate thermal regime, i.e. 15–20 °C annual average temperature. This area belongs to the carob tree forest located in a saline depression between the annual 300–400 mm isohyets (Peña-Zubiate et al., 1998). The soil is calcareous, has a franc-sandy texture and moderate salinity (8000 mΩ cm−2 electrical conductivity at the surface) (Anderson et al., 1970).
Pods were collected at random from 1000 plants in late summer (second week of March 2003) and stored at 4 °C until used 2–6 months later in the experiments. Seeds were selected visually for uniform size and healthy aspect. They were chemically scarified with concentrated sulfuric acid for 10 min and then washed overnight (12 h) under flowing tap water. Before sowing, seeds were rinsed three times in distilled water and placed in sterile Petri dishes with two discs of filter paper saturated with distilled water for control treatments [osmotic potential (Ψo) = 0·0 MPa] and the corresponding solutions of NaCl, Na2SO4, KCl, K2SO4, NaCl + Na2SO4, KCl + K2SO4, PEG 6000 and mannitol (Sigma Chemicals) in concentrations calculated to obtain the following Ψo: −0·4, −0·8, −1·2, −1·5, −1·9 and −2·2 MPa (Table 1). These osmotic potentials were verified with a vapour pressure osmometer (Model 5500, Wescor Inc., Logan, UT, USA). Bisaline solutions were prepared mixing equal volumes of their respective monosaline solutions at each corresponding osmotic potential. Solutions were renewed every 48 h under sterile conditions to ensure relatively constant Ψo in the treatments. In Prosopis species, germination occurs in a wide range of temperatures (25–40 °C) (Passera, 2000), so optimum germination requirements were as previously determined (data not shown). Seeds were allowed to germinate under controlled conditions in a Conviron G 30 germination chamber (Winnipeg, Manitoba, Canada) at 30 ± 1 °C and at 80 % r.h.
Table 1.
Concentrations of salts, mannitol and PEG required to obtain the desired osmotic potentials, each of which were verified with a vapour pressure osmometer
| Ψo (MPa) |
NaCl or KCl (mol L−l) |
Na2SO4 or K2SO4 (mol L−l) |
Mannitol (mol L−l) |
PEG 6000 (% w/v) |
|---|---|---|---|---|
| −0·4 | 0·1 | 0·088 | 0·100 | 12 |
| −0·8 | 0·2 | 0·178 | 0·180 | 18 |
| −1·2 | 0·3 | 0·266 | 0·340 | 22 |
| −1·5 | 0·4 | 0·333 | 0·425 | 26 |
| −1·9 | 0·5 | 0·400 | 0·540 | 31 |
| −2·2 | 0·6 | 0·480 | 0·610 | 33 |
A simple aleatory design (Steel and Torrie, 1995) with nine treatments was used. It consisted of a distilled-water control, six different salt treatments, a PEG treatment and a mannitol treatment. For statistical analysis, each osmotic potential obtained with each salt or osmotic agent was considered a different treatment. Thirty seeds were placed in each Petri dish with six replicates per salt treatment and five replicates for PEG and mannitol. The complete experiment was performed three times.
The number of germinated seeds in each dish was recorded every 24 h, with a last determination at 168 h (7 d) after the initiation of the experiment, according to the average germination time for most Prosopis species (Arce and Balboa, 1988; Catalán et al., 1994). Seeds were considered germinated when the radicle was 5 mm long.
Data were analysed using STATA™ version 4·0. (StataCorp, 1995). Germination data as percentage of total number of seeds were arcsine transformed. Two-way general linear model ANOVA was used to determine the effect of each salt composition at each osmotic potential at 168 h. Thus, the factors considered for two-way ANOVA were (1) type of salt solution and (2) concentration (Ψo). Also, a one-way ANOVA procedure was used to compare the effect of different salts composition at the same Ψo. Normality was verified with the Shapiro–Wilk test. Homogeneity of variance was verified with the Bartlett test. As some treatments had different numbers of replicates, one-tailed Scheffé test for multiple comparisons was used as post-hoc analysis to determine differences between treatments and between treatments and controls. P values below 0·05 were considered statistically significant.
RESULTS
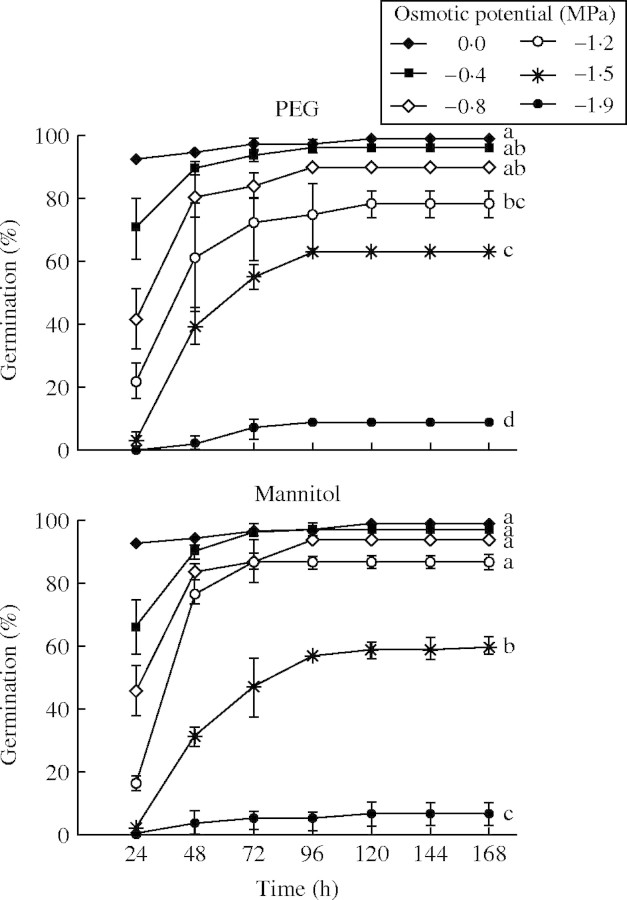
Different responses with different treatments at iso-osmotic concentrations gave evidence of the existence of specific ionic effects. An external Ψo of −0·4 MPa had no effect on germination percentage independently of the osmoticum used. At Ψo of −0·8MPa, germination percentage was inhibited in all salt treatments, except NaCl (Figs 1 and 2).
Fig. 1.
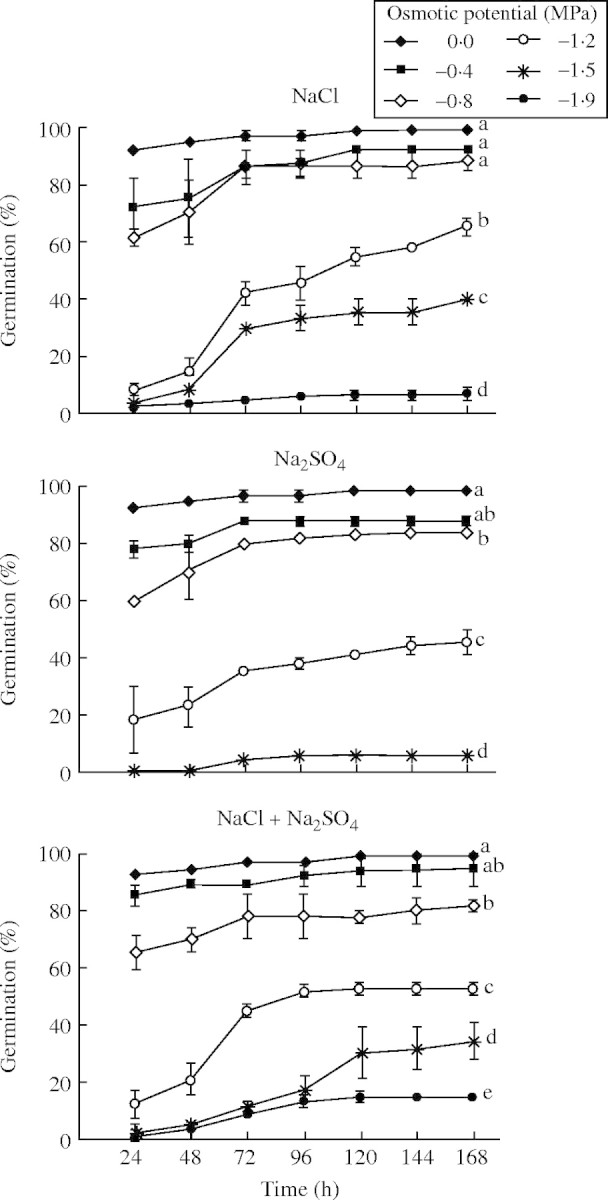
Percentage germination (mean ± s.e.) over time of P. strombulifera seeds exposed to different osmotic potentials of Na salts. No germination occurred at −1·9 MPa Na2SO4, so these results were not included in the graph. All treatments at −2·2 MPa inhibited all germination. Values with the same letter at 168 h are not significantly different at P > 0·05, Scheffé test.
Fig. 2.
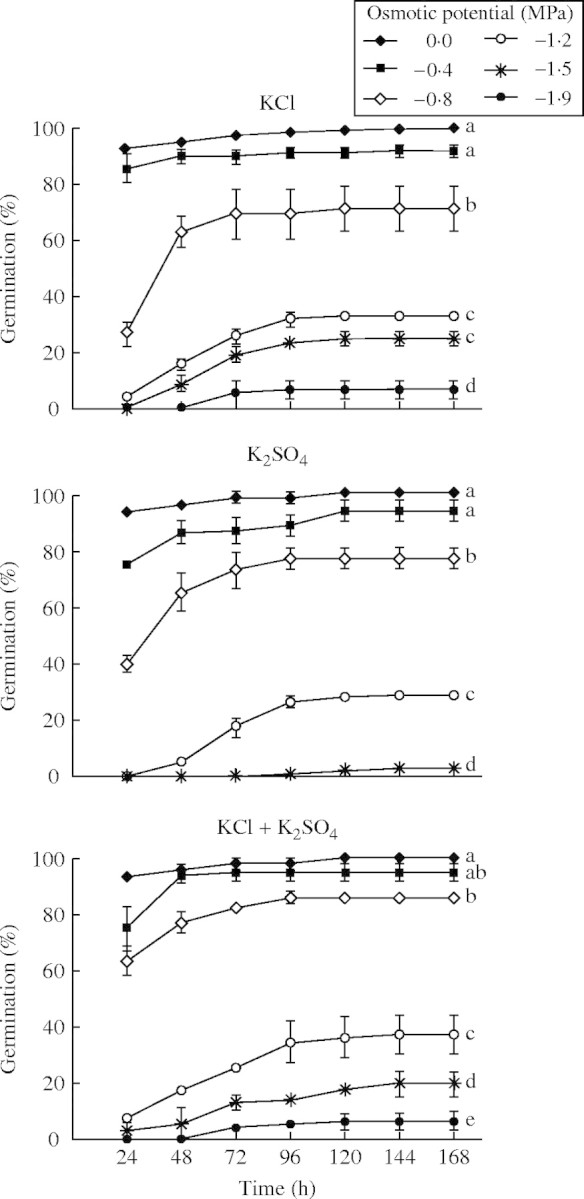
Percentage germination (mean ± s.e.) over time of P. strombulifera seeds exposed to different osmotic potentials of K salts. No germination occurred at −1·9 MPa K2SO4, so these results were not included in the graph. All treatments at −2·2 MPa inhibited all germination. Values with the same letter at 168 h are not significantly different at P > 0·05, Scheffé test.
For the Na salts, NaCl at −1·2 MPa caused a significant inhibition (P < 0·001) of germination percentage relative to the 0·0 MPa treatment (35 % after 168 h), whereas −1·2 MPa Na2SO4 inhibited 60 % after 168 h (P < 0·001) (Fig. 1). In −1·5MPa NaCl, germination percentage decreased by 60 % relative to the 0·0 MPa treatment after 168 h (P < 0·001), whereas Na2SO4 solution inhibited it by 93 % after 168 h (P < 0·001) (Fig. 1). Thus, 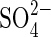 in monosaline Na-based solutions with osmotic potentials of −1·2 MPa and lower, was considerably more inhibitory than Cl− at iso-osmotic concentrations (P < 0·05). This
in monosaline Na-based solutions with osmotic potentials of −1·2 MPa and lower, was considerably more inhibitory than Cl− at iso-osmotic concentrations (P < 0·05). This 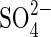 inhibition was reduced in the salt mixture (NaCl + Na2SO4) for treatments at −1·5 MPa and −1·9 MPa (P < 0·05) (Figs 1 and 4).
inhibition was reduced in the salt mixture (NaCl + Na2SO4) for treatments at −1·5 MPa and −1·9 MPa (P < 0·05) (Figs 1 and 4).
Unexpectedly, KCl caused a greater inhibition of germination percentage than NaCl (e.g. 26 % vs. 65 % germination after 24 h at −0·8 MPa), which was accentuated at −1·2 and −1·5 MPa (P < 0·001) (Figs 2 and 4). The different responses with different K salts at iso-osmotic concentrations provide evidence of the existence of specific ionic effects, similar to what happened with Na salts. Thus, 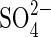 in monosaline K-based solutions with Ψo −1·5MPa and −1·9 MPa were also more inhibitory than Cl− anions, at iso-osmotic concentrations (P < 0·05). However, the bisaline solution (KCl + K2SO4), showed a less pronounced reversion of
in monosaline K-based solutions with Ψo −1·5MPa and −1·9 MPa were also more inhibitory than Cl− anions, at iso-osmotic concentrations (P < 0·05). However, the bisaline solution (KCl + K2SO4), showed a less pronounced reversion of 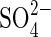 inhibition with increasing concentrations (Fig. 2), than the reversion obtained with the Na bisaline solution.
inhibition with increasing concentrations (Fig. 2), than the reversion obtained with the Na bisaline solution.
Responses of germination to treatments with PEG and mannitol were similar, showing higher germination percentages than all salt treatments at −0·8 MPa and lower. At −1·2 MPa, germination percentage was 76 % in PEG and 85 % in mannitol, being higher than in any of the salts. In Na salts at −1·2 MPa the germination percentages were in NaCl (63 %), followed by 50 % germination in the bisaline solution (NaCl + Na2SO4) and 40 % in Na2SO4. In K salts at −1·2MPa, germination percentages were 30 % in KCl, 25 % in K2SO4 and 37 % in the bisaline solution.
When Ψo was decreased to −1·5 MPa, low germination percentages were obtained with all salt treatments (maximum 40 % with NaCl), but 60 % germination was obtained with both non-ionic treatments. At −1·9 MPa, the osmotic factor strongly inhibited germination, as demonstrated by the inhibition imposed by PEG and mannitol. There was no germination at all in both sulfate monosaline solutions. Nevertheless, the germination percentages obtained with both bisaline solutions showed again a reduction of 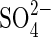 toxicity, giving no significant differences with their respective Cl salts (P < 0·05).
toxicity, giving no significant differences with their respective Cl salts (P < 0·05).
Given that inhibition was more noticeable in Ψo −1·2 MPa and lower in all salt treatments, the germination percentages at −1·2, −1·5 and −1·9 MPa after 168 h incubation in PEG, mannitol, monosaline and bisaline solutions were summarized in a new figure, Fig. 4. The figure also highlights the differences between the two bisaline solutions (P < 0·05) showing that K+ was more inhibitory than Na+.
All treatments at −2·2 MPa inhibited all germination, except for mannitol, in which the germination percentage was similar to that at −1·9 MPa (Fig. 3).
Fig. 4.
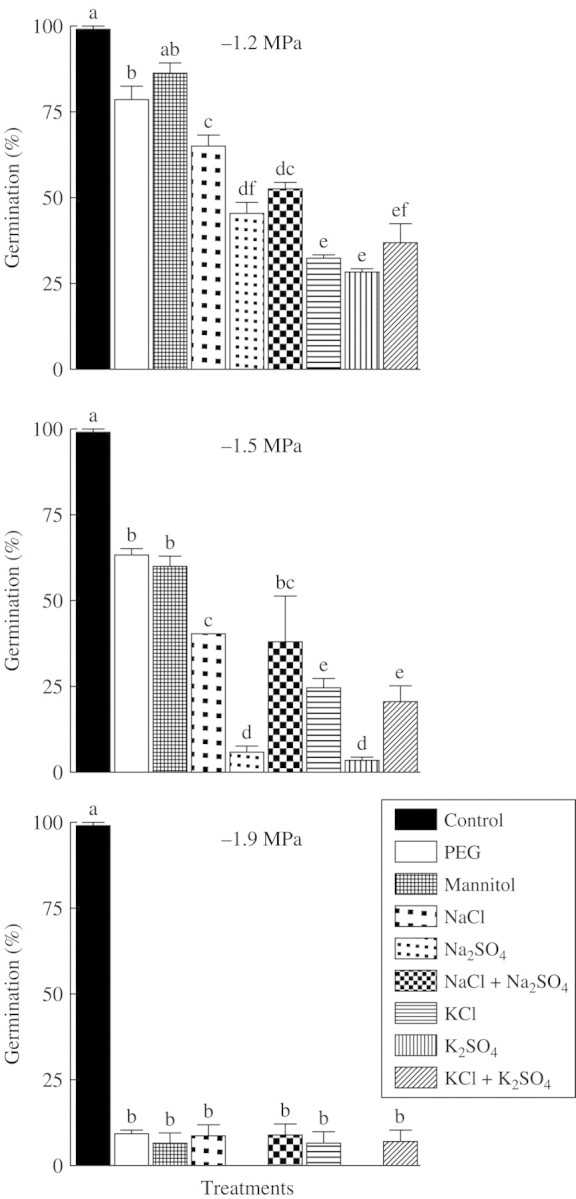
Percentage germination (mean ± s.e.) of P. strombulifera seeds at −1·2, −1·5 and −1·9 MPa with monosaline and bisaline solutions after 168 h of treatment. Data from Figs 1–3. Values with the same letter are not significantly different at P > 0·05, Scheffé test.
Fig. 3.
Percentage germination (mean ± s.e.) over time of P. strombulifera seeds exposed to PEG and mannitol. Values with the same letter at 168 h are not significantly different at P > 0·05, Scheffé test.
DISCUSSION
The threshold salinity for a significant reduction in germination varies between species (Gulzar and Khan, 2001). The germination response of P. strombulifera seeds to NaCl salinity indicates that this species is within the tolerance limits cited for the majority of Prosopis species, which is between −1·5 and −1·9 MPa (Passera, 2000).
Some reports interpret the effects of salinity on the germination of seeds of different plant species in terms of Ψo, while others explain the reduced germination as toxic effects of ionic elements (Poljakoff-Mayber et al., 1994; Al-Karaki, 2001). The present results show a general inhibition imposed by osmotic effect especially noticeable at Ψo −1·2 MPa and lower in all treatments. At Ψo −0·4 and −0·8 MPa NaCl the seeds could adjust osmotically reaching to final germination percentages equal to the controls. Nevertheless, in all the other salts used, the adjustment did not occur at −0·8 MPa, as evidenced by the statistically significant difference between K+ and  salt treatments and NaCl, PEG, mannitol and control treatments.
salt treatments and NaCl, PEG, mannitol and control treatments.
In monosaline solutions  was considerably more inhibitory than Cl− at iso-osmotic concentrations. Similar results were obtained by Perez and Tambelini (1995) in Prosopis juliflora seeds germinated in monosaline solutions of NaCl, CaCl2 and Na2SO4 at Ψo from −0·3 to −1·5 MPa, in which the germination percentage was also more affected by Na2SO4 than NaCl.
was considerably more inhibitory than Cl− at iso-osmotic concentrations. Similar results were obtained by Perez and Tambelini (1995) in Prosopis juliflora seeds germinated in monosaline solutions of NaCl, CaCl2 and Na2SO4 at Ψo from −0·3 to −1·5 MPa, in which the germination percentage was also more affected by Na2SO4 than NaCl.
Notable toxic effects of  were also observed in P. strombulifera plants which had been cultured hydroponically with increasing concentrations of Na2SO4 as the sole salinizing agent. They showed strong growth inhibition, lower leaf number, chlorosis and precocious senescence compared with control plants (Sosa et al., 2002). Notwithstanding,
were also observed in P. strombulifera plants which had been cultured hydroponically with increasing concentrations of Na2SO4 as the sole salinizing agent. They showed strong growth inhibition, lower leaf number, chlorosis and precocious senescence compared with control plants (Sosa et al., 2002). Notwithstanding, 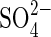 concentrations in root and leaf tissues were low when compared to Na+: 6g 100g−1d. wt
concentrations in root and leaf tissues were low when compared to Na+: 6g 100g−1d. wt 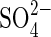 vs. 21 g 100 g−1d. wt Na+ in leaves and 4 g 100 g−1d. wt
vs. 21 g 100 g−1d. wt Na+ in leaves and 4 g 100 g−1d. wt 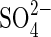 vs. 9 g 100 g−1d. wt Na+ in roots of plants growing at 531 mm Na2SO4 (Reginato et al., 2004).
vs. 9 g 100 g−1d. wt Na+ in roots of plants growing at 531 mm Na2SO4 (Reginato et al., 2004).
It has been reported recently that H+/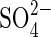 co-transport in the root cells is the first step for
co-transport in the root cells is the first step for 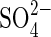 uptake of plants from the environment, and a gene family of
uptake of plants from the environment, and a gene family of 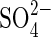 transporters has been identified with examples from many different plant species (Buchner et al., 2004). The availability of clones of some of these genes has enabled expression of the high-affinity
transporters has been identified with examples from many different plant species (Buchner et al., 2004). The availability of clones of some of these genes has enabled expression of the high-affinity 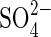 transporters SHST1 and SHST2 from Stylosanthes and HVST1 from barley to be shown to be regulated at transcriptional level by the external
transporters SHST1 and SHST2 from Stylosanthes and HVST1 from barley to be shown to be regulated at transcriptional level by the external 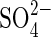 supply. Restriction of the external
supply. Restriction of the external 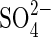 supply resulted in a rapid increase in the capacity of these plants to take up
supply resulted in a rapid increase in the capacity of these plants to take up 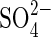 . Upon resupplying
. Upon resupplying 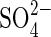 to such plants,
to such plants, 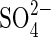 uptake rates declined, thus showing that transcription of the genes encoding
uptake rates declined, thus showing that transcription of the genes encoding 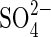 transporters is closely controlled by a negative feedback (Smith et al., 2000). It is considered that if such a regulatory mechanism were universal, permeability to
transporters is closely controlled by a negative feedback (Smith et al., 2000). It is considered that if such a regulatory mechanism were universal, permeability to 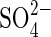 would be blocked and
would be blocked and 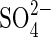 inhibition of germination in salt solutions could be explained by the rapid build-up of
inhibition of germination in salt solutions could be explained by the rapid build-up of 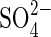 in the cell walls of the seeds, causing deleterious effects on water uptake.
in the cell walls of the seeds, causing deleterious effects on water uptake.
The germination percentages with bisaline solutions in the present experiment do not allow an unequivocal elucidation of the existence of a specific ionic effect of Cl− apart from those of SO42−. Notwithstanding, the 60 % of germination inhibition in −1·5MPa NaCl after 168-h incubation may be a consequence of both, an osmotic effect plus a specific anion toxicity, taking into account that in −1·5MPa PEG and mannitol the germination inhibition was only 40 %.
Another interesting result of the present work was that K+, a macronutrient, was more inhibitory than Na+ on germination of this species independently of the accompanying anion.
Potassium is known as an important osmotic agent but is also involved in more specific metabolic roles including protein and starch syntheses and enzyme activation (Al-Karaki, 2001). Nevertheless, cases of K+ toxicity have been reported in glycophytes (Rathert, 1982; El-Haddad and O'Leary, 1994), and in halophytes (El-Haddad and O'Leary, 1994; Kefu et al., 1995; Egan and Ungar, 1998). Interesting results have been reported in relation to specific K+ effects on the synthesis of osmoprotectants. In one report the results indicated that alfalfa cell cultures were unable to accumulate proline in response to K+ salt stress (Chaudhary et al., 1997), and in the other K+ salts were found to be inhibitory to the production of glycinebetaine at all salt concentrations in Atriplex prostrata plants, which produced less biomass and had lower survival percentages than plants treated with Na+ salts (Egan et al., 2001). The latter authors proposed that it is possible that plants exposed to K+ salts, in contrast to Na+ treatments, were not able to transport K+ into the vacuoles, causing a specific ion toxicity in the cytoplasm that inhibited both growth and glycinebetaine production.
The present study clearly demonstrates that the germination of P. strombulifera seeds is influenced not only by the salt concentrations (or the osmotic potential) but also by the nature of the ions in the salt solutions and their interactions. The marked differences in germination percentages obtained with monosaline and bisaline solutions, PEG and mannitol at the same osmotic potentials indicate specific ionic effects and point to shortcomings in the hypothesis that germination is solely controlled by the osmotic potential of the solutes or by the gradient by which water moves into seeds. This is in agreement with the results obtained by Katembe et al. (1998) who demonstrated that at high concentrations NaCl was more inhibitory to water uptake, germination and seedling root elongation of Atriplex prostrata than iso-osmotic PEG. The present results are in agreement with the suggestion by Poljakoff-Mayber et al. (1994) that germination inhibition is due to the combination of osmotic as well as ionic effects, but not with their assertion that the former are more important at low concentrations while toxic effects are greater at high concentrations. In the present study, in P. strombulifera seed germination, ionic effects as well as osmotic effects were noticeable from −0·8 MPa and lower. Moreover, considering the results with PEG and mannitol, it could be stated that ionic effects were observed in lower salt concentrations than osmotic effects.
An equally important contribution of this study is given by the results obtained with salt mixtures. It was demonstrated that the germination response is different when the major salts present in most salinized soils of America are used in a separate way, than when they are used in isoosmotic mixed solutions which can mimic what happens in the field (for the situation in saline soils from central Argentina, see the Introduction). Reversion of toxicity by salt mixtures was reported when Securigera securidaca seed germination was inhibited by −0·6 MPa NaCl and −1·0 MPa MgSO4 (Al-Jibury and Clor, 1986). These authors observed that a combination of these salts increased the tolerance limits for seed germination up to −1·4 and −1·5 MPa, respectively, suggesting that monosaline salt effects can be considerably alleviated in nature due to synergistic interactions between salts. The comparative studies of Cl− and 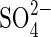 effects and the interaction between
effects and the interaction between 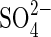 and Cl− in salt mixtures indicate that extrapolation of results obtained with monosaline solutions in laboratory experiments to field conditions may be speculative, since saline soils can have substantial amounts of Cl−,
and Cl− in salt mixtures indicate that extrapolation of results obtained with monosaline solutions in laboratory experiments to field conditions may be speculative, since saline soils can have substantial amounts of Cl−, 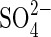 and HCO3− (Grattan and Grieve, 1999). Notwithstanding, the different responses obtained with bisaline solutions in the present work may be due to the lower concentration of
and HCO3− (Grattan and Grieve, 1999). Notwithstanding, the different responses obtained with bisaline solutions in the present work may be due to the lower concentration of 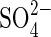 per se, or possible alleviation by addition of Cl−.Very few researchers have dealt with the effects of sulfate salts, and the interaction between
per se, or possible alleviation by addition of Cl−.Very few researchers have dealt with the effects of sulfate salts, and the interaction between 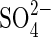 and Cl− in salinity requires further study. The present findings let us propose that future salinity studies, regardless of experimental scale or objectives, should be conducted with more realistic ion ratios, in agreement with Grattan and Grieve (1999).
and Cl− in salinity requires further study. The present findings let us propose that future salinity studies, regardless of experimental scale or objectives, should be conducted with more realistic ion ratios, in agreement with Grattan and Grieve (1999).
Supplementary Material
Acknowledgments
This study was supported with funds from CONICET (PEI No. 0480/97) and from SECYT-UNRC, Universidad Nacional de Río Cuarto, Argentina, to V.L.
LITERATURE CITED
- Al-Jibury LK, Clor MA. 1986. Interaction between sodium, calcium and magnesium chlorides affecting germination and seedling growth of Securigera securidaca Annals of Arid Zones 25: 105–111. [Google Scholar]
- Al-Karaki G. 2001. Germination, sodium, and potassium concentrations of barley seeds as influenced by salinity. Journal of Plant Nutrition 24: 511–522. [Google Scholar]
- Anderson DL, Del Aguila JB, Bernardon AE. 1970.Las formaciones vegetales en la provincia de San Luis. Buenos Aires, Argentina: Revista de Investigación Agropecuaria, INSTA. [Google Scholar]
- Apse MP, Blumwald E. 2002. Engineering salt tolerance in plants. Current Opinion in Biotechnology 13: 146–150. [DOI] [PubMed] [Google Scholar]
- Arce P, Balboa O. 1988. Some aspects of the biology of Prosopis growing in Chile. In: Habit MA, ed. The current state of knowledge on Prosopis juliflora. Rome: FOA, 313–322. [Google Scholar]
- Bal AR, Chattopadhyay NC. 1985. Effect of NaCl and PEG 6000 on germination and seedling growth or rice (Oryza sativa L.). Biologia Plantarum 27: 65–69. [Google Scholar]
- Bazzaz FA. 1973. Seed germination on relation to salt concentration in three populations for Prosopis farcta Oecologia 13: 73–80. [DOI] [PubMed] [Google Scholar]
- Blum A. 1988.Plant breeding for stress environments. Boca Raton, FL: CRC Press. [Google Scholar]
- Buchner P, Takahashi H, Hawkesford MJ. 2004. Plant sulphate transporters: co-ordination of uptake, intracellular and long-distance transport. Journal of Experimental Botany 404: 1765–1773. [DOI] [PubMed] [Google Scholar]
- Burkart A. 1976. A monograph of the genus Prosopis (Leguminosae subfamily Mimosoideae). Journal Arnold Arboretum 57: 450–523. [Google Scholar]
- Carter CT, Ungar IA. 2003. Germination response of dimorphic seeds of two halophyte species to environmentally controlled and natural conditions. Canadian Journal of Botany 81: 918–926. [Google Scholar]
- Catalán L, Balzarini M, Taleisnik E, Sereno R, Karlin U. 1994. Effects of salinity on germination and seedling growth of Prosopis flexuosa (D.C). Forest Ecology and Management 63: 347–357. [Google Scholar]
- Chaudhary MT, Merrett MJ, Wainwright SJ. 1997. Growth, ion content and proline accumulation in NaCl-selected and non-selected cell lines of lucerne cultured on sodium and potassium salts. Plant Science 127: 71–79. [Google Scholar]
- Cisneros JM, Cantero JJ, Cantero Gutiérrez A. 1997. Relaciones entre la fluctuación del nivel freático, su salinidad y el balance hídrico, en los suelos salinos-sódicos del centro de Argentina. Revista Universidad Nacional de Río Cuarto 17: 23–35. [Google Scholar]
- Cony MA, Trione SO. 1998. Inter- and intraspecific variability in Prosopis flexuosa and P. chilensis: seed germination under salt and moisture stress. Journal of Arid Environments 40: 307–317. [Google Scholar]
- Curtin D, Steppuhn H, Selles F. 1993. Plant responses to sulphate and chloride salinity: growth and ionic relations. Soil Science Society of America Journal 47: 1304–1310. [Google Scholar]
- Dafni A, Negbi M. 1978. Varability in Prosopis farcta in Israel: seed germination as affected by temperature and salinity. Israel Journal of Botany 27: 147–157. [Google Scholar]
- EganTP, Ungar IA, Meekins JF. 1997. The effects of NaCl, KCl, Na2SO4 and K2NO4 on the germination of Atriplex prostrata (Chenopodiaceae). Journal of Plant Nutrition 20: 1723–1730. [Google Scholar]
- Egan TP, Ungar I. 1998. Effect of different salts of sodium and potassium on the growth of Atriplex prostata (Chenopodiaceae). Journal of Plant Nutrition 21: 2193–2205. [Google Scholar]
- Egan TP, Dewald HD, Ungar I. 2001. The effect of different salts of sodium and potassium on the accumulation of glycinebetaine in Atriplex prostate Biologia Plantarum 44: 595–597. [Google Scholar]
- El-Haddad EHM, O'Leary JW. 1994. Effect of salinity and K/Na ratio of irrigation water on growth and solute content of Atriplex amnicola and Sorghum bicolor Journal of Applied Irrigation Science 14: 127–133. [Google Scholar]
- Fan Z, Chang Q, Tian C, Yabaki S, Okada A, Liu C. 1993. Genesis and characteristics of salt-affected soils in Tarim Basin. In: Ichikuni M, ed. Proceedings of the Japan-China International Symposium on the Study of the Mechanism of Desertification. Tokyo: Science and Technology Agency, 219–226. [Google Scholar]
- Grattan SR, Grieve CM. 1999. Salinity–mineral nutrient relations in horticultural crops. Scientia Horticulturae 78: 127–157. [Google Scholar]
- Gulzar S, Khan MA. 2001. Seed germination of a halophytic grass Aeluropus lagopoides Annals of Botany 87: 319–324. [Google Scholar]
- Holmberg N, Bülow L. 1998. Improving stress tolerance in plants by gene transfer. Trends in Plant Science 3: 61–66. [Google Scholar]
- Jarrell WM, Virginia RA. 1990. Response of mesquite to nitrate and salinity in a simulated phreatic environment: water use, dry matter and mineral nutrient accumulation. Plant and Soil 125: 185–196. [Google Scholar]
- Katembe WJ, Ungar IA, Mitchell JP. 1998. Effect of salinity on germination and seedling growth of two Atriplex species (Chenopodiaceae). Annals of Botany 82: 167–175. [Google Scholar]
- Kefu Z, Hai F, Harris PJC. 1995. The physiological basis of growth inhibition of halophytes by potassium. In: Khan MA, Ungar IA, eds. Biology of salt tolerant plants. Chelsea, MI: Crafters. [Google Scholar]
- López Villagra GM, Galera FM. 1992. Soil salinity-sodicity effects on germination, survival and development in four populations of Prosopis strombulifera (Lam) Benth. (Fabaceae: Mimosoideae). In: Dutton RW, ed. Prosopis species: aspects of their value, research and development. CORD: University of Durham. NC, 219–223. [Google Scholar]
- Noble CL. 1983. The potential for breeding salt tolerant plants. Proceedings of the Royal Society of Victoria 95: 133–161. [Google Scholar]
- Passera CB. 2000. Fisiología de Prosopis spp In: Actas de la III Reunión Nacional de la Asociación Argentina de Prosopis. Mendoza, Argentina. [Google Scholar]
- Peña-Zubiate CA, Anderson DL, Demmi MA, Saenz JL, D'Hiriart A. 1998.Carta de suelos y vegetación de la Provincia de San Luis. San Luis, Argentina: Secretaría de Agricultura, Ganadería, Pesca y Alimentación, INTA. [Google Scholar]
- Perez A, Tambelini C. 1995. Effect of saline and water stress and early aging on the ‘Algarroba’ seed germination. Pesquisa Agropecuária Brasileira 30: 1289–1295. [Google Scholar]
- Poljakoff-Mayber A, Somers GF, Werker E, Gallagher JL. 1994. Seeds of Kosteletzkya virginica (Malvaceae): their structure, germination, and salt tolerance. II. Germination and salt tolerance. American Journal of Botany 81: 54–59. [Google Scholar]
- Rathert G. 1982. Influence of extreme K:Na ratios and high substrate salinity on plant metabolism of crops differing in salt tolerance. Journal of Plant Nutrition 5: 183–193. [Google Scholar]
- Reginato M, Llanes A, Sosa L, Luna V. 2004. Variación del contenido de iones en plántulas de Prosopis strombulifera cultivadas en soluciones isoosmóticas de Na2SO4 y NaCl. Actas XII Reunión Argentina de Fisiología Vegetal. La Pampa, Argentina, 22−24 September 2004. [Google Scholar]
- Reinoso H, Sosa L, Colombo C, Ochoa F, Luna V. 2000. Morphological and physiological responses of Prosopis strombulifera (Lam.) Benth. to increasing salt conditions. In: Bush D, Cosgrove D, Hangarter R, Jorgensen R, Delmer D, Springer P, Lucas W, Schnell D, eds. Plant Biology 2000: American Society of Plant Physiologists. Annual Meeting, San Diego, CA, 15–19 July 2000, 201–202 (Abstract) [Google Scholar]
- Reinoso H, Sosa L, Ramírez L, Luna V. 2004. Salt-induced changes in the vegetative anatomy of Prosopis strombulifera (Leguminosae). Canadian Journal of Botany 82: 618–628. [Google Scholar]
- Romo JT, Haferkamp MR. 1978. Effects of osmotic potential, potassium chloride, and sodium chloride on germination of Greasewood (Sarcobatus vermiculatus). Great Basin Naturalist 47: 110–116. [Google Scholar]
- Ryan J, Miyamoto S, Stroehlein JL. 1975. Salt and specific ion effects on germination of four grasses. Journal of Range Management 28: 61–64. [Google Scholar]
- Smith FW, Rae AL, Hawkesford JM. 2000. Molecular mechanisms of phosphate and sulphate transport in plants. Biochimica et Biophysica Acta 1465: 236–245. [DOI] [PubMed] [Google Scholar]
- Sosa L, Reginato M, Reinoso H, Luna V. 2002. Efectos de cloruro y sulfato de Na sobre el crecimiento de plántulas de Prosopis strombulifera. Actas XI Reunión Latinoamericana de Fisiología Vegetal. Punta del Este, Uruguay, 22−25 October 2002. [Google Scholar]
- StataCorp LP. 1995.Stata Statistical Software: Reference Manual Release 4·0. College Station, Texas: Stata Corporation. [Google Scholar]
- Steel RGD, Torrie JH. 1995.Bioestadística: Principios y Procedimientos, 2nd edn. Spain: McGraw-Hill. [Google Scholar]
- Tobe K, Li X, Omasa K. 2000. Seed germination and radicle growth of a halophyte, Kalidium capsicum (Chenopodiaceae). Annals of Botany 85: 391–396. [Google Scholar]
- Ungar I. 1978. Halophyte seed germination. Botanical Review 44: 233–264. [Google Scholar]
- Ungar I. 1991.Ecophysiology of vascular halophytes. Boca Raton, FL: CRC Press. [Google Scholar]
- Villagra PE. 1997. Germination of Prosopis argentina and P. alpataco seeds under saline conditions. Journal of Arid Environments 37: 261–267. [Google Scholar]
- Yeo A. 1998. Molecular biology of salt tolerance in the context of whole-plant physiology. Journal Experimental Botany 49: 915–929. [Google Scholar]
- Zhu JK. 2001. Plant salt tolerance. Trends in Plant Science 6: 66–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.