Abstract
• Background and Aims Reactive oxygen species (ROS) are involved in triggering cell death. To visualize mitochondrial behaviour under ROS stress, transgenic arabidopsis plants possessing mitochondrial-targeted GFP (S65T) were studied.
• Methods Arabidopsis leaves were treated with ROS and ROS-inducing chemicals such as hydrogen peroxide, paraquat and menadione. Microscopic observations were carried out using a confocal laser scanning microscope system, and electrolyte leakage was also monitored.
• Key Results After treatment, mitochondria showed morphological changes from a bacillus-like to a round shape. The size of mitochondria treated with H2O2 decreased by half compared with controls. Concurrently, cytoplasmic streaming was blocked and mitochondria eventually swelled. Treatment of leaves with butanedione monoxime, an inhibitor of myosin ATPase, resulted in similar behaviour of mitochondria to that under ROS stress.
• Conclusions The results indicate that morphological changes of mitochondria and cessation of cytoplasmic streaming may interact, and this phenomenon is one of the features of ROS stress-induced cell death.
Keywords: Mitochondria, cytoplasmic streaming, arabidopsis
INTRODUCTION
Reactive oxygen species (ROS) are generated under various biotic and abiotic stresses, and trigger cell death. For instance, within minutes of plant-pathogen infection, a burst in oxidative metabolism produces ROS such as hydrogen peroxide (H2O2) and superoxide anion (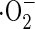 ) through an NADPH-dependent oxidase (Lamb and Dixon 1997). Consequently, accumulated ROS trigger hypersensitive cell death (Levine et al., 1994; Jabs et al., 1996). The NADPH oxidase inhibitor diphenylene iodonium partially blocks elicitor-induced cell death in arabidopsis (Jabs et al., 1996) and rice cells (Matsumura et al., 2003). In other cases, ROS generation is also reported under stresses such as ozone (Rao and Davis 1999), high light (Karpinski et al., 1997), heat shock (Vacca et al., 2004) and chilling (O'Kane et al., 1996).
) through an NADPH-dependent oxidase (Lamb and Dixon 1997). Consequently, accumulated ROS trigger hypersensitive cell death (Levine et al., 1994; Jabs et al., 1996). The NADPH oxidase inhibitor diphenylene iodonium partially blocks elicitor-induced cell death in arabidopsis (Jabs et al., 1996) and rice cells (Matsumura et al., 2003). In other cases, ROS generation is also reported under stresses such as ozone (Rao and Davis 1999), high light (Karpinski et al., 1997), heat shock (Vacca et al., 2004) and chilling (O'Kane et al., 1996).
In animal cells, a variety of key events in cell death focus on mitochondria. For instance, mitochondria are a major source of ROS, and cell death is preceded by disruption of the mitochondrial membrane potential (Green and Reed, 1998). Mitochondria also have a role in plant cell death. Yao et al. (2002) reported that victorin (a host-selective toxin secreted by Cochliobolus victoriae) caused a mitochondrial oxidative burst in oat cells, followed by a breakdown of the mitochondrial membrane potential. Moreover, cyclosporin A (a blocker of the mitochondrial permeability transition pore) prevented H2O2-induced ROS generation and cell death in cultured arabidopsis cells (Tiwari et al., 2002). These data indicate that ROS stress intensely affects mitochondrial function and stimulates further ROS generation.
In this study, H2O2, paraquat and menadione were used as ROS-inducing chemicals. These chemicals are widely used as cell death inducers in plant (Sun et al., 1999; Sweetlove et al., 2002; Fujibe et al., 2004) and mammalian cells (Park et al., 2004). Transgenic arabidopsis possessing mt-GFP [mitochondrial-targeted GFP (S65T)] under a 35S promoter was treated with these ROS-inducing chemicals, and mitochondrial behaviour was investigated. We demonstrate here that morphological changes of mitochondria and cessation of cytoplasmic streaming occur at an early stage of ROS stress. In addition, butanedione monoxime (BDM), an inhibitor of cytoplasmic streaming, induced cell death. These results demonstrated that morphological change to mitochondria is one of the early indicators of whether cells are affected by ROS stress.
MATERIALS AND METHODS
Plant materials
Transgenic Arabidopsis thaliana ecotype Col-0, possessing mt-GFP (Niwa et al., 1999), was grown at 23°C under continuous light.
Chemicals
Hydrogen peroxide was diluted with distilled water to 50–150 mm. Paraquat (nacalai tesque) and menadione (Sigma) were dissolved in DMSO. During treatment, the final DMSO concentration was never higher than 0·2%, which had no effect on arabidopsis leaves. In the present study, 30–100 µm menadione and 0·2–0·6 µm paraquat were used.
Microscopic observation
Microscopic observation was carried out using a confocal laser scanning microscope system (MicroRadiance MR/AG-2; Bio-Rad). Three-week-old transgenic arabidopsis leaves treated with each chemical were placed on glass slides and examined with an argon ion laser (488 nm) for the observation of GFP.
Ion leakage measurement
Three leaf discs obtained from 3-week-old arabidopsis plants were floated on distilled water. After that, chemicals were added and vacuum-infiltrated for 5 min. Electrolyte leakage was monitored using an electrical conductivity meter (Horiba, B-173) (Kawai-Yamada et al., 2004). Electrical conductivities of the medium were measured in microsiemens per centimetre.
RESULTS
Morphological change of mitochondria by ROS-inducing chemicals
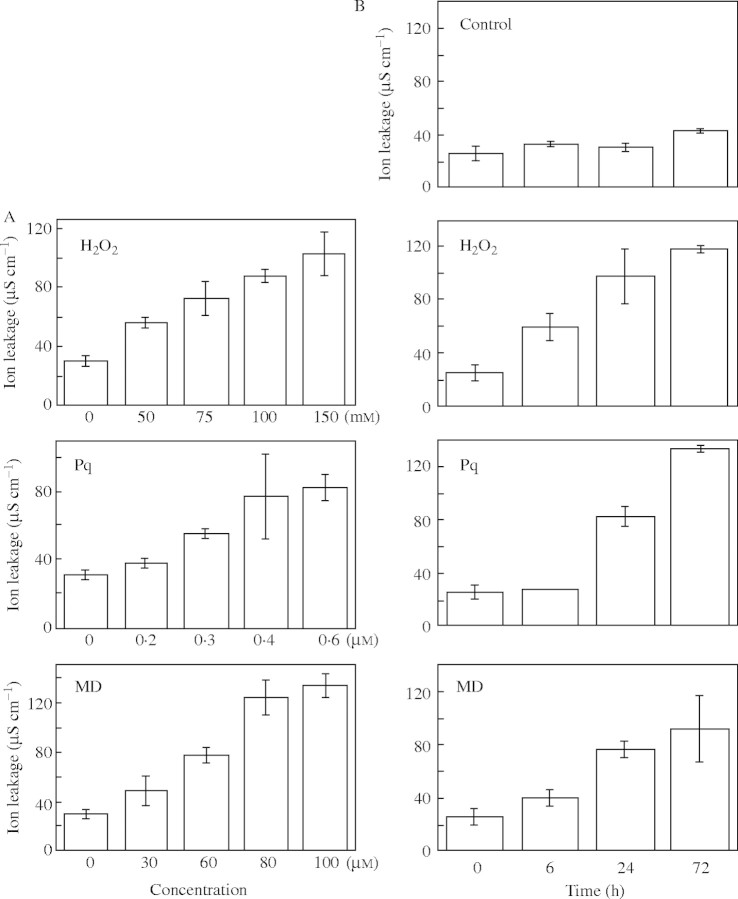
The accumulation of ROS causes cell death, which is demonstrated by electrolyte leakage from cells (Mitsuhara et al., 1999; Kawai-Yamada et al., 2004). To evaluate the effect of several ROS-inducing chemicals, arabidopsis leaves were treated with H2O2, paraquat and menadione. As shown in Fig. 1, a dose- and time-dependent increase in ion leakage was observed, and 100 mm H2O2, 0·3 µM paraquat and 60 µm menadione caused similar levels of ion leakage from arabidopsis leaf discs.
Fig. 1.
Evaluation of ion leakage in arabidopsis leaves treated with different ROS-inducing chemicals. (A) Effects of concentrations of ROS-inducing chemicals on ion leakage. Electrolyte leakage from leaf discs prepared from 3-week-old seedlings was measured after 24 h in H2O2 (0–150 mm), paraquat (0–0·6 µm) or menadione (0–100 µm). Data are means ± s.d. of three experiments. (B) Time course analysis of electrolyte leakage from leaf discs. Three leaf discs prepared from 3-week-old plants were treated with 100 mm H2O2, 0·3 µm paraquat or 60 µm menadione for 72 h. Controls were treated with water. Reported values are average ± s.d. of three or more experiments.
Mitochondrial dynamics were studied using transgenic arabidopsis plants possessing mt-GFP, in which GFP (S65T) was fused with the mitochondrial targeting sequence of the gamma-subunit of F1 ATPase under a CaMV 35S promoter (Niwa et al., 1999). As shown in Fig. 2A, without chemical treatment, mitochondria kept a bacilliform shape even at 72 h. On the other hand, each ROS-inducing chemical caused dramatic changes to mitochondria. When treated with 100 mm H2O2, 0·3 µm paraquat or 60 µm menadione, mitochondria became round. Treatment with H2O2 or paraquat caused the mitochondria to swell after 72 h. Morphological changes in mitochondria were quantified using Image-Pro® Plus version 4·0 software (Media Cybernetics). In H2O2-treated leaves, mitochondria were 49·1 % smaller in area and 48·3 % smaller in diameter than that of controls (Fig. 2B).
Fig. 2.
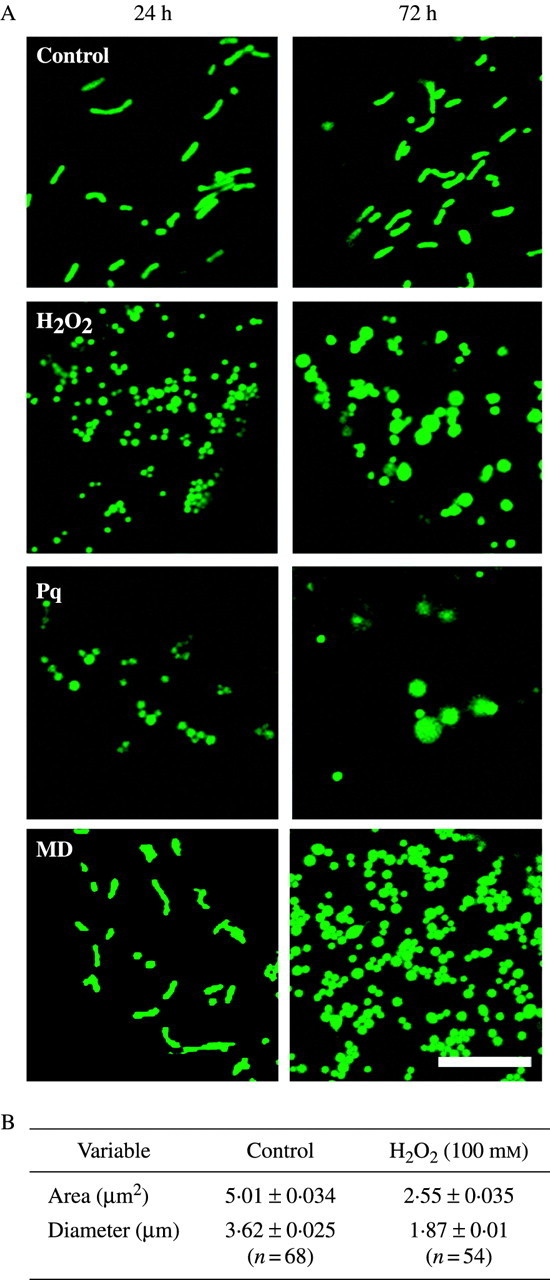
Mitochondrial changes under ROS stress. (A) Morphology of mitochondria incubated in ROS-inducing chemicals for 24 and 72 h. Epidermal cells of leaf discs obtained from transgenic mt-GFP arabidopsis were examined using a confocal laser scanning microscope. Leaf discs were treated with 100 mm H2O2, 0·3 µm paraquat or 60 µm menadione solutions and incubated at 23 °C under continuous light. Controls were treated with water. Scale bar = 10 µm. (B) Comparison of mitochondrial area and maximum diameter. The size and the area were quantified using Image-Pro® Plus version 4·0 (Media Cybernetics) based on the images of cells treated with 100 mm H2O2 (1 d).
Effects of a myosin ATPase inhibitor on mitochondrial movement
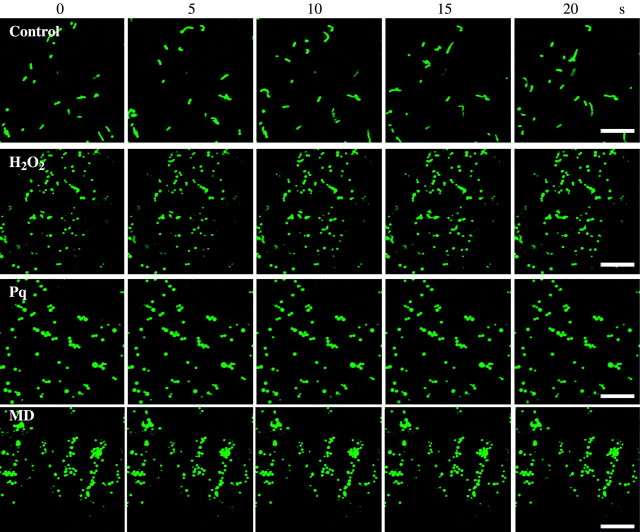
In addition to the morphological changes, ROS-inducing chemicals blocked mitochondrial movement (Fig. 3 and Supplementary movie at http://www.aob.oupjournals.org). Namely, when treated with H2O2 or paraquat for 24 h, or with menadione for 72 h, cessation of mitochondrial streaming was noted, whereas intensive streaming of organelles was seen in untreated samples.
Fig. 3.
Movement of mitochondria in epidermal cells of arabidopsis leaves expressing mt-GFP, which had been treated with ROS-inducing chemicals H2O2 (100 mm) or paraquat (0·3 µm) for 24 h or menadione (60 µm) for 72 h. Samples were observed using a confocal laser scanning microscope at 488 nm excitation wavelength to detect mt-GFP. Images were taken at 5-s intervals. Scale bars = 10 µm. Supplementary movie reproduces images of Fig. 3. The speed is 50-fold faster than actual.
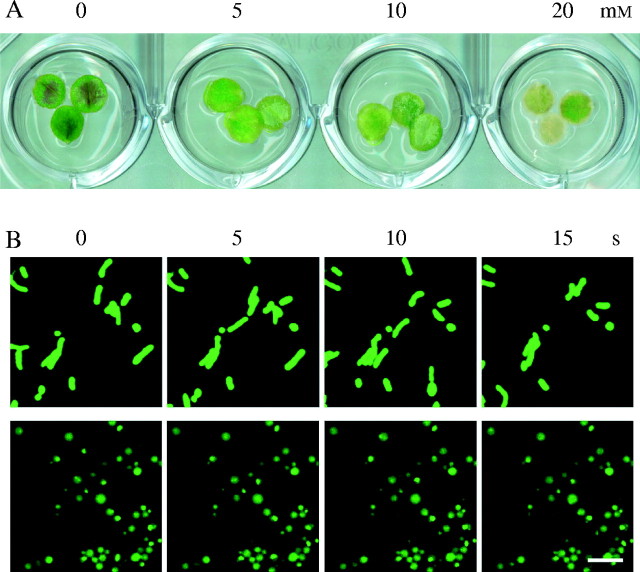
Mitochondrial movement is actomyosin-dependent in plant cells (Van Gestel et al., 2002). The leaves of mt-GFP plants were treated with butanedione monoxime (BDM), which is a myosin ATPase inhibitor (Herrmann et al., 1992; Van Gestel et al., 2002). After 3 d of treatment with BDM, the chlorosis of the leaf discs was shown in high-dose BDM treatment (Fig. 4A). Within 1h of BDM treatment, the streaming of mitochondria ceased and mitochondria became round or even swollen, similar to the case of H2O2-treated cells (Fig. 4B).
Fig. 4.
Effects of a myosin ATPase inhibitor on arabidopsis mitochondria. (A) Leaf discs 3 d after BDM treatment. Three leaf discs were submerged in BDM solution (0–20 mm) and incubated at 23 °C under continuous light. (B) Morphological changes of mitochondria in epidermal cells of arabidopsis leaves treated with BDM. Leaf discs obtained from 1-month-old mt-GFP plants were treated with 20 mm BDM for 1 h prior to observation by confocal laser scanning microscopy. Upper panels indicate controls without BDM treatment. Images were taken at 5-s intervals. Scale bar = 5 µm.
DISCUSSION
Exogenously supplied H2O2 induces cell death in suspension cultures of soybean (Levine et al., 1994), arabidopsis (Tiwari et al., 2002) and tobacco (Houot et al., 2001). These deaths are induced through a process similar to apoptosis, including cell shrinkage, chromatin condensation, and DNA fragmentation (Houot et al., 2001). Here, it is shown that ROS-inducing chemicals such as H2O2, paraquat and menadione cause morphological changes in mitochondria and cessation of cytoplasmic streaming in the early stages of cell death. Paraquat is a non-selective herbicide that disturbs proton translocation through the thylakoid membrane, leading to the production of ROS in chloroplasts (Babbs et al., 1989). Menadione is a redox-active quinone that generates intracellular superoxide. Reichheld et al. (1999) showed that menadione impairs the G1/S transition and delays the entry into mitosis in tobacco BY-2 cells, whereas higher concentrations of menadione cause cell death. Menadione also generates ROS and damages subunits of ATP synthase in arabidopsis cell cultures (Sweetlove et al., 2002). Oxidative stress has a significant effect on the mitochondrial proteome in arabidopsis cultures, and H2O2 and menadione reveal a very similar response (Sweetlove et al., 2002). The present findings also support the observation that ROS affect mitochondria, and morphological changes of mitochondria might be one of the common features of cells attacked by ROS stresses.
Van Gestel et al. (2002) reported that the myosin ATPase inhibitor BDM (butanedione monoxime) inhibits mitochondrial movement in tobacco BY-2 cells. The leaves of mt-GFP plants were also treated with BDM, which not only caused cessation of cytoplasmic streaming, but also morphological changes of mitochondria. In addition, morphological changes of mitochondria and cessation of streaming occurred at nearly the same time. As mentioned by Sellin and McArdle (1994), BDM possibly affects a number of mechanisms in plant cells. The relationship between morphological change and cytosolic streaming should be analysed futher. Kikuyama and Tazawa (1982) reported that cytoplasmic streaming was inhibited by cytosolic Ca2+ increase in plant cells. Furthermore Virolainen et al. (2002) reported that a high calcium concentration caused isolated wheat root mitochondria to swell. Thus ROS may downregulate cytoplasmic streaming through calcium, which causes morphological alterations to mitochondria in plant cells.
Several investigators demonstrated the importance of mitochondria in cell death for reasons other than generation of ROS. For instance, it is interesting that mitochondrial fission proteins (Dnm1, Mdv1 and Fis1) regulate cell death in animal cells and yeast (Fannjiang et al., 2004; Karbowski et al., 2004). The present study showed that mitochondrial size decreased by half under ROS stress. As in animal cells, this change may induce cell death in plant cells, and this morphological change may be one of the features of cell death widely conserved in living organisms.
Supplementary Material
Acknowledgments
We thank Ms Y. Takahashi for her help. This study was supported by Research for the Future from the Japan Society for the Promotion of Science and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
LITERATURE CITED
- Babbs CF, Pham JA, Coolbaugh RC. 1989. Lethal hydroxyl radical production in paraquat-treated plants. Plant Physiolgy 90: 1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannjiang Y, Cheng WC, Lee SJ, Qi B, Pevsner J, McCaffery JM, et al. 2004. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes and Development 18: 2785–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujibe T, Saji H, Arakawa K, Yabe N, Takeuchi Y, Yamamoto KT. 2004. A methyl viologen-resistant mutant of Arabidopsis, which is allelic to ozone-sensitive rcd1, is tolerant to supplemental ultraviolet-B irradiation. Plant Physiology 134: 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Reed JC. 1998. Mitochondria and apoptosis. Science 281: 1309–1312. [DOI] [PubMed] [Google Scholar]
- Herrmann C, Wray J, Travers F, Barman T. 1992. Effect of 2,3-butanedione monoxime on myosin and myofibrillar ATPases. An example of an uncompetitive inhibitor. Biochemistry 31: 12227–12232. [DOI] [PubMed] [Google Scholar]
- Houot V, Etienne P, Petitot AS, Barbier S, Blein JP, Suty L. 2001. Hydrogen peroxide induces programmed cell death features in cultured tobacco BY-2 cells in a dose-dependent manner. Journal of Experimental Botany 52: 1721–1730. [PubMed] [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL. 1996. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273: 1853–1856. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Arnoult D, Chen H, Chan DC, Smith CL, Youle RJ. 2004. Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. Journal of Cell Biology 164: 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM. 1997. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9: 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai-Yamada M, Ohori Y, Uchimiya H. 2004. Dissection of Arabidopsis Bax inhibitor-1 suppressing Bax-, hydrogen peroxide-, and salicylic acid-induced cell death. Plant Cell 16: 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuyama M, Tazawa M. 1982. Ca2+ ion reversibly inhibits the cytoplasmic streaming of Nitella Plotoplasma 113: 241–243. [Google Scholar]
- Lamb CJ, Dixon RA. 1997. The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology 48: 251–275. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. 1994. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593. [DOI] [PubMed] [Google Scholar]
- Matsumura H, Nirasawa S, Kiba A, Urasaki N, Saitoh H, Ito M, et al. 2003. Overexpression of Bax inhibitor suppresses the fungal elicitor-induced cell death in rice (Oryza sativa L) cells. The Plant Journal 33: 425–434. [DOI] [PubMed] [Google Scholar]
- Mitsuhara I, Malik KA, Miura M, Ohashi Y. 1999. Animal cell-death suppressors Bcl-x(L) and Ced-9 inhibit cell death in tobacco plants. Current Biology 9: 775–778. [DOI] [PubMed] [Google Scholar]
- Niwa Y, Hirano T, Yoshimoto K, Shimizu M, Kobayashi H. 1999. Non-invasive quantitative detection and applications of non-toxic, S65T-type green fluorescent protein in living plants. The Plant Journal 18: 455–463. [DOI] [PubMed] [Google Scholar]
- O'Kane D, Gill V, Boyd P, Burdon R. 1996. Chilling, oxidative stress and antioxidant responses in Arabidopsis thaliana callus. Planta 198: 371–337. [DOI] [PubMed] [Google Scholar]
- Park SY, Chang I, Kim JY, Kang SW, Park SH, Singh K, et al. 2004. Resistance of mitochondrial DNA-depleted cells against cell death: role of mitochondrial superoxide dismutase. Journal of Biological Chemistry 279: 7512–720. [DOI] [PubMed] [Google Scholar]
- Rao MV, Davis KR. 1999. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. The Plant Journal 17: 603–614. [DOI] [PubMed] [Google Scholar]
- Reichheld JP, Vernoux T, Lardon F, Van Montagu M, Inzé D. 1999. Specific checkpoints regulate plant cell cycle progression in response to oxidative stress. The Plant Journal 17: 647–656. [Google Scholar]
- Sellin LC, McArdle JJ. 1994. Multiple effects of 2,3-butanedione monoxime. Pharmacology and Toxicology 74: 305–313. [DOI] [PubMed] [Google Scholar]
- Sun YL, Zhao Y, Hong X, Zhai ZH. 1999. Cytochrome c release and caspase activation during menadione-induced apoptosis in plants. FEBS Letters 462: 317–321. [DOI] [PubMed] [Google Scholar]
- Sweetlove LJ, Heazlewood JL, Herald V, Holtzapffel R, Day DA, Leaver CJ, et al. 2002. The impact of oxidative stress on Arabidopsis mitochondria. The Plant Journal 32: 891–904. [DOI] [PubMed] [Google Scholar]
- Tiwari BS, Belenghi B, Levine A. 2002. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiology 128: 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca RA, de Pinto MC, Valenti D, Passarella S, Marra E, De Gara L. 2004. Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco Bright-Yellow 2 cells. Plant Physiology 134: 1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gestel K, Kohler RH, Verbelen JP. 2002. Plant mitochondria move on F-actin, but their positioning in the cortical cytoplasm depends on both F-actin and microtubules. Journal of Experimental Botany 53: 659–667. [DOI] [PubMed] [Google Scholar]
- Virolainen E, Blokhina O, Fagerstedt K. 2002. Ca2+-induced high amplitude swelling and cytochrome c release from wheat (Triticum aestivum L.) mitochondria under anoxic stress. Annals of Botany 90: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao N, Tada Y, Sakamoto M, Nakayashiki H, Park P, Tosa Y, Mayama S. 2002. Mitochondrial oxidative burst involved in apoptotic response in oats. The Plant Journal 30: 567–579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.