Abstract
• Background and Aims Although it was generally assumed that Maxillaria spp. do not produce nectar, in recent years, nectar has been reported for a number of these orchids. Nevertheless, our current understanding of nectary structure and nectar secretion in Maxillaria is based solely on M. coccinea (Jacq.) L.O. Williams ex Hodge, which, since it shows many features characteristic of ornithophilous flowers, is atypical of this largely entomophilous genus. The aim of the present paper is to describe, for the first time, nectar secretion in a presumed entomophilous species of Maxillaria.
• Methods The structure of the nectary of M. anceps Ames & C. Schweinf., nectar composition and the process of nectar secretion were investigated using light microscopy, scanning electron microscopy, transmission electron microscopy, histochemistry, refractometry and high performance liquid chromatography.
• Key Results and Conclusions Nectar appears as droplets that are exuded by modified stomata borne upon the labellar callus and collects upon the labellum and at the base of the column-foot. Although such stomata are known to occur in a number of angiosperm families, this is the first time for them to be observed in orchids. The callus consists largely of parenchyma with raphides and is supplied by eight to ten collateral bundles. This tissue, together with the single-layered epidermis, seemingly contains terpenoids. During the bud stage, the callus cells contain an organelle complement consistent with secretory cells whereas by day 4 of anthesis, much of the cell is occupied by a vacuole. The nectar is sucrose-dominant but also contains low concentrations of glucose, fructose, free amino acids and possibly terpenoids. The high sugar concentration (approx. 66 %) is consistent with melittophily and may indicate that, like the majority of Maxillaria spp., M. anceps is visited by stingless bees (Meliponini).
Keywords: Entomophily, HPLC, light microscopy, Maxillaria, melittophily, nectar, nectary, refractometry, SEM, stomata, TEM, terpenoids, ultrastructure
INTRODUCTION
Maxillaria Ruiz & Pav. is a large, morphologically and anatomically diverse genus of Neotropical orchids (Senghas, 1993; Holtzmeier et al., 1998; Davies, 1999). This diversity is reflected both in the pollination strategies employed by its members and in the type of floral reward offered to pollinators. This, perhaps, is hardly surprising since, on the basis of molecular evidence, Maxillaria, as it currently stands, is considered to be polyphyletic (Whitten et al., 2000). These rewards include pseudopollen (Janse, 1886; Porsch, 1905; van der Pjil and Dodson, 1969; Davies and Winters, 1998; Davies et al., 2000, 2003a; Davies and Turner, 2004; Matusiewicz et al., 2004), wax or a viscid, resinous material secreted by the labellum (Porsch, 1905; van der Pjil and Dodson, 1969; Senghas, 1993; Davies et al., 2003a, b; Flach et al., 2004; Matusiewicz et al., 2004; Singer and Koehler, 2004) and floral nectar (Davies et al., 2003a, b; Singer and Koehler, 2003, 2004; Davies and Turner, 2004; Stpiczyńska et al., 2004). However, a significant number of Maxillaria species seemingly offer no rewards whatsoever (Davies and Turner, 2004; Singer and Koehler, 2004).
In Maxillaria, pseudopollen is formed when multicellular, moniliform, labellar trichomes fragment forming short chains or individual cells containing rich reserves of protein and starch (Davies et al., 2000, 2003a; Davies and Turner, 2004) and these are gathered by stingless bees (Meliponini) that pollinate the flowers (R. B. Singer, pers. comm., 2002; Singer and Koehler, 2004). However, to date, there is no unequivocal evidence that pseudopollen is ingested by these insects. Likewise, Meliponini are thought to gather labellar wax, possibly for nest-building (van der Pjil and Dodson, 1969; Flach et al., 2004). The resinous, viscid material secreted by the labella of certain Maxillaria spp. contains lipoidal substances and aromatic amino acids and may thus be gathered for its nutritive value (Davies et al., 2003b). Flach et al. (2004), using gas chromatography–mass spectrometry and nuclear magnetic resonance analysis have examined these secretions further and found that triterpenoids form the major component.
Until recently, it was generally accepted that Maxillaria spp. do not produce nectar. However, nectar secretion is now known to occur in a number of species including M. imbricata Barb. Rodr., M. rigida Barb. Rodr., M. coccinea (Jacq.) L.O. Williams ex Hodge, M. pendens Pabst, M. parviflora (Poepp & Endl.) Garay and M. sophronitis (Rchb.f.) Garay (Roubik, 2000; Davies et al., 2003a, b; Singer and Koehler, 2003; Stpiczyńska et al., 2004).
Based upon data obtained from those Maxillaria spp. generally available in cultivation in the UK (Davies and Winters, 1998; Davies et al., 2000, 2003a, b; Davies and Turner, 2004) and supplemented by field data obtained by Singer and Koehler (2004), it is estimated that some 56 % of species do not reward pollinators, whereas 13 % produce wax or viscid material, 16 % produce pseudopollen and 8 % produce nectar. A further 7 % produce trichomes that do not closely resemble typical pseudopollen-producing hairs but may, nonetheless, function as pseudopollen (n = 100 species).
To date, the only species of Maxillaria to be studied in detail in terms of nectary structure and nectar secretion is M. coccinea (Stpiczyńska et al., 2004). The flowers of this species exhibit a number of features consistent with ornithophily and are presumed to be pollinated by hummingbirds. As such, this species is atypical for the genus. For example, the weakly zygomorphic flower is scarlet, shows diurnal anthesis and has a backwardly curved labellum. The floral tissues are tough and can withstand contact with a hard beak, and a strong fold in the labellum partly closes the floral tube at the level of the anther and stigma, thus forcing the visiting hummingbird to push its beak against the column so as to gain entry. A ‘faucet and sink’ arrangement occurs in this species and the nectary is represented by a small protuberance on the ventral surface of the column. Nectar collects in a semi-saccate reservoir formed by the fusion of the labellum and base of the column-foot. The nectary comprises a single-layered epidermis and three to four layers of small, subepidermal cells below which occur large parenchyma cells. The epidermal cells lack ectodesmata and it is thought that nectar passes along the apoplast and symplast and accumulates beneath the permeable, reticulate cuticle, which becomes distended prior to the discharge of nectar. The secretory cells are collenchymatous and have an organelle complement typical of cells involved in secretion, comprising nuclei, mitochondria, rough endoplasmic reticulum (ER) and plastids containing many plastoglobuli but few lamellae. Their thickened, cellulose walls contain numerous pits and plasmodesmata. Moreover, nectary cells often contain large, intravacuolar, protein bodies.
In previous papers (Davies et al., 2003a, b; Davies and Turner, 2004), it has been stated that M. jenischiana (Rchb.f.) C. Schweinf. also produces nectar and that this collects upon the labellum and at the base of the column-foot. However, it seems that this species is seldom seen in cultivation in the UK and, since no monograph has been published for the genus and some of the features present in the specimen examined were inconsistent with those described for M. jenischiana, it was not possible to identify the specimen with certainty. Consequently, further advice was sought and as a result, the specimen of M. jenischiana, and the subject of this present paper, has been redetermined as M. anceps Ames & C. Schweinf. (G. Carnevali, pers. comm., 2004). This plant shows diurnal anthesis and has strongly zygomorphic, relatively open, greenish-white flowers that last approx. 1 month in cultivation. The labellum is modified to form a landing platform and bears copious nectar upon its surface. However, no nectar guides were visible to the human eye but whether fragrance is present is less certain, although one of the authors could detect a sweet but unpleasant scent. Consequently, it is speculated that M. anceps is melittophilous and is perhaps more typical of nectariferous Maxillaria spp. than the presumed ornithophilous M. coccinea. Thus, the aim of this paper is to describe, for the first time, the secretion and chemical composition of nectar in a presumed entomophilous species of Maxillaria and to compare this with data previously obtained for M. coccinea (Stpiczyńska et al., 2004).
MATERIALS AND METHODS
Fresh flowers of M. anceps were examined by means of a hand lens, an Olympus SZX12 stereo-microscope and a TESLA BS-300 series scanning electron microscope (SEM) in order to locate the position of the nectary and the site of nectar secretion. Flowers were also examined microscopically when in bud (1 d before opening), at the commencement of anthesis (1–2 d after opening) and 3–4 d after opening. Hand-cut sections through nectar-secreting tissue were tested for starch and lipids using IKI and a saturated alcoholic solution of Sudan III, respectively. The tissue was fixed, cut and stained for both light microscopy and transmission electron microscopy (TEM) as previously described (Stpiczyńska et al., 2004). A Nikon Eclipse 600 microscope with screen measurement version 4·21 software was used for micrometry and photomicrography.
Preliminary tests for reducing sugars in the nectar of M. anceps using Clinistix (Beyer PLC) showed that the nectar of flowers prior to, or at the point of anthesis, whilst still tasting sweet, contained no glucose whereas that of older flowers gave a positive reaction for this substance. This test works on the principle that glucose (but not fructose) is oxidized in the presence of glucose oxidase to form gluconic acid and hydrogen peroxide. The latter, in the presence of peroxidase, oxidizes the chromogen system through purple to blue. On the basis of these results, it was suspected that initially the nectar of M. anceps mainly contains sucrose but that this, as anthesis progresses, is converted to glucose.
Flowers were observed for the first 4 d of anthesis and nectar samples were collected by means of calibrated, glass microcapillary tubes. The nectar was weighed using an analytical balance and its sugar concentration measured using a PZ0 RL-3 refractometer and expressed as a percentage (w/w solution). The concentration of amino acids was determined using the method described by Dafni (1992) and a saturated ethanolic solution of Sudan III was used to test for lipids. Terpenoids were detected by examining spots of fresh nectar using a Wood's Famed–1 L6/58 UV lamp, and these, together with other secretory products, display autofluorescence following exposure to UV or violet light (Roshchina, 2003). Finally, the sugar composition of nectar was determined by subjecting two pooled samples of nectar, weighing 13 mg and 27 mg, respectively, to high performance liquid chromatography (HPLC) (Rossomando, 1998).
RESULTS
Nectary structure
Maxillaria anceps has strongly zygomorphic, relatively open, greenish-white flowers with a well-developed labellum (Fig. 1). The nectary is represented by a simple, yellowish, labellar callus (Fig. 2). Nectar is secreted onto the labellar surface (Fig. 2) and may collect at the base of the column-foot. The callus consists largely of parenchyma (Fig. 3A and B). The secretory epidermal cells and subsecretory parenchyma cells measure 20·73 µm (range 15·32–27·07 µm) and 24·88 µm (range 13·92–33·34 µm) in diameter, respectively whereas those of the ground parenchyma are larger and have a mean diameter of 49·12 µm (range 34·68–61·43 µm). Some of the ground parenchyma cells contain raphides, whereas others contain flocculent, intravacuolar precipitates (Fig. 3C–E). Eight to ten collateral vascular bundles supply the callus (Fig. 3B and E). Staining with toluidine blue indicated that the walls of secretory cells consist of cellulose. Vacuoles of epidermal and subepidermal cells, as seen in semi-thin sections, stain an intense blue-green with this reagent and thus, probably contain terpenoids. These cells, in unstained sections, also contain yellow-grey, spherical bodies, some 7·42 µm (range 5·02–16·23 µm) in diameter and these, too, stain blue-green with toluidine blue (Fig. 3C).
Fig. 1.
Zygomorphic flower of Maxillaria anceps showing well-developed labellum. Scale bar = 5 mm.
Fig. 2.
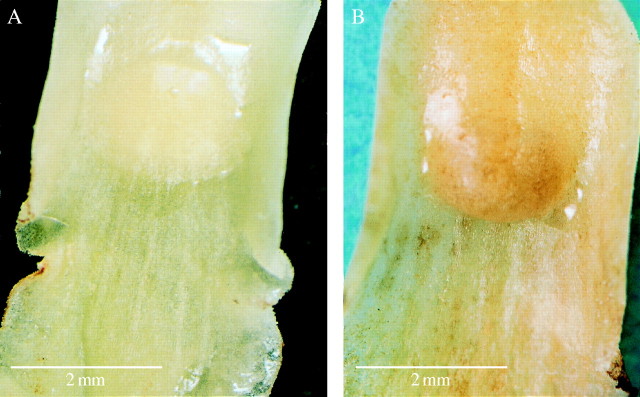
(A) Labellar surface during early anthesis showing simple callus and associated drops of nectar. Scale bar = 2 mm. (B). Labellar surface at day 4 with nectar accumulating at distal end of callus. Scale bar = 2 mm.
Fig. 3.
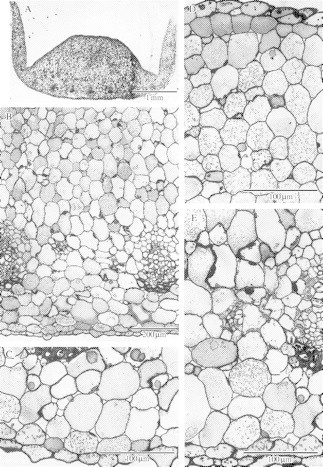
(A) Transverse section of labellum showing vertical, lateral lobes and central, adaxial callus with abaxial vascular bundles. Scale bar = 1 mm. (B) Detail of parenchymatous labellum with abaxial, collateral, vascular bundles. Scale bar = 200 µm. (C) Detail of abaxial, labellar parenchyma showing flocculent, intravacuolar precipitates and spherical bodies that are thought to contain terpenoids. Scale bar = 100 µm. (D) Similar section through adaxial surface of callus showing flocculent, intravacuolar precipitates and raphides. Scale bar = 100 µm. (E) Detail of abaxial, collateral, vascular bundle and adjacent tissues. Scale bar = 100 µm.
The adaxial, secretory epidermis bears relatively few stomata and these are absent from the abaxial surface. The circular, stomatal aperture is almost entirely covered by a thick cuticle (Fig. 4A) and transverse sections through stomata show that the thickened, outer, tangential walls of guard cells, adjacent to the stomatal aperture, have well-developed, cuticular projections (Fig. 4B) that form a peri-apertural ridge (Fig. 4A). Moreover, stomata of fresh material stain selectively with very dilute aqueous solutions of neutral red (0·03 %) and toluidine blue (0·005 % in 0·005 % sodium tetraborate solution). The callus epidermis is glabrous (Fig. 4C–E), whereas conical papillae are present (Fig. 4F) on other parts of the labellum, as previously described for this (as M. jenischiana) and a great number of other Maxillaria species (Davies and Turner, 2004). Peculiar, stalked glandular trichomes occur occasionally upon the adaxial surface of the callus and these appear to exude a colourless secretion (Fig. 5A).
Fig. 4.
(A) Detail of adaxial surface of labellar callus showing stoma. Note the peri-apertural ridge and the continuous, cuticular sheet that almost completely covers the stomatal aperture. Scale bar = 20 µm. (B) Transverse section through callus showing stoma. Note that the guard cells are nucleated, have dense cytoplasmic contents and thick, outer, tangential walls with cuticular projections adjacent to the stomatal aperture. Scale bar = 25 µm. (C) Labellar surface showing simple callus with glabrous surface. Scale bar = 100 µm. (D) Detail of callus surface of older flower. Here, compared with (C), the uninterrupted cuticle appears to have collapsed (arrow). Scale bar = 50 µm. (E) Detail of epidermal surface of callus showing a seemingly distended, cuticular layer. Scale bar = 20 µm. (F) Labellar surface remote from the callus showing typical, conical papillae. Scale bar = 50 µm.
Fig. 5.
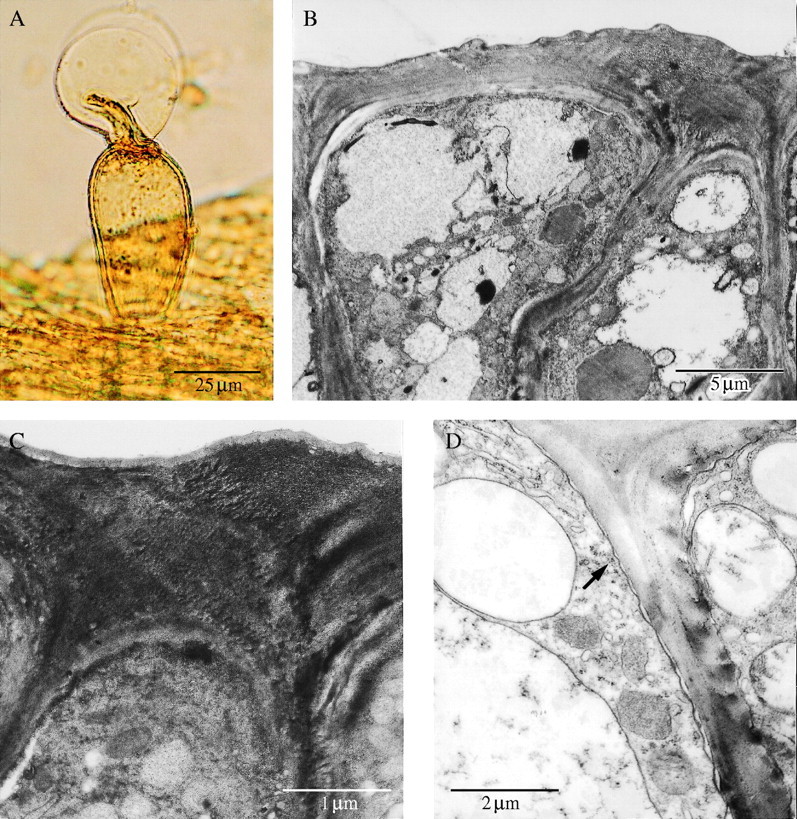
(A) Stalked, glandular trichome on surface of labellar callus with exudate that possibly contains terpenes. Scale bar = 25 µm. (B, C) TEM of cells of secretory epidermis showing thick, outer, tangential wall and uninterrupted cuticle. Scale bars = 5 µm and 1 µm, respectively. (D) Secretory callus tissue during bud stage showing mitochondria, rough ER, components of the vacuome, tonoplast and coated vesicles (arrow). Scale bar = 2 µm.
The cellulose cell walls of the secretory epidermis are thin (0·6–1·52 µm; mean 1·07 µm) but the outer, tangential walls (Fig. 5B and C) are much thicker (1·43–4·06 µm; mean 2·58 µm) and are covered by an uninterrupted cuticle (Fig. 4C) that becomes distended while the flower is in bud (Fig. 4C and E) but appears to collapse as anthesis progresses (Fig. 4D). Numerous plasmodesmata maintain cytoplasmic continuity between contiguous cells. During the bud stage, secretory cells contain dense cytoplasm with abundant mitochondria, dilated ER profiles, dictyosomes (Golgi bodies) and coated vesicles (Fig. 5D). Generally, smooth ER predominates although rough ER is also common. Abaxial, subepidermal cells contain amyloplasts with abundant starch (Fig. 6A and B) whereas their adaxial counterparts, as well as the adaxial epidermal cells themselves, contain plastids comprising a homogeneous stroma with few lamellae and plastoglobuli (Figs 5B and 6C). By day 4 of anthesis, the cytoplasm is less dense and has a parietal distribution, much of the cell being occupied by a single vacuole (Fig. 6C). During the early stages of anthesis, secretory cells contain small, intravacuolar, osmiophilic globular bodies (Fig. 5B) whereas, by day 4, these are larger (Fig. 6C) and the vacuolar precipitates form annular profiles (Fig. 6D).
Fig. 6.
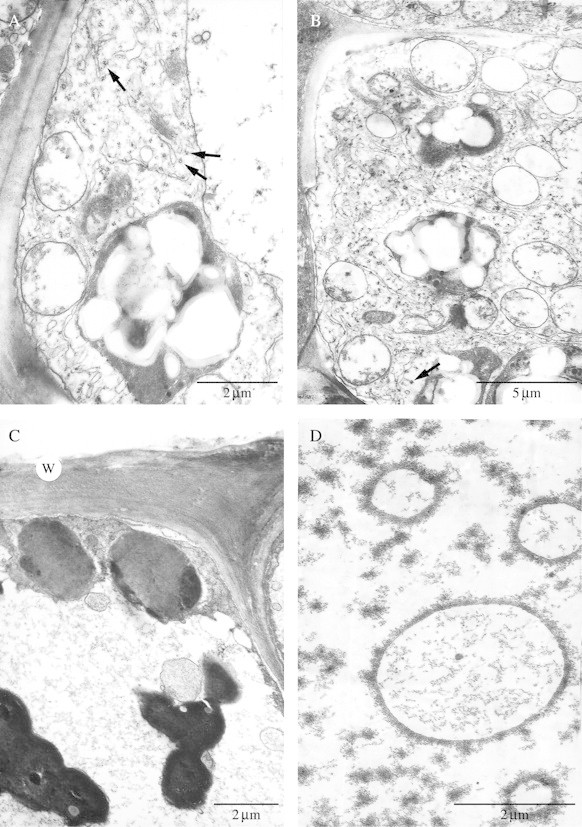
(A, B) Abaxial, subepidermal, labellar cells with mitochondria, rough ER, amyloplasts with starch grains, dictyosomes, components of the vacuole and coated vesicles (arrows). Scale bars = 2 µm and 5 µm, respectively. (C) Secretory, epidermal cell 4 d into anthesis showing outer tangential wall (w), parietal cytoplasm and much of the cell volume occupied by a vacuole. Note the plastids and intravacuolar, osmiophilic bodies and compare them with those found in cells at the early stages of flowering (Fig. 5B). In both cases, the plastids have a homogeneous matrix but few plastoglobuli and lamellae, whereas 4 d into anthesis, the osmiophilic bodies are larger and occur more frequently. Scale bar = 2 µm. (D) Secretory cells 4 d into anthesis showing intravacuolar, annular profiles. Scale bar = 2 µm.
Nectar secretion and composition
Traces of nectar appear while flowers are still in bud (1 d before anthesis). On the first day of anthesis, these appear as small pin-pricks of nectar but by day 2, the rate of nectar secretion has increased to such an extent that not only is nectar visible as droplets upon the callus, but is also seen to collect as a relatively large drop beneath the distal end of the callus, where the latter joins the labellar surface. These drops of nectar continue to increase in size and by day 4 of anthesis, the volume of nectar has reached its maximum (approx. 28 µL) and has gathered at the base of the column-foot.
Pooled samples of nectar (n = 5 flowers) gave a refractometer reading of 66·5 % (w/w solution) and HPLC analysis showed that the nectar contained 62·36 % sucrose, 0·61 % glucose and 0·27 % fructose, as well as 0·76–1·53 mg cm−3 of free amino acids. The nectar fluoresced blue-green under UV light indicating that it probably contains terpenoids but no lipids were detected.
DISCUSSION
Nectary structure
Nectar is the principal reward offered by orchid flowers (Dressler, 1990) and its presence confers considerable evolutionary advantage (Johnson and Bond, 1997; Neiland and Wilcock, 1998, 2000; Johnson and Nilsson, 1999). Indeed, notwithstanding the energy expenditure associated with nectar formation, it would appear that the production of nectar is the most effective way of increasing the incidence of successful pollination, even when there is a paucity of potential pollinators (Neiland and Wilcock, 1998).
The nectaries of orchids are very diverse. The most obvious type of nectary is the nectar spur, which arises from one of the perianth segments. Such structures occur in Calanthe R. Br., Comparettia Poepp. & Endl., Disperis Sw., Satyrium Sw. and Tipularia Nutt. (Dressler, 1990), but in many moth-pollinated orchids (e.g. Aerangis Rchb.f., Angraecum Bory, Gymnadenia R. Br. and Mystacidium Lindl.), the spur nectary arises as an outgrowth from the proximal part of the labellum, whereas in Spiranthinae it may be formed from the lateral sepals, the labellum and the column-foot. In Pelexia Poit. ex Rich., the spur is partially free, in contrast to Sarcoglottis C. Presl., where it has fused to a greater extent with the ovary (Singer and Sazima, 1999). Likewise, in Hexisea imbricata (Lindl.) Rchb.f., the nectary is represented by a saccate spur formed by the fusion of the column and proximal part of the labellum (Stpiczyńska et al., 2005). A similar arrangement is said to occur in Systeloglossum Schltr. (Dressler, 1990). Indeed, in orchids that have a column-foot (e.g. Dendrobium Sw. and Scaphyglottis Poepp. & Endl.), the nectar spur may arise either solely from that structure or from both the column-foot and the base of the labellum. In many Laeliinae such as Brassavola R. Br., Epidendrum L. and Rhyncholaelia Schltr., the spur is less obvious (cuniculus), lies parallel to and is fused with the ovary, and is only evident when the flower is cut longitudinally (Dressler, 1990).
Many orchids possess shallow, superficial nectaries upon their labella [e.g. Listera R. Br., Stelis Sw., Pleurothallis R. Br., Cirrhopetalum Lindl. (Dressler, 1990), Bulbophyllum ipanemense Hoehne, B. involutum Borba, Semir & F. Barros, B. weddellii (Lindl.) Rchb.f. (Teixeira et al., 2004), Epipactis atropurpurea Raf. (Pais, 1987) and the entomophilous Maxillaria parviflora (Poepp. & Endl.) Garay (Singer and Koehler, 2004)]. In Beadlea dutraei (Schltr.) Garay (syn. Cyclopogon dutraei Schltr.), Pelexia bonariensis (Lindl.) Schltr. and Stenorrhynchos orchioides (Sw.) Rich. (syn. Sacoila lanceolata (Aubl.) Garay), the nectaries occur as two symmetric glands upon the adaxial labellar surface (Galetto et al., 1997). In Maxillaria coccinea, the nectary is represented by a small protuberance upon the ventral surface of the column (Stpiczyńska et al., 2004) and nectar has been observed to collect at the base of the column of M. pendens Pabst and M. rigida Barb. Rodr. (Singer and Koehler, 2004), although no protuberance was reported for these species.
By contrast, in Maxillaria anceps, nectar is secreted by the labellar callus. The latter consists of parenchyma delimited by a single-layered epidermis that contains modified stomata. The incidence of minute droplets of nectar upon the callus would appear to correspond with stomatal distribution. Indeed, the stomata have an affinity for very dilute aqueous solutions of neutral red and toluidine blue, thereby indicating that they are probably involved in secretion. A number of reports relating to the exudation of nectar by modified stomata occur in the literature. This phenomenon is known to occur in a number of families including Rosaceae (Radice and Galati, 2003; Weryszko-Chmielewska et al., 2003), Brassicaceae (Davis et al., 1998), Scrophulariaceae (Gaffal et al., 1998; Nepi et al., 2003), Myrtaceae (O'Brien et al., 1996; Davis 1997) and Fabaceae (Waddle and Lersten, 1973; Teuber et al., 1980; Davis and Gunning, 1991; Razem and Davis, 1999). Members of these families, especially Fabaceae, however, have a greater stomatal density than M. anceps but like the latter species, possess stomata whose apertures become almost completely covered by a cuticular layer. Nectar-secreting stomata tend to differ from typical stomata in that, like hydathodes, they are unable to close fully since all the free surfaces of the guard cells are cuticularized. Also, ridges of circumferentially arranged microfibrils occur along the outer (and sometimes the inner) walls, and an unidentified osmiophilic wall material is often present (Davis and Gunning, 1991). However, whereas the stomatal guard cells borne upon the callus of M. anceps retain their nuclei and cytoplasm (Fig. 4B), much like those of typical stomata, the guard cells of hydathodes lose their living contents early in their development (Esau, 1965; Harder et al., 1970). The presence of cytoplasmic contents within the guard cells, together with observations that the stomata are not associated with epithema (distinct groups of small, parenchymatous cells lacking chlorophyll), nor occur at the ends of xylem strands, compel us to classify them as stomata rather than hydathodes (Esau, 1965; Harder et al., 1970) and it would appear that nectar probably passes from the phloem to the adaxial stomata along an apoplastic and/or a symplastic route (Fahn, 2000, and references therein). Although it is possible that nectar crosses the outer tangential wall of the epidermal cells and is secreted through the cuticle onto the surface of the callus, this hypothesis would not satisfactorily explain the selective uptake of stain by the stomata nor the more or less regular distribution of nectar droplets upon the callus surface. Sparse stomata also occur upon the nectary of M. coccinea but, in contrast to those of M. anceps, these are not involved in the secretion of nectar. Instead, in M. coccinea, nectar is secreted via a distended, permeable cuticle.
The secretory cells of M. anceps, like those of M. coccinea (Stpiczyńska et al., 2004), are nucleated and contain abundant mitochondria. However, they differ from those of the presumed ornithophilous species M. coccinea and Hexisea imbricata (Stpiczyńska et al., 2004, 2005) in that they are parenchymatous not collenchymatous, presumably since they are not required to withstand contact with a hard beak. Like the secretory cells of M. coccinea, those of M. anceps contain plastids with few plastoglobuli and lamellae, and the cell walls of both species contain plasmodesmata. Although rough ER is common in M. anceps (as in M. coccinea), it is the smooth type that predominates, as in Hexisea imbricata (Stpiczyńska et al., 2005). Intravacuolar protein bodies are lacking in M. anceps and, instead, the vacuoles contain annular profiles. Dictyosomes and coated vesicles also occur within the secretory cells of M. anceps but not those of M. coccinea, and the osmiophilic globular bodies observed in the former species during the very early stages of anthesis become larger as anthesis progresses. A similar complement of organelles also occurs in unrelated orchids such as Limodorum abortivum (L.) Sw. (Pais and Figueiredo, 1994) and H. imbricata (Stpiczyńska et al., 2005). Osmiophilic bodies have also been recorded for the nectary cells of Bulbophyllum spp. (Teixeira et al., 2004) but their function is not fully understood.
A distinctive feature of the nectary cells of M. anceps is the presence of presumed terpenoids. This may explain the yellow-grey, globular bodies seen in fresh material under light microscopy and the osmiophilic globules observed using TEM. This class of substance has also been recorded for the labella of other species of Maxillaria such as M. cerifera Barb. Rodr. and M. friedrichsthalii Rchb.f. (Flach et al., 2004) and it is likely that these compounds are the source of the blue-green fluorescence observed when nectar droplets of M. anceps are examined using UV light. Nectar fluorescence under UV light has also been reported for Allium porrum L. and Prunus persica (L.) Batch together with many other non-orchidaceous species (Thorp et al., 1975; Peumans et al., 1997; Radice and Galati, 2003), and it is possible that this fluorescence, whether produced by the nectar or by the perianth, enables pollinators to locate and recognize rewards and to distinguish between reward-bearing and reward-less flowers (Thorp et al., 1975). Remarkably, Holtzmeier et al. (1998) have reported bicellular and multicellular glandular hairs similar to those in Fig. 5A upon the leaves and pseudobulbs of a number of species of Maxillaria, and Dell and McComb (1978) have shown that the exudate produced by similar hairs often contains terpenes as well as essential oils. Although it is not possible to be certain of their role, Levin (1973) and Wagner (1991) have suggested that the primary function of such glandular trichomes is the production and storage of compounds that discourage herbivory.
Nectar composition
The nectar-sugar concentration of M. anceps relative to other bee-pollinated species (Baker and Baker, 1983, 1990; Cruden et al., 1983; Wyatt, 1983) is high (>66 %). However, it is known that certain Melipona spp. (stingless bees or Meliponini) such as M. fasciata (syn. M. panamica) from Costa Rica (one of the countries in which M. anceps occurs) can cope with nectar of comparable (>60 %) concentrations, but usually take nectar of lower sugar concentrations (20–50 %) (Roubik and Buchmann, 1984; Biesmeijer et al., 1999; Roubik, 2000). This may be particularly significant since Meliponini are thought to be the main pollinators of Maxillaria spp. (Singer and Cocucci, 1999; Roubik, 2000; Singer and Koehler, 2004). Similarly, the nectar-sugar concentration of melittophilous Beadlea dutraei also approaches 60 % (Galetto et al., 1997; Galetto and Bernardello, 2003). It is, of course, possible that the high nectar-sugar values recorded may simply be due to the way that the nectar accumulates and/or evaporation and that the sugar concentration of newly secreted nectar is much lower. Even so, potential pollinators must be capable of utilizing such elevated sugar concentrations, regardless of how they arise.
HPLC analysis revealed that during anthesis, sucrose is the predominant nectar-sugar in M. anceps but that glucose and fructose (as well as free amino acids) are also present at much lower concentrations. This confirms the results of preliminary tests using glucose-sensitive test-sticks. Such tests performed on nectar from newly-opened flowers proved negative, indicating that at first, glucose is probably either present at extremely low concentrations or absent, and that it is gradually produced from sucrose by microbial or invertase activity. A number of studies have suggested that a relationship exists between the chemical composition of nectar and the type of pollinator. For example, the nectar of flowers pollinated by Hymenoptera and sunbirds tends to be hexose-dominant whereas sucrose predominates in the nectar of sphingophilous and hummingbird-pollinated species (Heinrich, 1975; Cruden et al., 1983; Wyatt, 1983; Baker and Baker, 1990; van der Cingel, 2001; Nicolson and Fleming, 2003). That such a relationship also occurs amongst the Orchidaceae has been established for Epipactis atropurpurea Raf., Limodorum abortivum (L.) Sw. (Pais et al., 1986) and Mystacidium venosum Harv. ex Rolfe (Luyt and Johnson, 2001). However, there are exceptions. For example, the nectar of the bee-pollinated orchids Beadlea dutraei (Schltr.) Garay and Pelexia bonariensis (Lindl.) Schltr. is sucrose-dominant (Galetto et al., 1997; Galetto and Bernardello, 2003). Indeed, recent investigations of floral nectar in the tribe Synningieae (Perret et al., 2001) and in species of Ipomoea L. (Galetto and Bernardello, 2004) have revealed that the relationship between nectar composition and the type of pollinator is not as simple nor as clear as was once supposed in that the nectar of most species studied to date is sucrose-dominant. In fact, it would appear that the composition of nectar is phylogenetically biased, irrespective of the type of pollinator (Percival, 1961; Jeffrey et al., 1970; Bernardello et al., 1999; van Wyk, 2002).
The nectary of M. anceps thus, exhibits a remarkable set of important features. It would appear that in this species, nectar is secreted via modified stomata found upon the labellar callus and, although this feature has been observed for a number of plant species, it is the only example hitherto reported for the genus Maxillaria and, as far as is known, for any orchid. Although this is the first detailed account of nectary structure in a presumed melittophilous species of Maxillaria, it is predicted, given the apparently polyphyletic nature and diverse morphology of the genus, that nectaries of other nectariferous, bee-pollinated orchids currently assigned to Maxillaria will also reflect this diversity. The nectar of M. anceps fluoresces under UV light indicating that it probably contains terpenoids. This may help potential pollinators to recognize the nectar as a source of food and help them locate it within the flower. Since the resinous, labellar secretions found in a number of nectarless Maxillaria spp. are also known to contain triterpenoids (Flach et al., 2004), this may support the hypothesis that during the course of evolution, resinous labellar secretions may have replaced nectar as the main reward (Davies et al., 2003a).
Finally, the nectar, like that of Beadlea and Pelexia (Galetto et al., 1997; Galetto and Bernardello, 2003), is sucrose-dominant and, notwithstanding the complexity of nectar composition–pollinator relationships, this strengthens the possibility that M. anceps is melittophilous. Furthermore, the high sugar concentration would suggest that, like the majority of entomophilous Maxillaria spp., M. anceps is perhaps pollinated by stingless bees—a claim that can only be confirmed or refuted by careful field studies.
Supplementary Material
Acknowledgments
K.L.D. is grateful to the Stanley Smith (UK) Trust for their generous grant and we thank the Friends of the City of Swansea Botanical Complex, UK for partly funding this work. We also acknowledge the help of Mgr Janusz Matusiewicz of CLA AR, Lublin, Poland for making electron microscopy facilities available to us, and Dr Jacek Pielecki of the Department of Food Technology and Storage, AR, Lublin for HPLC facilities.
LITERATURE CITED
- Baker HG, Baker I. 1983. A brief historical review of the chemistry of floral nectar. In: Bentley B, Elias T, eds. The biology of nectaries. New York: Columbia University Press, 126–152. [Google Scholar]
- Baker HG, Baker I. 1990. The predictive value of nectar chemistry to the recognition of pollinator types. Israel Journal of Botany 39: 157–166. [Google Scholar]
- Bernardello G, Galetto L, Forcone A. 1999. Floral nectar chemical composition of some species from Patagonia. II. Biochemical Systematics and Ecology 27: 779–790. [Google Scholar]
- Biesmeijer JC, Smeets MAJP, Richter JAP, Sommeijer MJ. 1999. Nectar foraging by two species of Melipona in Costa Rica: botanical and climatological influences on sugar concentration of bee-collected nectar. Apidologie 30: 43–55. [Google Scholar]
- van der Cingel NA. 2001.An atlas of orchid pollination: America, Africa, Asia and Australia. Rotterdam: A.A. Balkema. [Google Scholar]
- Cruden RW, Herman SH, Peterson S. 1983. Patterns of nectar production and plant-pollinator co-evolution. In: Bentley B, Elias T, eds. The biology of nectaries. New York: Columbia University Press, 80–125. [Google Scholar]
- Dafni A. 1992.Pollination ecology. Oxford: Oxford University Press, 154–156, 158–159. [Google Scholar]
- Davies KL. 1999. A preliminary survey of foliar anatomy in Maxillaria Lindleyana 14: 126–135. [Google Scholar]
- Davies KL, Turner MP. 2004. Morphology of floral papillae in Maxillaria Ruiz & Pav. (Orchidaceae). Annals of Botany 93: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Winters C. 1998. Ultrastructure of the labellar epidermis in selected Maxillaria species (Orchidaceae). Botanical Journal of the Linnean Society 126: 349–361. [Google Scholar]
- Davies KL, Winters C, Turner MP. 2000. Pseudopollen: its structure and development in Maxillaria (Orchidaceae). Annals of Botany 85: 887–895. [Google Scholar]
- Davies KL, Turner MP, Gregg A. 2003. Atypical pseudopollen-forming hairs in Maxillaria Ruiz & Pav. (Orchidaceae). Botanical Journal of the Linnean Society 143: 151–158. [Google Scholar]
- Davies KL, Turner MP, Gregg A. 2003. Lipoidal labellar secretions in Maxillaria Ruiz & Pav. (Orchidaceae). Annals of Botany 91: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AR. 1997. Influence of floral visitation on nectar-sugar composition and nectary surface changes in Eucalyptus Apidologie 28: 27–42. [Google Scholar]
- Davis AR, Gunning BES. 1991. The modified stomata of the floral nectary of Vicia faba L. 2. Stomatal number and distribution as selection criteria for breeding for high nectar sugar production. Acta Horticulturae 288: 329–334. [Google Scholar]
- Davis AR, Pylatiuk JD, Paradis JC, Low NH. 1998. Nectar-carbohydrate production and composition vary in relation to nectary anatomy and location within individual flowers of several species of Brassicaceae. Planta 205: 305–318. [DOI] [PubMed] [Google Scholar]
- Dell B, McComb JA. 1978. Plant resins—their formation, secretion and possible functions. Advances in Botanical Research 6: 276–316. [Google Scholar]
- Dressler RL. 1990.The orchids—natural history and classification. London: Havard University Press. [Google Scholar]
- Esau K. 1965.Plant anatomy, 2nd edn. New York: John Wiley & Sons, 158–167, 315–317. [Google Scholar]
- Fahn A. 2000. Structure and function of secretory cells. Advances in Botanical Research 31: 37–75. [Google Scholar]
- Flach A, Dondon RC, Singer RB, Koehler S, Amaral Maria do Carmo E, Marsaioli AJ. 2004. The chemistry of pollination in selected Brazilian Maxillariinae orchids: floral rewards and fragrance. Journal of Chemical Ecology 30: 1045–1056. [DOI] [PubMed] [Google Scholar]
- Gaffal KP, Heimler W, el-Gammal S. 1998. The floral nectary of Digitalis purpurea L., structure and nectar secretion. Annals of Botany 81: 251–262. [Google Scholar]
- Galetto L, Bernardello G. 2003. Nectar sugar composition in angiosperms from Chaco and Patagonia (Argentina): an animal visitor's matter? Plant Systematics and Evolution 238: 69–86. [Google Scholar]
- Galetto L, Bernardello G. 2004. Floral nectaries, nectar production dynamics and chemical composition in six Ipomoea species (Convolvulaceae) in relation to pollinators. Annals of Botany 94: 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galetto L, Bernardello G, Rivera G. 1997. Nectar, nectaries, flower visitors, and breeding system in five terrestrial Orchidaceae from Central Argentina. Journal of Plant Research 110: 393–403. [Google Scholar]
- Harder R, Schumacher W, Fribas F, von Denffer D. 1970.Strasburger's textbook of botany. London: Longman, 88, 112. [Google Scholar]
- Heinrich B. 1975. Energetics of pollination. Annual Review of Ecology and Systematics 6: 139–170. [Google Scholar]
- Holtzmeier MA, Stern WL, Judd WS. 1998. Comparative anatomy and systematics of Senghas's cushion species of Maxillaria (Orchidaceae). Botanical Journal of the Linnean Society 127: 43–82. [Google Scholar]
- Janse JM. 1886. Imitirte pollenkörner bei Maxillaria sp. Deutsche Botanische Gesellschaft Berichte 4: 277–283. [Google Scholar]
- Jeffrey DC, Arditti J, Koopowitz H. 1970. Sugar content in floral and extrafloral exudates of orchids: pollination, myrmecology and chemotaxonomy implication. New Phytologist 69: 187–195. [Google Scholar]
- Johnson SD, Bond WJ. 1997. Evidence for widespread pollen limitation of fruiting success in Cape wildflowers. Oecologia 109: 530–534. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Nilsson LA. 1999. Pollen carryover, geitonogamy and the evolution of deception in orchids. Ecology 80: 2607–2619. [Google Scholar]
- Levin DA. 1973. The role of trichomes in plant defence. Quarterly Review of Biology 48: 3–15. [Google Scholar]
- Luyt R, Johnson SD. 2001. Hawkmoth pollination of the African epiphytic orchid Mystacidium venosum, with special reference to flower and pollen longevity. Plant Systematics and Evolution 228: 49–62. [Google Scholar]
- Matusiewicz J, Stpiczyńska M, Davies KL. 2004. Pseudopollen in the flowers of Maxillaria lepidota Lindl. (Orchidaceae). In: Polish Botanical Society, eds. Proceedings of the 53rd Meeting of the Polish Botanical Society, Toruń-Bydgoszcz, p. 14. [Google Scholar]
- Neiland MR, Wilcock CC. 1998. Fruit set, nectar reward, and rarity in the Orchidaceae. American Journal of Botany 85: 1657–1671. [PubMed] [Google Scholar]
- Neiland MR, Wilcock CC. 2000. Effects of pollinator behaviour on pollination of nectarless orchids: floral mimicry and interspecific hybridisation. In: Wilson KL, Morrison DA, eds. Monocots: systematics and evolution. Melbourne: CSIRO, 318–326. [Google Scholar]
- Nepi M, Pacini E, Nenci C, Collavoli E, Franchi GG. 2003. Variability of nectar production and composition in Linaria vulgaris (L.) Mill. (Scrophulariaceae). Plant Systematics and Evolution 238: 109–118. [Google Scholar]
- Nicolson SW, Fleming PA. 2003. Nectar as food for birds: the physiological consequences of drinking dilute sugar solutions. Plant Systematics and Evolution 238: 139–153. [Google Scholar]
- O'Brien SP, Loveys BR, Grant WJ. 1996. Ultrastructure and function of floral nectaries of Chamelaucium uncinatum (Myrtaceae). Annals of Botany 78: 189–196. [Google Scholar]
- Pais MS. 1987. Ultrastructure des nectaries floraux d'Epipactis atropurpurea et production du nectar. Annales des Sciences Naturel et Botanique 8: 17–28. [Google Scholar]
- Pais MS, Figueiredo ACS. 1994. Floral nectaries from Limodorum abortivum (L.) Sw. and Epipactis atropurpurea Rafin. (Orchidaceae): ultrastructural changes in plastids during the secretory process. Apidologie 25: 615–626. [Google Scholar]
- Pais MS, Chaves das Neves HJ, Vasconcelos M. 1986. Amino acid and sugar content of the nectar exudate from Limodorum abortivum (Orchidaceae). Comparison with Epipactis atropurpurea nectar composition. Apidologie 17: 125–136. [Google Scholar]
- Percival M. 1961. Types of nectar in angiosperms. New Phytologist 60: 235–281. [Google Scholar]
- Perret M, Chautems A, Spichiger R, Peixoto M, Savalainen V. 2001. Nectar sugar composition in relation to pollination syndromes in Synningieae (Gesneriaceae). Annals of Botany 87: 267–273. [DOI] [PubMed] [Google Scholar]
- Peumans WJ, Smets K, Van Nerum K, Van Leuven F, Van Damme EJM. 1997. Lectin and alliinase are the predominant proteins in nectar from leek (Allium porrum L.) flowers. Planta 201: 298–302. [DOI] [PubMed] [Google Scholar]
- van der Pijl L, Dodson CH. 1969.Orchid flowers: their pollination and evolution. Coral Gables, FL: University of Miami Press. [Google Scholar]
- Porsch O. 1905. Beiträge zur ‘histologischen’ Blütenbiologie I. Oesterreichische Botanische Zeitschrift 55: 165–173, 227–235, 253–260. [Google Scholar]
- Radice S, Galati BG. 2003. Floral nectary ultrastructure of Prunus persica (L.) Batch cv. Forastero (Newcomer), an Argentine peach. Plant Systematics and Evolution 238: 23–32. [Google Scholar]
- Razem FA, Davis AR. 1999. Anatomical and ultrastructural changes of the floral nectary of Pisum sativum L. during flower development. Protoplasma 206: 57–72. [Google Scholar]
- Roshchina VV. 2003. Autofluorescence of plant secreting cells as a biosensor and bioindicator reaction. Journal of Fluorescence 13: 403–420. [Google Scholar]
- Rossomando EF. 1998.High performance liquid chromatography in enzymatic analysis. New York: John Wiley & Sons. [DOI] [PubMed] [Google Scholar]
- Roubik DW. 2000. Deceptive orchids with Meliponini as pollinators. Plant Systematics and Evolution 222: 271–279. [Google Scholar]
- Roubik DW, Buchmann SL. 1984. Nectar selection by Melipona and Apis mellifera and the ecology of nectar intake by bee colonies in a tropical forest. Oecologia 61: 1–10. [DOI] [PubMed] [Google Scholar]
- Senghas K. 1993. Subtribus Maxillariinae. In: Breiger FG, Maatsch, R, Senghas, K, eds. Rudolph Schlechter: Die Orchideen Berlin: Blackwell Wissenschafts-Verlag, 28: 1727–1776. [Google Scholar]
- Singer RB, Cocucci AA. 1999. Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana 14: 47–56. [Google Scholar]
- Singer RB, Koehler S. 2003. Toward a phylogeny of Maxillariinae orchids: multidisciplinary studies with emphasis on Brazilian species. Lankesteriana 7: 57–60. [Google Scholar]
- Singer RB, Koehler S. 2004. Pollinarium morphology and floral rewards in Brazilian Maxillariinae (Orchidaceae). Annals of Botany 93: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RB, Sazima M. 1999. The pollination mechanism in the ‘Pelexia alliance’ (Orchidaceae: Spiranthinae). Botanical Journal of Linnean Society 131: 249–262. [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. 2004. Nectary structure and nectar secretion in Maxillaria coccinea (Jacq.) L.O. Williams ex Hodge (Orchidaceae). Annals of Botany 93: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. 2005. Comparative account of nectary structure in Hexisea imbricata (Lindl.) Rchb.f. (Orchidaceae). Annals of Botany 95: 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira SP, Borba EL, Semir J. 2004. Lip anatomy and its implications for the pollination mechanism of Bulbophyllum species (Orchidaceae). Annals of Botany 93: 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber LR, Albersten MC, Barnes DK, Heichel GH. 1980. Structure of floral nectaries of alfalfa (Medicago sativa L.) in relation to nectar production. American Journal of Botany 67: 433–439. [Google Scholar]
- Thorp RW, Briggs DL, Esters JR, Erickson EH. 1975. Nectar fluorescence under ultraviolet irradiation. Science 189: 476–478. [DOI] [PubMed] [Google Scholar]
- Waddle RM, Lersten NR. 1973. Morphology of discoid floral nectaries in Leguminosae, especially tribe Phaseoleae (Papilionoideae). Phytomorphology 23: 152–161. [Google Scholar]
- Wagner GJ. 1991. Secreting glandular trichomes: more than just hairs. Plant Physiology 96: 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weryszko-Chmielewska E, Masierowska ML, Konarska A. 2003. Characteristics of floral nectaries and nectar in two species of Crataegus (Rosaceae). Plant Systematics and Evolution 238: 33–42. [Google Scholar]
- Whitten WM, Williams NH, Chase MW. 2000. Subtribal and generic relationships of Maxillarieae (Orchidaceae) with emphasis on Stanhopeinae: combined molecular evidence. American Journal of Botany 87: 1842–1856. [PubMed] [Google Scholar]
- Wyatt R. 1983. Pollinator–plant interactions and the evolution of breeding systems, In: Real L, ed. Pollination biology. Orlando, FL: Academic Press, 51–96. [Google Scholar]
- van Wyk BE. 2002. The systematic value of nectar sugar composition in higher plants. In: University of Siena, Department of Environmental Sciences ‘G. Sarfatti’ and I.C.P.B.R., eds. Proceedings of an International Conference, Montalcino, Italy. Nectar and Nectary: From Biology to Biotechnology. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




