Abstract
• Background The plant cuticle is an extracellular lipophilic biopolymer covering leaf and fruit surfaces. Its main function is the protection of land-living plants from uncontrolled water loss. In the past, the permeability of the cuticle to water and to non-ionic lipophilic molecules (pesticides, herbicides and other xenobiotics) was studied intensively, whereas cuticular penetration of polar ionic compounds was rarely investigated.
• Recent Progress Recent work measuring cuticular penetration of inorganic and organic ions is presented; the effects of molecular size of ions, temperature, wax extraction, humidity and plasticizers strongly support the conclusion that ions penetrate cuticles via water-filled pores. The cuticle covering stomata and trichomes forms the preferential site of ion penetration. This indicates that cuticles possess a pronounced lateral heterogeneity: the largest fraction of the cuticle surface is covered by the lipophilic domains of cutin and wax, but to a certain extent polar domains are also present in the cuticle, which form preferential sites of penetration for polar compounds.
• The Future The chemical nature of these polar domains awaits detailed characterization, which will be of major importance in agriculture and green biotechnology, since polar paths of diffusion represent the most important transport routes for foliar-applied nutrients. Furthermore, many compounds acting as inducers of gene expression in transgenic plants are ionic and need to penetrate the cuticle via polar paths in order to be active.
Keywords: Cuticle, diffusion, foliar nutrition, leaf surface, permeability, polar pores, size selectivity, stomata, trichomes, wax
INTRODUCTION
About 500 million years ago plants conquered the land. As a prerequisite they developed the two structural cell wall polymers, lignin and cutin. The aromatic polymer lignin is incorporated into the secondary plant cell wall enhancing its mechanical stability, whereas the lipophilic cuticle is deposited as a two-dimensional polymer membrane on the plant surface covering all above-ground primary plant organs. The plant cuticle membrane is composed of the depolymerizable biopolymer cutin (Kolattukudy, 2001), the non-depolymerizable polymer cutan (Tegelaar et al., 1993) and associated soluble cuticular lipids also called cuticular waxes (Jenks and Ashworth, 2003). Waxes are predominantly linear, long-chain, aliphatic molecules with different functionalities (alkanes, alcohols, aldehydes, acids, etc.). They are solid, partially crystalline aggregates at room temperature (Reynhardt, 1997). Waxes can be found in the outer parts of the cutin polymer (intra-cuticular waxes) and on its surface (epicuticular waxes). Since the permeability of the cuticle to water and to organic compounds increases upon wax extraction by factors between 10 and 1000, it must be concluded that the cuticular transport barrier is largely formed by these cuticular waxes (Schönherr, 1976).
In the past decades many studies dealing with cuticular permeability have been conducted. The main function of the plant cuticle is the protection of plants from uncontrolled water loss, and thus the water-permeability of plant cuticles is of major ecological interest (Riederer and Schreiber, 2001). In agriculture, plant cuticles often represent the major barrier, which has to be overcome, when chemicals are sprayed on to leaf surfaces. Therefore, permeability of the cuticle to herbicides and pesticides was also studied intensively (Schönherr and Baur, 1994). Water and many synthetic compounds (pesticides, herbicides and other xenobiotics) are non-ionic and, with the exception of water, many of these molecules are lipophilic. Thus, there is now a very well-established theory as well as a series of experimental techniques available to predict and measure the permeability of the cuticle to water and to lipophilic molecules (Schönherr and Riederer, 1989; Niederl et al., 1998). In these models, plant cuticles are normally treated as solubility/mobility barriers. The permeability of the cuticle to water and to lipophilic molecules increases with mobility (diffusion coefficients) and solubility (partition coefficients) of these compounds within the transport-limiting barrier of the cuticles.
Significantly less is know about the permeability of the cuticle to ionic compounds. However, this is also of major significance in agriculture since, in foliar nutrition, elements such as calcium or nitrogen are sprayed as ions on to leaf surfaces and in order to be effective, these ions have to penetrate the lipophilic cuticle. Recently, significant progress has been achieved in measuring cuticular penetration quantitatively and setting up testable hypotheses about possible mechanisms of foliar uptake of polar ionic compounds (Schönherr, 2000, 2001, 2002; Schönherr and Schreiber, 2004; Schlegel et al., 2005). In the following, results on the permeability of the cuticle to non-ionic and ionic species will be described and compared with each other, and a model postulating two different routes of these physicochemically very different types of compound across the plant cuticle will be presented.
THE LIPOPHILIC PATH OF DIFFUSION
Experimentally as well as theoretically well-established models of the permeability of plant cuticles to non-ionic lipophilic compounds are available (Schönherr and Riederer, 1989). This approach treats the plant cuticle as a homogeneous mobility/solubility membrane, and two parameters (lipophilicity and mobility) are basically needed to describe cuticular permeability to non-ionic organic substances. Mobility describes the diffusion of the penetrating compounds across the transport-limiting barrier of the cuticle. This parameter strongly depends on the size of the diffusing molecules (Baur et al., 1997). Lipophilicity describes the solubility of the penetrating compounds within the transport-limiting barrier of the plant cuticle and it is normally described as the partition coefficient between the plant cuticle or the wax phase of the cuticle and the adjacent aqueous phase (Schönherr and Riederer, 1989).
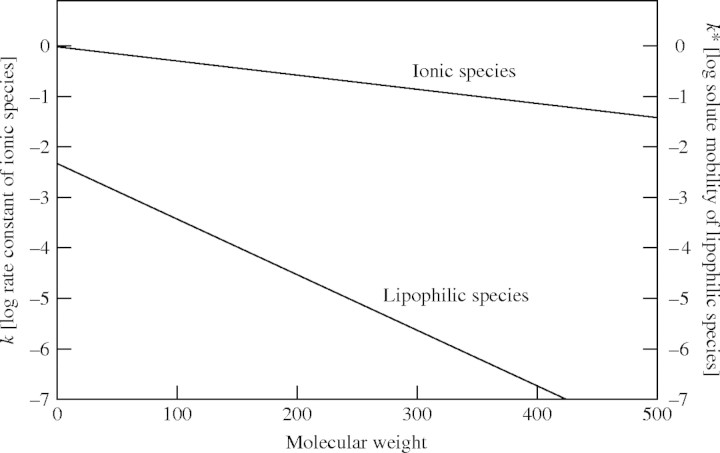
In a series of experiments transport of various lipophilic compounds across the cuticles of different plant species was measured and the effects of plasticizers, temperature and wax extraction on cuticular permeability to these compounds were determined (Schönherr and Riederer, 1989; Baur et al., 1997; Buchholz and Schönherr, 2000). The basic idea behind these experiments was to improve our knowledge of the foliar uptake of lipophilic, non-ionized substances (pesticides, herbicides and other xenobiotics). It was shown that mobilities of organic substances in the transport-limiting barrier of the plant cuticle had a pronounced size selectivity (Buchholz et al., 1998). A 4-fold increase in molecular weight resulted in a decrease of the mobility by a factor of >1000 (Fig. 1). This pronounced size selectivity indicates that lipophilic compounds must diffuse across lipophilic wax and cutin domains of the cuticle, forming highly ordered, solid and partially crystalline aggregates at room temperature (Riederer and Schreiber, 1995). Mobility in these domains can be increased significantly by the addition of lipophilic accelerators, which themselves are lipophilic compounds. They dissolve in cutin and wax domains and act as plasticizers by decreasing the barrier properties within the transport-limiting barrier of the cuticle (Buchholz and Schönherr, 2000). Furthermore, an increase in temperature and the extraction of cuticular waxes largely forming the transport-limiting barrier of the cuticle strongly increased cuticular permeability to lipophilic molecules (Schönherr and Riederer, 1989; Baur et al., 1997; Schreiber, 2002).
Fig. 1.
Correlation between the molecular weights of solutes and either k [= log of the rate constant (h−1) for penetration] for ionic species across isolated poplar (Populus canescens) cuticles (experimental data from Schönherr and Schreiber, 2004), or k* [= log of solute mobility (h−1) in poplar cuticles] for lipophilic species (experimental data from Buchholz et al., 1998).
EFFECT OF HUMIDITY ON CUTICULAR TRANSPIRATION
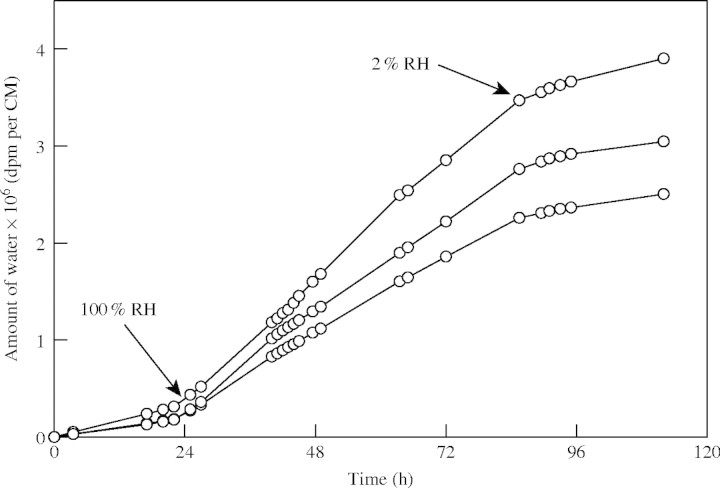
Water, as a small and polar but uncharged molecule, will diffuse across the plant cuticle using lipophilic cutin and wax domains as the transport path, where it will have a low solubility but a high mobility. It was shown that cuticular transpiration is highly correlated with cuticular permeability to lipophilic molecules (Niederl et al., 1998; Schreiber, 2002). Furthermore, permeability to water is affected in the same way by accelerators (Riederer and Schönherr, 1990), wax extraction (Riederer and Schreiber, 2001) and an increase in temperature (Schreiber, 2001), as is permeability to lipophilic molecules, which indicates that water largely uses the same transport paths across the cuticle. However, it was also shown that cuticular transpiration was significantly affected by humidity (Schreiber et al., 2001). When humidity at the outer side of the cuticle was increased from 0 to 100 %, cuticular transpiration was increased by a factor of 2–3 (Fig. 2). This indicates that water molecules can be absorbed by the lipophilic cuticle and cause swelling, which in turn leads to an increase of the cuticular transpiration.
Fig. 2.
Effect of humidity on rates of cuticular transpiration of isolated Prunus laurocerasus cuticles. Arrows indicate changes in the relative humidity from 2 % to 100 % and back to 2 %. CM = isolated cuticular membrane. Tritiated water (3H2O) was added as donor to the inner side of the CM mounted in transpiration chambers, and 3H2O that had diffused across the CM was collected in scintillation vials fixed to the outer side of the transpiration chambers, as described in detail by Schreiber et al. (2001).
If the plant cuticle were in fact a homogeneous mobility/solubility membrane (a model that can be used to analyse and describe cuticular permeability to lipophilic molecules), this effect of humidity should not be observable. This obvious contradiction can only be solved if it is postulated that there must be a lateral heterogeneity in cuticle structure and function. Besides lipophilic domains forming the largest fraction of the cuticular membrane, there are also polar domains in the transport-limiting barrier of the cuticle, which are sensitive to the absorption of water. With increasing humidity, increasing amounts of water are absorbed by these polar domains and, as a consequence, cuticular permeability increases.
THE POLAR PATH OF DIFFUSION
In contrast to large lipophilic molecules and small polar but still uncharged molecules like water, ionic compounds (mostly Ca2+ salts and glyphosate as an organic but charged molecules) have only recently been extensively analysed for cuticular permeability (Schönherr, 2000, 2001, 2002; Schönherr and Luber, 2001; Schönherr and Schreiber, 2004; Schlegel et al., 2005). In these experiments it was convincingly shown that charged molecules in fact can diffuse across isolated cuticles. Since charged molecules carry hydration shells (Stein, 1967), which cannot be shed, they will not be soluble in the lipophilic cutin and wax domains of the cuticles. Consequently, it must be concluded that charged compounds can cross isolated cuticles using alternative routes of diffusion, and it is postulated that charged molecules do so via aqueous polar pores traversing cuticular membranes.
From a series of different experiments there is very good evidence that these postulated polar paths of diffusion across cuticles must exist. Quite differently from water and lipophilic substances, inorganic ions and charged organic molecules penetrate isolated cuticular membranes independently of temperature (Schönherr, 2001; Schönherr and Luber, 2001) and plasticizers (Schönherr, 2000) and only weakly affected by wax extraction (Schönherr, 2000). However, penetration of ions, like that of water, was affected by humidity (Schönherr, 2000, 2001, 2002). Furthermore, size selectivity for the penetration of Ca2+ salts across isolated cuticles was significantly less pronounced than size selectivity for that of lipophilic molecules. A 4-fold increase in molecular weight resulted in a 5-fold decrease in rate of cuticular penetration of Ca2+ salts for Populus cuticular membranes (Fig. 1) and only a 2-fold decrease for Vicia faba leaves (Schlegel et al., 2005), whereas size selectivity of lipophilic molecules was three orders of magnitude higher in this range of molecular weights (Fig. 1). This very different behaviour of lipophilic and ionic compounds strongly suggests that these two groups of compounds (of physicochemically very different nature) penetrate plant cuticles via alternative paths. It must be concluded that ionic compounds use aqueous polar paths of diffusion, whereas lipophilic molecules diffuse along the lipophilic wax and cutin domains. Water, as a small but uncharged molecule, can use both paths.
The nature of the lipophilic paths across cuticles must be formed by the lipophilic cutin and wax domains. However, about the structure of the postulated polar paths, described here as polar aqueous pores, nothing is known at the moment, although a reasonable hypothesis can be formulated. The polar domains forming the postulated polar path of diffusion and those sites of the lipophilic cuticle sensitive to humidity could be formed by polar functional groups of cutin monomers. To a large extent, cutin is a polyester of esterified hydroxy-fatty acids. Polar domains within the lipophilic cutin polymer could be formed by non-esterified free carboxy and hydroxy groups of cutin monomers. This is supported by recent experiments showing that the effect of humidity on cuticular transpiration was reduced by about 50 % after methylation of carboxy groups in plant cuticles (Schreiber et al., 2001). Alternatively, polar transport paths within the lipophilic plant cuticle could be formed by carbohydrates extending from the outer epidermal cell walls into the cutin polymer and possibly to the outer surface as observed by Wattendorff and Holloway (1984). It is known that plant cuticles contain up to 20 % carbohydrates (Schreiber and Schönherr, 1990).
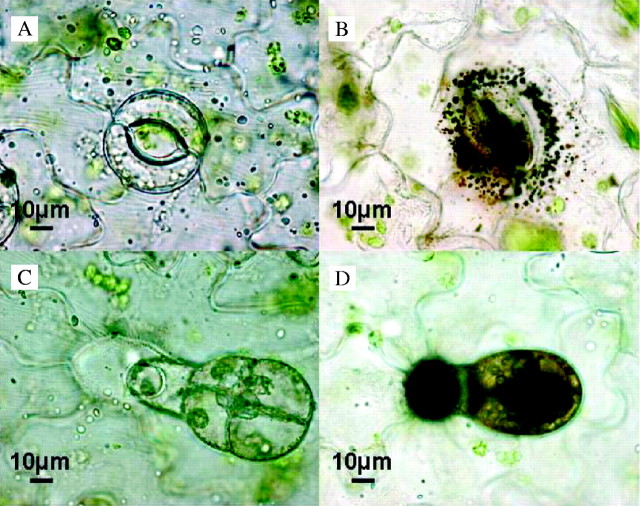
An alternative approach can be used to visualize the locations of these preferentially polar paths of diffusion within the leaf surface area. Ions like Ag+, forming visible black deposits after precipitation in the apoplast or the cytoplasm after having penetrated the cuticle, form good indicators for the localization of polar paths of diffusion within cuticles (Lord et al., 1979). This has already been shown in a series of experiments, in which it was observed that the cuticle covering stomatal cells obviously has a higher permeability to polar, ionized salts, like silver nitrate (Franke, 1964; Green and Bukovac, 1977; Schlegel and Schönherr, 2002). By this approach with Vicia faba leaves, it is evident that, in addition to the stomata, glandular trichomes themselves and the base of these trichomes can be identified as sites of polar paths of diffusion (Fig. 3). Thus, now good quantitative evidence from studies on size selectivity and good qualitative evidence from silver nitrate staining strongly support the view that there is a pronounced lateral heterogeneity in cutin structure and permeability. Owing to the preferential selection of lipophilic model compounds, these polar paths of diffusion have not been seen in many recent studies on cuticular permeability.
Fig. 3.
Light microscopic investigation of silver (Ag) deposits in stomata and trichomes of Vicia faba leaves after treatment with AgNO3. (A) Stoma of an untreated leaf surface. (B) Stoma after AgNO3 treatment. Characteristic silver deposits on the stoma and surrounding the guard cells are visible. (C) Trichome on an untreated leaf surface. (D) Trichome after AgNO3 treatment. Characteristic silver deposits in the base and the head of the trichome are visible.
FINAL CONCLUSIONS AND FUTURE APPROACHES
Although there is now good new evidence for the old hypothesis that there are polar paths of diffusion across plant cuticles, the chemical nature of these polar paths still remains unsolved. Some hypotheses about the nature of polar domains in plant cuticles have been raised, but future work on the permeability of plant cuticles will be needed to solve this question. A successful approach could be by chemical analysis trying to identify the exact chemical nature of these polar domains. Furthermore, it is very important to solve the question of to what extent polar paths of diffusion are characteristic of the cuticles of all plant species. Polar paths of diffusion became evident during work on cuticles of poplar (Populus canescens) and bean leaves (Vicia faba). However, in the past, cuticles isolated from other plants such as Citrus aurantium, ivy (Hedera helix) and Prunus laurocerasus had been used and the experimental data obtained did not indicate the presence of polar paths of diffusion.
Besides the academic interest of analysing the chemical nature of this pronounced lateral heterogeneity of pant cuticles, the fact that polar transport paths of diffusion across the cuticle can be characterized quantitatively should be most interesting for certain applications. Foliar nutrition is fully dependent on the uptake of ions via these polar pores in the cuticles. Furthermore, ionic herbicides and growth regulators are sprayed on to leaf surfaces and, in order to improve their uptake into the leaf, polar paths of diffusion will form preferential sites of uptake. The same argument accounts for promoters used to induce gene expression in transgenic plants. Potential promoters are chemically highly diverse (Gatz and Lenk, 1998) and those which are polar or charged will have to diffuse via polar pores in the cuticle. On the other side, these polar paths of diffusion would also form cuticular sites through which polar nutrients from the apoplast (such as sugars, ions and amino acids) are leaching to the leaf surface. These compounds often form the only source of nutrients for epiphyllic micro-organisms (Lindow and Leveau, 2002) and thus amounts and rates diffusing across these polar pores will determine epiphyllic growth and survival of micro-organisms.
Acknowledgments
The author gratefully acknowledges financial support by the DFG. This paper is dedicated to Jörg Schönherr on the occasion of his 65th birthday.
LITERATURE CITED
- Baur P, Buchholz A, Schönherr J. 1997. Diffusion in plant cuticles as affected by temperature and size of organic solutes: similarity and diversity among species. Plant, Cell and Environment 20: 982–994. [Google Scholar]
- Buchholz A, Baur P, Schönherr J. 1998. Differences among plant species in cuticular permeabilities and solute mobilities are not caused by differential selectivities. Planta 206: 322–328. [Google Scholar]
- Buchholz A, Schönherr J. 2000. Thermodynamic analysis of diffusion of non-electrolytes across plant cuticles in the presence and absence of the plasticiser tributyl phosphate. Planta 212: 103–111. [DOI] [PubMed] [Google Scholar]
- Franke W. 1964. Role of guard cells in foliar absorption. Nature 202: 1236–1237. [Google Scholar]
- Gatz C, Lenk I. 1998. Promoters that respond to chemical inducers. Trends in Plant Science 3: 152–358. [Google Scholar]
- Green DW, Bukovac MJ. 1977. Foliar penetration of naphthaleneacetic acid: enhancement by light and role of stomata. American Journal of Botany 64: 96–101. [Google Scholar]
- Jenks MA, Ashworth EN. 2003. Plant epicuticular waxes: function, production, and genetics. Horticultural Reviews 23: 1–68. [Google Scholar]
- Kolattukudy PE. 2001. Cutin from plants. In: Doi Y, Steinbüchel A, eds. Biopolymers. Vol. 3a. Polyesters I—Biological systems and biotechnological production. Weinheim: Wiley-VCH, 1–40. [Google Scholar]
- Lindow SE, Leveau JH. 2002. Phyllosphere microbiology. Current Opinion in Biotechnology 13: 238–243. [DOI] [PubMed] [Google Scholar]
- Lord WG, Greene DW, Emino ER. 1979. Absorption of silver nitrate and lead nitrate into leaves of McIntosh apple. Canadian Journal of Plant Science 59: 137–142. [Google Scholar]
- Niederl S, Kirsch T, Riederer M, Schreiber L. 1998. Co-permeability of 3H-labelled water and 14C-labelled organic acids across isolated plant cuticles: investigating cuticular paths of diffusion and predicting cuticular transpiration. Plant Physiology 116: 117–123. [Google Scholar]
- Reynhardt EC. 1997. The role of hydrogen bonding in the cuticular wax of Hordeum vulgare L. European Biophysics Journal 26: 195–201. [Google Scholar]
- Riederer M, Schönherr J. 1990. Effects of surfactants on water permeability of isolated plant cuticles and on the composition of their cuticular waxes. Pesticide Science 29: 85–94. [Google Scholar]
- Riederer M, Schreiber L. 1995. Waxes: transport barriers of plant cuticles. In: Hamilton RJ, ed. Waxes: chemistry, molecular biology and functions. Dundee: The Oily Press, 131–156. [Google Scholar]
- Riederer M, Schreiber L. 2001. Protecting against water loss: analysis of the barrier properties of plant cuticles. Journal of Experimental Botany 52: 2023–2032. [DOI] [PubMed] [Google Scholar]
- Schlegel TK, Schönherr J. 2002. Selective permeability of cuticles over stomata and trichomes to calcium chloride. Acta Horticulturae 549: 91–96. [Google Scholar]
- Schlegel TK, Schönherr J, Schreiber L. 2004. Size selectivity of aqueous pores in stomatous cuticles of Vicia faba leaves. Planta 10.1007/s00425-005-1480-1. [DOI] [PubMed] [Google Scholar]
- Schönherr J. 1976. Water permeability of isolated cuticular membranes: the effect of cuticular waxes on diffusion of water. Planta 131: 159–164. [DOI] [PubMed] [Google Scholar]
- Schönherr J. 2000. Calcium chloride penetrates plant cuticles via aqueous pores. Planta 212: 112–118. [DOI] [PubMed] [Google Scholar]
- Schönherr J. 2001. Cuticular penetration of calcium salts: effects of humidity, anions and adjuvants. Journal of Plant Nutrition and Soil Science 164: 225–231. [Google Scholar]
- Schönherr J. 2002. A mechanistic analysis of penetration of glyphosate salts across astomatous cuticular membranes. Pest Management Science 58: 343–351. [DOI] [PubMed] [Google Scholar]
- Schönherr J, Baur P. 1994. Modelling penetration of plant cuticles by crop protecting agents (CPA) and effects of adjuvants on rates of penetration. Pesticide Science 42: 185–208. [Google Scholar]
- Schönherr J, Luber M. 2001. Cuticular penetration of potassium salts: effects of humidity, anions and temperature. Plant and Soil 236: 117–122. [Google Scholar]
- Schönherr J, Riederer M. 1989. Foliar penetration and accumulation of organic chemicals in plant cuticles. Reviews of Environmental Contamination and Toxicology 108: 1–70. [Google Scholar]
- Schönherr J, Schreiber L. 2004. Size selectivity of aqueous pores in astomatous cuticular membranes isolated from Populus canescens (Aiton) Sm leaves. Planta 219: 405–411. [DOI] [PubMed] [Google Scholar]
- Schreiber L. 2001. Effect of temperature on cuticular transpiration of isolated cuticular membranes and intact leaf disks. Journal of Experimental Botany 52: 1893–1900. [DOI] [PubMed] [Google Scholar]
- Schreiber L. 2002. Copermeability of 3H-labelled water and 14C-labelled organic acids across isolated Prunus laurocerasus cuticles: effect of temperature on cuticular paths of diffusion. Plant, Cell and Environment 25: 1087–1094. [Google Scholar]
- Schreiber L, Schönherr J. 1990. Phase transitions and thermal expansion coefficients of plant cuticles: the effects of temperature on structure and function. Planta 182: 186–193. [DOI] [PubMed] [Google Scholar]
- Schreiber L, Skrabs M, Hartmann K, Diamantopoulos P, Simanova E, Santrucek J. 2001. Effect of humidity on cuticular transpiration of isolated cuticular membranes and leaf disks. Planta 214: 274–282. [DOI] [PubMed] [Google Scholar]
- Stein WD. 1967.The movement of molecules across cell membranes. New York, London: Academic Press. [Google Scholar]
- Tegelaar EW, Wattendorff J, de Leeuw JW. 1993. Possible effects of chemical heterogeneity in higher land plant cuticles on the preservation of its ultrastructure upon fossilization. Review of Palaeobotany and Palynology 77: 149–170. [Google Scholar]
- Wattendorff J, Holloway PJ. 1984. Periclinal penetration of potassium permanganate into mature cuticular membranes of Agave and Clivia leaves: new implications for plant cuticle development. Planta 161: 1–11. [DOI] [PubMed] [Google Scholar]