Abstract
• Backgroud and Aims Berchemiella wilsonii var. pubipetiolata (Rhamnaceae) is distributed in fragmented habitat patches in eastern China. It is highly endangered because of severe disturbance by anthropogenic activities. Information on genetic variation and structure is critical for developing successful conservation strategies for this species.
• Methods Allozyme variation of population genetic diversity and structure was investigated for a total of 98 individuals sampled from four extant populations using isoelectric focusing in thin-layer polyacrylamide slab gels.
• Key Results Based on 20 loci scored from the nine enzymes examined, a high genetic diversity was detected at both the species and population level, while there was a loss of low frequency alleles (<0·1) in all populations. Most loci showed deviation from Hardy–Weinberg equilibrium due to excess of heterozygotes in all populations, suggesting that selection for heterozygotes has occurred in this species. The genetic diversity was mainly found within populations with a moderate genetic differentiation (FST = 0·13), but the two geographically discontinuous population groups showed significant differences, with F-statistic values of 0·078 for the Zhejiang populations and 0·014 for the Anhui populations, respectively.
• Conclusions It appears most likely that this species has experienced a recent decrease in population size, and genetic drift in small populations has resulted in a loss of alleles occurring at low frequency. The differentiation into two population groups reflects a population genetic consequence that has been influenced by the different land-use in the two regions. Some conservation concerns are discussed together with possible strategies for implementing in situ and ex situ conservation.
Keywords: Berchemiella wilsonii var. pubipetiolata, allozyme, habitat fragmentation, genetic diversity, heterozygote excess, conservation genetics
INTRODUCTION
Habitat loss and fragmentation have become great concerns to conservation geneticists as anthropogenic activities and environmental deterioration have broken large, continuous populations into small and isolated ones. Populations in fragmented habitats are considered more vulnerable to demographic, environmental and genetic stochasticity, and therefore face a higher risk of local extinction (Boyce, 1992; Tilman et al., 1994, Lande, 1999).
Experimental and field investigations have demonstrated that fragmented populations may lose allelic richness or genetic diversity, and have increased population differentiation due to genetic drift and inbreeding depression (Buza et al., 2000; Shea and Furnier, 2002; Tomimatsu and Ohara, 2003). However, many studies based on allozymes have shown that even narrowly restricted species may maintain high levels of diversity (Ranker, 1994; Lewis and Crawford, 1995; Sharma et al., 2003). Young et al. (1993) found increased levels of gene flow among fragmented populations. Britten (1996) suggested that isolated populations might exhibit high heterozygosity due to strong selection pressures. Such a genetic structure was also detected in populations of a narrowly endemic plant established before and after fragmentation (Gonzalez-Astorga and Nunez-Farfan, 2001). The genetic consequences caused by habitat fragmentation are not yet well understood and further investigation is needed (Young et al., 1996). In the present study, an attempt was made to examine the impact of habitat fragmentation on the genetic diversity and population structure of Berchemiella wilsonii var. pubipetiolata, an endangered plant with a narrow distribution in eastern China. Such population genetic information is a prerequisite to understanding the species survival possibility in the short-term, so that an effective conservation strategy for long-term survival can be formulated and implemented.
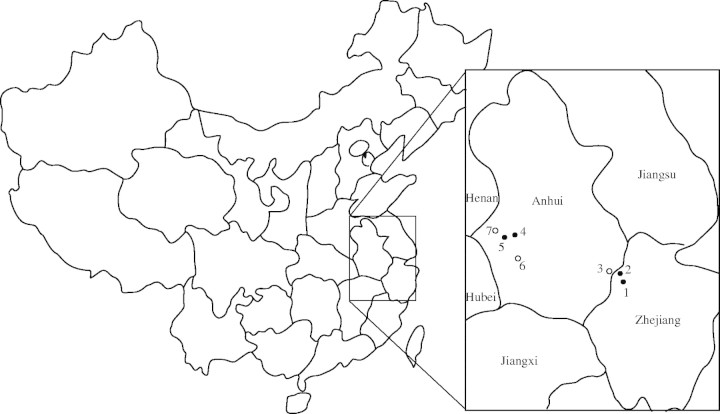
Berchemiella wilsonii was collected in the western Hubei Province, central China and described by E. H. Wilson in 1907 (Fu and Jin, 1992). Berchemiella wilsonii var. pubipetiolata was first described in 1988 (Qian, 1988). It is a deciduous tree up to 12 m tall which differs clearly from var. wilsonii in having pubescent petioles. Berchemiella wilsonii var. pubipetiolata is only found in two disjunct regions: the north-eastern Dabie Mountain in Anhui Province and the western Tianmu Mountain in Zhejiang Province (Li, 1988; Qian, 1988). Seven populations of the species were previously documented (Qian, 1988) (Fig. 1). However, the populations of B. wilsonii var. pubipetiolata have been severely disturbed by deforestation, road construction, tourism and urbanization in the past two decades. During extensive field surveys conducted in 2001 and 2002, only four sizeable populations were located, including two populations in the north-eastern Dabie Mountain and two populations in the western Tianmu Mountain. The species is a weak competitor and is scattered as a forest understorey tree in lowland drainage basins adjacent to streams and rivers at altitudes between 500 and 1200 m a.s.ll. In Zhejiang Province, B. wilsonii var. pubipetiolata individuals grow either in typical native forest of mixed evergreen and deciduous broad-leaved trees dominated by Liquidambar formosana, Acer davidiana and Cyclobalanopsis gracilis, or in the understorey of cultivated Carya cathayensis plantations. However, in Anhui Province, the populations are largely undisturbed and the dominant companion species usually are native Platycarya strobilacea, Acer davidiana and Cyclobalanopsis gracilis.
Fig. 1.
Map of the historical and extant populations of Berchemiella wilsonii var. pubipetiolata and sampling locations (open circles, extinct sites; filled circles, sampling sites): 1, Tuankou; 2, Maxiao; 3, Jixi; 4, Wanfushan; 5, Majiahe; 6, Jinzai; 7, Qianshan.
Little is know about the biology of the species. During the authors' field survey, mature trees were regularly found to produce hundreds of small hermaphrodite flowers (diameter approx. 4 mm) in May and June and are probably cross-pollinated. The fruits are mauve, long-ellipsoid drupes (approx. 8 mm long and approx. 3 mm in diameter) that ripen in August and are mostly dispersed by gravity. A recent study indicated that the both seed vigour and germination rate of this species were very low (37·8 % and 4 %, respectively) (Dang, 2004).
In this paper, allozyme analysis was used to examine the levels and patterns of genetic diversity in populations of B. wilsonii var. pubipetiolata to assess the effect of habitat fragmentation on population genetic structure of the species. The baseline genetic information should be useful for developing appropriate conservation strategies for B. wilsonii var. pubipetiolata.
MATERIALS AND METHODS
Populations sampled
About 25 plants of Berchemiella wilsonii (Schneid.) Nakai var. pubipetiolata Qian were sampled from four extant populations, excepting the Maxiao population site where some extant plants are not accessible on high mountain cliffs (Fig. 1 and Table 1), including two western Tianmu Mountain in Zhejiang Province (Tuankou population, 30°00′N, 119°03′E; Maxiao population, 30°09′N, 118°52′E) and two other populations in north-eastern Dabie Mountain in Anhui Province (Wanfushan population, 31°03′N, 116°34′E; Majiahe population, 31°05′N, 116°11′E). The Anhui populations are in a regional nature reserve within which logging was prohibited in 1992. Most individuals in Zhejiang had been logged by local farmers, and samples were collected from re-sprouts of the old root stumps. No seedlings or new ramets were found at the sites of the two Zhejiang populations. A few seedlings were observed in the two Anhui populations. In the present study, only adult individuals were exhaustively sampled but separated from each other by at least 5 m to avoid repeated sampling of the same tree.
Table 1.
Genetic diversity of Berchemiella wilsonii var. pubipetiolata within populations
| Population |
N |
A |
P |
Ho |
He |
|---|---|---|---|---|---|
| Tuankou | 25 | 1·85 (0·67) | 70·0 | 0·580 (0·430) | 0·349 (0·243) |
| Maxiao | 18 | 1·85 (0·67) | 70·0 | 0·592 (0·432) | 0·345 (0·235) |
| Wanfushan | 24 | 1·85 (0·49) | 75·0 | 0·563 (0·452) | 0·324 (0·217) |
| Majiahe | 31 | 1·80 (0·41) | 70·0 | 0·545 (0·431) | 0·308 (0·218) |
| Mean | 1·85 | 71·3 | 0·590 | 0·348 | |
| Overall | 2·10 (0·64) | 85·0 | 0·567 (0·384) | 0·378 (0·199) |
Standard errors are shown in parenthesis.
N, sample size; A, mean number of alleles per locus; P, percentage of polymorphic loci; Ho, observed heterozygosity; He, expected heterozygosity.
One-year-old twigs with mature buds were collected in spring 2003 before bud break. A total of 98 individuals was sampled, comprising 25 individuals at Tuankou, 18 at Maxiao, 24 at Wanfushan and 31 at Majiahe. Samples were stored at 4°C until electrophoresis was carried out.
Electrophoresis procedure
Twigs were soaked in water at room temperature (approx. 25°C) for 5 d and enzymes were extracted from the swollen buds. The extraction procedure follows Huang et al. (1994). Eletrophoresis was conducted using an isoelectric focusing polyacrylamide slab gel system (Mulcahy et al., 1981). Of the12 pre-screened enzyme systems, nine that resolved into clear banding patterns were then used for allozyme genetic analysis including acid phosphatase [ACP; EC (Enzyme Commission) 3.1.3.2], NAD(P)H-diaphorase (DIA; EC 1.6.2.2), esterase (EST; EC 3.1.1.–), isocitrate dehydrogenase (IDH; EC 1.1.1.41), malate dehydrogenase (MDH; EC 1.1.1.37), malic enzyme (ME; EC 1.1.1.40), phosphogluconate dehydrogenase (PGD; EC 1.1.1.44), phosphoglucoisomerase (PGI; EC 5.3.1.3) and shikimate dehydrogenase (SKD; EC 1.1.1.25). Gels were stained as described by Weeden and Wendel (1989) with minor modifications. A total of 20 loci was scored: Acp-1, Acp-2, Acp-3, Dia-1, Dia-2, Dia-3, Est-2, Est-3, Est-4, Idh-1, Idh-2, Mdh-1, Mdh-3, Mdh-4, Me, Pgd-1, Pgd-2, Pgi, Skd-1 and Skd-2.
Data analysis
A set of standard measures of genetic diversity were calculated using TFPGA version 1.3 (Miller, 1997), including allele frequencies for each locus, number of alleles per locus (A), percentage polymorphic loci (P, 0·95 criterion), observed heterozygosity (Ho), expected heterozygosity (He). Fixation indices (F) for each locus were estimated and tested for deviation from the Hardy–Weinberg equilibrium following the algorithm recommended by Levene (1949).
TFPGA was also used to determine F-statistics (Weir and Cockerham, 1984) at both the population and the group level. Statistical significance was tested using methods described by Workman and Niswander (1970). To determine the significance of the FST for each locus, the chi-square (χ2) test was used as χ2 = 2NFST(k − 1), with d.f. = (k − 1)(s − 1), where k is the number of alleles and s is the number of populations (Workman and Niswander, 1970). The 95 % confidence intervals of the F-statistics were estimated by 1000 bootstraps over loci for multi-locus, and by jackknifing over populations for the single-locus (Weir, 1990). Chi-square tests for heterozygeneity of allele frequencies across populations were performed using FSTAT version 2.9.1 (Goudet, 2000). A cluster analysis was conducted based on Nei's (1978) unbiased genetic distance/identity using unweighted pair group mean analysis (UPGMA).
RESULTS
A total of 42 alleles were scored in 20 loci, and the allele frequencies are presented in Table 2. Three loci (Dia-1, Mdh-1 and Pgd-1) were monomorphic in all four populations and the other 17 loci were polymorphic in at least one population. Allele frequencies were mostly heterogeneous across populations, except for Mdh-4 and Pgd-2 which were uniformly heterozygous in all four populations. Three alleles are exclusive to the Anhui populations, Dia-3b, Mdh-3b and Skd-1c, while another three alleles are exclusive to the Zhejiang populations, Acp-1c, Acp-3c and locus Skd-1a. Only one allele, Skd-2c, was found uniquely in the Tuankou population. There were no low frequency alleles (<0·1) observed in the Tuankou population, but three in each of the other three populations (Table 2).
Table 2.
Allele frequencies in Berchemiella wilsonii var. pubipetiolata at 20 allozyme loci
| Locus |
Allele |
Tuankou |
Maxiao |
Wanfushan |
Majiahe |
|---|---|---|---|---|---|
| Acp-1 | a | 0·440 | 0·083 | 0·500 | 0·500 |
| b | 0·400 | 0·639 | 0·500 | 0·500 | |
| c | 0·160 | 0·278 | 0·000 | 0·000 | |
| Acp-2 | a | 0·340 | 0·694 | 1·000 | 0·984 |
| b | 0·660 | 0·306 | 0·000 | 0·016 | |
| Acp-3 | a | 0·500 | 0·500 | 0·500 | 0·500 |
| b | 0·260 | 0·083 | 0·500 | 0·500 | |
| c | 0·240 | 0·417 | 0·000 | 0·000 | |
| Dia-1 | a | 1·000 | 1·000 | 1·000 | 1·000 |
| Dia-2 | a | 1·000 | 0·639 | 0·542 | 0·629 |
| b | 0·000 | 0·361 | 0·458 | 0·371 | |
| Dia-3 | a | 1·000 | 1·000 | 0·625 | 0·871 |
| b | 0·000 | 0·000 | 0·375 | 0·129 | |
| Est-2 | a | 0·480 | 0·500 | 0·500 | 0·500 |
| b | 0·520 | 0·500 | 0·500 | 0·500 | |
| Est-3 | a | 0·440 | 0·417 | 0·479 | 0·355 |
| b | 0·560 | 0·500 | 0·500 | 0·645 | |
| c | 0·000 | 0·083 | 0·021 | 0·000 | |
| Est-4 | a | 0·300 | 0·667 | 0·313 | 0·597 |
| b | 0·700 | 0·333 | 0·688 | 0·403 | |
| Idh-1 | a | 0·500 | 0·500 | 0·938 | 1·000 |
| b | 0·500 | 0·500 | 0·063 | 0·000 | |
| Idh-2 | a | 0·500 | 0·583 | 0·500 | 0·516 |
| b | 0·500 | 0·417 | 0·500 | 0·484 | |
| Mdh-1 | a | 1·000 | 1·000 | 1·000 | 1·000 |
| Mdh-3 | a | 1·000 | 1·000 | 0·896 | 0·823 |
| b | 0·000 | 0·000 | 0·104 | 0·177 | |
| Mdh-4 | a | 0·500 | 0·500 | 0·500 | 0·500 |
| b | 0·500 | 0·500 | 0·500 | 0·500 | |
| Me | a | 0·800 | 0·500 | 0·667 | 0·613 |
| b | 0·200 | 0·500 | 0·333 | 0·387 | |
| Pgd-1 | a | 1·000 | 1·000 | 1·000 | 1·000 |
| Pgd-2 | a | 0·500 | 0·500 | 0·500 | 0·500 |
| b | 0·500 | 0·500 | 0·500 | 0·500 | |
| Pgi | a | 0·500 | 0·333 | 0·833 | 0·903 |
| b | 0·500 | 0·667 | 0·167 | 0·097 | |
| Skd-1 | a | 0·580 | 0·500 | 0·000 | 0·000 |
| b | 0·420 | 0·500 | 0·500 | 0·500 | |
| c | 0·000 | 0·000 | 0·500 | 0·500 | |
| Skd-2 | a | 0·220 | 1·000 | 0·979 | 0·968 |
| b | 0·580 | 0·000 | 0·021 | 0·032 | |
| c | 0·200 | 0·000 | 0·000 | 0·000 |
Genetic diversity parameters within populations are listed in Table 1. The mean number of alleles per locus (A) at population and species level were 1·85 and 2·10, respectively. The overall value of P was 85·0 % for the species, while the average P at the population level was 71·3 %, ranging from 70·0 % for the Tuankou, Maxiao and Majiahe populations to 75·0 % for the Wanfushan population. The mean expected heterozygosity (He) was 0·378, ranging from 0·308 for the Majiahe population to 0·349 for the Tuankou population. However, the observed heterozygosity was very high with an average value of 0·590, ranging from 0·545 at Majiahe to 0·592 at Maxiao population. A significantly high deviation from Hardy–Weinberg equilibrium was found in all populations, reflected by the fact that most values of F indexes are near, or equal, to −1 at 17 polymorphic loci (Table 3). This result indicates that an excess of heterozygotes existed in all populations examined.
Table 3.
Fixation indices (F) and chi-square tests for Hardy–Weinberg equilibrium in four populations of Berchemiella wilsonii var. pubipetiolata
| Loci |
Tuanlou |
Maxiao |
Wanfushan |
Majiahe |
|---|---|---|---|---|
| Acp-1 | −0·418** | −0·094* | −1·000** | −1·000** |
| Acp-2 | −0·337NS | −0·440* | – | −0·016NS |
| Acp-3 | −0·600** | −0·756** | −1·000** | −1·000** |
| Dia-2 | – | −0·565* | −0·846** | −0·590** |
| Dia-3 | – | – | −0·600** | −0·148NS |
| Est-2 | −0·923** | −1·000** | −1·000** | −1·000** |
| Est-3 | −0·786** | −0·756** | −0·923** | −0·550** |
| Est-4 | −0·429* | −0·500* | −0·261NS | −0·676** |
| Idh-1 | −1·000** | −1·000** | −0·067NS | – |
| Idh-2 | −1·000** | −0·714** | −1·000** | −0·937** |
| Mdh-3 | – | – | −0·116NS | −0·215NS |
| Mdh-4 | −1·000** | −1·000** | −1·000** | −1·000** |
| Me | −0·250NS | −1·000** | −0·500* | −0·632** |
| Pgd-2 | −1·000** | −1·000** | −1·000** | −1·000** |
| Pgi | −1·000** | 0·000NS | 1·000** | −0·107NS |
| Skd-1 | −0·724** | −1·000** | −1·000** | −1·000** |
| Skd-2 | 0·235** | – | −0·021NS | −0·033NS |
–, Not applied.
P < 0·05;
P < 0·01;
not significant.
Partitioning of population genetic diversity based on the 17 polymorphic isozyme loci revealed that a significant population differentiation occurred in the natural habitat of B. wilsonii var. pubipetiolata, as demonstrated by mean FST = 0·130, suggesting that about 13 % of allozyme diversity occurred among populations (Table 4). Chi-square tests showed that 12 of the 17 polymorphic loci were significantly different in allele frequencies across populations (Table 4). Further examination of the population genetic structures in the two different provinces and testing of the significance of population differentiation showed that Zhejiang populations had a significant FST of 0·078, while the differentiation in Anhui populations was negligible (FST = 0·014) (Table 5). Indirect estimation of gene flow for the overall populations is Nm = 1·68, but Nm = 2·97 for Zhejiang populations and Nm = 17·28 for Anhui populations, respectively.
Table 4.
Wright's F-statistics for Berchemiella wilsonii var. pubipetiolata populations
| Locus |
FIS |
FIT |
FST |
|---|---|---|---|
| Acp-1 | −0·614 | −0·472 | 0·091** |
| Acp-2 | −0·374 | 0·161 | 0·389** |
| Acp-3 | −0·823 | −0·639 | 0·101** |
| Dia-2 | −0·671 | −0·434 | 0·148** |
| Dia-3 | −0·454 | −0·144 | 0·213** |
| Est-2 | −0·981 | −0·980 | 0·000 |
| Est-3 | −0·760 | −0·737 | 0·013NS |
| Est-4 | −0·473 | −0·312 | 0·109** |
| Idh-1 | −0·902 | −0·362 | 0·284** |
| Idh-2 | −0·914 | −0·905 | 0·005NS |
| Mdh-3 | −0·177 | −0·076 | 0·086** |
| Mdh-4 | −1·000 | −1·000 | 0·000 |
| Me | −0·634 | −0·551 | 0·051* |
| Pgd-2 | −1·000 | −1·000 | 0·000 |
| Pgi | −0·173 | 0·109 | 0·240** |
| Skd-1 | −0·932 | −0·514 | 0·217** |
| Skd-2 | 0·195 | 0·605 | 0·509** |
| Overall† | −0·717 (0·059) | −0·494 (0·110) | 0·130** (0·040) |
| 95 % CI | −0·871–0·634 | −0·731–0·316 | 0·057–0·214 |
P < 0·05;
P < 0·01;
not significant.
Standard deviations in parentheses.
Table 5.
Wright's F-statistics estimated for each population groups and overall populations of Berchemiella wilsonii var. pubipetiolata
|
FIT |
FST |
FIS |
Nm |
|||||
|---|---|---|---|---|---|---|---|---|
| Overall populations | −0·494 | 0·130** | −0·717 | 1·68 | ||||
| Within groups | ||||||||
| Zejiang group | −0·553 | 0·078* | −0·683 | 2·97 | ||||
| Anhui group | −0·728 | 0·014NS | −0·753 | 17·28 | ||||
P < 0·05;
P < 0·01;
not significant.
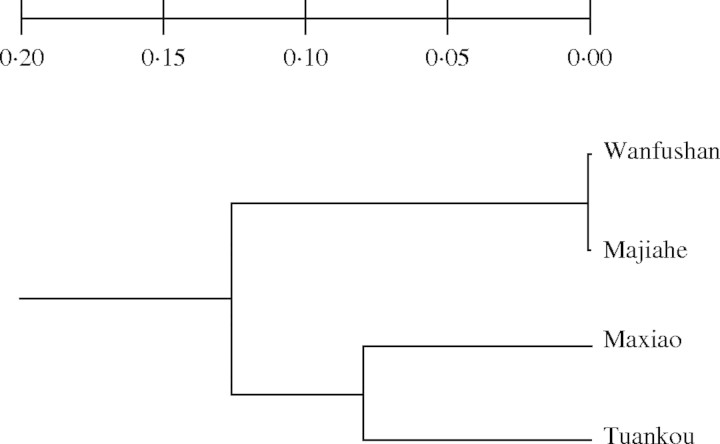
Mean genetic identity among the four populations was 0·905, ranging from I = 0·991 found between the Majiahe and Wanfushan populations, to I = 0·857 between the Tuankou and Wanfushan populations. The UPGMA cluster analysis further revealed two distinct clusters representing the Zhejiang and Anhui groups (Fig. 2).
Fig. 2.
UPGMA dendrogram showing genetic relationship of four populations of Berchemiella wilsonii var. pubipetiolata, based on Nei's unbiased genetic distance (Nei, 1978).
DISCUSSION
Genetic diversity
Many investigations of allozyme variation have demonstrated that small and fragmented plant populations have low levels of genetic diversity (Godt et al., 1997; Jones and Gliddon, 1999; Kollmann et al., 2000; Cheon et al., 2002). This is obviously not the case in B. wilsonii var. pubipetiolata. The values of P and He at the species level (P = 85·0, He = 0·378) were much higher than the average values previously found in other narrowly distributed woody species (P = 61·5, He = 0·165), while A was comparable to values for narrowly distributed species (A = 2·08) (Hamrick et al., 1992). Genetic diversity maintained within a species is considered as to a result of both historical events and recent evolutionary processes (Gonzalez-Astorga et al., 2003). The present genetic diversity retaining in populations of B. wilsonii var. pubipetiolata appears to be related to life history of the species. Being a member of a Tertiary relic genus (Li, 1988), B. wilsonii var. pubipetiolata may retain a high diversity which is in agreement with Witter and Carr (1988) who proposed that older taxa might be more diverse. Additionally, this species has experienced a recent decline in population size and local population extinctions during the past 20 years (Fig. 1); therefore, the current results might just reflect the historical patterns of genetic diversity in B. wilsonii var. pubipetiolata.
Although B. wilsonii var. pubipetiolata had high diversity at both population and species level in the present study, all populations lack rare alleles (<0·1) (Table 2). This may be explained as a result of random genetic drift caused by the recent reduction in population size. In general, low frequency allozyme alleles are far more abundant than alleles at intermediate frequency in undisturbed natural populations (Chakraborty et al., 1980). However, alleles at low frequencies are prone to be lost more rapidly than the loss of overall genetic variation when populations experience a rapid reduction in size (Nei et al., 1975; Leberg, 1992). Thus overall heterozygosity may not decrease substantially unless the population remains small for several generations (Nei et al., 1975; Allendorf, 1986). Such a loss of rare alleles has been reported in the tree Eucalyptus albens (Prober and Brown, 1994), the endangered daisy Rutodosis leptorrhynchoides (Young et al., 1999) and the endemic shrub Brongniartia vazquezii (Gonzalez-Astorga and Nunez-Farfan, 2001). Apparently, habitat fragmentation in natural populations could result in an immediate loss of rare alleles and a reduction of allele richness rather than a reduction of overall genetic heterozygosity. It is suggested that allele composition and richness should be monitored in genetic assessments of newly fragmented populations, as these estimators may be initial signs of deterioration of the population genetic dynamics.
Genetic fixation and excess of heterozygotes
Negative values of fixation index (F) were found at almost all loci across all four populations of B. wilsonii var. pubipetiolata, indicating an extreme excess of heterozygotes. A possible explanation for this pattern is that natural selection might favour heterozygotes that can cope with environment changes in these highly fragmented populations. Two factors are usually involved in driving selection for heterozygotes, environmental stress and inbreeding depression. For instance, Luijten et al. (2000) found excess of heterozygotes in small populations of Arnica montana when population size decreased, suggesting that the heterozygous individuals in small populations are survivors from formerly larger populations which have relatively high fitness. However, inbreeding depression often results in low levels of heterozygosity. Such an example is Piper cernuum (Mariot et al., 2002) in which heterozygote excess was revealed in all but the smallest populations, which was probably attributable to the effects of accentuated genetic drift. Accordingly, the occurrence or distribution of heterozygote excess seems to be species or case specific. In a fragmented population that has become successively smaller, random genetic drift should have a noticeable effect on allele composition and in reducing the proportion of heterozygotes. However, if a small population is the result of a recent and sudden reduction in size, inbreeding may not yet have had an effect on genetic structure. In tree species, heterozygote excess is quite common in adult stages or in old age classes (Bush and Smouse, 1992), and it is most likely that selection favouring heterozygotes and/or favouring against homozygotes is a common phenomenon in natural plant populations. Doligez and Joly (1997) used allozyme analysis for genotyping seeds and adults in a tropical tree Carapa procera, and found excess homozygotes in seeds and excess heterozygotes in adults, suggesting a strong selection for heterozygotes. Similar results have also been detected in other long-lived perennials (Arnica montana, Luijten et al., 2000; Piper cernuum, Mariot et al., 2002; Dioon edule, Gonzalez-Astorga et al., 2003).
Furthermore, in small and fragmented populations, the homozygous seeds produced by inbreeding may have lower germination and higher mortality than heterozygous seeds by outbreeding. Also, homozygous seedlings may have lower vigour than heterozygous ones. A high proportion of heterozygous seedlings has been observed in many plant populations (Bush and Smouse, 1992; Lee et al., 2000). Theoretically, inbreeding will result in an excess of homozygotes, but the proportion may be reduced at different life stages. For example, lethals and sublethals expressed at an early stage would leave more heterozygous individuals to be detected in surviving seeds and result in heterozygote excess (Keller and Waller, 2002). In a parallel study, it was found that the seed vigour of newly collected seeds was only 37·8 %, and the germination rate was only 4·0 % in the natural habitat. The highest germination rate obtained so far was less than 13·0 %, in spite of the fact that various treatments were attempted in experimental conditions (Dang, 2004). It is hypothesized that most homozygous seeds caused by inbreeding were aborted and could not germinate, which suggests that genotyping analysis for seeds and seedlings at different ages should be considered in future.
Genetic differentiation
The value of FST, a measure of the degree of differentiation among populations, was 0·130 for B. wilsonii var. pubipetiolata, revealing that about 87 % of the total genetic diversity resides within populations. This value is slightly higher than the average GST for narrowly long-lived woody species (GST = 0·124) but almost equal to that of species with gravity dispersed seeds (GST = 0·131) (Hamrick et al., 1992). The theory of population genetics predicts that, as a population becomes progressively smaller and more isolated, genetic differentiation caused by genetic drift and inbreeding depression should increase (Templeton et al., 1990). However, the genetic consequences of habitat fragmentation are complicated because of different life-history characteristics and pre-fragmentation abundances (Young et al., 1996). It appears that the moderate genetic differentiation and substantial gene diversity in the populations of B. wilsonii var. pubipetiolata documented in the present study reflect recent fragmentation of habitat and population decline. Although the geographical distance between the two Anhui populations is farther than that between the two Zhejiang populations, a much higher gene flow rate occurred between the former than between the latter, suggesting that seed and pollen dispersal is more efficient between the Anhui populations. This is consistent with the field observations that the numbers of individuals within populations were higher in the Anhui region, and also that re-generating seedlings were found in this region while no seedlings were found in Zhejiang region. Moreover, the population differentiation in Zhejiang and Anhui is significantly different (Table 5), reflecting the different land-use histories in the two regions. In fact, the insignificant FST of 0·014 detected among the Anhui populations could be considered negligible, suggesting a less disturbed habitat or a good recovery of B. wilsonii var. pubipetiolata populations since the area was designated as a nature reserve and logging was prohibited. The fact that some seedlings were observed in the region is promising. The two Zhejiang populations have been disturbed by road construction, agricultural practice and tourist exploitation over the past 50 years, and the habitat deterioration still continues. Berchemiella wilsonii var. pubipetiolata usually grows sparsely under stands of Chinese walnuts, which form the major economic resource for local residents. With increasing demand for walnuts, plantations have replaced the natural habitat of B. wilsonii var. pubipetiolata and local extinction of the species can be expected if no further conservation efforts are made.
Implications for conservation
The present results indicate that B. wilsonii var. pubipetiolata is not genetically depauperate in general. It appears that demographic stochasticity is a more immediate threat to the species than reduction of overall genetic diversity (Lande, 1999), and the main factor responsible for the reduction in population size is anthropogeneic activity. Habitat protection of the Zhejiang populations is particularly urgent since they are located in farmland. Conflicts between conservation and local interests is a difficult issue. There is evidence that tourism has severe impacts on threatened plants (Kelly et al., 2003). With the promotion of ‘ecotourism’ by local government, there have been rapidly increasing numbers of visitors in the Zhejiang region in recent years, the effects of which need to be investigated and monitored. Since most individuals grow on the sides of gullies in scenic sites (except for Majiahe population), B. wilsonii var. pubipetiolata is vulnerable to trampling and destruction by the infrastructure construction associated with tourism.
On the other hand, the genetic threat should not be neglected. The allozyme evidence obtained in the present study reflects the history of the population and the current status of genetic diversity remaining in B. wilsonii var. pubipetiolata. The genetic dynamics in the populations or the long-term survivability are still not understood, although it is suspected that population fragmentation will result in loss of rare alleles. The large percentage of aborted seeds and little seedling recruitment also observed raise great concern for long-term survival and suggests that immediate conservation measures need to be taken. However, the causes of seed abortion need further studies. Further studies should also focus on the reproductive biology of B. wilsonii var. pubipetiolata. Finally, it is suggested that appropriate numbers of seeds should be sampled from all populations in an ex situ conservation programme.
Acknowledgments
This work is supported by KIP Pilot Project of Chinese Academy of Sciences (KSCX2-SE-104) and National Natural Sciences Foundation of China (30470185). We thank Dr Hong Qian and Dr Desmond Layne for their discussion on the manuscript, Mrs Yuanyuan Chen for her laboratory assistance and Mr Haishan Dang for his help in field collection. We also thank Dr Mikael Hedren and another anonymous reviewer for their valuable comments and suggestions.
LITERATURE CITED
- Allendorf FW. 1986. Genetic drift and the loss of alleles versus heterozygosity. Zoo Biology 5: 181–190. [Google Scholar]
- Boyce MS. 1992. Population viability analysis. Annual Review of Ecology and Systematics 23: 481–506. [Google Scholar]
- Britten SB. 1996. Meta-analysis of the association between multilocus heterozygosity and fitness. Evolution 50: 2158–2164. [DOI] [PubMed] [Google Scholar]
- Bush RM, Smouse PE. 1992. Evidence for the adaptive significance of allozymes in forest trees. New Forests 6: 176–196. [Google Scholar]
- Buza L, Young A, Thrall P. 2000. Genetic erosion, inbreeding and reduced fitness in fragmented populations of the endanged tetraploid pea Swainsonia[?] recta Biological Conservation 93: 177–186. [Google Scholar]
- Chakraborty R, Fuerst PA, Nei M. 1980. Statistical studies on protein polymorphism in natural populations. III. Distribution of allele frequencies and the number of alleles per locus. Genetics 94: 1039–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon CP, Chung MY, Chung SG, Chung MG. 2002. Allozyme variation of a small subshrub Ardisia japonica (Mysinaceae) in north eastern Asia. Silvae Genetica 51: 1–6. [Google Scholar]
- Dang HS. 2004.Studies on the characteristics and physiology of seed germination of Berchemiella wilsonii var pubipetiolata, an endangered species. Masters Thesis, The Graduate School of Chinese Academy of Sciences. [Google Scholar]
- Doligez A, Joly HI. 1997. Genetic diversity and spatial structure within a natural stand of a tropical forest tree species, Carapa procera (Meliaceae), in French Guiana. Heredity 79: 72–82. [Google Scholar]
- Fu LK, Jin JM. 1992.China plant red data book—rare and endangered plants. Beijing: Science Press, 538–539. [Google Scholar]
- Godt MJW, Race T, Hamrick JL. 1997. A population analysis of Ziziphus celata, an endangered florida shrub. Journal of Heredity 88: 531–533. [Google Scholar]
- Gonzalez-Astorga J, Nunez-Farfan J. 2001. Effect of habitat fragmentation on the genetic structure of the narrow endemic Brongniaria vazquezii Evolutionary Ecology Research 3: 861–872. [Google Scholar]
- Gonzalez-Astorga J, Vovides AP, Ferrer MM, Iglesias C. 2003. Population genetics of Dioon edule Lindl. (Zamiaceae, Cycadales): biogeographical and evolutionary implications. Biological Journal of the Linnean Society 80: 457–467. [Google Scholar]
- Goudet J. 2000. FSTAT, a program to estimate and test gene diversities and fixation indices version 2·9.1. http://www.unil.ch/izea/softwares/fstat.html. [Google Scholar]
- Hamrick JL, Godt MJW, Sherman-Broyles SL. 1992. Factors influencing levels of genetic diversity in woody plant species. New Forests 6: 95–124. [Google Scholar]
- Huang H, Dane F, Norton JD. 1994. Allozyme diversity in Chinese, Seguin and American chestnut (Castanea spp.). Theoretical and Applied Genetics 88: 981–985. [DOI] [PubMed] [Google Scholar]
- Jones B, Gliddon C. 1999. Reproductive biology and genetic structure in Lloydia serotina Plant Ecology 141: 151–161. [Google Scholar]
- Keller LF, Waller DM. 2002. Inbreeding effects in wild populations. Trends in Ecology and Evolution 17: 230–241. [Google Scholar]
- Kelly CL, Pickering CM, Buckley RC. 2003. Impacts of tourism on threatened plant taxa and communities in Australia. Ecological Management and Restoration 4: 37–44. [Google Scholar]
- Kollmann J, Steinger T, Roy BA. 2000. Evidence of sexuality in European Rubus (Rosaceae) species based on AFLP and allozyme analysis. American Journal of Botany 87: 1592–1598. [PubMed] [Google Scholar]
- Lande R. 1999. Extinction risk from anthropogenic, ecological and genetic factors. In: Landweber, LA, Dobson, AP eds. Genetics and extinction of species. Princeton, NJ: Princeton University Press, 1–22. [Google Scholar]
- Leberg PL. 1992. Effects of a population bottleneck on genetic variation as measured by allozyme electrophoresis. Evolution 46: 477–494. [DOI] [PubMed] [Google Scholar]
- Lee SL, Wickneswari R, Mahani MC, Zakri AH. 2000. Genetic diversity of a tropical tree species, Shorea leprosula Miq. (Dipterocarpaceae), in Malaysia: implications for conservation of genetic resources and tree improvement. Biotropica 32: 213–224. [Google Scholar]
- Levene H. 1949. On a matching problem in genetics. The Annals of Mathematical Statistics 20: 91–94. [Google Scholar]
- Lewis PO, Crawford DJ. 1995. Pleistocene refugium endemics exhibit greater diversity than widespread congeners in the genus Polygonella (Polygonaceae). American Journal of Botany 82: 141–149. [Google Scholar]
- Li SC. 1988.Berchemiella (Rhamnaceae). In: Anhui Flora, Vol. 3. Wuhu: Prospect Press, 396. [Google Scholar]
- Luijten SH, Dierick A, Gerard J, Oostermeijer B, Raijmann LEL, denNijs HCM. 2000. Population size, genetic variation, and reproductive success in a rapidly declining, self-incompatible perennial (Arnica montana) in the Netherlands. Conservation Biology 14: 1776–1787. [DOI] [PubMed] [Google Scholar]
- Mariot A, Stasi LC, dos Reis MS. 2002. Genetic diversity in natural populations of Piper cernuum Journal of Heredity 93: 365–369. [DOI] [PubMed] [Google Scholar]
- Miller MP. 1997. Tools for Population Genetic Analyses (TFPGA) 1·3: a Windows program for the analysis of allozyme and molecular population genetic data. Computer software distributed by the author. [Google Scholar]
- Mulcahy DL, Robinson RW, Ihara M, Kesseil R. 1981. Gametophytic transcription for acid phosphatase in pollen of Cucurbita species hybrids. Journal of Heredity 72: 353–354. [Google Scholar]
- Nei M. 1978. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Maruyama T, Chakraborty R. 1975. The bottleneck effect and genetic variability in populations. Evolution 29: 1–10. [DOI] [PubMed] [Google Scholar]
- Prober SM, Brown ADH. 1994. Conservation of the grassy white box woodlands: population genetics and fragmentation of Eucalyptus albens Conservation Biology 8: 1003–1013. [Google Scholar]
- Qian H. 1988. A study on the genus Berchemiella Nakai (Rhamnceae) endemic to eastern Asia. Bulletin of Botanical Research 8: 119–126. [Google Scholar]
- Ranker TA. 1994. Evolution of high genetic diversity in the rare Hawaiian fern Adenophorus periens and implications for conservation management. Biological Conservation 70: 19–24. [Google Scholar]
- Sharma IK, Jones DL, French CJ. 2003. Unusually high genetic variability revealed through allozymic polymorphism of an endemic endangered Australian orchid, Pterostylis aff picta (Orchidaceae). Biochemical Systematics and Ecology 31: 613–626. [Google Scholar]
- Shea KL, Furnier GR. 2002. Genetic variation and population strucure in central and isolated populations of balsam fir, Abies balsamea (Pinaceae). American Journal of Botany 89: 783–791. [DOI] [PubMed] [Google Scholar]
- Templeton AR, Shaw K, Routman E, Davia SK. 1990. The genetic consequences of habitat fragmentation. Annals of the Missouri Botanical Gardens 77: 13–27. [Google Scholar]
- Tilman D, May RM, Lehman CL, Nowak MA. 1994. Habitat destruction and the extinction debt. Science 371: 65–66. [Google Scholar]
- Tomimatsu H, Ohara M. 2003. Genetic diversity and local population structure of fragmentation of Trillium camschatcense Biological Conservation 109: 249–258. [Google Scholar]
- Weeden NF, Wendel JF. 1989. Visualization and interpretations of plant isozymes. In: Soltis DS, Soltis PS, eds. Isozymes in plant biology. Portland, OR: Dioscorides Press, 46–72. [Google Scholar]
- Weir BS. 1990.Genetic data analysis. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Weir BS, Cocherham, CC. 1984. Estimating F statistics for the analysis of population structure. Evolution 38: 1358–1370. [DOI] [PubMed] [Google Scholar]
- Witter MS, Carr GD. 1988. Adaptive radiation and genetic differentiation in the Hawaiian silversword alliance (Compositae: Madiinae). Evolution 42: 1278–1287. [DOI] [PubMed] [Google Scholar]
- Workman PL, Niswander JD. 1970. Population studies on southwestern Indian Tribes. II. Local genetic differentiation in the Papago. American Society of Human Genetics 22: 24–49. [PMC free article] [PubMed] [Google Scholar]
- Young AG, Boyle T, Brown T. 1996. The population genetic consequences of habitat fragmentation in plants. Trends in Ecology and Evolution 11: 413–418. [DOI] [PubMed] [Google Scholar]
- Young AG, Brown AHD, Zich FA. 1999. Genetic structure of fragmented populations of the endangered daisy Rutodosis leptorrhynchoides Conservation Biology 13: 256–265. [Google Scholar]
- Young AG, Merriam HG, Warwick SL. 1993. The effects of forest fragmentation on genetic variation in Acer saccharum Marsh. (sugar maple) populations. Heredity 71: 277–289. [Google Scholar]