Abstract
• Background and Aims The purpose of this study was to investigate the basis of the optimal hydration status for cryopreservation of intermediate oily seeds using Citrus as a model.
• Methods The relationships between equilibrium relative humidity (RH), seed water content, presence of freezable water as determined by DSC analysis, and germination percentage after immersion in liquid nitrogen (LN) were investigated in Citrus aurantifolia, C. grandis, C. madurensis and C. reticulata. The relationship between the lipid content of seeds and their unfrozen water content was also investigated.
• Key Results Independent of their level of seed desiccation tolerance, the optimal desiccation RH for seed tolerance to LN exposure was 75–80 % in the four species studied. This optimal hydration status always coincided with that at which presence of frozen water could not be detected in seed tissues during the cooling/thawing process. The unfrozen water content of seeds was variable between species and negatively correlated to seed lipid content. Using the present data, those obtained previously in seven coffee species and those reported by other authors for five other species, a significant linear relationship was found between the lipid content and the unfrozen water content of seeds.
• Conclusions This study provides additional evidence that intermediate oily seeds do not withstand the presence of freezable water in their tissues during the cooling/warming process. Moreover, it offers two important applied perspectives: (1) independent of their level of desiccation tolerance, testing germination of seeds of a given oily seed species after equilibration in 75–80 % RH at 25 °C and LN exposure, gives a rapid and reliable evaluation of the possibility of cryopreserving whole seeds of this given species; (2) it is now possible to calculate the interval of water contents in which non-orthodox oily seeds of a given species are likely to withstand LN exposure as a function of their lipid content.
Keywords: Seed, intermediate, non-orthodox, Citrus, cryopreservation, water activity, relative humidity, desiccation, lipid content, DSC, ice, unfrozen water
INTRODUCTION
Three categories of seed storage behaviour are generally recognized among plant species: orthodox, intermediate and recalcitrant (Roberts, 1973; Ellis et al., 1990). Intermediate seeds can withstand partial dehydration, but they cannot be stored under conventional genebank conditions because they are cold-sensitive and desiccation does not increase their longevity (Ellis et al., 1990). The intermediate category contains numerous important tropical cash crops, such as oil palm (Elaeis guinensis) or coffee (Coffea arabica), and important locally used species, such as the multi-purpose neem tree (Azadirachta indica). About 20 % of the non-orthodox species compiled in the Electronic Seed Storage Behaviour (ESSB) Compendium (Hong et al., 1996) have been classified in the intermediate category. Moreover, a high proportion of tropical rain forest species produce intermediate seeds (Ouédraogo et al., 1999).
For non-orthodox seed species, cryopreservation is the only technique available for long-term germplasm conservation. In the case of intermediate seed-propagated species, seeds are partially desiccation tolerant and, therefore, the option which has to be always tested first is whole seed cryopreservation. Using nine different coffee species, the basis of the limits of the hydration window for intermediate seed cryopreservation has been investigated recently (Dussert et al., 2001). When expressed in terms of water content, the higher limit of the hydration window was highly variable but corresponded always to the seed unfrozen water content (Vertucci, 1990), as determined by DSC analysis, suggesting that seed survival strictly depended on avoidance of intracellular ice formation. This result was perfectly consistent with observations made in soybean seeds by Vertucci (1989a). Because they offer interesting applied perspectives, two other results of this study on coffee-seed tolerance to liquid nitrogen (LN) exposure have to be underlined (Dussert et al., 2001): (1) the unfrozen water content was negatively correlated with seed lipid content; (2) when expressed in terms of water activity, the interspecific variability for the higher limit was very low, suggesting that desiccating seeds below 75–85 % relative humidity (RH) allows them to reach directly the optimal hydration level for cryopreservation of non-orthodox oily seeds.
To verify whether these findings could be of general interest, tolerance to LN exposure of seeds of another genus containing intermediate seed species, the genus Citrus, was investigated. The relationships between equilibrium RH, seed water content, presence of freezable water and germination percentage after immersion in LN were investigated in Citrus aurantifolia, C. grandis, C. madurensis and C. reticulata. The relationship between the lipid content of seeds and their unfrozen water content was also determined and plotted against data found in other species.
MATERIALS AND METHODS
Plant material
Seeds were extracted manually from freshly harvested mature fruits of Citrus aurantifolia, C. grandis, C. madurensis and C. reticulata. After extraction, seeds were carefully rinsed with tap water and immediately surface dried with paper cloth. The testa of seeds was then removed by hand and seeds were directly placed under desiccation conditions. The whole process was always completed within 1 d.
Desiccation, cooling, thawing and germination procedures
Using saturated KOH, NaCl and KNO3 solutions, a preliminary experiment was carried out to determine the time to reach equilibrium at 25 °C as a function of the desiccation RH. The water content of ten C. reticulata seeds was measured after 0, 1, 2, 3, 6, 8, 10, 14, 17, 22, 27 and 29 days of desiccation. Seed dry weight was measured after 1 d of drying in an oven at 103 °C. This preliminary trial clearly showed that the time to reach equilibrium, as determined by the reduction and stabilization of the standard deviation/mean ratio, increased with increasing desiccation RH (Fig. 1). The time to equilibrium was 6, 14 and 27 days for 8, 75 and 95 % RH, respectively. Since 95 % was the highest RH tested for desiccation and LN-exposure tolerance trials, a desiccation period of 4 weeks was subsequently applied for all RHs employed. No fungal growth was observed, even at the highest RH employed.
Fig. 1.
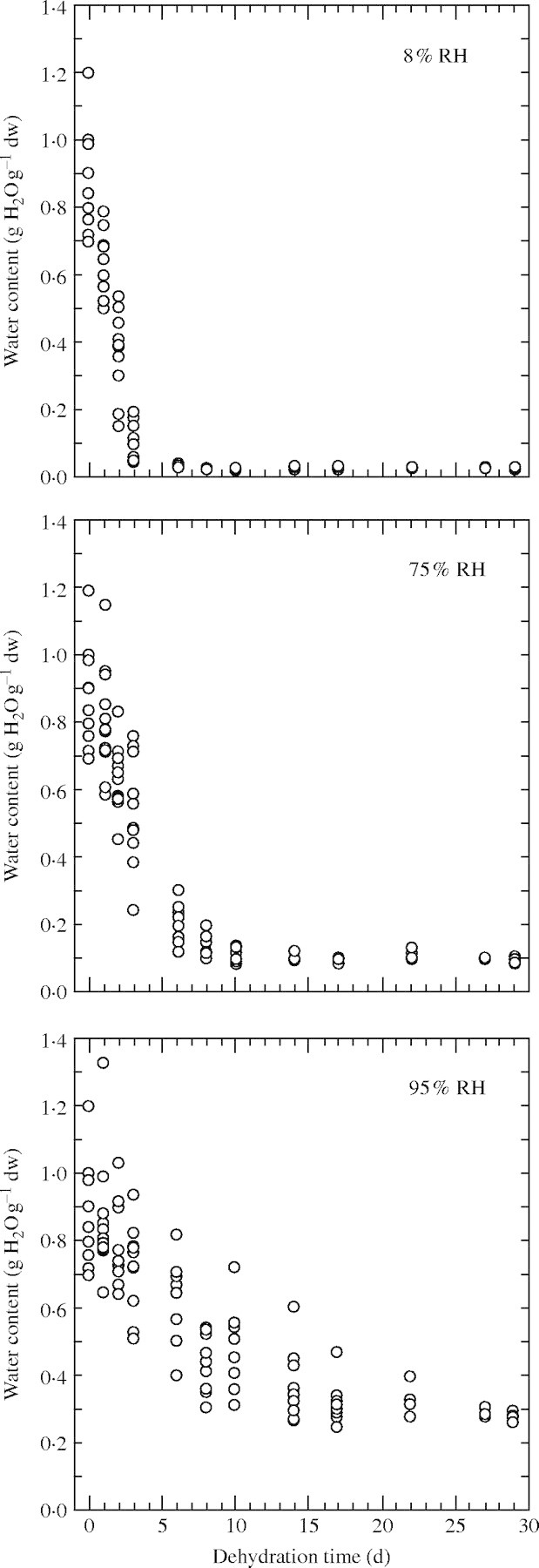
Evolution of water content of C. reticulata seeds with dehydration time under three different relative humidities. Each point corresponds to one seed.
To study tolerance to desiccation and LN exposure, seeds were desiccated by equilibration over ten saturated salt solutions (KOH, K acetate, K2CO3, NH4NO3, NaCl, NH4Cl, (NH4)2SO4, KCl, BaCl2, KNO3) providing an RH spectrum between 8 and 95 %. The water content (WC) of seeds at equilibrium (expressed in g H2O g−1 d. wt) was estimated using ten replicates of one single seed. Sorption isotherms were determined using the simplified D'arcy and Watt model, previously proposed in Dussert et al. (1999):
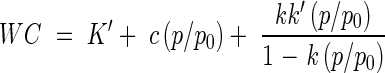 |
where WC is the seed water content, p/p0 is the relative vapour pressure (RH/100) and K′, c, k and k′ are specific parameters.
Before direct immersion in LN, seeds were placed in screw-capped 2-ml polypropylene cryotubes. Thawing was carried out by plunging the tubes in a 37 °C water-bath for 5 min. After desiccation or thawing, seeds were directly placed under germination conditions. For germination, seeds were placed above humid sand in closed plastic boxes at 25 °C.
For each combination of treatments, germination percentage was assessed using 100 seeds, using the criterion of normal seedling development. Quantification of desiccation sensitivity was performed using the quantal response model developed previously (Dussert et al., 1999):
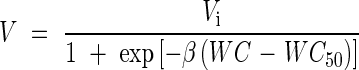 |
where V is the proportion of seeds producing at least one normal seedling, WC is the seed water content, WC50 is the water content at which half of the initial germination percentage (Vi) is lost and β describes the seed-to-seed variation for desiccation tolerance.
Lipid content determination
Seeds dried for 10 d over silica gel (final water content of about 0·03 g H2O g−1 d. wt) were used for lipid analysis. Total lipids were solvent-extracted from 1 g of ground seeds using a modified Folch method (Folch et al., 1957) with methylene chloride instead of chloroform as previously described in Dussert et al. (2001). Extracted lipids were quantified gravimetrically after complete solvent evaporation under a nitrogen stream at 40 °C. Seed lipid content was determined in triplicate for each species.
Differential scanning calorimetry
To obtain a broad range of seed water contents for DSC analysis, seeds were equilibrated over the saturated salt solutions used for measuring seed desiccation tolerance. After equilibration, some seeds were also partially rehydrated (in a 100 % RH atmosphere) prior to DSC analysis. Thermal transitions in seeds were measured using a TA Q100 DSC (TA instruments, New Castle, DE, USA). Seed samples (about 15 mg d. wt) sealed in aluminium pans were cooled to −120 °C at the maximal cooling rate of the apparatus (about 200 °C min−1), then heated at 10 °C min−1 from −120 to +20 °C. Only heating thermograms were recorded. After DSC analysis, pans were punctured and the sample dry weight was determined. In all species, 5-mg samples of total seed lipid extract, obtained as described above, were analysed to confirm the identification of endothermic lipid transitions in seed sample thermograms. Heating thermograms were analysed for the determination of the peak and onset temperatures, and the enthalpy of endothermic transitions measured. Transition enthalpies, expressed in joules per gram of dry weight, were determined from the area above the baseline and plotted against seed sample water content, expressed in g H2O g−1 d. wt, for the determination of unfrozen water content. Two lines of regression were then determined for each species according to the method described by Dussert et al. (2001).
RESULTS
Water sorption isotherms
Fitted water sorption isotherms are given in Fig. 2. The curves follow the expected shape in the range of relative humidities studied, i.e. a linear curve for p/p0 values between 0·1 and 0·7, and a sharp increase for higher values (>0·7). The very high proportion of variance explained by the model (98–99 %) demonstrated the goodness-of-fit of the simplified water sorption model for p/p0 values between 0·1 and 1 (Table 1). For the four Citrus species studied, K′, c, k and k′ values were consistent with values previously observed in other non-orthodox (Dussert et al., 1999) and orthodox (Vertucci and Leopold, 1987) oily seeds. The numerical expression of aw as a function of WC could thus be achieved.
Fig. 2.
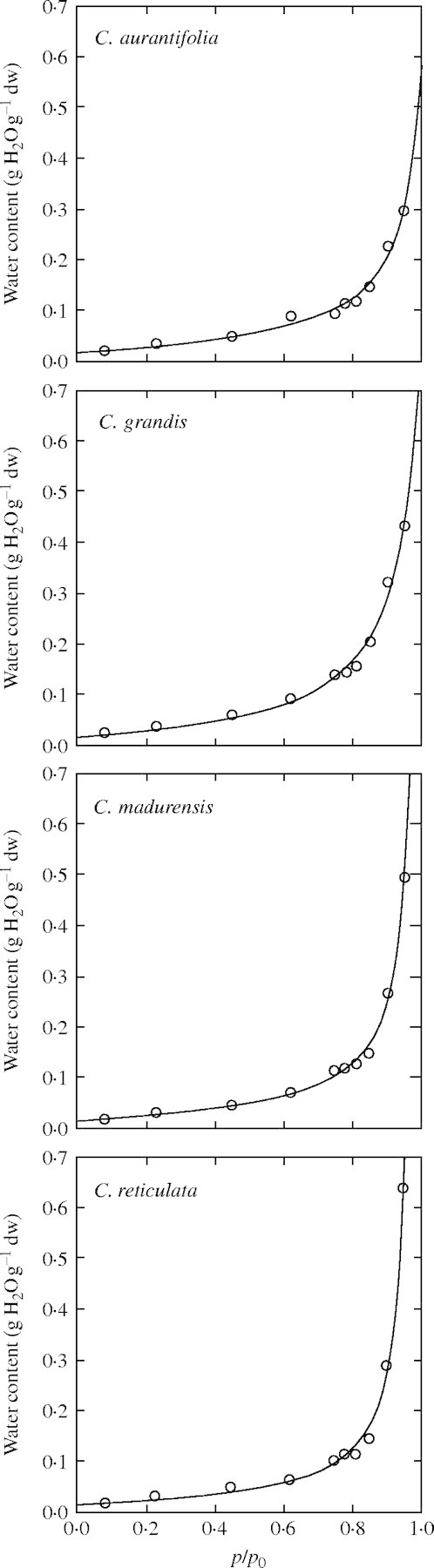
Sorption isotherms of the four Citrus species studied at 25 °C. The line corresponds to the fitted pattern using the simplified D'arcy and Watt model proposed in Dussert et al. (1999).
Table 1.
Specific parameters of the simplified water sorption model, K′, c, k and k′, and proportion of variance explained by the regression model, R2
| Species |
K′ |
c |
k |
k′ |
R2 (%) |
|---|---|---|---|---|---|
| C. aurantifolia | 0·015 | 0·027 | 0·952 | 0·028 | 99 |
| C· grandis | 0·017 | 0·003 | 0·946 | 0·047 | 99 |
| C. madurensis | 0·016 | 0·022 | 1·001 | 0·024 | 98 |
| C. reticulata | 0·015 | 0·010 | 1·014 | 0·023 | 99 |
Desiccation sensitivity
A great variability was observed among the four species studied for the level of desiccation tolerance of their seeds, as measured after equilibration under various RHs (Table 2 and Fig. 3). C. aurantifolia seeds exhibited no decline in germination percentage when desiccated in a RH as low as 8 % (water content of 0·02 g H2O g−1 d. wt). Among the three other species, aw50 varied from 0·67 in C. grandis, to 0·85 in C. madurensis (WC50 ranged from 0·10 to 0·17 g H2O g−1 d. wt).
Table 2.
Water content (WC50), water activity (aw50) and water potential (Ψ50) at which half of the initial germinability was lost, inverse to the seed-to-seed variation (β) total lipid content, unfrozen water content (WCu), and corresponding water activity (awu) and water potential (Ψu), of seeds of the four Citrus species studied
| Species |
WC50 (g g−1) |
aw50 |
β |
Ψ50 (MPa) |
Lipid content (% d. wt) |
WCu (g g−1) |
awu |
Ψu (MPa) |
|---|---|---|---|---|---|---|---|---|
| C. aurantifolia | <0·02 | <0·08 | – | <−340 | 52·4 | 0·09 | 0·74 | −41 |
| C. grandis | 0·10 | 0·67 | 35·0 | −54 | 37·1 | 0·14 | 0·77 | −36 |
| C. madurensis | 0·16 | 0·85 | 20·4 | −22 | 46·2 | 0·11 | 0·77 | −35 |
| C. reticulata | 0·14 | 0·82 | 27·5 | −27 | 51·8 | 0·09 | 0·74 | −41 |
Fig. 3.
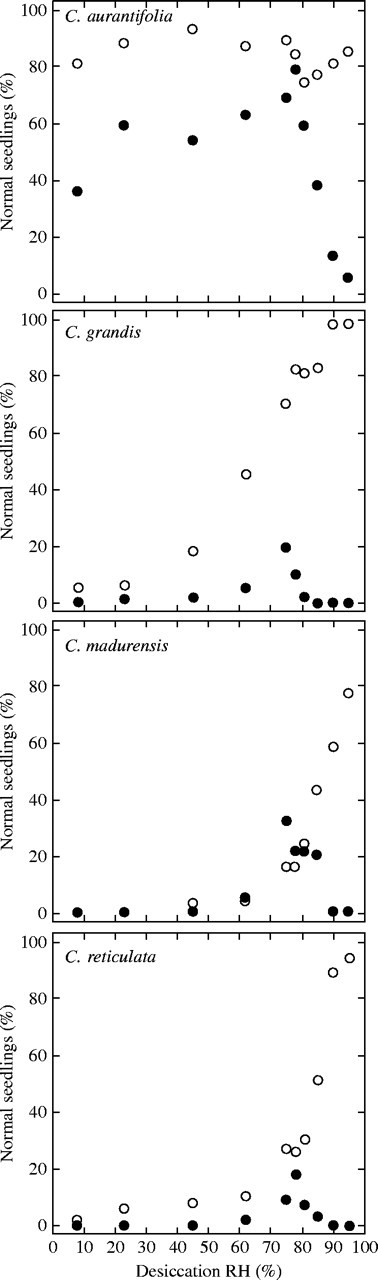
Relationship between desiccation RH (%) and germination percentage after desiccation (open circles) or desiccation and LN exposure (filled circles) in C. aurantifolia, C. grandis, C. madurensis and C. reticulata.
Sensitivity to LN exposure
In the four species studied, independent of their level of seed desiccation tolerance, the same biphasic response was observed for seedling recovery after LN exposure (Fig. 3). No survival was achieved at the highest RHs tested, a sharp increase in seedling recovery percentage was noted with lowering the desiccation RH down to 75–80 %, and then a slow decline in seed germination percentage with decreasing desiccation RH from 75 to 8 %. In all species studied, optimal RH values for seed cryopreservation were thus within the narrow 75–80 % window.
Seed response to LN exposure differed in the four species studied by two other characteristics: (1) in C. grandis, germination percentage of seeds exposed to LN at the optimal RH was significantly lower than that obtained after desiccation only, while no significant additional detrimental effect of LN exposure was observed at the optimal RH in the three other species (Fig. 3); (2) C. aurantifolia could be distinguished from C. madurensis and C. reticulata by the very high level of desiccation tolerance of its seeds, allowing high germination percentages after freezing even at the lowest RHs tested (Fig. 3). However, in C. aurantifolia, a significant decline in seed tolerance to LN exposure was observed with decreasing desiccation RHs from 75 % to 8 % (Fig. 3).
Total lipid content
The total lipid content of seeds of the four Citrus species studied was very high, ranging from 37·1 in C. grandis to 52·4 % d. wt in C. aurantifolia (Table 2).
Endotherm identification in heating thermograms
In the four Citrus species, similar global changes were observed in heating thermograms with varying seed water contents. Heating thermograms of four C. reticulata samples at different water contents are given in Fig. 4 in order to illustrate these global changes. At low water contents (<0·08 g H2O g−1 d. wt), three main endotherms (peaks 1, 2 and 3) were detected without any apparent increase in their size nor change in their onset temperatures with varying seed water contents (example given in Fig. 4: 0·07 g H2O g−1 d. wt). At higher water contents, another endotherm (peak 4) appeared between peaks 1 and 2 (example given in Fig. 4: 0·13 g H2O g−1 d. wt). With continuing seed rehydration, the size and the onset temperature of peak 4 increased (example given in Fig. 4: 0·28 g H2O g−1 d. wt) and, at the highest water contents experimented, peaks 1 and 3 appeared only as small shoulders of peak 4, while peak 2 was completely covered by peak 4 (example given in Fig. 4: 0·41 g H2O g−1 d. wt). DSC analysis of seed lipid extracts revealed the presence of several endotherms whose characteristics were identical to those of peaks identified in thermograms of seeds at low water contents. Heating thermograms of a C. grandis seed sample at 0·07 g H2O g−1 d. wt and of a C. grandis lipid sample are given in Fig. 4B to illustrate these comparisons. Using all these criteria (change in temperature and area with varying seed water content, comparison with the calorimetric characteristics of extracted lipid), the different endotherms identified in seed samples could be easily attributed to the melting transition of either lipids or ice.
Fig. 4.
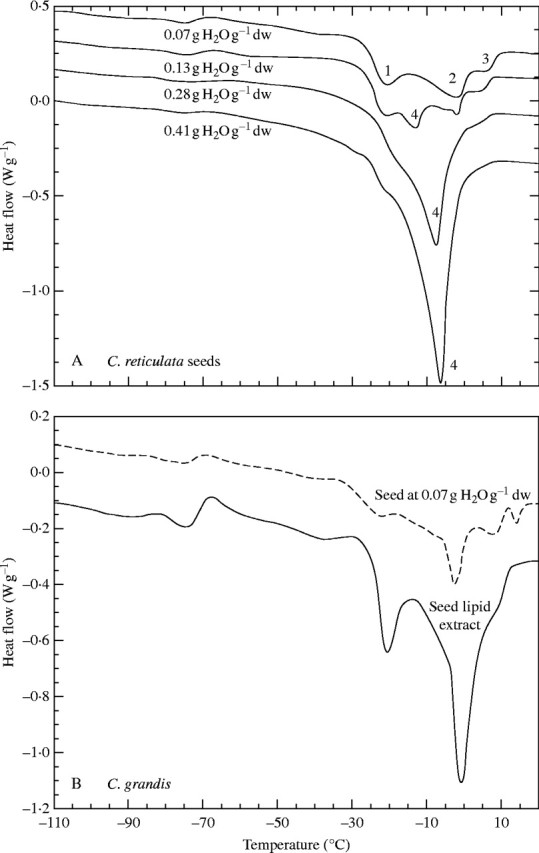
DSC heating thermograms of (A) seed samples of C. reticulata at various water contents and of (B) a seed sample of C. grandis at 0·07 g H2O g−1 d. wt and a sample of total lipids extracted from dry C. grandis seeds.
Relationships between seed unfrozen water content, lipid content and optimal water status for tolerance to LN exposure
Because the lipid and ice melting events always overlapped, the sum of all peak areas was used for regression against seed sample water content as performed by Dussert et al. (2001) in coffee seeds and Vertucci (1989a, b) in sunflower and soybean seeds. With each of the four species studied, this method led to the determination of two regression lines (Fig. 5) whose characteristics (slope and y value at origin) are given in Table 3. The y value at the origin of the regression line obtained at low water contents was always significant, and significantly correlated to seed lipid content, indicating the high sensitivity of the measurements done. Values of the heat of fusion of freezable water were consistent with those observed in other lipid-rich seeds (Vertucci, 1989a; Dussert et al., 2001).
Fig. 5.
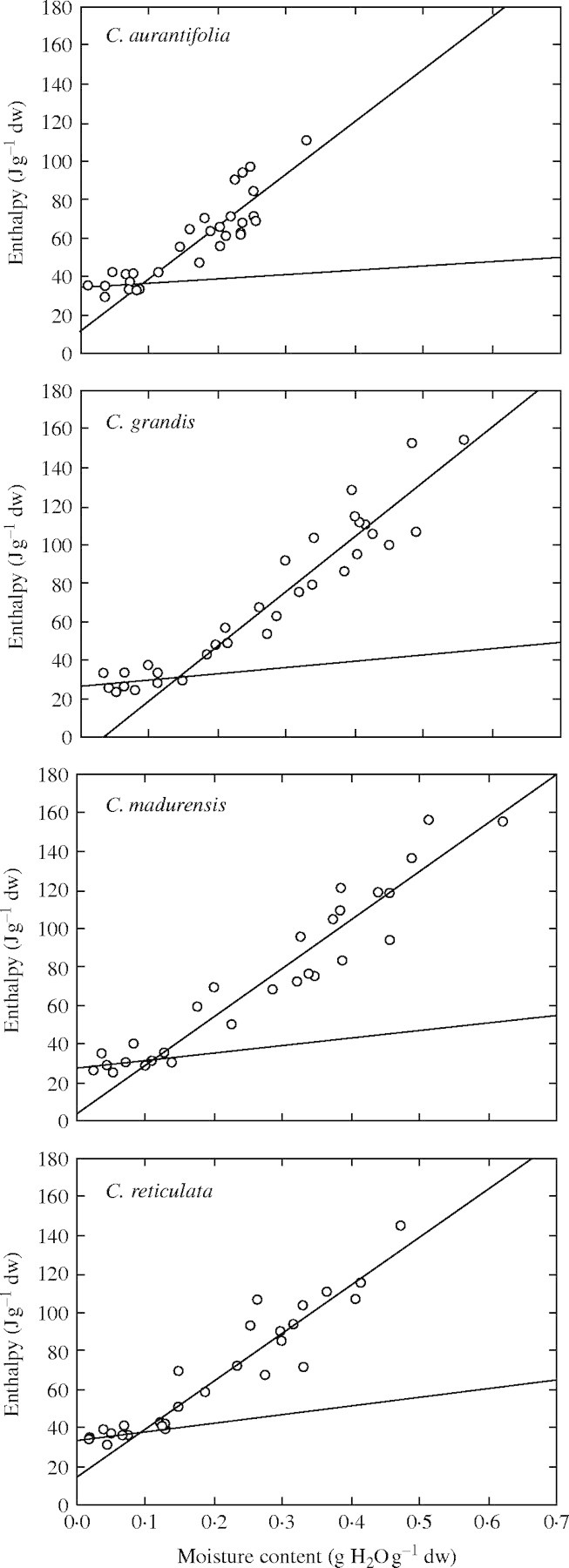
Determination of the unfrozen water content of seeds of the four Citrus species studied as calculated by the x-intercept of the intersection between the two lines of regression which best fitted the relationship between water content and enthalpy of the melting transitions of seed samples dried to various water contents.
Table 3.
Regression parameters, within the two regions of water contents (absence/presence of freezable water in seed tissues), of the relationship between water content and enthalpy of melting transitions of seed samples of the four Citrus species studied, proportion of variance explained by the regression, R2, and probability P of significance of each parameter
| Linear regression at low water contents |
Linear regression at high water contents |
Heat of fusion of freezable water (J g−1 H2O) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
y value at origin |
Slope (J g−1 H2O) |
y value at origin |
Slope (J g−1 H2O) |
R2 (%) |
|||||
| C. aurantifolia | 34·4 | 22·0 | 11·0 | 270·6 | 59 | 248·5 | |||
| P | <0·0001 | 0·7205 | 0·3055 | <0·0001 | |||||
| C. grandis | 26·5 | 32·2 | −9·8 | 282·3 | 83 | 250·1 | |||
| P | 0·0001 | 0·4668 | 0·3590 | <0·0001 | |||||
| C. madurensis | 27·9 | 38·0 | 3·5 | 250·1 | 81 | 212·1 | |||
| P | <0·0001 | 0·3405 | 0·7748 | <0·0001 | |||||
| C. reticulata | 33·8 | 44·6 | 15·2 | 246·8 | 84 | 202·2 | |||
| P | <0·0001 | 0·1512 | 0·0653 | <0·0001 | |||||
A high variability was found among the four Citrus species for the unfrozen water content of their seeds, WCu, as determined by the x-intercept of the intersection between the two regression lines. It was 0·14 g H2O g−1 d. wt for C. grandis, 0·12 g H2O g−1 d. wt for C. madurensis and 0·09 g H2O g−1 d. wt for C. aurantifolia and C. reticulata (Table 2).
Using the fitted sorption isotherm equations, water activity and water potential corresponding to WCu were calculated in each species. These two parameters were almost constant among the four Citrus species studied (Table 2): awu was about 0·75 and Ψu was about −38 MPa. These values were remarkably consistent with those at which seed tolerance to LN exposure was maximal (Fig. 3).
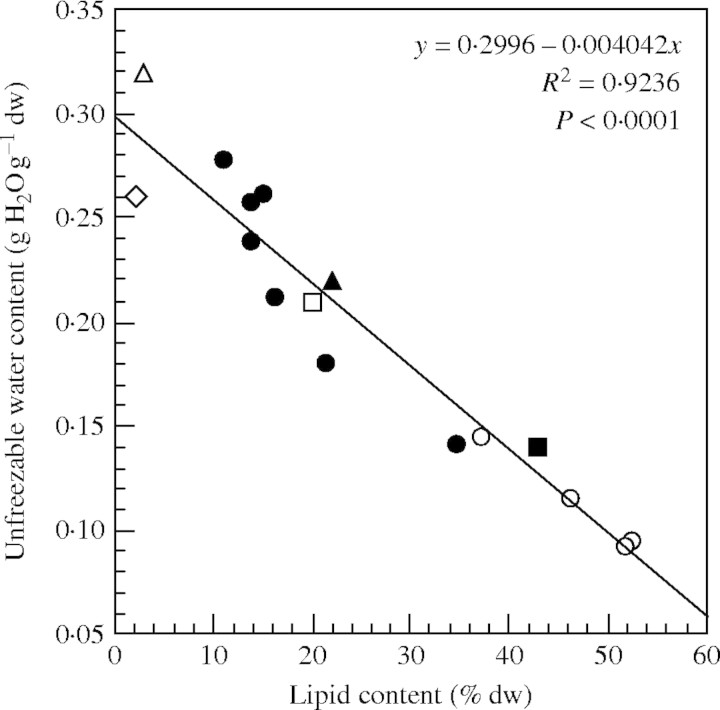
A negative relationship (R2 = 0·99, P = 0·0031) was found between the unfrozen water content and the lipid content of seeds of the four Citrus species studied (Table 2). When combining values obtained in the present study, those previously found in seven coffee species (Dussert et al., 2001), and those reported by other authors for different species (Azadirachta indica, Sacandé et al., 2000; pea, Vertucci, 1989a; Quercus rubra, Pritchard and Manger, 1998; Quercus robur, Sun, 1999; soybean, Vertucci, 1989a), a highly significant correlation was observed between these two traits. The high R2 value of the regression line obtained indicates the predictive value of the equation found:
 |
where WCu (g H2O g−1 d. wt) is the seed unfrozen water content and LC is the seed lipid content (% d. wt).
DISCUSSION
A great variability was observed between the four Citrus species studied for the level of desiccation tolerance of their seeds, independent of the variable used to quantify it, water content or water potential. This result is consistent with previous reports in which different groups of Citrus species have been studied (Normah and Serimala, 1995; Saipari et al., 1998; Lambardi et al., 2004). Although they display very different levels of seed desiccation tolerance, C. aurantifolia, C. grandis and C. reticulata have been classified in the intermediate seed storage category (Hong and Ellis, 1995). Therefore, as previously concluded when studying seeds of different Coffea species (Dussert et al., 1999, 2004), the present study highlights the difficulty in determining the boundaries, in terms of level of desiccation sensitivity, of each category of storage behaviour.
Independent of their level of seed desiccation tolerance, the four species studied shared a main common feature for the response of their seeds to LN exposure, which is that their optimal desiccation RH was 75–80 %. Since these values are very similar to those at which no more freezable water was detected, as determined by DSC analysis, the present results offer additional evidence that lipid-rich seeds do not withstand the presence of freezable water in their tissues during the cooling/thawing process. By comparison with pea seeds, which tolerate LN exposure after equilibration in RHs as high as 92 %, Vertucci (1989a) suggested that, in oily seeds, the interaction between storage lipids and water somehow promoted formation of ice crystals large enough to cause lethal damage.
In C. aurantifolia, a decline in seed tolerance to LN exposure was observed with decreasing desiccation RHs from 75 to 8 %, while desiccated seeds exhibited no injury. This particular pattern has already been observed in some orthodox lipid-rich seed species, such as sesame (Stanwood, 1987), soybean and sunflower (Vertucci, 1989b), when a high cooling rate (≥200 °C min−1) was employed. The cooling regime used in the present study corresponds to a cooling rate of about 200 °C min−1. Therefore, it is possible that the decline in seed tolerance to LN exposure observed in C. aurantifolia at low water contents is due to the sensitivity of lipid-rich seeds to rapid cooling. It is difficult to ascertain whether this detrimental effect of a rapid cooling regime participates in the difference in seed germination percentage observed in C. grandis between control and frozen seeds within the 23–75 % RH interval. In Coffea arabica, whose seed response to LN exposure is similar to that observed in C. grandis, the beneficial effect of a low cooling rate was only moderate, though significant (Dussert et al., 2001).
The fact that, in different species producing lipid-rich seeds, such as Citrus, coffee and soybean, maximal tolerance to immersion in LN was always reached after equilibration in an RH between 75 and 80 % highlights an important applied perspective. Independent of their level of desiccation tolerance, testing germination of seeds of a given species after equilibration over saturated NaCl, NH4Cl and (NH4)2SO4 solutions (75, 78 and 81 % RH at 25 °C, respectively) and LN exposure, gives a rapid and reliable evaluation of the possibility of cryopreserving whole seeds of this given oily species. It should be noted that the fact that seeds tolerate desiccation under 75–80 % RH is not a sufficient condition for them to tolerate exposure to LN temperature after such desiccation. This is perfectly illustrated by the difference in germination percentage observed between control and frozen C. grandis seeds after desiccation in 75 % RH.
The unfrozen water content in Citrus species was found to be negatively correlated to seed lipid content. Interestingly, using the data obtained in the present study, those collected previously with seeds of various coffee species (Dussert et al., 2001), and all data found in the literature (Vertucci, 1989a; Pritchard and Manger, 1998; Sun, 1999; Sacandé et al., 2000), it was possible to cover a very large interval of seed lipid contents and to show that a significant general negative correlation exists between the two traits. The existence of such a negative correlation is not new (e.g. Vertucci, 1989a) but, until now, not enough data were available to characterize it. After the studies performed with a relatively high number of coffee and Citrus species, it is now possible to have a good estimate of the unfrozen water content of seeds as a function of their lipid content using the equation given in Fig. 6. Assuming that oily seeds do not withstand the presence of freezable water in their tissues during the cooling/thawing process, it is now possible to predict a narrow interval of water contents in which oily seeds of a given species are likely to withstand LN exposure as a function of their lipid content. This can be very useful for germplasm curators who use desiccation methods other than equilibration in controlled RH (e.g. using silica-gel, air flow), or for manipulating seeds for which a detrimental effect of a low drying rate, such as that obtained by equilibration, has been shown. After compilation of seed high moisture freezing limit (HMFL) and lipid content (LC) data published for 13 different species, Pritchard (1995) found a significant negative correlation between the two traits, whose equation was
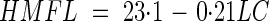 |
where HMFL is expressed in percentage of the fresh weight (% f. wt). Interestingly, when expressing WCu values given in Fig. 6 in % f. wt, the equation of the regression line is
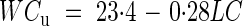 |
Fig. 6.
Relationship between the lipid content and the unfrozen water content of seeds of the four Citrus (open circles) species analysed in the present study, seven coffee (filled circles) species (Dussert et al., 2001), Azadirachta indica (Sacandé et al., 2000; filled square), pea (Vertucci, 1989a; open diamond), Quercus rubra (Pritchard and Manger, 1998; open triangle), Quercus robur (Sun, 1999; open square) and soybean (Vertucci, 1989a; filled triangle).
Even if the slope is slightly different, the similarity of the two equations indicates that extending the approach developed in the present study to an even greater number of intermediate oily species should allow to provide, in the near future, tropical germplasm curators with routine simple methods to determine the optimal hydration status for cryopreservation of any given intermediate oily seed species.
Acknowledgments
This work is the result of a collaborative study between University of Putra Malaysia (UPM), Institut de Recherche pour le Développement (IRD) and International Plant Genetic Resources Institute (IPGRI). This study has been supported in part by the International Plant Genetic Resources Institute (Serdang, Malaysia and Rome, Italy) and the Commission of the European Communities, specific Cooperative Research programme Quality of Life and Management of Living Resources, QLK5-2002-1279. The authors gratefully acknowledge Gérard Rocquelin (IRD) and Hervé Rimbaud (Calytherm SA, Montpellier, France) for their precious help with lipid and DSC analysis, respectively.
Footnotes
Present address: Sunway University College, Bandar Sunway, 46150 Petaling Jaya, Selangor, Malaysia.
Present address: Pôle Biodiversité Antilles, Cirad, Station de Roujol, 97170 Petit-Bourg, Guadeloupe, French West Indies.
LITERATURE CITED
- Dussert S, Chabrillange N, Engelmann F, Hamon S. 1999. Quantitative estimation of seed desiccation sensitivity using a quantal response model: application to nine species of the genus Coffea L. Seed Science Research 9: 135–144. [Google Scholar]
- Dussert S, Chabrillange N, Rocquelin G, Engelmann F, Lopez M, Hamon S. 2001. Tolerance of coffee (Coffea spp.) seeds to ultra-low temperature exposure in relation to calorimetric properties of tissue water, lipid composition and cooling procedure. Physiologia Plantarum 112: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussert S, Engelmann F, Louarn J, Noirot M. 2004. Inheritance of seed desiccation sensitivity in a coffee inter-specific cross: evidence for polygenic determinism. Journal of Experimental Botany 55: 1541–1547. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry 226: 497–509. [PubMed] [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. 1990. An intermediate category of seed storage behaviour? I. Coffee. Journal of Experimental Botany 41: 1167–1174. [Google Scholar]
- Hong TD, Ellis RH. 1995. Interspecific variation in seed storage behaviour within two genera—Coffea and Citrus Seed Science and Technology 23: 165–181. [Google Scholar]
- Hong TD, Linington S, Ellis RH, eds. 1996.Compendium of information on seed storage behaviour. Rome: International Plant Genetic Resources Institute. [Google Scholar]
- Lambardi M, De Carlo A, Biricolti S, Puglia AM, Lombardo G, Siragusa M, De Pasquale F. 2004. Zygotic and nucellar embryo survival following dehydration/cryopreservation of Citrus intact seeds. CryoLetters 25: 81–90. [PubMed] [Google Scholar]
- Normah MN, Sirimala SD. 1995. Cryopreservation of seeds and embryonic axes of several Citrus species. In: Ellis RH, Black M, Murdoch AJ, Hong TD, eds. Basic and applied aspects of seed biology. Dordrecht: Kluwer, 817–823. [Google Scholar]
- Ouédraogo AS, Thomsen K, Engels JMM, Engelmann F. 1999. Challenges and opportunities for enhanced use of recalcitrant and intermediate tropical forest tree seeds through improved handling and storage. In: Marzalina M, Khoo KC, Tsan FY, Krishnapillay B, eds. Seed Symposium 1998 ‘Recalcitrant Seeds’, 12–15 October 1998. Kuala Lumpur: IUFRO, 227–234. [Google Scholar]
- Pritchard HW. 1995. Cryopreservation of seeds. In: Day JG, Mc Lellan MR, eds. Methods in molecular biology, Vol. 38: Cryopreservation and freeze-drying protocols. Totowa, NJ: Humana Press, 133–144. [Google Scholar]
- Pritchard HW, Manger KR. 1998. A calorimetric perspective on desiccation stress during preservation procedures with recalcitrant seeds of Quercus robur L. Cryo-Letters Supp 1: 23–30. [Google Scholar]
- Roberts EH. 1973. Predicting the storage life of seeds. Seed Science and Technology 1: 499–514. [Google Scholar]
- Sacandé M, Buitink J, Hoekstra FA. 2000. A study of water relations in neem (Azadirachta indica) seed that is characterized by complex storage behaviour. Journal of Experimental Botany 51: 635–643. [DOI] [PubMed] [Google Scholar]
- Saipari E, Goswami AM, Dadlani M. 1998. Effect of seed drying on germination behaviour in Citrus Scientia Horticulturae 73: 185–190. [Google Scholar]
- Stanwood PC. 1987. Survival of sesame seeds at the temperature (–196 °C) of liquid nitrogen. Crop Science 27: 327–331. [Google Scholar]
- Sun WQ. 1999. State and phase transition behaviors of Quercus rubra seed axes and cotyledonary tissues: relevance to the desiccation sensitivity and cryopreservation of recalcitrant seeds. Cryobiology 38: 372–385. [DOI] [PubMed] [Google Scholar]
- Vertucci CW. 1989. Relationship between thermal transitions and freezing injury in pea and soybean seeds. Plant Physiology 90: 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci CW. 1989. Effects of cooling rate on seeds exposed to liquid nitrogen temperatures. Plant Physiology 90: 1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci CW. 1990. Calorimetric studies of the state of water in seed tissues. Biophysical Journal 58: 1463–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci CW, Leopold AC. 1987. Water binding in legume seeds. Plant Physiology 85: 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]