Abstract
• Background and Aims Floral design in self-compatible plants can influence mating patterns. This study investigated Narcissus longispathus, a self-compatible bee-pollinated species with wide variation in anther–stigma separation (herkogamy), to determine the relationship between variation in this floral trait and the relative amounts of cross- and self-fertilization.
• Methods Anther–stigma separation was measured in the field in six populations of N. longispathus from south-eastern Spain. Variation in herkogamy during the life of individual flowers was also quantified. Multilocus outcrossing rates were estimated from plants differing in herkogamy using allozyme markers.
• Key Results Anther–stigma separation varied considerably among flowers within the six populations studied (range = 1–10 mm). This variation was nearly one order of magnitude larger than the slight, statistically non-significant developmental variation during the lifespan of individual flowers. Estimates of multilocus outcrossing rate for different herkogamy classes (tm range = 0·49–0·76) failed to reveal a monotonic increase with increasing herkogamy.
• Conclusions It is suggested that the lack of a positive relationship between herkogamy and outcrossing rate, a result that has not been previously documented for other species, could be mostly related to details of the foraging behaviour of pollinators.
Keywords: Allozymes, Amaryllidaceae, anther–stigma separation, floral design, herkogamy, intra- and interpopulation variation, Narcissus longispathus, outcrossing rates
INTRODUCTION
The avoidance of inbreeding has been a major driving force shaping floral design and the evolution of plant mating systems (Darwin, 1877; Charlesworth and Charlesworth, 1987; Richards, 1997; Barrett, 2003). In self-compatible, animal-pollinated species, the spatial segregation of anthers and stigmas within the same flower (herkogamy) is usually considered an adaptive feature that functions to limit inbreeding by reducing the intensity of self-pollination. Indeed, there is some empirical evidence to support this assumption. Studies examining the relationship between the degree of herkogamy and outcrossing rate have generally found a monotonically increasing relationship both within (e.g. Nicotiana rustica, Breese, 1959; Mimulus ringens, Karron et al., 1997; Aquilegia caerulea, Brunet and Eckert, 1998; Datura stramonium, Motten and Stone, 2000) and among (e.g. Clarkia temblorensis, Holtsford and Ellstrand, 1992; Turnera ulmifolia, Belaoussoff and Shore, 1995) populations. Although in some species individual differences in anther–stigma separation may largely reflect plastic responses to variation in environmental factors (Elle and Hare, 2002; Weinig, 2002), investigations on the genetic basis of variation in herkogamy have generally found moderate to high heritabilities, and a rapid response to artificial selection (Breese, 1959; Shore and Barrett, 1990; Holtsford and Ellstrand, 1992; Motten and Stone, 2000; Lendvai and Levin, 2003). Selection on herkogamy variation therefore provides a simple mechanism for adjusting mating patterns in plant populations.
However, the functional role of herkogamy in promoting outcrossing is not always straightforward. Many species that possess this trait are self-incompatible and are therefore already protected from the harmful effects of selfing. In such cases herkogamy may function primarily to reduce interference between female and male sexual functions and/or to enhance pollen export efficiency (Webb and Lloyd, 1986; Barrett, 2002; Cesaro et al., 2004). Moreover, herkogamy commonly occurs in self-compatible species with large floral displays and, in these situations, the trait may be ineffective as a means of avoiding inbreeding, because extensive selfing can occur as a consequence of pollen dispersal between flowers of the same plant (Robertson, 1992; Harder and Barrett, 1995; Brunet and Eckert, 1998; Eckert, 2000; Montaner et al., 2001; Williams et al., 2001; Elle and Hare, 2002; Karron et al., 2004). To isolate clearly the functional role of herkogamy in promoting outcrossing requires a self-compatible species with limited floral display that maintains considerable phenotypic variation in anther–stigma variation in natural populations.
Here the influence of natural variation in herkogamy on outcrossing rate in the self-compatible, mostly single-flowered daffodil Narcissus longispathus Pugsley (Amaryllidaceae) is examined. This species exhibits considerable within-population variation in anther–stigma separation and a previous study (Barrett et al., 2004) identified variable allozyme markers that can be used to estimate multilocus outcrossing rates. Moreover, this study also found that selfed progeny rarely survive to maturity because of high inbreeding depression. Therefore, any influence of anther–stigma separation on outcrossing rate is likely to translate into differential contributions to the next generation, providing evidence for natural selection on herkogamy. The present investigation of N. longispathus addressed three specific questions: (1) What is the magnitude of the variation in anther–stigma separation within and between populations? (2) To what extent do developmental changes in anther–stigma position contribute to variation in herkogamy? (3) Is there a monotonically increasing relationship between the degree of herkogamy exhibited by plants within a population and their outcrossing rates, as found for other species?
MATERIALS AND METHODS
Study species
Narcissus longispathus (Amaryllidaceae) is a bee-pollinated, perennial herb endemic to a few mountain ranges in south-eastern Spain (Moreno Saiz and Sainz Ollero, 1992; Herrera et al., 1999). This large-flowered daffodil (corolla length approx. 50 mm) commonly produces a single flower per inflorescence and is self-compatible, producing equivalent amounts of seed following experimental self- and cross-pollinations (Herrera, 1995). This contrasts with other species in the genus, most of which are moderately to strongly self-sterile (reviewed by Barrett et al., 1996; Sage et al., 1999). In the Sierra de Cazorla mountains, where this study was conducted (see below), N. longispathus is represented by scattered populations confined to stream margins or poorly drained meadows around springs at 1000–1500 m. Flowering occurs from late February to mid-April, a period characterized by cool, rainy weather that frequently limits the activity of the species' main pollinator (Andrena bicolor, Andrenidae). Despite low pollinator visitation, most flowers of N. longispathus are pollinated and seed production is only weakly pollen limited. The species has a mixed mating system producing significant amounts of both outcrossed and selfed seed [range of population mean outcrossing rates (tm) = 0·54–0·77; N = 6 populations; Barrett et al. (2004)].
Field methods
The present study was conducted in six N. longispathus populations located in the Sierra de Cazorla mountain range, Jaén province, south-eastern Spain. Plants were sampled at the same (five sites), or very close to (one site), locations studied by Barrett et al. (2004), where further details of localities can be found. In March 2003, anther–stigma separation was measured in the field on 30–35 flowers (from as many individual plants) per population. Digital calipers were used and, after slitting the corolla, the shortest distance to the nearest 0·1 mm between the rim of the flat stigma and the nearest anther was measured. Given that in N. longispathus the stigma is always above the anthers, variation in herkogamy was positive in all cases. To minimize any possible effect of flower age-related variation in anther–stigma separation, flowers chosen for measurement were all of the same developmental stage: corolla with fully expanded corona, and pollen sacs undehisced or newly dehisced. In the latter case, only flowers with convex anthers that still presented most or all pollen grains were chosen for measurement.
Flowers of N. longispathus are long-lived, lasting for 16·5 d on average when exposed to natural pollination (Herrera, 1995). To assess variation in anther–stigma separation during the life of individual flowers, two different kinds of data were obtained in 2004. At the Cuevas Bermejas population, 25 newly opened flowers were individually marked on 12 March and scored using the following six classes of anther–stigma separation: 0–2 mm, 2–4 mm, 4–6 mm, 6–8 mm, 8–10 mm and >10 mm. One week later this procedure was repeated. In addition, seven bulbs with sprouting scapes bearing flower buds were excavated. They were potted with local soil, and kept outdoors until their flowers opened. The herkogamy class of each flower was then recorded daily from opening until the flowers withered or the anthers had most pollen removed.
To investigate the influence of herkogamy on outcrossing rate, 45 flowers in a population at Cuevas Bermejas were individually marked. Each flower was assigned to one of the six classes of anther–stigma separation indicated above. These discrete herkogamy classes, rather than direct measurements, were used because destructive manipulation of the corolla needed for accurate measurements would have most likely affected pollinator visitation and mating patterns. These flowers were left exposed to natural pollination, and the fruits produced (N = 32) collected at maturity in early June. Observed fruit set (71 %) fell within the limits commonly observed for this species (Herrera, 1995). After collection seed progenies were kept separate in paper bags at ambient temperature until electrophoretic analyses.
Electrophoresis
Variation was assayed at allozyme loci from the seed families (N = 32 families/637 seeds) using horizontal starch gel electrophoresis following methods detailed in Barrett et al. (2004). For each seed family genotypes were scored for 6–38 seeds (mean = 19·9). Seeds were prepared for electrophoretic analysis by soaking overnight in water, and homogenized in a 0·02 m Na2HPO4 buffer (pH 7·4) containing dl-dithiothreitol (1 mg ml−1). The crude extract was immediately absorbed onto chromatography paper wicks (Whatman 3MM™), and kept at −80°C until electrophoresis was performed on 11 % starch gels. Based on previous work (Barrett et al., 2004), six variable loci which were known to be polymorphic in the Cuevas Bermejas population were resolved. Two buffer systems were used: lithium-borate (pH 8·3) to resolve aspartato amino transferase, diaphorase and cytosol aminopeptidase; and histidine-citrate (pH 6·5) to resolve aconitase, acid phosphotase and phosphoglucose isomerase. All loci were diallelic. Genotypes were inferred based on segregation patterns of either dimeric or monomeric codominant enzymes. Polymorphic loci conformed to Mendelian expectations for segregation in the analysed progeny arrays.
Estimates of mating system and inbreeding
We estimated the multilocus outcrossing rate (tm) and the parental inbreeding coefficient (f) for the Cuevas Bermejas population (all herkogamy classes combined) using the computer program MLTR for Windows by K. Ritland (version 2·4, November 2002, available from the Internet at http://genetics.forestry.ubc.ca/ritland/programs.html; see also Ritland, 1990a). This program uses the methods of Ritland and Jain (1981) for multilocus maximum likelihood estimation of outcrossing rates, and implements Ritland's extensions (Ritland, 2002) to the mixed mating model to incorporate correlated mating. Estimates of tm were also computed for each herkogamy class using the MLTR program. There was extensive spatial intermingling in the studied population of flowers in different herkogamy classes; hence it seemed reasonable to assume that all families shared the same outcross pollen pool irrespective of the herkogamy class they belonged to. This assumption was incorporated into the computations by running the MLTR program on the whole data set (N = 32 families) and treating each herkogamy class as a different group of families. In this way, a single tm figure (and standard error, see below) was obtained for the entire set of progeny in each herkogamy class. This procedure was deemed preferable to estimating the outcrossing rate for each seed family and then relating these values to herkogamy using regression or analysis of variance approaches. The difficulties involved in obtaining accurate family-level estimates of outcrossing in naturally pollinated plants have been repeatedly emphasized in the literature, and Ivey and Wyatt (1999) went on to conclude that current family-level estimators for outcrossing are possibly inadequate. The different estimation methods available produce inconsistent estimates, involve very large estimation errors, fail to converge on reasonable estimates for some families, and lead to spurious correlations with environmental or other parameters (e.g. Morgan and Barrett, 1990; Cruzan et al., 1994; Leclerc-Potvin and Ritland, 1994; Cruzan, 1998; Ivey and Wyatt, 1999; Ritland, 2002).
Inbreeding depression (δ = 1 − fitness of selfed progeny/fitness of outcrossed progeny) for survival from seed to reproductive maturity for the Cuevas Bermejas population was calculated following Barrett et al. (2004), by substituting the population estimates of multilocus outcrossing rate and parental inbreeding coefficient into Ritland's equilibrium estimator of inbreeding (Ritland, 1990b). This estimator allows calculation of inbreeding depression when information is available from one generation, but requires an assumption of inbreeding equilibrium. Standard errors for all parameters were calculated by bootstrapping 5000 times over progeny arrays, using the N = 32 families as the units of resampling.
RESULTS
Variation in herkogamy
Variation in anther–stigma separation over the life of individual N. longispathus flowers was relatively minor and statistically non-significant. In the set of 25 flowers scored twice for herkogamy class, mean anther–stigma separation (±1 s.d.) changed from 4·8 ± 2·8 mm to 5·5 ± 2·0 mm over 1 week. This increase was not statistically significant (P = 0·36, randomization test for paired data on class midpoints). A similar result was obtained for the flowers of potted plants kept outdoors and monitored daily for a 10-d period. In this group of flowers, anther–stigma separation increased from 3·3 ± 1·8 mm at the time of flower opening to 4·7 ± 2·1 mm at flower withering or pollen exhaustion (P = 0·38, randomization test for paired data).
Anther–stigma separation varied considerably among flowers within the six populations studied, ranging from 1 to 10 mm (Table 1). In all populations, the range of variation observed was nearly one order of magnitude broader than the slight, statistically non-significant developmental variation recorded during the lifespan of individual flowers. There was significant heterogeneity among populations in mean anther–stigma separation (F5,179 = 8·88, P < 0·0001), but variation among populations was relatively minor in absolute terms, accounting for only 20·8 % of the total variance. Most observed variation in anther–stigma separation occurred among flowers of the same population (79·2 % of total variance).
Table 1.
Variation in anther–stigma separation within and among six Narcissus longispathus populations in the Sierra de Cazorla mountain range, south-eastern Spain
| Population |
N |
Range (mm) |
Mean ± s.d. (mm) |
|---|---|---|---|
| Cuevas Bermejas | 30 | 2·2–10·4 | 6·7 ± 2·2 |
| Tornillos de Gualay – A | 30 | 1·1–7·3 | 4·5 ± 1·6 |
| Tornillos de Gualay – C | 35 | 0·5–10·4 | 5·1 ± 2·2 |
| Valdecuevas | 30 | 3·5–10·3 | 7·1 ± 1·6 |
| Valdetrillos – 1 | 30 | 1·5–9·5 | 6·1 ± 2·0 |
| Valdetrillos – 2 | 30 | 1·8–7·7 | 5·1 ± 1·5 |
N = number of flowers measured.
Population-level mating and genetic parameters
The mean multilocus outcrossing rate (±1 s.e.) for the Cuevas Bermejas population in 2003, all herkogamy classes pooled, was tm = 0·598 ± 0·011 (95 % range of bootstrap estimates = 0·504–0·699). The inbreeding coefficient of parent plants (± 1 s.e.) was f = −0·098 ± 0·011, which did not differ significantly from zero (95 % range of bootstrap estimates = −0·200–0·009). The estimate of inbreeding depression obtained using Ritland's equilibrium estimator (Ritland, 1990b) was δ = 1·265, which was significantly >0 and did not differ significantly from unity (95 % range of bootstrap estimates = 0·97–1·58).
Anther–stigma separation and outcrossing rate
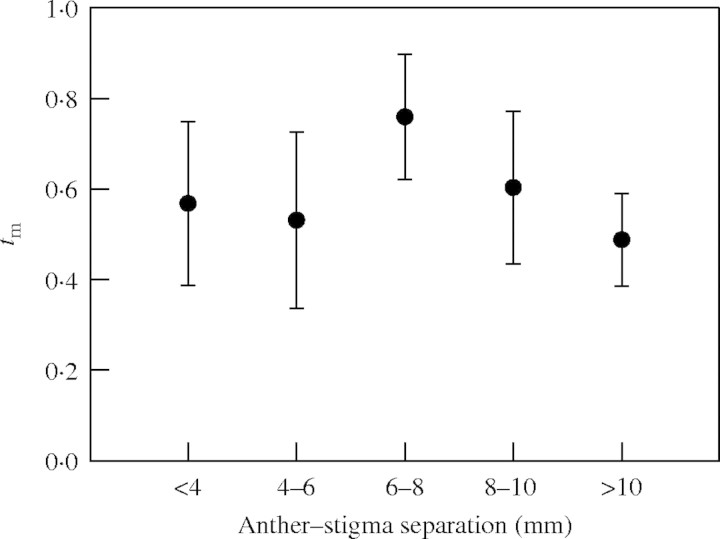
Mean multilocus outcrossing rates for the different herkogamy classes are illustrated in Fig. 1. The number of families and seeds available for the smallest herkogamy class (0–2 mm) was insufficient for separate analysis, and this class was therefore combined with the contiguous one (2–4 mm) to form a single category (<4 mm). Standard errors are similarly large for all herkogamy classes, as would be expected from the relatively small number of progenies examined per class (Fig. 1). There is some suggestion that mean outcrossing rate may vary among herkogamy classes, as there is a statistically significant difference between the means for the two classes exhibiting the extreme values: tm = 0·758 for the 6–8 mm class, and 0·488 for the >10 mm class (P = 0·050; comparison based on 5000 bootstrap repetitions). Nevertheless, this comparison is not statistically significant after adjustment of the significance level to account for the fact that the test was an unplanned comparison (e.g. Steel and Torrie, 1980).
Fig. 1.
Mean multilocus outcrossing rates (tm) for five herkogamy classes in the Cuevas Bermejas population of Narcissus longispathus, Sierra de Cazorla mountains, south-eastern Spain. Vertical segments extend over ±1 s.e. (obtained by bootstrapping). Number of families (Nf) and seeds (Ns) in each herkogamy class are as follows: <4 mm, Nf = 7, Ns = 106; 4–6 mm, Nf = 5, Ns = 95; 6–8 mm, Nf = 7, Ns = 173; 8–10 mm, Nf = 7, Ns = 120; >10 mm, Nf = 6, Ns = 143.
The pattern of variation in mean outcrossing rate with increasing herkogamy illustrated in Fig. 1 does not support a monotonically increasing relationship, as the two variables are uncorrelated (r = −0·134, P = 0·82, randomization test). Instead, there is a discernible trend for flowers with intermediate anther–stigma separation (6–8 mm) to be at a relative outcrossing advantage over those having either smaller or larger anther–stigma separation. This hypothesis was evaluated by comparing the values of class-specific outcrossing rates in 5000 randomly generated bootstrap samples. Statistical significance was assessed by determining the proportion of bootstrapped samples where either tm for the central 6–8 mm class was larger than the average tm for the remaining four classes, or the mean tm for the three central classes (4–6 mm, 6–8 mm and 8–10 mm) was larger than the mean tm for the two extreme classes (<4 mm and >10 mm). The results of this analysis support the hypothesis (at P = 0·028) of a relative outcrossing advantage to flowers with intermediate herkogamy in the N. longispathus population studied. It is unclear how the P-level should be properly adjusted to account for the unplanned nature of this ad hoc test, but any adjustment would lead to a more stringent P-value and, therefore, to statistical non-significance.
DISCUSSION
Herkogamy is a character that is frequently subject to ontogenetic change during a flower's lifespan, particularly in species in which anthers and stigmas mature at different times (Webb and Lloyd, 1986). Repeated measurement of anther–stigma separation on the same flowers of N. longispathus indicated that herkogamy increased with flower age. However, the temporal change in sex-organ separation was generally very small (0·72 ± 1·72 mm) and did not differ significantly from zero. This result clearly indicates that the within-population variation in anther–stigma separation that was observed mainly reflects differences between individual plants, rather than any heterogeneity of flower ages. Accordingly, it is valid to consider the ecological and evolutionary consequences of herkogamy variation in populations of N. longispathus.
Mating consequences of herkogamy variation
Individual variation in herkogamy was considerable in each of the six N. longispathus populations studied. The observed range is among the broadest so far reported for species with continuous herkogamy variation. In absolute terms, within-population ranges of anther–stigma separations in N. longispathus are comparable to those reported for Datura stramonium (Motten and Stone, 2000) and Aquilegia caerulea (Brunet and Eckert, 1998), and broader than those of several other species with herkogamy variation (e.g. Mimulus ringens, Karron et al., 1997; Ipomoea purpurea, Epperson and Clegg, 1987; Nicotiana rustica, Breese, 1959; Turnera ulmifolia, Shore and Barrett, 1990). The occurrence of this range of herkogamy raises the question of what selective forces maintain the variation in populations of N. longispathus. Clearly, understanding the reproductive consequences of herkogamy variation in terms of pollination and mating is critical for addressing this issue.
Previous studies that have investigated how anther–stigma separation influences outcrossing rate have generally found a monotonically increasing relationship between the two variables (see references in the Introduction). Logically, it would have been expected that the closer the anthers and stigmas are located in a flower the more likely autonomous and/or pollinator facilitated self-pollination would occur. The present results for N. longispathus depart from earlier investigations and this simple functional expectation. Outcrossing rates did not increase monotonically with anther–stigma separation in the population studied. There was some indication, however, that mean outcrossing rate did vary among herkogamy classes, and that flowers with intermediate values of herkogamy may be at a relative outcrossing advantage over those with either smaller or larger physical distances separating anthers and stigmas. Nevertheless, tests of these relationships did not reach statistical significance if more-stringent significance levels were used to account for the unplanned nature of comparisons. Low statistical power is a well-known limitation inherent in investigations comparing mating-system parameters among experimental treatments, populations, or groups of plants (Leclerc-Potvin and Ritland, 1994; Cruzan, 1998; Ivey and Wyatt, 1999). Unless sample sizes are very large, standard errors of tm estimates are generally too large to allow for the detection of among-group differences, and results of the present investigation are clearly no exception to this pattern. Under these circumstances, it is not unreasonable to consider the present failure to obtain fully significant test results as an instance of Type II error derived from intrinsically low statistical power.
The proximate mechanism underlying the lack of a consistent, monotonically increasing relationship between herkogamy and outcrossing rate can only be conjectured at present, but it is most likely related to details of the foraging behaviour of pollinators. In the N. longispathus populations studied, variation in herkogamy mainly depends on variation in style length, and anther–stigma separation is inversely correlated with distance from the stigma to the rim of the corona (M. Medrano and C. M. Herrera, unpubl. res.). The most herkogamous flowers tend to be also those with longest styles and with stigmas located closest to the rim of the corona. After visiting the shady interior of a N. longispathus flower for nectar and pollen, ectothermic pollinating bees quite often stop at the sunlit corona border to bask (Herrera, 1995), while at the same time cleaning themselves of pollen from legs and head and reorganizing the pollen load. This behaviour implies that the closer the stigma is to the border of the corona (i.e. the most herkogamous the flower), the more likely will be the fortuitous contact between the stigma and the body of a departing bee covered with self-pollen (Fig. 2). It is suggested that flowers of intermediate herkogamy have the highest outcrossing rates because their stigmas are neither too close to the stamens (where the bees spend much time collecting pollen and will frequently induce autogamy) nor too close to the border of the corona (where the bees also predictably spend some time before departure). This hypothesized scenario of bee-mediated stabilizing selection will be tested in future studies designed to dissect the relative importance of variation in herkogamy and degree of stigma exsertion as determinants of outcrossing rates.
Fig. 2.
A female Halictus xanthopus Kirby (Halictidae) briefly basking before departure on the border of the corona of a very herkogamous, relatively long-styled flower of Narcissus longispathus which it has just visited for pollen collection. The body of the pollen-laden bee is contacting the stigma. This occasional pollinator (approx. 11 mm long) is slightly larger than Andrena bicolor (9 mm), the main pollinator of N. longispathus. It is suggested that flowers with large anther–stigma separations experience low outcrossing rates because of the increased probability of self-pollination derived from a greater proximity of the stigma to the border of the corona, where pollinators frequently stop to thermoregulate before flying (Herrera, 1995).
The present estimates of inbreeding depression over the whole life cycle in the Cuevas Bermejas population confirmed earlier work on this population and other populations of N. longispathus, indicating that few selfed offspring reach reproductive maturity (Barrett et al., 2004). Assuming that inbreeding depression is uniformly strong and unrelated to individual differences in anther–stigma separation (Carr et al., 1997; Chang and Rausher, 1999; but see Stone and Motten, 2002; Takebayashi and Delph, 2000), variation in outcrossing rates should translate into differential survival prospects for the resulting progeny and, therefore, to natural selection on anther–stigma separation. The shape of the selection function (e.g. directional, stabilizing) would then be expected to mimic the shape of the relationship linking outcrossing rate and anther–stigma distance. Monotonic increases in outcrossing rate with increasing anther–stigma separation of the sort found in most species will thus likely translate into directional selection favouring increased herkogamy. In contrast, results of this study point to the possibility of pollinator-mediated stabilizing selection on anther–stigma separation in the Cuevas Bermejas population. This scenario would imply the existence of some outcrossing disadvantage when anther–stigma separation increases beyond a certain optimum level, a pattern that does not seem to have been previously documented for other species. On the other hand, however, the hypothesis of pollinator-mediated stabilizing selection on anther–stigma separation is difficult to reconcile with the broad intrapopulation variability in herkogamy observed. Corroborating the generality of the results of this study will thus require further work.
Stability of mixed mating and inbreeding equilibrium
Narcissus longispathus has a mixed mating system with populations consistently exhibiting moderate levels of selfing (Barrett et al., 2004). Remarkably, the mean multilocus outcrossing rate for the Cuevas Bermejas population reported in this study (tm = 0·598) is virtually identical to the estimate obtained by Barrett et al. (2004) for seed progenies collected in the same population 13 years earlier in 1990 (tm = 0·595). The estimates of the inbreeding coefficient of parent plants in this population were also similar and not significantly different from zero (1990, f = 0·031; 2003, f = −0·098). These values are considerably lower than the expected level of inbreeding in a population at equilibrium with the observed outcrossing rate, fexp = 0·251 [computed as fexp = (1 − tm)/(1 + tm); e.g. Spiess, 1989]. This indicates a consistently greater heterozygosity of adult plants relative to seeds in the two study years. Although these comparisons involve data for only 2 years, they suggest that the mating system and inbreeding parameters remained close to invariant over more than one decade in the Cuevas Bermejas population. As a consequence of similarities in tm and f, inbreeding depression estimates over the whole life cycle (δ) obtained using Ritland's equilibrium estimator (Ritland, 1990b) were also similar and close to unity in the two study years. Collectively, these results would support the view that the Cuevas Bermejas N. longispathus population is in inbreeding equilibrium, and that selection against selfed progeny is consistently strong in the population.
Similarly strong selection against selfed progeny in other animal-pollinated species with mixed mating systems has recently been reported: e.g. Decodon verticillatus (Eckert and Barrett, 1994), Shorea leprosula (Lee et al., 2000), Sagittaria latifolia (Dorken et al., 2002), Aquilegia canadensis (Herlihy and Eckert, 2002), Daphne laureola (Medrano et al., 2004). The high inbreeding depression estimates for the whole life cycle detected in these studies imply that few, if any, selfed progeny survive long enough to enter the adult reproductive stage and therefore self seeds make little or no genetic contribution to the next generation (reviewed by Barrett, 2003). However, these marker-based approaches for measuring inbreeding depression using the Ritland estimator (Ritland, 1990b) are based on the critical but usually untested assumption that populations are at inbreeding equilibrium. The present studies of the Cuevas Bermejas population of N. longispathus indicate that, at least for this population, this assumption is reasonable.
Acknowledgments
We thank Conchita Alonso for field assistance; Marina García and Rocío Requerey for conducting most laboratory work; Bill Cole for advice on electrophoresis; Kermit Ritland for making the MLTR program available; María Clara Castellanos, Alexander F. Motten, Víctor Parra and Judy Stone for comments on the manuscript; and the Consejería de Medio Ambiente, Junta de Andalucía, for permission to work in Cazorla and providing invaluable facilities there. M.M. was supported by a post-doctoral contract from the I3P Program of the Consejo Superior de Investigaciones Científicas, financed by the Fondo Social Europeo. This work was partly supported by grant BOS2000-1122-C03-01 from Ministerio de Ciencia y Tecnología to C.M.H. and an NSERC Discovery Grant to S.C.H.B.
LITERATURE CITED
- Barrett SCH. 2002. Sexual interference of the floral kind. Heredity 88: 154–159. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. 2003. Mating strategies in flowering plants: the outcrossing-selfing paradigm and beyond. Philosophical Transactions of the Royal Society of London B 358: 991–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH, Cole WW, Herrera CM. 2004. Mating patterns and genetic diversity in the wild daffodil Narcissus longispathus (Amaryllidaceae). Heredity 92: 459–465. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Lloyd DG, Arroyo J. 1996. Stylar polymorphisms and the evolution of heterostyly in Narcissus In: Lloyd DG, Barrett SCH, eds. Floral biology: studies on floral evolution in animal-pollinated plants. New York: Chapman and Hall, 339–376. [Google Scholar]
- Belaoussoff S, Shore JS. 1995. Floral correlates and fitness consequences of mating-system variation in Turnera ulmifolia Evolution 49: 545–556. [DOI] [PubMed] [Google Scholar]
- Breese EL. 1959. Selection for differing degrees of out-breeding in Nicotiana rustica Annals of Botany NS 23: 331–344. [Google Scholar]
- Brunet J, Eckert CG. 1998. Effects of floral morphology and display on outcrossing in blue columbine, Aquilegia caerulea (Ranunculaceae). Functional Ecology 12: 596–606. [Google Scholar]
- Carr DE, Fenster CB, Dudash MR. 1997. The relationship between mating-system characters and inbreeding depression in Mimulus guttatus Evolution 51: 363–372. [DOI] [PubMed] [Google Scholar]
- Cesaro AC, Barrett SCH, Maurice S, Vaissiere BE, Thompson JD. 2004. An experimental evaluation of self-interference in Narcissus assoanus: functional and evolutionary implications. Journal of Evolutionary Biology 17: 1367–1376. [DOI] [PubMed] [Google Scholar]
- Chang SM, Rausher MD. 1999. The role of inbreeding depression in maintaining the mixed mating system of the common morning glory, Ipomoea purpurea Evolution 53: 1366–1376. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. 1987. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics 18: 237–268. [Google Scholar]
- Cruzan MB. 1998. Genetic markers in plant evolutionary ecology. Ecology 79: 400–412. [Google Scholar]
- Cruzan MB, Hamrick JL, Arnold ML, Bennett BD. 1994. Mating system variation in hybridizing irises: effects of phenology and floral densities on family outcrossing rates. Heredity 72: 95–105. [Google Scholar]
- Darwin C. 1877.The different forms of flowers on plants of the same species. London: John Murray. [Google Scholar]
- Dorken ME, Friedman J, Barrett SCH. 2002. The evolution and maintenance of monoecy and dioecy in Sagittaria latifolia (Alismataceae). Evolution 56: 31–41. [DOI] [PubMed] [Google Scholar]
- Eckert CG. 2000. Contributions of autogamy and geitonogamy to self-fertilization in a mass flowering, clonal plant. Ecology 81: 532–542. [Google Scholar]
- Eckert CG, Barrett SCH. 1994. Inbreeding depression in partially self-fertilizing Decodon verticillatus (Lythraceae): population-genetic and experimental analyses. Evolution 48: 952–964. [DOI] [PubMed] [Google Scholar]
- Elle E, Hare JD. 2002. Environmentally induced variation in floral traits affects the mating system in Datura wrightii Functional Ecology 16: 79–88. [Google Scholar]
- Epperson BK, Clegg MT. 1987. First-pollination primacy and pollen selection in the morning glory, Ipomoea purpurea Heredity 58: 5–14. [Google Scholar]
- Harder LD, Barrett SCH. 1995. Mating cost of large floral displays in hermaphrodite plants. Nature 373: 512–515. [Google Scholar]
- Herlihy CR, Eckert CG. 2002. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature 416: 320–323. [DOI] [PubMed] [Google Scholar]
- Herrera CM. 1995. Floral biology, microclimate, and pollination by ectothermic bees in an early-blooming herb. Ecology 76: 218–228. [Google Scholar]
- Herrera CM, Hernández-Bermejo E, Luque P, Benavente A. 1999.Narcissus longispathus Pugsley. In: Blanca G, Cabezudo B, Hernández-Bermejo E, Herrera CM, Molero Mesa J, Muñoz J, Valdés B, eds. Libro rojo de la flora silvestre amenazada de Andalucía. I. Especies en peligro de extinción. Sevilla: Consejería de Medio Ambiente, Junta de Andalucía, 191–194. [Google Scholar]
- Holtsford TP, Ellstrand NC. 1992. Genetic and environmental variation in floral traits affecting outcrossing rate in Clarkia tembloriensis (Onagraceae). Evolution 46: 216–225. [DOI] [PubMed] [Google Scholar]
- Ivey CT, Wyatt R. 1999. Family outcrossing rates and neighborhood floral density in natural populations of swamp milkweed (Asclepias incarnata): potential statistical artifacts. Theoretical and Applied Genetics 98: 1063–1071. [Google Scholar]
- Karron JD, Jackson RT, Thumser NN, Schlicht SL. 1997. Outcrossing rates of individual Mimulus ringens genets are correlated with anther–stigma separation. Heredity 79: 365–370. [Google Scholar]
- Karron JD, Mitchell RJ, Holmquist KG, Bell JM, Funk B. 2004. The influence of floral display size on selfing rates in Mimulus ringens Heredity 92: 242–248. [DOI] [PubMed] [Google Scholar]
- Leclerc-Potvin C, Ritland K. 1994. Modes of self-fertilization in Mimulus guttatus (Scrophulariaceae)—a field experiment. American Journal of Botany 81: 199–205. [Google Scholar]
- Lee SL, Wickneswari R, Mahani MC, Zakri AH. 2000. Mating system parameters in a tropical tree species, Shorea leprosula Miq. (Dipterocarpaceae), from Malaysian lowland dipterocarp forest. Biotropica 32: 693–702. [Google Scholar]
- Lendvai G, Levin DA. 2003. Rapid response to artificial selection on flower size in Phlox Heredity 90: 336–342. [DOI] [PubMed] [Google Scholar]
- Medrano M, Alonso C, Herrera CM. 2004. Mating system, sex ratio, and persistence of females in the gynodioecious shrub Daphne laureola L. (Thymelaeaceae). Heredity 94: 37–43. [DOI] [PubMed] [Google Scholar]
- Montaner C, Floris E, Alvarez JM. 2001. Geitonogamy: a mechanism responsible for high selfing rates in borage (Borago officinalis L.). Theoretical and Applied Genetics 102: 375–378. [Google Scholar]
- Moreno Saiz JC, Sainz Ollero H. 1992.Atlas corológico de las monocotiledóneas endémicas de la Península Ibérica y Baleares. Madrid: ICONA, Ministerio de Agricultura. [Google Scholar]
- Morgan MT, Barrett SCH. 1990. Outcrossing rates and correlated mating within a population of Eichhornia paniculata (Pontederiaceae). Heredity 64: 271–280. [Google Scholar]
- Motten AF, Stone JL. 2000. Heritability of stigma position and the effect of stigma-anther separation on outcrossing in a predominantly self-fertilizing weed, Datura stramonium (Solanaceae). American Journal of Botany 87: 339–347. [PubMed] [Google Scholar]
- Richards AJ. 1997.Plant breeding systems. London: Chapman and Hall. [Google Scholar]
- Ritland K. 1990. A series of FORTRAN computer programs for estimating plant mating systems. Journal of Heredity 81: 235–237. [Google Scholar]
- Ritland K. 1990. Inferences about inbreeding depression based on changes of the inbreeding coefficient. Evolution 44: 1230–1241. [DOI] [PubMed] [Google Scholar]
- Ritland K. 2002. Extensions of models for the estimation of mating systems using n independent loci. Heredity 88: 221–228. [DOI] [PubMed] [Google Scholar]
- Ritland K, Jain S. 1981. A model for the estimation of outcrossing rate and gene frequencies using n independent loci. Heredity 47: 35–52. [Google Scholar]
- Robertson AW. 1992. The relationship between floral display size, pollen carryover and geitonogamy in Myosotis colensoi (Kirk) Macbride (Boraginaceae). Biological Journal of the Linnean Society 46: 333–349. [Google Scholar]
- Sage TL, Strumas F, Cole WW, Barrett SCH. 1999. Differential ovule development following self- and cross-pollination: the basis of self-sterility in Narcissus triandrus (Amaryllidaceae). American Journal of Botany 86: 855–870. [PubMed] [Google Scholar]
- Shore JS, Barrett SCH. 1990. Quantitative genetics of floral characters in homostylous Turnera ulmifolia var. angustifolia Willd. (Turneraceae). Heredity 64: 105–112. [Google Scholar]
- Spiess EB. 1989.Genes in populations, 2nd edn. New York: Wiley. [Google Scholar]
- Steel RGD, Torrie JH. 1980.Principles and procedures of statistics. A biometrical approach, 2nd edn. New York: McGraw-Hill. [Google Scholar]
- Stone JL, Motten AF. 2002. Anther–stigma separation is associated with inbreeding depression in Datura stramonium, a predominantly self-fertilizing annual. Evolution 56: 2187–2195. [DOI] [PubMed] [Google Scholar]
- Takebayashi N, Delph LF. 2000. An association between a floral trait and inbreeding depression. Evolution 54: 840–846. [DOI] [PubMed] [Google Scholar]
- Webb CJ, Lloyd DG. 1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. II. Herkogamy. New Zealand Journal of Botany 24: 163–178. [Google Scholar]
- Weinig C. 2002. Phytochrome photoreceptors mediate plasticity to light quality in flowers of the Brassicaceae. American Journal of Botany 89: 230–235. [DOI] [PubMed] [Google Scholar]
- Williams CF, Ruvinsky J, Scott PE, Hews DK. 2001. Pollination, breeding system, and genetic structure in two sympatric Delphinium (Ranunculaceae) species. American Journal of Botany 88: 1623–1633. [PubMed] [Google Scholar]