Abstract
• Background and Aims Flowers of Commelina coelestis and C. dianthifolia provide pollen alone as a floral reward, and rely on visual cues to attract pollinators. Three stamen types, all producing pollen, occur in each of these species: two cryptically coloured lateral stamens, a single cryptically coloured central stamen and three bright yellow staminodes that sharply contrast with the blue to purple corolla. The objective was to compare the stamen structure and pollen characteristics of each of the three stamen types, and to test the hypothesis that the staminodes are poor contributors of viable pollen for the siring of seed. The pollination roles of the three stamen types and the breeding systems of both species were also explored.
• Methods Light, fluorescence and scanning electron microscopy were utilized to examine stamen morphology and pollen structure and viability. Controlled hand pollinations were used to explore the breeding system of each species. Filament and style lengths were measured to investigate herkogamy and autogamy.
• Key Results Pollen from all stamen morphs is viable, but staminode pollen has significantly lower viability. Pollen polymorphism exists both (a) between the lateral and central stamens and the staminodes, and (b) within each anther. Lateral and central stamens have thicker endothecia with a greater number of secondary cell wall thickenings than the staminodes.
• Conclusions Both species are entomophilous and facultatively autogamous. Lateral stamen pollen is important for cross-pollination, central stamen pollen is utilized by both species as a pollinator reward and for delayed autogamy in C. dianthifolia, and the staminodes mimic, by means of both colour and epidermal features, large amounts of pollen to attract insects to the flowers. Pollen from all three anther morphs is capable of siring seed, although staminode pollen is inferior. The thin staminode endothecium with fewer secondary thickenings retards staminode dehiscence.
Keywords: Commelina coelestis, C. dianthifolia, floral mimicry, deception, staminode, pollen polymorphism, pollen viability, autogamy, endothecium, raphide crystals
INTRODUCTION
The pollen flowers of Commelina coelestis, a perennial indigenous to Mexico (Hooker and Jackson, 1895) and Commelina dianthifolia, a perennial native to Mexico and south-western United States (Brashier, 1966), rely on visual characteristics to attract insect pollinators. Three stamen types occur in both C. coelestis and C. dianthifolia: two cryptically coloured lateral stamens, a single cryptically coloured central stamen, and three brightly coloured staminodes that sharply contrast with the corolla make up the androecia of the study species.
Anther dimorphism in the Commelinaceae is common, and indicates the division of labour between stamens modified for pollinator attraction and those producing pollen for pollination (Vogel, 1978; Faden, 1992). Walker-Larson and Harder (2000) list pollinator attraction through visual cues and/or by providing floral rewards, a role commonly filled by staminodes. The visually apparent staminodes in C. coelestis may serve to mimic large quantities of pollen and draw the attention of visiting insects to the centre of the flower, away from the cryptically coloured lateral anthers that would then deposit pollen to be vectored to the stigmas of neighbouring flowers on the abdomen of insects (Vogel, 1978; Brantjes, 1980). The staminodes may be involved in pollinator deception, but may also provide a small amount of fodder pollen as a floral reward (Vogel, 1978). Pollen produced solely as a floral reward may be sterile (Simpson and Neff, 1983; Lloyd, 2000) and should be inferior for siring seed.
Despite the general knowledge and interest in the various stamen types in Commelina flowers, relatively little information is available on the stamen structure in the genus. The present objective was to compare the stamen structure and pollen characteristics, including pollen polymorphism between and within anthers, of each of the three stamen types (yellow staminode, and the cryptically coloured central and lateral stamens) per flower of C. coelestis and C. dianthifolia, using light, fluorescence and scanning electron microscopy (SEM), and to perform hand-pollinations to test the hypothesis that the staminodes themselves are relatively poor contributors of viable pollen for the siring of Commelina seed. The pollination roles of the three stamen types and the breeding systems of both species were also explored.
MATERIALS AND METHODS
Plant material
Seeds of Commelina coelestis Willd. (catalogue no. 1469) and C. dianthifolia Delile. (catalogue no. 1421) obtained from Thompson & Morgan (Poplar Lane, Ipswich, UK) were sown into Sunshine Mix 1 (Sun Gro Horticulture, Seba Beach, AB, Canada) and placed in a growth chamber with 14-h days (180 μE m−2 s−1) at 23 °C, and at 15 °C for 10 h darkness. At the 2–3-leaf stage, seedlings were transplanted into individual 15-cm pots and watered as needed with a 20 : 20 : 20 fertilizer mix. Spent flowers were deadheaded regularly to promote further blooming. Voucher specimens of both species were placed in the WP Fraser Herbarium (SASK) at the University of Saskatchewan, Saskatoon, SK, Canada.
Filament and gynoecium length
One flower from each of ten plants from both C. coelestis and C. dianthifolia was removed and examined under a dissecting microscope at ×10. Petals were removed and the length of the central filament, left-most lateral filament and left-most staminode were measured using ‘Force 6’ Electronic Digital Callipers (House of Tools, Saskatoon, SK, Canada). Filament length was recorded as the distance between the pedicel–receptacle junction and the filament–anther junction (Ushimaru and Nakata, 2002).
Gynoecium length was determined similar to filament length, from the pedicel–receptacle junction to the stigma, but flowers were not removed from the plant so as to prevent hindering the normal curling of the style. The pistil lengths of two flowers, one pollinated by hand from the central stamen and one left unpollinated, from each of ten plants of both species, were measured. Gynoecia were first measured at 0730 h shortly after anthesis, and again every half hour until 1400 h (C. coelestis) or 1300 h (C. dianthifolia).
Anther and staminode size
The central stamen, left lateral stamen and left-most staminode were dissected from one recently opened flower from each of ten plants from both C. coelestis and C. dianthifolia. The length and width at the point of the filament–anther junction of each anther viewed at ×10 were measured using digital callipers. Approximate areas were calculated by multiplying length and width.
Paraffin embedding, sectioning and staining
Individual stamens of all three morphs and whole flowers from both species were dissected from open flowers shortly after anthesis and from mature buds approx. 12 h prior to anthesis. Samples were fixed overnight in a 2 % glutaraldehyde solution in 25 mM Na phosphate buffer, pH 6·8, then rinsed with buffer followed by 50 % ethanol, before being dehydrated in a butan-1-ol series. Tissues were embedded in Paraplast® (Oxford Labware, St Louis, MO, USA), and sectioned at 8 µm using a Leitz Wetzlar rotary microtome. Sections were mounted onto slides coated with Mayer's adhesive (Baker and Silverton, 1976), affixed with heat, and stained with 0·05 % toluidine blue O (O'Brien and McCully, 1981). The paraffin was removed with xylene before mounting coverslips with Permount® (Fisher Scientific, Fair Lawn, NJ, USA).
Filament measurements
Ten measurements were taken along the length from five stamens of each type, spaced evenly from the filament base to the filament–anther junction, to provide comparison between the filaments of the three stamen types within a species. Where possible, transverse sections of filaments from whole flowers were selected to ensure the entire length of the filament was sampled. Oblique sections were not measured. Using an ocular micrometer on an Olympus light microscope, measurements of filaments (×40) and vascular bundles (×100) were taken of the longest (l) and shortest (w) diameters from the transverse sections, and their areas calculated using the formula for area of an ellipse [A = π(0·5l) × (0·5w)].
Endothecium
Endothecium thickness and number of secondary wall thickenings were measured from mature, predehiscent anthers of both species embedded in paraffin, sectioned and stained as described above. Transverse sections were examined at ×100. For measuring endothecium thickness with an ocular micrometer, the microscope field of view was divided into six equal segments by an eyepiece with three dissecting lines, meeting in the middle (i.e. lines connecting the following numbers on a clock: 12 and 6; 2 and 8; 4 and 10). The centre of the field of view was centred in the locule, and at each point where a line crossed the endothecium the thickness was measured. When measuring number of secondary wall thickenings, the field of view was divided into three equal segments (lines starting at 12, 4 and 8 on a clock and meeting in the middle) and in each segment the number of secondary thickenings in a 50-µm length of endothecium was counted. For both endothecium thickness and number of secondary thickenings, ten sections spaced evenly along the length of five anthers of each anther type were measured.
Thickness measurements taken from the dorsal side of the anther (where the connective tissue meets the locule) and measurements opposite this point (near the septum) were recorded as the thickest and thinnest areas of the endothecium, respectively. Standard deviation and mean endothecium thickness for the thickest, thinnest and entire endothecium were determined. Student's t-tests were used to compare endothecium thickness between the thickest and thinnest areas within each anther type, to compare endothecia between anther types, and to compare number of thickenings of the three anther types.
Pollen dimensions and viability
Pollen grain size was determined by excising stamens from recently opened flowers and dissecting the pollen under a dissecting microscope, on a glass slide, in a mixture of 3 : 1 lactic acid : glycerine to prevent grain swelling prior to measuring (McKone and Webb, 1988). Coverslips were lowered and secured using clear nail polish. The polar axis (length) and equatorial axis (width) of 105 grains were measured (×40) on the same day as mounting. Polar : equatorial ratios (P : E) were calculated, and pollen grain volumes determined using the equation for volume of an ellipsoid (an ellipse rotated about its major axis) (V = 4/3πab2, where a = 1/2 polar length, b = 1/2 equatorial length).
Pollen viability was determined using the fluorochromatic reaction (FCR) test involving fluorescein diacetate as described in Shivanna and Rangaswamy (1992). A gradient of fluorescence was observed, and only those pollen grains that fluoresced brightly were scored as viable. Percentage viability was calculated for both large and small pollen grains of all three anther types, and Student's t-tests were used to compare pollen viability between large and small grains within each anther type, and to compare pollen viability between anther types.
Pollen quantity per anther was determined by dissecting undehisced anthers, from flowers 1 d prior to anthesis, in a drop of distilled water on a glass slide under a dissecting microscope. Three transfers of the dissected anther into fresh water drops were required to remove all pollen. Coverslips were secured with nail polish before slides were viewed (×10) such that all pollen grains were counted. Large and small pollen grains were counted separately, and average grain number calculated for both grain sizes for each anther type. Student's t-tests were used to determine differences in pollen quantity between anther types. Pollen was counted in ten lateral stamens, 13 central stamens and 22 staminodes for C. coelestis, and 15 lateral stamens, ten central stamens and 18 staminodes for C. dianthifolia.
Scanning electron microscopy
Individual stamens of all three morphs from both C. coelestis and C. dianthifolia were dissected from open flowers shortly after anthesis and from flowers approx. 12 h prior to anthesis. Whole flowers of both species also were examined. Samples were fixed overnight in a 2 % glutaraldehyde solution, rinsed with 25 mm Na phosphate buffer, and post-fixed in 1 % OsO4 for 2 h. After samples were rinsed with buffer and then distilled water, they were dehydrated in an acetone series. Specimens were critical-point dried (Polaron Instruments, Watford, UK), mounted onto aluminum stubs with double-sided tape, gold coated with an Edwards S150B sputter coater, and viewed with a Philips SEM 505 at 30 kV. SEM preparation of pollen grains utilized OsO4 vapour as a post-fixing agent, and bypassed dehydration and critical-point drying, following the procedures in Smith and Tiedt (1991).
Photography
All light microscopy images (paraffin sections and pollen) were taken with a Zeiss Universal Large Research Microscope equipped with a 35-mm MC photomicrographic camera using Kodak Ektachrome 100ISO or Fujifilm Superia colour print film 100ISO. The slide film was scanned using an Epson Perfection 3200 photo scanner and colour print film was scanned at time of processing. Images were edited using Adobe Photoshop 5·0.
Hand-pollinations
Stigmas of fully opened flowers immediately after anthesis were examined with a hand lens (approx. ×1·5) early in the morning to determine if they were free of pollen grains. If pollen was absent, the flower was carefully emasculated before pollen from the appropriate stamen was dusted onto the stigma to complete the desired cross. The flower was tagged and seeds, if produced, were collected prior to fruit dehiscence. Five flowers of each species were utilized according to the following three treatments:
Treatment I (controls). No pollinations were performed by hand. Flowers were left intact, completely emasculated, or had only two of the three anther types (lateral, central or staminode) removed. Buds exhibiting environmental cleistogamy (Uphof, 1938) did not open, possibly due to thrips damage, and were observed to determine the occurrence of bud pollination.
Treatment II. After flowers were completely emasculated, the stigma was hand-pollinated by touching it with an anther of only one of the three stamen types. Either stamens from the same flower (self-pollination) or from other flowers of the same species (cross-pollination) were utilized as the pollen source.
Treatment III. After flowers of C. coelestis were completely emasculated, the possibility of hybridization between the two species was investigated by introducing pollen from the three stamen types of C. dianthifolia. Reciprocal crosses were also performed for the three stamen types of C. coelestis, separately.
RESULTS
Inflorescence
A spathe, or folded bract, enclosed the inflorescences of both species. Within each spathe of C. coelestis there is a single cyme comprising approximately six buds (Fig. 2C). The inflorescence of C. dianthifolia comprises two cymes; a lower one with approximately six buds is enclosed by the spathe, and an upper cyme with usually two, sometimes three buds, is raised above the spathe by a thin peduncle. Each cyme may possess one open flower per day (Fig. 1).
Fig. 2.

Whole flowers and anther macrophotography of C. coelestis (B, C, E, G and I) and C. dianthifolia (A, D, F and H). (A) Transverse section showing arrangement of lateral stamens (ls), central stamen (cs), staminodes (st), ovary (ov), petals (p) and sepals (s). Arrowheads indicate ovary trichomes. Scale bar = 0·5 mm. (B) Transverse section of flower showing arrangement of lateral stamens (ls), central stamen (cs) and staminodes (st). Arrowheads indicate ovary trichomes. Scale bar = 0·5 mm. (C) Inflorescence with petals and sepals of open flower removed. Lateral stamens (ls), central stamen (cs) and staminodes (st). Maturing buds at right. (D) SEM of whole flower with petals and sepals removed. Lateral stamens (ls), central stamen (cs), staminodes (st), stigma (sg) and style (sy). Trichomes (arrowheads) are visible on the ovary wall. Scale bar = 1 mm. (E) Adaxial (left) and abaxial (right) view of dehisced lateral stamens showing abundant yellow pollen. (F) Abaxial view of lateral stamen. (G and H) Central stamen at dehiscence. Note patches of purple and white. (I) Two of three staminodes from a single flower in abaxial (left) and adaxial (right) view showing arrangement of sterile lobes (sl) and fertile lobes (fl).
Fig. 1.

Flowers of Commelina coelestis (A) and C. dianthifolia (B), shortly after anthesis.
Floral morphology
The flowers of both species are zygomorphic, with three equal petals coloured intense blue, purple or white (C. coelestis) (Fig. 1A) or blue (C. dianthifolia) (Fig. 1B), and six conspicuous polymorphic stamens, three upper and three lower (Fig. 2A–D).
Two lower lateral stamens with long filaments have coloration that matches the filament and connective tissue to the corolla (Figs 1 and 2E and F). At anthesis, the anther's epidermis fractures along the stomium (Fig. 3B). Thecae opened adaxially (Fig. 3C, E, F), presenting pollen only to the inner side of the flower (introrse dehiscence) (Fig. 1). Prior to anther dehiscence, both the abaxial and adaxial sides of the stamen were relatively flat (Figs 2D and 3A, D), but after dehiscence only the adaxial surface was completely hidden by pollen (Fig. 2E).
Fig. 3.

SEM of lateral (A–F) and central (G–K) stamens of C. coelestis (B, D, E, I and J) and C. dianthifolia (A, C, F–H and K). (A) Adaxial side of pre-dehiscent anther and connective tissue between thecae. Scale bar = 0·25 mm. (B) Stomium of pre-dehiscent anther beginning to open (arrowhead). Scale bar = 0·1 mm. (C) Adaxial side of dehisced lateral stamen. Reflexed anther walls, residual pollen grains (po) and remnants of septum (se) are evident. Scale bar = 0·25 mm. (D) Abaxial side of pre-dehiscent anther. Scale bar = 0·25 mm. (E) Apical view of dehiscent anther. Scale bar = 0·25 mm. (F) Abaxial side of dehiscent anther showing reflexed walls. Scale bar = 0·25 mm. (G) Pre-dehiscent, curved anther. Scale bar = 0·25 mm. (H) Abaxial view of pre-dehiscent anther. Scale bar = 0·25 mm. (I) Lip cells (lc) at stomium. Scale bar = 50 μm. (J) Side view of dehisced anther. Scale bar = 0·25 mm. (K) Side view of fully dehisced anther, showing reflexed walls centrally, residual pollen grains (po), and remnants of septum (se). Scale bar = 0·25 mm.
The third lower, central stamen had an intermediate length filament that matched the petal colour and curved inward slightly, positioning the anther above the style at anthesis (Fig. 1). The connective tissue of the central stamen was not uniformly coloured (Fig. 2G, H), the epidermis of the thecae matching the corolla colour, but otherwise with patches of white. Thecae were curved (Figs 2D and 3G, H) and opened wide laterally to abaxially (Fig. 3J, K), presenting pollen outside the flower more conspicuously than the lateral stamen. Smaller epidermal cells lined the stomium region of the central anther (Fig. 3I) than the lateral ones (Fig. 3B).
The three upper stamens, termed ‘fodder androecia’, or ‘dummy anthers’ by Vogel (1978), have been reduced to staminodes. They have short filaments matching the petal colour, but bright yellow (Fig. 1), six-lobed antherodes (Fig. 2I). Of the six lobes, the two upper and two lower are sterile and comprise connective tissue with large intercellular spaces, and the central lobes are the thecae where small amounts of pollen are produced (Fig. 6A). Although staminodes of C. coelestis were usually larger than those of C. dianthifolia (Fig. 5A, B vs. Figs 5C, D and 6E), staminode size and shape varied in both species, even within the same flower (Fig. 2I). Within a staminode, the sterile lobes attached below the fertile thecae commonly were larger than those lobes above (Figs 2I and 5C, D). Alternatively, the sterile lobes on one side of the staminode dwarfed those at the other (Figs 2I left and 5B). The sterile lobes also differed in their orientation within a staminode, being parallel or perpendicular to the plane occupied by the fertile thecae (Fig. 5A). Variation was observed between staminodes at different positions but no clear trend was apparent. The epidermis of the sterile lobes consisted of isodiametric, conical cells (Fig. 5G) exhibiting a characteristic cuticular pattern (Fig. 5H).
Fig. 6.

Paraffin sections of staminodes of C. coelestis (A and C–G) and C. dianthifolia (B and E). (A) Longitudinal section showing four sterile lobes of connective tissue (sl) around two fertile thecae (fl). Undulating filament (ft) with solitary vascular bundle. Scale bar = 0·25 mm. (B) Transverse section of staminode filament with single vascular bundle. Scale bar = 0·1 mm. (C) Longitudinal section of fertile theca with pollen (po) in locule (lo) lined by endothecium (en). Scale bar = 0·1 mm. (D) Near tangential section of layer of endothecium (en) lining the same locule (lo). Scale bar = 0·1 mm. (E) Transverse sections through fertile lobes of dehisced staminode of C. coelestis (bottom) and pre-dehiscent staminode of C. dianthifolia (top). Scale bar = 0·5 mm. (F) Transverse section of dehisced fertile lobe. Scale bar = 0·1 mm. (G) Close-up of anther wall in transverse section, showing endothecium (en) below nucleated epidermis (ep). Scale bar = 25 μm.
Fig. 5.
SEM of staminodes of C. coelestis (A, B and E–H) and C. dianthifolia (C and D). (A and B) Staminodes showing the variation present between staminodes within a flower. Upper and lower lobes are sterile (sl) and the central lobes are fertile (fl). Scale bars = 0·25 mm. (C) Newly dehiscent and (D) fully dehiscent staminodes. Scale bars = 0·25 mm. (E) Pre-dehiscent and (F) post-dehisced fertile lobes. Reflexed theca walls expose pollen (po) within. (G) Surface of sterile lobe with isodiametric papillae. Scale bar = 10 μm. (H) Higher magnification of sterile lobe showing surface detail. Scale bar = 10 μm.
The fertile lobes of the staminode occasionally exceeded the size of the sterile lobes (Figs 2D, left, and 5A), but typically the reverse predominated (Figs 2I and 5B–D). Figures 5E and 5F are lateral views of the pollen-bearing lobes pre- and post-dehiscence, respectively; fracturing occurred along a longitudinal stomium (Fig. 5C, F). Epidermal cells of the fertile thecae were elongate and flattened (Fig. 5E, F); thus, the fertile lobes did not reflect light to the same degree as the sterile lobes (Fig. 2I).
Table 1 illustrates the difference in anther size between stamen types. For both species the anther of the central stamen was the largest, the staminodes were intermediate in size, and the lateral anthers were smallest. These differences are significant in C. coelestis, but in C. dianthifolia there was no significant difference between the sizes of the central and staminode anthers (Table 1).
Table 1.
Average anther dimensions of the three stamen types of C. coelestis and C. dianthifolia
|
C. coelestis |
C. dianthifolia |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stamen type |
Height (mm) |
Width (mm) |
Area (mm2) |
Height (mm) |
Width (mm) |
Area (mm2) |
||||
| Lateral | 2·22 ± 0·07 | 1·26 ± 0·04 | 2·83 ± 0·15c | 1·43 ± 0·07 | 0·85 ± 0·03 | 1·22 ± 0·08b | ||||
| Central | 2·84 ± 0·30 | 1·64 ± 0·21 | 4·66 ± 0·80a | 2·09 ± 0·08 | 1·17 ± 0·06 | 2·46 ± 0·19a | ||||
| Staminode | 1·68 ± 0·09 | 2·07 ± 0·11 | 3·53 ± 0·31b | 1·18 ± 0·06 | 1·79 ± 0·08 | 2·11 ± 0·15a | ||||
Values averaged across measurements of ten of each anther type ± standard error for both species.
Different superscript letters within a column indicate significantly different values at P < 0·05.
Differences in filament length between the three anther types were significant (P < 0·05) in all cases for both species (Table 2). Significant differences in the girth of filaments and the solitary vascular bundle per filament (Table 2) existed between the three stamen types of C. coelestis, with the central stamen (Figs 2B and 4D) being the thickest and the staminode the thinnest (Fig. 2B and C). Similarly, the staminode filament and its vascular bundle (Fig. 6B) of C. dianthifolia was significantly thinner than the other stamen types (Figs 1B and 2A, D), but no significant difference existed between the central and lateral stamens for filament or vascular bundle areas (Table 2).
Table 2.
Average length, cross-sectional area, and area of the vascular bundle in filaments of C. coelestis and C. dianthifolia
|
C. coelestis |
C. dianthifolia |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stamen type |
Filament length (mm) |
Filament cross-sectional area (mm2) |
Vascular bundle area (mm2) |
Filament length (mm) |
Filament cross-sectional area (mm2) |
Vascular bundle area (mm2) |
||||
| Lateral | 10·47 ± 0·31a | 5·12 × 10−2 ± 6·11 × 10−3b | 5·42 × 10−3 ± 3·52 × 10−4b | 8·97 ± 0·27a | 3·77 × 10−2 ± 2·17 × 10−3a | 3·65 × 10−3 ± 1·91 × 10−4a | ||||
| Central | 8·68 ± 0·21b | 1·17 × 10−1 ± 9·86 × 10−3a | 6·31 × 10−3 ± 3·72 × 10−4a | 7·17 ± 0·26b | 4·35 × 10−2 ± 3·96 × 10−3a | 3·91 × 10−3 ± 3·29 × 10−4a | ||||
| Staminode | 6·29 ± 0·15c | 1·39 × 10−2 ± 1·10 × 10−3c | 1·08 × 10−3 ± 5·70 × 10−5c | 6·22 ± 0·28c | 9·80 × 10−3 ± 5·87 × 10−4b | 1·24 × 10−3 ± 6·90 × 10−5b | ||||
Values averaged across measurements of filaments of ten of each stamen type ± standard error for both species.
Different superscript letters within a column indicate significantly different values at P < 0·05.
Fig. 4.
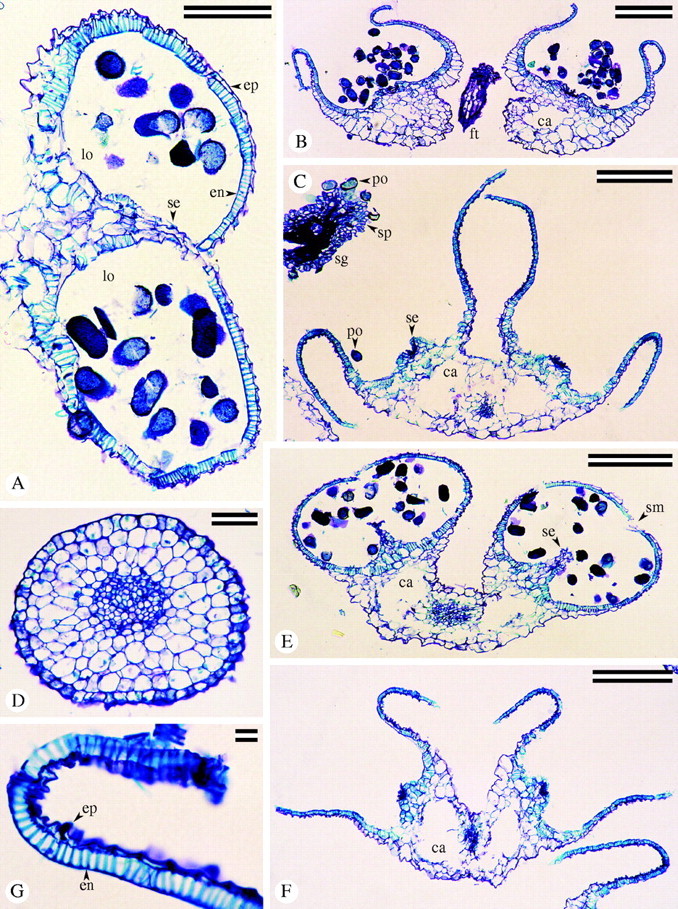
Paraffin sections of lateral (A–C) and central (D–G) stamens of C. coelestis (B, D and G) and C. dianthifolia (A, C, E and F). (A) Half of pre-dehiscent anther in transverse section with locules (lo) separated by intact septum (se); endothecium (en) and epidermis (ep). Scale bar = 0·1 mm. (B) Dehisced anther with cavity (ca) in connective tissue. Walls reflexing. Filament (ft). Scale bar = 0·25 mm. (C) Fully dehisced anther in transverse section with residual pollen (po) and remnants of septa (se). Cavities (ca) in connective tissue are visible interior to both septa. Section of stigma (sg) shows stigmatic papillae (sp) and adhering pollen grains (po). Scale bar = 0·25 mm. (D) Filament of central stamen with single vascular bundle. Scale bar = 50 μm. (E) Pre-dehiscent anther with ruptured stomium (sm), partially degraded septa (se), and connective tissue cavities (ca) bordering vascular bundle. Scale bar = 0·1 mm. (F) Fully dehisced anther with connective tissue cavities (ca). Upper walls reflexed whereas lower walls maintain horizontal position. Curled anther wall of adjacent lateral stamen at bottom right. Scale bar = 0·25 mm. (G) Curled upper wall of anther. Note endothecium (en) and epidermis (ep). Scale bar = 10 μm.
Anther anatomy
Each half of an anther of the lateral and central stamens from mature buds possessed two microsporangia separated by a septum and held together by connective tissue (Fig. 4A). In anthers of staminodes, however, no septa were identified within the fertile lobes (Fig. 6E, F). In all anthers, the connective tissue comprised relatively large parenchyma cells (Figs 4A–C, E, F and 6A, C–E). The vascular bundles of the filament continued into the connective tissue (Figs 4C, E, F and 6A, E). A large intercellular space had formed on both sides of this bundle in lateral (Fig. 4B, C) and central (Fig. 4E, F) stamens, but was not apparent there in staminodes (Fig. 6E).
During maturation of the thecae of the lateral and central stamens, the intervening septum (Fig. 4A) deteriorated acropetally, from the stomium region toward the reinforced locule base (Fig. 4E), leaving only the base of the septum intact (Fig. 4C, F) and recognizable by SEM (Fig. 3C, K).
The endothecium developed as a reinforced, subepidermal layer readily distinguished from the epidermis by its turquoise bands of secondary wall (Figs 4A–C, E–G and 6G). The endothecium varied greatly within each anther and between anther types, for both species. For all anthers of both species, the region of thickest endothecium was the back of the locule opposite the stomium, and the area of thinnest endothecium immediately surrounded the stomium (Figs 4A, E and 6E, F). In C. coelestis, overall the endothecium of the lateral stamens was thickest (21·2 µm), whereas the endothecium of the central stamen was thickest (23·7 µm) for C. dianthifolia (Table 3). The endothecium of the staminodes was the thinnest (13·5 µm and 11·3 µm for C. coelestis and C. dianthifolia, respectively). With the exception of the endothecium of the lateral and central stamens of C. coelestis, the difference was significant in all cases (Table 3).
Table 3.
Average endothecium thickness and number of secondary wall thickenings per anther type for C. coelestis and C. dianthifolia
| Average endothecium thickness (µm) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Stamen type |
Thick region |
Thin region |
Entire endothecium |
Average no. thickenings per 50 µm |
||||
| C. coelestis | ||||||||
| Lateral | 33·05 ± 1·87a | 13·40 ± 0·35a | 21·20 ± 1·42a | 11·33 ± 0·20a | ||||
| Central | 30·32 ± 1·42a | 12·76 ± 0·39a | 19·63 ± 1·19a | 11·43 ± 0·17a | ||||
| Staminode | 18·69 ± 0·78b | 9·83 ± 0·31b | 13·52 ± 0·65b | 8·17 ± 0·20b | ||||
| C. dianthifolia | ||||||||
| Lateral | 34·57 ± 1·45b | 12·26 ± 0·36a | 20·40 ± 1·34b | 11·89 ± 0·23a | ||||
| Central | 43·68 ± 1·71a | 11·83 ± 0·30a | 23·74 ± 1·75a | 11·76 ± 0·21a | ||||
| Staminode | 14·06 ± 0·47c | 10·05 ± 0·34b | 11·26 ± 0·41c | 7·89 ± 0·19b | ||||
Values averaged across measurements of endothecium of ten of each stamen type ± standard error for both species.
Different superscript letters within a column indicate significantly different values at P < 0·05.
The number of secondary wall thickenings per 50 µm was not significantly different between lateral and central stamens (Fig. 4G), but staminodes (Fig. 6G) had significantly fewer secondary wall thickenings (Table 3). Endothecium secondary thickening patterning for all three stamen types was U-shaped, and even apparent below the primary wall of the endothecium by SEM (Fig. 7F). The highly magnified transverse section of the central stamen endothecium (Fig. 4G), the longitudinal sections of staminode locule (Fig. 6C), and particularly the tangential view of the endothecium layer (Fig. 6D), clearly show the secondary wall patterning.
Fig. 7.

Pollen and features of the gynoecium of C. coelestis (A, E, G, H and K–M) and C. dianthifolia (B–D, F, I and J). (A and B) Dimorphic pollen of central stamen. Scale bars = 50 μm. (C) Dimorphic pollen of staminode. Scale bar = 50 μm. (D) SEM of dimorphic pollen of lateral stamen. Scale bar = 10 μm. (E) Large pollen grain of central stamen, treated with OsO4 vapour. Scale bar = 10 μm. (F) Small pollen grain inside locule of central stamen. Secondary thickenings (arrowheads) are visible through primary wall of endothecium (en) lining locule. Scale bar = 10 μm. (G) Large pollen grain of staminode with adhering raphide crystals (r). Treated with OsO4 vapour. Scale bar = 10 μm. (H) Small pollen grain of central stamen with adhering raphide crystals (r). Treated with OsO4 vapour. Scale bar = 10 μm. (I and J) Fluorochromatic reaction (FCR) test on lateral stamen pollen. Large pollen (L), small pollen (S) and intermediately sized pollen (I). (I) Pollen viewed under normal light and (H) viewed under fluorescence. Large pollen grains fluoresced brightly but small and intermediate grains did not fluoresce. Scale bars = 0·1 mm. (K) Autogamy mechanism, with the stigma pushed against the dehisced central stamen. Scale bar = 1 mm. (L) Pollen (left) adhering to three-lobed stigma pushed against anther of central stamen; stigmatic papillae evident at right. Scale bar = 0·1 mm. (M) Glandular trichome on ovary wall. Scale bar = 10 μm.
Patterns of anther-wall curling after dehiscence varied among the three stamen types. The central walls of the anther of the lateral stamen of both species typically straightened out (Fig. 3E) but only partially reflexed back on themselves (Figs 3C and 4B, C), while the outer walls curled back (Fig. 3E, F) to expose the inner surface of the thecae (Fig. 4B, C). The opposite occurred in the central stamen, with the central walls reflexing back toward the centre of the anther (Figs 3J, K and 4F, G) and the lateral or outer walls straightening but not curling back (Figs 3K and 4F). Figure 4F shows the central stamen in a section of a whole flower with the outer walls of an adjacent lateral stamen visible in the lower right. Fertile lobes of the staminodes frequently opened in the growth chamber (Fig. 5C, D) but not as wide as central and lateral stamens. Thecae walls did not curl in any paraffin-sectioned samples (Fig. 6E, F), but the stomial groove opened and widened slightly. In one SEM specimen the anther walls did curl back (Fig. 5D), but in all other SEM samples of staminodes dissected post-anthesis, only a widening of the stomial groove was seen (Fig. 5F).
Even in the widely exposed locules, a small quantity of pollen grains remained in all stamen types: lateral (Figs 3C and 4C), central (Fig. 3K) and staminode (Figs 5D and 6E right).
Pollen morphology, viability and production
Pollen from all three anther types, for both study species, was yellow, matching the colour of the connective tissue of the staminodes (Fig. 2E, G–I). Under SEM the verrucose–spinulose surface pattern of both large and small grains was evident (Fig. 7D–H).
Pollen dimorphism was observed both between anther types and within the same anther for both C. coelestis (Fig. 7A, E, H) and C. dianthifolia (Fig. 7B–D). Within each anther the pollen was divided into large and small pollen grains, the small having polar and equatorial lengths approximately half that of the large grains (Table 4). Large grains were the primary pollen grains, but small grains making up 3 % (C. coelestis lateral stamen) to 14 % (C. dianthifolia staminode) of the total per anther were found in all three anther types (Table 5). A few intermediately sized pollen grains were observed (Fig. 7I), but their presence was infrequent.
Table 4.
Dimensions and viability of large (L) and small (S) pollen grains of C. coelestis and C. dianthifolia
| Stamen type |
Grain size |
Average polar length (µm) |
Average equatorial length (µm) |
Average P : E ratio (µm) |
Average volume (µm3) |
Viability (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. coelestis | ||||||||||||
| Lateral | L | 83·36 ± 0·52b | 51·10 ± 0·24a | 1·64 ± 0·01b | 1·14 × 105 ± 1·27 × 103a | 85·26 ± 1·85a | ||||||
| S | 40·98 ± 0·62c | 27·17 ± 0·39c | 1·53 ± 0·03c | 1·63 × 104 ± 5·60 × 102b | 0·00 ± 0·00c | |||||||
| Central | L | 84·12 ± 0·53b | 50·98 ± 0·28a | 1·66 ± 0·02b | 1·15 × 105 ± 1·35 × 103a | 85·05 ± 1·57a | ||||||
| S | 40·71 ± 0·58c | 26·81 ± 0·41c | 1·56 ± 0·03c | 1·57 × 104 ± 5·25 × 102bc | 0·60 ± 0·53c | |||||||
| Staminode | L | 89·98 ± 0·74a | 48·14 ± 0·46b | 1·89 ± 0·02a | 1·10 × 105 ± 2·28 × 103a | 46·16 ± 2·99b | ||||||
| S | 38·79 ± 0·53d | 25·45 ± 0·57d | 1·61 ± 0·04c | 1·40 × 104 ± 6·90 × 102c | 1·66 ± 1·15c | |||||||
| C. dianthifolia | ||||||||||||
| Lateral | L | 81·05 ± 0·56a | 49·93 ± 0·31a | 1·63 ± 0·02a | 1·06 × 105 ± 1·50 × 103a | 90·70 ± 0·79a | ||||||
| S | 35·67 ± 0·47c | 24·40 ± 0·40c | 1·50 ± 0·03c | 1·15 × 104 ± 4·02 × 102c | 0·56 ± 1·05d | |||||||
| Central | L | 80·55 ± 0·69a | 49·40 ± 0·34a | 1·64 ± 0·02a | 1·04 × 105 ± 1·94 × 103a | 93·15 ± 0·68b | ||||||
| S | 34·79 ± 0·37c | 25·26 ± 0·43c | 1·42 ± 0·03cd | 1·20 × 104 ± 4·70 × 102c | 0·85 ± 1·06d | |||||||
| Staminode | L | 76·02 ± 0·77b | 49·86 ± 0·60a | 1·54 ± 0·02b | 1·02 × 105 ± 3·10 × 103a | 30·41 ± 3·59c | ||||||
| S | 35·17 ± 0·33c | 26·43 ± 0·43b | 1·37 ± 0·03d | 1·33 × 104 ± 4·85 × 102b | 0·55 ± 2·01d | |||||||
Size, shape and volume values averaged across measurements of 50 grains of each grain size per species ± standard error.
Viability values averaged across eight of each anther type per species ± standard error.
Within columns for each species separately, different superscript letters indicate significantly different values at P < 0·05.
Table 5.
Average number of pollen grains per anther for C. coelestis and C. dianthifolia
| Species |
Stamen type |
Large grains |
Small grains |
Total pollen count per anther |
|---|---|---|---|---|
| C. coelestis | Lateral | 3017 ± 174 | 103 ± 13·0 | 3120 ± 183a |
| Central | 3551 ± 195 | 162 ± 47·5 | 3713 ± 318a | |
| Staminode | 268 ± 18·2 | 11·1 ± 2·3 | 279 ± 19·7b | |
| C· dianthifolia | Lateral | 1889 ± 146 | 99·4 ± 31·0 | 1989 ± 146b |
| Central | 2399 ± 228 | 165 ± 71·3 | 2564 ± 195a | |
| Staminode | 249 ± 25·1 | 37·7 ± 10·6 | 287 ± 22·6c |
Within columns for each species separately, different superscripts indicate significantly different values at P < 0·05.
Large pollen grains in the staminodes of C. coelestis had significantly longer polar and thinner equatorial axes than large pollen grains produced in lateral and central stamens, and the small grains in the staminodes had both shorter polar and equatorial axes than the small grains in the other anthers (Table 4). Large pollen grains in the staminodes of C. dianthifolia have similar equatorial lengths but significantly shorter polar axis than the large grains in the other stamens, and the small grains have the same polar length but a longer equatorial axis.
In all cases large pollen is significantly more viable than small pollen (Table 4). In C. coelestis, large pollen in lateral and central stamens had high viability (>85 %) but the large pollen in the staminode was significantly less viable (46 %). Similarly in C. dianthifolia, large pollen in lateral and central stamens was highly viable (>90 %), yet the large pollen in the staminode was significantly less viable (30 %). Most small pollen grains were sterile; there was no significant difference in small pollen viability between stamen types for either species. Figures 7I and 7J display the FCR test comparing large and small pollen grains in lateral stamens of C. dianthifolia.
Central stamens produced a greater amount of pollen than lateral stamens and staminodes, lateral stamens produced an intermediate quantity of pollen, and staminodes produced the fewest pollen grains (Table 5). For total pollen counts, the differences were significant in all cases except lateral vs. central stamens in C. coelestis. Staminodes produced only 7·5 % (C. coelestis) to 11·1 % (C. dianthifolia) as much pollen as the central stamen. In C. coelestis and C. dianthifolia, collectively the three staminodes accounted for only 7·8 % and 11·6 %, respectively, of a flower's total pollen (Table 5).
Raphide crystals (Fig. 7G, H) were observed on all pollen grains from both species when viewed under SEM and light microscopy. Although not quantified, crystals were more frequently observed on pollen from the staminodes. Raphide crystals were only observed on pollen prepared with osmium tetroxide vapour as outlined by Smith and Tiedt (1991). Conventional SEM procedures used to prepare anthers and other tissue apparently washed away or dissolved raphide crystals.
Gynoecium characteristics
Following anthesis, gynoecium length remained constant for both pollinated and non-pollinated flowers of C. coelestis until approx. 1130 h, after which the style began to curl rapidly, causing a reduction in the receptacle–stigma distance (Fig. 8A). By 1330 h for non-pollinated flowers and 1400 for pollinated flowers, the stigma approached the height of the average central stamen (Fig. 8A) and on flowers with petals removed, the stigma was frequently in contact with the central anther (Fig. 7K). Commelina dianthifolia styles began to curl rapidly shortly after anthesis (Fig. 2D), quickly shortening the receptacle–stigma distance (Fig. 8B). By 0900 h for pollinated flowers and 0930 h for non-pollinated ones, the stigma matched the height of the central stamen (Fig. 8B). Commelina dianthifolia styles continued to curl into tight coils for the duration of the experiment, brushing the stigma past the lower side of the central stamen several times until measurements were stopped. For both species, there was no significant difference in the rate of gynoecium shortening/curling between pollinated and non-pollinated treatments.
Fig. 8.
Change in gynoecial length over time compared with filament lengths of the lateral and central stamens: (A) Commelina coelestis; (B) C. dianthifolia.
The ovary of both species had three locules (Fig. 2A), with two maturing ovules in each of the dehiscent locules nearest the central and lateral filaments, and only one maturing ovule in the upper, indehiscent locule adjacent to the staminodes, for a total of five potential seeds per flower. The stigmas of both species were topped with papillae to which multiple pollen grains adhered (Figs 4C and 7L). Multicellular trichomes (‘glandular micro-hairs’; Tomlinson, 1969) occurred on the ovary surfaces of both C. coelestis (Figs 2B and 7M) and C. dianthifolia (Fig. 2A, D).
Breeding system
The resulting seed-set following controlled hand-pollinations are recorded for C. coelestis and C. dianthifolia in Table 6. Treatment I for C. coelestis resulted in very little seed produced: only three out of a possible 25 seeds were produced by non-emasculated, not pollinated flowers, and a single seed in an unopened bud. Treatment II resulted in seed production from both selfing and cross pollinating within species when lateral and central stamen pollen was used, but no seeds matured from pollination using staminode pollen. Similar seed-set resulted from pollen from C. dianthifolia in treatment III, except that a single seed resulted from pollination with staminode pollen.
Table 6.
Hand-pollinations of C. coelestis and C. dianthifolia
| No. of seeds produced by |
||||
|---|---|---|---|---|
| Pollen donor |
C. coelestis |
C. dianthifolia |
||
| Treatment I: controls | ||||
| Non-pollinated, complete emasculation | 0 | 0 | ||
| Non-pollinated, no emasculation | 3 | 13 | ||
| Non-pollinated, only lateral stamens remained | 0 | 1 | ||
| Non-pollinated, only central stamen remained | 0 | 14 | ||
| Non-pollinated, only staminodes remained | 0 | 0 | ||
| Unopened flower | 1 | 8 | ||
| Treatment II: same-species pollination | ||||
| Lateral stamen (self-pollinated) | 9 | 19 | ||
| Central stamen (self-pollinated) | 19 | 22 | ||
| Staminode (self-pollinated) | 0 | 4 | ||
| Lateral stamen (cross-pollinated) | 11 | 19 | ||
| Central stamen (cross-pollinated) | 11 | 12 | ||
| Staminode (cross-pollinated) | 0 | 3 | ||
| Treatment III: interspecies hybridization | ||||
| Lateral stamen | 14 | 12 | ||
| Central stamen | 11 | 12 | ||
| Staminode | 1 | 0 | ||
Five flowers were tested for each trial; therefore, a maximum of 25 seeds could have matured.
Similar trends were observed in the controlled pollinations of C. dianthifolia in treatments II and III. Seed-set in treatment I was higher for non-emasculated, non-pollinated flowers and for partially emasculated flowers with the central stamen alone left intact. Unopened C. dianthifolia flowers also produced more seed than the C. coelestis flowers did.
DISCUSSION
Floral morphology
The arrangement of staminodes and fertile stamens in C. coelestis and C. dianthifolia is typical of pollen flowers that possess both feeding anthers and pollinating anthers (Ronse Decraene and Smets, 2001), and characterizes a division of labour between pollinating and fodder stamens (Vogel, 1978; Brantjes, 1980; Faden, 1985, 1992). Vogel (1978) proposed that the staminodes or ‘dummy’ anthers of C. coelestis and other Commelina species with modified staminodes served to mimic large quantities of pollen. He stated the larger, pollen laden lower stamens (lateral stamens) were not visited by insects directly, but that their pollen was loaded onto the abdomens of insects harvesting, or attempting to harvest, pollen as a food source from the upper staminodes. The fertile pollen from the lateral stamens could then be vectored to the stigmas of neighbouring flowers.
Lateral anther thecae open longitudinally on the adaxial surface (Fig. 3C, E, F). Pollen is displayed primarily on the adaxial surface and is not readily visible to insects approaching from the front of the flower. This apparent deception is furthered by the small anther size and cryptic coloration of the connective tissue, filaments and style. Assuming that the pollen from the lateral stamens is not intended as a reward for pollinators, the close proximity of the lateral anthers with the stigma (Fig. 8) would facilitate outcrossing as described by Vogel (1978). Ushimaru et al. (2003) found this to be true for the lateral stamens of Commelina communis. Deyrup (1988) observed Poecilognathus punctipennis (Diptera: Bombyliidae) pollen-feeding on the cryptic lateral stamens of Commelina erecta, but noted that the bright central stamen often distracted the flies.
The pollen of the large central anther is presented in wide-opening, longitudinal thecae on the lateral to abaxial face of the anther (Fig. 3J, K). This manner of pollen display, coupled with the curvature of the anther and the filament (Fig. 3G, H), placing the anther above the style despite the insertion of the filament base below the ovary, probably makes the anther very conspicuous to approaching insects. The connective of the central anther has patches of white and a colour that closely matches the corolla. These contrasting marks may not be cryptic coloration, but may increase the conspicuousness of the central stamen (Faden, 1992).
Stamen placement within a flower determines whether pollen will be fed upon or used for pollination (Lloyd, 2000). In many Commelina species, the central anther and staminodes occupy a similar position in the flower, with the pollen of the former matching the colour of the connective tissue of the latter (Faden, 2000). Ushimaru et al. (2003) found that the central stamen of C. communis provided pollen as a reward for visiting insects, and McCollum et al. (1984) called the central anther of C. erecta a feeding anther and proposed it to be analogous to nectar, serving only to provide a reward for pollinators and having very little to do with fertilization outside this role. Stamens producing fodder pollen in heterantherous flowers usually produce non-viable pollen (Ronse Decrane and Smets, 2001), but this is not the case for the central anthers of C. coelestis or C. dianthifolia. The primary role of the central anther may be to provide a nutritional reward for visiting pollinators, but the high viability of the pollen suggests it may function in other capacities also, as discussed in later sections.
The roles of the staminodes in C. coelestis and C. dianthifolia are less clear. Staminodes that produce a pollen reward are generally sterile (Yeo, 1993) and Faden (2000) reported that anthers responsible for pollinator attraction may contain less reward than pollinating anthers. Staminode functions can be both nutritive and attractive (Ronse Decraene and Smets, 2001), the former being pollen production for food and the latter being the connective's bright yellow colour matching that of the pollen in C. coelestis and C. dianthifolia. The above statements closely reflect the characteristics of the staminodes in C. coelestis and C. dianthifolia. The staminodes are intermediate in size between the lateral and central stamens, and their bright coloration and lobed appearance are striking against the blue background of the corolla, making them the most conspicuous members of the androecia. The staminodes of Commelina spp. probably evolved directly from functional stamens, taking on other functions (Walker-Larson and Harder, 2000; Ronse Decraene and Smets, 2001).
The epidermes of the staminode connective tissue of both species have a highly differentiated papillose pattern with isodiametric cells and a rugose epidermal texture, lacking in the epidermal cells of both central and lateral stamens. Papillose patterns and rugose epidermes, similar to the ones found on the staminode connective tissue, predominate on the petals of insect-pollinated species (Christensen and Hansen, 1998), and serve to increase the visibility of floral parts (Gale and Owens, 1983). The papillose surface on petals decreases surface reflection and directs light specifically through pigments to increase colour saturation (Kay et al., 1981); similar surface textures combined with a rugose epidermis creates a glistening appearance (Gale and Owens, 1983), and may increase the visibility of staminodes to insects. Highly differentiated papillae along a petal draw the attention of an insect into the centre of a flower (Christensen and Hansen, 1998), and such a mechanism may direct the reward seekers' attention away from the lateral stamens which match the corolla colour.
Some insects, including the honey bee (Apis mellifera), are able to distinguish epidermal patterns on petals and learn which patterns are associated with rewards (Kevan and Lane, 1985). Previous encounters with C. coelestis and C. dianthifolia flowers could enable the bees to learn that staminodes have relatively low floral rewards and the mimicry syndrome would be ineffective. The staminodes of C. erecta were found to have no effect on bee behaviour in the flower (McCollum et al., 1984), but the insects were also feeding on the central anthers of neighbouring flowers and may have previously learned to discount the presence of the staminodes. Petalous papillae also have been proposed to provide footholds for flower-visiting insects searching for pollen or nectar (Kay et al., 1981; Christensen and Hansen, 1998). Staminode papillae may serve a similar function during the foraging of the central stamen; Brantjes (1980) observed bumble bees buzz pollinating Commelina flowers [reported as C. communis but identified as C. coelestis by R. Faden (pers. comm.)] by grabbing hold of the staminodes and vibrating the entire flower.
Despite the proposed mimetic role of the staminodes (Vogel, 1978), few reports of insects attempting to gather pollen from the connective lobes or the small thecae of the staminodes have been found. The primary role of staminodes in C. coelestis and C. dianthifolia may be the initial attraction of insects, leading potential pollinators to the central anther where the food reward is, and drawing attention away from the cryptic lateral anthers.
Endothecium characteristics
Differences in the endothecia likely cause the different patterns in anther dehiscence observed between the three stamen types; however, endothecial characteristics affecting anther dehiscence are relatively unknown (Matsui and Omasa, 2002). Cell lysis results in stomium opening (Keijzer et al., 1996), and this process is independent of the presence or absence of secondary wall thickenings (Dawson et al., 1999). Staminode stomia of both C. coelestis and C. dianthifolia appear to rupture frequently, but anther walls typically do not curl back to expose the pollen within. Anther dehiscence results from differential desiccation between tissues with and without secondary thickenings, thus causing the reflexing of the locule wall (Keijzer et al., 1996; Dawson et al., 1999). In maize, U-shaped thickenings are found regularly in the endothecial cells at the anther top, and only irregularly in endothecial cells in the two rows directly adjacent to the stomium in the rest of the locule (Keijzer et al., 1996). At anther dehiscence, only the top of each locule opens up, and anthers opened artificially along their entire length did not display locule wall reflexion. In Arabidopsis thaliana, locules of anthers without endothecial secondary thickenings did not open, despite the rupturing of the stomium (Dawson et al., 1999), and irregularly arranged endothecium cells prevented anther dehiscence in Lycopersicon esculentum (Sato et al., 2002). These results confirm the data in this study, that suggests the reduction in endothecium thickness and the number of secondary wall thickenings in the locule walls of the fertile lobes of the staminode inhibit staminode dehiscence, despite the rupturing of the stomium.
Interestingly, endothecial curling patterns also differed between the lateral and central stamen, despite no large differences in endothecium characteristics. The mechanism involved is unknown, but original curvature of the central anther may counteract the wall forces that lead to rolling back of the outer walls. Nevertheless, the resulting differences in curling patterns cause pollen to be presented more visually on the central anther.
Pollen morphology, viability and production
Pollen dimorphism is known in many genera (Ong and Rao, 1973) and is common in Commelina spp. between the lateral and central stamens (Faden, 1992, 2000), displayed as differences in colour, size or shape. The pollen of the lateral and central stamens in both study species is very similar, with the exception of a small but significant reduction in the viability of lateral stamen pollen of C. dianthifolia. The dimorphism between stamens exists in the pollen of lateral and central stamens and that of the staminodes. In most studies, staminode pollen in Commelina spp. has not been considered beyond its viability (Vogel, 1978; Poole and Hunt, 1980; Faden, 1992; Ushimaru et al., 2003), but McCollum et al. (1984) noted that in C. erecta, the staminode pollen was about one-sixth the size of the lateral and central stamen pollen and was otherwise normal. Relative size of large grains between anthers was no different, but the shape indicated by the P : E ratio, and the significantly lower staminode pollen viability, revealed a significant dimorphism between staminodes and the lateral and central stamens. Grain contents of large staminode pollen grains appeared lighter under light microscopy (Fig. 7C), and stained lighter (Fig. 6E, F) than that of lateral and central stamens. Staminode pollen had an irregular outline apparent in some sectioned specimens (Fig. 6E, F) but not in samples prepared for light microscopy (Fig. 7C). A detailed, ultrastructural study of the pollen is required to determine the significance of these differences. The staminodes' intensely coloured connective tissue, coupled with a reduced pollen production with low viability, suggests that staminode pollen serves primarily as fodder pollen.
More surprising, however, is the observation of dimorphic pollen within individual anthers, a characteristic that seemingly has not been mentioned previously in the literature for Commelina species. Small pollen grains are sterile, and production is relatively low but still a significant proportion of the total pollen produced. The small grains may be aborted pollen grains, but the high numbers of sterile large pollen grains in the staminodes and the high proportion of sterile small grains would indicate that two abortive processes occur during development. A detailed study of microsporogenesis and microgametogenesis is needed to determine the origins and functions of the small pollen grains. The infrequently observed intermediate grains are assumed to be under-developed large pollen grains because of their sterility.
Interestingly, staminode pollen of C. coelestis had a higher viability than that of C. dianthifolia (Table 4), but was unsuccessful at siring seed in the controlled hand-pollinations (Table 6). Discrepancies between viability testing and apparent viability based on seed siring indicate the need for a pollen germination assay for Commelina pollen; attempts using Brewbaker and Kwack's medium (Brewbaker and Kwack, 1963) (10 % sucrose) failed.
Pollen production in the staminodes and the lateral stamens corresponds with attractive or fodder androecia producing less of a reward than pollinating androecia, as discussed above. However, the central stamens of both species produced more pollen, with equal or higher viability than the lateral stamens as indicated in both the FCR viability tests and the controlled hand-pollinations, suggesting that the central stamens may function as much more than just feeder stamens.
Raphide crystals
Raphide crystals of calcium oxalate, originating in the plasmodium, have been observed in other species of Commelinaceae (Hardy et al., 2000). Raphides in the anther may be involved in tissue degradation at the stomium to hasten anther dehiscence (Horner and Wagner, 1992), may enhance pollination by providing needed calcium for pollen germination and the elongation of the generative cell (D'Arcy et al., 1996; Iwano et al., 2004), or may form to sequester excess calcium in older anthers (Iwano et al., 2004), playing a regulatory role in the maintenance of ionic equilibrium (Dickison, 2000). Calcium oxalate itself may also be an attractant and a floral reward (D'Arcy et al., 1996). In Petunia hybrida, Iwano et al. (2004) observed calcium crystals beneath the stomium adhering to pollen grains at the time of anther dehiscence. Sterile staminodes in Schizanthus have locules containing raphide crystals instead of pollen; at dehiscence, the display of calcium oxalate crystals is thought to play a role in pollinator attraction (D'Arcy et al., 1996). The higher concentration of raphide crystals observed on the staminode pollen could give staminodes the appearance of having greater rewards than they do, further distracting insect visitors from the lateral stamens. Another role commonly attributed to raphides is the discouragement of herbivory (Dickison, 2000), including pollen robbery (D'Arcy et al., 1996). Given the higher abundance of raphides in the staminode pollen, and that Commelina spp. provide exclusively pollen as a floral reward, this role seems contradictory.
Gynoecium morphology and breeding system
All floral parts in C. coelestis and C. dianthifolia are glabrous except for the stigma and the ovary. Glandular trichomes (‘micro-hairs’; Tomlinson, 1969) on the ovary have no apparent secretory function; there is no distinguishable floral odour and no nectar production. Faden (1992) suggested the trichomes may have a protective function.
The trilobed stigmas of both C. coelestis and C. dianthifolia are comprised of papillae. Pollen grains landing on the stigma contact a secretion released by cells beneath the papillae and germinate rapidly (Owens and Horsfield, 1982), and the pollen tubes travel parallel with the papillae before entering the style.
The pollen : ovule (P : O) ratio, including the pollen produced by the staminodes, is 2158 ± 149 and 1480 ± 111 for C. coelestis and C. dianthifolia, respectively. Both values are between the ranges set by Cruden (1977) for facultative xenogamy and xenogamy (796·6 and 5859·2, respectively); discounting pollen produced in the staminodes does not move the P : O value out of this range. The anther and pistil arrangement implies entomophily but the coiling behaviour of the style suggests autogamy may be a factor in successful seed production, as discussed below.
In Commelina erecta, a plant with similar floral structures, Halictus sp., Bombus sp. and Apis mellifera were observed to feed on pollen in the central stamen (McCollum et al., 1984), with their abdomen extending between the two lateral anthers, frequently coming in contact with both the pollen-laden lateral anthers and the stigma. Other smaller bees were seen feeding on the central stamen, and several fly species fed on both the central and lateral anthers, but these were too small to contact the lateral anthers or the stigma at the same time. Deyrup (1988) observed a bee fly, Poecilognathus punctipennis, feeding on the pollen of lateral and central stamens of C. erecta. These flies had pollen adhering to the body hairs, and frequently moved from one flower to another, possibly contributing to cross-pollination, but Deyrup (1988) and Faden (1992) suggest that syrphids and bees play a more important role in the pollination of Commelina species. Deyrup (1988) made no mention of insect behaviour regarding the staminodes. Brantjes (1980) observed honey bees and syrphid flies (Episyrphus balteatus, Eristalis spp. and Helophilus spp.) preferentially feeding on the staminodes, with the abdomen frequently contacting the lateral anthers and the stigma in C. coelestis. Brantjes (1980) also noted that individuals within a species had varied behaviour; some individuals focused solely on the staminodes while others turned their attention directly to the lateral stamens.
Andromonoecy (having perfect and male flowers on the same plant) is common in Commelina (Maheshwari and Maheshwari, 1955; McCollum et al., 1984; Faden, 1985, 2000). In Commelina erecta (McCollum et al., 1984) and C. forskalaei (Maheshwari and Maheshwari, 1955), pollination of the initial flowers in each inflorescence appeared to cause the abortion of the gynoecium in subsequent flowers, resulting in functionally male flowers with a sterile pistilode; removal of spent flowers of the species in the present study may have inhibited normal development of male flowers, preventing the possibility of exploring differences between male and perfect flowers.
Many members of Commelinaceae, including C. coelestis and C. dianthifolia, are self compatible (Uphof, 1938; Maheshwari and Maheshwari, 1955; Owens, 1981; Simpson et al., 1986), confirmed in the present study. If mimicry is utilized, autogamy should be facultative and delayed to first allow the possibility of cross-pollination by insect pollinators (Simpson et al., 1986). Delayed autogamy was observed in Commelina benghalensis and C. forskalaei (Maheshwari and Maheshwari, 1955), C. coelestis (Faegri and van der Pijl, 1979), C. communis (Ushimaru et al., 2003) and C. erecta (McCollum et al., 1984), and a similar mechanism of style coiling causing autogamy was seen in the unrelated species, Mirabilis nyctaginea and M. jalapa (Nyctaginaceae) (Cruden, 1973). In flowers exhibiting autogamous selfing, the stigma and anthers are usually in close proximity (Cruden, 1973; Ushimaru and Nakata, 2002). At the time of anthesis the anthers in closest proximity to the stigma belong to the lateral stamens, but the treatment I-controlled pollinations clearly indicate that the central stamen is responsible for autogamy in C. dianthifolia. The styles of perfect, chasmogamous flowers of C. benghalensis and C. forskalaei coil, brushing the stigma against the fertile central stamen and effecting pollination (Maheshwari and Maheshwari, 1955); this mechanism is also observed in C. dianthifolia. As the flowers of C. coelestis and C. dianthifolia approach the end of anthesis, the style curls inward, and the petals wither into a soft mass. In C. coelestis, however, petals wilted prior to the style curling in toward the central stamen, and often enclosed the stamens, creating a barrier that prevented contact between the stigma and central stamen, resulting in low seed-set in non-pollinated, non-emasculated flowers of C. coelestis.
‘Pseudocleistogamy’, or ‘ecological cleistogamy’, occurs when environmental factors such as temperature, light availability, nutrition and others prevent normally chasmogamous flowers from opening (Uphof, 1938), and has been observed in both C. benghalensis and C. forskalaei (Maheshwari and Maheshwari, 1955). In C. coelestis and C. dianthifolia grown in the growth chamber, pseudocleistogamy may have resulted from damage when thrips populations were not controlled. In such cases, successful pollination was rare for C. coelestis but more common in C. dianthifolia, suggesting that bud pollination is possible.
No obvious differences occurred in seed-set between self- and cross-pollinated flowers (treatment II). Staminode pollen sired a small amount of seed in C. dianthifolia but not in C. coelestis, indicating that staminode pollen germinability may be lower than estimated with the FCR viability test. Although C. coelestis is self-compatible, it appears to rely on insects for cross-pollination. Commelina dianthifolia, however, self-pollinates readily due to the coiling nature of the style shortly after anthesis. The controlled pollinations indicate that the central stamen provides pollen important for selfing, while the lateral stamen pollen is utilized for outcrossing.
The results of treatment III indicated the potential for hybridization between C. coelestis and C. dianthifolia. Commelina coelestis is hexaploid (2n = 90) (Darlington and Wylie, 1955), and the successful seed production from interspecies crosses indicates that C. dianthifolia may also be hexaploid (R. Faden, pers. comm.). The seeds of these crosses appeared normal, but seed viability or germination tests were not carried out. Hybridizations between these or other Commelina spp. have not been mentioned in previous studies.
CONCLUSIONS
Both C. coelestis and C. dianthifolia are entomophilous and self-compatible, and C. dianthifolia is also facultatively autogamous. Lateral stamen pollen is important for cross-pollination, central stamen pollen is utilized by both species as a pollinator reward and for delayed autogamy in C. dianthifolia, and the staminodes mimic, by means of colour, epidermal features and raphide crystals, large amounts of pollen to attract insects to the flowers. Evidently, deception is employed by both species to draw the attention of pollinators away from the lateral stamens whose colour matches the petals, but may break down with visitor experience or when small pollen-feeding bees and flies harvest pollen from both the central and lateral stamens without contacting the stigma and effecting pollination.
Pollen from all three anther morphs is capable of siring seed, although the small quantity and poor viability of staminode pollen makes the staminodes inferior for this purpose. The pollen dimorphism between the staminodes and the lateral and central stamens is common between anthers in flowers with both feeding and fertile stamens, but the purpose and origin of the dimorphism that occurs within each stamen remains unknown.
The thin staminode endothecium with fewer secondary thickenings retards staminode dehiscence, but the cause of the differences in anther wall reflexion between the lateral and central anthers remains unexplained.
Acknowledgments
We thank Dr Robert Faden and an anonymous reviewer for their detailed reviews and helpful suggestions, and the former for providing the article and English translation of Brantjes (1980). We also thank Dennis Dyck for photography and assistance with scanning images. This research was funded by an NSERC Undergraduate Student Research Award to W.C.H. and an NSERC Discovery Grant to A.R.D.
LITERATURE CITED
- Baker FJ, Silverton RE. 1976.Introduction to medical laboratory technology, 5th edn. London: Butterworths. [Google Scholar]
- Brantjes NBM. 1980. Individualisme bij bloembezoekende insekten op Commelina [Individualism by flower-seeking insects on Commelina]. De Levende Natuur 82: 9–16. [Google Scholar]
- Brashier CK. 1966. A revision of Commelina (Plum.) L. in the U.S.A. Bulletin of the Torrey Botanical Club 93: 1–19. [Google Scholar]
- Brewbaker JL, Kwack BH. 1963. The essential role of calcium ion in pollen germination and pollen tube growth. American Journal of Botany 50: 859–865. [Google Scholar]
- Christensen KI, Hansen HV. 1998. SEM-studies of epidermal patterns of petals in the angiosperms. Opera Botanica 135: 1–91. [Google Scholar]
- Cruden RW. 1973. Reproductive biology of weedy and cultivated Mirabilis (Nyctaginaceae). American Journal of Botany 60: 802–809. [Google Scholar]
- Cruden RW. 1977. Pollen–ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution 31: 32–46. [DOI] [PubMed] [Google Scholar]
- D'Arcy WG, Keating RC, Buchmann SL. 1996. The calcium oxalate package or so-called resorption tissue in some angiosperm anthers. In: D'Arcy WG, Keating RC, eds. The anther: form, function, and phylogeny. Cambridge: Cambridge University Press, 159–191. [Google Scholar]
- Darlington CD, Wylie AP. 1955.Chromosome atlas of flowering plants, 2nd edn. London: George Allen and Unwin. [Google Scholar]
- Dawson J, Sozen E, Vizir I, Van Waeyenberge S, Wilson ZA, Mulligan BJ. 1999. Characterization and genetic mapping of a mutation (ms35) which prevents anther dehiscence in Arabidopsis thaliana by affecting secondary wall thickening in the endothecium. New Phytologist 144: 213–222. [Google Scholar]
- Deyrup MA. 1988. Pollen-feeding in Poecilognathus punctipennis (Diptera: Bombyliidae). Florida Entomologist 71: 597–609. [Google Scholar]
- Dickison WC. 2000.Integrative plant anatomy. San Diego: Academic Press. [Google Scholar]
- Faden RB. 1985. Commelinaceae. In: Dahlgren RMT, Clifford HT, Yeo PF, eds. The families of the monocotyledons: structure, evolution, and taxonomy. Berlin: Springer-Verlag, 381–387. [Google Scholar]
- Faden RB. 1992. Floral attraction and floral hairs in the Commelinaceae. Annals of the Missouri Botanical Garden 79: 46–52. [Google Scholar]
- Faden RB. 2000. Floral biology of Commelinaceae. In: Wilson KL, Morrison DA, eds. Monocots: systematics and evolution. Melbourne: CSIRO Publishing, 309–317. [Google Scholar]
- Faegri K, van der Pijl L. 1979.Principles of pollination ecology, 3rd edn. New York: Pergamon Press. [Google Scholar]
- Gale RMO, Owens SJ. 1983. Cell distribution and surface morphology in petals, androecia, and styles of Commelinaceae. Botanical Journal of the Linnean Society 87: 247–262. [Google Scholar]
- Hardy CR, Stevenson DW, Kiss HG. 2000. Development of the gametophytes, flower, and floral vasculature in Dichorisandra thyrsiflora (Commelinaceae). American Journal of Botany 87: 1228–1239. [PubMed] [Google Scholar]
- Hooker JD, Jackson BD. 1895.Index kewensis. An enumeration of the genera and species of flowering plants, 1st edn. Oxford: Clarendon Press. [Google Scholar]
- Horner HT, Wagner BL. 1992. Association of four different calcium crystals in the anther connective tissue and hypodermal stomium of Capsicum annuum (Solanaceae) during microsporogenesis. American Journal of Botany 79: 531–541. [Google Scholar]
- Iwano M, Entani T, Shiba H, Takayama S, Isogai A. 2004. Calcium crystals in the anther of Petunia: the existence and biological significance in the pollination process. Plant Cell Physiology 45: 40–47. [DOI] [PubMed] [Google Scholar]
- Kay QON, Daoud HS, Stirton CH. 1981. Pigment distribution, light reflection and cell structure in petals. Botanical Journal of the Linnean Society 83: 57–84. [Google Scholar]
- Keijzer CJ, Leferink-Ten Klooster HB, Reinders MC. 1996. The mechanics of the grass flower: anther dehiscence and pollen shedding in maize. Annals of Botany 78: 15–21. [Google Scholar]
- Kevan PG, Lane MA. 1985. Flower petal microtexture is a tactile cue for bees. Proceedings of the National Academy of Sciences of the USA 82: 4750–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DG. 2000. The selection of social actions in families. III. Reproductively disabled individuals and organs. Evolutionary Ecology Research 2: 29–40. [Google Scholar]
- McCollum TM, Estes JR, Sullivan JR. 1984. Reproductive biology of Commelina erecta (Commelinaceae). In: Horner NV, ed. Festschrift for Walter W. Dalquist in honour of his sixty-sixth birthday. Wichita Falls: Midwestern State University, Department of Biology, 57–66. [Google Scholar]
- McKone MJ, Webb CJ. 1988. A difference in pollen size between the male and hermaphrodite flowers of two species of Apiaceae. Australian Journal of Botany 36: 331–337. [Google Scholar]
- Maheshwari P, Maheshwari JK. 1955. Floral dimorphism in Commelina forskalaei Vahl and C. benghalensis L. Phytomorphology 5: 413–422. [Google Scholar]
- Matsui T, Omasa K. 2002. Rice (Oryza sativa L.) cultivars tolerant to high temperature at flowering: anther characteristics. Annals of Botany 89: 683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien TP, McCully ME. 1981.The study of plant structure. Principles and selected methods. Melbourne: Termarcarphi. [Google Scholar]
- Ong ET, Rao AN. 1973. Pollen dimorphism in certain angiosperms. Journal of Palynology 9: 142–151. [Google Scholar]
- Owens SJ. 1981. Self-incompatibility in the Commelinaceae. Annals of Botany 47: 567–581. [Google Scholar]
- Owens SJ, Horsfield NJ. 1982. A light and electron microscopic study of stigmas in Aneilema and Commelina species (Commelinaceae). Protoplasma 112: 26–36. [Google Scholar]
- Poole MM, Hunt DR. 1980. Pollen morphology and the taxonomy of the Commelinaceae: an exploratory survey. American Commelinaceae: VIII. Kew Bulletin 34: 639–660. [Google Scholar]
- Ronse Decrane LP, Smets EF. 2001. Staminodes: their morphological and evolutionary significance. The Botanical Review 67: 351–402. [Google Scholar]
- Sato S, Peet MM, Thomas JF. 2002. Determining critical pre- and post-anthesis periods and physiological processes in Lycopersicon esculentum Mill. exposed to moderately elevated temperatures. Journal of Experimental Botany 53: 1187–1195. [DOI] [PubMed] [Google Scholar]
- Shivanna KR, Rangaswamy NS. 1992.Pollen biology. A laboratory manual. New Delhi: Narosa Publishing House. [Google Scholar]
- Simpson BB, Neff JL. 1983. Evolution and diversity of floral rewards. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold, 142–159. [Google Scholar]
- Simpson BB, Neff JL, Dieringer G. 1986. Reproductive biology of Tinantia anomala (Commelinaceae). Bulletin of the Torrey Botanical Club 113: 149–158. [Google Scholar]
- Smith GF, Tiedt LR. 1991. A rapid, non-destructive osmium tetroxide technique for preparing pollen for scanning electron microscopy. Taxon 40: 185–200. [Google Scholar]
- Tomlinson PB. 1969.Anatomy of the monocotyledons. Vol. 3: Commelinales–Zingiberales. Oxford: Clarendon Press. [Google Scholar]
- Uphof JCT. 1938. Cleistogamic flowers. The Botanical Review 4: 21–49. [Google Scholar]
- Ushimaru A, Nakata K. 2002. The evolution of flower allometry in selfing species. Evolutionary Ecology Research 4: 1217–1227. [Google Scholar]
- Ushimaru A, Itagaki T, Ishii HS. 2003. Variation in floral organ size depends on function: a test with Commelina communis, an andromonoecious species. Evolutionary Ecology Research 5: 615–622. [Google Scholar]
- Vogel S. 1978. Evolutionary shifts from reward to deception in pollen flowers. In: Richards AJ, ed. The pollination of flowers by insects. New York: Academic Press, 89–104. [Google Scholar]
- Walker-Larson J, Harder LD. 2000. The evolution of staminodes in angiosperms: patterns of stamen reduction, loss, and functional re-invention. American Journal of Botany 87: 1367–1384. [PubMed] [Google Scholar]
- Yeo PF. 1993.Secondary pollen presentation. Form, function, and evolution. New York: Springer-Verlag. [Google Scholar]




