Abstract
• Background and Aims Helianthemum marifolium and H. caput-felis are two endangered plant species of the western Mediterranean. Several aspects of the reproduction of both species were examined to determine whether their rarity could be related to factors causing reproductive limitation.
• Methods The flowering and fruiting phenology of both species in two non-sympatric island populations (Mallorca, Balearic Islands, western Mediterranean) were compared. Hand-pollination experiments were conducted to determine their fruit and seed production under different pollen sources. The composition of the pollinator assemblage and the effect of temporal variation and sun exposure on reproductive output and seedling survival were also investigated.
• Key Results Flowering periods were longer for H. marifolium than for H. caput-felis in the populations studied. Helianthemum marifolium is mostly an outbreeder, i.e. fruit and seed set was three-fold higher when pollen came from other plants. In H. caput-felis, neither fruit nor seed set was affected by pollination treatments. Flower visitors were more diverse for H. caput-felis than for H. marifolium. In both species, most floral visits were made by hymenopterans. The total number of pollinator visits varied significantly between years, decreasing more than two-fold from 2001 to 2002, in both species. In agreement with its outbreeder character, variation in reproductive output between years was also observed in H. marifolium. It showed a 50 % decrease in fruit set in 2002, a pollinator-poor year. Finally, seedling survival increased three- to six-fold from 2001 to 2002. A correlation between seedling size and survival had also been detected.
• Conclusions Reproductive limitations were detected for neither species (i.e. fruit and seed set, pollination service and seedling survival on natural populations). Hence, the increasing rarity of these two species is probably a direct result of the destruction of their habitat.
Keywords: Breeding system, conservation, Helianthemum, pollinator assemblage, reproductive output, seedling survival, threatened species, western Mediterranean
INTRODUCTION
Information on the reproductive biology of endangered plants is crucial for predicting their survival capacity and establishing the appropriate measures for their conservation (Schemske et al., 1994; Delanoë et al., 1996; Bernardello et al., 2001). Such studies may help to identify the factors that reduce the reproductive capacity of individual plants and the subsequent maintenance or regeneration of populations.
Successful plant propagation generally involves three consecutive events that are modulated by three different factors: pollination, seed dispersal (including seed predation) and seedling establishment. Pollination and seed dispersal often depend on mutualistic interactions with animals (Herrera and Pellmyr, 2002). As such, a reduction in pollinator service can directly affect reproductive output, decreasing the quantity and/or quality of fruit and seed set (Ågren, 1996) and promoting selfing in self-compatible species (Erhardt and Jäggi, 1995; Washitani, 1996; Traveset et al., 1998). In addition, seed dispersal may be limited, thus decreasing the probabilities of seed germination, escape from seed predators and/or seedling establishment (Rey and Alcántara, 2000; Traveset et al., 2003). In a broad sense, an increase in selfing rate or a reduction of seed dispersal results in a reduction in gene flow within and/or among populations and may thus cause an increase in inbreeding depression (O'Brien, 1994; Buza et al., 2000). Finally, seedling survival is considered one of the most critical stages in the life cycle (not only for threatened species) in Mediterranean and arid ecosystems (Escudero et al., 1999; Rey and Alcántara, 2000; Traveset et al., 2003). In such habitats, seedling survival depends strongly on biotic interactions (such as competition and facilitation; Callaway and Walker, 1997) as well as on abiotic factors (e.g. stochastic rainfall patterns; Turner, 1990).
Habitat fragmentation caused by human disturbance is currently considered one of the main factors reducing population viability of rare plants (Schemske et al., 1994). Reduced size may subsequently affect the performance of small plant populations (Kearns et al., 1998). In the Mediterranean basin in particular, over the last 30 years, coastal ecosystems have suffered a massive reduction of size and quality as a consequence of the disproportionate urban development (Greuter, 1995). This process is particularly accentuated in the Balearic Islands due to dramatically increasing tourism (Mus, 1995).
The present work focuses on the study of two endangered plant species of Helianthemum (Cistaceae). Helianthemum marifolium (L.) Mill. is a small shrub distributed along the east and south Iberian Peninsula and on the Balearic Islands. All existing populations in the Balearic Islands belong to an endemic subspecies, H. marifolium subsp. origanifolium (Lam.) G. López (Alomar et al., 1997). Such populations are located in coastal areas, mainly in the Pityusic islands (Eivissa and Formentera), at up to 100 m a.s.l., with a single population in the south-west of Mallorca. This population is close to Palma, the largest city of the island, and it is severely threatened by urban development. Helianthemum marifolium subsp. origanifolium and its habitat are protected by local and regional laws, and it is considered as ‘vulnerable’ according to the International Union for the Conservation of Nature (IUCN) (Alomar et al., 1997).
The second species, H. caput-felis Boiss., is a rare small shrub distributed throughout the western Mediterranean (Balearic Islands, eastern Iberian peninsula, Sardinia, south Italy and North Africa). In the Balearics it is located only at coastal sites, in the south of Mallorca (Alomar et al., 1997). Pujol (2001) recently reported a dramatic reduction of the Iberian Peninsula populations which he attributed to the direct pressure of coastal urban development. Fortunately, all Balearic populations are located in protected areas, making its local conservation status more favourable. Helianthemum caput-felis is included in the Annex of the Berne Convention (1991) and it is protected by European legislation (Habitats Directive, European Community 1992). It is categorized as ‘rare’ by the IUCN (Alomar et al., 1997).
This paper aims to identify the critical stages in the life history of these two endangered species. The work of the present paper was included as a possible recovery plan for H. marifolium. Besides, the comparison with another related species, H. caput-felis, could be interesting because a priori both species may share the same reproductive limitations. Both species are related phylogenetically and live in similar habitats (Alomar et al., 1997). The following questions are specifically addressed: (a) What is the flowering phenology of these two species? Does it vary between them? (b) Does the reproductive output of both species change under different pollen sources? (c) Does the pollinator assemblage and visitation rate differ between the two species? (d) Do biotic (plant traits) and abiotic factors (sun exposure and year) influence their reproductive potential and seedling survival? The data gathered in this study will be useful for designing more adequate management and conservation practices for these two species.
MATERIALS AND METHODS
Study species
Helianthemum marifolium subsp. origanifolium and H. caput-felis are perennial shrubs, with a height up to 35 cm. Flowers are arranged in inflorescences at the tip of new branches. For a summary of floral traits see Table 1. Flowers are yellow and hermaphroditic, open at dawn and close at dusk, and have short lifespans (2–3 d for H. marifolium and 3–4 d for H. caput-felis; pers. obs.). The anthers are sensitive to touch and spread outwards when the flower is visited by pollinators (pers. obs.), a trait shown by other species in the genus (Proctor et al., 1996). For both species, the main floral reward to pollinators is pollen (Herrera, 1992; Proctor et al., 1996). Fruits are capsules that detach at maturation. Germination takes place in autumn, at the onset of the rainy season (pers. obs.). Seed germination varies widely for both species, being quite high for H. marifolium (73 %; Pons, 2002), but very low for H. caput-felis (4 %; Tébar et al., 1997), despite the high seed viability of the latter (96 %; Tébar et al., 1997). The low germination of H. caput-felis has been attributed to physical exogenous dormancy (impermeable coat), a widespread trait among the Cistaceae (Thanos et al., 1992).
Table 1.
Floral traits of H. marifolium and H. caput-felis
|
H. marifolium |
H. caput-felis |
|||||
|---|---|---|---|---|---|---|
| Floral traits |
n |
Mean ± s.e. |
n |
Mean ± s.e. |
||
| No. of inflorescences | 50 | 16·9 ± 2·4 | 50 | 85·0 ± 12·3 | ||
| Flowers/inflorescence | 50 | 7·3 ± 1·4 | 50 | 3·0 ± 1·0 | ||
| Flower mass (mg) | 45 | 7·3 ± 0·4 | 50 | 52·8 ± 1·9 | ||
| No. of petals | 45 | 4·9 ± 0·0 | 50 | 5·0 ± 0·0 | ||
| Petal length (mm) | 45 | 3·93 ± 0·06 | 50 | 7·94 ± 0·05 | ||
| No. of sepals | 45 | 3·0 ± 0·0 | 50 | 5·0 ± 0·0 | ||
| Sepal length (mm) | 45 | 3·23 ± 0·05 | 50 | 6·91 ± 0·05 | ||
| No. of stamens | 45 | 35·4 ± 1·0 | 50 | 46·6 ± 1·0 | ||
| Stamen length (mm) | 45 | 1·70 ± 0·04 | 50 | 3·64 ± 0·04 | ||
| Anther length (mm) | 45 | 0·46 ± 0·01 | 50 | 0·49 ± 0·01 | ||
| No. of ovules | 31 | 10·2 ± 1·2 | 49 | 6·0 ± 0·0 | ||
| Gyneceum length (mm) | 45 | 1·78 ± 0·05 | 50 | 4·80 ± 0·06 | ||
| Ovarium length (mm) | 45 | 1·04 ± 0·02 | 50 | 1·44 ± 0·03 | ||
Sample size (n), plant mean (±1 s.e.) are given for each trait.
Flowers/inflorescence was determined on a sample of up to 50 inflorescences per plant. Ovule number was estimated for at least 15 flowers per plant. All other measures were taken on one fresh flower per plant. Plant means for flowers/inflorescence and ovule number were averaged for each plant and then across all plants. Flower mass was measured as fresh mass.
Study sites
The population of H. marifolium is located in a protected area called Es Carnatge, just outside the city of Palma de Mallorca (39°33′N, 2°42′E) and contains approx. 250 adult plants. The annual precipitation (starting in September of the previous year) was 576 and 974 mm for 2001 and 2002, respectively (data from the Balearic Meteorological Institute). This population is found in a relictual thyme scrubland (approx. 200 m2 in area) that stands on a fixed dune. Vegetation is dominated by Thymbra capitata, Satureja microphylla ssp. rodriguezii, Fumana thymifolia ssp. laevis, Cistus clusii, Micromeria filiformis and H. marifolium. In 2001, the flowering period of H. marifolium overlapped with 19 co-occurring plant species.
The population of H. caput-felis is located in a dune system near the town of Sa Ràpita, also in the south of Mallorca, and approx. 30 km south-east of Es Carnatge (39°22′N, 2°58′E). The annual precipitation (same period as above) was 588 and 1201 mm for 2001 and 2002, respectively. Vegetation is dominated by Juniperus phoenicea and Pinus halepensis; H. caput-felis occurs in clearings, accompanied by Helicrysum dunense and Cistus clusii. In 2001, H. caput-felis flowered simultaneously with one co-occurring species (Cistus clusii).
Flowering and fruiting phenology
On 15 March 2001, 45 plants of H. marifolium and 59 of H. caput-felis were tagged in natural populations. An inflorescence was randomly chosen on each plant and periodically examined (every 2–3 d in H. marifolium and 3–4 d in H. caput-felis) to record the number of receptive flowers (those with petals remaining in the flower). For H. caput-felis, most inflorescences produce fewer than three flowers (Table 1), so inflorescences with at least three flowers were tagged, in order to quantify more accurately the flowering phenology.
Breeding system
The reproductive biology of both species was examined in 2002 under controlled conditions. In October 2001, nine and eight adult plants of H. marifolium and H. caput-felis, respectively, were transplanted into pots filled with soil from the original localities and moved to an experimental greenhouse (free of pollinators) in Esporles (Mallorca). On each plant, four to 26 inflorescences (depending on availability on each plant) were randomly tagged and only one of the treatments assigned to each inflorescence (i.e. to every single flower on that inflorescence). To each treatment and species, one to seven inflorescences were assigned (both species), using 10·0 ± 0·5 and 2·9 ± 0·1 (mean ± s.e.) flowers per treatment and plant (H. marifolium and H. caput-felis, respectively). The experiment was conducted as a complete block design, with the treatments nested within each plant. The treatments were (1) no manipulation (control or spontaneous self-pollination); (2) hand-pollination with pollen of the same flower (induced self-pollination), (3) hand-pollination using pollen from the same plant, but from another flower (individual geitonogamy) and (4) hand-pollination using pollen from a different plant (xenogamy). All flowers used for treatments 3 and 4 were emasculated before they had become receptive. Hand-pollinations were performed once on each a flower as it became receptive.
Fruits from all treatments were harvested as soon as they ripened (mainly during May) and dissected in the laboratory. In both species, and especially for H. caput-felis, both developed and undeveloped fruits remain attached to the inflorescence for long periods of time (pers. obs.), hence dissection was necessary to distinguish developed and empty fruits with certainty. To avoid the effect of inflorescence variation, fruits from all inflorescences within each individual plant and treatment were pooled, i.e. within-plant variation among inflorescences was ignored.
Flower visitors
Direct observations of pollinators of H. marifolium and H. caput-felis were made on three different dates in 2001 and 2002 (with a total of 4·75 and 18·0 h for H. marifolium and 12·5 and 12·25 h for H. caput-felis, for 2001 and 2002, respectively), to determine the pollinator assemblage and the rate of flower visitation by the different visitors. Observations were made on a total of 42 and 85 plants of H. marifolium and H. caput-felis, respectively. For each individual plant, observation hours always varied across different observation days. Each observation was mostly performed on sunny days, and lasted for 15 min, during which the following were recorded: (a) the pollinator species, (b) the number of visits made by each pollinator species, (c) the number of contacted flowers per visit, (d) the flower handling time (only for flying pollinators) and (e) exposure of the plant to sun (‘sun’ or ‘shade’). At the end of each census, the number of receptive flowers per plant was noted. Nocturnal observations were not performed because flowers of both species are closed at night.
Factors determining reproductive potential
A total of 50 plants of each species of Helianthemum were tagged on 15 March 2001, coinciding with those most used for phenology. For each plant, the plant size (average between the maximum and its orthogonal crown diameter) was measured to the nearest centimetre and recorded sun exposure (‘sun’ vs. ‘shade’). Fruits of each plant (17·6 ± 2·0 for H. marifolium and 26·5 ± 1·2 for H. caput-felis, respectively) were collected to determine fruit and seed set in the laboratory. Fruit set was estimated afterwards by dissecting fruits and counting the total of developed fruits in relation to collected fruits. Developed fruits were then used to measure: (a) fruit mass (to the nearest 0·1 mg), (b) seed mass (mean mass of one seed), (c) fruit length (to the nearest 0·01 mm) and (d) seed set (i.e. number of seed per total number of ovules), since aborted ovules are easily distinguishable in both species. In 2002, total inflorescence and flower production per plant were also measured in both species (using a subsample of 50 inflorescences per plant to estimate flower production in H. caput-felis).
Seedling survival
In February 2001, 200 seedlings of H. marifolium and 240 of H. caput-felis were individually tagged in their natural populations, their maximum crown size and number of leaves measured, and their exposure to sun (‘sun’ vs. ‘shade’) recorded. As the age of the seedlings could not be determined, seedlings with more than ten leaves were not considered in order to exclude seedlings that were possibly 2 or more years old (pers. obs.). Seedling survival was determined once in October 2001. In February 2002, a new cohort of 130 seedlings of H. marifolium and 214 of H. caput-felis (14 and 19 of them respectively had been tagged in 2001) were individually tagged; seedling survival was determined in November 2002.
Data analyses
The pattern of flowering phenology of both species was tested using failure-time analysis. A Cox proportional hazard regression model was fitted to the number of days between inflorescence tagging and the opening (receptivity) of each one of the flowers on the inflorescence. Using the parametric accelerated failure-time model, results were identical. Each individual plant was added as a random or ‘frailty’ effect in the model. Ties were estimated using the efron method, using the program S-Plus 2000 (Mathsoft, 1999).
Unless otherwise stated, all further analyses were performed using the GENMOD procedure of SAS 9.0 statistical package (SAS Institute, 2000). Differences between both species of Helianthemum in the number of receptive flowers at flowering peak was assessed by fitting a generalized linear model (GLIM) with a Poisson distribution and a logarithmical link function. Deviances from the model were scaled using the root-square of the ratio deviance/degrees of freedom to correct the over-dispersion of data.
The effects of the different pollination treatments on fruit and seed set within each plant (i.e. proportion of flowers producing fruits, and the proportion of ovules producing seeds within each fruit) were analysed separately for each species, using GLIMs with binomial error distributions and logit link functions. As all pollination treatments were applied to each individual plant, a repeated measures design was used, with individual plant as random factor and treatment as (within-subject) fixed factor. For seed set, aborted fruits (without seeds) were excluded for further calculations and analyses. Whenever significant differences between treatments were detected, multiple pairwise contrasts were performed, using likelihood ratio statistics and Bonferroni corrections.
Differences in the number of pollinator visits and the number of flowers visited per visit per plant were assessed using GLIMs, with poisson distributions and a logarithmical link functions. Deviances from the model were scaled as above. For the number of visits, pollinator group, year and sun exposure were included as fixed factors and the number of receptive flowers per plant as continuous covariates. For the number of flowers visited per pollinator visit, pollinator group was included as a fixed factor and the number of receptive flowers as a continuous covariate. Flower handling-time per pollinator visit was tested using GLIMs, with normal distribution and a logarithmical link function. The independent variables were: pollinator taxon for H. caput-felis; year for H. marifolium. Only plants that were visited by pollinators were considered for this analysis. In all the previous analyses for pollinators, only differences between orders of flying insects were considered (Diptera vs. Hymenoptera), in order to have a big enough sample size. In these analyses each plant species was analysed separately.
Difference in reproductive traits in field conditions between years and sun exposure (fixed and independent variables) were tested separately for each plant species. Mean plant size was included as a covariate, performing a previous analysis to detect a homogeneity of slopes between independent variables and the covariate. These analyses were assessed with GLIMs, using binomial distribution and logit link functions for fruit and seed set, and normal distributions and logarithmical link functions for the rest of the variables (fruit mass, seed mass and fruit length). For further references to these analyses applied in an ecological context, see Herrera (2000).
GLIMs were also used to analyse the potential effect of seedling size (number of leaves and maximum crown), year and sun exposure on seedling survival, using a binomial response variable and a logit link function. To choose between both continuous variables and evaluate whether (and which type) a model with heterogeneous slopes was required, all possible models were fitted using the various combinations of these variables and their interactions, and the best model selected based on their AIC (Akaike information criterion; estimates the variation explained by the model but penalizes for the number of parameters estimated by the model; see Akaike, 1973). For this best model, significant factor effects based on type III likelihood-ratio tests were reported. These analyses were performed using STATISTICA 6.0 (Statsoft Inc., 2001) because this program can select the best model based on their AIC.
Unless otherwise stated, average values are reported as mean ± standard error (±1 s.e.).
RESULTS
Flowering and fruiting phenology
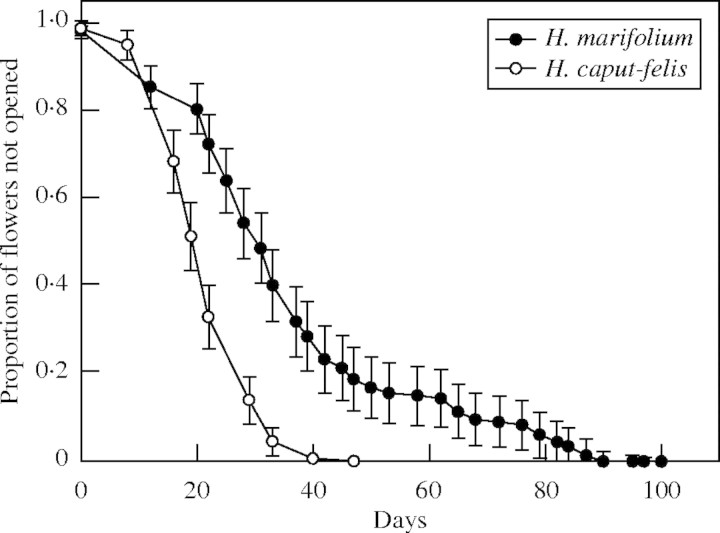
Helianthemum marifolium at Es Carnatge started flowering on 24 February and ended on 4 June, whereas H. caput-felis at Sa Ràpita began flowering on 25 March and finished by 11 May. Therefore, the flowering period is roughly twice as long in H. marifolium as for H. caput-felis (103 vs. 47 d, respectively). With regards to flowering pattern, flowers of H. marifolium showed a significantly lower chance of opening than flowers of H. caput-felis (index of flowering rate: −0·675 ± 0·081; χ2 = 68·9, d.f. = 1·0, P < 0·0001; Fig. 1), e.g. H. marifolium had a longer flowering phenology than H. caput-felis.
Fig. 1.
Survivorship curves of the flowering pattern of H. marifolium and H. caput-felis, showing the cumulative proportion of receptive flowers that are last to open on census days (mean ± s.e.). Census day was considered as the number of days between the tagging day of each inflorescence (for each species) and the opening of each one of the flowers on each inflorescence. Survival curves were fitted using the Kaplan–Meier estimation.
At the peak of flowering of both species (29 March in H. marifolium and 16 April in H. caput-felis), H. caput-felis showed a significantly higher number of receptive flowers per inflorescence than H. marifolium (1·4 ± 1·7 vs. 0·4 ± 0·7; χ2 = 17·07, d.f. = 1, P < 0·0001), in spite of the higher flower production per inflorescence of H. marifolium (Table 1).
Breeding system
Fruit set in H. marifolium differed marginally between treatments (χ2 = 6·27, d.f. = 3, P = 0·099): outcrossed flowers had a higher fruit set than the rest of the treatments (Table 2). However, significant differences were not detected in seed set (χ2 = 5·13, d.f. = 3, P = 0·167).
Table 2.
Fruit set (proportion of flowers that produced fruits on each plant) and seed set (proportion of flowers that produced seeds within each fruit) of H. marifolium and H. caput-felis for the different treatments in the hand-pollination experiment (mean ± s.e.)
|
H. marifolium |
H. caput-felis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source of pollen |
n |
Fruit set |
Seed set |
n |
Fruit set |
Seed set |
||||
| SelfS | 102 | 0·153 ± 0·078 a | 0·646 ± 0·132 a | 21 | 0·833 ± 0·105 a | 0·450 ± 0·097 a | ||||
| SelfI | 95 | 0·166 ± 0·070 a | 0·371 ± 0·031 a | 23 | 0·952 ± 0·048 a | 0·288 ± 0·055 a | ||||
| Geit | 70 | 0·045 ± 0·030 a | 0·219 ± 0·081 a | 23 | 0·929 ± 0·071 a | 0·413 ± 0·055 a | ||||
| Xen | 102 | 0·561 ± 0·071 b | 0·560 ± 0·039 a | 43 | 0·975 ± 0·025 a | 0·666 ± 0·056 a | ||||
Treatments with the same letter do not differ significantly (likelihood pairwise contrasts; P < 0·05).
SelfS, not manipulation; SelfI, pollination with pollen of the same flower; Geit, hand-pollination using pollen from the same plant, but from another flower; Xen, hand-pollination using pollen from a different plant; n, number of pollinated flowers per treatment.
Mean values (1 ± s.e.) are shown for each treatment.
In H. caput-felis, pollination treatment did not affect fruit set or seed set, e.g. H. caput-felis produced the same number of seeds and fruits whether or not a pollinator visit took place (χ2 = 3·44, d.f. = 3, P = 0·329; χ2 = 6·25, d.f. = 3, P = 0·100, for fruit and seed set respectively).
Flower visitors
Helianthemum marifolium received a lower diversity of flower visitors than H. caput-felis (Table 3). In both species, hymenopterans performed most visits (96 % in H. marifolium and 66 % in H. caput-felis), Apis mellifera being the foremost (92 % in H. marifolium and 64 % in H. caput-felis).
Table 3.
Taxonomic affiliation (species, order and family) and visiting frequency per year of the different flower visitors of H. marifolium and H. caput-felis
| Order |
Family |
Species |
2001 |
2002 |
||||
|---|---|---|---|---|---|---|---|---|
| H. marifolium | ||||||||
| Coleoptera | Coleoptera spp. 1 | 0 | 1 (0·06) | |||||
| Coleoptera spp. 2 | 0 | 5 (0·28) | ||||||
| Coleoptera spp. 3 | 0 | 1 (0·06) | ||||||
| Hymenoptera | Apiidae | Apis mellifera | 34 (7·16) | 59 (3·28) | ||||
| Halictidae | Halictus sp. | 0 | 4 (0·22) | |||||
| H. caput-felis | ||||||||
| Diptera | Syrphidae | Eristalinus aeneus | 15 (1·20) | 0 | ||||
| Eupeodes corollae | 17 (1·36) | 0 | ||||||
| Sphaerophoria scripta | 2 (0·16) | 0 | ||||||
| Eristalix tenax | 9 (0·72) | 0 | ||||||
| Syrphidae spp. 1 | 1 (0·08) | 1 (0·08) | ||||||
| Hymenoptera | Apiidae | Apis mellifera | 89 (7·12) | 2 (0·16) | ||||
| Halictidae | Halictidae spp. 1 | 3 (0·24) | 0 | |||||
| Lepidoptera | Lepidoptera spp. 1 | 1 (0·08) | 0 | |||||
Numbers in parenthesis refer to the number of visit per hour per each species of pollinator.
Numbers of hours censused totalled 4·75 and 18·0 h for H. marifolium and 12·5 and 12·25 h for H. caput-felis, for 2001 and 2002, respectively.
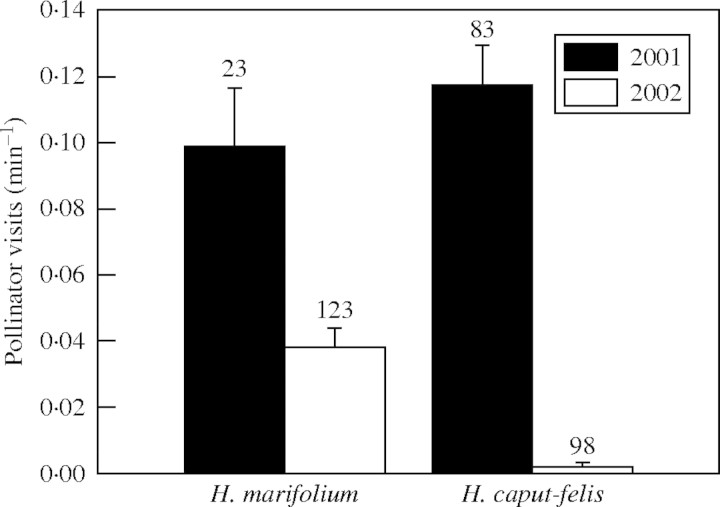
The number of pollinator visits was significantly affected by the year in both plant species (H. marifolium: χ2 = 9·45, d.f. = 1, P = 0·002; H. caput-felis: χ2 = 345·9, d.f. = 1, P < 0·0001), being higher in 2001 than 2002 (Fig. 2). In H. marifolium, exposure to sun had only a marginal effect (sun: 0·86 ± 0·12; shade: 0·39 ± 0·11; χ2 = 3·78, d.f. = 1, P = 0·052) but not with the ‘sun exposure’ × year interaction (χ2 = 0·98, d.f. = 1, P = 0·322). In H. caput-felis, however, plants in shade received significantly more visits (1·26 ± 0·27; χ2 = 7·56, d.f. = 1, P = 0·006) than plants in sun (0·67 ± 0·10). For this species, differences between years were not tested due to the low number of visits in 2002 (n = 3); for this reason both years were pooled, the effect of year not being considered in further analyses. In separate analyses, the number of flowers visited was significantly different between pollinator groups (Hymenoptera vs. Diptera; χ2 = 4·74, d.f. = 1, P = 0·030) for H. caput-felis, being higher for Diptera than for Hymenoptera (2·6 ± 0·4, n = 46 and 2·1 ± 0·2, n = 90, respectively, both years pooled). In H. marifolium, virtually all pollinators were hymenopterans; hence, differences between pollinator groups were not tested. Helianthemum marifolium showed significant differences between years (χ2 = 3·99, d.f. = 1, P = 0·046), with 2001 having the greater visitation (2001: 2·8 ± 0·3, n = 34; 2002: 2·5 ± 0·2, n = 64).
Fig. 2.
Visitation rate (number of pollinator visits per minute; mean ± s.e.) for each species of Helianthemum and year. Numbers over the columns indicate the number of observations (15 min) during which plants were observed for each species and years. For each species, years are significantly different (P < 0·05).
Comparing the two pollinator groups in H. caput-felis, flower handling time was significantly higher for dipterans (40·4 ± 8·7s, n = 46) than for hymenopterans (6·8 ± 0·8s, n = 90; χ2 = 60·40, d.f. = 1, P < 0·001). In H. marifolium, differences between years were detected (χ2 = 20·91, d.f. = 1, P < 0·0001), i.e. hymenopterans showed higher handling times in 2001 (16·4 ± 20·7, n = 34) than in 2002 (10·0 ± 1·9, n = 60).
Factors determining reproductive potential
Reproductive traits varied between sun exposure for H. caput-felis but not for H. marifolium (Table 4). Helianthemum caput-felis produced more inflorescences per plant, more flowers per inflorescence and larger fruits in sun-exposed plants.
Table 4.
Mean (1 ± s.e.) and results of generalized linear models of the sun exposure (sun vs. shade) and year variation (2001 vs. 2002) on reproductive variables of H. marifolium and H. caput-felis
| Source of variation |
H. marifolium |
H. caput-felis |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sun/2001 |
Shade/2002 |
d.f. |
χ2 |
P |
Sun/2001 |
Shade/2002 |
d.f. |
χ2 |
P |
|||||||||||
| Inflorescence production | ||||||||||||||||||||
| Sun exposure | 13·7 ± 1·6 (29) | 21·3 ± 5·1 (21) | 1, 47 | 0·40 | 0·525 | 95·1 ± 14·9 (36) | 58·8 ± 20·5 (14) | 1, 47 | 19·87 | <0·001 | ||||||||||
| Plant crown | 1, 47 | 59·27 | <0·001 | 1, 47 | 131·38 | <0·001 | ||||||||||||||
| Flower production | ||||||||||||||||||||
| Sun exposure | 77 ± 10 (29) | 117 ± 32 (21) | 1, 47 | 0·10 | 0·750 | 306 ± 63 (36) | 172 ± 56 (14) | 1, 47 | 28·74 | <0·001 | ||||||||||
| Plant crown | 1, 47 | 57·88 | <0·001 | 1, 47 | 218·21 | <0·001 | ||||||||||||||
| One seed mass | ||||||||||||||||||||
| Sun exposure | 0·35 ± 0·02 (22) | 0·39 ± 0·02 (19) | 1, 57 | 0·16 | 0·693 | 0·81 ± 0·017(36) | 0·88 ± 0·04 (14) | 1, 80 | 3·57 | 0·059 | ||||||||||
| Year | 0·36 ± 0·02 (31) | 0·38 ± 0·02 (31) | 1, 57 | 0·02 | 0·898 | 0·84 ± 0·03 (37) | 0·83 ± 0·02 (42) | 1, 80 | 0·84 | 0·361 | ||||||||||
| Fruit weight | ||||||||||||||||||||
| Sun exposure | 3·11 ± 0·21 (22) | 3·05 ± 0·29 (19) | 1, 58 | 0·17 | 0·677 | 4·74 ± 0·12 (36) | 4·14 ± 0·22 (14) | 1, 80 | 6·23 | 0·013 | ||||||||||
| Year | 3·12 ± 0·21 (32) | 3·19 ± 0·22 (31) | 1, 58 | 0·02 | 0·881 | 4·79 ± 0·19 (37) | 4·47 ± 0·13 (42) | 1, 80 | 1·49 | 0·222 | ||||||||||
| Fruit length | ||||||||||||||||||||
| Sun exposure | 3·15 ± 0·07 (23) | 3·17 ± 0·10 (19) | 1, 59 | 0·06 | 0·813 | 3·36 ± 0·03 (36) | 3·17 ± 0·06 (14) | 1, 81 | 15·07 | <0·0001 | ||||||||||
| Year | 3·18 ± 0·08 (33) | 3·19 ± 0·07 (31) | 1, 59 | 0·92 | 0·337 | 3·27 ± 0·05 (38) | 3·32 ± 0·03 (48) | 1, 81 | 0·40 | 0·592 | ||||||||||
| Plant crown | 1, 59 | 3·58 | 0·059 | 1, 81 | 5·20 | 0·023 | ||||||||||||||
| Fruit set | ||||||||||||||||||||
| Sun exposure | 0·590 ± 0·070 (25) | 0·523 ± 0·036 (19) | 1, 62 | 1·91 | 0·168 | 0·476 ± 0·032 (36) | 0·506 ± 0·059 (14) | 1, 88 | 0·05 | 0·819 | ||||||||||
| Year | 0·742 ± 0·058 (35) | 0·352 ± 0·022 (32) | 1, 62 | 13·18 | <0·001 | 0·516 ± 0·045 (44) | 0·463 ± 0·031 (49) | 1, 88 | 0·56 | 0·454 | ||||||||||
| Plant crown | 1, 62 | 4·03 | 0·045 | 1, 88 | 0·08 | 0·771 | ||||||||||||||
| Seed set | ||||||||||||||||||||
| Sun exposure | 0·516 ± 0·047 (22) | 0·383 ± 0·054 (19) | 1, 58 | 2·04 | 0·153 | 0·351 ± 0·023 (36) | 0·307 ± 0·035 (14) | 1, 81 | 1·05 | 0·306 | ||||||||||
| Year | 0·478 ± 0·048 (32) | 0·432 ± 0·040 (31) | 1, 58 | 0·10 | 0·751 | 0·398 ± 0·029 (38) | 0·309 ± 0·022 (48) | 1, 81 | 3·61 | 0·056 | ||||||||||
Inflorescence and flower production were only compared in 2002 because both variables were only measured in that year.
The numbers in parenthesis refer to sample size for each category of sun exposure and year.
The interaction between variables and/or the covariate (plant crown) were included in the table whenever they were significant (P > 0·05).
However, these differences did not result in higher fruit or seed sets in plants located in sunny sites for either species (Table 4). Considering differences between years, fruit set was significantly lower in 2002 for H. marifolium (2001: 0·74 ± 0·06, n = 35; 2002: 0·35 ± 0·02, n = 32), but not for H. caput-felis. None of the interactions between year and sun exposure were statistically significant.
Considering the effect of plant size on reproductive traits, inflorescence and flower production were significantly correlated in both species, and for fruit length for H. caput-felis and fruit set for H. marifolium (Table 4). None of the reproductive variables showed a significant interaction between sun exposure and/or year with the covariate (plant crown), except for inflorescence and flower production for sun exposure (χ2 < 60·18, d.f. = 1, P < 0·001, for four analyses). In H. caput-felis, as would be expected, plants located in the sun showed a higher slope value in the correlation between plant size and inflorescence and flower production (i.e. as plant size increased, plants in the sun produced proportionally more inflorescences and flowers than plants under vegetation). However, H. marifolium showed an inverse trend: larger plants under vegetation produced proportionally higher numbers of inflorescences and flowers.
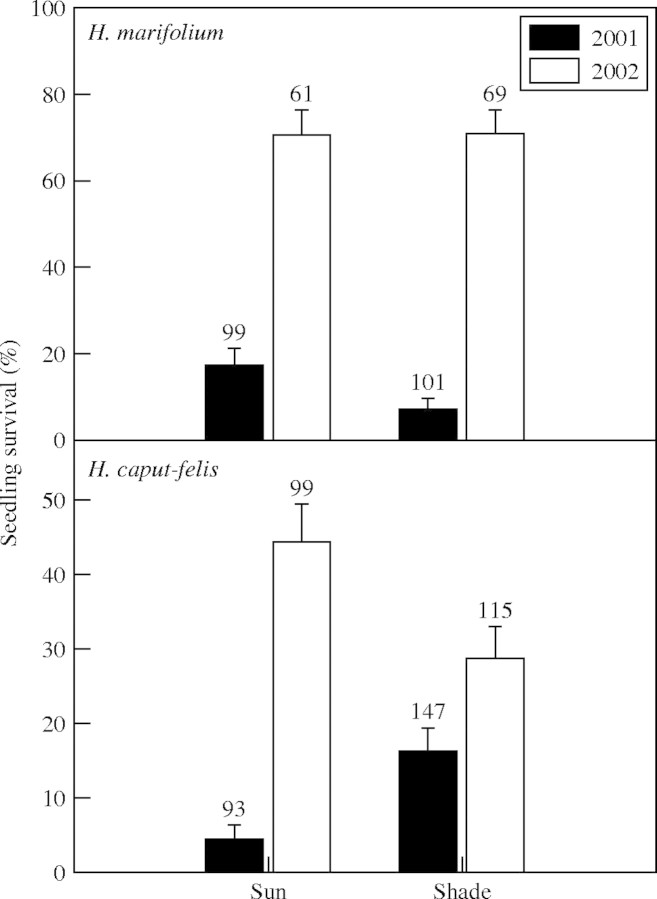
Seedling survival
Seedling survival varied significantly between years for both species (Table 5), being three- to six-fold higher in 2002 for both species (Fig. 3). Though sun exposure did not significantly affect in seedling survival in either species, a significant interaction between year and sun exposure was observed in H. marifolium (Fig. 3 and Table 5). As would be expected, maximum seedling crown was positively correlated with seedling survival in both species (Table 5), while the number of leaves was positively correlated with survival in H. caput-felis seedlings (Table 5), but not in H. marifolium.
Table 5.
Results of generalized linear models analysing the effect of leaf number, maximum seedling crown, sun exposure and year on the success of seedling survival in H. marifolium and H. caput-felis
| Species |
Independent variables |
d.f. |
χ2 |
P |
|---|---|---|---|---|
| H. marifolium | Maximum seedling crown | 1 | 8·23 | 0·004 |
| Year | 1 | 137·5 | <0·001 | |
| Sun exposure | 1 | 2·83 | 0·093 | |
| Year × sun exposure | 1 | 5·83 | 0·016 | |
| H· caput-felis | Leaf number | 1 | 9·08 | 0·002 |
| Maximum seedling crown | 1 | 8·41 | 0·004 | |
| Year | 1 | 39·5 | <0·001 |
Variables shown are those included in the model with the lowest AIC score. Non-significant variables are omitted from tables.
Fig. 3.
Percentage of seedling survival per species, year and sun exposure (mean ± s.e.). Numbers over the columns indicate the number of tagged seedlings per species, year and sun exposure.
DISCUSSION
The reproductive stages studied for these two species of Helianthemum do not limit the plant reproductive performance of the populations and years studied. So, at least for the short and medium term, both species are able to persist in their natural populations. However, the variation between years detected in seed production, pollinator service and seedling survival, suggests that reproductive output may be affected in adverse years.
Helianthemum marifolium is self-compatible, but mostly an outbreeder, i.e. outcrossed flowers produced three times more fruits than self-pollinated ones (i.e. those receiving pollen from the same flower or plant). Helianthemum caput-felis shows a comparable fruit and seed set in self-pollinated and outcrossed flowers; hence, fruit and seed set do not depend on the pollen source. Previous studies have suggested that self-incompatibility is common in the Cistaceae (Herrera, 1992; Talavera et al., 1993), and it is generally attributed to gametophytic incompatibility (Nettancourt, 1977). Apparently, this mechanism does not operate so effectively in H. caput-felis (as suggested also by Tébar et al., 1997).
The reliance on a single pollinator species in H. marifolium, where 95 % of the visits are performed by A. mellifera, increases the vulnerability of this species. However, A. mellifera is one of the most widespread and generalist insect pollinators (even as an alien species, in many ecosystems); hence, it is more reliable than most other pollinators (Richardson et al., 2000). Considering each pollinator, hymenopterans visited more flowers per plant than dipterans. This does not necessary imply an increase of fruit and seed set, as it could favour geitonogamy and thus increase selfing rates. Such risk is higher for H. caput-felis, given its higher number of receptive flowers per day and higher self-compatibility. A comparable case has been described for Hormatophylla spinosa (Gómez and Zamora, 1996). In this Mediterranean high-mountain shrub, 90 % of pollinator flights are produced among flowers of the same plant, suggesting that wind pollination may increase the outcrossing rate of this species. Although the flower handling time was five- to six-fold longer for dipterans than for hymenopterans, dipterans are unlikely to be more effective pollinators in H. caput-felis; instead, they rarely touch the stigma surface, often acting as pollen robbers (pers. obs.). Unfortunately, data about pollinator efficiency (pollen load) was not collected and this hypothesis cannot be verified.
The differences between years in plant reproductive performance (decreased fruit set in H. marifolium and seed set in H. caput-felis, both in 2002), are probably related to the lower number of pollinator visits observed that year, at least for H. marifolium whose reproductive output relies on crossing pollinations. Sun exposure appeared to affects flower production, but not reproductive output in H. caput-felis; sun-exposed plants produced a larger flower display, but the same fruit and seed set as shaded plants [similar to report by Herrera (1991) for Lavandula latifolia]. In contrast, the floral display of H. marifolium did not differ between sun exposure, but fruit set was higher in sunny sites; this difference cannot be explained by variation in pollinator visits, since these were not more frequent in sun-exposed sites.
Rainfall patterns determine one of the main resources affecting seedling survival in arid and Mediterranean regions (Fowler, 1988; Escudero et al., 1999; Traveset et al., 2003). In the present study, higher seedling survival of both species coincides with one of the years with the highest rainfall (2002). In contrast to other species (Rey and Alcántara, 2000; Traveset et al., 2003), seedling survival of both species did not vary with exposure to sun. Larger seedlings of both species showed a higher summer survival rate, as has been previously observed in the congeneric H. squamatum, an endemic gypsophile from semi-arid Spain (Escudero et al., 1999).
In short, none of the factors controlling the reproduction of the two endangered species of Helianthemum are important for their reproductive performance. In other words, neither biotic or abiotic limitation affects the conservation of these two species. Instead, what seems crucial to explain their rarity is the increasing destruction of their habitat. A large part of the seacoast of the Mediterranean region has been degraded by tourism facilities (19 %); this effect is most dramatic in the island of Mallorca (48 %; Blondel and Aronson, 1999). Thus, the preservation of such habitats is the most critical aspects in any strategies for the conservation of these two species and, more generally, of the Mediterranean coastal flora and fauna.
Supplementary Material
Acknowledgments
Amparo Lázaro, Núria Riera and Joan Carles Salom provided assistance during some of the fieldwork. Enrique Descals revised the English text, Angeles Marcos-García identified the syrphid flies and Luis Santamaría provided very useful comments and statistical advice. I am especially grateful to Anna Traveset for her supervision of the design and write up of this work. Thanks are also due to three anonymous reviewers for comments on the final version of the manuscript. This work was partially funded by the Spanish DGIC (PB97-1174) and by a research grant from the Town-Hall of the Ciutat de Palma 2000.
LITERATURE CITED
- Akaike H. 1973. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, eds. Proceedings of the second international symposium on information theory. Budapest, Hungary: Akademiai Kiado, 267–281. [Google Scholar]
- Alomar G, Mus M, Rosselló JA. 1997.Flora endèmica de les Balears. Palma de Mallorca: Consell Insular de Mallorca. [Google Scholar]
- Ågren J. 1996. Population size, pollinator limitation, and seed set in the self-incompatible herb Lythrum salicaria Ecology 77: 1779–1790. [Google Scholar]
- Bernardello G, Anderson GJ, Stuessy T, Crawford D. 2001. A survey of floral traits, breeding system, floral visitors, and pollination systems of the angiosperms of the Juan Fernández Islands (Chile). Botanical Review 67: 255–308. [Google Scholar]
- Blondel J, Aronson J. 1999.Biology and wildlife of the Mediterranean Region. Oxford: Oxford University Press. [Google Scholar]
- Buza L, Young A, Thrall P. 2000. Genetic erosion, inbreeding and reduced fitness in fragmented populations of the endangered tetraploid pea Swainsona recta Biological Conservation 93: 177–186. [Google Scholar]
- Callaway RM, Walker L. 1997. Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology 78: 1958–1965. [Google Scholar]
- Delanoë O, Montmollin B de, Olivier L, the IUCN/SCC Mediterranean Islands Plant Specialist Group. 1996.Conservation of Mediterranean island plants. 1. Strategy for action. Gland, Switzerland: IUCN. [Google Scholar]
- Erhardt A, Jäggi B. 1995. From pollination by Lepidoptera to selfing: the case of Dianthus glacialis (Caryophyllaceae). Plant Systematics and Evolution 195: 67–76. [Google Scholar]
- Escudero A, Somolinos RC, Olano JM, Rubio A. 1999. Factors controlling the establishment of Helianthemum squamatum, an endemic gypsophile of semi-arid Spain. Journal of Ecology 87: 290–302. [Google Scholar]
- Fowler NL. 1988. What is a safe site? Neighbour, litter, germination date, and patch effects. Ecology 69: 947–961. [Google Scholar]
- Gómez JM, Zamora R. 1996. Wind pollination in high-mountain populations of Hormathophylla spinosa (Cruciferae). American Journal of Botany 83: 580–585. [Google Scholar]
- Greuter W. 1995. Origin and peculiarities of Mediterranean island floras. Ecologia Mediterranea 21: 1–10. [Google Scholar]
- Herrera CM. 1991. Dissecting factors responsible for individual variation in plant fecundity. Ecology 72: 1436–1448. [Google Scholar]
- Herrera CM. 2000. Flower-to-seedling consequences of different pollination regimes in an insect-pollinated shrub. Ecology 81: 15–29. [Google Scholar]
- Herrera CM, Pellmyr O, eds. 2002.Plant animal interactions: an evolutionary approach. Oxford: Blackwell Publishing. [Google Scholar]
- Herrera J. 1992. Flower variation and breeding systems in the Cistaceae. Plant Systematics and Evolution 179: 245–255. [Google Scholar]
- Kearns CA, Inouye DW, Waser NM. 1998. Endangered mutualisms: the conservation of plant–pollinator interactions. Annual Review of Ecology and Systematics 29: 83–112. [Google Scholar]
- Mathsoft. 1999.S-Plus 2000. Guide to Statistics, Vol. 2. Seattle: Mathsoft Inc. [Google Scholar]
- Mus M. 1995. Conservation of flora in the Balearic Islands. Ecologia Mediterranea 21: 185–194. [Google Scholar]
- Nettancourt D de. 1977.Incompatibility in angiosperms. Berlin: Springer. [Google Scholar]
- O'Brien SJ. 1994. A role for molecular genetics in biological conservation. Proceedings of the National Academy of Sciences of the USA 91: 5748–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons MA. 2002.Estudis sobre la biologia de la reproducció de les brolles i les timonedes (Rosmarinion officinalis Br.-Bl. et Molinier 1934) de les Pitïuses. PhD Thesis, University of the Balearic Islands, Spain. [Google Scholar]
- Proctor M, Yeo P, Lack A. 1996.The natural history of pollination. London: Harper Collins. [Google Scholar]
- Pujol JA. 2001. La urbanización de la costa alicantina marca el declive de la jarilla de cabeza de gato. Quercus 188: 43–46. [Google Scholar]
- Rey P, Alcántara J. 2000. Recruitment dynamics of a fleshy-fruited plant (Olea europaea): connecting patterns of seed dispersal to seedling establishment. Journal of Ecology 88: 622–633. [Google Scholar]
- Richardson DM, Allsopp N, D'Antonio CM, Milton SJ, Rejmánek M. 2000. Plant invasions—the role of mutualisms. Biological Review 75: 65–93. [DOI] [PubMed] [Google Scholar]
- SAS Institute. 2000.SAS/STAT® software: User's guide. Cary, NC: SAS Institute. [Google Scholar]
- Schemske DW, Husband BC, Ruckelshaus MH, Goodwillie C, Parker IM, Bishop JG. 1994. Evaluating approaches to the conservation of rare and endangered plants. Ecology 75: 584–606. [Google Scholar]
- Statsoft. 2001.STATISTICA for Windows Tulsa: Statsoft Inc. (http://www.statsoft.com). [Google Scholar]
- Talavera S, Gibbs PE, Herrera J. 1993. Reproductive biology of Cistus ladanifer (Cistaceae). Plant Systematics and Evolution 186: 123–134. [Google Scholar]
- Tébar FJ, Gil L, Llorens L. 1997. Reproductive biology of Helianthemum apenninum (L.) Mill. and H. caput-felis Boiss. (Cistaceae) from Mallorca (Balearic Islands, Spain). Acta Botanica Malacitana 22: 53–63. [Google Scholar]
- Thanos CA, Georghiou K, Kadis C, Panzati C. 1992. Cistaceae: a plant family with hard seeds. Israel Journal of Botany 29: 22–44. [Google Scholar]
- Traveset A, Gulias J, Riera N, Mus M. 2003. Transition probabilities from pollination to establishment in a rare dioecious shrub species (Rhamnus ludovici-salvatoris) in two habitats. Journal of Ecology 91: 427–437. [Google Scholar]
- Traveset A, Willson MF, Sabag C. 1998. Effect of nectar-robbing birds on fruit set of Fuchsia magellanica in Tierra del Fuego: a disrupted mutualism. Functional Ecology 12: 459–464. [Google Scholar]
- Turner RM. 1990. Long-term vegetation change of a fully protected Sonoran Desert site. Ecology 71: 464–477. [Google Scholar]
- Washitani I. 1996. Predicted genetic consequences of strong fertility selection due to pollinator loss and in isolated population of Primula sieboldii Conservation Biology 10: 59–64. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.