Abstract
• Background and Aims Air seeding has long been regarded as a quick and successful measure for vegetation rehabilitation in China. However, seedling emergence of often-used species including Agriophyllum squarrosum, Artemisia sphaerocephala, Artemisia ordosica, Hedysarum fruticosum, Caragana korshinskii and Medicago sativa is low. Experiments were conducted under controlled conditions to study the effects of sowing depth and water supply on seedling emergence, in order to understand the requirements for increasing seedling emergence.
• Methods Seeds were exposed to different environments of burial and water supply regimes in PVC pots (7 cm in diameter and 11 cm in height) under the same light intensity and alternating temperature regimes in a growth chamber.
• Key Results Seedlings of three species (Agriophyllum squarrosum, Artemisia sphaerocephala, Artemisia ordosica) with relatively light seeds emerged well at a 0·5 cm sowing depth under a 7·5 and 10 mm water supply regime. However, few seedlings of these species emerged when the sowing depth was over 1 cm or when water supply was 5 mm. Seedlings of Caragana korshinskii, Hedysarum fruticosum and Medicago sativa emerged from sowing depths of 0·5–4 cm, 0·5–3 cm, and 0·5–4 cm, respectively, under both 7·5 and 10 mm water supply regimes. Under a 5 mm water supply regime, seedlings of these species also emerged at over 1 cm sowing depth. Seeds of all six species sown on the surface of sand did not germinate, and seedlings did not emerge when they were sown at depths greater than 6 cm.
• Conclusions Based on these experiments, a 0·5 cm sowing depth resulted in the highest seedling emergence and it is concluded that this is the optimal sowing depth for seedling emergence of all six species.
Keywords: Air seeding, desertification, Mu Us sandy land, seedling emergence, semi-arid area, sowing depth, vegetation rehabilitation
INTRODUCTION
Desertification affects 27·3 % of the land area of China, including 471 counties in 18 provinces and autonomous regions (China National Committee for the Implementation of the UN Convention to Combat Desertification, 1992). To rehabilitate vegetation in these desertified areas, one long-adopted strategy has been dispersal of seeds with aircraft (air seeding), which is labour-saving compared with transplanting nursery seedlings (Qi, 1998). Six species among those widely used for air-seeding are Agriophyllum squarrosum, Artemisia sphaerocephala, Artemisia ordosica, Hedysarum fruticosum, Caragana korshinskii and Medicago sativa. These species are of significant ecological and practical value in vegetation rehabilitation (Zheng et al., 2003). However, their seedling emergence rate is low when using air seeding (Wen, 1992) and the underlying causes are unclear. Therefore, it is necessary to examine seedling emergence in more detail in order to improve vegetation rehabilitation with these species when air seeding is used.
Many factors, including temperature, light and soil moisture, can affect seed germination (Agami, 1986; Devilliers et al., 1994; Khan and Ungar, 1996; Baskin and Baskin, 1998). Seedling emergence from soil is more complex than just a matter of seed germination (Maun, 1994). For example, sowing depth has been cited as a determinant of vegetation distribution and composition in coastal sand dune communities (Van der Valk, 1974; Maun and Lapierre, 1986). In fact, sand deposition has been recognized as a major selective force in the evolution of seed size, seed germination, seedling emergence, and survival of seedlings and mature plants (Maun, 1994). The germination of seeds may be directly related to the depth at which seeds are sown (Zhang and Maun, 1994). Sowing at shallow depths generally stimulates more seed germination than for seeds on the surface, because the former provides a moist environment around them and prevents seeds and seedlings from drying out (Harper and Obeid, 1967), as well as preventing herbivory by insects. However, excessive sowing depth may prevent seedlings from emerging above the sand surface and thus prevent their survival. It has been reported that seeds from sand dune species sown at 2–6 cm depths could germinate and seedlings emerge, while seedlings failed to emerge from seeds sown deeper than 6 cm (Maun, 1981; Maun and Lapierre, 1986). Huang and Gutterman (1999, 2000) also reported that no seedlings of Artemisia ordosica emerged for seeds sown at 2 cm. They also found that the higher the sand moisture content, from 1·7 % to 14·7 %, the higher and earlier the germination; while for 19·4 % or higher moisture content germination and emergence was delayed.
For the six species mentioned above, Zheng et al. (2003) demonstrated that exposure to light accounted for lower germination of three species, Agriophyllum squarrosum, Artemisia sphaerocephala and Artemisia ordosica, while germination of Hedysarum fruticosum, Caragana korshinskii and Medicago sativa was independent of such exposure. Furthermore, by taking into consideration the adverse impact of high temperature on seedling survival at the surface of sandy soil, it is apparent that optimal sowing depth of seeds combined with suitable soil moisture is necessary for seed germination and seedling development for all six species.
In this study, experiments were specifically designed to explore how these six species respond to different sowing depths in sand and to soil moisture, in terms of seedling emergence. The intention was that the experimental results should improve our understanding of the performance of these species in natural ecosystems and allow us to make recommendations for improving the results of air seeding.
MATERIALS AND METHODS
The study area was at the Mu Us sandy land on the Ordos Plateau in China, which has an annual mean precipitation of 345·2 mm and annual mean temperature of 6·7 °C. The monthly mean temperatures are below 5 °C from November to March, and between 7·4 and 21·9 °C from April to October. Mean precipitation from April to October is 321·8 mm, which accounts for about 93 % of annual precipitation.
As discussed in the previous section, the six species studied have significant ecological and economical value for vegetation rehabilitation in the study area (Zheng et al., 2003). Seeds of these species were collected from June to October, 2000, depending on the different species, and were randomly chosen from the whole plant population to get an adequate representation of genetic variation. For each species, a set of 20 square plots (100 m2 per plot) was set up. After the seeds matured, depending on the size and weight of the seed, varied amounts (50–100 g) of legumen (for Hedysarum fruticosum Maxim., Caragana korshinskii Kom. and Medicago sativa L.), seeds (for Agriophyllum squarrosum (L.) Moq.) and achenes (for Artemisia sphaerocephala Krasch. and Artemisia ordosica Krasch.) were removed directly from plants in each plot by hand. A total 1000–2000 g of seeds was collected for each species. Seeds were spread on tables at room temperature until dried, and then they were threshed through screens by hand. A small fanning mill was used to separate seeds from chaff (Gul and Weber, 1999; Khan et al., 2001; Zheng et al., 2003). Seeds were then transported to Japan, and stored at 4 °C until they were used in the experiments. The experiments were conducted in Japan in 2002.
Germination experiments were carried out within automatic temperature- and light-controlled growth chambers. The chambers were set for daily photoperiods (14 h light, 10 h dark) using cool white fluorescent lights. The temperatures were set to 15/25 °C (night/day) under 600 µmol m−2 s−1 light flux density. This alternating temperature regime was chosen because it was close to field conditions and suitable for many species (Khan and Ungar, 1997; Naidoo and Naicker, 1992), and it is well known that experiments conducted at constant temperature might have different effects on germination compared with alternating temperatures (Washitani and Takenaka, 1984; Ghersa et al., 1992).
The raw sand used in this study was collected from riverbeds in Japan, washed several times with tap water, dried in an oven for 3 d at 80 °C, and then sifted through several sieves to remove debris. Next, the sand was separated into four groups based on particle size: >0·5 mm, 0·5–0·25 mm, 0·25–0·1 mm and 0·1–0·05 mm. Finally, the prepared sand used for the experiments was mixed from different particle size groups with proportions similar to field conditions in the study area. The composition percentages (percent of gravimetric content) for the four size groups were 3·3 ± 1·1, 45·0 ± 3·7, 47·3 ± 2·4 and 2·4 ± 0·5, for >0·5, 0·5–0·25, 0·25–0·1 and 0·1–0·05 mm, respectively.
PVC pots (7 cm in diameter and 11 cm in height) were first filled with prepared sand to the specific depth at which seeds were to be placed. Then the pots were filled with additional sand. The drainage outlet at the bottom of the pots was covered with strips of nylon mesh to prevent the loss of sand whilst allowing drainage of excess water.
There were eight sowing depth treatments (0, 0·5, 1·0, 1·5, 2, 3, 4, 6 cm), and five replicates per treatment for each species. In each replicate, 25 randomly selected seeds were utilized. The PVC pots with sand and seeds were placed in a random arrangement on a table in a growth chamber and the locations of pots were changed every day. The pots were watered immediately after planting and then watered once every 3 d with tap water. Three water supply regimes were applied, 5 mm every 3 d, 7 mm every 3 d, or 10 mm every 3 d, which was equivalent to 50, 75, and 100 mm per month, respectively. The water regimes of 50 and 100 mm per month were designed to match precipitation field in June and July, respectively, in Mu Us sandy land, based on the 30 year average of microenvironment data. The precipitation in June and July was used as a reference because these two months are the most critical for seedling establishment and growth in the study area. Water was applied gently to avoid disturbing the seeds, especially those at 0 cm (Maun and Lapierre, 1986). Emerged seedlings, with their cotyledons visible on the sand surface, were counted daily. The tests continued until no additional seedlings emerged (Tobe et al., 2000).
For every water treatment, eight extra pots filled with prepared sand were utilized for monitoring soil moisture. Five of them were used to measure gravimetric soil water content. The weight of each of the five pots was measured every day using an electronic balance (Mettler PM4600, Mettler Instrument AG, Germany). For each pot, soil moisture within different layers (0·5, 1·0, 1·5, 2·0, 3·0, 4·0, 5·0, 6·0, 7·0 cm) was first measured by weighing the sand of every layer, and then each layer was dried in an oven and weighed again. In this way, soil moisture for different layers in the final day and in the whole pot on each day could be calculated (Figs 1, 2). The other three pots were used to monitor the volumetric water content (Fig. 3) using a sensor (ECH2O-10, Decagon Devices, Inc. Pullman, WA, USA). The relationship between gravimetric soil water content and volumetric water content could be described by the following equation:
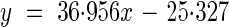 |
where y is gravimetric soil water content (g water 100 g−1 soil) and x is volumetric water content (m3 m−3) (R2 = 0·93).
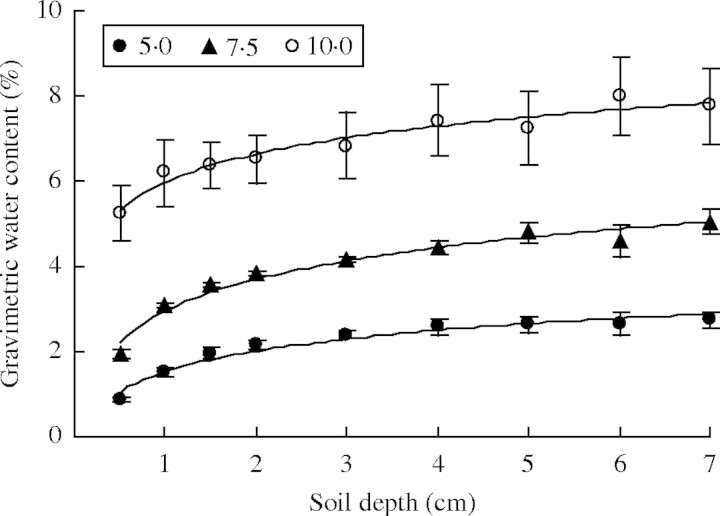
Fig. 1.
Gravimetric soil water content (g water 100 g−1 soil, ±s.e.) when 5·0, 7·5, 10·0 mm water was supplied every 3 d. n = 5.
Fig. 2.
Gravimetric soil water content (g water 100 g−1 soil, ±s.e.) at different layers when 5·0, 7·5, 10·0 mm water was supplied every 3 d. n = 5. The fitted regression lines are as follws: 5·0 mm water supply, y = 0·9538 ln(x) + 5·9757, R2 = 0·9526; 7·5 mm water supply, y = 1·0767 ln(x) + 2·9493, R2 = 0·9678; 10·0 mm water supply, y = 0·6961 ln(x) + 1·5294, R2 = 0·9599.
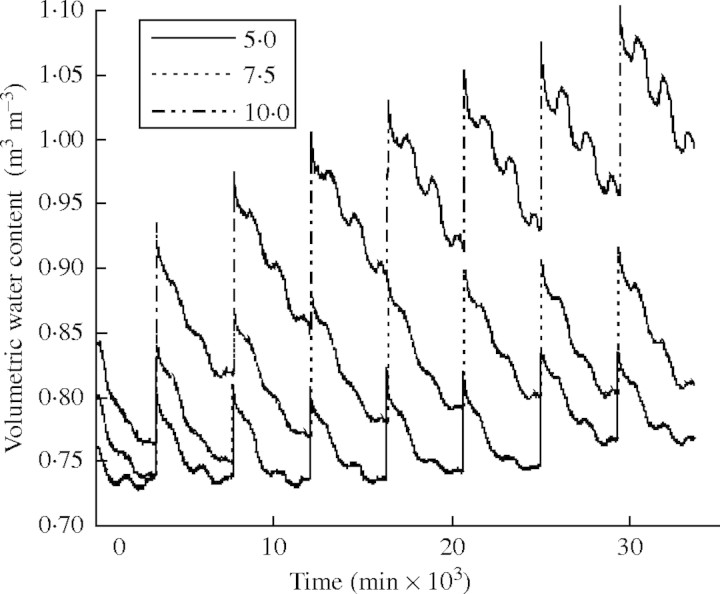
Fig. 3.
Volumetric water content of soil (m3 m−3) when 5·0, 7·5, 10·0 mm water was supplied every 3 d. n = 3. The data were automatically recorded at 10-min intervals.
Emergence was measured using two indices: final percentage emergence and emergence rate. The final percentage emergence was defined as the percentage of seedlings emerged at the end of the experiment (25 d). The emergence rate was estimated with a modified Rozema index of germination rate (Rozema, 1975), Σ(100Gi/(nti)), where n is the number of seeds used in an experiment, and Gi is the number of seedlings emerged on day ti (ti = 0, 1, 2, 3 …).
Values of final percentage emergence were arcsine transformed before statistical analysis to ensure homogeneity of variance (Zar, 1984). Values of emergence rate were not transformed because variances were homogeneous. The transformed values of final percentage emergence and untransformed data of emergence rate were analysed using a three-way analysis of variance (ANOVA). If significant differences were found, Tukey's test was used to determine mean differences between treatments (Chen and Maun, 1999). All statistical analyses, including the test for homogeneity of variance, were performed using the SPSS 10·0 package (SPSS, 2000).
RESULTS
The three-way ANOVA indicated that water supply, sowing depth, species and their combinations significantly affected final seedling emergence percentage. It was found that, in general, final seedling emergence percentage of the six species was significantly higher at higher water supply, lower at deeper sowing, and varied with different combinations of the two factors (Tables 1, 2; Fig. 4). Few seeds on the surface of the sand germinated for any of the six species, under every watering condition. For all other levels of sowing depth and water supply regimes, the six species can be divided into three groups based on their final seedling emergence response.
Table 1.
Results of three-way ANOVA of characteristics of final seedling emergence percentage and emergence rate of six species in relation to water supply and sowing depth
| Dependent variables |
|||
|---|---|---|---|
| Independent variables |
Final emergence percentage |
Emergence rate |
|
| Species (S) | 325·0*** | 535·2*** | |
| Water supply (W) | 31·6*** | 39·7*** | |
| Burial depth (D) | 254·8*** | 342·4*** | |
| S × W | 3·2** | 3·9*** | |
| S × D | 37·9*** | 47·9*** | |
| W × D | 27·4*** | 28·3*** | |
| S × W × D | 2·4*** | 2·6*** | |
There were three levels of water supply (5·0, 7·5, 10·0 mm every 3 d) and eight sand burial depths (0·0, 0·5, 1·0, 1·5, 2·0, 3·0, 4·0, 6·0 cm). Each treatment had five replicates. Figures represent F-values.
P < 0·01,
P < 0·001.
Table 2.
Results of multi-variable comparison of water supply and sowing depth effects on final emergence percentage and emergence rate of six species (Tukey test)
| Water supplying regimes |
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Burial depth |
Agriophyllum squarrosum |
Artemisia sphaerocephala |
Artemisia ordosica |
Caragana korshinskii |
Hedysarum fruticosum |
Medicago sativa |
||||||||||||||||||||||||||||||
| 5·0 |
7·5 |
10·0 |
5·0 |
7·5 |
10·0 |
5·0 |
7·5 |
10·0 |
5·0 |
7·5 |
10·0 |
5·0 |
7·5 |
10·0 |
5·0 |
7·5 |
10·0 |
|||||||||||||||||||
| Final emergence percentage | ||||||||||||||||||||||||||||||||||||
| 0·0 | Aa | Aa | Aac | Aa | Abcd | Abc | Aa | Aac | Ab | Aa | Aa | Aad | Aa | Aa | Aad | Aa | Aa | Aad | ||||||||||||||||||
| 0·5 | Aa | Bbce | Bbe | Bcd | Bbce | Be | Aad | Bbcf | Bbe | ACad | Bbe | Bbe | Bbd | Bce | Bbe | Bad | Bef | Bbe | ||||||||||||||||||
| 1·0 | Ba | Ab | BCa | BCb | ACb | ACb | Ab | Ab | Cb | Bac | Bac | BCac | Cc | BCac | Bc | Cc | Bac | Cc | ||||||||||||||||||
| 1·5 | Aa | Aa | ACac | BCa | Ca | Da | Aa | Aa | Ca | Bbc | Bbc | BCbcd | Cbd | CEce | Bbde | Cd | Bd | Cd | ||||||||||||||||||
| 2·0 | Aa | Aac | ACac | ACa | Ca | Da | Aac | Aa | Ca | BDb | Bb | Abce | Bb | CBbde | Bbd | Cd | Bd | BCd | ||||||||||||||||||
| 3·0 | Aa | Aab | Aa | Aa | Ca | Da | Aa | Aa | Ca | BCbc | BCcde | ABc | Bcde | Ccd | Aac | Cde | Be | BCde | ||||||||||||||||||
| 4·0 | Aa | Aa | Aa | Aa | Ca | Da | Aa | Aa | Ca | CDbc | ACac | ABbc | Aa | AEac | Aa | Cd | Bd | Dd | ||||||||||||||||||
| 6·0 | Aa | Aa | Aa | Aa | Ca | Da | Aa | Aa | Ca | Aa | Aa | Aa | Aa | Aa | Aa | Aa | Aa | Aa | ||||||||||||||||||
| Emergence rate | ||||||||||||||||||||||||||||||||||||
| 0·0 | Aa | Aa | Aac | Aa | Abc | Abc | Aa | Aab | Ab | Aa | Aa | ADac | Aa | Aa | Aac | Aa | Aa | Aac | ||||||||||||||||||
| 0·5 | Aa | Bbf | Bcf | Badi | Bfgh | Bbch | Aa | Bfgih | Bcf | ACad | Bbce | Bce | Bdf | Bcf | Bbceg | Badi | Be | Bce | ||||||||||||||||||
| 1·0 | Ba | ACb | BCa | Bb | ACb | Ab | Ab | Ab | Cb | Bad | Bad | BCad | Cac | Bad | Bac | Cc | Bcd | Cc | ||||||||||||||||||
| 1·5 | Aa | ACab | ACbe | Bab | Ca | Ca | Aab | Aab | Cab | Bcg | Bcg | BCcdg | Ccdg | Beg | Bcg | Dcf | Bf | BCdf | ||||||||||||||||||
| 2·0 | Aae | Cace | ACacd | Bae | Cae | Cae | Ae | Ae | Cae | BDbd | Bb | ACbc | Dbc | Bb | Bbd | Df | Bf | Cf | ||||||||||||||||||
| 3·0 | Aa | ACa | Aa | Aa | Ca | Ca | Aa | Aa | Ca | CDbc | Bb | ACb | BDbc | Bbc | Aac | Dd | Bd | BCd | ||||||||||||||||||
| 4·0 | Aa | Aa | Aa | Aa | Ca | Ca | Aa | Aa | Ca | Cb | Aab | ACbc | Aa | Aac | Aa | Cd | Cd | Dd | ||||||||||||||||||
| 6·0 | Aa | Aa | Aa | Aa | Ca | Ca | Aa | Aa | Ca | Aa | Aa | Da | Aa | Aa | Aa | Aa | Aa | Aa | ||||||||||||||||||
Seeds were exposed to three levels of water supply regime (5·0, 7·5, 10·0 mm at 3-d intervals) and eight levels of burial depth (0·0, 0·5, 1·0, 1·5, 2·0, 3·0, 4·0, 6·0 cm). Each treatment had five replicates.
Different capital letters in every column indicates significant difference of final emergence percentage and emergence rate with same water supply. Different lower case letters in every row indicates significant difference of final germination percentage and emergence rate of different species with different water supply under the same sand burial, P < 0·05.
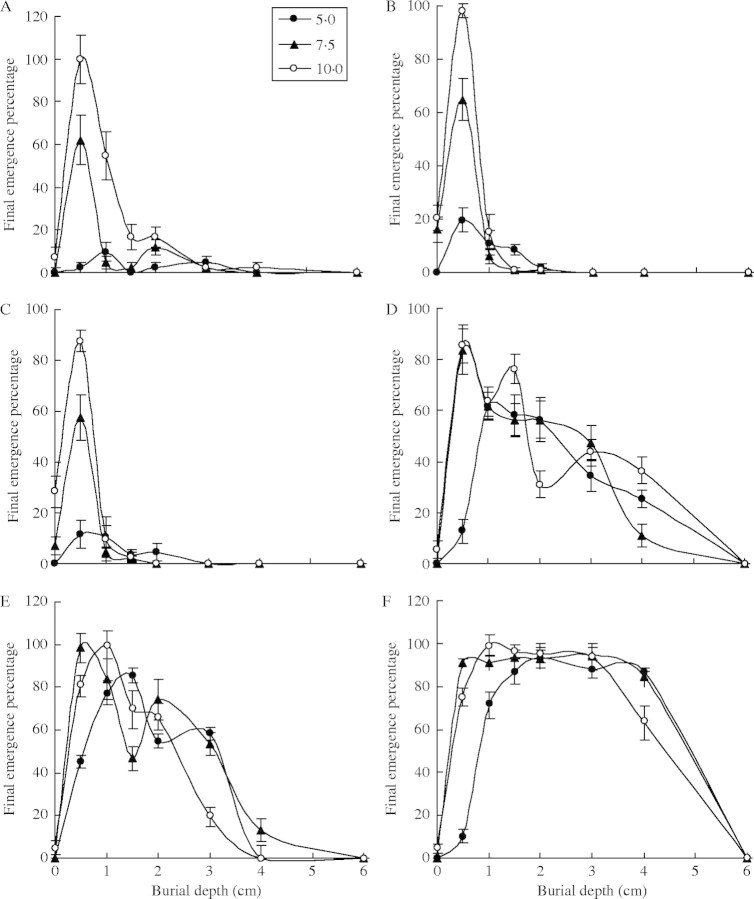
Fig. 4.
Final percentage emergence of six species at different sowing depths (0·0, 0·5, 1·0, 1·5, 2·0, 3·0 4·0, 6·0 cm) and water supplying regimes (5·0, 7·5, 10·0 mm at 3-d intervals). Each point represents the mean of five replicates (±s.e.). (A) Agriophyllum squarrosum; (B) Artemisia sphaerocephala; (C) Artemisia ordosica; (D) Caragana korshinski; (E) Hedysarum fruticosum; (F) Medicago sativa.
Agriophyllum squarrosum, Artemisia sphaerocephala and Artemisia ordosica belong to one group (Fig. 4A–C). For this first group, the highest percentage emergence occurred at sowing depths of 0·5 cm, and a significant decrease in seedling emergence occurred at 1 cm. With regard to responses to water supply, final seedling emergence was highest at or above 7·5 mm. A 5 mm water supply led to significantly lower final seedling emergence than a 10 mm supply.
Within this group, individual species also showed some differences. For Agriophyllum squarrosum, seedling emergence was 100 % at 0·5 cm depth, but this was followed by a significant decrease in emergence to 54·8 % at 1 cm depth, 2·4 % at 4 cm, and 0 % at 6 cm depth under the 10 mm water supply regime. For Artemisia sphaerocephala, seedlings failed to emerge at or below 3 cm depth. Its seedling emergence was 98·3 % at 0·5 cm depth, also followed by a significant decrease to 15·4 % at 1 cm depth and 0·9 % at 2 cm under the 10 mm water supply regime. For Artemisia ordosica, the emergence was 87·6 % at 0·5 cm depth, only 9·7 % at 1 cm, 4·4 % at 2 cm, and 0 % at 3 cm or deeper under the 10 mm water supply regime.
Caragana korshinskii and Hedysarum fruticosum belong to the second group (Fig. 4D, E). For Caragana korshinskii, the highest seedling emergence percentage occurred at 0·5 cm sowing depth under 7·5 mm and 10 mm water supply. However, for 5 mm water supply seedling emergence was significantly lower, and the highest value occurred at 1 cm depth. For all three water supply regimes, below the depth with highest seedling emergence, seedling emergence gradually decreased with sowing depth. There was no significant difference between final seedling emergence percentages from 1–3 cm under 5 mm water supply. Similarly, final seedling emergence percentages from 0·5–3 cm and from 0·5–1·5 cm depth showed no significant difference under 7·5 mm and 10 mm water supply, respectively. In fact, except at 0·5 cm sowing depth, there was no significant difference for final seedling emergence percentage between different water supply regimes at the same depth.
For Hedysarum fruticosum, a relatively higher seedling emergence occurred at 0·5–2 cm sowing depths under all water supply regimes than for C. korshkinski. No significant differences were noted among seedling emergence at 1–1·5 cm depth under 5 mm water supply, 0·5–1 cm depth under 7·5 mm water supply, or 0·5–2 cm depth under 10 mm water supply. The highest final seedling emergence percentage under 5 mm water occurred at deeper sowing depths than that under 7·5 or 10 mm water supply. When sowing depth was over 3 cm, the final seedling emergence percentage under 10 mm water supply was lower than that under 5 or 7·5 mm water supply.
The third group had one species, i.e. Medicago sativa (Fig. 4F). Its final seedling emergence percentage was higher from 0·5–4 cm sowing depths, except at 0·5 cm under 5 mm water supply. There was no significant difference between final seedling emergence percentages at 1–4 cm depth under 5 mm water supply, 0·5–4 cm depth under 7·5 mm water supply, or 1–3 cm depth under 10 mm water supply. Except at 0·5 cm sowing depth, there was no significant difference between final seedling emergence percentages under different water supply regimes.
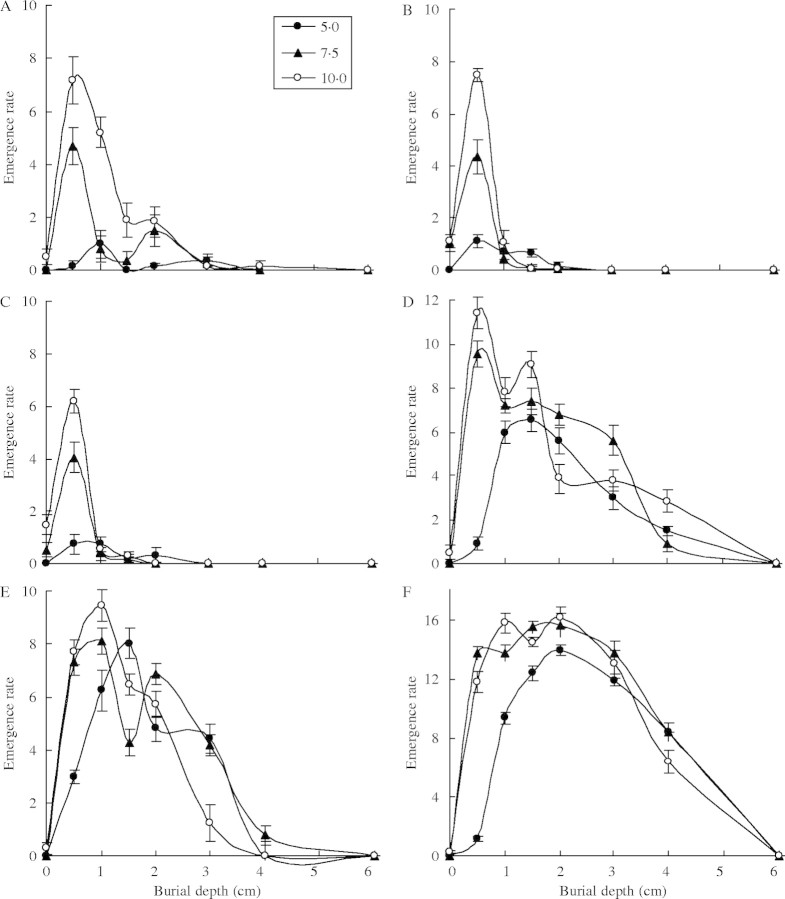
The three-way ANOVA of seedling emergence rates indicated significant effects of sowing depth, water supply regimes, species and their interactions (Tables 1, 2; Fig. 5). Emergence rate responded to water supply regimes and sowing depth in a similar pattern to final seedling emergence percentage, with the six species falling into the same three groups. Generally, the 10 mm water supply regime was associated with more rapid seedling emergence.
Fig. 5.
Emergence rate of six species under different sowing depths (0·0, 0·5, 1·0, 1·5, 2·0, 3·0 4·0, 6·0 cm) and water supplying regimes (5·0, 7·5, 10·0 mm at 3-d intervals). Each point represents the mean of five replicates (±s.e.). Figure order is the same as Fig. 4.
DISCUSSION
In sand dune habitats, build-up of overlaying sand imposes an abiotic stress similar to other stresses, such as herbivory, high or low temperatures, or desiccation. Under regular sand accretion, such natural burial acts as a strong selective force and many species have adapted genetically, morphologically and physiologically to withstand it. Some species have become so specialized that they do not survive without coverage by sand to maintain high vigour (Eldred and Maun, 1982). In dune communities, coverage by sand is a major factor controlling the distribution and composition of vegetation (Ranwell, 1958; Van der Valk, 1974; Maun and Lapierre 1984, 1986), because the deposition of sand brings about a change in physical factors such as moisture, temperature, aeration, and other characteristics of the soil–plant microenvironment (Maun, 1998), which in turn affects seedling emergence. For the six species used in our experiment, all of them needed coverage by sand for successful seedling emergence, as evidenced by the experimental results and field trials (Wen, 1992).
Maun (1985) marked ten microsites containing seeds of Calamovilfa longifolia in autumn and measured the amount of natural sand accretion on these seeds during one winter. In his experiments, seeds were placed at depths ranging from 1–29 cm during the winter. The seeds showed four possible seedling emergence patterns: (1) the seeds germinated and emerged as seedlings; (2) they germinated but did not emerge due to decomposition resulting from the activity of microorganisms; (3) the seeds did not germinate and were destroyed by biotic agents; and (4) the seeds underwent enforced or innate dormancy and become part of the seed bank. For our experiment, seeds without seedling emergence were due mainly to reasons (2) and (4). It was noticed that some seeds of all species did not germinate or germinated but did not emerge under deeper burial, but such detailed information was unavailable because we focused on seedling emergence in this investigation.
In our experiments, more water supply resulted in increased seedling emergence. For sowing depth, seedlings of three species emerged primarily at 0·5 cm depth, two species emerged from 0·5–3 cm, and the other species emerged from 0·5–4 cm depth. The results for seedling emergence of Caragana korshinskii and Hedysarum fruticosum in all treatments were consistent with those reported for Calamovilfa longifolia by Maun and Riach (1981), where the highest percentage emergence and rate of emergence of seedlings occurred from 1–2 cm depths, and as the depth increased from 2 to 12 cm there was a significant decrease in both seed germination and percentage emergence. A similar seedling emergence response was also observed at a mean optimal depth of 4·73 cm and a maximum depth of 11 cm, where no emergence occurred in the field (Zhang and Maun, 1990). Huang and Gutterman (1999, 2000) reported that seedlings of three Artemisia species did not emerge when they were sown at over 2 cm.
In terms of seedling emergence rate, seedlings with shallow sowing depth emerged earlier, while at deeper sowing it was delayed. Zhang and Maun (1990) reported seedlings from 0, 2, and 4 cm sowing depths began to emerge 5–6 d after sowing and reached their maximum emergence percentage about 11–15 d after sowing. As the depth of seeds increased, so did the length of time taken by the seedlings to emerge from sand. Seedlings at 8 cm depth began to emerge after 17 d. In our study, for three species with small seeds, seedlings of Agriophyllum squarrosum emerged from deeper soil than those of the two Artemisia species. This implies that Agriophyllum squarrosum is more adapted to a shifting dune environment than the two Artemisia species. That is indeed true in the field. Agriophyllum squarrosum is a pioneer species in the dune environment, while Artemisia sphaerocephala and Artemisia ordosica are mid- to late-successional species (Zheng et al., 2003).
As a general rule, seeds exposed on the surface showed very poor germination. Excessive light, temperature fluctuations and desiccation of seeds after watering may have contributed to this, but the most likely cause is exposure of seeds to evaporation stress (Zhang and Maun, 1990). In our study, it was noted that a few seeds (especially for the two Artemisia species) germinated and emerged on the surface of sand, probably because seeds of these species were covered with mucilaginous material, which easily sticks to sand after absorbing water. The frequent watering shifted the surface sand and led to a few millimetres of sand coverage for a few seeds in the 0 cm burial treatment and induced them to germinate and emerge (Maun and Lapierre, 1986). Except for this, few seeds on the surface of the sand germinated and emerged. We propose that for Caragana korshinskii, Hedysarum fruticosum and Medicago sativa desiccation of seeds after watering is the main reason why they did not germinate and emerge, while for Agriophyllum squarrosum, Artemisia sphaerocephala and Artemisia ordosica light exposure was another harmful factor (Zheng et al., 2003).
Why the deeply sown seedlings did not emerge may be due to various causes. First, it may be argued that following seed germination, the emergence of seedlings depends on the energy contained in the endosperm or cotyledons of a seed (Zhang and Maun, 1993) as well as the depth at which a seed is sown. Secondly, sowing decreases the concentration of oxygen in the root zone. When soil in the root zone has excessive moisture, air is driven out of the soil pore spaces and the only source of oxygen for the plant is that present in soil water (Armstrong, 1979). It is well known that oxygen is essential for root growth, and if roots are deprived of oxygen, they soon perish (Armstrong, 1979), primarily due to a lack of ATP (essential for active uptake of nutrients) and accumulation of toxic substances (Armstrong, 1979; Drew and Lynch, 1980). Insufficient diffusion of oxygen to the root zone and higher moisture levels may also increase the populations of some soil-borne pathogens. In addition, sowing in sand may create a physical overburden above apical meristems, thus retarding their upward growth (Maun, 1998). Another reason may be due to the dormancy level of seeds. A small proportion of seeds may remain dormant when they are deeper in the soil. The suggested causes of lengthened dormancy are higher soil moisture, lower temperature, poor aeration and higher CO2 levels (Harper and Obeid, 1967). A longer seed dormancy at greater depths would be ecologically advantageous because seeds would survive in the dormant state in the seed bank (Maun, 1998). Especially in a desert environment, when wind removes surface layers of sand and reduces the depth of seeds, if seeds are exposed to a suitable environment, they may germinate and emerge.
The emergence of seedlings is also related to the weight of seeds, and hence to the amount of energy reserves within them (Van der Valk, 1974; Maun and Riach, 1981; Weller, 1985). In this study, the weight of 1000 seeds of Agriophyllum squarrosum, Artemisia sphaerocephala, Artemisia ordosica, Hedysarum fruticosum, Caragana korshinskii and Medicago sativa were 0·83 ± 0·00, 0·66 ± 0·00, 0·60 ± 0·00, 9·81 ± 0·02, 40·91 ± 0·04, 2·28 ± 0·02 g (mean ± s.e.), respectively. The light seeds of the first three species might be one of the causes for their high emergence rate at 0·5 cm depth, while the other three species with heavier seeds could emerge from relatively greater depths. However, it is not clear why Medicago sativa had higher seedling emergence than Hedysarum fruticosum and Caragana korshinskii, since its seeds are lighter than those of the two latter species. One possible reason is that these two species are more likely to be attacked by fungi than Medicago sativa when seeds were deeper in the soil, according to our observations during in the experiments.
CONCLUSIONS
These experiments explored the appropriate requirements for successful seedling emergence for the six species. The results suggest that being covered by sand leads to improved seedling emergence. For Agriophyllum squarrosum, Artemisia sphaerocephala and Artemisia ordosica with relatively light seeds, there was little or no seedling emergence at sowing depths over 0·5 cm under a 5 mm water supply. Under the same 5 mm water supply regime, for Hedysarum fruticosum, Caragana korshinskii and Medicago sativa with relatively heavy seeds, seedlings emerged at 1–4 cm burial depths. The 5 mm water supply regime was quite similar to the natural precipitation in the field during the main germinating season for these seeds. However, soil in the field has more moisture than that in our experiment. In the field, the soil of the surface layer usually contained 3–5 % water, and 5–15 % after rain in the spring. In our experiment, the soil water content was only 3·4 %, 5·2 % and 6·5 % for 5 mm, 7·5 mm and 10 mm water supply, respectively, after watering the first time. Under the 5 mm water supply regime, the water content reached 4·8 % after 20 d of watering (Fig. 1). It was also noted that the soil water content varied from 0·8–5·3 % at 0·5 cm depth for different water supply regimes (Fig. 2). The better moisture conditions in the field lead us to believe that seeds of the six species should be able to germinate and their seedlings emerge at 0·5 cm burial depth in spring in the field. This may require air seeding to be conducted in late May to enable proper cover by sand (0·5 cm), because wind is the natural agent for covering seeds and the prevailing wind in Mu Us sandy land starts from March and gradually decreases in late May or early June. However, the recommendation for timing of air seeding will need more field trials. It is suggested that further investigations are needed to test our laboratory results, including field trials after air seeding to assess the seeding rate per unit area, seedling emergence, depth at which seeds were located after various periods, moisture content of the substrate and probable causes of seed loss, low germination and seedling mortality.
Supplementary Material
Acknowledgments
We thank the Fund for Field Station in Resource and Environment Fields, Chinese Academy of Sciences, the Fund of President of Chinese Academy of Sciences, and National Key Basic Research Program (G2000018600) for supporting seed collection in China. We also appreciate the Association of International Research Initiatives for Environmental Studies, Japan for funding this research. The National Institute for Environment Studies supplied all necessary equipment and is deeply appreciated.
LITERATURE CITED
- Agami M. 1986. The effects of different soil water potentials, temperature and salinity on germination of seeds of the desert shrub Zygophyllum dumosum Physiologia Plantarum 67: 305–309. [Google Scholar]
- Armstrong W. 1979. Aeration in higher plants. Advances in Botanical Research 7: 225–231. [Google Scholar]
- Baskin CC, Baskin JM. 1998.Seeds. Ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press. [Google Scholar]
- Chen H, Maun MA. 1999. Effects of sand burial depth on seed germination and seedling emergence of Cirsium pitcheri Plant Ecology 140: 53–60. [Google Scholar]
- China National Committee for the Implementation of the UN Convention to Combat Desertification. 1992.China national action program to combat desertification, Beijing: Ministry of Forestry. [Google Scholar]
- Devilliers AJ, Rooyen MWV, Theron GK, Deventer HAV. 1994. Germination of three Namaqualand pioneer species, as influencing by salinity, temperature, and light. Seed Science and Technology 22: 427–433. [Google Scholar]
- Drew MC, Lynch JM. 1980. Soil anaerobiosis, micro-organisms and root function. Annual Review of Phytopathology 18: 37–66. [Google Scholar]
- Eldred RA, Maun MA. 1982. A multivariate approach to the problem of decline in vigour of Ammophila Canadian Journal of Botany 60: 1371–1380. [Google Scholar]
- Ghersa CM, Arnold RLB, Martinezghersa MA. 1992. The role of fluctuating temperatures in germination and establishment of sorghum-halepense regulation of germination at increasing depths. Functional Ecology 6: 460–468. [Google Scholar]
- Gul B, Weber DJ. 1999. Effects of salinity, light and temperature on germination in Allenrolfea occidentalis Canadian Journal of Botany. 77: 240–246. [Google Scholar]
- Harper JL, Obeid M. 1967. Influence of seed size and depth of sowing on the establishment and growth of varieties of fiber and oils seed flax. Crop Sciences 7: 527–532. [Google Scholar]
- Huang ZY, Gutterman Y. 1999. Germination of Artemisia sphaerocephala (Asteraceae), occurring in the sandy desert areas of Northwest China. South African Journal of Botany 65: 187–196. [Google Scholar]
- Huang ZY, Gutterman Y. 2000. Comparison of germination strategies of Artemisia ordosica with its two congeners from deserts of China and Israel. Acta Botanica Sinica 42: 71–80. [Google Scholar]
- Khan MA, Gul B, Weber DJ. 2001. Seed germination characteristics of Halogeton glomeratus Canadian Journal of Botany 79: 1189–1194. [Google Scholar]
- Khan MA, Ungar IA. 1996. Influence of salinity and temperature on the germination of Haloxylon recurvum Annals of Botany 78: 547–551. [Google Scholar]
- Khan MA, Ungar IA. 1997. Effects of light, salinity, and thermoperiod on the seed germination of halophytes. Canadian Joutnal of Botany 75: 835–841. [Google Scholar]
- Maun MA. 1981. Seed germination and seedling establishment of Calamovilfa longifolia on Lake Huron sand dunes. Canadian Journal of Botany 59: 460–469. [Google Scholar]
- Maun MA. 1985. Population biology of Ammophila breviligulata and Calamovilfa longifolia on Lack Huron sand dunes. I. Habitat, growth form, reproduction, and establishment. Canadian Journal of Botany 63: 113–124. [Google Scholar]
- Maun MA. 1994. Adaptations enhancing survival and establishment of seedlings on coastal dune systems. Vegetatio 111: 59–70. [Google Scholar]
- Maun MA. 1998. Adaptations of plants to burial in coastal sand dunes. Canadian Journal of Botany 76: 713–738. [Google Scholar]
- Maun MA, Lapierre J. 1984. The effects of burial by sand on Ammophila breviligulata Journal of Ecology 72: 827–839. [Google Scholar]
- Maun MA, Lapierre J. 1986. Effects of burial by sand on seed germination and seedling emergence of four dune species. American Journal of Botany 73: 450–455. [Google Scholar]
- Maun MA, Riach S. 1981. Morphology of caryopses, seedlings and seedling emergence of the grass Calamovilfa longifolia from various depths in sand. Oecologia 49: 137–142. [DOI] [PubMed] [Google Scholar]
- Naidoo G, Naicker K. 1992. Seed germination in the coastal halophytes Triglochin bulbosa and Triglochin striata Aquatic Botany 42: 217–229. [Google Scholar]
- Qi J. 1998.Aerial sowing for sand control in China. Beijing: Science Press. [Google Scholar]
- Ranwell DS. 1958. Movement of vegetated sand dunes at Newborough Warren, Anglesey. Journal of Ecology 46: 83–100. [Google Scholar]
- Rozema J. 1975. The influence of salinity, inundation and temperature on germination of some halophytes and non-halophytes. Oecologia Plantarum 10: 341–353. [Google Scholar]
- SPSS. 2000.SPSS 10.0 for windows. SPSS Inc. USA. [Google Scholar]
- Tobe K, Li X, Omasa K. 2000. Seed germination and radicle growth of a Halophyte, Kalidium caspicum (Chenopodiaceae). Annals of Botany 85: 391–396. [Google Scholar]
- Van Der Valk AG. 1974. Environmental factors controlling the distribution of forbs on coastal foredunes in Cap Hatteras National Seashore. Canadian Journal of Botany 52: 1057–1073. [Google Scholar]
- Washitani I, Takenaka A. 1984. Germination responses in non-dormant seed population of Amarathus patulus to constant temperatures in the sub-optimal range. Plant Cell and Environment 7: 353–358. [Google Scholar]
- Weller SG. 1985. Establishment of Lithospermum caroliniense on sand dune: the role of nutlet mass. Ecology 66: 1893–1901. [Google Scholar]
- Wen Y. 1992. A study on the effect of rainfall on aerial seeding. In: Wang JX, ed. Collected Papers of the Maowusu Sands Exploitation and Control Research Centre. Hohhot, China: Inner Mongolia University Press, 43–46 (in Chinese). [Google Scholar]
- Zar JH. 1984.Biostatistical analysis, Englewood: Prentice-Hall, Inc. [Google Scholar]
- Zhang J, Maun MA. 1990. Effects of sand burial on seed germination, seedling emergence, survival, and growth of Agropyron psammophilum Canadian Journal of Botany 68: 304–310. [Google Scholar]
- Zhang J, Maun MA. 1993. Seed mass and seedling size relationship in Calamovilfa longifolia Canadian Journal of Botany 71: 551–557. [Google Scholar]
- Zhang J, Maun MA. 1994. Potential for seed bank formation in seven Great lakes and dune species. American Journal of Botany 81: 387–394. [Google Scholar]
- Zheng YR, Xie ZX, Gao Y, Shimizu H, Jiang LH, Yu Y. 2003. Ecological restoration in northern china: germination characteristics of 9 key species in relation to air seeding. Belgian Journal of Botany 136: 129–138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.