Abstract
• Background and Aims Natural regeneration of white spruce (Picea glauca) after disturbance has been reported to be very poor. Here a study was made to determine whether C compounds released from understorey species growing together with white spruce could be involved in this regeneration failure, either by (1) changing soil nutrient dynamics, (2) inhibiting germination, and/or (3) delaying seedling growth.
• Methods Foliage leachates were obtained from two shrubs (Ledum palustre and Empetrum hermaphroditum) and one bryophyte (Sphagnum sp.) with high phenolic compound concentrations that have been reported to depress growth of conifers in boreal forests, and, as a comparison, one bryophyte (Hylocomium splendens) with negligible phenolic compounds. Mineral soil from a white spruce forest was amended with plant leachates to examine the effect of each species on net N mineralization. Additionally, white spruce seeds and seedlings were watered with plant leachates to determine their effects on germination and growth.
• Key Results Leachates from the shrubs L. palustre and E. hermaphroditum contained high phenolic compound concentrations and dissolved organic carbon (DOC), while no detectable levels of C compounds were released from the bryophytes Sphagnum sp. or H. splendens. A decrease in net N mineralization was determined in soils amended with L. palustre or E. hermaphroditum leachates, and this effect was inversely proportional to the phenolic concentrations, DOC and leachate C/N ratio. The total percentage of white spruce germination and the growth of white spruce seedlings were similar among treatments.
• Conclusions These results suggest that the shrubs L. palustre and E. hermaphroditum could negatively affect the performance of white spruce due to a decrease in soil N availability, but not by direct effects on plant physiology.
Keywords: Boreal ecosystem, DOC, phenolics, regeneration, soil N cycling, Picea glauca, Ledum palustre, Sphagnum sp., Empetrum hermaphroditum, Hylocomium splendens
INTRODUCTION
White spruce (Picea glauca) is one of the most common species in boreal forests in North America. This species fails to regenerate naturally in managed forests because of a low seed availability and inadequate seedbed conditions after clear-cutting (Timoney and Peterson, 1996; Greene et al., 1999; Wurtz and Zasada, 2001). The presence of understorey species has also been associated with decreases in coniferous forest regeneration (Ponge et al., 1998). Besides the negative interactions that may occur between species due to resource competition, some authors have argued that the presence of allelopathic compounds leached from the understorey, such as phenolic compounds, could partially explain the low regeneration of conifers in boreal forests after clear-cut disturbance (Ponge et al., 1998).
Phenolic compounds are carbon-based secondary metabolites widespread in plants that have been described to play an important role in the interaction of vegetation with its environment (Waterman and Mole, 1994; Inderjit, 1996; Hättenschwiler and Vitousek, 2000; Kraus et al., 2003). Phenolics can be leached out by rainfall from green foliage and decomposing litter, and thus reach the soil underneath the canopy (Kuiters and Sarink, 1986; Inderjit and Mallik, 1996; Gallet and Pellisier, 1997). Once in the soil, they can affect soil nutrient dynamics by forming complexes with proteins and delaying organic matter decomposition and mineralization (Horner et al., 1988; Nicolai, 1988; Hättenschwiler and Vitousek, 2000; Castells et al., 2004), and by increasing the soil microbial activity and N immobilization (Sparling et al., 1981; Blum and Shafer, 1988; Sugai and Schimel, 1993). These processes result in a decrease of the inorganic N available for plant uptake.
Phenolics have also been described as allelopathic agents affecting the performance of target vegetation, either by inhibiting seed germination, root elongation or plant growth (Nilsson and Zackrisson, 1992; Gallet, 1994; Zhu and Mallik, 1994; Inderjit, 1996). Because the specific mechanisms of allelopathy have not been extensively described, some controversy has arisen about whether phenolics do indeed affect plant physiological processes under natural conditions (Michelsen et al., 1995; Wardle and Nilsson, 1997). Some studies support the hypothesis that negative interactions among plants mediated by organic compounds released from foliage, including phenolics, are actually caused by changes in soil nutrient dynamics (Michelsen et al., 1995; Schmidt et al., 1997) and not by direct effects on the target species. Although both mechanisms may operate simultaneously (Inderjit and Del Moral, 1997), determining what process prevails in natural conditions is rather complex. Phenolics comprise a large group of secondary metabolites with a wide range of chemical properties, from the low molecular weight phenolic acids to the high molecular weight condensed tannins, and the fate of these compounds when released to the nearby soil may highly depend on the type of compound (Inderjit, 1996; Hättenschwiler and Vitousek, 2000). Because the quantity and quality of phenolics in plants is strongly determined by genetics (Hamilton et al., 2001), the specific mechanisms affected by phenolics in soil–plant or plant–plant interactions may vary among donor species. Moreover, other polar compounds such as carbohydrates are frequently leached out from the canopy together with the phenolics (Horner et al., 1988). Carbohydrates have been shown to increase soil N immobilization when microbes use them as a C source (Sparling et al., 1981; Blum and Shafer, 1988; Sugai and Schimel, 1993; Castells et al., 2004) and they should also be considered because in some cases the effects of carbohydrates eclipse the changes produced by phenolics (Castells et al., 2004).
Here we studied whether phenolic-containing species commonly associated with white spruce forests in Interior Alaska could negatively interfere with the performance of white spruce, and thus potentially affect its regeneration through the release of C compounds by rainfall. We selected two shrubs (Ledum palustre and Empetrum hermaphroditum) and one bryophyte (Sphagnum sp.) with high concentrations of phenolic compounds (Nilsson and Zackrisson, 1992; Rasmunsen et al., 1995; Castells et al., 2003) that had been reported to exert negative effects on conifers in natural conditions. As a comparison, a bryophyte (Hylocomium splendens) with negligible phenolic compound concentrations was selected. The reported negative effects of these species on boreal ecosystems include decreases in growth of Picea mariana and Picea glauca at sites dominated by Ledum sp. (Inderjit and Mallik, 1996; Cole et al., 2003), inhibition of Pinus sylvestris regeneration post-fire in sites dominated by Empetrum hermaphroditum (Zackrisson et al., 1997), and suppression of vascular plant growth in Sphagnum-dominated bogs (Van Breemen, 1995).
Leachates from all four species (Ledum palustre, Empetrum hermaphroditum, Sphagnum sp., and Hylocomium splendens) were obtained in the laboratory and applied to mineral soils, white spruce seeds and seedlings. The aim was to ascertain whether compounds released from each species could negatively affect white spruce performance at three levels: (1) at the soil level by changing net N mineralization and thus N availability; (2) at the seed level by inhibiting germination; and (3) at the plant level by decreasing seedling growth. The processes that may be relevant in white spruce regeneration failure for each of the studied species are discussed, differentiating between indirect effects on plant growth through changes in nutrient availability and direct allelopathic effects on plant physiology during germination and seedling growth.
MATERIALS AND METHODS
Leachate preparation
Green leaves from two evergreen boreal shrubs, Ledum palustre (Labrador tea) and Empetrum hermaphroditum (crowberry), and the non-decomposing layer of two bryophytes, Sphagnum sp. and Hylocomium splendens, were sampled near Fairbanks, Alaska (64·8°N, 148·0°W) during July 1998. The plant material was sampled from several individuals and combined together within species to obtain a single leachate pool. Although this approach does not consider the intraspecific variability of the phenolic and organic carbon concentrations, it has the advantage of incorporating an averaged chemical composition while minimizing the number of treatment replicates. Leachate for each of the species was obtained by shaking fresh plant material (50 g equivalent dry weight) in 1 L distilled water for 48 h at room temperature (Zackrisson et al., 1996) and filtering through Whatman 42 filter paper. A control of distilled water only was prepared following the same procedure used for the plant leachates. A subsample of each leachate was analysed for total water-soluble phenolics by the Folin–Ciocalteu method (Marigo, 1973) using gallic acid as a standard, and for dissolved organic carbon (DOC) (Shimadzu TOC 5000). Dissolved organic nitrogen (DON) was analysed by digesting 20 mL of leachate with concentrated H2SO4. The resulting 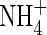 as well as the
as well as the 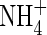 and
and 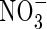 present in the non-digested leachates were analysed using a modified Technicon Autoanalyzer II. Dissolved organic nitrogen was estimated by subtracting the
present in the non-digested leachates were analysed using a modified Technicon Autoanalyzer II. Dissolved organic nitrogen was estimated by subtracting the 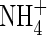 of the intact leachate from the total
of the intact leachate from the total  obtained in the digested samples. The C/N ratio was calculated by dividing DOC by DON. Leachates were stored at −20 °C and thawed before use.
obtained in the digested samples. The C/N ratio was calculated by dividing DOC by DON. Leachates were stored at −20 °C and thawed before use.
Soil sampling and incubation
The A soil horizon was sampled from four locations in a closed white spruce forest (Picea glauca/Hylocomium splendens community) at Bonanza Creek Experimental Forest, Fairbanks. This mature stand is dominated by white spruce, but occasional Betula papyrifera and Populus tremuloides persist beneath the spruce canopy. The forest floor is primarily covered by a moss mat of Hylocomium splendens. Scattered shrubs of Alnus crispa and Viburnum edule make up less than 1 % cover. Herbaceous cover is also low and consists primarily of Calamagrostis canadensis, Geocaulon lividum and Pyrola secunda. Soils are Alfic cryochrept, silt loam, well-drained with no permafrost, very friable and with abundant roots. The depth range for the A horizon was 6–15 cm. Soils were bulked together among locations, sieved through 2 mm mesh and kept at 4 °C. Total organic C (3·2 % C) and total N (0·16 % N) were analysed by an elemental combustion analyser (LECO CNS-2000, St. Joseph, MI).
The mineral soil (100 g FW) was placed into 250 mL jars 24 h before starting the incubation in order to minimize soil perturbation effects. Six replicates per treatment were used (Ledum, Empetrum, Sphagnum, Hylocomium and distilled water leachates), with 30 jars in total. Each jar was supplied with leachate until field capacity was reached (17 mL), and incubated at 15 °C for 4 weeks. Field capacity was determined by adding excess water to six replicates of 50 g FW soil subsamples and applying a vacuum at 0·33 bar for 1·5 h. Soils were weighed before and after vacuum treatment to calculate the amount of moisture necessary to reach field capacity. For the determination of net N mineralization, three 15 g FW replicates of soil before the incubation (initial) and three replicates per jar at the end of the incubation (final) were extracted with 75 mL of 2 N KCl for 1 h, filtered through Whatman 42 filter paper and analyzed for 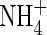 and
and  as described above. Subsamples of the initial and final soils for each jar were dried at 65 °C for 48 h to determine water content. Potential soil net N mineralization rates were calculated as the difference between initial and final extractable
as described above. Subsamples of the initial and final soils for each jar were dried at 65 °C for 48 h to determine water content. Potential soil net N mineralization rates were calculated as the difference between initial and final extractable  and
and 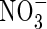 concentrations and expressed per unit of soil dry weight. Nitrification was not detectable for any of the treatments.
concentrations and expressed per unit of soil dry weight. Nitrification was not detectable for any of the treatments.
Seed germination experiment
Picea glauca (Moench) Voss seeds were obtained from cones sampled during summer 1998 in Fairbanks. To study the effects of plant leachates on seed germination, 80 seeds per treatment were placed in eight 9-cm diameter petri dishes (ten seeds each) on Whatman 42 filter paper. Each Petri dish was watered on the initial day with 1·5 mL of leachate (Ledum, Empetrum, Hylocomium, Sphagnum or distilled water). Seeds were kept at room temperature and high humidity for 1 week, and the appearance of the radicle was checked daily.
Seedling growth experiment
Fifty 1-year-old Picea glauca seedlings (3·24 ± 0·09 g DW) from a local nursery were forced from dormancy in a glasshouse under a 24 h light photoperiod and 18–30 °C. Seedlings were transplanted to individual conical containers (‘Cone-tainers’ single cell system, Stuewe and Sons, Corvallis, OR) with the mineral soil sampled at Bonanza Creek Experimental Forest, Fairbanks, AK. Leachate treatments were assigned to each seedling following a randomized block design, with ten seedlings per treatment. Seedlings were watered twice a week with 10 mL of leachate. On the days leachates were not applied, the seedlings were irrigated as needed. At day 47, seedling shoots were harvested and new growth and old growth were separated. Both fractions were oven-dried at 65 °C for 48 h and weighed. Seedling growth was expressed as the ratio between shoot new growth and old growth.
Statistical analyses
The effects of leachates on net N mineralization, seed germination and seedling growth were tested by a one-way ANOVA. A linear correlation was calculated between net N mineralization and organic C and between net N mineralization and total phenolics. All statistical analyses were conducted using Statistica 6·0 (Statsoft, Inc., Tulsa, USA).
RESULTS AND DISCUSSION
Chemical composition of the leachates greatly differed between the shrubs and the bryophytes. Leachates of the shrub L. palustre had the highest concentrations of total water-soluble phenolics, DOC, 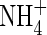 ,
,  and C/N ratio among all analysed species (Table 1). The shrub E. hermaphroditum also presented significant amounts of total water-soluble phenolics and DOC, although it had negligible concentrations of
and C/N ratio among all analysed species (Table 1). The shrub E. hermaphroditum also presented significant amounts of total water-soluble phenolics and DOC, although it had negligible concentrations of  and
and 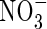 . Dissolved organic nitrogen concentrations were similar between leachates of L. palustre and E. hermaphroditum. The bryophytes Sphagnum sp. and H. splendens had no significant concentrations of total water-soluble phenolics, DOC, DON,
. Dissolved organic nitrogen concentrations were similar between leachates of L. palustre and E. hermaphroditum. The bryophytes Sphagnum sp. and H. splendens had no significant concentrations of total water-soluble phenolics, DOC, DON,  and
and  in the leachates compared with the distilled water control. Sphagnum is reported to contain phenolic acids, including the genus-specific sphagnum acid (Verhoeven and Liefveld, 1997). Although some phenolics in Sphagnum are attached to the cell walls, the majority are found in a free water-soluble form and can be excreted into the bog water around the mosses (Rasmussen et al., 1995). However, in our study no significant release of phenolic compounds or DOC from Sphagnum was found, and thus no effect on soil N cycling, white spruce germination or growth was reported. The effects of Sphagnum in creating a wet, acidic, nutrient-poor and anoxic environment are expected to be more dramatic in the suppression of vascular plant growth than the potential effect of the released organic compounds (Van Breemen, 1995).
in the leachates compared with the distilled water control. Sphagnum is reported to contain phenolic acids, including the genus-specific sphagnum acid (Verhoeven and Liefveld, 1997). Although some phenolics in Sphagnum are attached to the cell walls, the majority are found in a free water-soluble form and can be excreted into the bog water around the mosses (Rasmussen et al., 1995). However, in our study no significant release of phenolic compounds or DOC from Sphagnum was found, and thus no effect on soil N cycling, white spruce germination or growth was reported. The effects of Sphagnum in creating a wet, acidic, nutrient-poor and anoxic environment are expected to be more dramatic in the suppression of vascular plant growth than the potential effect of the released organic compounds (Van Breemen, 1995).
Table 2.
Effects of plant leachates on Picea glauca seedling growth
| Treatments |
Species |
Relative growth (new growth/old growth) |
|---|---|---|
| Control | 0·37 ± 0·054 | |
| Shrubs | Ledum plaustre | 0·50 ± 0·053 |
| Empetrum hermaphroditum | 0·43 ± 0·062 | |
| Bryophytes | Sphagnum sp. | 0·41 ± 0·073 |
| Hylocomium splendens | 0·49 ± 0·054 |
Values are mean ± s.e., n = 10. No significant differences were found for any of the treatments compared to the control.
Table 1.
Chemical characteristics of leaf leachates from the four studied species
| Treatment |
Species |
Phenolics (mg mL−1) |
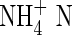 (mg L−1) (mg L−1) |
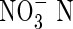 (mg L−1) (mg L−1) |
DON (mg mL−1) |
DOC (mg mL−1) |
C/N |
|---|---|---|---|---|---|---|---|
| Control | 0·02 | 0·00 | 0·04 | 0·004 | 0·03 | 7·2 | |
| Shrubs | Ledum palustre | 0·77 | 14·93 | 0·15 | 0·011 | 1·48 | 140·3 |
| Empetrum hermaphroditum | 0·39 | 0·23 | 0·04 | 0·010 | 0·74 | 72·5 | |
| Bryophytes | Sphagnum sp. | 0·02 | 0·00 | 0·01 | 0·006 | 0·05 | 9·7 |
| Hylocomium splendens | 0·02 | 0·07 | 0·04 | 0·007 | 0·04 | 6·9 |
A single leachate pool per treatment was obtained by combining the foliage from several individuals.
DON = dissolved organic nitrogen, DOC = dissolved organic carbon.
The analyses of total phenolic compounds using the Folin–Ciocalteau assay have been shown to present some problems when estimating concentrations from different species (Appel et al., 2001). Because each species contains a particular phenolic profile, from the low molecular weight phenolic acids to the high molecular weight condensed tannins, the measure of total phenolic concentrations can be either over- or under-estimated, if only the reducing capacities of the phenolic aromatic hydroxyls are considered as performed by the Folin–Ciocalteu assay (Appel et al., 2001). In our study, however, the use of this method does not seem to present strong limitations because we aimed to determine the biological activity of phenolics on soil N cycling, seed germination and seedling growth. At least for some of these processes, for instance when phenolics form bonds with proteins present in the soil, the hydroxyl groups are directly involved (Appel, 1993) and thus the use of Folin–Ciocalteu assay seems adequate. Moreover, since the analyses were performed on the water-soluble phenolics leached from foliage, we would expect a more homogeneous composition compared to the whole phenolic fraction.
The addition of leachates from the shrubs L. palustre and E. hermaphroditum to mineral soils decreased net N mineralization compared with the control (Fig. 1). This effect could be caused by an increase in N immobilization due to the presence in the leachates of high DOC concentrations and a high C/N ratio. Additions of C are expected to increase soil microbial biomass (Bradley et al., 1997) and thus to stimulate microbial turnover, increasing gross mineralization as well as immobilization rates (Clein and Schimel, 1995). Castells et al. (2003) showed that leachates of L. palustre decreased the soil net N mineralization by increasing N immobilization, but no distinction was made regarding the type of C compounds that caused this effect. Both phenolics and carbohydrates have been reported to increase N immobilization when microbes used labile C compounds as a substrate (Sparling et al., 1981; Shafer and Blum, 1991; Sugai and Schimel, 1993; Schimel et al., 1996; Castells et al., 2004). Additionally, phenolics can also decrease net N mineralization by forming complexes with proteins and delaying organic matter decomposition and gross N mineralization (Hättenschwiler and Vitousek, 2000). The nature of the compounds released by leaching will influence what process is taking place. Thus, the high molecular weight phenolics, such as condensed tannins, were more involved in linking organic matter and slowing decomposition, while the low molecular-weight phenolics and carbohydrates were more easily degraded by micro-organisms when used as a C source (Fierer et al., 2001; Castells et al., 2004). The inversely proportional relationship between changes in net N mineralization and DOC or phenolic concentrations in our study (Fig. 2) suggests that the quantitative differences between L. palustre and E. hermaphroditum on net N mineralization are more related to the concentration of labile C compounds present in the leachates rather than differences in biological activities of the compounds released from each species. The release of 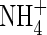 in L. palustre leachate may also favour the increase of soil N immobilization. Because there is a prevalence of N immobilization over gross N mineralization in the organic horizon from boreal forests during the growing season (Jonasson et al., 1993; Castells et al., 2003), the N mineralized in the mineral horizons can be a major source of N for plant uptake, and a decrease in net N mineralization may diminish the inorganic N available for vegetation and so potentially affect white spruce growth. Previous studies have shown that plant leachates may affect soil nutrient dynamics not only over a short term but also over a longer term, with a corresponding potentially higher impact on ecosystem functioning. Thus, Ledum sp. leachates changed soil chemical properties, increasing the C/N ratio of the organic matter (Castells et al., 2003), and also K and
in L. palustre leachate may also favour the increase of soil N immobilization. Because there is a prevalence of N immobilization over gross N mineralization in the organic horizon from boreal forests during the growing season (Jonasson et al., 1993; Castells et al., 2003), the N mineralized in the mineral horizons can be a major source of N for plant uptake, and a decrease in net N mineralization may diminish the inorganic N available for vegetation and so potentially affect white spruce growth. Previous studies have shown that plant leachates may affect soil nutrient dynamics not only over a short term but also over a longer term, with a corresponding potentially higher impact on ecosystem functioning. Thus, Ledum sp. leachates changed soil chemical properties, increasing the C/N ratio of the organic matter (Castells et al., 2003), and also K and 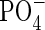 concentrations (Inderjit and Mallik, 1997). Moreover, soils sampled underneath Ledum palustre had a lower net N mineralization and soil C/N ratio compared with soils not associated with L. palustre, and the carbon compounds released from the canopy were at least partially responsible for this effect (Castells et al., 2003).
concentrations (Inderjit and Mallik, 1997). Moreover, soils sampled underneath Ledum palustre had a lower net N mineralization and soil C/N ratio compared with soils not associated with L. palustre, and the carbon compounds released from the canopy were at least partially responsible for this effect (Castells et al., 2003).
Fig. 1.
Net N mineralization from mineral soils incubated with distilled water (control), leachates of the shrubs Ledum palustre (LED) and Empetrum hermaphroditum (EMP), and leachates of the bryophytes Sphagnum sp. (SPH) and Hylocomium splendens (HYL). n = 6. Statistical differences between control and each leachate treatment are shown: **P < 0·01; ns, not significant.
Fig. 2.

Correlations between net N mineralization of soils amended with leachates from Ledum palustre, Empetrum hermaphroditum, Sphagnum sp., Hylocomium splendens and distilled water (control) with (A) the dissolved organic carbon (DOC) and (B) total phenolic concentrations present in the leachates.
When white spruce seeds were watered with L. palustre or E. hermaphroditum lechates, germination was significantly lower from the second to the fourth day compared with the control (Fig. 3). However, no differences in final germination were found after 1 week of treatment. Leachates of L. palustre and E. hermaphroditum had no effect on white spruce seedling growth. The reported changes caused by L. palustre and E. hermaphroditum leachates on soil net N mineralization, and thus an expected lower N availability for the treated white spruce seedlings, did not diminish growth. White spruce seedlings can accumulate storage reserves during the growing season and retranslocate nutrients to produce new tissue (Chapin et al., 1990), especially after winter when the root system has not yet regenerated (McAlister and Timmer, 1998). McAlister and Timmer (1998) found that net nutrient uptake of white spruce seedlings during the first year of growth was small or negative, and nutrients allocated to new growth were mostly from internal rather than external sources. The seedlings used in our experiment were dormant before being transplanted under favourable conditions for growth. Thus, they could use stored nutrients for allocating to new growth and so become more independent of soil nutrient availability. The effects of leachates on N mineralization, however, are expected to become significant for seedling growth over a longer term when seedlings have to rely on external nutrient sources.
Fig. 3.
Germination of white spruce seeds amended with distilled water (control) and leachates of the shrubs Ledum palustre (LED) and Empetrum hermaphroditum (EMP) and leachates of the bryophytres Sphagnum sp. (SPH) and Hylocomium splendens (HYL). n = 80. Significant differences between the control and each of the two shrubs are shown: *P < 0·05, **P < 0·01. No differences were found between the control and the bryophyte treatments.
In conclusion, soluble compounds released from the shrubs L. palustre and E. hermaphroditum could indirectly affect the performance of white spruce during regeneration by decreasing the soil N availability. Phenolic compounds from the bryophytes Sphagnum sp. and H. splendens do not play a role in the performance of white spruce. Our results do not support the occurrence of allelopathic effects exerted by phenolics involving inhibition of germination or early seedling growth. However, other questions should be addressed before rejecting the presence of allelopathy in this system. First, the temporal variation in plant chemical composition should be taken into account. The concentrations of phenolic compounds are known to vary with plant phenology and season (Kraus et al., 2003), and thus differences in the quantitative effects of leachates on plant–soil and plant–plant interactions may be expected over time. Second, other sources of phenolics released to the nearby soil and surrounding vegetation should be considered. Leaf litter has proved to be a major source of phenolic compounds during decomposition (Hättenschwiler and Vitousek, 2000; Kraus et al., 2003) and its different chemical profile compared to green leaves, for instance, with a major presence of insoluble condensed tannins (Hättenschwiler and Vitousek, 2000), opens the possibility of additional biological activities that were not considered here. Determining the importance of green leaf and litter leachates across different seasons on soil N cycling, germination and seedling growth will offer a more accurate picture on the effects of the understorey on white spruce regeneration.
Supplementary Material
Acknowledgments
We greatly appreciate the ideas and support of F.S. Chapin in conducting this project. E.C. had a predoctoral fellowship (FPI) from the Ministerio de Educacion y Cultura (Spain) in collaboration with Carburos Metalicos S.A. We are also grateful for financial support from MEC (Spain) in the form of grants to J.P.
Footnotes
Present address: Department of Entomology, University of Illinois, 320 Morrill Hall, 505 S. Goodwin, Urbana, IL 61801, USA.
LITERATURE CITED
- Appel HM. 1993. Phenolics in ecological interactions: the importance of oxidation. Journal of Chemical Ecology 19: 1521–1552. [DOI] [PubMed] [Google Scholar]
- Appel HM, Governor HL, D'Ascenzo M, Siska E, Schultz JC. 2001. Limitations of the Folin assays of foliar phenolics in ecological studies. Journal of Chemical Ecology 27: 761–778. [DOI] [PubMed] [Google Scholar]
- Blum U, Shafer SR. 1988. Microbial populations and phenolic acids in soil. Soil Biology and Biochemistry 20: 793–800. [Google Scholar]
- Bradley RL, Fyles JW, Titus B. 1997. Interactions between Kalmia humus quality and chronic low C inputs in controlling microbial and soil nutrient dynamics. Soil Biology and Biochemistry 29: 1275–1283. [Google Scholar]
- Castells E, Peñuelas J, Valentine DW. 2003. Influence of the phenolic compound bearing species Ledum palustre on soil N cycling in a boreal hardwood forest. Plant and Soil 251: 155–166. [Google Scholar]
- Castells E, Peñuelas J, Valentine DW. 2004. Are phenolic compounds released from the Mediterranean shrub Cistus albidus responsible for changes in N cycling in siliceous and calcareous soils? New Phytologist 162: 187–195. [Google Scholar]
- Chapin FSI, Schulze ED, Mooney HA. 1990. The ecology and economics of storage in plants. Annual Review of Ecology and Systematics 21: 423–447. [Google Scholar]
- Clein JS, Schimel JP. 1995. Nitrogen turnover and availability during succession from Alder to Poplar in Alaskan taiga. Soil Biology and Biochemistry 27: 743–752. [Google Scholar]
- Cole E, Youngblood A, Newton M. 2003. Effects of competing vegetation on juvenile white spruce (Picea glauca (Moench) Voss) growth in Alaska. Annals of Forest Science 60: 573–583. [Google Scholar]
- Fierer N, Schimel JP, Cates RG, Zou J. 2001. Influence of balsam poplar tannin fractions on carbon and nitrogen dynamics in Alaskan taiga floodplain soils. Soil Biology and Biochemistry 33: 1827–1839. [Google Scholar]
- Gallet C. 1994. Allelopathic potential in bilberry-spruce forests: influence of phenolic compounds on spruce seedlings. Journal of Chemical Ecology 20: 1009–1024. [DOI] [PubMed] [Google Scholar]
- Gallet C, Pellissier F. 1997. Phenolic compounds in natural solutions of a coniferous forest. Journal of Chemical Ecology 23: 2401–2412. [Google Scholar]
- Greene DF, Zasada JC, Sirois L, Kneeshaw D, Morin H, Charron I, Simard D. 1999. A review of the regeneration dynamics of North American boreal forest tree species. Canadian Journal of Forest Research 29: 824–839. [Google Scholar]
- Hamilton JG, Zangerl AR, DeLucia EH, Berenbaum MR. 2001. The carbon-nutrient balance hypothesis: its rise and fall. Ecology Letters 4: 86–95. [Google Scholar]
- Hättenschwiler S, Vitousek PM. 2000. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends in Ecology and Evolution 15: 238–243. [DOI] [PubMed] [Google Scholar]
- Horner JD, Gosz JR, Cates RG. 1988. The role of carbon-based plant secondary metabolites in decomposition in terrestrial ecosystems. The American Naturalist 132: 869–883. [Google Scholar]
- Inderjit. 1996. Plant phenolics and allelopathy. The Botanical Review 62: 186–202. [Google Scholar]
- Inderjit, Del Moral R. 1997. Is separating resource competition from allelopathy realistic? The Botanical Review 63: 221–230. [Google Scholar]
- Inderjit, Mallik AU. 1996. Growth and physiological responses of black spruce (Picea mariana) to sites dominated by Ledum groenlandicum Journal of Chemical Ecology 22: 575–585. [DOI] [PubMed] [Google Scholar]
- Inderjit, Mallik AU. 1997. Effects of Ledum groenlandicum amendments on soil characteristics and black spruce seedling growth. Plant Ecology 133: 29–36. [Google Scholar]
- Jonasson S, Havström M, Jensen M, Callaghan TV. 1993.In situ mineralization of nitrogen and phosphorus of artic soils after perturbations simulating climate change. Oecologia 95: 179–186. [DOI] [PubMed] [Google Scholar]
- Kraus TEC, Dahlgren RA, Zasoski RJ. 2003. Tannins in nutrient dynamics of forest ecosystems. Plant and Soil 256: 41–66. [Google Scholar]
- Kuiters AT, Sarink HM. 1986. Leaching of phenolic compounds from leaf and needle litter of several deciduous and coniferous trees. Soil Biology and Biochemistry 18: 475–480. [Google Scholar]
- McAlister JA, Timmer VR. 1998. Nutrient enrichment of white spruce seedlings during nursery culture and initial plantation establishment. Tree Physiology 18: 195–202. [DOI] [PubMed] [Google Scholar]
- Marigo G. 1973. Sur une méthode de fractionnement et d'estimation des composés phénoliques chez les végétaux. Analusis 2: 106–110. [Google Scholar]
- Michelsen A, Schmidt IK, Jonasson S, Dighton J, Jones HE, Callaghan TV. 1995. Inhibition of growth, and effects on nutrient uptake of arctic graminoids by leaf extracts: allelopathy or resource competition between plants and microbes? Oecologia 103: 407–418. [DOI] [PubMed] [Google Scholar]
- Nicolai V. 1988. Phenolic and mineral content of leaves influences decomposition in European forest ecosystems. Oecologia 75: 575–579. [DOI] [PubMed] [Google Scholar]
- Nilsson MC, Zackrisson O. 1992. Inhibition of scots pine seedling establishment by Empetrum hermaproditum Journal of Chemical Ecology 18: 1857–1870. [DOI] [PubMed] [Google Scholar]
- Ponge JF, André J, Zackrisson O, Bernier N, Nilsson MC, Gallet C. 1998. The forest regeneration puzzle. Biological mechanisms in humus layer and forest vegetation dynamics. Bioscience 48: 523–530. [Google Scholar]
- Rasmussen S, Wolff C, Rudolph H. 1995. Compartmentalization of phenolic constituents in Sphagnum Phytochemistry 38: 35–39. [Google Scholar]
- Schimel JP, van Cleve K, Cates RG, Clausen TP, Reichardt PB. 1996. Effects of balsam poplar (Populus balsamifera) tannins and low molecular weight phenolics on microbial activity in taiga floodplain soil: implications for changes in N cycling during succession. Canadian Journal of Botany 74: 84–90. [Google Scholar]
- Schmidt IK, Michelsen A, Jonasson S. 1997. Effects of labile soil carbon on nutrient partitioning between an arctic graminoid and microbes. Oecologia 112: 557–565. [DOI] [PubMed] [Google Scholar]
- Shafer SR, Blum U. 1991. Influence of phenolic acids on microbial populations in the rhizosphere of cucumber. Journal of Chemical Ecology 17: 369–388. [DOI] [PubMed] [Google Scholar]
- Sparling GP, Ord BG, Vaughan D. 1981. Changes in microbial biomass and activity in soils amended with phenolic acids. Soil Biology and Biochemistry 13: 455–460. [Google Scholar]
- Sugai SF, Schimel JP. 1993. Decomposition and biomass incorporation of 14C-labeled glucose and phenolics in taiga forest-floor: effect of substrate quality, successional state, and season. Soil Biology and Biochemistry 25: 1379–1389. [Google Scholar]
- Timoney KP, Peterson G. 1996. Failure of natural regeneration after clearcut logging in Wood Buffalo National Park, Canada. Forest Ecology and Management 87: 89–105. [Google Scholar]
- Van Breemen N. 1995. How Sphagnum bogs down other plants. Trends in Ecology and Evolution 10: 270–275. [DOI] [PubMed] [Google Scholar]
- Verhoeven JTA, Liefveld WM. 1997. The ecological significance of organochemical compounds in Sphagnum Acta Botanica Neerlandica 46: 117–130. [Google Scholar]
- Wardle DA, Nilsson MC. 1997. Microbe–plant competition, allelopathy and arctic plants. Oecologia 109: 291–293. [DOI] [PubMed] [Google Scholar]
- Waterman PG, Mole S. 1994.Analysis of ohenolic olant metabolites. Oxford: Blackwell Scientific Publications. [Google Scholar]
- Wurtz TL, Zasada JC. 2001. An alternative to clear-cutting in the boreal forest of Alaska: a 27-year study of regeneration after shelterwood harvesting. Canadian Journal of Forest Research 31: 999–1011 [Google Scholar]
- Zackrisson O, Nilsson MC, Dahlberg A, Jäderlund A. 1997. Interference mechanisms in conifer–Ericaceae–feathermoss communities. Oikos 78: 209–220. [Google Scholar]
- Zackrisson O, Nilsson MC, Wardle DA. 1996. Key ecological function of charcoal from wildfire in the Boreal forest. Oikos 77: 10–19 [Google Scholar]
- Zhu H, Mallik AU. 1994. Interactions between Kalmia and black spruce: isolation and identification of allelopathic compounds. Journal of Chemical Ecology 20: 407–421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




