Significance
Latent viral infection is a major obstacle for effective antiviral treatment and presents a continuous risk to the host. The dormant viral genome during latent infection provides few therapeutic targets other than itself for antiviral drug development. This study demonstrates the clearance of latent Epstein–Barr virus genomes in a subpopulation of Burkitt’s lymphoma patient-derived cells with clustered regularly interspaced short palindromic repeat/Cas9 nuclease. Viral genome destruction leads to proliferation arrest and apoptosis in Epstein–Barr virus-infected cells, with no observed cytotoxicity to noninfected cells. Although many hurdles remain before this approach could be used in the clinic, this strategy may lead to a generalized approach to cure latent viral infections.
Keywords: genome editing, latency, herpes virus
Abstract
Latent viral infection is a persistent cause of human disease. Although standard antiviral therapies can suppress active viral replication, no existing treatment can effectively eradicate latent infection and therefore a cure is lacking for many prevalent viral diseases. The prokaryotic immune system clustered regularly interspaced short palindromic repeat (CRISPR)/Cas evolved as a natural response to phage infections, and we demonstrate here that the CRISPR/Cas9 system can be adapted for antiviral treatment in human cells by specifically targeting the genomes of latent viral infections. Patient-derived cells from a Burkitt’s lymphoma with latent Epstein–Barr virus infection showed dramatic proliferation arrest and a concomitant decrease in viral load after exposure to a CRISPR/Cas9 vector targeted to the viral genome.
The herpesviridae virus family consists of some of the most widespread human pathogens in the world. More than 90% of adults have been infected with at least one of the eight subtypes of herpes viruses, and latent infection persists in most people (1). These herpes virus subtypes infect a wide range of cells, including epithelium, neuron, monocyte, and lymphocyte, and the consequences can be either mild (herpes simplex by HSV-1) or severe [cancer by Epstein–Barr virus (EBV) and Kaposi’s sarcoma-associated herpes virus]. HSV infection is also a known risk factor for HIV (2). In its latent state, the viral genome persists within the host cells and it has not been possible to find therapeutic approaches that completely eradicate such infections.
Since its discovery 50 y ago, EBV has been a closely studied member of the herpesviridae. As one of the most common human viruses, EBV causes infectious mononucleosis and is associated with certain forms of lymphoma. To date, however, no EBV vaccine or treatment exists. EBV is highly efficient at transforming quiescent human B lymphocytes; the resulting lymphoblastoid cell lines are now commonly used for human genetic studies, and it is possible to use patient-derived cells that propagate directly in culture because of the viral infection and require no other manipulation. The EBV genome encodes about 85 genes, several of which are essential for lytic or latent infection. During latency, the EBV genome circularizes and resides in the cell nucleus as an episome. EBV latency usually progresses through three programs, with protein production decreasing from full sets of EBV nuclear antigens (EBNAs) and latent membrane proteins to just EBNA1. EBNA1 binds to the EBV origin of replication (oriP) to maintain viral episomes; it also regulates expression of other viral genes.
Most current antiviral drug development programs are focused on protein targets and are only effective in preventing active viral replication. It has been recognized that it would be useful to target latent infections with viral genome-specific nucleases (3–5), but the challenges of engineering sequence-specific nucleases have hampered progress. Clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9 is a naturally occurring bacterial immune system that uses a novel nuclease system to protect bacteria from phage infection (6–9), and it has recently been harnessed for a variety of genome-engineering applications (10–17). DNA sequence recognition requires only a single 20-nt guide RNA and a protospacer adjacent motif, which enables one to rapidly engineer and test a large number of DNA cleavage sites (17–20). Here we demonstrate a therapeutic strategy for herpes virus by targeting the CRISPR/Cas9 system directly to essential viral genome sequences.
Results
A Natural Model of Latent EBV Infection.
As the first discovered human tumor virus, EBV was initially isolated from cultured Burkitt’s lymphoma samples (21). The Raji cell line was the first established long-term culture from Burkitt’s lymphoma patients (22, 23), and is one of the most extensively studied EBV models. The close relationship between the Raji EBV genome and the EBV reference (24) provides a straightforward blueprint for genome engineering. We therefore used Raji cells for most of the work that follows.
Design of EBV-Targeting CRISPR/Cas9.
Plasmids consisting of a U6 promoter-driven chimeric guide RNA (sgRNA) and a ubiquitous promoter-driven Cas9 were obtained from Addgene, as described by Cong et al. (11). An EGFP marker fused after the Cas9 protein allowed selection of Cas9+cells (Fig. 1A). We adapted a modified chimeric guide RNA design for more efficient Pol-III transcription and more stable stem-loop structure (25).
Fig. 1.
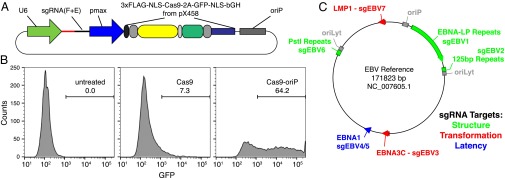
EBV-targeting CRISPR/Cas9 designs. (A) Scheme of CRISPR/Cas plasmids, adapted from ref. 11. (B) Effect of oriP on transfection efficiency in Raji cells. Both Cas9 and Cas9-oriP plasmids have a scrambled guide RNA. (C) CRISPR guide RNA targets along the EBV reference genome. Green, red, and blue represent three different target sequence categories.
Lymphocytes are known for being resistant to lipofection, and therefore we used nucleofection for DNA delivery into Raji cells. We chose the Lonza pmax promoter to drive Cas9 expression because it offered strong expression within Raji cells. Twenty-four hours after nucleofection, we observed obvious EGFP signals from a small proportion of cells through fluorescent microscopy. The EGFP+ cell population decreased dramatically after that, however, and we measured <10% transfection efficiency 48 h after nucleofection (Fig. 1B). We attributed this transfection efficiency decrease to the plasmid dilution with cell division. To actively maintain the plasmid level within the host cells, we redesigned the CRISPR plasmid to include the EBV origin of replication sequence, oriP. With active plasmid replication inside the cells, the transfection efficiency rose to >60% (Fig. 1B).
To design guide RNA targeting the EBV genome, we relied on the EBV reference genome from strain B95-8. We targeted six regions with seven guide RNA designs for different genome-editing purposes (Fig. 1C and Table S1). EBNA1 is crucial for many EBV functions, including gene regulation and latent genome replication. We targeted guide RNA sgEBV4 and sgEBV5 to both ends of the EBNA1 coding region to excise this whole region of the genome. Guide RNAs sgEBV1, -2, and -6 fall in repeat regions, so that the success rate of at least one CRISPR complex is multiplied. These “structural” targets enable systematic digestion of the EBV genome into smaller pieces. EBNA3C and latent membrane protein-1 are essential for host cell transformation, and we designed guide RNAs sgEBV3 and sgEBV7 to target the 5′ exons of these two proteins, respectively.
EBV Genome Editing.
The double-strand DNA breaks generated by CRISPR are repaired with small deletions. These deletions will disrupt the protein coding and hence create knockout effects. SURVEYOR assays confirmed efficient editing of individual sites (Fig. S1). Beyond the independent small deletions induced by each guide RNA, which disable individual genes, large deletions between targeting sites can systematically destroy the EBV genome. Guide RNA sgEBV2 targets a region with 12 125-bp repeat units (Fig. 2A). PCR amplification of the whole repeat region gave an ∼1.8-kb band (Fig. 2B). After 5 or 7 d of sgEBV2 transfection, we obtained ∼0.4-kb bands from the same PCR amplification (Fig. 2B). The ∼1.4-kb deletion is the expected product of repair ligation between cuts in the first and the last repeat unit (Fig. 2A).
Fig. 2.
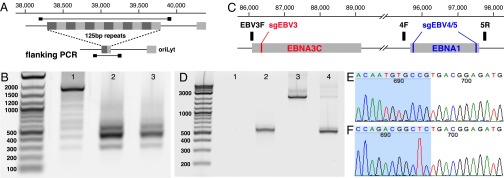
CRISPR/Cas9 induced large deletions. (A) Genome context around guide RNA sgEBV2 and PCR primer locations. (B) Large deletion induced by sgEBV2. Lanes 1–3 are before, 5 d after, and 7 d after sgEBV2 treatment, respectively. (C) Genome context around guide RNA sgEBV3/4/5 and PCR primer locations. (D) Large deletions induced by sgEBV3/5 and sgEBV4/5. Lanes 1 and 2 are 3F/5R PCR amplicons before and 8 d after sgEBV3/5 treatment. Lanes 3 and 4 are 4F/5R PCR amplicons before and 8 d after sgEBV4/5 treatment. (E and F) Sanger sequencing confirmed genome cleavage and repair ligation 8 d after sgEBV3/5 (E) and sgEBV4/5 (F) treatment. Blue and white background highlights the two ends before repair ligation.
We further demonstrated that it is possible to delete regions between unique targets (Fig. 2C). Six days after sgEBV4-5 transfection, PCR amplification of the whole flanking region (with primers EBV4F and -5R) returned a shorter amplicon, together with a much fainter band of the expected 2 kb (Fig. 2D). Sanger sequencing of amplicon clones confirmed the direct connection of the two expected cutting sites (Fig. 2F). A similar experiment with sgEBV3-5 also returned an even larger deletion, from EBNA3C to EBNA1 (Fig. 2 D and E).
Cell Proliferation Arrest with EBV Genome Destruction.
Two days after CRISPR transfection, we flow-sorted EGFP+ cells for further culture and counted the live cells daily. As expected, cells treated with Cas9 plasmids that lacked oriP or sgEBV lost EGFP expression within a few days and proliferated with a rate similar rate to the untreated control group (Fig. 3A). Plasmids with Cas9-oriP and a scrambled guide RNA maintained EGFP expression after 8 d, but did not reduce the cell proliferation rate. Treatment with the mixed mixture sgEBV1–7 resulted in no measurable cell proliferation and the total cell count either remained constant or decreased (Fig. 3A). Flow-cytometry scattering signals clearly revealed alterations in the cell morphology after sgEBV1–7 treatment, as the majority of the cells shrank in size with increasing granulation (Fig. 3 B–D) (population P4 to P3 shift). Cells in population P3 also demonstrated compromised membrane permeability by DAPI staining (Fig. 3 E–G). To rule out the possibility of CRISPR cytotoxicity, especially with multiple guide RNAs, we performed the same treatment on two other samples: the EBV− Burkitt’s lymphoma cell line DG-75 (Fig. S2) and primary human lung fibroblast IMR90 (Fig. S3). Eight and 9 d after transfection, the cell proliferation rates did not change from the untreated control groups, suggesting negligible cytotoxicity.
Fig. 3.
Cell proliferation arrest with EBV genome destruction. (A) Cell proliferation curves after different CRISPR treatments. Five independent sgEBV1–7 treatments are shown here. (B–D) Flow-cytometry scattering signals before (B), 5 d after (C), and 8 d after (D) sgEBV1–7 treatments. (E–G) Annexin V Alexa647 and DAPI staining results before (E), 5 d after (F), and 8 d after (G) sgEBV1–7 treatments. Blue and red correspond to subpopulation P3 and P4 in (B–D). (H and I) Microscopy revealed apoptotic cell morphology after sgEBV1–7 treatment. (J–M) Nuclear morphology before (J) and after (K–M) sgEBV1–7 treatment. (Scale bars: 10 μm.)
Previous studies have attributed the tumorigenic ability of EBV to its interruption of host-cell apoptosis (26). Suppressing EBV activity may therefore restore the apoptosis process, which could explain the cell death observed in our experiment. Annexin V staining revealed a distinct subpopulation of cells with intact cell membrane but exposed phosphatidylserine, suggesting cell death through apoptosis (Fig. 3 E–G). Bright-field microscopy showed obvious apoptotic cell morphology (Fig. 3 H and I) and fluorescent staining demonstrated drastic DNA fragmentation (Fig. 3 J–M). Taken together, these data suggest restoration of the normal host-cell apoptosis pathway after EBV genome destruction.
Complete Clearance of EBV in a Subpopulation.
To study the potential connection between cell proliferation arrest and EBV genome editing, we quantified the EBV load in different samples with digital PCR targeting EBNA1. Another Taqman assay targeting a conserved human somatic locus served as the internal control for human DNA normalization. On average, each untreated Raji cell has 42 copies of EBV genome (Fig. 4A). Cells treated with a Cas9 plasmid that lacked oriP or sgEBV did not have an obvious difference in EBV load difference from the untreated control. Cells treated with a Cas9-plasmid with oriP but no sgEBV had an EBV load that was reduced by ∼50%. In conjunction with the prior observation that cells from this experiment did not show any difference in proliferation rate, we interpret this result as likely caused by competition for EBNA1 binding during plasmid replication. The addition of the guide RNA mixture sgEBV1–7 to the transfection further reduced the EBV load. The live and dead cells have ∼65% EBV and 85% decreases, respectively, compared with the untreated control, and as previously discussed these cells did not proliferate.
Fig. 4.
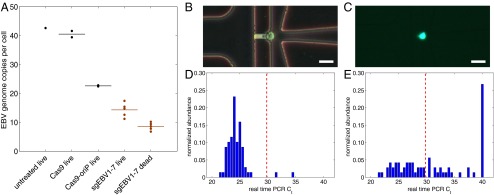
EBV load quantitation after CRISPR treatment. (A) EBV load after different CRISPR treatments by digital PCR. Cas9 and Cas9-oriP had two replicates, and sgEBV1–7 had five replicates. (B and C) Microscopy of captured single cells for whole-genome amplification. (D) Histogram of EBV quantitative PCR Ct values from single cells before treatment. (E) Histogram of EBV quantitative PCR Ct values from single live cells 7 d after sgEBV1–7 treatment. Red dash lines in D and E represent Ct values of one EBV genome per cell. (Scale bars: 25 μm.)
Although we provided seven guide RNAs at the same molar ratio, the plasmid transfection and replication process is likely quite stochastic. Some cells will inevitably receive different subsets or mixtures of the guide RNA mixture, which might affect the treatment efficiency. To control for such effects, we measured EBV load at the single-cell level by using single-cell whole-genome amplification with an automated microfluidic system. We loaded freshly cultured Raji cells onto the microfluidic chip and captured 81 single cells (Fig. 4B). For the sgEBV1–7 treated cells, we flow-sorted the live cells 8 d after transfection, and captured 91 single cells (Fig. 4C). Following the manufacturer’s instructions, we obtained ∼150-ng amplified DNA from each single-cell reaction chamber. For quality-control purposes, we performed four-loci human somatic DNA quantitative PCR on each single-cell amplification product (27) and required positive amplification from at least one locus. Sixty-nine untreated single-cell products passed the quality control and displayed a log-normal distribution of EBV load (Fig. 4D), with almost every cell displaying significant amounts of EBV genomic DNA. We calibrated the quantitative PCR assay with a subclone of Namalwa Burkitt’s lymphoma cells, which contain a single integrated EBV genome. The single-copy EBV measurements gave a Ct of 29.8, which enabled us to determine that the mean Ct of the 69 Raji single cell samples corresponded to 42 EBV copies per cells, in concordance with the bulk digital PCR measurement. For the sgEBV1–7 treated sample, 71 single-cell products passed the quality control and the EBV load distribution was dramatically wider (Fig. 4E). Although 22 cells had the same EBV load as the untreated cells, 19 cells had no detectable EBV and the remaining 30 cells displayed dramatic EBV load decrease from the untreated sample.
Essential Targets for EBV Treatment.
The seven guide RNAs in our CRISPR mixture target three different categories of sequences that are important for EBV genome structure, host cell transformation, and infection latency, respectively. To understand the most essential targets for effective EBV treatment, we transfected Raji cells with subsets of guide RNAs. Although sgEBV4/5 reduced the EBV genome by 85%, they could not suppress cell proliferation as effectively as the full mixture (Fig. 3A). Guide RNAs targeting the structural sequences (sgEBV1/2/6) could stop cell proliferation completely, despite not eliminating the full EBV load (26% decrease). Given the high efficiency of genome editing and the proliferation arrest (Fig. 2), we suspect that the residual EBV genome signature in sgEBV1/2/6 was not because of intact genomes but because of free-floating DNA that has been digested out of the EBV genome (i.e., as a false positive). We conclude that systematic destruction of EBV genome structure appears to be more effective than targeting specific key proteins for EBV treatment.
Discussion
We report here what is, to our knowledge, the first application of the CRISPR/Cas9 system to latent viral infection treatment. A Burkitt’s lymphoma cell line with latent EBV infection served as a natural model. EBV-specific guide RNAs induced effective genome editing, especially large deletions. Transfection of a guide RNA mixture completely suppressed virally induced cell proliferation and restored the apoptosis pathway in the cells. Around 25% of the treated cells were completely cleared of EBV with no detectable viral DNA, and another 50% of cells showed substantial EBV load decrease relative to the untreated sample. Because any practical therapeutic could be administered in multiple doses, it is not necessary to obtain complete elimination of all virus-infected cells with a single treatment; the geometric reduction effect from multiple doses will amplify the efficacy of any such treatment. It is also quite possible that partial viral elimination, either on a per cell or a per patient basis, will provide a synergistic opportunity for the sensitized immune system of the patient to more effectively control or eliminate residual latent infection.
Latent infection is one of the major obstacles for antiviral treatment. Existing therapies suppress active viral replication and alleviate symptoms, but latent infection can evade immune surveillance and reactivate the lytic cycle at any time; this creates a persistent risk throughout the host lifetime. The availability of viral genomes as therapeutic targets inspired the engineering of DNA-cleavage enzymes for targeted DNA mutagenesis. Prior studies of the efficacy of endonuclease treatment of herpesviridae infections, however, had significant limitations. It was shown that meganuclease treatment of cell lines could prevent infection by HSV (28), but the effects of previously existing latent infections were not explored. Another study explored latency treatment using a meganuclease combined with an exonuclease in an HSV model system (5). The cell lines were derived from fibroblasts and not neurons, which are the natural targets of HSV, and the ultimate relevance is still unknown because it was not shown that the virus could be completely eliminated even in this model system, where the cell biology may be distant from the naturally occurring infection. Thus far, the lack of an adequate in vitro model for HSV infection has hampered antiviral research. The continuous Burkitt’s lymphoma cell lines derived from different patients provide a convenient and naturally occurring renewable source of material for EBV studies, which is in contrast to the lack of easily available HSV models.
The high viral mutation rate also hampers the wide application of most sequence-specific DNA-cleavage enzymes, as each sequence-specific enzyme requires sophisticated designing. The CRISPR/Cas system has attracted great attention as a simple and efficient genome-engineering technique whose sequence specificity can be easily programmed by an RNA molecule. Although most applications of CRISPR have focused mainly on gene-function studies, its original role as a prokaryotic immune system could inspire a new approach for next-generation antiviral therapy. With the appropriate guide RNA, the Cas9 enzyme can recognize and cleave virtually any viral genome (29). However, off-target cleavage is a major concern for any nuclease therapy. Single-molecule microscopy (30) and immunoprecipitation assays (31, 32) have measured the off-target sites binding behavior of the Cas9 endonuclease. ChiP-seq further allowed unbiased detection of genome-wide Cas9 binding (31, 32). Together with recent improvements on CRISPR target specificity with dual-guide RNA designs (33–35), these studies show that off-target effects are not a substantial impediment to human genome-engineering applications and certainly do not appear to be an obstacle for applications that target nonhuman genomes (36).
When used for gene-function studies, CRISPR usually targets key coding regions, aiming to disrupt protein production. We designed guide RNAs for key EBV protein disruption, including the double incision of the master regulator EBNA1. Despite efficient DNA cleavage, we were surprised to see that the subsequently repaired EBV genomes could still drive host-cell proliferation. This finding could be because of functional redundancy from other proteins or perhaps noncoding RNA in the massive EBV genome. We obtained the best results by systematically destroying the entire EBV genome using guide RNAs targeting several repeat regions, thus maximizing the chance of DNA cleavage. Even with the presence of DNA repair processes, we estimated that the possibility of reconstructing the full genome from many smaller pieces is extremely low. Indeed, cleaving these repeat regions suppressed host-cell proliferation as efficiently as using the full guide-RNA mixture.
Further development of the strategy reported here into a practical therapeutic will require more sophisticated approaches to delivery. Although that is beyond the scope of this study, the next steps in developing a therapy will take advantage of the vast existing literature on gene therapy, as well as the numerous delivery technologies that have been developed for that purpose. Adenovirus and the related adeno-associeated virus are promising potential approaches because they do not integrate into the host’s genome. One could also imagine using a delivery approach that took advantage of the fact that many herpesvirus infections are highly localized and would not necessarily require systemic treatment. For example, HSV-1 latent infections are found only in a few sensory neurons in the trigeminal ganglia, HSV-2 latent infections are found only in genital dorsal root ganglia, and EBV latent infections are found only in B cells. One could try to engineer delivery viruses that mimic the cell-type tropism of the target virus, design targeted delivery vehicles that are cell type specific, or one could apply the delivery material directly to the affected tissues.
Materials and Methods
Cell Culture.
Burkitt’s lymphoma cell lines Raji, Namalwa, and DG-75 were obtained from ATCC and cultured in RPMI 1640 supplemented with 10% (vol/vol) FBS and 1× PSA, following ATCC recommendations. Human primary lung fibroblast IMR-90 was obtained from Coriell and cultured in Advanced DMEM/F-12 supplemented with 10% (vol/vol) FBS and 1× PSA.
CRISPR/Cas9 Treatment.
EBV-specific CRISPR/Cas9 plasmids were modified from pX458 (11), to include the pmax promoter from Lonza, sgRNA(F+E) from ref. 25, and EBV replication origin oriP from strain B95-8. We performed DNA transfection with the Lonza Nucleofector II.
Flow Cytometry Analysis and Cell Sorting.
We conducted flow cytometry experiments on the BD FACSAria II machines in Stanford Stem Cell FACS Core. Annexin V Alex647 (Life Technologies) was used following the manufacturer’s recommendations.
DNA Analysis.
Genomic DNA from flow-sorted cells was used for downstream DNA analysis. Digital PCR was performed on the Fluidigm BioMark system. Single-cell whole-genome amplification was performed on the Fluidigm C1 system.
A complete description of the materials and methods is provided in SI Materials and Methods. See Table S2 for PCR primers.
Supplementary Material
Acknowledgments
We thank Jill Chinen and Dalong Qian for laboratory management; Lolita Penland and Christopher Emig for cloning suggestions; Patricia Lovelace, Jennifer Ho, and Evan Chen for flow cytometry guidance; Brian Yu for assistance with single-cell whole-genome amplification; and Charles Gawad, Michael Rothenberg, and Shang Cai for helpful discussions. This project was supported by National Institutes of Health Grants U54CA151459, U01HL099995, P01CA139490, and U01HL099999.
Footnotes
Conflict of interest statement: Stanford University has applied for a patent based on this work.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410785111/-/DCSupplemental.
References
- 1.Hjalgrim H, Friborg J, Melbye M. In: Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Arvin A, et al., editors. Cambridge: Cambridge Univ Press; 2007. pp. 929–959. [PubMed] [Google Scholar]
- 2.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: A meta-analysis. J Infect Dis. 2002;185(1):45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 3.Wayengera M. Identity of zinc finger nucleases with specificity to herpes simplex virus type II genomic DNA: Novel HSV-2 vaccine/therapy precursors. Theor Biol Med Model. 2011;8:23. doi: 10.1186/1742-4682-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qu X, et al. Zinc-finger-nucleases mediate specific and efficient excision of HIV-1 proviral DNA from infected and latently infected human T cells. Nucleic Acids Res. 2013;41(16):7771–7782. doi: 10.1093/nar/gkt571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubert M, et al. In vitro inactivation of latent HSV by targeted mutagenesis using an HSV-specific homing endonuclease. Mol Ther Nucleic Acids. 2014;3:e146. doi: 10.1038/mtna.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horvath P, Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010;327(5962):167–170. doi: 10.1126/science.1179555. [DOI] [PubMed] [Google Scholar]
- 7.Terns MP, Terns RM. CRISPR-based adaptive immune systems. Curr Opin Microbiol. 2011;14(3):321–327. doi: 10.1016/j.mib.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhaya D, Davison M, Barrangou R. CRISPR-Cas systems in bacteria and archaea: Versatile small RNAs for adaptive defense and regulation. Annu Rev Genet. 2011;45:273–297. doi: 10.1146/annurev-genet-110410-132430. [DOI] [PubMed] [Google Scholar]
- 9.Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482(7385):331–338. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]
- 10.Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jinek M, et al. RNA-programmed genome editing in human cells. Elife (Cambridge) 2013;2:e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mali P, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi LS, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152(5):1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert LA, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154(2):442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H, et al. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154(6):1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153(4):910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014;343(6166):80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shalem O, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343(6166):84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, et al. High-throughput screening of a CRISPR/Cas9 library for functional genomics in human cells. Nature. 2014;509(7501):487–491. doi: 10.1038/nature13166. [DOI] [PubMed] [Google Scholar]
- 21.Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet. 1964;1(7335):702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 22.Pulvertaft JV. Cytology of Burkitt’s tumour (African lymphoma) Lancet. 1964;1(7327):238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- 23.Epstein MA, et al. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji) J Natl Cancer Inst. 1966;37(4):547–559. [PubMed] [Google Scholar]
- 24.Hatfull G, Bankier AT, Barrell BG, Farrell PJ. Sequence analysis of Raji Epstein–Barr virus DNA. Virology. 1988;164(2):334–340. doi: 10.1016/0042-6822(88)90546-6. [DOI] [PubMed] [Google Scholar]
- 25.Chen B, et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155(7):1479–1491. doi: 10.1016/j.cell.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruf IK, et al. Epstein-barr virus regulates c-MYC, apoptosis, and tumorigenicity in Burkitt lymphoma. Mol Cell Biol. 1999;19(3):1651–1660. doi: 10.1128/mcb.19.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Fan HC, Behr B, Quake SR. Genome-wide single-cell analysis of recombination activity and de novo mutation rates in human sperm. Cell. 2012;150(2):402–412. doi: 10.1016/j.cell.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grosse S, et al. Meganuclease-mediated inhibition of HSV1 Infection in cultured cells. Mol Ther. 2011;19(4):694–702. doi: 10.1038/mt.2010.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebina H, Misawa N, Kanemura Y, Koyanagi Y. Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep. 2013;3:2510. doi: 10.1038/srep02510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507(7490):62–67. doi: 10.1038/nature13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu X, et al. Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian cells. Nat Biotechnol. 2014;32(7):670–676. doi: 10.1038/nbt.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuscu C, Arslan S, Singh R, Thorpe J, Adli M. Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nat Biotechnol. 2014;32(7):677–683. doi: 10.1038/nbt.2916. [DOI] [PubMed] [Google Scholar]
- 33.Ran FA, et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154(6):1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol. 2014;32(3):279–284. doi: 10.1038/nbt.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai SQ, et al. Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat Biotechnol. 2014;32(6):569–576. doi: 10.1038/nbt.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



