Significance
Targeting more than one molecular cause implied in the pathogenesis of Alzheimer’s disease (AD) with a sole drug is considered a promising challenge, because it may address the numerous failures that recently occurred during clinical trials that were conducted in this area. Donecopride has been designed by us as a multitarget-directed ligand, targeting both serotonin subtype 4 receptor and acetylcholinesterase with excellent in vitro activities. The latter seems able to not only restore the cholinergic neurotransmission altered in AD but also, promote the secretion of a neurotrophic protein that is detrimental to the neurotoxic amyloid-β peptide. With its excellent drugability, donecopride further displayed significant procognitive effects in mice and generated a promising lead for a previously unidentified approach in AD treatment.
Abstract
RS67333 is a partial serotonin subtype 4 receptor (5-HT4R) agonist that has been widely studied for its procognitive effect. More recently, it has been shown that its ability to promote the nonamyloidogenic cleavage of the precursor of the neurotoxic amyloid-β peptide leads to the secretion of the neurotrophic protein sAPPα. This effect has generated great interest in RS67333 as a potential treatment for Alzheimer’s disease (AD). We show herein that RS67333 is also a submicromolar acetylcholinesterase (AChE) inhibitor and therefore, could contribute, through this effect, to the restoration of the cholinergic neurotransmission that becomes altered in AD. We planned to pharmacomodulate RS67333 to enhance its AChE inhibitory activity to take advantage of this pleiotropic pharmacological profile in the design of a novel multitarget-directed ligand that is able to exert not only a symptomatic but also, a disease-modifying effect against AD. These efforts allowed us to select donecopride as a valuable dual (h)5-HT4R partial agonist (Ki = 10.4 nM; 48.3% of control agonist response)/(h)AChEI (IC50 = 16 nM) that further promotes sAPPα release (EC50 = 11.3 nM). Donecopride, as a druggable lead, was assessed for its in vivo procognitive effects (0.1, 0.3, 1, and 3 mg/kg) with an improvement of memory performances observed at 0.3 and 1 mg/kg on the object recognition test. On the basis of these in vitro and in vivo activities, donecopride seems to be a promising drug candidate for AD treatment.
Among the large family of serotonin receptors (5-HTR), some of them, such as the subtype 4 (5-HT4R), are of particular interest in improving memory performance and therefore, decreasing memory deficits, such as those that occur in Alzheimer's disease (AD). In the CNS, they are located in structures that are primarily involved in cognitive functions, like the olfactory tubercles, basal ganglia, septum, substantia nigra, superior colliculi, hippocampus, and cortex. Several compounds act as agonists to 5-HT4R (BIMU1, BIMU8, RS17017, SL65.0155, VRX-03011, prucalopride, RS67333, and RS67506). One of the most affine (pKi = 7.88) and selective vs. other receptors is RS67333, which acts as a partial agonist (1). With respect to the potential therapeutic modulation of 5-HT4R with RS67333 and excluding its putative antidepressant-like activity (2–4), most studies focused on the promnesic or antiamnesic actions of this compound. These effects on cognitive functions that concern learning and memory are probably, in part, because of the fact that the pharmacological stimulation of these receptors increases the release of ACh in the hippocampus and cortex, and it also increases serotonin, dopamine, and GABA release (5–13). Concerning the selective aspects of memory functions, RS67333 has been shown to improve object recognition in adult (14, 15) and aged animals (16, 17) and place recognition (10) in rodents. It also increases spatial learning on the Morris water maze task in rodents, and it even reverses the deleterious effect of atropine (18) or scopolamine during this same task.
Based on the structural analogy existing between RS67333 and donepezil (Fig. 1), we postulated that RS67333 could improve learning and memory by not only activating 5-HT4R but also, inhibiting acetylcholinesterase (AChE) activity. Indeed, several early studies reported that the inhibition of AChE improved cognition and that this effect is the main reason for the initial use of donepezil, galantamine, and rivastigmine as cognitive enhancers in AD (19, 20). AChE inhibition has also been reported to improve performances in healthy rodents or animal models of memory deficiency (21). The hypothesis of an involvement of AChE inhibition by RS67333 in memory improvement is an issue that has never been tested.
Fig. 1.
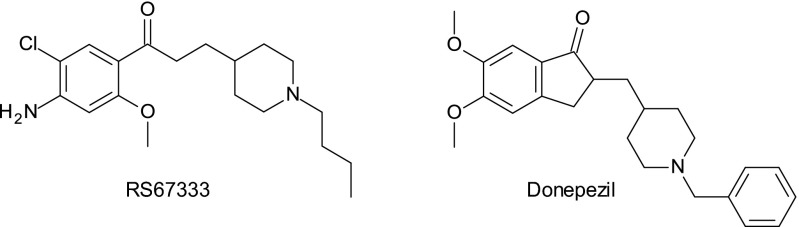
RS67333 and donepezil are chemically close.
Furthermore, such a pharmacological profile could be also exploited to lead to pleiotropic compounds that are theoretically useful in AD treatment. Indeed, today, it is well-established that 5-HT4R activation not only favors ACh release but also, is involved in the nonamyloidogenic cleavage of amyloid precursor protein (APP) in the neurotrophic sAPPα fragment, with secretion that is detrimental to amyloid-β peptide (Aβ) production (22–24). However, inhibiting the catalytic activity of AChE is widely used to restore cholinergic neurotransmission in AD, and interacting with the peripheral anionic site (PAS) of this enzyme could also reduce amyloid aggregation, for which AChE would be responsible (25). These activities seem to be synergistic when they are associated in an AD animal model, which we have recently shown in mice (26). A second pharmacological approach based on the fact that a single compound may be able to hit multiple targets is now emerging. This concept, called multitarget-directed ligands (MTDLs) (27, 28), would have inherent advantages over a combination of drugs called multiple medication therapy. It would specially obviate the problems linked to the complexity of the pharmacokinetic profile of the combined drugs and the risk of drug–drug interactions. Moreover, MTDL could also alleviate compliance difficulties associated with multiple medication therapy. It has also been shown that MTDLs generally show a higher synergistic effect than that observed with a combination of drugs. Numerous examples of MTDL against AD have been recently described (27). Most of them associate an AChE inhibitory effect with another activity hitting another molecular target of AD, such as antioxidant effect, monoamine oxidase inhibition, calcium channel blocking effect, metal chelating activity, etc. However, no MTDLs associating an inhibition of AChE and 5-HT4R agonist effect have been hitherto described. Among the different ways to synthesize such MTDLs, one of them is to merge the frameworks of two selective starting compounds, each one exerting an activity toward a sole target (28). This goal is even more easily reached if the starting compounds are structurally close, and it is the reason why we considered the structural analogy between donepezil and RS67333 as a good starting point to design MTDLs displaying both activities (AChE inhibition and 5-HT4R agonist activities). We will, thus, provide proof of this concept with the synthesis and biological evaluation of MR31147 (donecopride) as conceived from the pharmacomodulation of RS67333.
Results
RS67333 Is a Submicromolar (h)AChE Inhibitor.
RS67333 displayed remarkable inhibitory effects toward (h)AChE (IC50 = 403 nM), which have not been described previously. This submicromolar activity seems to be highly linked to a structural family effect, because the compound RS67506—another potent 5-HT4R agonist (pKi = 8.8) that is chemically similar to RS67333—also strongly inhibits (h)AChE (IC50 = 211 nM). These compounds share common intracyclic nitrogen able to engage a Π-cation interaction with a Phe in the aromatic gorge of AChE (29). Conversely, some other 5-HT4R agonists belonging to other structural families are devoid of such activity (Table 1).
Table 1.
AChE(h) inhibitory activity for some known 5-HT4R ligands
| AChE(h) IC50 (nM) or percent inhibition at 10−5 M | |
| RS67333 | 403 ± 86 (n = 2) |
| RS67506 | 211 ± 37 (n = 2) |
| Prucalopride | 46% |
| Zacopride | 34% |
| ML10302 | 48% |
| CJ033466 | 49% |
| SL65.0155 | 22% |
| BIMU8 | 18% |
On the basis of these results, RS67333 was chosen as a hit to perform a hit-to-lead chemistry study. Numerous derivatives of RS67333 were synthesized. Among them, the compound MR31147 (donecopride) was selected for its outstanding in vitro activity toward both (h)5-HT4eR and (h)AChE. The cyclohexyl moiety, introduced into the structure of donecopride, seems to be a compromise between the N-butyl group of RS67333 and the bulky benzyl group of donepezil and is able to potentiate both activities.
Donecopride Is a Potent, Selective, and Partial (h)5-HT4eR Agonist.
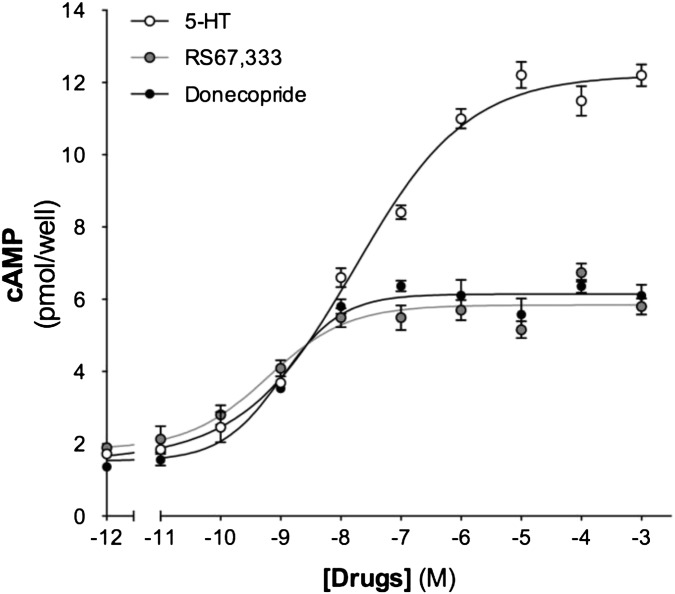
Donecopride (Fig. 2) is an (h)5-HT4eR ligand that is as potent as RS67333 (Table 2). It behaves like a partial agonist, with its intrinsic activity of 48% vs. 49% for RS67333 (Fig. 3). This profile is in accordance with our objective, because prolonged exposure of G protein-coupled receptors to an agonist can result in receptor desensitization, which is often dependent on the degree of intrinsic activity (30). However, the selectivity of donecopride toward 5-HT4R is quite good, because among a panel of 42 common neurotransmitter receptors and transporters, only 5-HT2BR and σ2R are more sensitive than 5-HT4R (Table 2). Concerning 5-HT2BR, donecopride behaves like an inverse agonist, thus avoiding the potential risk of valvulopathy that the activation of these receptors could provoke. Concerning σ2R, they are found in many peripheral tissues, where their role remains unclear. However, they are overexpressed in a wide variety of human and murine tumor cell lines and solid tumors and seem to be valuable biomarkers of cell proliferation (31). This σ2 profile for donecopride should not theoretically interfere with its central activity.
Fig. 2.
Donecopride (MR31147) is issued from RS67333 through a hit-to-lead chemistry process.
Table 2.
(h)5-HT4eR activity and selectivity for RS67333 and donecopride
| (h)5-HT4eR | 5-HT2BR Ki (nM) | σ2R Ki (nM) | ||
| Ki (nM) | Percent of control agonist response | |||
| Donecopride | 8.5 ± 0.3 (n = 2) | 48.3 ± 0.9 (n = 3) | 1.6 | 1.6 |
| RS67333 | 13 ± 4 (n = 3) | 48.7 ± 5.2 (n = 3) | 11 | 1.26 (1) |
Fig. 3.
RS67333 and donecopride act as (h)5-HT4eR partial agonists.
Donecopride Is a Potent, Selective, and Mixed Type Competitive (h)AChE Inhibitor.
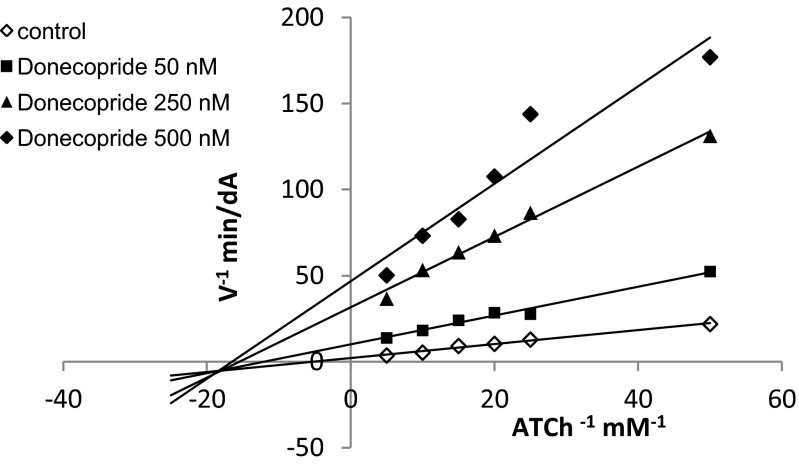
Donecopride is also a potent catalytic (h)AChE inhibitor with the same order of magnitude as donepezil. It seems further selective toward this enzyme vs. (eq) butyrylcholinesterase (BuChE) (Table 3). Furthermore, it also seems to be able to interact with the PAS of AChE, which was suggested by its significant ability to displace propidium iodide from the latter (percent inhibition of the fluorescence of propidium bound to AChE = 24% at 10 μM); donepezil was used as a control (21.5% at 10 μM) (Table 3) (32). This result was confirmed by the mixed type competitive AChE inhibitor profile of donecopride (Fig. 4) and accounts for its potential inhibitory effect against AChE-induced Aβ aggregation (33).
Table 3.
(h)AChE inhibitory activity and selectivity vs. (eq)BuChE and the propidium iodide competition assay for donecopride and donepezil
| (h)AChE IC50 (nM) | (eq)BuChE IC50 (nM) | Propidium iodide displacement percent inhibition at 10−5 M | |
| Donecopride | 16 ± 5 (n = 2) | 3,530 ± 50 (n = 2) | 24 ± 2 (n = 2) |
| Donepezil | 6 ± 0.6 (n = 5) | 7,000 (30) | 21.5 ± 1.5 (n = 2) |
Fig. 4.
Lineweaver Burk plots of inhibition kinetics show that donecopride acts as a mixed type competitive AChE inhibitor.
Donecopride Promotes the sAPPα Secretion in an in Vitro Cellular Model.
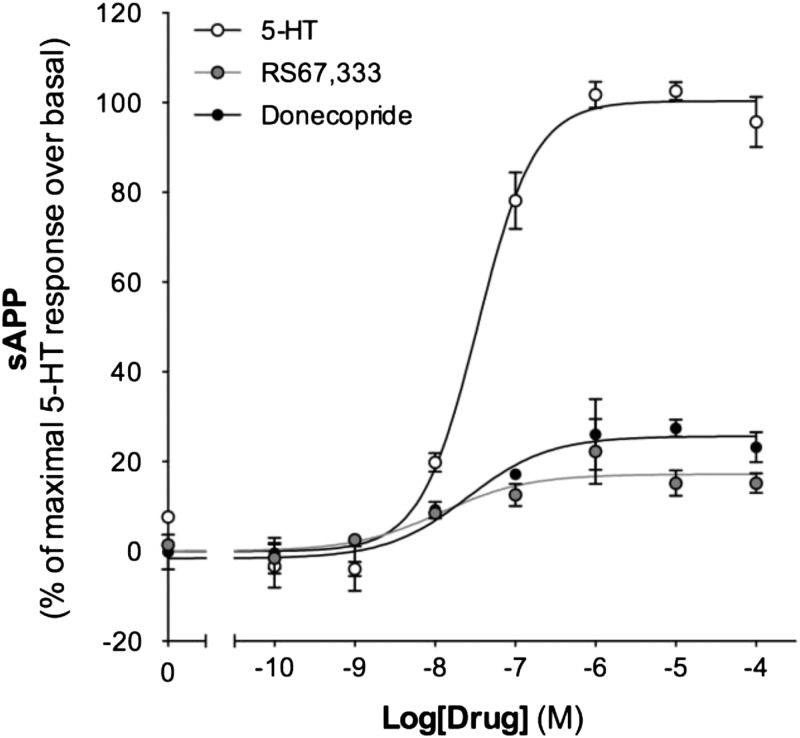
5-HT4R activation induces the nonamyloidogenic processing of APP and sAPPα release in vivo and in vitro (34, 35). Interestingly, we have recently shown that the early and chronic administration of RS67333 is able to reduce amyloid plaque formation, and it also prevents cognitive deficits in a transgenic mouse model of AD (36). The in vitro ability of donecopride to promote sAPPα secretion was evaluated on COS-7 cells that were transiently expressing 5-HT4R. Similar to RS67333, donecopride induced a clear dose-dependent sAPPα release (Fig. 5 and Table 4).
Fig. 5.
Donecopride promotes sAPPα secretion in an in vitro cellular model to a similar degree as RS67333.
Table 4.
sAPPα release induced by donecopride and RS67333
| sAPPα | ||
| EC50 (nM) | ECmax (percent of maximum 5-HT response over basal) | |
| 5-HT | 39.9 ± 3.3 (n = 6) | 100 (n = 6) |
| Donecopride | 11.3 ± 10.0 (n = 3) | 22.5 ± 1.4 (n = 7) |
| RS67333 | 27.2 ± 10.6 (n = 3) | 20.5 ± 2.1 (n = 5) |
Donecopride Exhibits Good Drugability Parameters.
Before performing an in vivo study of donecopride, several drugability parameters were established, including its water solubility and blood–brain barrier (BBB) cross-membrane penetration. The thermodynamic water solubility of donecopride at 48 h was determined at various pH levels according to the classic shake-flask method and miniaturized UV spectrometric quantification (Table 5) (37).
Table 5.
Donecopride solubility (micromolar)
| Sint (pH 12) | Sphysio (pH 7.4) | Sw (water) |
| 0.7 ± 0.4 | 18.2 ± 1.1 | 0.9 ± 0.5 |
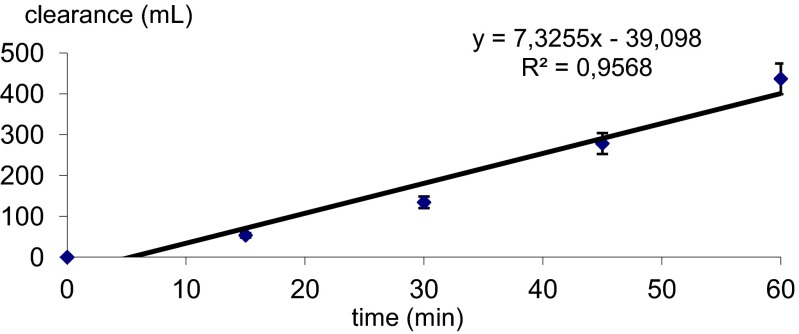
Particular attention has been paid to the ability of the fumarate salt of donecopride (chosen among a series of salts) to cross the BBB. According to a parallel artificial membrane permeability assay experiment (38), the drug (Mr = 508 g⋅mol−1) was classified among the compounds as having good brain penetration with logPe = −4.43 ± 0.10 (Pe represents the PAMPA effective permeability coefficient). This result was fully confirmed after performing a cellular test using bovine brain capillary endothelial cells and rat glial cells with Pe > 2 × 10−3 cm/min (Fig. 6). This good permeability seems to be in accordance with the calculated log P of donecopride (log P = 4.4; MarvinSketch 5.11.1).
Fig. 6.
Donecopride fumarate exhibits good brain penetration according to a cellular in vitro test.
Because the poor brain penetration of 5-HT4R ligands is often linked to P-glycoprotein (P-gp) -mediated efflux (30), we evaluated the ability of donecopride to inhibit the P-gp on NCI/ADR-res cells at 1 and 10 μM. The results confirmed that donecopride inhibits rhodamine123 efflux to a lesser degree than cyclosporin (∼140% vs. 400%). Cyclosporin is a reference substrate of P-gp, and it markedly promoted the accumulation of rhodamine123 into cells, whereas donecopride had only a modest effect at concentrations ranging from 1 to 10 µM. This result clearly suggested that donecopride was not a potent substrate for P-gp and therefore, is not actively effluxed from cells (Table 6).
Table 6.
Rhodamine123 efflux inhibition by cyclosporin and donecopride in NCI/ADR-res cells
| Percentage | |
| NCI/ADR-res | 100 ± 23 (n = 4) |
| Cyclosporin 50 µM | 400 ± 134 (n =4) |
| Donecopride 10 µM | 147 ± 19 (n = 4) |
| Donecopride 1 µM | 138 ± 28 (n = 4) |
Some other toxicological parameters have been also established for donecopride including mutagenicity, cytotoxicity, and human ether-à-go-go related gene (hERG) affinity. It did not exhibit mutagenicity, cytotoxicity, or significant hERG affinity (Table 7).
Table 7.
Mutagenicity, cytotoxicity, and hERG affinity for donecopride
| Mutagenicity | Cytotoxicity on MRC5 cell (inhibition percentage at 10−6 M) | hERG affinity (affinity percentage at 10−6 M) |
| No mutagenicity | 6 ± 11 (n = 3) | 27 |
All of these parameters account for a suitable drugability that enabled the preliminary study of donecopride in vivo.
Donecopride Is Procognitive in Mice.
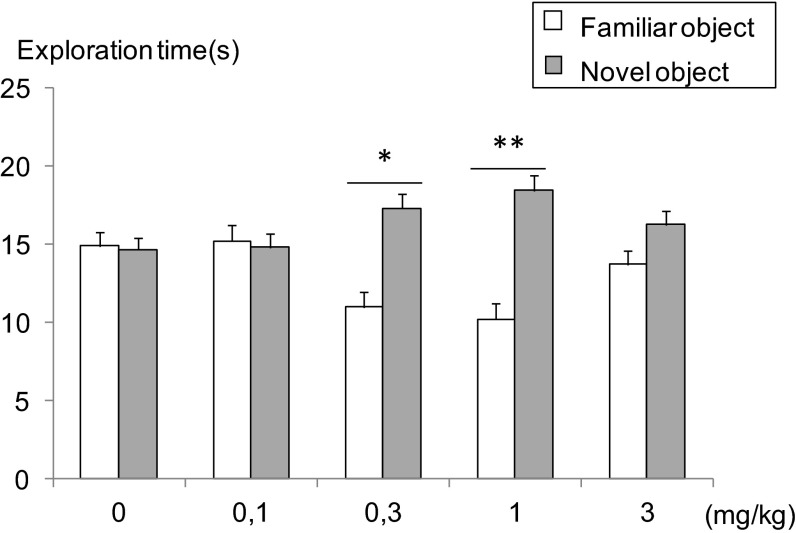
The aim of this study was to draw a dose–effect curve of donecopride on the behavioral performances observed during an object recognition task. Therefore, we examined the impact of the i.p. administration of donecopride (given 30 min just before an acquisition session) on long-term memory performances, which were evaluated after 72 h. The required time to reach the time criterion of objects exploration during training did not significantly differ between the different groups. After a 72-h intertrial interval, two-way ANOVA showed an objects effect and a group × object interaction [F(1,44) = 12.944, P = 0.0008 and F(4,44) = 3.547, P = 0.0136, respectively]. Indeed, only mice treated with either 0.3 or 1 mg/kg donecopride showed an objects effect [one-way ANOVA: F(1,9) = 7.263, P = 0.0246 and F(1,8) = 12.244, P = 0.0081, respectively]. Additionally, posthoc analysis using the Student Newman–Keuls test (α = 0.05) indicated that mice treated with either 0.3 or 1 mg/kg donecopride showed a significantly higher discrimination index (DI; DI = 6.300 ± 1.647 and 8.222 ± 2.350, respectively) than the control group (DI = −0.222 ± 1.839) (Fig. 7). However, the absence of procognitive effect at the highest dose (3 mg/kg) may easily be attributed to the appearance of a cholinergic side effect, classically observed while using high doses of AChE inhibitors (39).
Fig. 7.
Donecopride exhibits a significant procognitive effect during the object recognition task. Significant differences between novel and familiar object exploration times (posthoc Student Newman–Keuls test) are shown. *P < 0.05; **P < 0.01.
Discussion
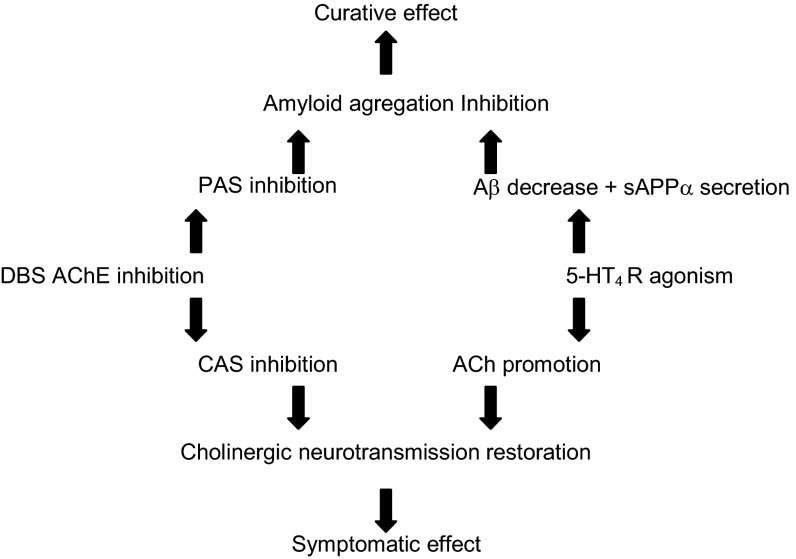
The discovery of the AChE inhibitory activity of RS67333 seems to be of a great interest, because this compound has been widely studied for its in vivo procognitive effect. The in vivo procognitive effect was hitherto only imputed to the 5-HT4R agonist activity and therefore, the resulting 5-HT4R–related ACh (and perhaps, sAPPα) release enhancement. The influence of AChE inhibition and therefore, the contribution of the resulting increase in synaptic ACh concentration on this procognitive effect will have to be assessed. Furthermore, RS67333 can be considered as a hit to design pleiotropic agents with a dual 5-HT4R agonist/AChE inhibition activity. With this goal in mind, we performed the synthesis of a series of novel analogs of RS67333. Our first objective was to confirm whether modulation of the chemical structure of RS67333 could enhance its activity toward both targets. For the synthesized compound, we were pleased to identify donecopride, which validates our hypothesis. This compound conserves its affinity toward 5-HT4R in vivo, with a Ki of 10.4 nM, but it also retains the partial agonist activity of RS67333. We have further proven that donecopride is able to promote the secretion of the neurotrophic protein, sAPPα, in an in vitro cellular model. In addition, more interestingly, we have also shown that a simple chemical modification, such as the replacement of the n-butyl chain of RS67333 by a methylenecyclohexyl group, dramatically increases the potency of our compound toward AChE. Indeed, donecopride inhibits the catalytic active site (CAS) of AChE with an IC50 of 16 nM, which is comparable with donepezil and 25-fold better than RS67333. Donecopride is also able to display a dual binding site (DBS) inhibitory effect on AChE. This MTDL profile seems to confer to donecopride the ability to display improvements in both cholinergic neurotransmission (through the catalytic inhibition of AChE and the release of ACh) and inhibition of Aβ accumulation and aggregation. This second effect would be possible through the nonamyloidogenic 5-HT4R–dependent cleavage of the precursor of Aβ as well as the interaction of donecopride with the PAS of AChE, which is able to counteract the chaperone role played by the latter in Aβ aggregation. Finally, these complementary activities could exert both symptomatic and disease-modifying effects for AD treatment (Fig. 8).
Fig. 8.
Pleiotropic interest of DBS AChEI/5-HT4R agonists. CAS, catalytic active site; PAS, peripheral anionic site.
Interest in such a dual effect has been recently shown with the synergistic coadministration of subactive doses of donepezil and RS67333, which resulted in a significantly procognitive effect (26). Donecopride, which combines the activities of donepezil and RS67333 in a single compound, possesses excellent drugability parameters with a good bioavailability; it also has the ability to cross the BBB and features a nontoxic profile. Finally, donecopride exerted a significant procognitive effect in mice in vivo. It will be interesting to compare the in vivo effects of drugs bearing only one activity (ACh inhibition or 5-HT4R stimulation) with donecopride in AD mouse models. Considering that chronic treatments with RS67333 have already shown their ability to slow down AD pathology and prevent cognitive deficits in mice (36), donecopride opens the door to a previously unidentified class of compounds that could take advantage of the well-known properties of AChE inhibitors, and it could also enhance the serotonergic transmission that seems to be more and more relevant for a cure for AD (40). Undeniably, serotonin signaling is associated with a lowering of Aβ levels in either soluble form or deposits, a decrease that has a positive outcome on cognitive performance in mice and may prevent deleterious amyloid accumulation in AD (40–42). However, activation of 5-HTR (5-HT2A, 5-HT2C, and 5-HT4) has been shown to also induce an increase in soluble form of APPα (34, 36, 43). Whereas the precise identification of the soluble fragment is still pending for 5-HT2A/2CR, 5-HT4R agonists undoubtedly induce the secretion of the neuroprotective sAPPα fragment and the activation on the nonamyloidogenic processing of APP (36). Thus, to the symptomatic cholinergic effects, donecopride and related MTDLs should add capacities to impact APP metabolism by activating 5-HT4R. Additional in vivo experiments are required to precisely examine the beneficial effect of this two-target combination and whether it could slow down AD progression.
Considering that the recent failures that have occurred in numerous clinical trials conducted with novel drugs for AD treatment were sometimes attributed to their great selectivity toward a unique target (44, 45), the development of an MTDL, such as donecopride, could be viewed as a promising challenge.
Materials and Methods
Donecopride fumarate was obtained in six steps starting from 3-amino-4-chloro-2-anisic acid. Inhibitory capacity of compounds on AChE was evaluated through the use of the spectrometric method of Ellman et al. (46). Details describing synthesis and characterization of donecopride, acetyl and butyrylcholinesterase assays, propidium competition assay, serotonin and other receptors assays, sAPPα production, parallel artificial membrane permeability assay and in vitro BBB assay, water solubility determination, cytotoxicity assay, P-gp efflux pump inhibition, hERG assay, mutagenicity assay, and novel object recognition task can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by funding from the Conseil Régional de Basse Normandie, France (Dispositif de Soutien aux Projets de Recherche Emergents), French Agence Nationale de la Recherche Projects MALAD ANR-12-JS007-0012-01 and ADAMGUARD ANR-12-BSV4-008-01, and Ligue Européenne Contre la Maladie d’Alzheimer Grant 12721.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410315111/-/DCSupplemental.
References
- 1.Eglen RM, Bonhaus DW, Johnson LG, Leung E, Clark RD. Pharmacological characterization of two novel and potent 5-HT4 receptor agonists, RS 67333 and RS 67506, in vitro and in vivo. Br J Pharmacol. 1995;115(8):1387–1392. doi: 10.1111/j.1476-5381.1995.tb16628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gómez-Lázaro E, et al. Effects of a putative antidepressant with a rapid onset of action in defeated mice with different coping strategies. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(2):317–327. doi: 10.1016/j.pnpbp.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Lucas G, et al. Selective serotonin reuptake inhibitors potentiate the rapid antidepressant-like effects of serotonin4 receptor agonists in the rat. PLoS ONE. 2010;5(2):e9253. doi: 10.1371/journal.pone.0009253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas G, et al. Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron. 2007;55(5):712–725. doi: 10.1016/j.neuron.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Kilbinger H, Wolf D. Effects of 5-HT4 receptor stimulation on basal and electrically evoked release of acetylcholine from guinea-pig myenteric plexus. Naunyn Schmiedebergs Arch Pharmacol. 1992;345(3):270–275. doi: 10.1007/BF00168686. [DOI] [PubMed] [Google Scholar]
- 6.Consolo S, Arnaboldi S, Giorgi S, Russi G, Ladinsky H. 5-HT4 receptor stimulation facilitates acetylcholine release in rat frontal cortex. Neuroreport. 1994;5(10):1230–1232. doi: 10.1097/00001756-199406020-00018. [DOI] [PubMed] [Google Scholar]
- 7.Ge J, Barnes NM. 5-HT4 receptor-mediated modulation of 5-HT release in the rat hippocampus in vivo. Br J Pharmacol. 1996;117(7):1475–1480. doi: 10.1111/j.1476-5381.1996.tb15309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucas G, et al. Neurochemical and electrophysiological evidence that 5-HT4 receptors exert a state-dependent facilitatory control in vivo on nigrostriatal, but not mesoaccumbal, dopaminergic function. Eur J Neurosci. 2001;13(5):889–898. doi: 10.1046/j.0953-816x.2000.01453.x. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto M, et al. Evidence for involvement of central 5-HT(4) receptors in cholinergic function associated with cognitive processes: Behavioral, electrophysiological, and neurochemical studies. J Pharmacol Exp Ther. 2001;296(3):676–682. [PubMed] [Google Scholar]
- 10.Bianchi C, Rodi D, Marino S, Beani L, Siniscalchi A. Dual effects of 5-HT4 receptor activation on GABA release from guinea pig hippocampal slices. Neuroreport. 2002;13(17):2177–2180. doi: 10.1097/00001756-200212030-00003. [DOI] [PubMed] [Google Scholar]
- 11.Orsetti M, Dellarole A, Ferri S, Ghi P. Acquisition, retention, and recall of memory after injection of RS67333, a 5-HT(4) receptor agonist, into the nucleus basalis magnocellularis of the rat. Learn Mem. 2003;10(5):420–426. doi: 10.1101/lm.67303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Licht CL, Knudsen GM, Sharp T. Effects of the 5-HT(4) receptor agonist RS67333 and paroxetine on hippocampal extracellular 5-HT levels. Neurosci Lett. 2010;476(2):58–61. doi: 10.1016/j.neulet.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Steward LJ, et al. Ability of 5-HT4 receptor ligands to modulate rat striatal dopamine release in vitro and in vivo. Br J Pharmacol. 1996;117(1):55–62. doi: 10.1111/j.1476-5381.1996.tb15154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotte M, Dauphin F, Freret T, Boulouard M, Levallet G. A biphasic and brain-region selective down-regulation of cyclic adenosine monophosphate concentrations supports object recognition in the rat. PLoS ONE. 2012;7(2):e32244. doi: 10.1371/journal.pone.0032244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levallet G, Hotte M, Boulouard M, Dauphin F. Increased particulate phosphodiesterase 4 in the prefrontal cortex supports 5-HT4 receptor-induced improvement of object recognition memory in the rat. Psychopharmacology (Berl) 2009;202(1-3):125–139. doi: 10.1007/s00213-008-1283-8. [DOI] [PubMed] [Google Scholar]
- 16.Lamirault L, Simon H. Enhancement of place and object recognition memory in young adult and old rats by RS 67333, a partial agonist of 5-HT4 receptors. Neuropharmacology. 2001;41(7):844–853. doi: 10.1016/s0028-3908(01)00123-x. [DOI] [PubMed] [Google Scholar]
- 17.Lamirault L, Guillou C, Thal C, Simon H. Combined treatment with galanthaminium bromide, a new cholinesterase inhibitor, and RS 67333, a partial agonist of 5-HT4 receptors, enhances place and object recognition in young adult and old rats. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(1):185–195. doi: 10.1016/S0278-5846(02)00351-2. [DOI] [PubMed] [Google Scholar]
- 18.Fontana DJ, Daniels SE, Wong EH, Clark RD, Eglen RM. The effects of novel, selective 5-hydroxytryptamine (5-HT)4 receptor ligands in rat spatial navigation. Neuropharmacology. 1997;36(4-5):689–696. doi: 10.1016/s0028-3908(97)00055-5. [DOI] [PubMed] [Google Scholar]
- 19.Musiał A, Bajda M, Malawska B. Recent developments in cholinesterases inhibitors for Alzheimer’s disease treatment. Curr Med Chem. 2007;14(25):2654–2679. doi: 10.2174/092986707782023217. [DOI] [PubMed] [Google Scholar]
- 20.Anand P, Singh B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch Pharm Res. 2013;36(4):375–399. doi: 10.1007/s12272-013-0036-3. [DOI] [PubMed] [Google Scholar]
- 21.Yuede CM, Dong H, Csernansky JG. Anti-dementia drugs and hippocampal-dependent memory in rodents. Behav Pharmacol. 2007;18(5-6):347–363. doi: 10.1097/FBP.0b013e3282da278d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho S, Hu Y. Activation of 5-HT4 receptors inhibits secretion of β-amyloid peptides and increases neuronal survival. Exp Neurol. 2007;203(1):274–278. doi: 10.1016/j.expneurol.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Lezoualc’h F. 5-HT4 receptor and Alzheimer’s disease: The amyloid connection. Exp Neurol. 2007;205(2):325–329. doi: 10.1016/j.expneurol.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Russo O, et al. Design, synthesis, and biological evaluation of new 5-HT4 receptor agonists: Application as amyloid cascade modulators and potential therapeutic utility in Alzheimer’s disease. J Med Chem. 2009;52(8):2214–2225. doi: 10.1021/jm801327q. [DOI] [PubMed] [Google Scholar]
- 25.Inestrosa NC, et al. Acetylcholinesterase accelerates assembly of amyloid-β-peptides into Alzheimer’s fibrils: Possible role of the peripheral site of the enzyme. Neuron. 1996;16(4):881–891. doi: 10.1016/s0896-6273(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 26.Freret T, et al. Synergistic effect of acetylcholinesterase inhibition (donepezil) and 5-HT(4) receptor activation (RS67333) on object recognition in mice. Behav Brain Res. 2012;230(1):304–308. doi: 10.1016/j.bbr.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Cavalli A, et al. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008;51(3):347–372. doi: 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- 28.Morphy R, Rankovic Z. The physicochemical challenges of designing multiple ligands. J Med Chem. 2006;49(16):4961–4970. doi: 10.1021/jm0603015. [DOI] [PubMed] [Google Scholar]
- 29.Sopkova-de Oliveira Santos J, et al. Virtual screening discovery of new acetylcholinesterase inhibitors issued from CERMN chemical library. J Chem Inf Model. 2010;50(3):422–428. doi: 10.1021/ci900491t. [DOI] [PubMed] [Google Scholar]
- 30.Brodney MA, et al. Identification of multiple 5-HT₄ partial agonist clinical candidates for the treatment of Alzheimer’s disease. J Med Chem. 2012;55(21):9240–9254. doi: 10.1021/jm300953p. [DOI] [PubMed] [Google Scholar]
- 31.Bai S, et al. Synthesis and structure-activity relationship studies of conformationally flexible tetrahydroisoquinolinyl triazole carboxamide and triazole substituted benzamide analogues as σ2 receptor ligands. J Med Chem. 2014;57(10):4239–4251. doi: 10.1021/jm5001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberry TL, Mallender WD, Thomas PJ, Szegletes T. A steric blockade model for inhibition of acetylcholinesterase by peripheral site ligands and substrate. Chem Biol Interact. 1999;119-120:85–97. doi: 10.1016/s0009-2797(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 33.Sugimoto H, Yamanishi Y, Iimura Y, Kawakami Y. Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr Med Chem. 2000;7(3):303–339. doi: 10.2174/0929867003375191. [DOI] [PubMed] [Google Scholar]
- 34.Maillet M, et al. Crosstalk between Rap1 and Rac regulates secretion of sAPPalpha. Nat Cell Biol. 2003;5(7):633–639. doi: 10.1038/ncb1007. [DOI] [PubMed] [Google Scholar]
- 35.Cochet M, et al. 5-HT4 receptors constitutively promote the non-amyloidogenic pathway of APP cleavage and interact with ADAM10. ACS Chem Neurosci. 2013;4(1):130–140. doi: 10.1021/cn300095t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giannoni P, et al. Early administration of RS 67333, a specific 5-HT4 receptor agonist, prevents amyloidogenesis and behavioral deficits in the 5XFAD mouse model of Alzheimer’s disease. Front Aging Neurosci. 2013;5:96. doi: 10.3389/fnagi.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bard B, Martel S, Carrupt PA. High throughput UV method for the estimation of thermodynamic solubility and the determination of the solubility in biorelevant media. Eur J Pharm Sci. 2008;33(3):230–240. doi: 10.1016/j.ejps.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Avdeef A, et al. PAMPA—critical factors for better predictions of absorption. J Pharm Sci. 2007;96(11):2893–2909. doi: 10.1002/jps.21068. [DOI] [PubMed] [Google Scholar]
- 39.Moser PC, et al. SL65.0155, a novel 5-hydroxytryptamine(4) receptor partial agonist with potent cognition-enhancing properties. J Pharmacol Exp Ther. 2002;302(2):731–741. doi: 10.1124/jpet.102.034249. [DOI] [PubMed] [Google Scholar]
- 40.Sheline YI, et al. An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice. Sci Transl Med. 2014;6(236):236re4. doi: 10.1126/scitranslmed.3008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cirrito JR, et al. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc Natl Acad Sci USA. 2011;108(36):14968–14973. doi: 10.1073/pnas.1107411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fowler SW, et al. Genetic modulation of soluble Aβ rescues cognitive and synaptic impairment in a mouse model of Alzheimer’s disease. J Neurosci. 2014;34(23):7871–7885. doi: 10.1523/JNEUROSCI.0572-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nitsch RM, Deng M, Growdon JH, Wurtman RJ. Serotonin 5-HT2a and 5-HT2c receptors stimulate amyloid precursor protein ectodomain secretion. J Biol Chem. 1996;271(8):4188–4194. doi: 10.1074/jbc.271.8.4188. [DOI] [PubMed] [Google Scholar]
- 44.Rosini M, Simoni E, Minarini A, Melchiorre C. Multi-target design strategies in the context of Alzheimer’s disease: Acetyl-cholinesterase inhibition and NMDA receptor antagonism as the diving forces. Neurochem Res. 2014 doi: 10.1007/s11064-014-1250-1. [DOI] [PubMed] [Google Scholar]
- 45.Citron M. Alzheimer’s disease: Strategies for disease modification. Nat Rev Drug Discov. 2010;9(5):387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- 46.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.