Significance
High-altitude cerebral edema, a serious and often fatal condition, results from lowered oxygen supply. This study explores the mechanisms by which hypoxia induces cerebral edema. We show that hypoxia induced cerebral edema and neuronal apoptosis associated with increased expression of the neuropeptide corticotrophin releasing factor (CRF) and its type 1 receptor (CRFR1), the water channel aquaporin-4 (AQP4), and endothelin-1; these effects could be blocked by the CRFR1 antagonist. In cultured astrocytes, CRF, acting through CRFR1, triggers intracellular signaling and contributes to phosphorylation and expression of AQP4 to enhance water influx into cells. These data provide an understanding of the development of cerebral edema by high-altitude hypoxia and suggest that CRFR1 might be a target molecule for prevention of this disorder.
Keywords: water permeability, high altitude, acute mountain sickness
Abstract
Cerebral edema is a potentially life-threatening illness, but knowledge of its underlying mechanisms is limited. Here we report that hypobaric hypoxia induces rat cerebral edema and neuronal apoptosis and increases the expression of corticotrophin releasing factor (CRF), CRF receptor type 1 (CRFR1), aquaporin-4 (AQP4), and endothelin-1 (ET-1) in the cortex. These effects, except for the increased expression of CRF itself, could all be blocked by pretreatment with an antagonist of the CRF receptor CRFR1. We also show that, in cultured primary astrocytes: (i) both CRFR1 and AQP4 are expressed; (ii) exogenous CRF, acting through CRFR1, triggers signaling of cAMP/PKA, intracellular Ca2+, and PKCε; and (iii) the up-regulated cAMP/PKA signaling contributes to the phosphorylation and expression of AQP4 to enhance water influx into astrocytes and produces an up-regulation of ET-1 expression. Finally, using CHO cells transfected with CRFR1+ and AQP4+, we show that transfected CRFR1+ contributes to edema via transfected AQP4+. In conclusion, hypoxia triggers cortical release of CRF, which acts on CRFR1 to trigger signaling of cAMP/PKA in cortical astrocytes, leading to activation of AQP4 and cerebral edema.
Approximately 140 million people around the world live at altitudes of >2,500 m, including >8 million who live on the Qinghai–Tibet plateau of China at an average elevation of >4,000 m. Living at these altitudes requires physiological adaptations to compensate for the lower partial pressure of oxygen (1). In travelers who ascend to altitudes too high or too quickly (2–4) or in people with fatigue and infection or psychological stress (3, 4), hypoxia can induce acute mountain sickness (AMS) that can develop into high-altitude cerebral edema (HACE), a serious and often fatal condition (2–6). Today’s ability to travel rapidly to high altitudes means that, every year, millions of people are exposed to the risk of AMS and HACE (7).
In human volunteers exposed for 32 h to hypobaric hypoxia (corresponding to 4,572 m altitude), brain volume increases by 2.77% (8), whereas normobaric hypoxia (12% O2, corresponding to a simulated altitude of 4,500 m) for 16 h produces 50% AMS, headache, a mild increase in brain volume, and cytotoxic cerebral edema (9). A recent study reported that seven of nine male students exposed to isobaric hypoxia (inhaled room air enriched with N2 for 6 h to obtain arterial saturation values of 75–80%) developed AMS; the mean apparent diffusion coefficient (ADC) increased by 2.12% in these subjects, indicating mild extracellular (vasogenic) cerebral edema. The ADC changes were negatively correlated with AMS scores, suggesting that severe AMS is associated with intracellular (cytotoxic) cerebral edema in addition to vasogenic edema (10).
In parallel with the increasing brain water content, hypoxia acutely activates the hypothalamic–pituitary–adrenal axis in rats (11–13), increasing corticotropin-releasing factor (CRF) and endothelin-1 (ET-1) expression in the paraventricular nucleus of the hypothalamus (14). We previously showed that these effects are mediated by CRF receptor 1 (CRFR1) (12, 14). These observations led us to speculate that other CRF systems in the brain (15, 16) might also be involved in the response to hypoxia.
Aquaporin-4 (AQP4), a water channel protein, is expressed in astrocytes, especially in the foot processes (17), and is a primary influx route for water during brain edema in pathologies such as brain injury, stroke, and brain tumors. Because both CRF and its receptor are widely distributed in the central and peripheral nervous systems (18–21), including in astrocytes, microglia, and neurons of the cortex (22), we hypothesized that cortical astrocytes that express both CRFR1 and AQP4 might be involved in hypoxia-induced brain edema.
In this study, we addressed the hypothesis that, during high-altitude hypoxia, CRF mediates cerebral edema through CRFR1 signaling and AQP4 activation in cortical astrocytes.
Results
Hypoxia-Induced Cerebral Edema and CRF, CRFR1, and AQP4 Activation.
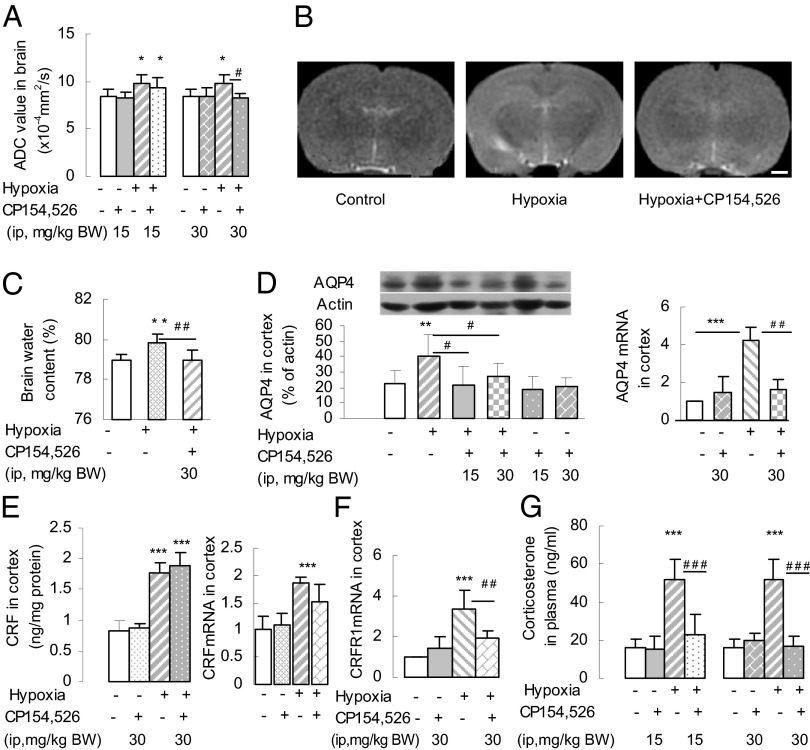
To determine whether high-altitude hypoxia induces HACE, rats were exposed to simulated high-altitude hypoxia, simulating an altitude of 7,000 m (equivalent to 7.8% O2 at sea level) for 8 h to acutely induce hypoxic brain edema. To study the role of CRFR1, the CRFR1-specific antagonist CP154,526 [N-butyl-N-ethyl-4,9-dimethyl-7-(2,4,6-trimethylphenyl)-3,5,7-triazabicyclo[4.3.0]nona-2,4,8,10-tetraen-2-amine] (15 or 30 mg per kg of body weight) was injected s.c. 30 min before the rats were subjected to hypoxia. The hypoxia resulted in brain edema, detected by magnetic resonance imaging (MRI) as a significant increase in ADC of the brain (Fig. 1 A and B) and by an increase in whole-brain water content (Fig. 1C). These effects were blocked by CP154,526 at 30 mg/kg, but not at 15 mg/kg.
Fig. 1.
Simulated high-altitude hypoxia induced brain edema in rats. (A) Increases in ADC values determined by MRI after hypoxia were abolished by pretreatment with the CRFR1 antagonist CP154,526 (30 mg/kg) (n = 6 or 7 per group). *P < 0.05 (vs. control); #P < 0.05 (vs. hypoxia). (B) Representative MRI images of rat brain: control, hypoxia, and pretreatment with CP154,526 + hypoxia (hypoxia+CP154,526). (Scale bar: 1 mm.) (C) Elevation of brain water content after hypoxia was blocked by CP154,526 (n = 6 or 7). **P < 0.01 (vs. control); ##P < 0.01 (vs. hypoxia). (D) Hypoxia increased the expression of AQP4 and AQP4 mRNA in the cortex, and the increase of AQP4 protein and AQP4 mRNA was blocked by CP154,526 (n = 6 or 7). **P < 0.01; ***P < 0.001 (vs. control); #P < 0.05; ## P < 0.01 (hypoxia vs. antagonist + hypoxia). (E) Hypoxia-induced increases in CRF content and CRF mRNA expression in cortex were not abolished by CP154,526 (n = 6 or 7). ***P < 0.001 (vs. control). (F) CRFR1 mRNA expression in the cortex was increased by hypoxia, and this increase was abolished by CP154,526 (n = 6 or 7). ***P < 0.001 (vs. control); ##P < 0.01 (vs. hypoxia). (G) Hypoxia increased the plasma concentration of corticosterone and this effect was abolished by CP154,526 (n = 6 or 7). ***P < 0.001 (vs. control); ###P < 0.001 (vs. hypoxia).
We then measured the effects of hypoxia on the expression of CRF, CRF mRNA, CRFR1 mRNA, AQP4, and AQP4 mRNA in the frontal cortex. The levels of all were markedly increased by hypoxia (Fig. 1 D–F), and, as expected, the plasma concentration of corticosterone was also increased (Fig. 1G). Pretreatment with CP154,526 (30 mg/kg) blocked the increases in CRFR1 mRNA, AQP4, and AQP4 mRNA expression and the increase in corticosterone secretion, without affecting the basal level (Figs. 1G and 2B). We omitted glucocorticoid replacement to probe the mechanisms underlying HACE, because cortisol levels are not correlated with the severity of AMS in humans (23). Pretreatment with CP154,526 had no significant effect on CRF content or CRF mRNA expression in the cortex (Fig. 1E). Thus, we hypothesized that, under hypoxia, the local increases in the expression and release of CRF play a major role in the elevated expression of AQP4 in the cortex, acting via CRFR1 receptors.
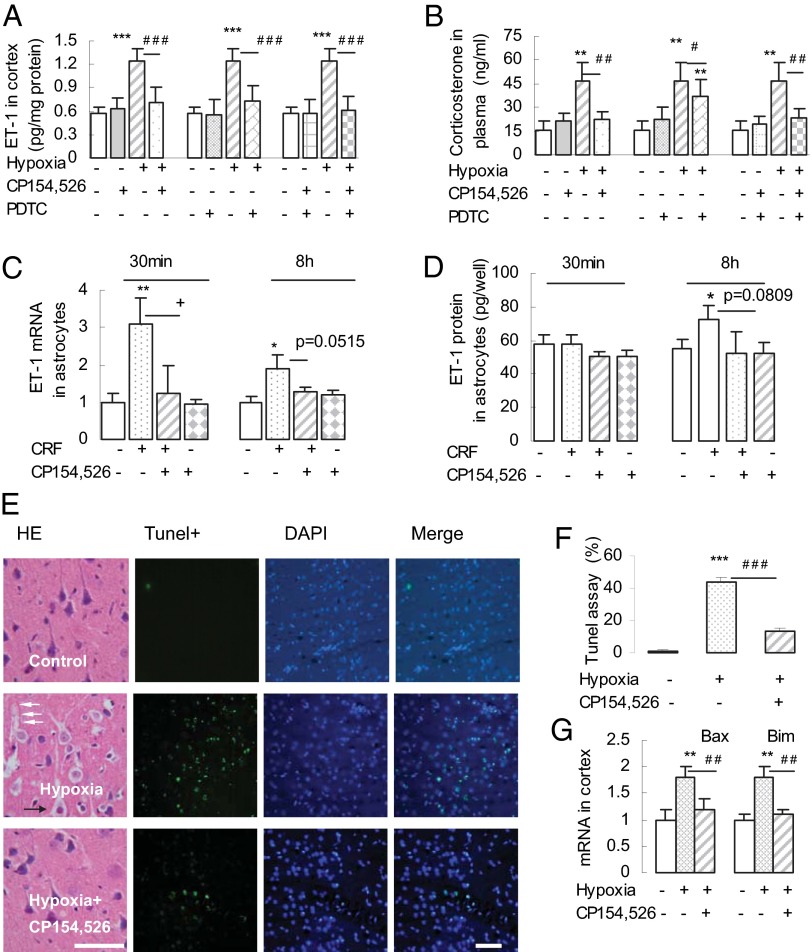
Fig. 2.
Hypoxia-activated CRFR1-mediated up-regulation of ET-1, neuronal apoptosis and apoptotic gene activation in cortex. (A and B) Hypoxia enhanced ET-1 levels in cortex (A) and corticosterone concentration in plasma (B). These increases were abolished by pretreatment with CP154,526, PDTC (NF-κB inhibitor), and a combination of CP154,526 + PDTC (n = 6 or 7). **P < 0.01; ***P < 0.001 (vs. control); #P < 0.05; ##P < 0.01; ###P < 0.001 (vs. hypoxia). (C and D) CRF induced up-regulated expression of ET-1 mRNA (C) and protein (D), which was also blocked by CRFR1 antagonist. *P < 0.05; **P < 0.01 (vs. control); +P < 0.05 (CRF vs. CRF + CP154,526); P = 0.0515 (C, CRF vs. CRF + CP154,526); P = 0.0809 (D, CRF vs. CRF + CP154,526). (E and F) CRFR1-mediated neuron swelling and apoptosis in the cortex. (E) H&E-stained brain sections revealed swollen pyramidal cells, enlarged intercellular space, and perivascular vacuoles, and the effects were reduced by pretreatment with CP154,526 (30 mg/kg). White arrows indicate capillary vessels, and black arrows indicate pyramidal cells. (Scale bar: 50 μm.) (E and F) TUNEL staining showed an increase in apoptotic signals in the cortex of hypoxic rats, but significantly reduced signals in CRFR1 antagonist-pretreated cortex (n = 6 or 7). [Scale bar: 60 μm (E, rightmost column).] ***P < 0.001 (vs. control); ###P < 0.001 (hypoxia + CRFR1 antagonist vs. hypoxia). (G) Hypoxia-activated mRNA expression of apoptotic genes Bax and Bim in the cortex, which was blocked by pretreatment with CP154,526 (n = 6 or 7). **P < 0.01 (vs. control); ##P < 0.01 (vs. hypoxia).
Hypoxia-Induced Apoptosis and Apoptotic Gene Expression and ET-1 Activation.
Accompanying brain edema and the increased expression of CRF and CRF mRNA, we found significantly increased ET-1 content in the cortex and increased corticosterone in plasma after hypoxia. This increase was also blocked by pretreatment with 30 mg/kg CP154,526, by pretreatment with pyrrolidine dithiocarbamate (PDTC; 150 mg/kg), an inhibitor of nuclear factor kappa B (NF-κB), and by pretreatment with a combination of both (Fig. 2 A and B).
We went on to test whether this effect also reflected a local, direct effect of CRF on cortical astrocytes. In cultured astrocytes, 30-min exposure to CRF induced an increased expression of ET-1 mRNA, and the increase of ET-1 mRNA was blocked in the presence of CP154,526. After 8-h exposure to CRF, there was significantly increased ET-1 protein expression, and again this increase of ET-1 protein was blocked by CP154,526 (100 nM; P = 0.08; Fig. 2 C and D).
Hypoxia produced pyramidal cell swelling, enlargement of the intercellular space, and perivascular vacuoles as shown in H&E-stained sections. Neuronal apoptosis was evident from increased TUNEL labeling (Fig. 2 E and F), and increased mRNA expression of the apoptotic genes BCL2-associated X (Bax) and Bcl-2-interacting mediator of cell death (Bim) was demonstrated by real-time PCR (Fig. 2G). All of these effects were blocked by CP154,526, indicating that CRFR1 not only mediates cerebral edema, but also activates apoptotic genes and neuronal apoptosis.
CRFR1 Signaling-Involved AQP4 Phosphorylation and Expression.
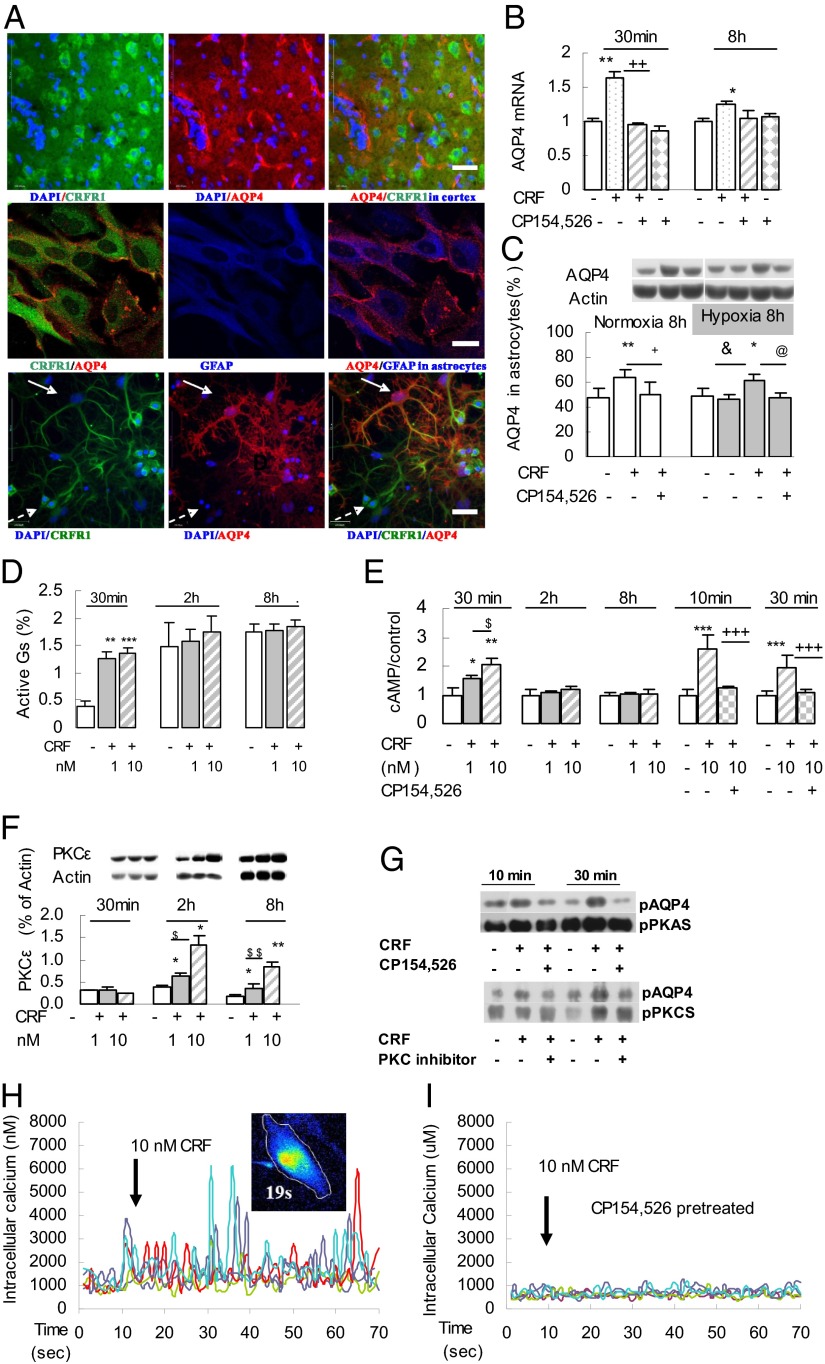
Using laser-scanning confocal microscopy and triple-labeling immunofluorescence, we identified both CRFR1 and AQP4 expression in the frontal cortex and in cultured purified primary astrocytes, as well as in cultured nonpurified mixed astrocytes (Fig. 3A). As shown by PCR, cortical neurons expressed CRFR1, but not AQP4, whereas astrocytes expressed both (Fig. S1A), suggesting that an interaction between CRFR1 and AQP4 involves cerebral astrocytes.
Fig. 3.
CRFR1-activated signaling pathways induced phosphorylation and transcription of AQP4 in primary cerebral astrocytes. (A) Confocal micrographs showing coexistence of AQP4 and CRFR1 in rat cerebral cortex sections (Top), cultured astrocytes (Middle), and mixed cultures of hippocampal neurons and astrocytes (Bottom). The white arrow indicates an astrocyte that expresses both CRFR1 and AQP4; the dashed white arrow indicates a neuron that expresses CRFR1 but not AQP4. [Scale bars: 100 μm (Top and Bottom); 40 μm (Middle).] (B) Exposure to CRF (10 nM for 30 min or 8 h) increased AQP4 mRNA expression in cultured astrocytes, and this increase was blocked by CP154,526. (C) CRF (10 nM for 8 h) enhanced AQP4 protein expression in astrocytes in both normoxic and hypoxic conditions that was blocked by CP154,526 (100 nM) (n = 3). *P < 0.05; **P < 0.01 (CRF vs. control); +P < 0.05; ++P < 0.05 (CRF vs. CRF antagonist); &P < 0.05 (1.0% O2 vs. CRF + 1.0% O2); @P < 0.05 (CRF + 1.0% O2 vs. CRF + 1.0% O2 + CRFR1 antagonist). (D and E) Active Gs (co-IP) and cAMP significantly increased after incubation of primary astrocytes with 1 or 10 nM CRF (30 min); CRF-induced cAMP accumulation was blocked by pretreatment with 100 nM CP154,526 (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001 (CRF vs. control); $P < 0.05 (1 nM CRF vs. 10 nM CRF); +++P < 0.001 (10 nM CRF vs. 10 nM CRF + 100 nM CP154,526). (F) CRF (1 or 10 nM) induced increased expression of PKCε isoforms after 2- or 8-h incubation (n = 3). *P < 0.05; **P < 0.01 (CRF vs. control); $P < 0.05; $$P < 0.01 (CRF 10 nM vs. CRF 1 nM). (G) CRF (10 nM for 10 and 30 min) activated CRFR1-targeted AQP4 phosphorylation by PKA and PKC signaling, as shown by co-IP, and this pAQP4 was reduced by CP154,526 (100 nM; n = 3). (H and I) CRF (10 nM) activated [Ca2+]i oscillations in cultured primary astrocytes (n = 5). (H) Arrows indicate CRF added in Ca2+-free medium. (I) CRF-induced oscillations in [Ca2+]i were blocked by preincubation with CP154,526 (100 nM) in Ca2+-free medium for 30 min.
To clarify how CRFR1 signaling results in changed permeability and enhanced water influx into astrocytes, we investigated the activation of intracellular signaling pathways in cultured astrocytes in response to CRF challenge under normoxic (21% O2, 74% N, 5% CO2) and hypoxic (1% O2, 94% N, 5% CO2) conditions. Phosphorylation sites on the C-terminal of AQP4 for protein kinase A (PKA), PKC, and calcium/calmodulin-dependent protein kinase II (CaMKII) binding are all known to contribute to the action of AQP4 in inducing brain edema (24).
In cultured astrocytes, exposure to 10 nM CRF for 30 min increased AQP4 mRNA expression, and this increase could be blocked by CP154,526 (Fig. 3B). After 8-h exposure, the expression of AQP4 protein was also significantly increased, and the increase of AQP4 protein was completely blocked by pretreatment with CP154,526 in both normoxic or hypoxic conditions (Fig. 3C).
Exposure to CRF (1 or 10 nM) for 30 min activated Gs expression (Fig. 3D) and increased cAMP concentrations, and the cAMP increase was blocked by preincubation with CP154,526 (Fig. 3E). Expression of the PKC-ε isoform was enhanced after exposure to CRF for 2 or 8 h (Fig. 3F), but that of PKC-βII or PKC-γ was not changed. Finally, CRF triggered the phosphorylation of AQP4 protein at the PKA and PKC binding sites, and the AQP4 phosphorylation, too, could be blocked by CP154,526 (Fig. 3G).
Exposure of cultured primary astrocytes to CRF resulted in intracellular Ca2+ ([Ca2+]i) oscillations observed with confocal microscopy. The oscillations reached a peak within several seconds, thereafter gradually dropping to basal level by 50 s (Fig. S1B). The CRF-triggered oscillations could be observed in Ca2+-free medium (Fig. 3H), as well as in medium with 1.25 mmol/L Ca2+ (Fig. S1C), indicating that they arose from mobilization of intracellular stores. The oscillations were abolished by preincubation with CP154,526 (100 nM) 30 min before CRF loading (Fig. 3I). The [Ca2+]i oscillations depended on CRF concentration and were reduced by 100 nM CRF (Fig. S1D).
HIF-1α Is Not Involved in Hypoxia-Induced Astrocyte Swelling.
We then tested whether cellular hypoxia activates the inducible nitric oxide synthase (iNOS)–NO–PKG signaling pathway (25) through hypoxia inducible factor 1α (HIF-1α) to regulate AQP4 in cortical astrocytes. Hypoxia (1% O2, 30 min) increased HIF-1α mRNA expression in purified astrocytes (Fig. S2A) but had no effect on iNOS mRNA expression or on PKGI (∼85 kDa), PKGII (∼75 kDa) (Fig. S2C), or phosphorylated AQP4 (Fig. S2D) in these cells, and a PKG inhibitor had no significant effect (Fig. S2C). By contrast, in nonpurified astrocytes, hypoxia significantly increased iNOS mRNA expression (Fig. S2B); Thus, hypoxia-activated HIF-1α did not directly contribute to the activation of iNOS, PKG, and AQP4 in cultured purified astrocytes, but it contributed to the up-regulation of iNOS in mixed cultures, potentially via the activation of microglia (26). Acute hypoxia induces hypocapnia (27, 28), which causes brain injury (29); NO production in cerebral endothelial cells is decreased, but unchanged, in astrocytes during hypocapnia independent of pH (30), suggesting that endothelial cells may also play a role in modulating NOS activity.
CRF-Induced Cell Swelling in Transgenic CHO Cells Transfected with CRFR1+ and AQP4+.
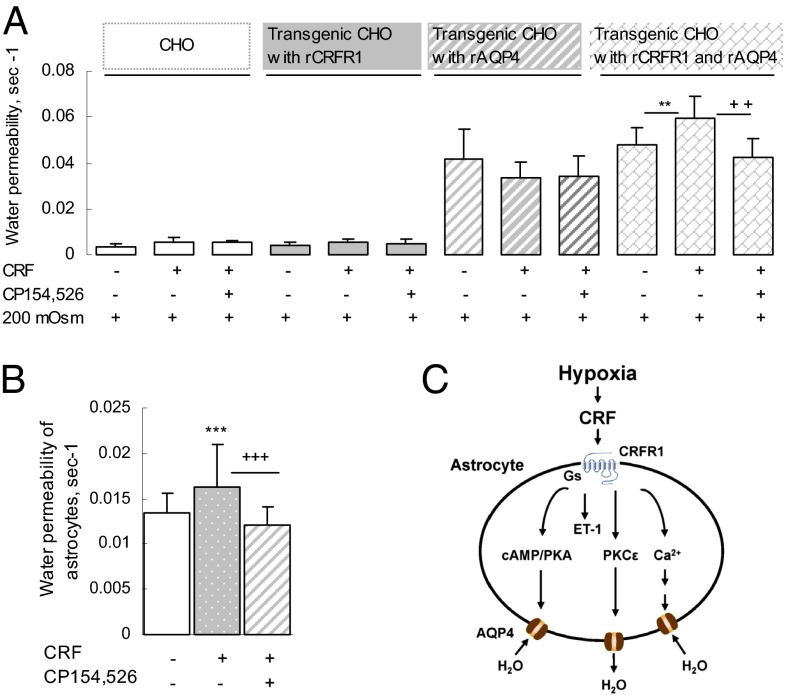
To confirm that CRFR1 signaling activates AQP4 and thereby induces cell swelling, we established a transgenic CHO cell model. CHO cells were transfected with rCRFR1+ (rat CRFR1+) and/or rAQP4+ (rat AQP4+) to generate CHO–rCRFR1−/rAQP4−, CHO–rCRFR1+/rAQP4−, CHO–rCRFR1−/rAQP4+, and CHO–rCRFR1+/rAQP4+ cells. We identified these cells by quantitative real-time PCR and assessed their swelling in response to switching the culture medium from 300 mOsm PBS to hypoosmotic (200 mOsm) PBS, with or without CRF (10 nM) and CP154,526.
The CHO–rCRFR1+/rAQP4+ cells were significantly more swollen than the CHO–rCRFR1−/rAQP4+ cells when challenged by CRF (10 nM), and this difference was completely abolished by CP154,526 (Fig. 4A). Thus, the transfected rCRFR1 plays a stimulatory role in activating the transfected rAQP4, leading to enhanced water permeability of the CHO–rCRFR1+/rAQP4+cells. Exposure to CRF (10 nM) immediately increased the water permeability of cultured primary cortical astrocytes, and the water permeability was fully abolished by preincubation with CP154,526 (100 nM) (Fig. 4B) or 5-chloro-4-[N-(cyclopropyl)methyl-N-propylamino]-2-methyl-6-(2,4,6-trichlorophenyl) aminopyridine (NBI; 100 nM; another CRFR1 antagonist) for 30 min (Fig. S3B).
Fig. 4.
Activation of CRFR1+ by CRF induces cell swelling in transgenic CHO cells transfected with CRFR1+ and AQP4+. (A) In CHO cells transfected with either CRFR1+ or AQP4+ alone, CRF (10 nM) had no effect on the rate of swelling induced by exposure to hypoosmotic medium (200 mOsm). By contrast, CRF increased the rate of induced swelling of CHO cells that had been transfected with both CRFR1+ and AQP4+, and this effect was blocked by CP154,526 (100 nM; n = 50–60 cells). **P < 0.01 (CRF treatment vs. control); ++P < 0.01 [CRF treatment vs. CRF treatment + CRFR1 antagonist in hypoosmotic medium (200 mOsm)]. (B) CRF (10 nM) significantly increased water permeability through AQP4 in cultured rat primary astrocytes, and the increase of water permeability was blocked by CP154,526 (n = 33–35 cells). ***P < 0.001 (CRF vs. control); +++P < 0.001 (CRF vs. CRF + CRFR1 antagonist). (C) Model illustrating how hypoxia induces cerebral edema: Hypoxia stimulates CRF release that activates CRFR1 to initiate a signaling cascade that activates AQP4 and causes cell swelling.
CRFR1-Activated ET-1 Expression in Astrocytes.
In cultured cortical astrocytes, CRF stimulated an increase in ET-1 mRNA expression and AQP4 mRNA expression by 30 min and up-regulated ET-1 protein by 8 h; all of these effects were blocked by NBI (Fig. S3 B and C). Thus, CRFR1 mediates the observed increases in ET-1 and ET-mRNA expression as well as the increase in AQP4 mRNA expression.
Discussion
Overview.
Here, we showed that exposure to 7.8% O2 (equivalent to 7,000 m altitude) for 8 h induced cerebral edema and neuronal apoptosis, apoptotic gene expression in rats that depend upon CRFR1 activation, because it was blocked by a CRFR1 antagonist. This hypoxia-induced edema is consistent with clinical reports that high-altitude hypoxia may induce human brain edema. We suggest that there may be a “window of opportunity” for therapy or prevention of hypoxia-induced cerebral edema and apoptosis.
CRFR1 Involved in Brain Edema, Apoptosis, and ET-1 Expression.
ET-1 receptors (A and B) are widely expressed in the endothelial cells of the cerebral microvasculature as well as in populations of neurons, astrocytes and microglia, and ET-1 is thought to mediate interactions between astrocytes and the cerebral microvasculature (31, 32) that have an important role in ischemia or stroke-induced brain edema and injury (31). Ischemia induces up-regulation of expression of ET-1/3 and its receptors-A/B in the rat cortex (33), and activation of ET-1 by ischemia induces brain edema via AQP4 in rat astrocytic end-feet (34). In the present study, hypobaric hypoxia-activated cortical ET-1 is also involved in cerebral edema and apoptosis through activation of both CRFR1 and NF-κB, suggesting that antagonists for CRFR1 and ET-1R might be appropriate for treating hypoxia-induced disorders.
We found that hypoxia up-regulates ET-1 and induces apoptosis in the cortex and that CRF stimulates ET-1 expression in cultured cerebral astrocytes. All of these effects could be blocked by treatment with a CRFR1 antagonist, suggesting that CRF and its receptor CRFR1 have a key role in hypoxic cerebral edema and apoptosis. In humans, ET-1 levels >5.5 fmol/mL in serum are linked to severe brain edema in acute stroke patients (35). ET-1A plays a protective role during brain edema (36), but ET-1B receptors also regulate astrocytic function (31); in an experimental rat model of stroke, an ET-1B antagonist could reduce brain edema, serum ET-1, and astrocyte swelling (37). Recently, ET-1 has been shown to promote NO production and astrocyte migration by activation of ET-1B and NF-κB, inducing iNOS (38). Importantly, hypoxia elicited a parallel increase of both ET-1 and AQP4 expression in rat cortex, which depended on the activation of both CRFR1 and the NF-κB pathway.
CRFR1 Involved in Brain Edema and AQP4 Expression.
AQP4, the most abundant water channel in the brain, is expressed in astrocytes, particularly in the end feet, and promotes water clearance from swelling cells (39). Increased expression of AQP4 in the brain is associated with cerebral edema and related neurological disorders (40–43), and AQP4-deficient mice survive better than wild-type mice in a model of brain edema (41).
AQP4 activity is regulated in the short-term by phosphorylation, trafficking, binding with inhibitor, and protein–protein interactions, and in the long-term by changes in mRNA expression, protein synthesis, and degradation (24). We found that 8-h hypoxia (7.8% O2) up-regulated AQP4 protein and AQP4 mRNA expression in rat cortex, along with increasing expression of CRF, CRF mRNA, and CRFR1 mRNA. By contrast, hypoxia (1% O2) or 10 nM CRF challenge for 30 min did not up-regulate AQP4 expression in cultured primary astrocytes, but both triggered AQP4 phosphorylation, leading to enhancement of permeability and astrocyte swelling. Thus, in the cerebral cortex, locally released CRF acts via CRFR1 expressed on astrocytes to activate AQP4, which then contributes to cerebral edema.
Hypobaric hypoxia-induced brain edema, including CRFR1-mediated cytotoxic astrocyte edema and vasogenic edema, suggested an integrative working model for hypoxia-induced cerebral edema, because CRFR1 and AQP4 are expressed in astrocytes and cerebral microvessel endothelial cells (Fig. 4C). A review by Verkman indicates that both cytotoxic and vasogenic brain edema can occur independently or together (44).
CRFR1 Signaling Up-Regulated AQP4.
In cultured primary astrocytes, CRF challenge elicited up-regulation of Gs, cAMP/PKA, and PKCε, and triggered [Ca2+]i oscillations, which could be abolished by a CRFR1 antagonist (Fig. 3 D–I). These effects resulted in the activation of phosphorylated AQP4, as confirmed by coimmunoprecipitation (co-IP), resulting in astrocyte swelling. Activation of CRFR1 also triggered [Ca2+]i oscillations in cultured astrocytes (Fig. 3 H and I), which may contribute to phosphorylation of AQP4 via PKG, because PKG produces phosphorylated AQP4 (pAQP4) when NO is provided (45).
It has been reported that AQP4 induces Ca2+ spike signaling through activation of P2 purinergic receptors in a model of cortical astrocytic edema induced by hypoosmotic stress (46). However, in cultured primary astrocytes challenged by either CRF or hypoxia, we found no activation of PKGI, PKGII, iNOS, or pAQP4, although HIF-1α was up-regulated (Fig. S2A). iNOS expression was up-regulated in mixed cultures that contained both astrocytes and microglia (Fig. S2B). Because CRF can stimulate NO production in cultured rat microglia (26), PKG may contribute to astrocyte swelling via AQP4 in the presence of CRF-evoked NO release from microglia.
CRF binding to CRFR1 can induce PKC signaling, but at least 12 isoforms of PKC have been described (47, 48). We found that PKC-ε, but not PKC-βII or PKC-γ, is up-regulated in cultured primary astrocytes after CRF challenge (Fig. 3F); notably, this PKC-ε up-regulation occurred at 2 and 8 h after the onset of CRF stimulation but not after 30 min. By contrast, phosphorylated PKC-bound AQP4 was detectable after 30-min exposure to CRF, and this pAQP4 level was partly reduced by the CRFR1 antagonist (Fig. 3G). Thus, we suggest that, in the early stage of hypoxia, AQP4 is phosphorylated by cAMP/PKA, producing acute astrocyte swelling, but subsequently is phosphorylated by PKC-ε to reverse water influx. This pAQP4 by PKC-ε is consistent with studies showing that activation of PKC can protect cortical neurons and astrocytes from ischemia-like injury, hypoxia, and glucose deprivation (49).
Conclusions
Our results demonstrate that hypoxia induces rat brain edema and apoptosis (Fig. 4C). These effects involve activation of CRF and CRFR1 mRNA, CRFR1, which signals through PKA, PKC-ε, and [Ca2+]i oscillations in cerebral astrocytes. Phosphorylation of AQP4 by PKA contributes to astrocytic edema at the early stage and phosphorylation by PKC-ε to achieve restoration at the later stage. The activated [Ca2+]i does not directly induce edema through PKG in astrocytes, but may do so in the intact brain in the presence of microglia. Hypoxia-activated CRFR1 and ET-1, modulated by CRFR1 and NF-κB, contribute to the activation of apoptosis and apoptotic genes.
Materials and Methods
Adult male Sprague–Dawley rats (300 ± 20 g) in healthy, clean-grade condition (certification no. SCXK2008-0033) were purchased from the Experimental Animal Center of Zhejiang Province (China) and maintained under a 12/12-h light/dark cycle (lights on 06:00–18:00 h) at room temperature (20 ± 2 °C), with free access to food and water. Rats were housed in groups of six and adapted to these conditions for 1 wk before experimental manipulation. All animal procedures were performed according to National Institutes of Health guidelines under protocols approved by the Institutional Animal Care and Use Committee of Zhejiang University. The methods used in this study (animal breeding, MRI, confocal imaging, measurement of [Ca2+]i and signal imaging, cell culture, plasmid construction, transient transfection, quantitative real-time RT-PCR, Western blotting, and osmotic water permeability measurements) are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Profs. I. C. Bruce (Department of Physiology, School of Medicine, Zhejiang University) and Rajiv R. Ratan (Weill Medical College, Cornell University) for their critical reading of the manuscript. This work was supported by National Basic Research Program (973) of the Ministry of Science and Technology of China Grants 2012CB518200 and 2006CB504100.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404493111/-/DCSupplemental.
References
- 1.Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: Regional and life-cycle perspectives. Am J Phys Anthropol. 1998;(Suppl 27):25–64. doi: 10.1002/(sici)1096-8644(1998)107:27+<25::aid-ajpa3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Wilson MH, Newman S, Imray CH. The cerebral effects of ascent to high altitudes. Lancet Neurol. 2009;8(2):175–191. doi: 10.1016/S1474-4422(09)70014-6. [DOI] [PubMed] [Google Scholar]
- 3.Basnyat B, Murdoch DR. High-altitude illness. Lancet. 2003;361(9373):1967–1974. doi: 10.1016/S0140-6736(03)13591-X. [DOI] [PubMed] [Google Scholar]
- 4.Hackett PH, Roach RC. High altitude cerebral edema. High Alt Med Biol. 2004;5(2):136–146. doi: 10.1089/1527029041352054. [DOI] [PubMed] [Google Scholar]
- 5.Firth PG, et al. Mortality on Mount Everest, 1921-2006: Descriptive study. BMJ. 2008;337:a2654. doi: 10.1136/bmj.a2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu T, et al. Ataxia: An early indicator in high altitude cerebral edema. High Alt Med Biol. 2006;7(4):275–280. doi: 10.1089/ham.2006.7.275. [DOI] [PubMed] [Google Scholar]
- 7.Roach RC, Hackett PH. Frontiers of hypoxia research: Acute mountain sickness. J Exp Biol. 2001;204(Pt 18):3161–3170. doi: 10.1242/jeb.204.18.3161. [DOI] [PubMed] [Google Scholar]
- 8.Mórocz IA, et al. Volumetric quantification of brain swelling after hypobaric hypoxia exposure. Exp Neurol. 2001;168(1):96–104. doi: 10.1006/exnr.2000.7596. [DOI] [PubMed] [Google Scholar]
- 9.Kallenberg K, et al. Magnetic resonance imaging evidence of cytotoxic cerebral edema in acute mountain sickness. J Cereb Blood Flow Metab. 2007;27(5):1064–1071. doi: 10.1038/sj.jcbfm.9600404. [DOI] [PubMed] [Google Scholar]
- 10.Schoonman GG, et al. Hypoxia-induced acute mountain sickness is associated with intracellular cerebral edema: A 3 T magnetic resonance imaging study. J Cereb Blood Flow Metab. 2008;28(1):198–206. doi: 10.1038/sj.jcbfm.9600513. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Du JZ. Hypoxia effects on hypothalamic corticotropin-releasing hormone and anterior pituitary cAMP. Zhongguo Yao Li Xue Bao. 1996;17(6):489–492. [PubMed] [Google Scholar]
- 12.Xu JF, Chen XQ, Du JZ, Wang TY. CRF receptor type 1 mediates continual hypoxia-induced CRF peptide and CRF mRNA expression increase in hypothalamic PVN of rats. Peptides. 2005;26(4):639–646. doi: 10.1016/j.peptides.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Chen XQ, Kong FP, Zhao Y, Du JZ. [High-altitude hypoxia induces disorders of the brain-endocrine-immune network through activation of corticotropin-releasing factor and its type-1 receptors] Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2012;28(6):481–487. [PubMed] [Google Scholar]
- 14.He JJ, Chen XQ, Wang L, Xu JF, Du JZ. Corticotropin-releasing hormone receptor 1 coexists with endothelin-1 and modulates its mRNA expression and release in rat paraventricular nucleus during hypoxia. Neuroscience. 2008;152(4):1006–1014. doi: 10.1016/j.neuroscience.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 15.Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11(3):585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potter E, et al. The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci USA. 1992;89(9):4192–4196. doi: 10.1073/pnas.89.9.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen S, et al. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17(1):171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: Yet more partners discovered. Trends Pharmacol Sci. 2002;23(2):71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- 19.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 20.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: Implications for physiology and pathophysiology. Endocr Rev. 2006;27(3):260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- 21.Hauger RL, et al. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003;55(1):21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 22.Kapcala LP, Dicke JA. Brain corticotropin-releasing hormone receptors on neurons and astrocytes. Brain Res. 1992;589(1):143–148. doi: 10.1016/0006-8993(92)91174-d. [DOI] [PubMed] [Google Scholar]
- 23.Woods DR, et al. The cortisol response to hypobaric hypoxia at rest and post-exercise. Horm Metab Res. 2012;44(4):302–305. doi: 10.1055/s-0032-1304322. [DOI] [PubMed] [Google Scholar]
- 24.Gunnarson E, Zelenina M, Aperia A. Regulation of brain aquaporins. Neuroscience. 2004;129(4):947–955. doi: 10.1016/j.neuroscience.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 25.Murad F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling. Biosci Rep. 1999;19(3):133–154. doi: 10.1023/a:1020265417394. [DOI] [PubMed] [Google Scholar]
- 26.Bi YH, Song TT, Chen XQ, Du JZ. [Corticotropin-releasing hormone modulates NO production, TNF-alpha and IL-6 release in rat primary microglia] Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2013;29(4):323–325. [PubMed] [Google Scholar]
- 27.Jacobson L, Dallman MF. ACTH secretion and ventilation increase at similar arterial PO2 in conscious rats. J Appl Physiol (1985) 1989;66(5):2245–2250. doi: 10.1152/jappl.1989.66.5.2245. [DOI] [PubMed] [Google Scholar]
- 28.Du JZ, Li QF. Effect of simulated hypoxic acclimation on organism, organ and hematology in Ochotoniae curzoniae and rats. Acta Theriologica Sinica. 1982;2(1):35–42. [Google Scholar]
- 29.Fathi AR, et al. Carbon dioxide influence on nitric oxide production in endothelial cells and astrocytes: Cellular mechanisms. Brain Res. 2011;1386:50–57. doi: 10.1016/j.brainres.2011.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curley G, Kavanagh BP, Laffey JG. Hypocapnia and the injured brain: More harm than benefit. Crit Care Med. 2010;38(5):1348–1359. doi: 10.1097/CCM.0b013e3181d8cf2b. [DOI] [PubMed] [Google Scholar]
- 31.Koyama Y, Michinaga S. Regulations of astrocytic functions by endothelins: Roles in the pathophysiological responses of damaged brains. J Pharmacol Sci. 2012;118(4):401–407. doi: 10.1254/jphs.11r13cp. [DOI] [PubMed] [Google Scholar]
- 32.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10(11):1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 33.Li JJ, et al. Endothelins-1/3 and endothelin-A/B receptors expressing glial cells with special reference to activated microglia in experimentally induced cerebral ischemia in the adult rats. Neuroscience. 2010;167(3):665–677. doi: 10.1016/j.neuroscience.2010.02.062. [DOI] [PubMed] [Google Scholar]
- 34.Lo AC, et al. Endothelin-1 overexpression leads to further water accumulation and brain edema after middle cerebral artery occlusion via aquaporin 4 expression in astrocytic end-feet. J Cereb Blood Flow Metab. 2005;25(8):998–1011. doi: 10.1038/sj.jcbfm.9600108. [DOI] [PubMed] [Google Scholar]
- 35.Moldes O, et al. High serum levels of endothelin-1 predict severe cerebral edema in patients with acute ischemic stroke treated with t-PA. Stroke. 2008;39(7):2006–2010. doi: 10.1161/STROKEAHA.107.495044. [DOI] [PubMed] [Google Scholar]
- 36.Matsuo Y, Mihara Si, Ninomiya M, Fujimoto M. Protective effect of endothelin type A receptor antagonist on brain edema and injury after transient middle cerebral artery occlusion in rats. Stroke. 2001;32(9):2143–2148. doi: 10.1161/hs0901.94259. [DOI] [PubMed] [Google Scholar]
- 37.Moldes O, et al. Neuroprotection afforded by antagonists of endothelin-1 receptors in experimental stroke. Neuropharmacology. 2012;63(8):1279–1285. doi: 10.1016/j.neuropharm.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 38.Wang HH, Hsieh HL, Yang CM. Nitric oxide production by endothelin-1 enhances astrocytic migration via the tyrosine nitration of matrix metalloproteinase-9. J Cell Physiol. 2011;226(9):2244–2256. doi: 10.1002/jcp.22560. [DOI] [PubMed] [Google Scholar]
- 39.Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18(11):1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- 40.Jung JS, et al. Molecular characterization of an aquaporin cDNA from brain: Candidate osmoreceptor and regulator of water balance. Proc Natl Acad Sci USA. 1994;91(26):13052–13056. doi: 10.1073/pnas.91.26.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manley GT, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6(2):159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 42.Mao X, Enno TL, Del Bigio MR. Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur J Neurosci. 2006;23(11):2929–2936. doi: 10.1111/j.1460-9568.2006.04829.x. [DOI] [PubMed] [Google Scholar]
- 43.Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry. 2002;72(2):262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verkman AS. Aquaporins in clinical medicine. Annu Rev Med. 2012;63:303–316. doi: 10.1146/annurev-med-043010-193843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunnarson E, et al. Identification of a molecular target for glutamate regulation of astrocyte water permeability. Glia. 2008;56(6):587–596. doi: 10.1002/glia.20627. [DOI] [PubMed] [Google Scholar]
- 46.Thrane AS, et al. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc Natl Acad Sci USA. 2011;108(2):846–851. doi: 10.1073/pnas.1015217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krotova KY, Zharikov SI, Block ER. Classical isoforms of PKC as regulators of CAT-1 transporter activity in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;284(6):L1037–L1044. doi: 10.1152/ajplung.00308.2002. [DOI] [PubMed] [Google Scholar]
- 48.Breitkreutz D, Braiman-Wiksman L, Daum N, Denning MF, Tennenbaum T. Protein kinase C family: On the crossroads of cell signaling in skin and tumor epithelium. J Cancer Res Clin Oncol. 2007;133(11):793–808. doi: 10.1007/s00432-007-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Bright R, Mochly-Rosen D, Giffard RG. Cell-specific role for epsilon- and betaI-protein kinase C isozymes in protecting cortical neurons and astrocytes from ischemia-like injury. Neuropharmacology. 2004;47(1):136–145. doi: 10.1016/j.neuropharm.2004.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.