Significance
In recent years, there has been a vigorous debate among ecologists over the merits of two contrasting models of biodiversity: the niche and neutral theories of ecology. Using two different theoretical models of ecological dynamics, we show that there is a transition between a selection-dominated regime (the niche phase) and a drift-dominated regime (the neutral phase). This is analogous to the phase diagram of water, which can be in the solid, liquid, or gas phase, depending on the temperature and pressure. Our results demonstrate how the niche and neutral theories both emerge from the same underlying ecological principles.
Keywords: neutral theory, disordered systems, phase transitions, niche theory
Abstract
An ongoing debate in ecology concerns the impacts of ecological drift and selection on community assembly. Here, we show that there is a transition in diverse ecological communities between a selection-dominated regime (the niche phase) and a drift-dominated regime (the neutral phase). Simulations and analytic arguments show that the niche phase is favored in communities with large population sizes and relatively constant environments, whereas the neutral phase is favored in communities with small population sizes and fluctuating environments. Our results demonstrate how apparently neutral populations may arise even in communities inhabited by species with varying traits.
The success of the neutral theory of biodiversity and biogeography (1, 2) at explaining patterns in biodiversity has resulted in a vigorous debate on the processes underlying the assembly, dynamics, and structure of ecological communities (1, 3–12). Starting with the pioneering work of MacArthur (13–15), ecologists have emphasized the roles of interspecific competition and environmental interactions in community assembly and dynamics. These niche-based models emphasize ecological selection as the driving force of community assembly, whereas neutral models of biodiversity assume a functional equivalence between species and emphasize the role of ecological drift (i.e., stochasticity) in community dynamics (1, 2, 16, 17). The success of both types of models at explaining ecological data highlights the crucial need for understanding the impacts of ecological drift and selection in community ecology (18).
Hypothesis
We begin with a hypothesis that a diverse ecological community with many species can be either neutral or nonneutral, depending on the state of its environment. We call the regime in which a community is well described using neutral models the “neutral phase” and the regime in which the community behaviors are inconsistent with neutrality the “niche phase.” The dynamics in the neutral phase are dominated by stochasticity whereas the dynamics in the niche phase are dominated by selection. Our goal in this paper is to demonstrate that these two phases naturally emerge from simple probabilistic models of ecological dynamics and that a community may transition from one phase to the other as its environment is altered (Fig. 1).
Fig. 1.
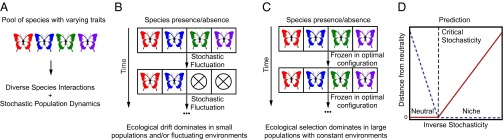
A schematic illustrating the intuition underlying our hypothesis for a phase transition between the neutral and niche regimes in ecology. (A) The important ingredients of our model are a large pool of diverse species, implying a diversity of species interactions, subject to stochastic population dynamics. (B) Stochastic ecological drift will dominate the dynamics of communities with small population sizes and/or fluctuating environments. (C) By contrast, stabilizing selective forces will cause a community with a large population size and a constant environment to freeze into a unique, optimal configuration. (D) We predict that there is a transition between a drift-dominated (neutral) phase and a selection-dominated (niche) phase. That is, the community behaves exactly neutral when the inverse stochasticity is less than a critical threshold, and the deviation from neutrality rises quickly once the inverse stochasticity is larger than the critical threshold. The red line represents an order parameter based on the distance from neutrality, the dashed blue line represents an order parameter based on the niche phase, and the dashed black line denotes the critical stochasticity.
Historically, ecological neutrality is based on the assumption of functional equivalence, which states that trophically similar species are essentially identical in terms of their vital characteristics, such as birth and death rates (19). Ecological neutrality, however, is generally not a measurable feature of a community. Therefore, we adopt a pragmatic definition of neutrality: We say that a community is “statistically neutral” if its multivariate distribution of species abundances cannot be distinguished from a distribution constructed under the assumption of ecological neutrality. In other words, the multivariate species abundance distributions of statistically neutral communities are indistinguishable from those of communities of functionally identical species. Note that ecological neutrality implies statistical neutrality, but statistical neutrality does not necessarily imply ecological neutrality. We can now restate our hypothesis more precisely: As the characteristics of an ecosystem change (e.g., carrying capacity, immigration rate), there will be a transition between a neutral phase where the ecosystem behaves as if it is effectively neutral and a niche phase where the multivariate species abundance distribution is inconsistent with statistical neutrality.
Background on Phase Transitions in Disordered Systems
Our hypothesis that ecological systems are likely to exhibit multiple phases is based on an analogy with disordered systems in physics. For this reason, we briefly provide some background on phase transitions in disordered systems. A phase transition refers to an abrupt change in the qualitative behavior of a system as one of its characteristics, or a characteristic of its environment, is altered (20). The most well-known example may be the behavior of water, which can be found as a solid, liquid, or gas depending on temperature and pressure. Disordered systems often display a more complicated type of phase transition, labeled the freezing transition, where the system configuration gets “frozen” into a particular state.
One illustrative example of a disordered physical system is a protein (21). A protein can be thought of as a disordered system in which the different amino acids along the protein chain interact heterogeneously. The diversity of interactions in a protein distinguishes natural proteins from homopolymers and is what allows some proteins to fold to a stable native structure, while causing others (like prions or amyloids) to misfold. In ecology, this is analogous to the observation that the diversity of interactions between the species in a community distinguishes niche-like communities from neutral communities. To continue our analogy, at high temperatures, a typical polypeptide sequence will be in an unfolded phase where it samples different configurations randomly. If the temperature is lowered below a critical value, the polypeptide will freeze into a single structure (the folded state). This phase transition occurs when the stochasticity impacting the dynamics (i.e., the temperature) is smaller than the energetic differences in the interactions of the amino acids along the chain. If we take this analogy seriously, we should expect to find a critical amount of stochasticity, compared with the diversity of species traits, that separates neutral and niche communities.
Theoretical Models for Studying Community Assembly
To test our hypothesis regarding the niche-to-neutral transition, we analyzed two models of ecological dynamics: (i) a generalized Lotka–Volterra (LV) system including immigration and stochasticity and (ii) a binary model for the presence/absence (PA) of the species in a community. Each of these models has advantages and disadvantages. The LV model is a widely used and interpretable model of many ecological phenomena. However, in general, it is intractable to perform analytic calculations using the LV model and one must rely on numerical simulations. In contrast to the LV system, the PA model is amenable to analytical arguments but this comes at the expense of ignoring species abundances. Both models assume well-mixed populations, although relaxing this assumption is an important avenue of future research. These two models correspond to extreme cases of functional responses (22, 23). The functional response in the PA model is essentially a step function in which species interact only when their abundances are above a threshold. By contrast, the LV model corresponds to linear functional responses. We expect that real communities lie somewhere in between these models.
Parameterizing Ecosystem Characteristics
To construct ecological phase diagrams, it is necessary to parameterize ecosystem characteristics. Because we are interested in stochastic community assembly, we must introduce parameters that reflect the impact of stochasticity as well as parameters that capture variation in species traits. Due to the similarity of the two models, we use the same symbols for analogous parameters with an added tilde for parameters in the PA model (e.g., K denotes carrying capacity in the LV model and denotes carrying capacity in the PA model).
There are two potential sources of stochasticity in the ecological dynamics: “demographic stochasticity” resulting from random births and deaths in small populations and “environmental stochasticity” caused by random variations in the environmental conditions. Although there is no doubt that the origin of the stochasticity is important for making quantitative ecological predictions (24), extensive numerical simulations suggest that the qualitative phase diagrams are insensitive to these details (SI Appendix). For this reason, we parameterize the amount of stochasticity by a single parameter, the noise strength ω .
We must also introduce parameters describing species traits. In principle, each species in the community has a unique immigration rate, a unique carrying capacity, and some set of parameters that describes how it interacts with other species. In the main text, we restrict ourselves to the case where all species have the same immigration rate, λ , and the same carrying capacity K (see SI Appendix for relaxation of these assumptions). We assume that the regional species pool is large so that there is a separation of timescales between the dynamics of the local community and those of the regional species pool. As a result our model does not explicitly include speciation, even though speciation is ultimately required to maintain diversity over longer timescales relevant to the species pool. Following May’s seminal work (25), we randomly draw symmetric interaction coefficients from a probability distribution and focus on describing the average behavior of ecosystems. Specifically, the interaction matrix C —with element cij characterizing the strength of interaction between species i and j—is drawn from a Gamma distribution with mean μ/S and variance or “interaction diversity”, σ2/S , where S is the number of species (see SI Appendix for results with other distributions).
Stochastic Lotka–Volterra Dynamics
The first model that we analyze is a system of stochastic LV equations including immigration. Niche-based models of community assembly frequently use LV equations as a simplified description of ecological dynamics within a well-mixed community (13, 26–28). Here, we study a system of LV equations incorporating immigration and multiplicative noise (i.e., stochasticity). The rate of change in the abundance (xi) of species i = 1, … , S is
| [1] |
The first term (λ) is the rate that species i immigrates into the local community from an infinitely large regional species pool. The second term (xi(K − xi)) limits the population of species i to its carrying capacity (K) in the absence of immigration and species interactions. The third term describes the effects that other species in the community have on species i according to their interaction coefficients (cij). All of these deterministic terms (i.e., λ, K, and cij) collectively represent the effects of ecological selection on the abundance of species i. Ecological drift is incorporated into our model through the last term , which represents stochasticity using a Gaussian “white noise” ηi(t), with mean 〈ηi(t)〉 = 0, variance 〈ηi(t)ηj(t′)〉 = δijδ(t − t′), and strength .
Dynamics of Presence/Absence Model
In the PA model, a species i is described by a binary variable si with si = 1 if species i is present in a community and si = 0 if it is absent. The stochastic dynamics of species PA are defined by two rates: the rate at which a species immigrates into a community (i.e., the rate that si = 0 becomes si = 1) and the rate at which a species becomes extinct once it is in the community (i.e., the rate that si = 1 becomes si = 0). Thus, a species immigrates into a community and lives there for some time before it dies out, only to reimmigrate back into the community later, and so on. We assume that the rate of immigration is simply , and we model the rate of extinction as . Therefore, in the absence of any interactions a species goes extinct at a rate that is exponentially slow in its carrying capacity , and competitive species interactions effectively decrease carrying capacity through (13). The master equation describing the dynamics of with these rates is discussed in detail in SI Appendix. After an initial transient period, the community reaches a steady state where the immigration and extinction processes are balanced. Due to the simplicity of this model, we can derive an analytic expression for the steady-state probability distribution:
| [2] |
Here, is a normalizing constant such that the total probability sums to one.
Measuring the Neutrality of a Community
To test our hypothesis that communities can exhibit a niche-to-neutral transition, it is necessary to define “order parameters” that distinguish the niche and neutral phases. By convention, an order parameter is chosen so that it is zero in one phase and greater than zero in the other. Recall that the dynamics in the neutral phase are dominated by stochasticity and multivariate species abundance distributions in this phase are indistinguishable from those obtained from a neutral model with functionally equivalent species. By contrast, the niche phase is dominated by interactions and multivariate species abundance distributions are peaked around the equilibrium value they would have in the absence of stochasticity.
Using these intuitions we can define order parameters for both the LV model and the PA model. In the LV model, we define an order parameter that measures the distance (i.e., Kullback–Leibler divergence) between the multivariate species abundance distribution resulting from LV dynamics and the multivariate species abundance distribution resulting from purely neutral dynamics (see below). This order parameter is zero in the neutral phase and nonzero in the niche phase. For the PA model, it is convenient to consider a different order parameter, the Shannon entropy, of the steady-state PA probability distribution. The Shannon entropy is zero in the niche phase and nonzero in the neutral phase. We now discuss both of these order parameters in more detail.
Measuring Neutrality in LV Models.
Early studies attempting to quantify the neutrality of a community focused on the shape of the marginal species abundance distribution, i.e., a histogram indicating the number of species with 10 individuals in the community, the number of species with 20 individuals in the community, and so on. However, recent studies have shown that both and neutral and nonneutral ecological models give rise to similar marginal species distributions (12). For this reason, to measure neutrality in the LV model we use the multivariate species abundance distribution. In contrast to previous studies on marginal species abundance distributions in niche and neutral communities (e.g., ref. 12), using the multivariate species abundance distribution allows us to study the effects of different species interactions on correlations in species abundances.
In particular, we quantify statistical neutrality in our LV simulations by measuring the distance between the steady-state distributions of species abundances obtained from the LV model and purely neutral dynamics . The measure of distance that we use is called the Kullback–Leibler divergence, (29). One interpretation of KL(PLV∥PN) is defined as the amount of information about the true multivariate species abundance distribution [i.e., ] that is lost by approximating the distribution with one obtained from a neutral model [i.e., ]. The KL divergence ranges from zero to infinity, with KL(PLV∥PN) = 0 implying that the simulated distribution is identical to the distribution obtained under the assumption of neutrality. We study the average of the KL divergence over many random realizations of the species interactions, i.e., 〈KL(PLV∥PN)〉. We expect 〈KL(PLV∥PN)〉 ∼ 0 in the neutral phase, whereas 〈KL(PLV∥PN)〉 ≫ 0 in the niche phase. Similar results are obtained with distance measures other than the KL divergence (SI Appendix).
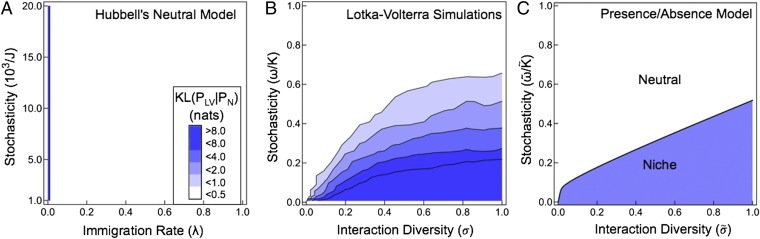
In principle, it is possible to use an explicit formula for from a specific neutral ecological model. However, many variations of neutral ecological models have been proposed and it is unclear which neutral model to use to calculate our order parameter. To circumvent this problem, we exploit the observation that the multivariate species abundance distributions of all neutral models share several features. Because we have restricted ourselves to considering LV systems where all species have the same immigration rate, we also restrict ourselves to considering neutral models where this assumption holds. The implications of nonuniform immigration rates are discussed in SI Appendix. With this caveat in mind, we observe that ecologically neutral models are also statistical neutral. Namely, the time-averaged moments of the abundance of species i are the same as the time-averaged moments of the abundance of species j. Moreover, the correlation in the abundances of species i and j is the same as the correlation in the abundances of species k and l (SI Appendix). Simulations shown in Fig. 2A demonstrate that this is the case, at least for Hubbell’s neutral model (Materials and Methods), where the KL divergence equals zero for all positive immigration rates. Finally, we note that although ecological neutrality implies statistical neutrality, statistical neutrality does not necessarily imply ecological neutrality. Thus, our use of statistical neutrality is consistent with the interpretation of ecological neutrality as a type of null model that allows one to identify communities in which selection is important.
Fig. 2.
Phase diagram of neutral and competitive ecosystems. (A) Communities simulated according to Hubbell’s neutral model are statistically neutral with a KL divergence equal to zero for all positive immigration rates and community sizes (J). (B) Simulations of competitive LV communities with immigration display two phases: a statistically neutral phase with 〈KL(PLV∥PN)〉 ∼ 0 and a niche phase with 〈KL(PLV∥PN)〉 ≫ 0. Note that the colors represent exponential growth in the KL divergence. The critical stochasticity defining the phase boundary scales with interaction diversity (σ). Simulations were performed with μ = 1.0 and λ = 0.01. (C) The phase diagram calculated from the presence/absence model has a statistically neutral phase and a niche phase and a phase boundary that scales with interaction diversity . The phase diagram was calculated with and .
Measuring Neutrality in the Presence/Absence Model.
In the PA model, we do not have access to species abundances. For this reason, it is convenient to define a different order parameter that measures the fluctuations in the binary vector of community composition, : the entropy, , of the steady-state probability distribution . In the absence of stochasticity, will “freeze” into a unique configuration resulting from ecological selection and H[PPA] = 0. In contrast, if the dynamics are entirely random, then each species will randomly flip between being absent and present in the community and H[PPA] = S ln 2. For diverse ecosystems with S ≫ 1, we can define the boundary between the neutral phase and the niche phase as the points where 〈H[PPA]/S〉 = 0, with angular brackets denoting averaging over random realizations of interaction coefficients.
Phase Diagrams for Ecological Dynamics
Phase Diagrams.
Armed with the order parameters discussed in the previous section, we can construct phase diagrams for both the LV and PA models. Fig. 2 shows the KL divergence and entropy as a function of stochasticity and interaction diversity for the two models. First, we note that the phase diagram determined using LV simulations is remarkably similar to the phase diagram calculated using our PA model (compare Fig. 2 B and C), which suggests that our results are fairly robust to model details. Fig. 2B shows that there is a large neutral regime in which 〈KL(PLV∥PN)〉 ∼ 0 in the LV simulations. The distance from neutrality rises once the stochasticity is lowered below a critical value. That is, 〈KL(PLV∥PN)〉 increases for small ω/K; note that the colors in Fig. 2B represent exponential growth in 〈KL(PLV∥PN)〉.
Fig. 2C shows the phase diagram for the PA model. In the limit the number of species S becomes large, and the entropy is strictly zero in the niche phase (blue shaded area) and different from zero in the neutral phase (white area). In particular, we find that the PA of the species in a community freezes into a small number of configurations determined by the species traits if the stochasticity is lowered below a critical value. This freezing is indicative of a phase transition from neutrality to niche-dominated ecological dynamics in the PA model.
It is important to recall that the neutral phase refers to a regime in which the multivariate species abundance distribution is well described by a neutral model, even if the underlying community is not ecologically neutral. For example, one special case that illustrates this relation is a community of species that have become so differentiated that they do not interact at all; i.e., μ = 0 and σ = 0. Because there is no disorder, this community will reside in the statistically neutral phase even though the species are all highly differentiated. Nevertheless, the species in a community must be differentiated for the community to reside in the niche phase.
Scaling Relation for the Niche–Neutral Phase Boundary.
We can explicitly calculate the phase boundary separating the niche and neutral phases, using the PA model. For diverse ecosystems with many species S ≫ 1, the relation defining the phase boundary can be derived by mapping the problem to the random energy model in physics (30, 31) (SI Appendix). Using this mapping we can derive a simple scaling relation that indicates when an ecological community will transition between the niche and neutral phases (SI Appendix):
The niche phase is favored when the interaction diversity is large relative to the impact of stochasticity on the dynamics of the population. By contrast, the neutral phase is favored when the interaction diversity is small relative to the impact of stochasticity on the dynamics of the population. This confirms the basic intuitions about ecological dynamics that were suggested by the analogy with protein folding discussed in the Introduction.
On the Nature of the Transition.
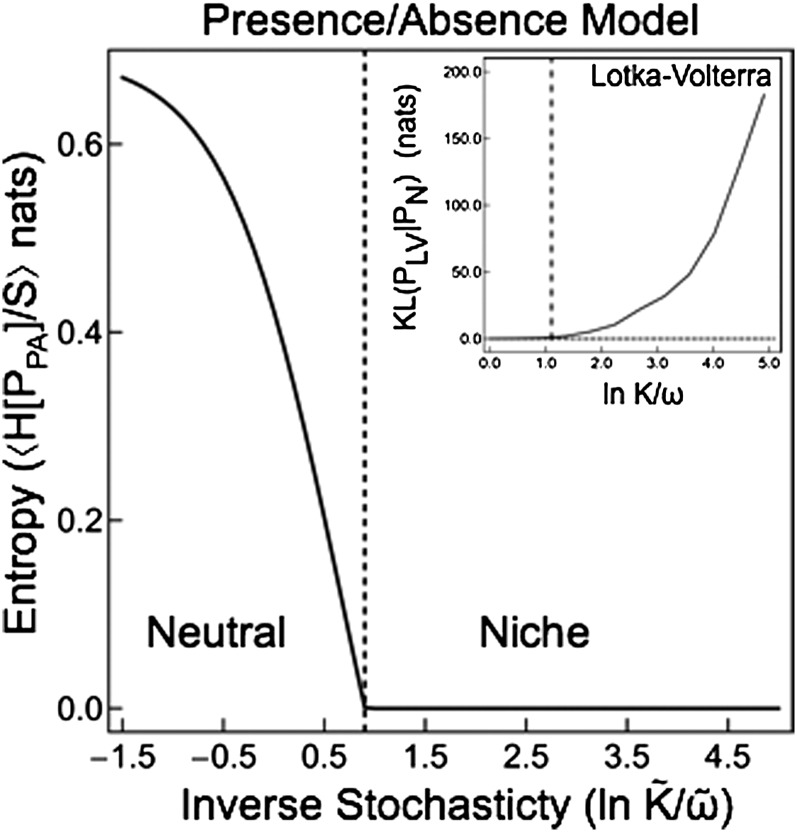
The transition between the niche and neutral phases in the PA model is sharp (Fig. 3). In the LV model the distance from neutrality (〈KL(PLV∥PN)〉) increases when the stochasticity is lowered below the critical value. However, in the PA model, the derivative of the entropy with respect to stochasticity is undefined along the phase boundary, the signature of a freezing phase transition in the theory of disordered systems. Comparing the two models, the niche-to-neutral transition in the PA model appears to be sharper than in the LV model. Technically, a phase transition occurs at a point with an undefined derivative, whereas the term “crossover” is used to describe transitions between qualitative regimes without this feature. Our numerical results do not distinguish whether the transition in the LV model is a crossover or a true phase transition. This difference arises due to the differences in the functional responses of the two models. These two models were chosen, in part, because they represent the two extremes of possible species functional responses (linear vs. step function). We expect the functional responses of real ecological communities lie somewhere in between these two models. For this reason, we expect that real ecological communities will also exhibit a transition between the niche and neutral phases.
Fig. 3.
The nature of the transition between the niche and neutral phases. Note that the order parameters for the two models are different: The order parameter for the PA model is zero in the niche phase and greater than zero in neutral phase, whereas the order parameter for the LV model is greater than zero in the niche phase and zero in the neutral phase. The average entropy 〈H[PPA]/S〉, which is a measure of fluctuations in the community composition, is positive in the neutral phase and zero in the niche phase, illustrating the freezing transition in the PA model. (Inset) In LV simulations the distance from neutrality 〈KL(PLV∥PN)〉 is essentially zero in the neutral phase and rises to large values in the niche phase. Parameters: , , , μ = 1.0, σ ∼ 0.4, λ = 0.01.
Ecological Implications
The Prevalence of Neutral and Niche Communities.
Our model suggests that neutral communities and niche-like communities both correspond to large volumes of the ecological phase diagram. Moreover, our model is such that species always have real differences in traits, but these differences in species traits leave no trace on the equilibrium multivariate species abundance distributions in the statistically neutral regime. This does not preclude the possibility that one could observe the effects of species trait variation on other types of observations. This may explain the success of neutral models at explaining many large-scale patterns in ecology, even though selective forces are well documented, and ubiquitous, on local scales. Furthermore, the crossover region surrounding the phase boundary corresponding to “nearly neutral” communities occupies only a small volume of the phase diagram. As a result, we predict that nearly neutral communities should actually be quite rare, as long as there is not an external force (e.g., group selection) driving communities toward the niche–neutral boundary.
Ecological Disturbances.
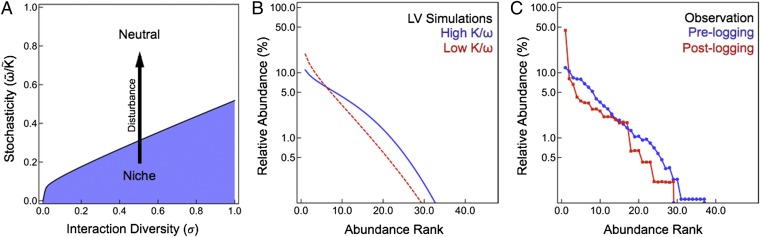
One of our main predictions is that the apparent neutrality of an ecological community is a function of both the inhabiting species and the environment. As a result, it is possible to drive a community between the niche and neutral phases by changing the environmental conditions. As an example, we consider the effects of selective logging on a population of butterflies in a tropical forest on Buru, Indonesia (32). Through habitat destruction, logging essentially moves the butterfly community from a position with high K/ω to one with low K/ω, tracing a path along the stochasticity axis in the phase diagram (Fig. 4A). Our model predicts that when a diverse community within the niche phase is placed under a stress that lowers K/ω to the critical value, it will undergo a transition to the neutral regime. LV simulations show that this transition results in a collapse of biodiversity and leads to an increase in the skewness of the species abundance distribution (SI Appendix). The increase in skewness of the species abundance distribution calculated from LV simulations is evident in a steeper curve in the rank-abundance plot for low K/ω compared with high K/ω (Fig. 4B). Similarly, the observed data display an increase in the skewness of the rank-abundance curve of the logged forest relative to the unlogged forest, consistent with a loss of biodiversity accompanying a niche-to-neutral transition (Fig. 4C). As our model predicts a transition in the shape of the rank-abundance curve as a function of increasing stochasticity (or decreasing carrying capacity), observations of rank-abundance curves as a function of deforestation, i.e., where the amount of deforestation varies continuously, could provide a more stringent test of our model than only the two endpoints discussed here. Nevertheless, this example demonstrates the potential of ecological phase diagrams for predicting the qualitative effects of community-wide disturbances and for capturing the characteristics that contribute to community resilience.
Fig. 4.
Temporal variation in stochasticity and biodiversity in disturbed habitats. (A) An environmental disturbance that decreases carrying capacity may cause a community to shift from the niche phase to the neutral phase. (B) A community with a high carrying capacity (K = 1.0: blue) has a less skewed species abundance distribution than a community with a low carrying capacity (K = 0.1: red), as shown by the steeper red curve in the rank-abundance plot obtained from LV simulations. Simulations were run with μ = 1.0, σ = 0.5, λ = 0.02, and ω = 0.6. (C) Similarly, rank-abundance plots of butterfly species in a tropical Indonesian forest before (blue) and after (red) logging reflect an increase in skewness of the species abundance distribution following the disturbance (32).
Conclusion
In summary, we have argued that the niche and neutral perspectives of ecology naturally emerge from stochastic models for the dynamics of diverse populations as distinct phases of an ecological community. Population dynamics in the niche phase are dominated by ecological selection, whereas population dynamics in the neutral phase are dominated by ecological drift. Furthermore, we have derived a simple scaling relation for determining whether an ecological community will be well described by neutral models.
Our hypothesis can be experimentally tested using synthetic microbial communities in which the immigration rates, carrying capacities, and interaction coefficients can be controlled to search for a transition as one moves from one region of the phase diagram to another (33). Alternatively, connections to island biogeography discussed in SI Appendix suggest that our hypothesis could be tested by calculating the KL divergence from the multivariate species abundance distributions on a chain of islands as a function of their distance to the mainland (34). Observation of a transition in the shape of the rank-abundance curve of a community along a disturbance gradient would also provide evidence of the niche–neutral transition.
In this work, we made some simplifications that are unrealistic for natural ecological communities. For example, we restricted our analysis to well-mixed communities with purely competitive interactions. It will be necessary to generalize our results to include the effects of dispersal, mutualism, predator–prey interactions, etc., to obtain a more quantitative model of natural communities. Nevertheless, we conjecture that the presence of a niche–neutral phase transition is robust to these model perturbations. However, disordered systems with complex interactions display additional phases (35), which suggests that more complex ecological communities may also exhibit additional phases with novel characteristics.
Materials and Methods
We simulated Hubbell’s neutral model with a local community of J individuals connected to an infinitely large metacommunity containing S = 50 equally abundant species. In each time step, with probability λ, an individual randomly drawn from the metacommunity replaced a randomly chosen individual in the local community, or with probability 1 − λ, one randomly chosen individual in the local community replaced another randomly chosen individual in the local community. The simulations were run for 5 × 107 steps. Ten simulations were run for each set of parameters, and the results were averaged.
LV simulations with S = 50 species were performed over the parameter ranges specified in Figs. 1–4. In each case, the competition coefficients were sampled randomly, and then the stochastic Lotka–Volterra equations (Eq. 1) were forward integrated for 5 × 107 steps of size δt = 0.005, using the Milstein method. Ten simulations were run for each set of parameters, and the results were averaged.
Supplementary Material
Acknowledgments
We thank Jeff Gore, Alex Lang, Javad Noorbakhsh, Daniel Segre, and Les Kauffman for useful discussion and the Alfred Sloan Foundation for funding.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1405637111/-/DCSupplemental.
References
- 1.Rosindell J, Hubbell SP, He F, Harmon LJ, Etienne RS. The case for ecological neutral theory. Trends Ecol Evol. 2012;27(4):203–208. doi: 10.1016/j.tree.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography (MPB-32) Princeton: Princeton Univ Press; 2001. [Google Scholar]
- 3.Ricklefs RE, Renner SS. Global correlations in tropical tree species richness and abundance reject neutrality. Science. 2012;335(6067):464–467. doi: 10.1126/science.1215182. [DOI] [PubMed] [Google Scholar]
- 4.Wootton JT. Field parameterization and experimental test of the neutral theory of biodiversity. Nature. 2005;433(7023):309–312. doi: 10.1038/nature03211. [DOI] [PubMed] [Google Scholar]
- 5.McGill BJ. A test of the unified neutral theory of biodiversity. Nature. 2003;422(6934):881–885. doi: 10.1038/nature01583. [DOI] [PubMed] [Google Scholar]
- 6.Ricklefs RE. The unified neutral theory of biodiversity: Do the numbers add up? Ecology. 2006;87(6):1424–1431. doi: 10.1890/0012-9658(2006)87[1424:tuntob]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Dornelas M, Connolly SR, Hughes TP. Coral reef diversity refutes the neutral theory of biodiversity. Nature. 2006;440(7080):80–82. doi: 10.1038/nature04534. [DOI] [PubMed] [Google Scholar]
- 8.Jeraldo P, et al. Quantification of the relative roles of niche and neutral processes in structuring gastrointestinal microbiomes. Proc Natl Acad Sci USA. 2012;109(25):9692–9698. doi: 10.1073/pnas.1206721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tilman D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc Natl Acad Sci USA. 2004;101(30):10854–10861. doi: 10.1073/pnas.0403458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volkov I, Banavar JR, Hubbell SP, Maritan A. Inferring species interactions in tropical forests. Proc Natl Acad Sci USA. 2009;106(33):13854–13859. doi: 10.1073/pnas.0903244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haegeman B, Loreau M. A mathematical synthesis of niche and neutral theories in community ecology. J Theor Biol. 2011;269(1):150–165. doi: 10.1016/j.jtbi.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Chisholm RA, Pacala SW. Niche and neutral models predict asymptotically equivalent species abundance distributions in high-diversity ecological communities. Proc Natl Acad Sci USA. 2010;107(36):15821–15825. doi: 10.1073/pnas.1009387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macarthur R, Levins R. The limiting similarity, convergence, and divergence of coexisting species. Am Nat. 1967;101:377–385. [Google Scholar]
- 14.Chase JM, Leibold MA. Ecological Niches: Linking Classical and Contemporary Approaches. Chicago: Univ of Chicago Press; 2003. [Google Scholar]
- 15.Tilman D. Resource Competition and Community Structure. Vol 17. Princeton: Princeton Univ Press; 1982. [PubMed] [Google Scholar]
- 16.Volkov I, Banavar JR, Hubbell SP, Maritan A. Neutral theory and relative species abundance in ecology. Nature. 2003;424(6952):1035–1037. doi: 10.1038/nature01883. [DOI] [PubMed] [Google Scholar]
- 17.Azaele S, Pigolotti S, Banavar JR, Maritan A. Dynamical evolution of ecosystems. Nature. 2006;444(7121):926–928. doi: 10.1038/nature05320. [DOI] [PubMed] [Google Scholar]
- 18.Vellend M. Conceptual synthesis in community ecology. Q Rev Biol. 2010;85(2):183–206. doi: 10.1086/652373. [DOI] [PubMed] [Google Scholar]
- 19.Hubbell SP. Neutral theory in community ecology and the hypothesis of functional equivalence. Funct Ecol. 2005;19(1):166–172. [Google Scholar]
- 20.Kadanoff LP. Statistical Physics: Statics, Dynamics and Renormalization. Singapore: World Scientific; 2000. [Google Scholar]
- 21.Bryngelson JD, Wolynes PG. Spin glasses and the statistical mechanics of protein folding. Proc Natl Acad Sci USA. 1987;84(21):7524–7528. doi: 10.1073/pnas.84.21.7524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holling CS. Some characteristics of simple types of predation and parasitism. Can Entomol. 1959;91:385–398. [Google Scholar]
- 23.Abrams P. The functional responses of adaptive consumers of two resources. Theor Popul Biol. 1987;32:262–288. [Google Scholar]
- 24.Lande R, Engen S, Sæther BE. Stochastic Population Dynamics in Ecology and Conservation. Oxford: Oxford Univ Press; 2003. [Google Scholar]
- 25.May RM. Will a large complex system be stable? Nature. 1972;238(5364):413–414. doi: 10.1038/238413a0. [DOI] [PubMed] [Google Scholar]
- 26.MacArthur R. Species packing and competitive equilibrium for many species. Theor Popul Biol. 1970;1(1):1–11. doi: 10.1016/0040-5809(70)90039-0. [DOI] [PubMed] [Google Scholar]
- 27.Chesson P. MacArthur’s consumer-resource model. Theor Popul Biol. 1990;37(1):26–38. [Google Scholar]
- 28.Chesson P, Kuang JJ. The interaction between predation and competition. Nature. 2008;456(7219):235–238. doi: 10.1038/nature07248. [DOI] [PubMed] [Google Scholar]
- 29.Kullback S, Leibler RA. On information and sufficiency. Ann Math Stat. 1951;22:79–86. [Google Scholar]
- 30.Derrida B. Random-energy model: Limit of a family of disordered models. Phys Rev Lett. 1980;45(2):79–82. [Google Scholar]
- 31.Derrida B. Random-energy model: An exactly solvable model of disordered systems. Phys Rev B. 1981;24:2613–2626. [Google Scholar]
- 32.Hill JK, Hamer KC, Lace LA, Banham WMT. Effects of selective logging on tropical forest butterflies on Buru, Indonesia. J Appl Ecol. 1995;32:754–760. [Google Scholar]
- 33.Hekstra DR, Leibler S. Contingency and statistical laws in replicate microbial closed ecosystems. Cell. 2012;149(5):1164–1173. doi: 10.1016/j.cell.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 34.MacArthur RH, Wilson EO. An equilibrium theory of insular zoogeography. Evolution. 1963;17(4):373–387. [Google Scholar]
- 35.Kirkpatrick S, Sherrington D. Infinite-ranged models of spin-glasses. Phys Rev B. 1978;17:4384–4403. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.