Significance
Vaccination is the most effective means of attaining protection against influenza viruses. However, the constantly evolving nature of influenza viruses enables them to escape preexisting immune surveillance, and thus thwarts public health efforts to control influenza annual epidemics and occasional pandemics. One solution is to elicit antibodies directed against highly conserved epitopes, such as those within the stem region of influenza HA, the principal target of virus-neutralizing antibody responses. This study shows that annual influenza vaccines induce antibody responses that are largely directed against the highly variable HA head region. In contrast, heterologous immunization with HA derived from influenza strains that are currently not circulating in humans (e.g. H5N1) can substantially increase HA stem-specific responses.
Keywords: stalk, breadth, immunoglobulin, neutralization
Abstract
The emergence of pandemic influenza viruses poses a major public health threat. Therefore, there is a need for a vaccine that can induce broadly cross-reactive antibodies that protect against seasonal as well as pandemic influenza strains. Human broadly neutralizing antibodies directed against highly conserved epitopes in the stem region of influenza virus HA have been recently characterized. However, it remains unknown what the baseline levels are of antibodies and memory B cells that are directed against these conserved epitopes. More importantly, it is also not known to what extent anti-HA stem B-cell responses get boosted in humans after seasonal influenza vaccination. In this study, we have addressed these two outstanding questions. Our data show that: (i) antibodies and memory B cells directed against the conserved HA stem region are prevalent in humans, but their levels are much lower than B-cell responses directed to variable epitopes in the HA head; (ii) current seasonal influenza vaccines are efficient in inducing B-cell responses to the variable HA head region but they fail to boost responses to the conserved HA stem region; and (iii) in striking contrast, immunization of humans with the avian influenza virus H5N1 induced broadly cross-reactive HA stem-specific antibodies. Taken together, our findings provide a potential vaccination strategy where heterologous influenza immunization could be used for increasing the levels of broadly neutralizing antibodies and for priming the human population to respond quickly to emerging pandemic influenza threats.
The emergence of novel influenza virus strains poses a continuous public health threat (1, 2). The World Health Organization estimates that influenza viruses infect one-billion people annually, with three- to five-million cases of severe illness, and up to 500,000 deaths worldwide (3). Following influenza virus infection, humoral immune responses against the viral hemagglutinin (HA) protein may persist for decades in humans (4). These anti-HA responses correlate strongly with protection against influenza infection (5). Serological memory is maintained by antibody-secreting long-lived plasma cells and reinforced by memory B cells, which can rapidly differentiate into antibody-secreting cells upon antigen reexposure (6).
Influenza vaccine efficacy is constantly undermined by antigenic variation in the circulating viral strains, particularly in the HA and neuraminidase (NA) proteins. Current influenza vaccination strategies rely on changing the HA and NA components of the annual human vaccine to ensure that they antigenically match circulating influenza strains (7, 8). Developing an influenza vaccine that is capable of providing broad and long-lasting protective antibody responses remains the central challenge for influenza virus research.
HA is a trimer, with each monomer comprised of two subunits: HA1, which includes the HA globular head, and HA2, whose ectodomain together with the N- and C-terminal parts of HA1 constitute the HA stem region (9). Phylogenetically, the 18 HA subtypes characterized so far are divided into two groups. Among strains that have recently caused disease in humans, H1 and H5 HAs belong to group 1, whereas H3 and H7 HAs belong to group 2 (10). Conventional anti-HA neutralizing antibodies primarily target a few immunodominant epitopes located in proximity to the receptor-binding domain within the globular head region of the molecule (11, 12). Although these antibodies are potentially protective, they are strain-specific because of the high variability of such epitopes, and thus lack, in general, the much-desired broad neutralizing activity. Recently, broadly neutralizing human (13–18) and murine (19) monoclonal antibodies (mAbs) directed against distinct epitopes within the HA stem region have been extensively characterized. These mAbs were shown to interfere with the influenza viruses’ life cycle in different ways (20). By generating monoclonal antibodies from plasmablasts isolated ex vivo, we demonstrated that these broadly neutralizing antibodies could be retrieved from patients infected with or vaccinated against the pandemic H1N1 2009 influenza virus (18, 21). Recent observations that HA stem epitopes are accessible on the majority of HA trimers on intact virions (22), and that a stable HA stem protein that is immunologically intact could be produced (23), provided further hope for the feasibility of a stem-based universal influenza vaccine (24).
Notably, HA stem-specific mAbs isolated from humans showed a high degree of affinity maturation, suggesting a memory B-cell origin. These results raised two important questions that we address in the current study. First, what are the baseline levels of broadly cross-reactive stem-binding antibodies and memory B cells? Second, using current influenza vaccines, to what extent can HA stem-specific responses be boosted in comparison with those directed against the HA globular head?
Structural studies have clearly demonstrated that the main neutralizing antibody epitopes within the HA stem region are conformation-dependent, and that the integrity of these epitopes requires the presence of the HA1 subunit in addition to the HA2 subunit, which constitute the bulk of the HA stem (16, 17). To be able to directly measure HA stem-reactive antibodies and memory B cells, we used a chimeric HA molecule that expresses the globular head of H9 HA on H1 backbone (25). Our data demonstrate that post-2009 trivalent inactivated vaccines (TIV) induced minimal stem-specific responses in comparison with head-specific responses. On the other hand, immunization with H5N1 generated relatively strong anti-HA stem responses, demonstrating that it is feasible to elicit broadly neutralizing responses in humans given the right immunogen design.
Results
TIV Immunization Induces Minimal Stem-Specific Plasmablast and Antibody Responses.
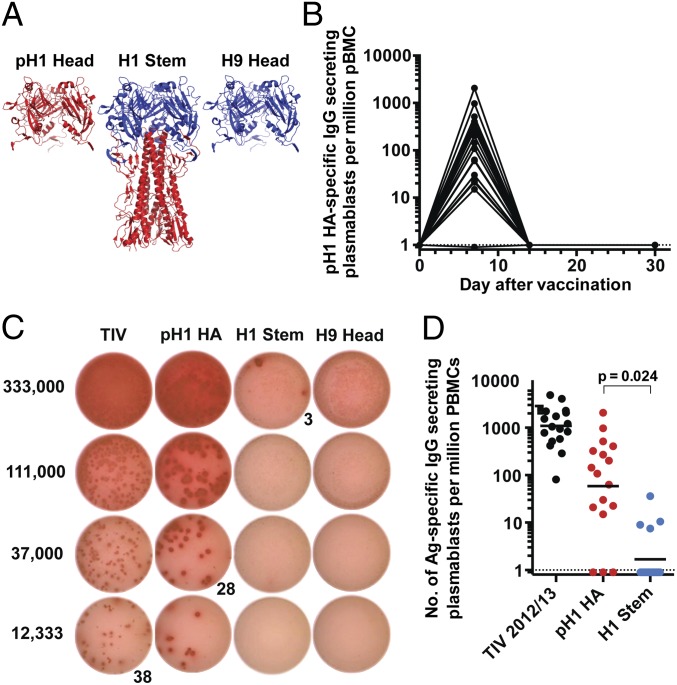
We used a recombinant, trimeric globular HA head protein from the 2009 pandemic H1N1 virus (pH1N1) and a chimeric HA that expresses the globular head of an H9 HA and H1 stem to measure vaccine-induced anti-pH1 head and stem antibody and B-cell responses, respectively (Fig. 1A) (25). The chimeric protein will hereafter be referred to as the H1 stem. We also used the trimeric H9 head protein to determine the chimeric H9/H1-specific signals/spots that are caused by H9 head binding. First, we wanted to determine the portion of the plasmablast response induced by TIV that is specific to the stem region. We examined B-cell responses in 17 healthy adult volunteers immunized with the 2012/13 TIV (Table 1). It is important to note that HA and NA from the 2009 pH1N1 virus constituted the H1N1 component of seasonal TIVs for the 2010–2014 influenza seasons. We determined the kinetics of anti-pH1 plasmablast responses in blood after vaccination by ELISPOT (Fig. 1B). Antigen-specific plasmablast response peaked at day 7 before returning to background levels by day 14 postvaccination (Fig. 1B). At the peak of the response, plasmablasts directed against the stem were barely detectable in comparison with those directed against the pH1 HA (Fig. 1C). The latter were detectable in all but three individuals at day 7 postvaccination, whereas H1 stem-specific plasmablasts were detectable in only four (of 17) individuals (Fig. 1D). In those four individuals the average frequency of H1 stem-specific antibody secreting cells (ASCs) was 10 per million peripheral blood mononuclear cells (PBMCs), which was 10-fold lower than the average frequency of ASCs directed against the pH1 HA (Fig. 1D).
Fig. 1.
Plasmablast responses after vaccination with the 2012/13 TIV are largely directed against the HA head region. Healthy adult volunteers were vaccinated with the 2012/13 TIV (n = 17). PBMCs were isolated at baseline and at days 7, 14, and 30 postvaccination. (A) Structural depiction of the recombinant proteins used to measure HA head vs. HA stem responses. As discussed in the Methods, the globular head region of the H1 stem protein was derived from H9 HA (blue) whereas the stem region is from H1 (red). (B) Kinetics of the pH1 HA-specific IgG-secreting plasmablasts. (C) A representative IgG-specific ELISPOT analysis of day 7 plasmablasts taken from one subject. ELISPOT wells were coated with the proteins displayed above each column. The numbers on the left represent the number of PBMCs plated on each row (threefold dilution). The number given below is the spot count for that well. (D) Day 7 plasmablast responses to the 2012/13 seasonal TIV detected by ELISPOT. Each symbol represents one individual (n = 17). Shown is the frequency of TIV-specific plasmablasts (black), pH1 HA-specific plasmablasts (red), and H1 stem-specific plasmablasts (blue). P values are from Student t tests. Dotted lines represent limit of detection.
Table 1.
Number of subjects, year of enrollment, and influenza vaccines used in the study
| Vaccine | Year | Vaccine strains | No. of subjects |
| TIV | 2010/11 | A/California/7/09 (H1N1) | 18 |
| A/Perth/16/2009 (H3N2) | |||
| B/Brisbane/60/2008 | |||
| TIV | 2011/12 | A/California/7/09 (H1N1) | 16 |
| A/Perth/16/2009 (H3N2) | |||
| B/Brisbane/60/2008 | |||
| TIV | 2012/13 | A/California/7/09 (H1N1) | 17 |
| A/Victoria/361/2011 (H3N2) | |||
| B/Wisconsin/1/2010 | |||
| TIV | 2013/14 | A/California/7/09 (H1N1) | 10 |
| A/Victoria/361/2011 (H3N2) | |||
| B/Massachusetts/2/2012 | |||
| H5N1 | 2008/09 | A/Vietnam/1203/2004 | 17 |
| A/Indonesia/05/2005 |
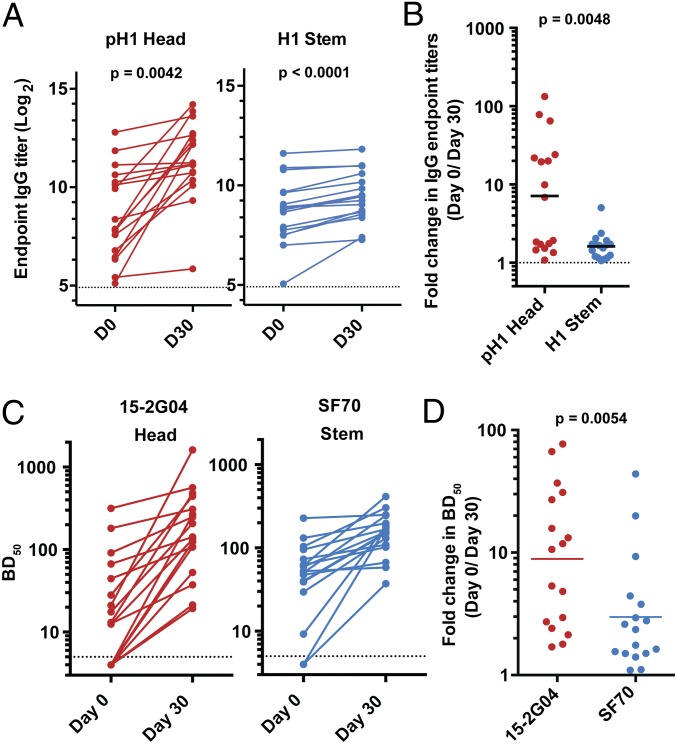
We next examined serum antibody responses to the pH1 globular head and H1 stem in those subjects. We observed an average of 1.6-fold increase in H1 stem-specific IgG antibody titers from prevaccination [geometric mean titer (GMT) = 410] to 30 d post-TIV vaccination (GMT = 626), and a sevenfold increase in anti-pH1 head-specific IgG antibody titers (GMT = 390 and 2,777 at prevaccination and 30 d postvaccination, respectively) (Fig. 2 A and B).
Fig. 2.
Serological analysis of HA head- and stem-specific antibody responses following immunization with the 2012/13 TIV. Pre- and 30 d postvaccination sera from individuals vaccinated with the 2012/13 TIV were examined by ELISA (A and B) and blocking-of-binding assay (C and D). (A) Pre- and 30 d postvaccination IgG antibody titers against the pH1 HA head (Left, red) or H1 stem (Right, blue). Each symbol represents one individual (n = 17). P values are from paired Student t tests. Dotted lines represent limit of detection. (B) Fold-change in IgG antibody titers against the pH1 HA head (red) and H1 stem (blue). (C) ELISA plates were coated with the pH1 HA and incubated with serial dilutions of the sera followed by either the HA head-binding biotinylated mAb 15-G04 (Left, red) or the HA stem-binding SF70 (Right, blue). The binding of the mAbs was detected by enzyme-conjugated streptavidin. Shown are the reciprocal serum dilutions that blocked 50% (BD50) of the mAb binding. Dotted lines represent limit of detection. (D) Fold-change in BD50 values against 15-2G04 (red) and SF70 (blue) after TIV vaccination.
To confirm these results, we used a competition-based approach that was recently described to measure the increase in serum antibody responses to epitopes within the HA head and stem regions (Fig. S1) (13). In this assay we measured the extent to which pre- and postvaccination sera blocked the binding of head- and stem-specific mAbs to the pH1 HA. For stem responses, we used a broadly neutralizing, stem-binding mAb, SF70, which was isolated from a 2009 pH1N1-infected patient (18). The epitope that SF70 binds to is highly similar to those described for the group 1 prototypic broadly neutralizing mAbs CR6261 and F10 (16, 17). For the HA head, we used 15-2G04 (21), a mAb that, unlike SF70, exhibits hemagglutination inhibition (HAI) activity against the pH1N1 virus, confirming its binding to an epitope within the pH1 globular head. After vaccination, there was a small (threefold) increase in the 50% blocking dilution or BD50 values against SF70 (Fig. 2 C and D). In contrast and consistent with the ELISA data, we observed a greater increase (eightfold) in BD50 titers against the HA head-binding mAb, 15-2G04 (Fig. 2 C and D). In summary, these data show that anti-HA stem plasmablast and antibody responses following TIV vaccination are modest in comparison with those directed against the HA globular head.
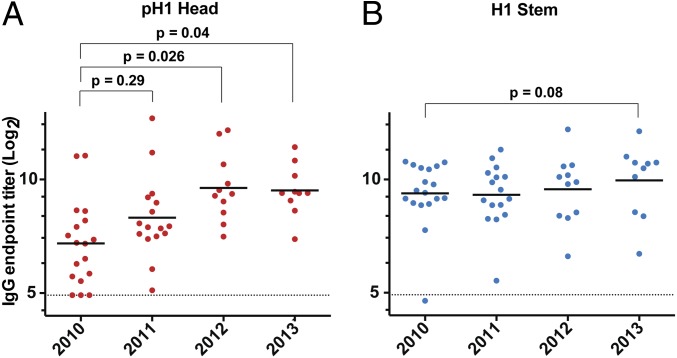
Before the emergence of the 2009 pH1N1 virus, the vast majority of young adults who constitute the various cohorts examined in the present study did not have any detectable HAI titers against this virus (26). We wanted to examine the baseline levels of the pH1 head- and H1 stem-specific serum antibodies over the 4-y period following the emergence of the pandemic. It is important to note here that the H1 component of the TIVs administered over this period was the pH1 HA and most of our study subjects had detectable baseline HAI titers against the pH1N1 virus. To this end, we determined anti-pH1 head and anti-H1 stem IgG titers in preimmunization sera collected from subjects enrolled during the 2010/11, 2011/12, 2012/13, and 2013/14 influenza seasons (Table 1). Serum anti-pH1 head IgG levels were lowest in the 2010/11 cohort (Fig. 3A). These levels showed only a slight increase in the 2011/12 cohort. However, in the 2012/13 season, we detected a significant (P = 0.026) increase in anti-pH1 HA head-specific antibody titers compared with the 2010/11 cohort. There was no significant gain in such titers between the 2012/13 and 2013/14 cohorts (Fig. 3A). In contrast, anti-H1 stem titers did not significantly change over the same period (Fig. 3B). These data confirm our earlier observation that immunization with TIV results in a greater antihead—in comparison to antistem—antibody response.
Fig. 3.
Analysis of the pH1 HA head- and H1 stem-specific IgG antibody titers over the 2010–2014 period. Prevaccination sera collected from subjects enrolled during the 2010/11 (n = 18), 2011/12 (n = 16), 2012/13 (n = 11), and 2013/14 (n = 10) influenza seasons. Geometrical mean IgG titers directed against the pH1 head (A, red) and H1 stem (B, blue) are depicted. Each symbol represents one individual. P values are from Student t tests. Dotted lines represent limits of detection.
Head-Specific Memory B-Cells Dominate After Immunization with TIV.
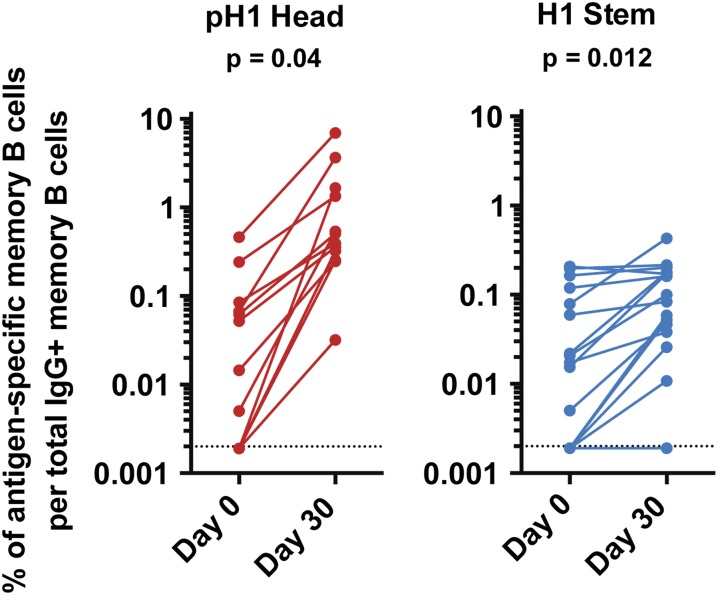
We next determined the baseline and post-TIV immunization frequency of blood memory B cells using the previously described memory B-cell assay (27). For detecting influenza HA-specific responses we used the antigens shown in Fig. 1A. We determined the frequency of IgG+ memory B cells that are directed against the pH1 head (n =12) and H1 stem (n =16) after TIV (2011/12 and 2012/13) immunization. Consistent with plasmablast and antibody responses, we observed a large increase in the frequency of anti-pH1 head IgG+ memory B cells (median = 0.033% and 0.45% at day 0 and day 30 postvaccination, respectively, P = 0.04) and a modest increase in anti-H1 stem IgG+ memory B cells (from 0.02% to 0.09% at days 0 and 30 postvaccination, respectively) (P = 0.012) (Fig. 4). These data show that although stem-specific IgG+ memory B cells are detectable in most individuals, they are minimally boosted by TIV immunization in comparison with the head-specific ones.
Fig. 4.
Memory B-cell responses induced following immunization with TIV. PBMCs isolated either before- or 30 d after immunization with either the 2011/12 or the 2012/13 TIV. The frequency of pre- and 30 d postvaccination levels of IgG+ memory B cells directed against the pH1 head (Left, red) or H1 stem region (Right, blue). P values are from paired Student t tests. Dotted lines represent limit of detection.
Enhanced Anti-HA Stem Antibody Responses After H5N1 Vaccination.
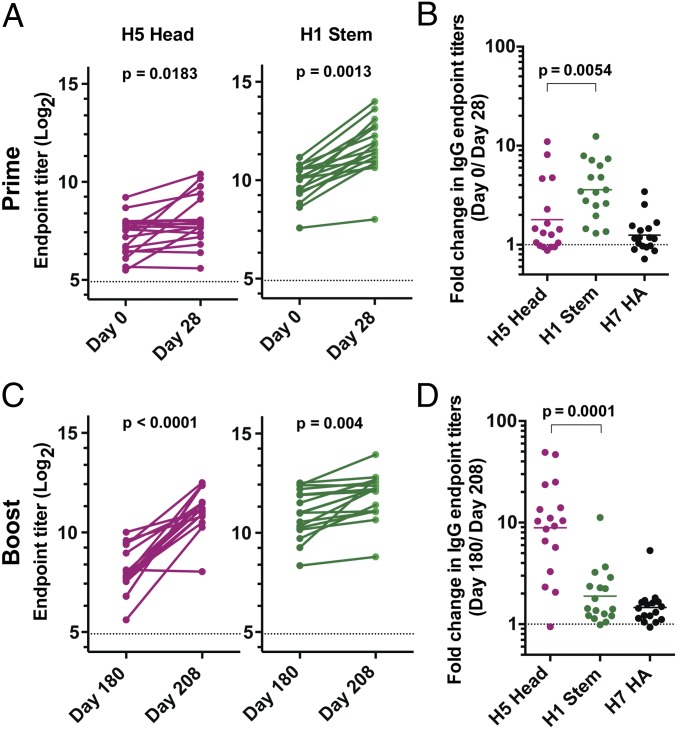
We have shown that cross-reactive B cells dominated the plasmablast response following the 2009 pH1N1 vaccination (21). We wanted to determine whether immunization with a similarly heterologous (relative to the seasonal antigens) influenza vaccine would affect the trend of serum antibody responses to the HA head vs. stem regions. Therefore, we determined anti-H5 HA head and anti-H1 stem antibody levels in 17 paired serum samples collected before and after immunization with an inactivated H5N1 vaccine derived from A/Vietnam/04/1203 or A/Indonesia/05/2005 (Table 1) (28). Those subjects received a booster H5N1 immunization with a vaccine that was derived from A/Indonesia/05/2005 6 mo later (28). Blood samples were analyzed at four time points; baseline, 28 d following the primary immunization and before the booster immunization, and 28 d after the booster immunizations. Both H5 and H1 belong to group 1 HAs and have a significant degree of homology in the amino acid sequence of their stem regions; therefore, we used the chimeric H9/H1 HA molecule to measure anti-H5 HA stem-specific antibody responses by ELISA. We also measured antibody titers against H7 HA, a representative of group 2 HAs. Interestingly, there was an enhanced (an average of fourfold) increase in stem-specific IgG antibody titers from prevaccination (GMT = 925) to day 28 postprimary H5N1 vaccination (GMT = 3,330, P = 0.0013). On the other hand, the increase in anti-H5 head antibody titers was modest (1.8-fold increase, GMT = 150 and 270 at prevaccination and 28 d postprimary vaccination, respectively; P = 0.018) (Fig. 5 A and B). The increase in stem-specific antibody responses was largely group 1-specific, as there was barely any increase (1.2-fold) in anti-H7 HA IgG antibody responses (Fig. 5B).
Fig. 5.
Robust antistem antibody responses following primary H5N1 immunization. Sera collected before- and 28 d after priming (A and B) and booster (C and D) H5N1 immunization. The time interval between the priming and the booster immunizations was 6 mo. (A) Pre- and 28 d postprimary immunization IgG endpoint titers directed against Indonesia H5 HA head region (Left, magenta) and H1 stem region (Right, green). P values are from Student t tests. (B) Fold-change in IgG antibody titers against the Indonesia H5 HA head (magenta), H1 stem (green), and H7 HA (black) after primary H5N1 immunization. (C) Pre- and 28 d postboost IgG endpoint titers directed against Indonesia H5 HA head region (Left, magenta) and H1 stem region (Right, green). P values are from Student t tests. (D) Fold-change in IgG antibody titers against the Indonesia H5 HA head (magenta), H1 stem (green), and H7 HA (black) after secondary H5N1 immunization.
Responses after the H5N1 booster immunization resembled those observed after TIV immunization; there was a strong anti-H5 head antibody response (ninefold increase, GMT = 265 and 2,360 at days 180 and 208 postprimary vaccination, respectively; P < 0.0001) and a feeble stem-specific response (1.8-fold increase, GMT = 2,000 and 3,780 at days 180 and 208 postprimary vaccination, respectively; P = 0.004) (Fig. 5 C and D). As with response after the priming immunization, minimal increase (1.4-fold) in anti-H7 HA titers were observed after the booster immunization (Fig. 5D).
Next, we compared the increase in group 1 neutralizing antibodies following a single TIV vs. H5N1 immunization. To avoid any interference from H1 head-, H5 head-, or N1 NA-specific antibodies, we used a chimeric H9N3 virus that was previously generated and characterized (25). After TIV immunization, we detected an increase in neutralizing antibody titers in only 2 of the 15 subjects tested (mean titers were 145.8 and 132.9 at days 0 and 30 postvaccination, respectively, P = 0.6) (Fig. S2A). In contrast, stem-directed neutralizing antibody titers modestly increased in 11 of the 16 subjects tested (mean titers were 198.6 and 397.3 at days 0 and 28 postvaccination, respectively, P = 0.042) (Fig. S2B).
In summary, these data show that the balance between vaccine-induced anti-HA head vs. stem antibody responses can be shifted in favor of stem responses by vaccination using HA molecules against which humans have minimal preexisting antibody titers/memory B cells, such as those derived from avian H5N1 viruses. However, upon boosting with the same HA, the dominance of antihead responses is restored.
Discussion
In this study we report three key findings in regards to human B-cell and antibody responses to the conserved influenza HA stem region: (i) baseline antibodies and memory B cells directed against the HA stem region are widely prevalent, albeit at low levels; (ii) although immunization with TIV did induce some B-cell and antibody responses to epitopes within the HA stem region, the responses were disproportionately higher against the HA globular head epitopes; and (iii) immunization with an inactivated H5N1 vaccine considerably improved the cross-reactive anti-HA stem responses. Clearly, these findings have important implications for the pursuit of HA stem-based “universal” influenza vaccines and for influenza immunization strategies in general.
Our data show that there is a clear bias in favor of the HA globular head over stem in terms of the magnitude of antibodies directed against either region in response to influenza vaccines in general. This finding is indicated by our observation that the overall anti-H1 stem-specific antibody levels, unlike their anti-pH1 HA head counterparts, did not significantly change over the 4 y following the emergence of the 2009 pH1N1 (Fig. 3).
We have previously proposed that broadly cross-reactive antibodies, such as those directed against the HA stem, are produced by low-frequency memory B cells that are specific to conserved but subdominant epitopes (21). However, after introduction of a relatively novel HA from a noncirculating flu strain, cross-reactive B-cell responses make up a relatively greater proportion of the humoral immune response. The relatively strong HA stem-specific responses after primary H5 vaccination provide additional support to this hypothesis. Additional lines of evidence for the enhanced antistem responses after H5 vaccination or infection in humans include: the isolation of stem-specific mAbs from combinatorial antibody libraries generated from the bone marrow of five survivors of an avian H5N1 outbreak (29); the increase in stem-binding antibodies after H5 immunization as reported by Ledgerwood et al. (30); and isolation, using a flow cytometry-based approach, of B cells expressing the broadly neutralizing HA stem-specific mAbs from an individual immunized with H5N1 vaccine (31). Finally, Khurana et al. observed that serum antibodies that bound to linear peptides from the HA2 subunit increased in individuals immunized with either unadjuvanted or alum-adjuvanted H5N1 vaccines (32).
Notably, all vaccines used in our study were unadjuvanted, and it has been proposed that adjuvants, such as the oil-in-water emulsion MF59, could expand the breadth of the antibody responses to influenza vaccines (32). In the latter study, the authors showed an increase in antibody titers directed against epitopes within the HA1 subunit in sera from individuals immunized with MF59-adjuvanted H5N1 vaccine. This result can be explained by the fact that they analyzed sera obtained after a second or a third booster immunization, whereas we observed potent antistem responses after primary H5N1 immunization. In general it is important to make a distinction between the ability of adjuvants to increase the overall magnitude of immune responses and their ability to qualitatively modulate an immune response with an already established immunodominance hierarchy. To test the latter point in context of influenza, the impact of adjuvants on the ratio and longevity of antihead vs. stem responses after vaccination should be examined.
The most probable explanation for our results with primary H5 vaccination is that vaccinees had relatively lower frequency of preexisting memory B cells specific to most of the prominent H5 HA head epitopes in comparison with the stem-specific ones. Thus, upon immunization more HA stem-specific memory B cells got recruited to the immune reaction and differentiated into ASCs. However, these conditions have probably changed before the H5N1 booster immunization, and the preexisting frequency of H5 head-specific memory B cells has relatively increased. Therefore, the booster immunization resulted in significantly larger head-specific responses in comparison with stem-specific ones. This concept has been clearly demonstrated in animal models (33, 34).
The effect of homologous boosting is another point raised by our study. Although this strategy does eventually lead to significant accumulation of HA-specific antibody titers, these antibodies are mostly directed against the strain-specific head region. Under this scenario, boosting the cross-reactive HA stem-specific responses is largely marginalized.
The high prevalence of HA stem-specific antibodies at baseline in tested subjects is consistent with earlier studies reporting the detection of baseline neutralizing antibody titers against H5N1 in healthy volunteers (13, 35). Our data indicate that most humans are capable of establishing a humoral immune memory that is specific to the conserved HA stem region. This finding is intriguing in light of what was recently suggested that antibodies directed against the HA2 subunit might enhance virus fusion and thus virus-induced pathology in the absence of virus-neutralizing responses (36). The latter suggestion indicates that other factors, such as the quantity and the exact epitopes targeted by antistem antibodies, play a role in determining the overall impact of such antibodies on immunity to influenza. It is important to note here that the main neutralizing epitope within the HA stem is conformational, and thus cannot be detected by measuring binding to linear peptides from the HA2 subunit. Overall, our data raise the important question of what would be the minimal “concentration” of antistem antibodies required to provide in vivo protection (37). Therefore, it will be important in future studies to determine the quantity of HA stem-specific antibodies or memory B cells that would positively correlate with better clinical outcomes against influenza infections (38).
In summary, the isolation and characterization of broadly neutralizing human mAbs directed against the conserved stem region of influenza HA represents a potentially important step toward developing a universal influenza vaccine. Our data show that low levels of antibodies and memory B cells directed against the HA stem region are widely prevalent in humans. However, TIVs induce B-cell responses that are largely directed against the HA head region. In contrast, heterologous immunization with HA derived from influenza strains that are currently not circulating in humans has greatly increased HA stem-specific responses. Our findings delineate a potential vaccination strategy where H5N1 or H7N9 immunization could be used not only for immunologically priming the population to quickly respond to serious pandemic influenza threats, but also for generating broadly neutralizing antibodies against influenza in humans.
Methods
All studies were approved by the Emory University institutional review board. Healthy adult volunteers were given either the TIV from the 2010/11, 2011/12, 2012/13, and 2013/14-influenza seasons or a monovalent inactivated H5N1 vaccine. Generation and characterization of the chimeric H9N3 virus was previously described (25). Direct ELISPOT to enumerate total and HA specific plasmablasts were performed as previously described (39). Recombinant HA-specific ELISA, as well as HAI (done with the chimeric H9N3 virus) were performed as previously described (40). More detailed materials and methods are presented in the SI Methods.
Supplementary Material
Acknowledgments
This work was funded in parts by National Institute of Allergy and Infectious Diseases Contracts HHSN266200700006C (to R.A. and P.C.W.) and HHSN26620070010C (to A.G.-S. and P.P.); Contract HHSN272200800005C (to M.J.M.), which supported the H5N1 vaccination trial; Grant 1P01AI097092 (to P.P., P.C.W., and R.A.); Grant 1U19AI109946-01 (to P.P.); the Georgia Research Alliance (M.J.M.); Children’s Healthcare of Atlanta (M.J.M.); the National Center for Advancing Translational Sciences of the National Institutes of Health under Award UL1TR000454 (to M.J.M.); Training Grant T32AI074492 from the National Institute of Allergy and Infectious Diseases (to A.H.E.); Erwin Schrödinger Fellowship J3232 from the Austrian Science Fund (to F.K.); a Canadian Institutes of Health Research Postdoctoral fellowship (to M.S.M.). Some of clinical studies were supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award UL1TR000454.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414070111/-/DCSupplemental.
References
- 1.Rappuoli R, Dormitzer PR. Influenza: Options to improve pandemic preparation. Science. 2012;336(6088):1531–1533. doi: 10.1126/science.1221466. [DOI] [PubMed] [Google Scholar]
- 2.Webby RJ, Webster RG. Are we ready for pandemic influenza? Science. 2003;302(5650):1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- 3.WHO 2008. Immunization, vaccines and biologicals: Influenza. Available at www.who.int/immunization/topics/influenza/en/. Accessed June 20, 2014.
- 4.Yu X, et al. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature. 2008;455(7212):532–536. doi: 10.1038/nature07231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis T., Jr Vaccination against influenza. Bull World Health Organ. 1953;8(5-6):725–741. [PMC free article] [PubMed] [Google Scholar]
- 6.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8(3):363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 7.Ellebedy AH, Webby RJ. Influenza vaccines. Vaccine. 2009;27(Suppl 4):D65–D68. doi: 10.1016/j.vaccine.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monto AS. Seasonal influenza and vaccination coverage. Vaccine. 2010;28(Suppl 4):D33–D44. doi: 10.1016/j.vaccine.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 9.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 10.Ellebedy AH, Ahmed R. Re-engaging cross-reactive memory B cells: The influenza puzzle. Front Immunol. 2012;3:53. doi: 10.3389/fimmu.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caton AJ, Brownlee GG, Yewdell JW, Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype) Cell. 1982;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- 12.Gerhard W, Yewdell J, Frankel ME, Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981;290(5808):713–717. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- 13.Corti D, et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120(5):1663–1673. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corti D, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333(6044):850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 15.Dreyfus C, et al. Highly conserved protective epitopes on influenza B viruses. Science. 2012;337(6100):1343–1348. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekiert DC, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324(5924):246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sui J, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16(3):265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wrammert J, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208(1):181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan GS, et al. A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J Virol. 2012;86(11):6179–6188. doi: 10.1128/JVI.00469-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandenburg B, et al. Mechanisms of hemagglutinin targeted influenza virus neutralization. PLoS ONE. 2013;8(12):e80034. doi: 10.1371/journal.pone.0080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li GM, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci USA. 2012;109(23):9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris AK, et al. Structure and accessibility of HA trimers on intact 2009 H1N1 pandemic influenza virus to stem region-specific neutralizing antibodies. Proc Natl Acad Sci USA. 2013;110(12):4592–4597. doi: 10.1073/pnas.1214913110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Y, Welsh JP, Swartz JR. Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc Natl Acad Sci USA. 2014;111(1):125–130. doi: 10.1073/pnas.1308701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nabel GJ, Fauci AS. Induction of unnatural immunity: Prospects for a broadly protective universal influenza vaccine. Nat Med. 2010;16(12):1389–1391. doi: 10.1038/nm1210-1389. [DOI] [PubMed] [Google Scholar]
- 25.Pica N, et al. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci USA. 2012;109(7):2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hancock K, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361(20):1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 27.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: A sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286(1-2):111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Belshe RB, et al. National Institute of Allergy and Infectious Diseases-Funded Vaccine and Treatment Evaluation Units Safety and immunogenicity of influenza A H5 subunit vaccines: Effect of vaccine schedule and antigenic variant. J Infect Dis. 2011;203(5):666–673. doi: 10.1093/infdis/jiq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashyap AK, et al. Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proc Natl Acad Sci USA. 2008;105(16):5986–5991. doi: 10.1073/pnas.0801367105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ledgerwood JE, et al. VRC 306 Study Team DNA priming and influenza vaccine immunogenicity: Two phase 1 open label randomised clinical trials. Lancet Infect Dis. 2011;11(12):916–924. doi: 10.1016/S1473-3099(11)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whittle JR, et al. Flow cytometry reveals that H5N1 vaccination elicits cross-reactive stem-directed antibodies from multiple Ig heavy-chain lineages. J Virol. 2014;88(8):4047–4057. doi: 10.1128/JVI.03422-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khurana S, et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci Transl Med. 2010;2(15):ra5. doi: 10.1126/scitranslmed.3000624. [DOI] [PubMed] [Google Scholar]
- 33.Krammer F, Palese P. Universal influenza virus vaccines: Need for clinical trials. Nat Immunol. 2014;15(1):3–5. doi: 10.1038/ni.2761. [DOI] [PubMed] [Google Scholar]
- 34.Krammer F, Pica N, Hai R, Margine I, Palese P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol. 2013;87(12):6542–6550. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sui J, et al. Wide prevalence of heterosubtypic broadly neutralizing human anti-influenza A antibodies. Clin Infect Dis. 2011;52(8):1003–1009. doi: 10.1093/cid/cir121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khurana S, et al. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Trans Med. 2013;5(200):200ra114. doi: 10.1126/scitranslmed.3006366. [DOI] [PubMed] [Google Scholar]
- 37.Yewdell JW. Viva la revolucion: Rethinking influenza a virus antigenic drift. Curr Opinion Virol. 2011;1(3):177–183. doi: 10.1016/j.coviro.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corti D, Lanzavecchia A. Broadly neutralizing antiviral antibodies. Annu Rev Immunol. 2013;31:705–742. doi: 10.1146/annurev-immunol-032712-095916. [DOI] [PubMed] [Google Scholar]
- 39.Crotty S, et al. Cutting edge: Long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171(10):4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 40.Wrammert J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453(7195):667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.