Significance
A number of highly potent and broadly neutralizing HIV-specific monoclonal antibodies have recently been isolated from B cells of infected individuals. However, the effects of these antibodies on the persistent viral reservoirs in HIV-infected individuals receiving antiretroviral therapy (ART) are unknown. We demonstrate that a select number of HIV-specific monoclonal antibodies potently suppressed entry into CD4+ T cells of HIV isolated from the latent viral reservoir as well as replication of reservoir virus in autologous CD4+ T cells derived from infected individuals receiving ART. These findings provide new opportunities for passive immunotherapy to prevent plasma viral rebound following discontinuation of antiretroviral drugs.
Keywords: latent HIV, HIV-specific antibodies, HIV envelope protein
Abstract
Several highly potent and broadly neutralizing monoclonal antibodies against HIV have recently been isolated from B cells of infected individuals. However, the effects of these antibodies on the persistent viral reservoirs in HIV-infected individuals receiving antiretroviral therapy (ART) are unknown. We show that several HIV-specific monoclonal antibodies—in particular, PGT121, VRC01, and VRC03—potently inhibited entry into CD4+ T cells of HIV isolated from the latent viral reservoir of infected individuals whose plasma viremia was well controlled by ART. In addition, we demonstrate that HIV replication in autologous CD4+ T cells derived from infected individuals receiving ART was profoundly suppressed by three aforementioned and other HIV-specific monoclonal antibodies. These findings have implications for passive immunotherapy as an approach toward controlling plasma viral rebound in patients whose ART is withdrawn.
The sustained suppression of HIV replication by antiretroviral therapy (ART) has dramatically improved the clinical outcome of infected individuals (1). In addition, research directed at potential pathways toward the development of an effective preventive HIV vaccine has provided insights into the nature of the immune response to HIV infection (2, 3). In this regard, recent advances in antibody-cloning technologies have led to the discovery of several highly potent and broadly neutralizing monoclonal antibodies against HIV from B cells of HIV-infected individuals (4–7). Of interest, several studies have demonstrated that certain broadly neutralizing HIV-specific monoclonal antibodies can prevent acquisition of the virus, suppress viral replication, delay and/or prevent plasma viral rebound following treatment interruption in infected animals (8–14), and block cell-to-cell transmission of laboratory-adapted HIV in vitro (15). However, it is unclear what in vivo effects these antibodies might have on HIV in humans and, in particular, what effects they may have on the virus contained in the persistently infected CD4+ T cells of individuals whose plasma viremia is controlled by ART. These infected CD4+ T cells are considered to be the major obstacle to viral eradication (16–18) as well as a potential source of plasma viral rebound following discontinuation of ART in patients whose viremia had been well controlled in therapy (1). In this regard, considerable efforts in current HIV therapeutic research have been focused on developing strategies aimed at achieving sustained virologic remission in the absence of ART (1). This focus is especially important given that viral rebound and sustained HIV replication has been observed in almost all infected individuals whose plasma viremia had been well controlled while receiving ART and whose ART was subsequently withdrawn (19). Therefore, it is important to determine which, if any, of the many recently characterized HIV-specific monoclonal antibodies can inhibit viral entry into CD4+ T cells of HIV isolated from the latent viral reservoir as well as replication of reservoir virus in autologous CD4+ T cells derived from infected individuals whose plasma viremia was well-controlled on ART. Such knowledge is critical to establishing novel opportunities for passive immunotherapy to prevent plasma viral rebound following discontinuation of antiretroviral drugs. We conducted the present study to address this issue.
Results
Binding of HIV-Specific Monoclonal Antibodies to Cell-Free Virions Derived from the Latent Viral Reservoir.
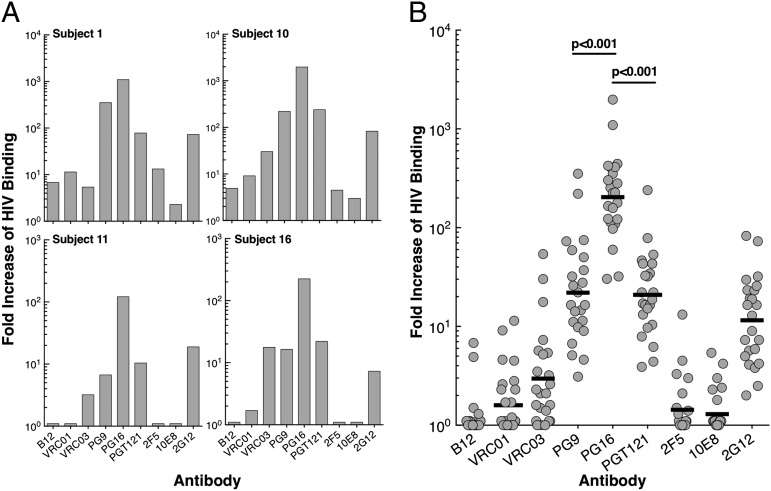
The capability of broadly neutralizing HIV-specific monoclonal antibodies to suppress HIV isolated from infected individuals was addressed by using several different approaches. We first measured the binding of nine HIV-specific monoclonal antibodies (CD4-binding site on gp120: B12, VRC01, and VRC03; V1V2 site on gp120: PG9 and PG16; glycan-V3 site on gp120: PGT121 and 2G12; and membrane proximal external region on gp41: 2F5 and 10E8) to virions induced from latently infected, resting CD4+ T cells of the study subjects whose plasma viremia was well-controlled on ART (Table S1). Cell-free virions equivalent to 50,000–100,000 copies of HIV RNA were incubated with the above antibodies that were conjugated to magnetic Protein A beads, and the number of bound virions was determined by Cobas Ampliprep/Cobas Taqman HIV-1 Test (Version 2.0; Roche Diagnostics). Fig. 1A shows representative (individual subjects) data, and Fig. 1B shows cumulative data of the virion-binding capacity for the nine HIV-specific monoclonal antibodies. Among the panel, PG16 exhibited the highest level of binding to virions. The geometric mean fold increase of HIV bound to PG16 over that bound to human IgG (the control for nonspecific binding) was 184 (range 30–1,094). The level of virions bound to PG16 was 9.3 and 9.8 times greater than the levels of virions bound to the next two closest in rank order, PG9 (P < 0.001) and PGT121 (P < 0.001), respectively (Table 1). These data suggest that HIV induced from the latent viral reservoirs of infected aviremic individuals preferentially bind to PG16 antibody.
Fig. 1.
Effect of broadly reactive HIV-specific monoclonal antibodies on virus isolated from latently infected resting CD4+ T cells obtained from HIV-infected individuals receiving ART. (A) Representative data showing fold increase of virions bound to a panel of broadly reactive HIV-specific monoclonal antibodies over negative control (human IgG). (B) Cumulative data showing fold increase of virions bound to a panel of broadly reactive HIV-specific monoclonal antibodies over negative control (human IgG). The fold increases of the binding of virions to HIV-neutralizing antibodies over the control antibody (human IgG) were compared by using the studentized range test. The geometric mean values are shown as black bars.
Table 1.
P values for comparisons of virion binding capacity of HIV-specific monoclonal antibodies
| Antibody | B12 | VRC01 | VRC03 | PG9 | PG16 | PGT121 | 2F5 | 10E8 | 2G12 |
| B12 | >0.5 | 0.01 | <0.001 | <0.001 | <0.001 | >0.5 | >0.5 | <0.001 | |
| VRC01 | 0.35 | <0.001 | <0.001 | <0.001 | >0.5 | >0.5 | <0.001 | ||
| VRC03 | <0.001 | <0.001 | <0.001 | 0.20 | 0.08 | <0.001 | |||
| PG9 | <0.001 | >0.5 | <0.001 | <0.001 | 0.25 | ||||
| PG16 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| PGT121 | <0.001 | <0.001 | 0.40 | ||||||
| 2F5 | >0.5 | <0.001 | |||||||
| 10E8 | <0.001 | ||||||||
| 2G12 |
Bold numbers indicate a significant P value.
Effect of HIV-Specific Monoclonal Antibodies on Entry into CD4+ T Cells of HIV Isolated from the Latent Viral Reservoir.
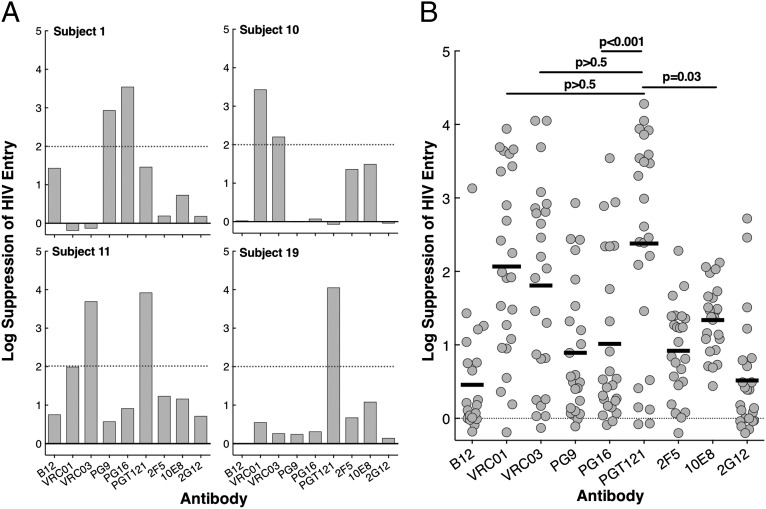
Given that binding of antibodies to HIV envelope (Env) does not necessarily indicate blocking of viral entry into susceptible target cells, we investigated whether these broadly reactive HIV-specific monoclonal antibodies could prevent viral entry into CD4+ T cells derived from HIV-uninfected healthy donors. To obtain sufficient quantities of virus for further investigation, the supernatants containing HIV used in the above experiments (isolated from the latent viral reservoir of the study subjects) were incubated with stimulated CD8-depleted CD4+ T cells from HIV-negative donors for up to 5 d. Supernatants containing virus (0.5–1.0 ng of HIV p24) were preincubated with the nine broadly reactive HIV-specific monoclonal antibodies and human IgG (control) for 90 min, and the virus–antibody conjugates were then added to highly enriched and activated CD4+ T cells obtained from HIV-uninfected healthy donors. Following a 2-d incubation, cells were extensively washed and trypsinized to remove surface-bound virions and lysed for DNA isolation and quantitation of cell-associated HIV DNA by droplet digital PCR (Bio-Rad Laboratories). This entry assay serves as an in vitro indicator of viral spread that might occur in vivo from the persistent viral reservoir upon discontinuation of ART. Representative and cumulative data on the capacity of the antibodies to block entry of virions from the study subjects into normal CD4+ T cells are shown in Fig. 2 A and B. In contrast to the observation of preferential PG16 binding to HIV virions among the panel of antibodies, viral entry was most potently suppressed by PGT121 and VRC01 (the mean of >2 log suppression over the control antibody; Fig. 2B). The highest mean log suppression of HIV entry was achieved by PGT121 (2.4 log), VRC01 (2.1 log), and VRC03 (mean 1.8 log). The mean differences in log suppression against all six other antibodies (B12, PG9, PG16, 2F5, 10E8, and 2G12) tested were statistically significant for PGT121 and significant against five (B12, PG9, PG16, 2F5, and 2G12) for VRC01 and two (B12 and 2G12) for VRC03 (Table 2). Of note, the viral isolates from 72%, 52%, and 44% of HIV-infected individuals we studied were neutralized (>2 log suppression) by PGT121, VRC01, and VRC03, respectively (Table 3). There was 32% overlap between PGT121 and either VRC01 or VRC03 antibodies. These data suggest that PGT121, VRC01, and VRC03 can dramatically suppress HIV entry into CD4+ T cells.
Fig. 2.
Effect of broadly reactive HIV-specific monoclonal antibodies on virus isolated from latently infected resting CD4+ T cells obtained from HIV-infected individuals receiving ART. (A) Representative data showing log suppression of viral entry into CD4+ T cells obtained from HIV-uninfected donors by HIV-specific monoclonal antibodies over negative control (human IgG). (B) Cumulative data showing log suppression of viral entry into CD4+ T cells obtained from HIV-uninfected donors by HIV-specific antibodies over negative control (human IgG). The log suppression of viral entry over the control antibody (human IgG) was compared by using the studentized range test. The mean values are shown as black bars.
Table 2.
P values for comparisons of inhibition of viral entry into CD4+ T cells by HIV-specific monoclonal antibodies
| Antibody | B12 | VRC01 | VRC03 | PG9 | PG16 | PGT121 | 2F5 | 10E8 | 2G12 |
| B12 | <0.001 | <0.001 | >0.5 | >0.5 | <0.001 | >0.5 | 0.08 | >0.5 | |
| VRC01 | >0.5 | 0.005 | 0.03 | >0.5 | 0.005 | 0.3 | <0.001 | ||
| VRC03 | 0.08 | 0.2 | >0.5 | 0.08 | >0.5 | 0.001 | |||
| PG9 | >0.5 | <0.001 | >0.5 | >0.5 | >0.5 | ||||
| PG16 | <0.001 | >0.5 | >0.5 | >0.5 | |||||
| PGT121 | <0.001 | 0.03 | <0.001 | ||||||
| 2F5 | >0.5 | >0.5 | |||||||
| 10E8 | 0.15 | ||||||||
| 2G12 |
Bold numbers indicate a significant P value.
Table 3.
Frequency of HIV-infected individuals whose virus was neutralized by HIV-specific antibodies
| Antibody | % |
| B12 | 4 |
| VRC01 | 52 |
| VRC03 | 44 |
| PG9 | 16 |
| PG16 | 24 |
| PGT121 | 72 |
| 2F5 | 4 |
| 10E8 | 8 |
| 2G12 | 16 |
Effect of HIV-Specific Monoclonal Antibodies on HIV Replication in Autologous CD4+ T Cells.
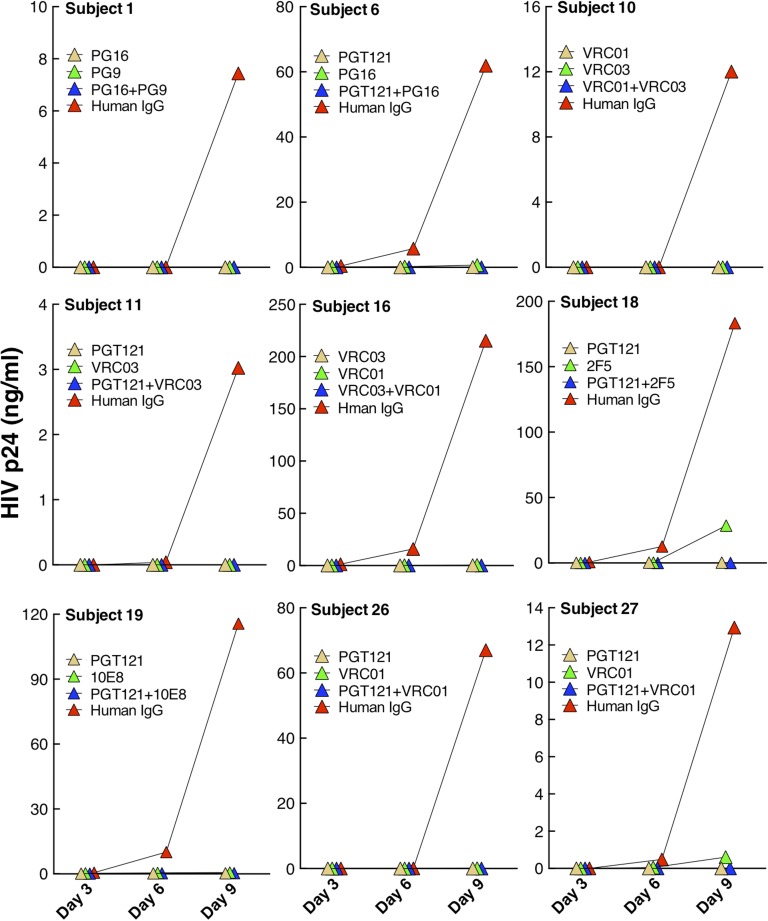
Finally, we investigated whether the HIV-specific monoclonal antibodies shown in Fig. 2 A and B could suppress viral replication in an autologous setting. Accordingly, highly enriched CD4+ T cells from nine representative study subjects whose plasma viremia was controlled by ART were stimulated with anti-CD3/CD28 antibodies in the presence of two HIV-specific antibodies (individually or in combination) that were most effective in blocking viral entry for each study subject. The autologous CD4+ T-cell cultures were maintained for 9 d, during which culture supernatants were collected every 3 d and assayed for HIV p24 ELISA. This assay, which uses purified autologous CD4+ T cells that contain at least a component of the persistent viral reservoir, serves as an indicator of in vivo HIV expression and propagation that occurs upon discontinuation of ART in an individual whose viremia had been well-controlled while receiving ART. As shown in Fig. 3, HIV replication in autologous CD4+ T cells was suppressed to below the limit of detection by all combinations of HIV-specific monoclonal antibodies tested, whereas viral replication was detected with control IgG in every subject. Consistent with the viral entry data, monoclonal antibodies PGT121, VRC01, and VRC03 potently suppressed HIV replication. However, other HIV-specific monoclonal antibodies (such as PG9, PG16, and 10E8) that were less frequently effective in blocking entry of the virus from the overall cohort of infected individuals were equally effective at suppressing viral replication in those individuals whose virus was sensitive to these antibodies. Of note, although most single antibodies also completely suppressed viral replication, two did not (2F5 in subject 18 and VRC01 in subject 27). Collectively, these data suggest that HIV-specific monoclonal antibodies can profoundly suppress HIV replication in stimulated autologous CD4+ T cells of infected individuals receiving ART.
Fig. 3.
Effect of broadly reactive HIV-specific monoclonal antibodies on viral replication in autologous CD4+ T cells of infected individuals whose viremia was controlled on ART. CD4+ T cells from nine study subjects were stimulated with anti-CD3/CD28 antibodies in the presence of two HIV-specific monoclonal antibodies (20 µg/mL; individually or in combination) that exhibited the highest levels of inhibition of HIV entry. The cultures were maintained for 9 d and the level of viral replication was determined by HIV p24 ELISA.
Discussion
In the present study, we investigated the effects of recently identified potent and broadly neutralizing HIV-specific monoclonal antibodies on viral isolates induced from the latent viral reservoir of HIV-infected individuals whose plasma viremia had been controlled on ART. The latent HIV reservoir has long been recognized as one of the major obstacles to eradicating the virus in infected individuals whose plasma viremia is controlled on ART (16–18). Consequently, there is a growing interest in developing novel therapeutic strategies to either completely eliminate the infected cells and/or achieve sustained virologic remission that is sustained by the natural host immune response and/or by immunotherapy following the discontinuation of ART (20). Given that there are significant challenges in achieving total eradication of replication-competent virus in a substantial proportion of infected individuals (21)*, therapeutic strategies designed to maintain sustained virologic remission in infected individuals without the requirement for indefinite ART may represent more realistic goals. In this regard, recently identified potent and broadly neutralizing HIV-specific monoclonal antibodies have been shown to suppress the virus in infected animals (11–14) and to inhibit cell-to-cell transmission of laboratory-adapted HIV in vitro (15). Clinical trials have been planned and/or are underway to determine the ability of these well-characterized monoclonal antibodies to prevent the acquisition of HIV infection and suppress HIV in infected individuals in vivo. In this regard, it would be of considerable interest to determine whether these antibodies are capable of suppressing replication-competent HIV that is contained within the persistent viral reservoir, and thus could be used as a potential modality to blunt the plasma viral rebound following the deliberate cessation of ART. Recently characterized HIV-specific antibodies have been shown to be broadly neutralizing—albeit with wide variations—against diverse panels of pseudotyped HIV grouped into tiers based on neutralization susceptibility (22). We demonstrated that certain HIV-specific monoclonal antibodies (some better than others) can efficiently bind virions that have been induced from the latent reservoir of HIV-infected individuals whose plasma viremia has been successfully controlled on ART. In addition, several of these antibodies block entry of HIV isolated from the latent pool into CD4+ T cells derived from HIV-uninfected individuals. Furthermore, we demonstrated that these same antibodies could completely block HIV replication in mitogen-stimulated autologous CD4+ T cells of infected individuals. Of note, despite the fact that PG9 and PG16 are closely related somatically, PG16 showed a greater capacity to bind to virions isolated from the latent viral reservoir of HIV-infected individuals receiving ART. It has been shown that the binding of PG9 and PG16 to V1–V2 of HIV Env is influenced by the composition of glycans in this region (23). Moreover, it has been shown that levels of glycosylation and the composition of glycans are influenced by the type of cells from which the HIV Env or virions is expressed or secreted (24). Given that the cell-free virions we used to perform the antibody–HIV binding assays were recovered from short-term stimulated autologous CD4+ T cells of the study subjects—a process that likely closely resembles HIV propagation in vivo—our findings underscore the importance of glycan composition of virions used to assess the therapeutic potential of glycan-dependent antibodies such as PG16. However, it is also noteworthy that PG16, the antibody that showed the highest degree of binding to the virions isolated from the latent viral reservoir, was not the most effective entry inhibitor of the viral isolates from a larger proportion of the study subjects. It is plausible that PG16 may bind to a region of V1–V2 of the HIV Env that is highly accessible but not necessarily critical for viral entry. It is also possible that a brief period of virus propagation, a necessary step for obtaining sufficient titers of infectious HIV to perform the viral entry assays, may have altered the viral envelope composition, such that virions used in the binding assay were different from virus propagated for the entry assay in ways that had a greater effect on glycan-dependent antibodies such as PG16. These observations underscore the potential importance of addressing the functional capabilities of HIV-specific antibodies that might be used in the context of immune-based therapy in physiologically relevant experimental systems.
Our findings have potentially important implications for the design of therapeutic strategies. A combination of HIV-neutralizing monoclonal antibodies, particularly PGT121, VRC01, and VRC03, may provide sustained virologic remission in infected individuals following the discontinuation of ART. The above approach is a realistic therapeutic strategy given the potent activities of these antibodies against HIV isolated from the persistent viral reservoir and their likely long-circulating half-lives (25). With the possibility of biochemical modification allowing for a more prolonged plasma half-life (25), it is conceivable that HIV-infected individuals could potentially maintain very low or undetectable plasma viremia in the absence of ART following relatively infrequent administration of these antibodies. Finally, clinical trials involving passive immunization should include prescreening of HIV isolates from the persistent viral reservoirs of infected individuals with a panel of HIV-specific antibodies, to identify those that manifest the most potent suppressive activity against the patient viral isolates.
Materials and Methods
Study Subjects.
Twenty-nine HIV-infected individuals receiving ART for a mean of 2.6 y were included in this study (Table S1). All study participants were receiving various combination antiretroviral drug regimens, and all maintained undetectable levels of plasma viremia (<50 copies per mL) at the time of study. Leukapheresed products were collected from the study subjects in accordance with clinical protocols approved by the Institutional Review Boards of the National Institute of Allergy and Infectious Diseases and the University of Toronto, Canada, and by the Office of Human Subjects Research at the National Institutes of Health. All study subjects provided informed consent.
Isolation of CD4+ T Cells.
Peripheral blood mononuclear cells (PBMCs) were obtained from leukapheresis by Ficoll–Hypaque density gradient centrifugation. Resting CD4+ T cells were isolated from the PBMCs of HIV-infected individuals by using an automated separation technique (StemCell Technologies) followed by depletion of CD25+, CD69+, and HLA-DR+ CD4+ T cells using microbeads (Miltenyi Biotech). The purity of enriched resting CD4+ T cells was generally >98% assessed by flow cytometry. Activated CD4+ T cells were isolated from PBMCs of healthy seronegative donors by using an automated separation technique (StemCell Technologies) following 2-d stimulation with anti-CD3 antibody.
Preparation of HIV Isolates from Latently Infected, Resting CD4+ T Cells.
Culture supernatants containing cell-free HIV were prepared by stimulating resting CD4+ T cells obtained from HIV-infected individuals whose plasma viremia was controlled on ART with plate-bound anti-CD3 and soluble CD28 antibodies for up to 5 d. The titer of the virus in the supernatant was determined by Cobas Ampliprep/Cobas Taqman HIV-1 Test (Version 2.0; Roche Diagnostics). To obtain sufficient amounts of viral stock for the viral entry assay, the above HIV isolates were briefly propagated in CD8-depleted anti-CD3 antibody-stimulated PBMCs from HIV-seronegative healthy donors. The concentration of the virus was determined by HIV p24 ELISA.
Detection of Virions Bound to HIV-Specific Mononuclear Antibodies.
A total of 5 µg of each HIV-specific monoclonal antibody to be evaluated was incubated with magnetic Protein A beads (Life Technologies) in 200 µL of PBS-0.02% Tween 20 at room temperature for 10 min. The antibody–bead conjugates were then washed with 100 µL of PBS-0.02% Tween 20 and incubated with the cell-free virions equivalent to 50,000–100,000 copies of HIV RNA at 4 °C for 90 min. Tubes containing antibody–bead–HIV were then placed in a magnet, and the contents in the tubes were washed four times with 500 µL of PBS containing 2% FBS to eliminate unbound virions. The antibody–virion complexes were then detached from magnetic Protein A beads by using 40 µL of 50 mM glycine buffer (pH 2.8) followed by neutralization of the solutions using 60 µL of PBS containing 4 µL of 1 M Tris (pH 8.0). The above steps were repeated, and 50% of the volume containing HIV was subjected to Cobas Ampliprep/Cobas Taqman HIV-1 Test (Version 2.0; Roche Diagnostics) to determine the copy number of virion-associated HIV RNA. Multiple control and validation experiments were carried out before formally conducting each assay by using patient HIV isolates. Each experiment was conducted once.
Assessment of Inhibition of Viral Entry by HIV-Specific Mononuclear Antibodies.
HIV (0.5–1ng of HIV p24) was incubated with each HIV-specific monoclonal antibodies and human IgG (20 µg/mL) as a control for 90 min. The antibody–HIV complex was then added to 1 × 106 activated CD4+ T cells from healthy donors and incubated for 2 d in 48-well plates. Each set of experiments was conducted by using activated CD4+ T cells from a single HIV-negative donor. The enriched CD4+ T cells were highly activated (judging by forward and side scatter on flow cytometry and the color of medium), and the viability of the target cells was generally >90–95% on the day of assay. Following the incubation period, cells were washed twice, trypsinized, and lysed for DNA isolation. To determine the frequency of CD4+ T cells carrying HIV DNA, 2.5 µg of DNA was digested with XbaI, and 500 ng of DNA was subjected to droplet digital PCR (Bio-Rad Laboratories) according to the manufacturer’s specifications. The amplification reaction was carried out by using HIV-specific primers and probe and RPP30 (housekeeping gene)-specific primers and probe. The following primers were used for amplification of HIV LTR: 5′-GGTCTCTCTGGTTAGACCAGAT-3′ (5′ primer) and 5′-CTGCTAGAGATTTTCCACACTG-3′ (3′ primer) along with the fluorescent probe 5′-6FAM-AGTAGTGTGTGCCCGTCTGTT-IABkFQ-3′. The following primers were used for amplification of RPP30: 5′-GATTTGGACCTGCGAGCG-3′ (5′ primer) and 5′-GCGGCTGTCTCCACAAGT-3′ (3′ primer) along with the fluorescent probe 5′-HEX-TTCTGACCTGAAGGCTCTGCGC-IABkFQ-3′. Multiple control and validation experiments were carried out before formally conducting each assay by using patient HIV isolates. Each experiment was conducted once.
Effect of HIV-Neutralizing Antibodies on Viral Replication in Autologous CD4+ T Cells.
To examine the capacity of HIV-neutralizing antibodies to block viral replication, highly enriched CD4+ T cells from each of nine study subjects were stimulated with plate-bound anti-CD3 and soluble CD28 antibodies with two different HIV-neutralizing (individually and in combination) and the control antibodies (20 µg/mL). Given that the frequency of cells carrying replication-competent HIV is relatively low in infected individuals receiving long-term ART, CD4+ T cells were isolated from the time points at which the study subjects became aviremic (<50 copies of HIV RNA per mL) for the first time. The culture supernatants were removed every 3 d for HIV p24 ELISA, and fresh medium containing antibodies and interleukin-2 was added.
Statistical Methods.
The fold changes of the binding of virions to HIV-neutralizing antibodies over the control antibody (human IgG) were compared by using the studentized range test, which adjusts the level of significance for multiple comparisons. The data were skewed and therefore logged. The log suppression of HIV entry over the control antibody (human IgG) was compared by using the studentized range test, which adjusts the level of significance for multiple comparisons.
Supplementary Material
Acknowledgments
We thank Dr. Dennis Burton and the National Institutes of Health AIDS Reagent Program for providing HIV-specific mononuclear antibodies. We also thank the study volunteers for participating in this study. This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
*Henrich TJ, et al., Conference on Retroviruses and Opportunistic Infections, March 3–6, 2014, Boston, MA, abstr. 144LB.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414148111/-/DCSupplemental.
References
- 1.Chun TW, Fauci AS. HIV reservoirs: Pathogenesis and obstacles to viral eradication and cure. AIDS. 2012;26(10):1261–1268. doi: 10.1097/QAD.0b013e328353f3f1. [DOI] [PubMed] [Google Scholar]
- 2.Burton DR, et al. A blueprint for HIV vaccine discovery. Cell Host Microbe. 2012;12(4):396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fauci AS, Marston HD. Ending AIDS—is an HIV vaccine necessary? N Engl J Med. 2014;370(6):495–498. doi: 10.1056/NEJMp1313771. [DOI] [PubMed] [Google Scholar]
- 4.Moir S, Malaspina A, Fauci AS. Prospects for an HIV vaccine: Leading B cells down the right path. Nat Struct Mol Biol. 2011;18(12):1317–1321. doi: 10.1038/nsmb.2194. [DOI] [PubMed] [Google Scholar]
- 5.Kwong PD, Mascola JR. Human antibodies that neutralize HIV-1: Identification, structures, and B cell ontogenies. Immunity. 2012;37(3):412–425. doi: 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou T, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329(5993):811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker LM, et al. Protocol G Principal Investigators Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6(2):207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura Y, et al. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: Implications for HIV-1 vaccine development. Proc Natl Acad Sci USA. 2003;100(25):15131–15136. doi: 10.1073/pnas.2436476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trkola A, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11(6):615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 11.Klein F, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492(7427):118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shingai M, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503(7475):277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barouch DH, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503(7475):224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horwitz JA, et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci USA. 2013;110(41):16538–16543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malbec M, et al. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J Exp Med. 2013;210(13):2813–2821. doi: 10.1084/jem.20131244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chun TW, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94(24):13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 18.Wong JK, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 19.Davey RT, Jr, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999;96(26):15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deeks SG, et al. International AIDS Society Scientific Working Group on HIV Cure Towards an HIV cure: A global scientific strategy. Nat Rev Immunol. 2012;12(8):607–614. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med. 2014;20(4):425–429. doi: 10.1038/nm.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwong PD, Mascola JR, Nabel GJ. Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning. Nat Rev Immunol. 2013;13(9):693–701. doi: 10.1038/nri3516. [DOI] [PubMed] [Google Scholar]
- 23.Pancera M, et al. Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1-V2-directed antibody PG16. Nat Struct Mol Biol. 2013;20(7):804–813. doi: 10.1038/nsmb.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonomelli C, et al. The glycan shield of HIV is predominantly oligomannose independently of production system or viral clade. PLoS ONE. 2011;6(8):e23521. doi: 10.1371/journal.pone.0023521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6(5):343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.