Significance
The metabolic costs of brain development are thought to explain the evolution of humans’ exceptionally slow and protracted childhood growth; however, the costs of the human brain during development are unknown. We used existing PET and MRI data to calculate brain glucose use from birth to adulthood. We find that the brain’s metabolic requirements peak in childhood, when it uses glucose at a rate equivalent to 66% of the body’s resting metabolism and 43% of the body’s daily energy requirement, and that brain glucose demand relates inversely to body growth from infancy to puberty. Our findings support the hypothesis that the unusually high costs of human brain development require a compensatory slowing of childhood body growth.
Keywords: neuroimaging, diabetes, human evolution, neuronal plasticity, anthropology
Abstract
The high energetic costs of human brain development have been hypothesized to explain distinctive human traits, including exceptionally slow and protracted preadult growth. Although widely assumed to constrain life-history evolution, the metabolic requirements of the growing human brain are unknown. We combined previously collected PET and MRI data to calculate the human brain’s glucose use from birth to adulthood, which we compare with body growth rate. We evaluate the strength of brain–body metabolic trade-offs using the ratios of brain glucose uptake to the body’s resting metabolic rate (RMR) and daily energy requirements (DER) expressed in glucose-gram equivalents (glucosermr% and glucoseder%). We find that glucosermr% and glucoseder% do not peak at birth (52.5% and 59.8% of RMR, or 35.4% and 38.7% of DER, for males and females, respectively), when relative brain size is largest, but rather in childhood (66.3% and 65.0% of RMR and 43.3% and 43.8% of DER). Body-weight growth (dw/dt) and both glucosermr% and glucoseder% are strongly, inversely related: soon after birth, increases in brain glucose demand are accompanied by proportionate decreases in dw/dt. Ages of peak brain glucose demand and lowest dw/dt co-occur and subsequent developmental declines in brain metabolism are matched by proportionate increases in dw/dt until puberty. The finding that human brain glucose demands peak during childhood, and evidence that brain metabolism and body growth rate covary inversely across development, support the hypothesis that the high costs of human brain development require compensatory slowing of body growth rate.
A prolonged period of childhood and juvenile growth is a defining feature of human life history (1–3). Compared with other great apes, human offspring are weaned early, leading to an extended period of dependence on procured resources rather than breast milk (1, 4). Although this unique human reproductive pattern is viewed as shortening the interbirth interval and thus increasing fertility (5, 6), what is less clear is why humans also grow so slowly during childhood. Although most primates grow slower than other mammals (7), human childhood and juvenile growth stand out as unusually slow even by primate and great ape standards, during which it proceeds at a pace more typical of reptiles than of mammals (8, 9). In humans, a sizeable percentage of preadult growth is deferred until the pubertal growth spurt, when growth rate markedly increases and adult size is achieved (1).
Many hypotheses have been proposed to explain this slow and prolonged preadult life-stage, with most pointing to the extra time and energy required for human learning and brain development (5, 10–12). It has long been assumed that human cultural practices are sufficiently complex that they take many years to learn, which could have selected for a slowing down and extension of preadult development (13). A recent variant of this idea notes the importance of complex extractive techniques to human foraging success, and proposes that humans have a long preadult stage to facilitate mastery of these skills (2).
Other hypotheses have focused on energetic trade-offs and viewed slowed growth as compensation for the brain’s high energetic needs (1, 14–19). Because of its large size, the human brain has unusually high energy costs (15, 20, 21), which are particularly elevated compared with the body’s metabolic budget early in the life cycle (18, 22). It has been estimated that the human brain accounts for between 44% and 87% of resting metabolic rate (RMR) during infancy, childhood, and adolescence (23–25), suggesting strong trade-offs with other functions. The human brain’s demand for energy is sufficiently high during these periods that it could require that the body expend less on growth, thus slowing and prolonging the preadult period (1, 14–18, 26). This reasoning leads to the expectation that the ages of slowest body growth will coincide developmentally with peak brain metabolic needs.
Although the energetic trade-off concept is widely cited, the ability to test it against alternative hypotheses (2, 5, 10, 11) has been hampered by limitations of prior measures of brain metabolism during human growth. Direct estimates of total brain energy use have been based upon the nitrous oxide (N2O) method, which quantifies brain oxygen consumption and estimates energy expenditure based on an assumption that glucose exclusively enters oxidative phosphorylation (24). However, recent work shows that the rate of glucose uptake exceeds oxygen consumption in the brain (27), with up to 30% of brain glucose not entering oxidative phosphorylation during childhood (28). This additional use of glucose, in aerobic glycolysis, contributes to protein synthesis associated with synaptic growth and other important developmental functions (28–30), yet is not reflected in measures of oxygen consumption like N2O, which therefore underestimate total brain glucose uptake and use.
Although past work points to high metabolic requirements of the growing human brain, data are available for only a small number of individuals of restricted age range, which limits their utility for evaluating trade-offs between brain metabolism and body growth during development. The only previous attempt to derive a growth curve for brain metabolism that we are aware of assumed that the mass-specific metabolic rate of the brain, measured using the N2O method (24), is stable at adult-like levels across development (25, 31). Using this method, it was estimated that the brain accounts for 87% of RMR at birth, and that this fraction then steadily declines as the brain-to-body mass ratio decreases with age (25). This finding, if correct, would not support the hypothesis that ages of slowest body growth, in childhood, coincide with ages of peak brain metabolic requirements.
In contrast with the assumption that the per-gram brain metabolic rate is stable with age, PET studies show that glucose uptake in the cerebral cortex is more than twice as high during early- to midchildhood than in adulthood (32). This dynamism reflects the additional energetic costs associated with overproliferation of neuronal processes and synapses before activity-dependent pruning in late childhood and adolescence (33, 34), along with aerobic glycolysis, which is thought to rise in support of synaptic growth (27–29). In contrast, at birth, before extensive postnatal synaptic proliferation and the corresponding rise in aerobic glycolysis, PET-derived glucose uptake is 20–30% lower than in adults (32). Although past PET studies provide insights into the dynamics of glucose use at various stages of development, these studies only report point estimates in specific brain structures (32, 35), thus leaving the costs of the entire human brain during development—and potential evolutionary trade-offs with body growth—uncertain.
To quantify the metabolic costs of the human brain, in this study we used a unique, previously collected age series of PET measures of brain glucose uptake spanning birth to adulthood (32), along with existing MRI volumetric data (36), to calculate the brain’s total glucose use from birth to adulthood, which we compare with body growth rate. We estimate total brain glucose uptake by age (inclusive of all oxidative and nonoxidative functions), which we compare with two measures of whole-body energy expenditure: RMR, reflecting maintenance functions only, and daily energy requirements (DER), reflecting the combination of maintenance, activity, and growth. We hypothesized that ages of peak substrate competition (i.e., competition for glucose) between brain and body would be aligned developmentally with the age of slowest childhood body growth, and more generally that growth rate and brain glucose use would covary inversely during development, as is predicted by the concept of a trade-off between brain metabolism and body growth in human life-history evolution.
Results
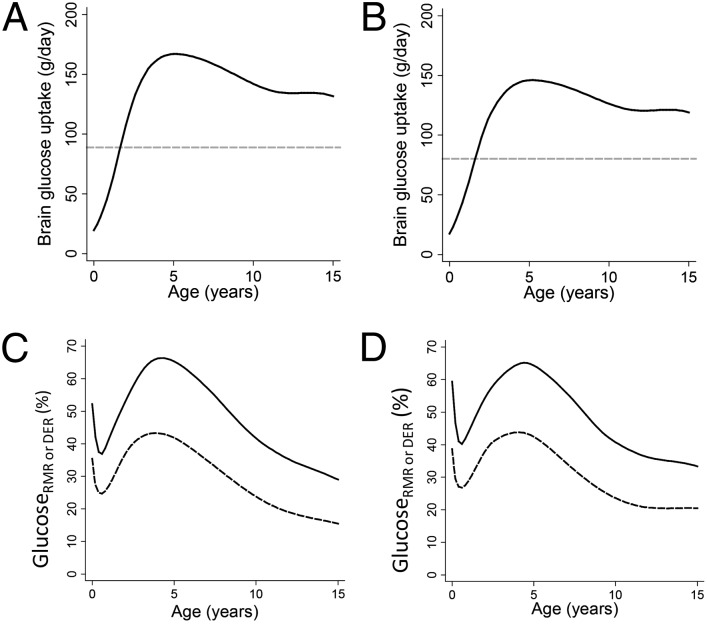
We first fit continuous functions to point estimates, inclusive of gray and white matter, of glucose uptake for the cerebrum, brainstem, and cerebellum in 29 individuals (32) ranging in age from birth to late adolescence (SI Appendix, Fig. S1). Regional glucose uptake rates were then multiplied by regional brain weights to obtain the daily grams of glucose consumed by the entire brain in both oxidative metabolism and aerobic glycolysis (for details, see Materials and Methods). Daily glucose use by the brain peaks at 5.2 y of age at 167.0 g/d and 146.1 g/d in males and females, respectively. These values represent 1.88- and 1.82-times the daily glucose use of the brain in adulthood (Fig. 1 A and B and SI Appendix, Fig. S2), despite the fact that body size is more than three-times as large in the adult.
Fig. 1.
Glucose use of the human brain by age. (A) Grams per day in males. (B) Grams per day in females; dashed horizontal line is adult value (A and B). (C) Glucosermr% (solid line) and glucoseder% (dashed line) in males. (D) Glucosermr% (solid line) and glucoseder% (dashed line) in females.
To evaluate developmental changes in the strength of substrate competition between the brain and body, we next calculated the body’s RMR and DER by entering the body weights (SI Appendix, Fig. S3) of the individuals for whom brain weights were obtained (37) into age- and sex-specific predictive equations (38). To allow direct comparison with brain glucose uptake, RMR (SI Appendix, Fig. S4) and DER (SI Appendix, Fig. S5) were first converted to grams of glucose equivalents (1 g glucose = 3.72 kcal of energy expenditure) (39). We expressed metabolic competition between brain and body as glucosermr% and glucoseder%, calculated as the ratios between brain glucose uptake and daily glucose-gram equivalent RMR or DER, respectively. These ratios do not indicate the percent of RMR or DER used by the brain because some brain glucose uptake (the numerator) is not used in energy production (28). Instead, glucosermr% and glucoseder% can be interpreted as the fraction of the body’s RMR or DER that could be met by the quantity of glucose consumed by the brain (see SI Appendix, SI Materials and Methods for details). In males and females, respectively, total brain glucose uptake is the equivalent of 52.5% and 59.8% of RMR at birth, drops to 37.5 and 40.8% of RMR in the first half-year, then rises to a lifetime peak of 66.3% and 65.0% of RMR by 4.2–4.4 y (Fig. 1 C and D and SI Appendix, Fig. S6). Although the brain accounted for a smaller fraction of DER, the pattern of glucose consumption relative to DER was very similar to that for RMR (Fig. 1 C and D): glucoseder% accounted for the equivalent of 35.4% and 38.7% of DER at birth, declined to 24.7% and 26.8% by 7 mo, before rising to peak levels of 43.3% at 3.8 y (males) and 43.8% at 4 y (females). Adult glucosermr% was 19.1% and 24.0% whereas glucoseder% was 10.9% and 15.0% in males and females, respectively.
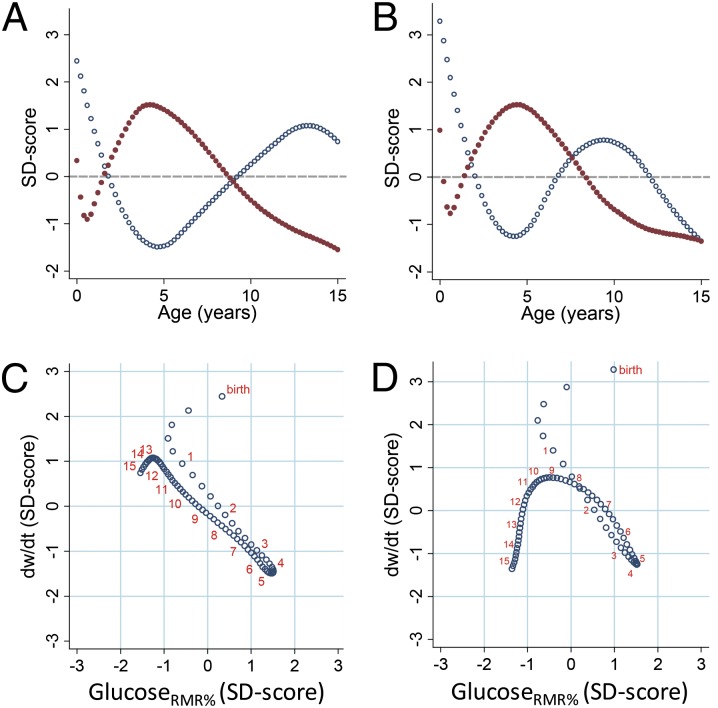
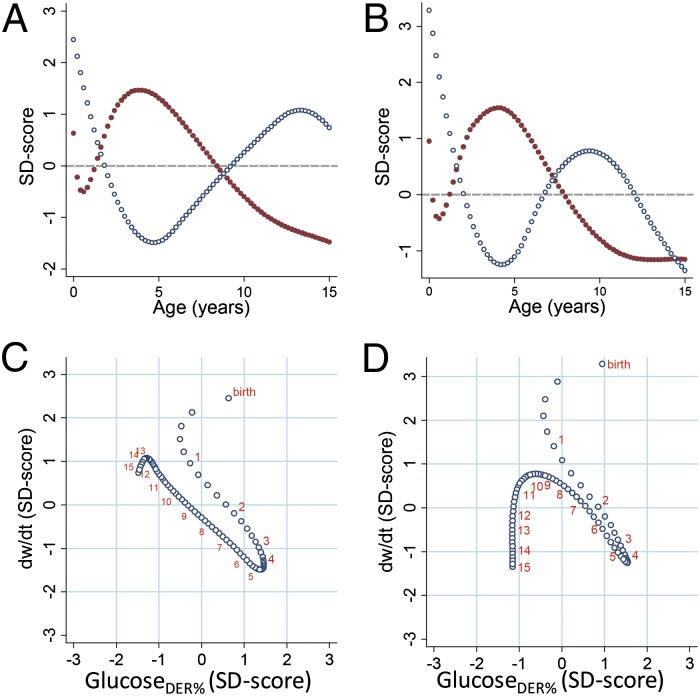
Finally, we compared age changes in glucosermr% and glucoseder% with body-weight growth velocity, calculated as the derivative (dw/dt) of the function linking body weight with age. Plotting unitless SD scores of glucosermr% and dw/dt against age reveals opposing age trends in the two measures starting at 6 mo of age (Fig. 2 A and B). When SD scores of dw/dt are plotted against those of glucosermr% (Fig. 2 C and D), the first 6 mo are characterized by a fast but decelerating rate of body-weight growth and a parallel reduction in glucosermr%. Starting at 6 mo, each increase in glucosermr% is matched by a proportionate decrease in dw/dt, with peak glucosermr% and the trough of dw/dt co-occurring. After the childhood peak in brain glucose demands, each subsequent age-related decline in glucosermr% is accompanied by a proportionate increase in dw/dt until onset of pubertal weight gain (∼12–13 y in males and ∼9–10 y in females), after which slower growth is accompanied by declining glucosermr% as both body size and brain metabolism approach adult levels. Very similar results were obtained when brain glucose was evaluated in relation to DER (Fig. 3). For reference, predicted values of key variables are reported for males and females in SI Appendix, Tables S1 and S2, respectively.
Fig. 2.
Glucosermr% and body-weight growth rate. Glucosermr% and weight velocities plotted as SD scores to allow unitless comparison. (A) Glucosermr% (red dots) and dw/dt (blue dots) by age in males. (B) Glucosermr% (red dots) and dw/dt (blue dots) by age in females. (C) Weight velocity vs. glucosermr% in males. (D) Weight velocity vs. glucosermr% in females. For reference, orange numbers indicate ages at yearly intervals (C and D).
Fig. 3.
Glucoseder% and body-weight growth rate. Glucoseder% and weight velocities plotted as SD scores to allow unitless comparison. (A) Glucoseder% (red dots) and dw/dt (blue dots) by age in males. (B) Glucoseder% (red dots) and dw/dt (blue dots) by age in females. (C) Weight velocity vs. glucoseder% in males. (D) Weight velocity vs. glucoseder% in females. For reference, orange numbers indicate ages at yearly intervals (C and D).
Discussion
Our findings agree with past estimates indicating that the brain dominates the body’s metabolism during early life (31). However, our PET-based calculations reveal that the magnitude of brain glucose uptake, both in absolute terms and relative to the body’s metabolic budget, does not peak at birth but rather in childhood, when the glucose used by the brain comprises the equivalent of 66% of the body’s RMR, and roughly 43% of total expenditure. These findings are in broad agreement with past clinical work showing that the body’s mass-specific glucose production rates are highest in childhood, and tightly linked with the brain’s metabolic needs (40). Whereas past attempts to quantify the contribution of the brain to the body’s metabolic expenditure suggested that the brain accounted for a continuously decreasing fraction of RMR as the brain-to-body weight ratio declined with age (25, 31), we find a more complex pattern of substrate trade-off. Both glucosermr% and glucoseder% decline in the first half-year as a fast but decelerating pace of body growth established in utero initially outpaces postnatal increases in brain metabolism. Beginning around 6 mo, increases in relative glucose use are matched by proportionate decreases in weight growth, whereas ages of declining brain glucose uptake in late childhood and early adolescence are accompanied by proportionate increases in weight growth. The relationships that we document between age changes in brain glucose demands and body-weight growth rate are particularly striking in males, who maintain these inverse linear trends despite experiencing threefold changes in brain glucose demand and body growth rate between 6 mo and 13 y of age. In females, an earlier onset of pubertal weight gain leads to earlier deviations from similar linear inverse relationships.
Our results shed light on several unique features of human life-history evolution. The long period of slow human childhood and juvenile growth has alternately been viewed as, among other hypotheses, necessary for learning of complex foraging skills (2), as a by-product of selection for lifespan extension (5), as allowing greater activity devoted to subsistence tasks that contribute to the family’s pooled energy budget (10), as reducing the dietary burden on human mothers raising multiple overlapping dependent offspring (11), or as compensation for high brain energy needs (1, 14–18). Our finding of a strong, inverse relationship between developmental changes in the brain’s glucose uptake and body-weight growth rate supports the last hypothesis. It is notable that the increase in body growth rate at puberty—which is unusually pronounced in humans—is deferred to an age when brain glucose uptake is greatly reduced (1, 26, 41). We interpret our finding of an inverse relationship between the brain’s demand for glucose and body-weight growth rate as support for the hypothesis that human brain development is sufficiently costly to require a compensatory reduction in expenditure on body growth, thus helping explain our unusually slow rate of childhood growth and resultant delay in attainment of adult size and sexual maturity compared with other closely related great apes.
The childhood peak in brain glucose uptake is greatest in the cerebrum, and less pronounced in the brainstem and cerebellum (32). These patterns suggest that peak brain glucose uptake during childhood reflects neuronal plasticity in the cerebral cortex (32), which involves overproliferation of energetically costly dendritic arbors and synapses before activity-dependent pruning (33, 34). Recent work shows that glucose uptake outpaces oxygen use in the human brain, and that this imbalance also peaks at the age of greatest glucose uptake in childhood, pointing to an important role of aerobic glycolysis in support of synaptic proliferation and growth (28, 30). Collectively, this large increase in glucose uptake corresponds closely with the age of slowest body-weight growth.
Although we find that body growth rate and brain substrate use covary inversely during human development, pointing to a likely trade-off between these functions, our findings lead to the more general prediction that other costly somatic or physiological expenditures will also be reduced at this age to free up energy and substrate to support brain development. Consistent with this perspective, recent studies of total energy expenditure (TEE) show that typical physical activity levels (PAL = TEE/RMR) for preschool age children (3–5 y) are lower than in later childhood and adolescence (38, 39, 42). These findings suggest that, like the body’s very low expenditure on somatic growth, activity-related (discretionary) expenditure during human development is also comparatively low at ages of peak brain metabolic demand.
Pontzer et al. (43) recently showed that humans and other primates have reduced TEEs for their body sizes, despite having basal metabolic rates (BMRs) consistent with those of other mammals. The authors conclude that a reduced rate of energy throughput helps explain some shared features of primate life histories, including slower growth compared with other mammals. Pontzer et al. speculate that high brain energy needs might account for the fact that primate BMRs are not similarly reduced, but they also note extensive life-history variation among primates that traces to differences in allocation priorities. Our findings complement these data and implicate the high energy and substrate requirements of the brain, especially during the ages of most rapid development and learning, as a likely cause of life-history differences between humans and other closely related primates, such as chimpanzees.
Because the costs of neocortical synaptic growth and metabolism comprise a major endpoint for glucose in the brain, examination of the generalizability of the brain energetics–body growth trade-off hypothesis can be informed by data on postnatal synaptogenesis in other primates, which are available for humans, chimpanzees, and macaques. In all three species neocortical synapse densities are doubled during growth, compared with densities at birth. In macaques the period of peak synaptic density occurs during infancy, whereas in humans and chimpanzees peak synaptic densities take place during the midjuvenile or (in humans) childhood period (44). Brain energetics are thus not likely to impose a strong constraint on later juvenile body growth in macaques, whereas in chimpanzees growth is predicted to be slowed in the juvenile period, but to a lesser degree than in humans because of their smaller brains and corresponding lower brain metabolic needs (22). Comparative growth data support this prediction (41), showing that, unlike humans, chimpanzees and other great apes do not display marked declines in weight velocities after infancy. Additionally, humans are not distinct in having a “growth spurt” but in delaying it until after a long period of slow childhood growth (41).
Our findings also lead to the prediction that a slowing of preadult growth and increase in brain metabolism coevolved in the course of human evolution. Estimates of body growth from fragmentary and limited fossil hominin remains can be complicated; nonetheless, current data on hominin dental emergence, enamel growth rate, and skeletal growth suggest that a more extended period between weaning and sexual maturity began to appear at least 1.5 million y ago in Homo erectus (45). Data on dental eruption point to coevolution between brain expansion and maturational delay (45, 46), with a fully modern pattern of delayed physical growth not emerging until the origin of anatomically modern humans (47, 48). Although these findings are generally consistent with the hypothesis that brain expansion has set the pace for changes in body-size growth in the hominin lineage, our results underscore the challenges of interpreting the strength of metabolic trade-offs related to human brain evolution from data on the cranial capacity of fossils alone (e.g., ref. 14). In the modern human sample evaluated here, an average gram of brain tissue at 4 y uses more than 2.5-times the glucose of a gram of brain at birth (see data in SI Appendix, Tables S1 and S2). Absolute brain glucose requirements peak at ∼5 y, several years before final brain size is achieved, and adult brain glucose requirements are only half those at age 5 y. This uncoupling of the energetic requirements of the brain from brain size reflects developmental dynamics in substrate-intensive processes related to neural plasticity and learning, which are not preserved in fossil samples. In this context, it is notable that, despite having similar cranial capacities, dental eruption data suggest that Neanderthals grew more rapidly than modern humans (48).
Although it is not possible to precisely evaluate the coevolution between brain metabolic requirements and slowing of body growth from the fossil record, the ontogeny of brain metabolism that we document provides clues into the ecological strategies that were likely required for the modern human pattern of brain development to evolve. It has been noted that the high and inflexible glucose requirements of the developing human brain make it susceptible to impairment from nutritional shortfall (18). Despite this finding, we find that peak brain glucose uptake occurs after the age of complete weaning from breast milk in most human societies (4), and when the energetic buffer of body fat stores are near their lifetime nadir (18). In light of this finding, it seems likely that the evolution of human encephalization required cultural or social strategies to buffer the energy intake of dependent offspring. Rudimentary hunting and gathering economies appeared with the emergence of the genus Homo and are believed to have been important in supporting early brain expansion (49–51). A shift to calorically dense and easily digested foods, and greater food sharing among social groups, would have increased the nutritional quality and stability of the diet (14, 15, 20). Although direct evidence for childcare strategies are not preserved in the fossil record, recent comparative analyses suggest that cooperative care in raising and provisioning young was likely important in achieving levels of encephalization seen in Homo sapiens (52, 53). A system in which extended social networks provisioned food for children, combined with shifts to calorically dense and easily digested foods procured through hunting (14, 15), would have allowed the costs of human brain development to be widely distributed and also buffered against shortfall. This shift in the social context of human child rearing, occurring together with a protracted postnatal development of cortical connectivity (54, 55), likely created new opportunities for imitative learning and cumulative, intergenerational development of cultural traditions to become central features of the human adaptive complex (56, 57).
Our results also highlight underappreciated implications of the high glucose requirements of the human brain for medicine and public health. Diabetes is a condition in which tissues (primarily muscle) fail to respond appropriately to insulin, thus reducing glucose uptake. Although an important precursor to type 2 diabetes, peripheral insulin resistance is also effective at shunting scarce glucose to high-demand tissues or organs, such as to the fetus during the mild diabetes of pregnancy (58), or to the brain during the insulin-resistance triggered by stress (59). Changes in insulin secretion are believed to help coordinate glucose delivery to the brain as brain size and energy requirements change during development (60, 61). The present finding that the brain’s requirement for glucose peaks after weaning, at an age when body fat stores are minimal (18), points to strong selection on physiologic mechanisms to redirect glucose delivery to the brain during nutritional stress. Studies report interactions between fetal undernutrition or small birth size and childhood weight gain as predictors of diabetes risk, suggesting an important role for childhood energetics and substrate availability in diabetes development (62, 63). Although normative data on developmental changes in insulin sensitivity during child development are lacking (64), our findings lead to the prediction that facultative buffering of substrate supply to the brain (e.g., insulin resistance induced acutely by stress) will be prominent at 4–5 y of age (59).
Measuring brain metabolism in living children poses unique challenges that warrant discussion. For ethical reasons, there is no study that includes PET and MRI data in the same healthy individuals spanning birth to adulthood. Consequently, we used brain volumetrics and glucose uptake rates obtained from two different United States samples deemed developmentally normal before screening (MRI) or after clinical assessments for possible abnormalities turned up negative (PET) (see Materials and Methods for more details). An additional limitation is that our analyses are based on cross-sectional brain and body-weight data, which pool fast- and slow-maturing individuals; this pooling can attenuate the apparent magnitude of the pubertal growth spurt while also obscuring the variable timings of its onset (65). Analytic techniques that account for variation in maturational tempo, such as centering of individual growth curves on maturational indices rather than chronological age (65), would require repeat PET imaging in healthy individuals, which, as noted, is not ethical. The age of the trough in body-weight growth rate estimated from the cross-sectional data used here is consistent with mixed-longitudinal reference data showing lowest rates of weight gain from 4 to 4.5 y (66), and the latest World Health Organization (WHO) report on human energy requirements similarly finds that caloric needs for body growth are lowest at 4–5 y (38). Importantly, the cross-sectional body weights that we use to calculate RMR and the brain masses used to calculate brain glucose uptake were obtained from the same individuals, suggesting that our approach provides a useful basis for evaluating the changing relationship between brain metabolism and body size across a large sample varying widely in age and developmental stage.
In sum, we find that the rate of glucose uptake by the human brain, in both absolute terms and relative to the body’s metabolic expenditure, does not peak at birth, when the size of the brain relative to the body is largest, but in childhood, when synaptic densities and related metabolic processes are maximal. These new estimates show that the ages when substrate trade-offs between the brain and body are greatest in humans coincide developmentally with the ages of slowest childhood body-weight growth, and that age-related changes in relative brain glucose needs and body-weight gain are tightly, inversely related from early infancy until puberty. These findings thus provide rare empirical support for the hypothesis that humans evolved a protracted period of slow preadult growth to compensate for the unusually high metabolic costs of brain development underlying the extraordinary human capacity for cultural learning.
Materials and Methods
PET Procedure.
As described elsewhere (32, 67), glucose uptake was measured by one of the authors (H.T.C.) using PET in 36 individuals, including 7 healthy young adult volunteers (ages 19–30 y; mean 24.4 y; five males, two females) and 29 children (age range: birth to 15.5 y). The lCMRGlc values in the 29 children were compared with those of the seven healthy young adult volunteers, whose detailed neurological and psychological examinations disclosed no abnormalities. Written informed consent for the PET procedures was obtained directly from adult participants, and for minors, from parents, with institutional review board oversight by the University of California at Los Angeles Human Subject Protection Committee. Additional details of the PET protocol and sample have been described previously (32) and are discussed in the SI Appendix, SI Methods and Materials.
Continuous functions were fit separately to glucose uptake from the cerebrum, brainstem, and cerebellum (SI Appendix, Fig. S1). We first evaluated the fit of quadratic and cubic polynomial models using the Akaike Information Criterion (AIC). This process yielded quadratic models as best fitting for cerebellum and brainstem glucose uptake. Glucose uptake in the cerebrum followed a more complex age trend, which we modeled using a cubic spline function. Using the mkspline statistic in Stata v.11, we varied the number of knots, which were placed at age quantiles, and used the AIC to evaluate overall goodness-of-fit (68), yielding a 3-knot curve. Predicted values were calculated at 0.2-y intervals to allow the PET data to be combined with volumetric data and to calculate derived variables.
Volumetric Brain MRI Data.
Total and regional brain volumes for 402 healthy males and females 4.6–20 y of age were obtained from the database established by the National Institutes of Health MRI Study of Normal Brain Development (36), of which one of us (N.L.) is an affiliate. Cerebrum was defined as whole-brain volume excluding the latter two structures. Longitudinal mixed-effects growth curve models were fit to these data, using the AIC for model selection, to yield the estimated regional volumes used here, with volumes predicted at 0.2-y increments. Additional details of the brain volumetry in this sample are described elsewhere (36) and in the SI Appendix, SI Materials and Methods.
We augmented the longitudinal MRI series with data from published and unpublished MRI studies that included total brain volume and volumes of the cerebrum, cerebellum, and brainstem in normal healthy subjects aged birth to 4.6 y (SI Appendix, Table S3). Because the MRI estimates before and after 4.6 y of age were derived from different populations with different body and brain sizes, it was not possible to splice absolute brain-region volumes to form single birth–adulthood growth curves. We therefore converted brain region volumetric data from the various studies to regional weights [specific gravities: cerebrum 1.0335; brainstem 1.0277; cerebellum 1.0375 (69)], allowing calculation of the percentage of totally brain weight (TBW) accounted for by cerebrum, cerebellum, and brainstem (SI Appendix, Fig. S7), which were then multiplied by brain weights from the Dekaban and Sadowsky (37) brain-weight series spanning birth to adulthood. This study reports brain weights from autopsy records limited to the subset in which the brain was weighed soon after death and there was no indication of brain pathology, growth disruption, or gross overweight (n = 1,004 from birth to 15 y). To our knowledge, this highly cited data series is the only one available that reports brain and body weights from the same individuals, and that spans birth to adulthood. Using these data, we first modeled TBW with Gompertz and polynomial functions (spliced at 3.6 and 2.6 y in males and females, respectively) (SI Appendix, Fig. S8), which were multiplied by percent TBW for each region, yielding age-specific estimates of cerebral, cerebellar, and brainstem weights normalized to the same study sample (SI Appendix, Fig. S9).
Calculation of Total Brain Glucose Use.
Glucose use by the cerebrum, cerebellum, and brainstem were calculated on 0.2-y intervals from the regional PET functions (μmol/min per 100 g), which were multiplied by cerebrum, cerebellum, and brainstem weights. Global brain glucose use was calculated as the sum of cerebral, cerebellar, and brainstem glucose use (SI Appendix, Fig. S2).
RMR and DER.
RMR (reflecting maintenance only) and DER (inclusive of maintenance, activity, and growth) were estimated from the body weights of the individuals included in the Dekaban and Sadowsky series (37). Weight data were first fit with cubic spline functions (SI Appendix, Fig. S3) with predicted values generated at 0.2-y intervals. We then used the most recent age- and sex-specific equations from the 2004 WHO recommendations on human energy requirements (38) to obtain RMR (SI Appendix, Fig. S4) and DER (SI Appendix, Fig. S5). To allow direct comparison with brain glucose uptake (only some of which is destined for use as an energy substrate; see below), we converted RMR and DER to glucose equivalents by multiplying by the conversion 1 kcal = 0.2688 g of glucose (39).
Calculation of Glucosermr% and Glucoseder%.
We quantify the strength of brain-body substrate trade-offs using the ratios glucosermr% and glucoseder%, calculated as the ratio of brain glucose uptake (grams per day) divided by the body’s RMR or DER (converted to daily glucose equivalents, assuming 1 kcal RMR or DER requires 0.2688 g glucose) × 100. These ratios may be interpreted as the percentage of the body’s RMR or DER that could be met by brain glucose uptake if all brain glucose were converted to energy via oxidative phosphorylation (see SI Appendix, SI Materials and Methods for detailed discussion). Because RMR was estimated from WHO age- and sex-specific predictive equations, there were discontinuities at the ages corresponding to changes in the predictive equations (at 3 and 10 y of age) (SI Appendix, Fig. S4). As a final step, we fit cubic spline functions to the male and female glucosermr% data (both 11-kn models), yielding continuous functions that minimized these irregularities (SI Appendix, Fig. S6).
Evaluating Trade-offs Between Brain Substrate Use and Body-Weight Growth.
The derivative of the cubic spline function relating weight to age (dw/dt) was calculated in STATA and used as an estimate of body-weight growth velocity in this cross-sectional sample, which was compared with age changes in glucosermr% and glucoseder%. All variables were first converted to SDscores to allow unitless comparisons.
Calculating Adult Values.
To allow comparison with values at younger ages, mean body weights and TBW from the 19–21 y age bin in Dekaban and Sadowsky (37) (n = 17 females and 64 males), PET data from seven adult healthy volunteers, and MRI volumetry data for 20 y old subjects from the MRI study (36) were used to derive young adult values of RMR, regional brain weights, and brain metabolic rates (38). Adult DER was estimated as adult RMR × a young adult PAL of 1.75 for males and 1.60 for females (38).
Supplementary Material
Acknowledgments
We thank William Johnson for providing statistical advice on cubic spline curve fitting; Kim Hill for providing critical feedback Yarrow Axford for providing manuscript comments; Paul Aljabar and the Centre for the Developing Brain (Kings College, London) for providing unpublished brain volume data for healthy newborns; and three anonymous reviewers for providing critical feedback that strengthened the manuscript. This study was funded in part by National Science Foundation Grant BCS-0827546 (to D.E.W.); National Science Foundation Grant BCS-0827531 (to C.C.S.); James S. McDonnell Foundation Grant 220020293 (to C.C.S.); and National Institutes of Health Brain Development Cooperative Group Grants N01 HD023343, N01 MH090002, N01 NS092314–NS002320 and NS034783.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The MRI data are available through the National Institutes of Health Brain Development Cooperative Group, www.pediatricmri.nih.gov.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323099111/-/DCSupplemental.
References
- 1.Bogin B. Patterns of Human Growth. 2nd Ed. Cambridge, UK: Cambridge Univ Press; 1999. [Google Scholar]
- 2.Kaplan H, Hill K, Lancaster J, Hurtado AM. A theory of human life history evolution: Diet, intelligence and longevity. Evol Anthropol. 2000;9(4):156–185. [Google Scholar]
- 3.Schultz AH. The Life of Primates. London: Weidenfeld & Nicolson; 1969. [Google Scholar]
- 4.Sellen D, Smay D. Relationship between subsistence and age at weaning in “preindustrial” societies. Hum Nat. 2001;12(1):47–87. doi: 10.1007/s12110-001-1013-y. [DOI] [PubMed] [Google Scholar]
- 5.Hawkes K, O’Connell JF, Jones NG, Alvarez H, Charnov EL. Grandmothering, menopause, and the evolution of human life histories. Proc Natl Acad Sci USA. 1998;95(3):1336–1339. doi: 10.1073/pnas.95.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuzawa CW, Bragg JM. Plasticity in human life history strategy: Implications for contemporary human variation and the evolution of genus Homo. Curr Anthropol. 2012;53(Suppl 6):S369–S382. [Google Scholar]
- 7.Charnov EL, Berrigan D. Why do female primates have such long lifespans and so few babies? or life in the slow lane. Evol Anthropol. 1993;1(6):191–194. [Google Scholar]
- 8.Case TJ. On the evolution and adaptive significance of postnatal growth rates in the terrestrial vertebrates. Q Rev Biol. 1978;53(3):243–282. doi: 10.1086/410622. [DOI] [PubMed] [Google Scholar]
- 9.Walker R, Hill K, Burger O, Hurtado AM. Life in the slow lane revisited: Ontogenetic separation between chimpanzees and humans. Am J Phys Anthropol. 2006;129(4):577–583. doi: 10.1002/ajpa.20306. [DOI] [PubMed] [Google Scholar]
- 10.Kramer KL, Ellison PT. Pooled energy budgets: Resituating human energy allocation trade-offs. Evol Anthropol. 2010;19(4):136–147. [Google Scholar]
- 11.Gurven M, Walker R. Energetic demand of multiple dependents and the evolution of slow human growth. Proc Biol Sci. 2006;273(1588):835–841. doi: 10.1098/rspb.2005.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bock J, Sellen D. Childhood and the evolution of the human life course. Hum Nat. 2002;13(2):153–159. doi: 10.1007/s12110-002-1006-5. [DOI] [PubMed] [Google Scholar]
- 13.Konner M. The Evolution of Childhood: Relationships, Emotion, Mind. Cambridge, MA: Belknap; 2010. [Google Scholar]
- 14.Foley RA, Lee PC. Ecology and energetics of encephalization in hominid evolution. Philos Trans R Soc Lond B Biol Sci. 1991;334(1270):223–231, discussion 232. doi: 10.1098/rstb.1991.0111. [DOI] [PubMed] [Google Scholar]
- 15.Leonard WR, Robertson ML. Nutritional requirements and human evolution: A bioenergetics model. Am J Hum Biol. 1992;4(2):179–195. doi: 10.1002/ajhb.1310040204. [DOI] [PubMed] [Google Scholar]
- 16.Navarrete A, van Schaik CP, Isler K. Energetics and the evolution of human brain size. Nature. 2011;480(7375):91–93. doi: 10.1038/nature10629. [DOI] [PubMed] [Google Scholar]
- 17.Leonard WR, Robertson ML, Snodgrass JJ, Kuzawa CW. Metabolic correlates of hominid brain evolution. Comp Biochem Physiol A Mol Integr Physiol. 2003;136(1):5–15. doi: 10.1016/s1095-6433(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 18.Kuzawa CW. Adipose tissue in human infancy and childhood: An evolutionary perspective. Am J Phys Anthropol. 1998;107(Suppl 27):177–209. doi: 10.1002/(sici)1096-8644(1998)107:27+<177::aid-ajpa7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Schuppli C, Isler K, van Schaik CP. How to explain the unusually late age at skill competence among humans. J Hum Evol. 2012;63(6):843–850. doi: 10.1016/j.jhevol.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Aiello LC, Wheeler P. The expensive-tissue hypothesis: The brain and the digestive system in human and primate evolution. Curr Anthropol. 1995;36(2):199–221. [Google Scholar]
- 21.Fonseca-Azevedo K, Herculano-Houzel S. Metabolic constraint imposes tradeoff between body size and number of brain neurons in human evolution. Proc Natl Acad Sci USA. 2012;109(45):18571–18576. doi: 10.1073/pnas.1206390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leigh SR. Brain growth, life history, and cognition in primate and human evolution. Am J Primatol. 2004;62(3):139–164. doi: 10.1002/ajp.20012. [DOI] [PubMed] [Google Scholar]
- 23.Durnin J. Basal Metabolic Rate in Man. Report of a Joint FAO/WHO/UNU Expert Consultation on Energy and Protein Requirements. Glasgow: Univ of Glasgow; 1981. [Google Scholar]
- 24.Kennedy C, Sokoloff L. An adaptation of the nitrous oxide method to the study of the cerebral circulation in children; Normal values for cerebral blood flow and cerebral metabolic rate in childhood. J Clin Invest. 1957;36(7):1130–1137. doi: 10.1172/JCI103509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holliday MA. Body composition and energy needs during growth. In: Falkner F, Tanner JM, editors. Human Growth: A Comprehensive Treatise, Vol 2 Postnatal Growth. 2 Ed. New York: Plenum; 1986. pp. 117–139. [Google Scholar]
- 26.Aiello LC, Wells JCK. Energetics and the evolution of the genus Homo. Annu Rev Anthropol. 2002;31:323–328. [Google Scholar]
- 27.Vaishnavi SN, et al. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci USA. 2010;107(41):17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19(1):49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lunt SY, Vander Heiden MG. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 30.Bauernfeind AL, et al. Aerobic glycolysis in the primate brain: Reconsidering the implications for growth and maintenance. Brain Struct Funct. 2014;219(4):1149–1167. doi: 10.1007/s00429-013-0662-z. [DOI] [PubMed] [Google Scholar]
- 31.Holliday MA. Metabolic rate and organ size during growth from infancy to maturity and during late gestation and early infancy. Pediatrics. 1971;47(Suppl 2):169–179. [PubMed] [Google Scholar]
- 32.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22(4):487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 33.Petanjek Z, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108(32):13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huttenlocher PR. Synaptic density in human frontal cortex —Developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 35.Chugani HT, Phelps ME. Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science. 1986;231(4740):840–843. doi: 10.1126/science.3945811. [DOI] [PubMed] [Google Scholar]
- 36.Brain Development Cooperative Group Total and regional brain volumes in a population-based normative sample from 4 to 18 years: The NIH MRI Study of Normal Brain Development. Cereb Cortex. 2012;22(1):1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dekaban AS, Sadowsky D. Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Ann Neurol. 1978;4(4):345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- 38.Food and Agriculture Organization & World Health Organization . Human Energy Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. Rome: Food and Agricultural Organization of the United Nations; 2004. [Google Scholar]
- 39.Institute of Medicine, Panel on Macronutrients . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 40.Bier DM, et al. Measurement of “true” glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes. 1977;26(11):1016–1023. doi: 10.2337/diab.26.11.1016. [DOI] [PubMed] [Google Scholar]
- 41.Leigh SR. Evolution of human growth spurts. Am J Phys Anthropol. 1996;101(4):455–474. doi: 10.1002/(SICI)1096-8644(199612)101:4<455::AID-AJPA2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 42.Butte NF, et al. Revision of dietary reference intakes for energy in preschool-age children. Am J Clin Nutr. 2014;100(1):161–167. doi: 10.3945/ajcn.113.081703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pontzer H, et al. Primate energy expenditure and life history. Proc Natl Acad Sci USA. 2014;111(4):1433–1437. doi: 10.1073/pnas.1316940111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bianchi S, et al. Synaptogenesis and development of pyramidal neuron dendritic morphology in the chimpanzee neocortex resembles humans. Proc Natl Acad Sci USA. 2013;110(Suppl 2):10395–10401. doi: 10.1073/pnas.1301224110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dean MC, Lucas VS. Dental and skeletal growth in early fossil hominins. Ann Hum Biol. 2009;36(5):545–561. doi: 10.1080/03014460902956725. [DOI] [PubMed] [Google Scholar]
- 46.Dean C, et al. Growth processes in teeth distinguish modern humans from Homo erectus and earlier hominins. Nature. 2001;414(6864):628–631. doi: 10.1038/414628a. [DOI] [PubMed] [Google Scholar]
- 47.Smith TM, et al. Earliest evidence of modern human life history in North African early Homo sapiens. Proc Natl Acad Sci USA. 2007;104(15):6128–6133. doi: 10.1073/pnas.0700747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith TM, et al. Dental evidence for ontogenetic differences between modern humans and Neanderthals. Proc Natl Acad Sci USA. 2010;107(49):20923–20928. doi: 10.1073/pnas.1010906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bunn H. Meat made us human. In: Unger P, editor. Evolution of the Human Diet: The Known, the Unknown, and the Unknowable. New York: Oxford Univ Press; 2006. pp. 191–211. [Google Scholar]
- 50.Plummer T. Flaked stones and old bones: Biological and cultural evolution at the dawn of technology. Am J Phys Anthropol. 2004;125(Suppl 39):118–164. doi: 10.1002/ajpa.20157. [DOI] [PubMed] [Google Scholar]
- 51.Pobiner BL, Rogers MJ, Monahan CM, Harris JW. New evidence for hominin carcass processing strategies at 1.5 Ma, Koobi Fora, Kenya. J Hum Evol. 2008;55(1):103–130. doi: 10.1016/j.jhevol.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Isler K, van Schaik CP. How our ancestors broke through the grey ceiling: Comparative evidence for cooperative breeding in early Homo. Curr Anthropol. 2012;53(Suppl 6):S453–S465. [Google Scholar]
- 53.Bogin B, Bragg J, Kuzawa C. Humans are not cooperative breeders but practice biocultural reproduction. Ann Hum Biol. 2014;41(4):368–380. doi: 10.3109/03014460.2014.923938. [DOI] [PubMed] [Google Scholar]
- 54.Miller DJ, et al. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci USA. 2012;109(41):16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bufill E, Agustí J, Blesa R. Human neoteny revisited: The case of synaptic plasticity. Am J Hum Biol. 2011;23(6):729–739. doi: 10.1002/ajhb.21225. [DOI] [PubMed] [Google Scholar]
- 56.Boyd R, Richerson PJ, Henrich J. The cultural niche: Why social learning is essential for human adaptation. Proc Natl Acad Sci USA. 2011;108(Suppl 2):10918–10925. doi: 10.1073/pnas.1100290108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hill K, Barton M, Hurtado AM. The emergence of human uniqueness: Characters underlying behavioral modernity. Evol Anthropol. 2009;18(5):187–200. [Google Scholar]
- 58.Haig D. Genetic conflicts in human pregnancy. Q Rev Biol. 1993;68(4):495–532. doi: 10.1086/418300. [DOI] [PubMed] [Google Scholar]
- 59.Kuzawa CW. Beyond feast-famine: Brain evolution, human life history, and the metabolic syndrome. In: Muehlenbein M, editor. Human Evolutionary Biology. Cambridge: Cambridge Univ Press; 2010. [Google Scholar]
- 60.Kubera B, et al. Energy allocation between brain and body during ontogenetic development. Am J Hum Biol. 2013;25(6):725–732. doi: 10.1002/ajhb.22439. [DOI] [PubMed] [Google Scholar]
- 61.Peters A, et al. The selfish brain: Competition for energy resources. Neurosci Biobehav Rev. 2004;28(2):143–180. doi: 10.1016/j.neubiorev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 62.Ong KK. Size at birth, postnatal growth and risk of obesity. Horm Res. 2006;65(Suppl 3):65–69. doi: 10.1159/000091508. [DOI] [PubMed] [Google Scholar]
- 63.Norris SA, et al. COHORTS Group Size at birth, weight gain in infancy and childhood, and adult diabetes risk in five low- or middle-income country birth cohorts. Diabetes Care. 2012;35(1):72–79. doi: 10.2337/dc11-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levy-Marchal C, et al. ESPE-LWPES-ISPAD-APPES-APEG-SLEP-JSPE; Insulin Resistance in Children Consensus Conference Group Insulin resistance in children: Consensus, perspective, and future directions. J Clin Endocrinol Metab. 2010;95(12):5189–5198. doi: 10.1210/jc.2010-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51(3):170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baumgartner RN, Roche AF, Himes JH. Incremental growth tables: Supplementary to previously published charts. Am J Clin Nutr. 1986;43(5):711–722. doi: 10.1093/ajcn/43.5.711. [DOI] [PubMed] [Google Scholar]
- 67.Chugani HT. Neuroimaging of developmental nonlinearity and developmental pathologies. In: Thatcher R, Lyon G, Rumsey J, Krasnegor N, editors. Developmental Neuroimaging: Mapping the Development of Brain and Behavior. San Diego: Academic; 1996. pp. 187–195. [Google Scholar]
- 68.Silverwood RJ, De Stavola BL, Cole TJ, Leon DA. BMI peak in infancy as a predictor for later BMI in the Uppsala Family Study. Int J Obes (Lond) 2009;33(8):929–937. doi: 10.1038/ijo.2009.108. [DOI] [PubMed] [Google Scholar]
- 69.Degos V, et al. Computed tomography-estimated specific gravity of noncontused brain areas as a marker of severity in human traumatic brain injury. Anesth Analg. 2006;103(5):1229–1236. doi: 10.1213/01.ane.0000237401.22688.22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.