Significance
The peripheral effect of ghrelin on energy metabolism has been controversial. Our study demonstrates a direct peripheral effect of ghrelin to increase de novo lipogenesis in hepatocytes. Moreover, we define mammalian target of rapamycin (mTOR)-peroxisome proliferator-activated receptor-γ (PPARγ) signaling pathway as the intracellular target of ghrelin in hepatocytes. The effect of ghrelin is mediated by the direct interaction between raptor and PPARγ. Our study identifies a previously unidentified pathway to treat NFALD via targeting hepatic ghrelin receptor/mTOR/PPARγ.
Keywords: NAFLD, gastric hormone, growth hormone secretagogue receptor, GHSR
Abstract
Although ghrelin has been demonstrated to stimulate energy intake and storage through a central mechanism, its effect on hepatic lipid metabolism remains largely uncharacterized. Ghrelin receptor antagonism or gene deletion significantly decreased obesity-associated hepatic steatosis by suppression of de novo lipogenesis, whereas exogenous ghrelin stimulated lipogenesis, leading to hepatic lipid accumulation in mice. The effects of ghrelin were mediated by direct activation of its receptor on hepatocytes. Cultured hepatocytes responded to ghrelin with increased lipid content and expression of lipogenesis-related genes. Ghrelin increased phosphorylation of S6, the downstream target of mammalian target of rapamycin (mTOR) signaling in cultured hepatocytes, whereas ghrelin receptor antagonism reduced hepatic phosphorylation of S6 in db/db mice. Inhibition of mTOR signaling by rapamycin markedly attenuated ghrelin-induced up-regulation of lipogenesis in hepatocytes, whereas activation of hepatic mTOR signaling by deletion of TSC1 increased hepatic lipogenesis. By interacting with peroxisome proliferator-activated receptor-γ (PPARγ), mTOR mediates the ghrelin-induced up-regulation of lipogenesis in hepatocytes. The stimulatory effect of ghrelin on hepatic lipogenesis was significantly attenuated by PPARγ antagonism in cultured hepatocytes and in PPARγ gene-deficient mice. Our study indicates that ghrelin activates its receptor on hepatocytes to promote lipogenesis via a mechanism involving the mTOR-PPARγ signaling pathway.
Triglyceride deposition in the liver, which is strongly associated with obesity, is the initial event in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). Over time, hepatic steatosis may progress to steatohepatitis, cirrhosis, and primary hepatocellular carcinoma (1). The current therapeutic strategy for NAFLD has been focused on reversal of hepatic steatosis, primarily through weight reduction. Treatment is often ineffective because of the difficulty in achieving sustained weight loss. Alternative approaches are needed but are limited by incomplete understanding of the mechanisms controlling the development of steatosis. Gastric hormones may be involved in regulation of lipid metabolism. Studies in both animals and humans demonstrate that ghrelin, a 28-aa peptide hormone secreted by X/A-like endocrine cells in the gastric fundus (2, 3), stimulates lipid accumulation in adipose tissue (4). Chronic infusion of ghrelin increases both adipose and hepatic lipid storage (5). Genetic disruption of either ghrelin or ghrelin receptor genes renders mice resistant to obesity and to the development of hepatic steatosis (6). Interestingly, the anabolic effect of ghrelin appears to be independent of its hyperphagic action. Chronic third intracerebroventricular infusion of ghrelin in diet-induced obese rats increases adiposity and gene expression of lipogenic enzymes in white adipose tissue while food intake remains unchanged (7). Most studies suggest that ghrelin increases lipid deposition in adipose tissue through an effect on hypothalamic neurons (8). However, the location and anatomical structure of the hypothalamus pose significant hurdles for therapies that target this organ, and peripheral targets would be appealing alternatives. In this study, we demonstrate that ghrelin stimulates lipogenesis and increases triglyceride content in liver by direct activation of its receptor on hepatocytes. This effect is mediated via the mammalian target of rapamycin (mTOR) and peroxisome proliferator-activated receptor-γ (PPARγ) signaling pathway. This study provides direct evidence that both pharmacological and genetic interventions directed at ghrelin receptor ameliorate the development of hepatic steatosis associated with obesity.
Results
Effects of GHSR1a Antagonism.
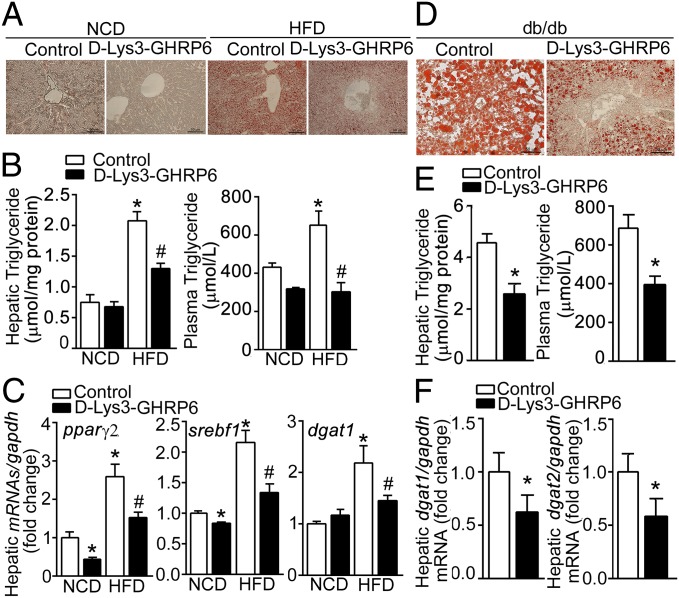
To determine whether the ghrelin receptor, GHSR1a, mediates the development of hepatic steatosis, we used d-Lys3-GHRP6, a GHSR1a antagonist without GHSR1a constitutive activity, to block the effects of endogenous ghrelin in diet-induced obese (DIO) mice and db/db mice. As shown in Fig. 1 A and B, GHSR1a antagonism significantly decreased hepatic steatosis induced by a high fat diet, as measured by oil-red staining and hepatic triglyceride content. The decrease was associated with a marked reduction in plasma triglyceride concentration. Transcription of genes related to hepatic lipogenesis, including nuclear receptors peroxisome proliferator-activated receptor gamma (pparγ2) and sterol regulatory element-binding protein (srebf1), as well as acyl CoA:diacylglycerol acyltransferase (dgat1), a key enzyme in triglyceride synthesis, were significantly decreased in DIO mice receiving d-Lys3-GHRP6 (Fig. 1C). Similar observations were made in db/db mice. As shown in Fig. 1 D and E, severe hepatic steatosis was detected in db/db mice. d-Lys3-GHRP6 significantly reduced hepatic lipid content (Fig. 1D) and plasma triglyceride levels (Fig. 1E). Moreover, transcription of dgat1 and dgat2 was markedly decreased in response to d-Lys3-GHRP6 administration, suggesting a reduction of triglyceride synthesis (Fig. 1F). Importantly, the effects of d-Lys3-GHRP6 were not due to reduced food intake. d-Lys3-GHRP6 demonstrated no effect on daily food intake in either C57BL/6J (Fig. S1A) or db/db mice (Fig. S1B), nor on expression of genes related to β-oxidation, such as carnitine palmitoyltransferase I α (cpt1α), acyl-Coenzyme A dehydrogenase (acadm), very-long-chain acyl-Coenzyme A dehydrogenase (acadvl) (Fig. S1C). In addition, respiratory quotient was unaffected by d-Lys3-GHRP6 in db/db mice (Fig. S1D).
Fig. 1.
Effects of GHSR1a antagonism on hepatic steatosis. (A–C) Six-week-old C57BL/6J mice were fed normal chow diet (NCD) or 45% high fat diet (HFD) for 12 wk. d-Lys3-GHRP6 was administrated i.p. (10 μmol/kg daily) for 1 wk. Shown are representative oil red staining of liver frozen sections (A), hepatic triglyceride content and plasma triglyceride levels (B), and levels of lipogenesis-related genes (C) in response to d-Lys3-GHRP6 (filled bars) relative to control (open bars) with gapdh as control. n = 6. *P < 0.05 vs. NCD control; #P < 0.05 vs. HFD control. (D–F) In 12-wk-old db/db mice, d-Lys3-GHRP6 significantly decreased hepatic steatosis measured by oil red staining (D), hepatic triglyceride content and plasma triglyceride levels (E), and expression of lipogenesis-related genes (F). n = 6. *P < 0.05 vs. control.
Effects of GHSR1a Gene Knockout.
Wild-type mice fed a 45% high fat diet for 16 wk demonstrated significant increases in body weight relative to animals fed normal chow (52.5 ± 2.3 g vs. 33.8 ± 0.9 g; P < 0.01) (Fig. 2A and Fig. S2A). GHSR1a−/− mice were resistant to high fat diet-induced obesity, with body weight significantly less than wild-type littermates (39.5 ± 1.8 g vs. 52.5 ± 2.3 g; P < 0.01) (Fig. S2A). Oil red staining revealed a significant reduction in lipid staining in livers (Fig. 2C), and hepatic triglyceride content and plasma triglyceride levels (Fig. 2D) from GHSR1a−/− mice fed high fat diet (HFD) relative to wild-type littermates. Decreased hepatic steatosis was associated with a significant reduction in expression of molecules critical for hepatic lipogenesis. Whereas hepatic PPARγ and SREBP1c increased substantially in wild-type littermates fed HFD, no elevation was detected in GHSR1a−/− mice (Fig. 2E). Likewise, HFD-induced increase in hepatic levels of dgat1 and dgat2 in wild-type mice was not detected in GHSR1a−/− mice (Fig. 2F). Transcription of genes related to β-oxidation and lipid uptake was not altered by GHSR1a gene deletion (Fig. S2 B and C).
Fig. 2.
Effects of GHSR1a gene deletion on steatosis. GHSR1a−/− mice and wild-type littermates were fed NCD or 45% HFD for 16 wk. Gross morphology of body (A) and liver (B), oil red staining (C), liver and plasma triglyceride content (D), and levels of PPARγ and SREBP1 (E) were shown. Expression of dgat1 and dgat2 (F) was analyzed by real-time RT-PCR, with gapdh as control. n = 6. *P < 0.05 vs. NCD control; #P < 0.05 vs. HFD control.
Hepatic Steatosis Increased by Exogenous Ghrelin.
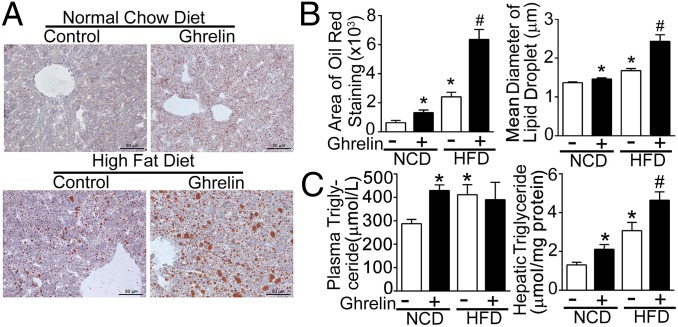
To examine the effect of exogenous ghrelin on hepatic steatosis, acyl-ghrelin was continuously infused for 2 wk into C57BL/6J mice fed with either normal chow diet (NCD) or HFD. Exogenous ghrelin had no effect on either nocturnal food intake or total daily food intake (Fig. S3A). Body weight gain was increased in mice fed HFD following ghrelin infusion (Fig. S3A). As shown in Fig. 3A, infusion of ghrelin increased hepatic lipid deposition measured by oil-red staining in mice fed NCD or HFD. Both the average area and diameter of lipid depots were significantly increased (Fig. 3B). These alterations were associated with increases in hepatic triglyceride content. Plasma triglyceride concentrations increased in the NCD group with ghrelin infusion (Fig. 3C). Exogenous ghrelin demonstrated no effect on the expression of hepatic β-oxidation–related genes (Fig. S3B).
Fig. 3.
Effects of exogenous ghrelin on hepatic lipid metabolism. Six-week-old C57BL/6J mice were fed either NCD or 45% HFD for 12 wk. Acyl-ghrelin was continuously infused (11 nmol/kg per d) for 2 wk. (A) Oil red staining was performed on liver frozen slices. (B) Total area and average diameter of lipid droplets were quantified by NIH ImageJ software and expressed as mean ± SEM. (C) Hepatic and plasma triglyceride content were assayed. At least five mice were included in each group. *P < 0.05 vs. NCD control; #P < 0.05 vs. HFD control.
Direct Effects of Ghrelin on Hepatocytes.
To determine whether ghrelin acts directly on hepatocytes, we confirmed expression of GHSR1a in liver. As shown in Fig. 4A, GHSR1a mRNA and protein were detected in liver and hypothalamus derived from wild-type mice, whereas GHSR1a−/− mice showed no expression. Cytoplasm membrane binding of rhodamine-labeled ghrelin was observed in cells stained positively for albumin (Fig. 4B). Because ghrelin receptor has been demonstrated to be desensitized by receptor internalization, we next examined its internalization. As shown in Fig. 4C, incubation of hepatocytes with rhodamine-ghrelin demonstrated rapid receptor internalization. Negligible at 0 and 5 min, rhodamine signals were detected intracellularly in a vesicular pattern at 15, 30, and 45 min. The direct effects of ghrelin on hepatic lipogenesis were further demonstrated by using cultured hepatocytes. As shown in Fig. 4 D and E, treatment of cultured hepatocytes from wild-type animals with ghrelin (10−8 M) for 36 h substantially increased lipid accumulation, whereas cells from GHSR1a−/− mice demonstrated no response to ghrelin. Ghrelin increased hepatic lipid content by up-regulating de novo lipogenesis. Transcription factors pparγ2 and srebf1, and key enzymes encoded by genes such as acetyl-CoA carboxylase 1 (acaca), fatty acid synthase (fasn), glycerol-3-phosphate acyltransferase 1 (gpam), and dgat1, increased in response to ghrelin in hepatocytes from wild-type mice, but not in cells from GHSR1a−/− animals (Fig. 4F). In contrast, ghrelin demonstrated no effect on hepatic β-oxidation–related genes (Fig. S3C).
Fig. 4.
Direct effects of ghrelin on hepatic lipid metabolism. (A) Existence of ghsr1a mRNA and protein in liver. Hypothalamus from wild-type mice was used as positive control. Results shown are representative of three individual experiments. (B) Rhodamine-labeled ghrelin (red) binding assay revealed binding sites on cells stained positively for albumin immunoreactivity (green). Rabbit IgG was used as negative control. (C) Rhodamine-labeled ghrelin (red) binding assays in different time points are shown. Nuclei were stained with Hoechst dye. (D and E) Effects of acyl-ghrelin (10−8 M, 36 h) on oil red staining (D) and triglyceride content (E) in cultured hepatocytes. Results were normalized to control and expressed as fold changes. (F) Effects of ghrelin on lipogenesis genes. Cultured hepatocytes from wild-type mice and GHSR1a−/− mice were treated with ghrelin (10−8 M, filled bars) for 3 h. n = 3–6. *P < 0.05 vs. control.
mTOR Signaling Mediates Hepatic Lipogenesis.
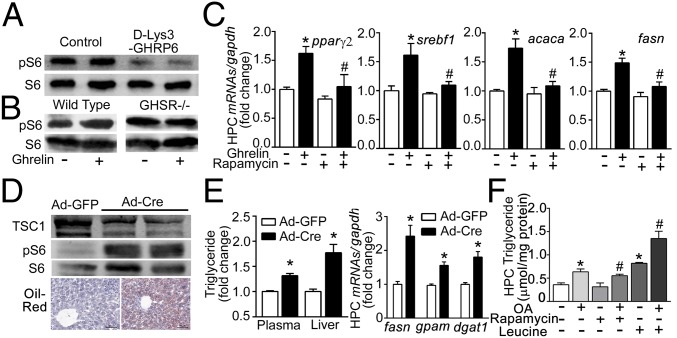
We next examined whether mTOR mediates the effects of ghrelin on hepatic lipid metabolism. As shown in Fig. 5A, antagonism of GHSR1a by d-Lys3-GHRP6 substantially decreased phosphorylation of S6, the downstream target of mTORC1 in liver. Treatment of cultured hepatocytes with ghrelin significantly stimulated phosphorylation of S6 in wild-type mice, while demonstrating no effect in hepatocytes derived from GHSR1a−/− animals (Fig. 5B). Ghrelin-induced up-regulation of hepatic lipogenesis-related transcriptional factors pparγ2 and srebf1, and enzymes encoded by acaca and fasn, was attenuated by rapamycin (20 nM), an inhibitor of mTOR signaling (Fig. 5C). Administration of Ad-CMV-Cre adenovirus into TSC1loxp/loxp mice significantly attenuated hepatic levels of TSC1 and increased phosphorylation of S6 (Fig. 5D), indicating activation of mTOR signaling in the liver. This was accompanied by a significant increase in hepatic lipid deposition, plasma and hepatic triglyceride levels, and transcription of lipogenesis genes such as fasn, gpam, and dgat1 (Fig. 5E). Hepatic expression of genes related to lipid β-oxidation and transport was unchanged by mTOR activation (Fig. S4A). The effect of mTOR signaling was further confirmed by experiments using hepatocytes. Pretreatment with rapamycin markedly attenuated the oleic acid-induced triglyceride accumulation (Fig. 5F). In contrast, leucine, which has been documented to activate mTOR signaling, significantly augmented the effect of oleic acid on triglyceride content.
Fig. 5.
mTOR mediation of effects of ghrelin on hepatic lipid metabolism. (A) Effect of d-Lys3-GHRP6 (10 μmol/kg) on phosphorylation of S6 in liver of db/db mice. (B) Hepatocytes from wild-type mice and GHSR1a−/− littermates treated with ghrelin for 12 h. Western blotting were performed to detect phosphorylation of S6. (C) Effects of rapamycin on ghrelin-induced transcriptional genes. Hepatocytes from C57BL/6J mice were treated with ghrelin (10−8 M) and/or rapamycin (20 nM) for 3 h. *P < 0.05 vs. control, #P < 0.05 vs. ghrelin group. (D and E) Eight-week-old TSC1loxp/loxp mice were injected the Ad-CMV-Cre (Ad-Cre) adenovirus (109 pfu) or Ad-CMV-GFP (Ad-GFP) control via the tail vein. At least eight mice were included in each group. (D) Hepatic samples were analyzed for TSC1, phosphor-S6, and S6. Hepatic lipid metabolism was evaluated by oil red staining (D), hepatic and plasma triglyceride content, and mRNA levels of lipogenesis-related enzymes (E). *P < 0.05 vs. Ad-GFP. (F) Hepatocytes from C57BL/6J mice were cultured with or without oleic acid (OA, 62.5 μM) for 36 h. Cells were treated with rapamycin (20 nM) and leucine (4 mM) for 24 h, and assayed for triglyceride content. Each experiment was repeated at least three times. *P < 0.05 vs. vehicle; #P < 0.05 vs. OA group.
Interaction Between mTOR and PPARγ.
The data presented in Figs. 1–5 suggest that ghrelin stimulates expression of PPARγ. This observation was confirmed by demonstrating that ghrelin increased levels of PPARγ protein only in hepatic tissues derived from wild-type animals, but not in those from GHSR1a−/− mice (Fig. 6A). Ghrelin-induced increment in PPARγ expression appears to occur at the transcriptional level. Analysis of peroxisome proliferator hormone response elements (PPRE) activity demonstrated that treatment of cultured hepG2 cells with ghrelin for 3 h significantly increased PPRE activity (Fig. 6A). We thus hypothesized that ghrelin stimulates hepatic lipogenesis by activating the mTOR-PPARγ signaling pathway. To test this hypothesis, we first examined the interaction between mTOR and PPARγ in hepatocytes. As shown in Fig. 6B, activation of mTOR signaling by injection of Ad-CMV-Cre adenovirus into TSC1loxp/loxp mice resulted in significant increments in hepatic PPARγ and SREBP1. Infection of hepatocytes isolated from TSC1loxp/loxp mice with Ad-CMV-Cre adenovirus augmented mRNA levels of pparγ and srebf1 (Fig. 6B). This effect was blocked by GW9662, a selective PPARγ antagonist (Fig. 6C). Consistent with this observation, leucine-induced increment in lipogenesis-related gene expression was blocked by GW9662 (Fig. 6D). β-Oxidation–related genes, acadm and acadvl, were unchanged by leucine (Fig. S4B). These data suggested a potential interaction between mTOR signaling and PPARγ in hepatocytes. To demonstrate the interaction, we used an antibody against the regulatory-associated protein of mTOR (raptor), a critical component of TORC1, to pull down its associated proteins. PPARγ was coimmunoprecipitated by raptor in hepatic tissue extracts (Fig. 6E). Activation of hepatic mTOR signaling by Ad-CMV-Cre–mediated deletion of TSC1 (Fig. 5D) significantly increased raptor expression and the amount of PPARγ pulled down by raptor, suggesting interaction between raptor and PPARγ. Experiments in cultured cells confirmed the interaction between raptor and PPARγ. Transfection of Clu122 cells, an immortalized hypothalamic cell line, with raptor plasmids significantly increased phosphorylation of S6. Raptor-induced activation of mTOR signaling was accompanied by substantial increments in PPARγ protein and mRNA expression as well as srebf1 transcripts (Fig. 6F).
Fig. 6.
Interaction between mTOR signaling and PPARγ. (A) Hepatocytes isolated from wild-type mice and GHSR1a−/− littermates were treated with ghrelin for 12 h. Western blotting detected PPARγ. HepG2 cells were transfected with PPREX3-TK-Luc plasmid or control pRL-CMV plasmid, then treated with ghrelin. Luciferase activity was measured, and results were expressed as mean ± SEM, *P < 0.05 vs. vehicle. (B) Liver proteins and mRNAs from TSC1loxp/loxp mice treated with Ad-CMV-Cre (Ad-Cre) or Ad-CMV-GFP (Ad-GFP) adenovirus (109 pfu) were used to detect alterations in PPARγ and SREBP1. *P < 0.05 vs. Ad-GFP. (C) Hepatic primary cells (HPCs) isolated from TSC1loxp/loxp mice were infected with Ad-Cre or Ad-GFP (106 pfu) for 36 h, then treated with GW9662 (20 μM) for 12 h. mRNAs were analyzed for expression of pparγ and srebf1. *P < 0.05 vs. Ad-GFP+DMSO; #P < 0.05 vs. Ad-Cre+DMSO group. (D) HPCs isolated from C57BL/6J mice were treated with leucine (4 mM) and GW9662 (20 μM) for 12 h. mRNAs was analyzed for expression of pparγ, srebf1, fasn, and dgat1. *P < 0.05 vs. vehicle; #P < 0.05 vs. leucine. (E) Liver proteins from TSC1loxp/loxp mice treated with Ad-Cre or Ad-GFP adenovirus (109 pfu) were immunoprecipitated by using antibody against raptor. Rabbit IgG was used as negative control. (F) Clu122 cells were transfected with raptor or EGFP plasmids for 36 h. Proteins and mRNAs were analyzed by Western blot and PCR. *P < 0.05 vs. EGFP.
Effects of PPARγ on Hepatic Lipogenesis.
We next examined whether PPARγ is involved in the regulation of hepatic lipogenesis by ghrelin. Treatment of cultured hepatocytes with GW9662 completely blocked ghrelin-induced increments in triglyceride content and levels of lipogenesis-related genes (Fig. 7A). To further determine the role of PPARγ, we infected cultured hepatocytes with Ad-PPARγ. As shown in Fig. 7 B and C, ghrelin substantially enhanced both basal and oleic acid-stimulated triglyceride synthesis in hepatocytes derived from wild-type littermates, but demonstrated no effect in cells isolated from GHSR1a null mice. Overexpression of PPARγ, which resulted in increased levels of srebf1, fasn, acaca, and dgat1 (Fig. 7D), significantly enhanced ghrelin-elicited increases in triglyceride synthesis (Fig. 7C). Furthermore, oleic acid dose-dependently increased triglyceride content in hepatocytes from wild-type mice, while demonstrating no effect in cells from PPARγ+/− heterozygous mice (Fig. 7E). These changes were accompanied by a significant reduction in levels of pparγ, pparγ2, and srebf1, and lipogenesis-related enzyme genes (Fig. 7E). Although ghrelin markedly augmented both basal and oleic acid-induced triglyceride synthesis in wild-type hepatocytes, its effect was completely attenuated in cells from PPARγ+/− heterozygous mice (Fig. 7F). Ghrelin increased mRNA levels of pparγ, acaca, and fasn in wild-type hepatocytes, while demonstrating no effect on hepatocytes from PPARγ+/− heterozygous mice (Fig. 7F).
Fig. 7.
PPARγ mediation of effects of ghrelin on hepatic lipid metabolism. (A) HPCs from C57BL/6J mice were treated with ghrelin (10−8 M) and/or GW9662 (20 μM) for 12 h. Levels of triglyceride and lipogenesis-related genes were assayed and *P < 0.05 vs. control; #P < 0.05 vs. ghrelin group. (B and C) HPCs isolated from wild-type or GHSR1a−/− littermates were infected with Ad-GFP or Ad-PPARγ. Where indicated, HPCs were treated with oleic acid (OA) for 24 h and ghrelin for 12 h. Cells were stained with oil-red dye (B) or harvested for triglyceride detection (C). *P < 0.05 vs. control; #P < 0.05 vs. ghrelin group. (D) HPCs isolated from C57BL/6J mice were infected with Ad-GFP (control) or Ad-PPARγ. Lipogenesis-related genes were analyzed. *P < 0.05 vs. Ad-GFP. (E) HPCs from wild-type (WT) or PPARγ+/− littermates were treated with OA for 36 h. Triglyceride and lipogenesis-related genes were analyzed. *P < 0.05 vs. basal or vs. wild type. (F) HPCs were cultured from WT or PPARγ+/− littermates. Where indicated, cells were treated with OA for 36 h and ghrelin for 12 h. Triglyceride and lipogenesis-related genes were measured. *P < 0.05 vs. basal or vs. control; #P < 0.05 vs. OA group.
Discussion
While multiple studies indicate that ghrelin acts through central mechanisms to increase caloric intake during short periods of negative energy supply (9), pathways mediating long-term effects on energy storage are less well-described. In white adipose tissue, ghrelin functions through hypothalamic neuronal circuits to increase lipogenesis while decreasing β-oxidation (10). These effects are mediated by activation of GHSR1a on hypothalamic agouti-related peptide/neuropeptide Y (AGRP/NPY) neurons that, in turn, promote blockade of melanocortin receptors 3 and 4 (11). This central neural circuit regulates peripheral lipogenesis through the sympathetic nervous system (10). Previous studies have also demonstrated a central effect of ghrelin on adipose lipid metabolism, exerted via orexigenic actions (8). These observations are consistent with the concept that the hypothalamic locus within the gut-brain axis dictates the actions of ghrelin in the regulation of glucose and lipid homeostasis. Because hypothalamic neurons account for multiple aspects of the biological function of ghrelin, including regulation of food intake and body weight (12, 13), control of adiposity (14) and modulation of blood pressure (15), targeting the hypothalamic ghrelin receptor/GHSR1a for treatment of metabolic dysfunction without systemic side effects is challenging. Recent studies suggest alternative pathways for lipid metabolism involving peripheral actions of ghrelin. The presence of functional GHSR1a has been demonstrated in adipocytes by pharmacological, physiological, and genetic studies (16, 17). Both GHSR1a-dependent and GHSR1a-independent mechanisms have been reported to mediate the effects of ghrelin on adipogenesis and lipid metabolism in adipocytes (6, 18). The present study defines a previously unidentified hepatocyte pathway for ghrelin in the regulation of hepatic lipogenesis. Both morphological and functional studies demonstrated the presence of ghrelin receptor in hepatocytes. Activation of GHSR1a by ghrelin increased triglyceride synthesis by promoting expression of lipogenesis-related genes in hepatocytes. In mice, inhibition or knock out of GHSR1a caused a reduction in hepatic steatosis in both diet-induced obese mice and genetically obese mice. Importantly, these interventions affected hepatic triglyceride content and circulating levels of triglyceride without significant alteration in food ingestion. These observations indicate a direct effect of ghrelin on hepatic lipogenesis through a mechanism independent of central stimulation of energy intake. Consistent with our observations, previous studies have shown that ghrelin stimulates adiposity independent of its hyperphagic effect (7). Importantly, peripheral administration of ghrelin at a dose demonstrated to have no effect on energy intake by our studies (Fig. S3A) and others (19) exerts significant effect on hepatic lipid profiles. In hypothalamic neurons, ghrelin activates NPY/AgRP neurons in the arcuate nucleus to stimulate food intake by both intracellular calcium signaling and the AMP-activated protein kinase (AMPK) signaling pathway (20). Other studies have shown that p53 is crucial for the adipogenic and lipogenic effect of ghrelin. Lack of p53 abolishes the stimulation of lipid storage induced by chronically administered ghrelin (21). Our study suggests that mTOR signaling is involved in ghrelin-activated regulation of hepatic triglyceride synthesis. Ghrelin increased phosphorylation of S6 only in wild-type hepatocytes; this effect was absent in cells isolated from GHSR1a−/− mice. Antagonism of GHSR1a significantly attenuated phosphorylation of S6 in obese mice. Inhibition of mTOR signaling by rapamycin blocked the ghrelin-induced up-regulation in hepatic lipogenesis-related transcriptional factors and enzymes. Consistent with this concept, activation of hepatic mTOR signaling, by deletion of TSC1 or by exposure of hepatocytes to leucine, increased lipid deposition. These data provide direct evidence that mTOR signaling mediates the effect of ghrelin on hepatic lipogenesis. Parallel changes in mTOR and PPARγ activity induced by ghrelin indicate a potential interaction between these two signaling molecules. This concept is supported by several observations. PPARγ antagonism blocked increased expression of hepatic PPARγ and lipogenesis-related gene expression induced by either genetic or pharmacological activation of mTOR signaling. Coimmunoprecipitation of PPARγ with raptor indicates a strong interaction between PPARγ and TORC1. Overexpression of raptor increased the expression of PPARγ and the interaction between these two molecules. Thus, PPARγ may function as the key downstream molecule mediating the effect of mTOR signaling on hepatic triglyceride synthesis. This concept is consistent with a previous report that the mTOR signaling pathway activates PPARγ to stimulate adipogenesis (22). Abundantly expressed in adipose tissue, PPARγ is a master regulator for adipogenesis, fat storage, and lipid metabolism (23). Basal expression of PPARγ is relatively low in normal human and rodent livers. The beneficial effect of PPARγ agonism in NAFLD has, therefore, long been considered to result from repartition of lipid to adipose tissue from the liver (24). However, emerging evidence suggests that hepatic PPARγ may also contribute to the regulation of de novo lipid metabolism and, thus, to the development of hepatic steatosis in obesity. In contrast to animal and human studies demonstrating improvement of NAFLD by PPARγ agonists rosiglitazone and pioglitazone, mice with liver-specific deletion of PPARγ are protected against the development of steatosis (25). Consistent with this observation, our study indicates that PPARγ mediates the ghrelin-stimulated increments in hepatic lipogenesis. Antagonism of PPARγ or PPARγ deficiency blocked up-regulation of lipogenesis-related transcriptional factors and enzymes induced by ghrelin in hepatocytes. In contrast, overexpression of PPARγ augmented the effects of ghrelin on hepatic triglyceride synthesis. Together with the previous finding that levels of PPARγ increase significantly in severe fatty liver associated with diabetes or obesity (26, 27), our study suggests that PPARγ is a potential target for treatment of NAFLD. In conclusion, our study suggests that ghrelin stimulates hepatic de novo lipogenesis by direct activation of its receptor on hepatocytes via a mechanism involving mTOR-PPARγ signaling. The clinical implication of this finding is that a previously unidentified pathway to treat NFALD may exist via targeting hepatic ghrelin receptor.
Materials and Methods
Animals and Treatments.
Animals.
Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health [(NIH) publication no. 85–23, revised 1996). All experimental protocols were approved by the Animal Care and Use Committee of Peking University (Permit Number: LA2012-60). Twelve-week-old male C57BL/6J lean mice, high fat diet-induced obese mice, and db/db mice were used. GHSR1a gene knockout mice in which exon 1 and exon 2 were deleted were obtained from the Shanghai Research Center for Biomodel Organisms (28). Mice were housed in standard plastic rodent cages and maintained in a regulated environment (24 °C, 12-h light and 12-h dark cycle with lights on at 0700 and off at 1900). Regular chow and water were available ad libitum unless specified otherwise.
Diets.
Where indicated, 4-wk-old mice were assigned to receive normal chow diet (control diet, D12450H; Research Diets) or a high-fat diet (45% fat, D12451; Research Diets) for 8–16 wk.
Surgery and implantation of osmotic minipumps.
Mice were anesthetized with isoflurane. A 1-cm incision was made in the back skin and mice were implanted s.c. with an Alzet osmotic minipump (model 1002) filled with vehicle or acyl-ghrelin (11 nmol⋅kg−1⋅d−1) for 14 d. Before implantation, pumps were filled with the test agent and then placed in a Petri dish with sterile 0.9% saline at 37 °C for at least 4 h before implantation to prime the pumps.
Rhodamine-Ghrelin Binding Assay.
Colocalization of rhodamine-ghrelin and albumin.
Isolated hepatocytes were washed with PBS three times and incubated with rhodamine-ghrelin for 10 min, then fixed by 4% (wt/vol) paraformaldehyde for 20 min, followed by treatment with 0.02% Triton X-100 for 30 min. Nonspecific sites were blocked by 1% BSA for 1 h. Cell suspensions were incubated with anti-albumin for 30 min at room temperature, then overnight at 4 °C. Cells were then incubated with chicken anti-rabbit fluorescein isothiocyanate-conjugated IgG (1:100) at room temperature for 2 h. Fluorescent signals were observed and photomicrographs taken under a confocal laser-scanning microscope (Leica).
Binding assay.
Isolated hepatocytes were washed with PBS three times and incubated with rhodamine-ghrelin at time points indicated, then fixed with 4% paraformaldehyde and stained with Hoechst for 15 min before detection.
Statistical Analysis.
All values are expressed as mean ± SEM. Statistical differences were evaluated by two-way ANOVA and Newman–Student–Keuls test. Comparisons between two groups involved use of the Student t test. P < 0.05 denotes statistical significance.
Supplementary Material
Acknowledgments
We thank Nanping Wang for the Ad-GFP adenovirus, Guozhi Xiao for the Ad-Cre adenovirus, and Youfei Guan for the Ad-PPARγ adenovirus and PPARγ+/− mice. This research was supported by National Natural Science Foundation of China Grants 81030012, 81330010, and 81390354, and American Diabetes Association Grant 1-13-BS-225.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1411571111/-/DCSupplemental.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Date Y, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141(11):4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 3.Ariyasu H, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86(10):4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez A, et al. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes (Lond) 2009;33(5):541–552. doi: 10.1038/ijo.2009.40. [DOI] [PubMed] [Google Scholar]
- 5.Sangiao-Alvarellos S, et al. Central ghrelin regulates peripheral lipid metabolism in a growth hormone-independent fashion. Endocrinology. 2009;150(10):4562–4574. doi: 10.1210/en.2009-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies JS, et al. Ghrelin induces abdominal obesity via GHS-R-dependent lipid retention. Mol Endocrinol. 2009;23(6):914–924. doi: 10.1210/me.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Tilve D, et al. Ghrelin-induced adiposity is independent of orexigenic effects. FASEB J. 2011;25(8):2814–2822. doi: 10.1096/fj.11-183632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theander-Carrillo C, et al. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest. 2006;116(7):1983–1993. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murakami N, et al. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol. 2002;174(2):283–288. doi: 10.1677/joe.0.1740283. [DOI] [PubMed] [Google Scholar]
- 10.Nogueiras R, et al. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007;117(11):3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen HY, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145(6):2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 12.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: Hypothalamic control of food intake and body weight. Neuron. 1999;22(2):221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 13.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444(7121):854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 14.Sainsbury A, Zhang L. Role of the hypothalamus in the neuroendocrine regulation of body weight and composition during energy deficit. Obesity Rev. 2012;13(3):234–257. doi: 10.1111/j.1467-789X.2011.00948.x. [DOI] [PubMed] [Google Scholar]
- 15.Dampney RA, et al. Long-term regulation of arterial blood pressure by hypothalamic nuclei: Some critical questions. Clin Exp Pharmacol Physiol. 2005;32(5-6):419–425. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim MS, et al. The mitogenic and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18(9):2291–2301. doi: 10.1210/me.2003-0459. [DOI] [PubMed] [Google Scholar]
- 17.Lin L, et al. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell. 2011;10(6):996–1010. doi: 10.1111/j.1474-9726.2011.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson NM, et al. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology. 2004;145(1):234–242. doi: 10.1210/en.2003-0899. [DOI] [PubMed] [Google Scholar]
- 19.Lippl F, et al. Low-dose ghrelin infusion—evidence against a hormonal role in food intake. Regul Pept. 2012;174(1-3):26–31. doi: 10.1016/j.regpep.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Kohno D, Sone H, Minokoshi Y, Yada T. Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem Biophys Res Commun. 2008;366(2):388–392. doi: 10.1016/j.bbrc.2007.11.166. [DOI] [PubMed] [Google Scholar]
- 21.Porteiro B, et al. Ghrelin requires p53 to stimulate lipid storage in fat and liver. Endocrinology. 2013;154(10):3671–3679. doi: 10.1210/en.2013-1176. [DOI] [PubMed] [Google Scholar]
- 22.Kim JE, Chen J. Regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes. 2004;53(11):2748–2756. doi: 10.2337/diabetes.53.11.2748. [DOI] [PubMed] [Google Scholar]
- 23.Ferré P. The biology of peroxisome proliferator-activated receptors: Relationship with lipid metabolism and insulin sensitivity. Diabetes. 2004;53(Suppl 1):S43–S50. doi: 10.2337/diabetes.53.2007.s43. [DOI] [PubMed] [Google Scholar]
- 24.Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARγ agonists: Time for a reassessment. TEM. 2012;23(5):205–215. doi: 10.1016/j.tem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Matsusue K, et al. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111(5):737–747. doi: 10.1172/JCI17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Memon RA, et al. Up-regulation of peroxisome proliferator-activated receptors (PPAR-alpha) and PPAR-gamma messenger ribonucleic acid expression in the liver in murine obesity: Troglitazone induces expression of PPAR-gamma-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology. 2000;141(11):4021–4031. doi: 10.1210/endo.141.11.7771. [DOI] [PubMed] [Google Scholar]
- 27.Bedoucha M, Atzpodien E, Boelsterli UA. Diabetic KKAy mice exhibit increased hepatic PPARgamma1 gene expression and develop hepatic steatosis upon chronic treatment with antidiabetic thiazolidinediones. J Hepatol. 2001;35(1):17–23. doi: 10.1016/s0168-8278(01)00066-6. [DOI] [PubMed] [Google Scholar]
- 28.Xu G, et al. Ghrelin contributes to derangements of glucose metabolism induced by rapamycin in mice. Diabetologia. 2012;55(6):1813–1823. doi: 10.1007/s00125-012-2509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.