Significance
Neurons develop processes called neurites to form defined networks. Neurite growth is regulated by many intracellular signaling pathways, among which signaling via phosphatase and tensin homolog (PTEN) is of particular relevance because it controls the translation of a substantial subset of mRNAs that encode proteins with a role in neurite growth. Previous studies indicated that the E3 ubiquitin ligases Nedd4-1 and Nedd4-2 may ubiquitinate and negatively regulate PTEN in various cell types, including Xenopus laevis retinal ganglion cells. We report a strikingly inverted scenario, according to which Nedd4s are dispensable for ubiquitination of PTEN in mammalian central nervous system neurons. Instead, Nedd4-1 mRNA is one of the most important targets of PTEN-dependent signaling in the regulation of neurite growth.
Abstract
Protein ubiquitination is a core regulatory determinant of neural development. Previous studies have indicated that the Nedd4-family E3 ubiquitin ligases Nedd4-1 and Nedd4-2 may ubiquitinate phosphatase and tensin homolog (PTEN) and thereby regulate axonal growth in neurons. Using conditional knockout mice, we show here that Nedd4-1 and Nedd4-2 are indeed required for axonal growth in murine central nervous system neurons. However, in contrast to previously published data, we demonstrate that PTEN is not a substrate of Nedd4-1 and Nedd4-2, and that aberrant PTEN ubiquitination is not involved in the impaired axon growth upon deletion of Nedd4-1 and Nedd4-2. Rather, PTEN limits Nedd4-1 protein levels by modulating the activity of mTORC1, a protein complex that controls protein synthesis and cell growth. Our data demonstrate that Nedd4-family E3 ligases promote axonal growth and branching in the developing mammalian brain, where PTEN is not a relevant substrate. Instead, PTEN controls neurite growth by regulating Nedd4-1 expression.
In the mammalian cerebral cortex, the development and function of postmitotic neurons are tightly regulated by multiple signaling mechanisms such as protein and lipid phosphorylation, Ca2+ signaling (1, 2), and protein ubiquitination (3, 4), which often intersect to regulate the same target process or effector protein. A paradigmatic example in this context is phosphatase and tensin homolog (PTEN). PTEN is a lipid phosphatase that converts phosphatidylinositol-3,4,5-trisphosphate (PtdInsP3) into phosphatidylinositol-4,5-bisphosphate (PtdInsP2) and antagonizes phosphoinositide 3-kinase (PI3K)–dependent signaling (5). PTEN plays a prominent role in neuronal development (6), acts as a major tumor suppressor, and regulates numerous additional cellular events in various cell types (5). In keeping with its prominent and diverse functions, PTEN is tightly regulated at the transcriptional and posttranslational levels (7). With regard to posttranslational modifications that regulate PTEN, ubiquitination is of particular interest. It is thought to involve K48-linked polyubiquitination and proteosomal degradation and K63-linked polyubiquitination or monoubiquitination, which were proposed to affect the function and subcellular compartmentalization of PTEN (8–10).
Nedd4-1 (neural precursor cell expressed, developmentally down-regulated 4-1) was the first E3 ligase to be implicated in PTEN ubiquitination. Initial evidence in this regard was based on in vitro biochemical assays (9). Additional studies indicated that Nedd4-1 may catalyze the mono- and polyubiquitination of PTEN, leading to nuclear import of PTEN and PTEN degradation, respectively (8). In the mammalian cortex, down-regulation of PTEN causes aberrant proliferation of neural progenitor cells, defects in neuronal polarity establishment, hypertrophy of neuronal cell bodies, and excessive neurite growth (11–14). On the other hand, KO of Nedd4-1 in postmitotic hippocampal and cortical neurons impairs dendrite growth (15), indicating that PTEN and Nedd4-1 may have antagonistic functions in the control of dendrite growth. Further studies based on RNAi-mediated knockdown (KD) of PTEN and Nedd4 supported the notion of a key role for Nedd4-mediated PTEN degradation in axon growth in Xenopus laevis retinal ganglion cells and mammalian peripheral nervous system neurons (16, 17). However, the issue of regulation of PTEN by Nedd4-1–mediated ubiquitination has been controversial. For example, our previous work showed that Nedd4-1 KO or Nedd4-1 KD does not affect the nuclear import or stability of PTEN (18), and several other groups identified alternative E3 ligases for PTEN, including XIAP, WWP2, and CHIP (19–21).
We demonstrate here that the Nedd4-family E3 ligases Nedd4-1 and Nedd4-2 promote axon growth in mammalian central nervous system neurons. This function does not involve changes in the expression, ubiquitination, localization, or phosphatase activity of PTEN. Instead, PTEN suppresses Nedd4-1 expression at the translational level by negatively regulating mTORC1 activity, and the regulation of neurite growth by PTEN and mTORC1 requires endogenous Nedd4-1.
Results
KO of Nedd4-1 and Nedd4-2 Causes Defects in Axon Growth.
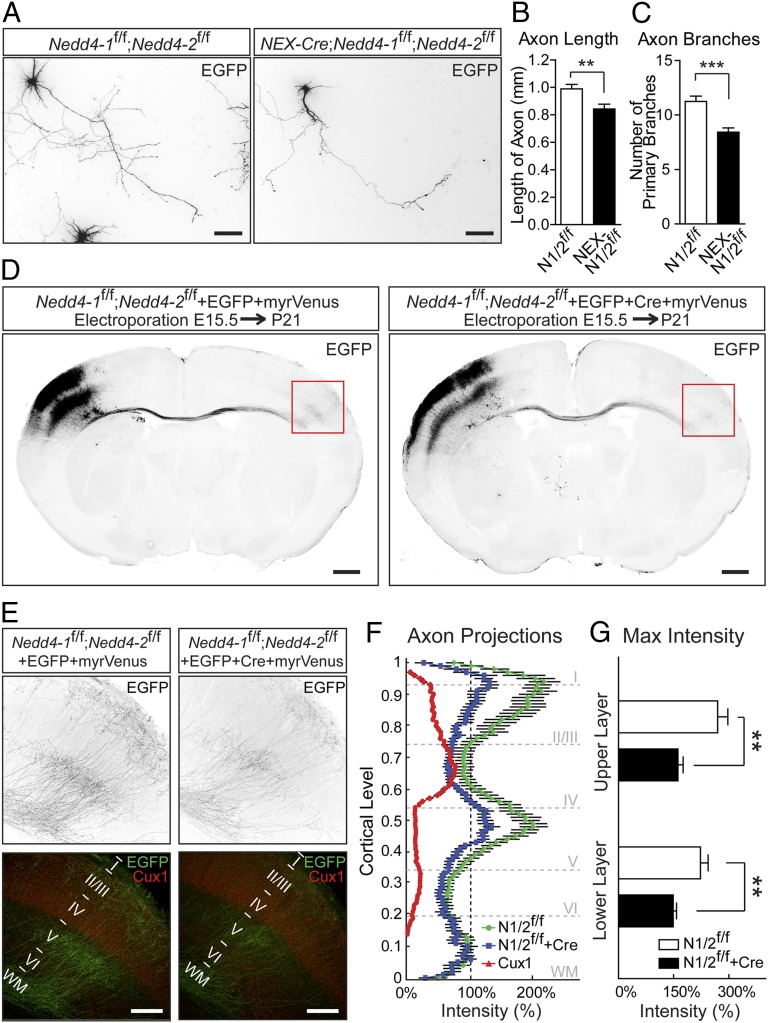
Mammalian Nedd4-1 promotes the growth and arborization of dendrites by monoubiquitinating Rap2 (15). An independent study indicated that X. laevis Nedd4, which is most homologous to mammalian Nedd4-2, controls axon branching in retinal ganglion cells by polyubiquitinating PTEN (16). Consistent with the latter study, deletion of Nedd4-2 resulted in a reduction of neurite complexity in mammalian neurons (Fig. S1). To study the roles of Nedd4-1 and Nedd4-2 in axonal development in mammalian neurons, we analyzed postmitotic neuron-specific Nedd4-1 and Nedd4-2 conditional double KO mice (NEX-Cre;Nedd4-1f/f;Nedd4-2f/f, hereafter NEX-N1/2f/f). The NEX-N1/2f/f mice are viable and show no obvious behavioral alterations in the cage environment. We prepared primary cultured hippocampal neurons from control and NEX-N1/2f/f mice to study axon morphology. At 7 d in vitro (DIV7), the length of the main axon shaft and the number of primary axonal branches showed a significant reduction in cultured NEX-N1/2f/f neurons (Fig. 1 A–C and Fig. S2 A–C). We further used in utero electroporation to study the roles of Nedd4-1 and Nedd4-2 in axonal morphogenesis in vivo. Axonal morphology was visualized by overexpressing a membrane-anchored variant of the Venus yellow fluorescent protein (myrVenus), and Nedd4-1/Nedd4-2 were eliminated by coexpression of Cre recombinase (Cre) in Nedd4-1f/f;Nedd4-2f/f embryos. Most of the myrVenus-expressing neurons migrated properly to the upper cortical layers of the ipsilateral side independent of Cre expression, indicating that Nedd4-1 and Nedd4-2 are dispensable for neuronal migration (Fig. 1D). Both the control and the Nedd4-1;Nedd4-2 double KO neurons projected axons to the contralateral side of the cortex, but the extent of axon invasion as assessed by myrVenus signal intensity was lower with Nedd4-1;Nedd4-2 double KO neurons (Fig. 1 D and E). Callosal axons branched specifically in layers II/III and V on the contralateral side (Fig. 1E). Interestingly, we observed a significant reduction of axonal branching in Nedd4-1;Nedd4-2 double KO neurons (Fig. 1 E–G). This reduced axonal branching in vivo was not due to a major dysfunction or increased apoptosis of neurons caused by Nedd4-1/Nedd4-2 deletion, because the number of neurons was normal in a given cortical area of NEX-N1/2f/f brains, although we observed a reduced cortical thickness (Fig. S2 D–G). Taken together, our in vitro and in vivo results indicate that the Nedd4-family E3 ligases Nedd4-1 and Nedd4-2 play an evolutionarily conserved role in the regulation of axon morphogenesis.
Fig. 1.
Nedd4-1 and Nedd4-2 regulate axon growth in mouse hippocampal and cortical neurons. (A) Representative images of control (Left) and Nedd4-1 and Nedd4-2 double KO (Right) cultured hippocampal neurons transfected with an EGFP-expressing vector. (Scale bars, 100 μm.) (B) Reduced length of main axon shafts in Nedd4-1;Nedd4-2 double KO neurons. N1/2f/f, 0.99 ± 0.033 mm, n = 92; NEX-N1/2f/f, 0.84 ± 0.035 mm, n = 81. **P = 0.0026, unpaired t test. (C) Reduced number of primary axonal branches in Nedd4-1;Nedd4-2 double KO neurons. N1/2f/f, 11 ± 0.5, n = 92; NEX-N1/2f/f, 8 ± 0.4, n = 81. ***P < 0.0001, unpaired t test. (D) Nedd4-1f/f;Nedd4-2f/f embryos were cotransfected with pFUGW (EGFP) and pCX-myrVenus (Left) or pFUGWiCre (EGFP+Cre) and pCX-myrVenus (Right) expression vectors using in utero electroporation. (Scale bars, 500 μm.) (E) High-magnification images of regions in the red boxes in D. Immunostaining for Cux1 was used to label layer II–IV neurons. (Scale bars, 200 μm.) (F) Quantification of the signal intensity from the cortical surface (cortical level 1) to the bottom of the white matter (WM) (cortical level 0) for axon projections at the contralateral side shown in D and E. The maximum signal in the white matter was set to 100% intensity. N1/2f/f, n = 19 from eight animals; NEX-N1/2f/f, n = 11 from five animals. (G) Significant reduction in maximum intensity of axonal projections of Nedd4-1;Nedd4-2 double KO neurons in the upper and lower layers of the cortex shown in D–F. Upper layer (cortical level 0.7–1): N1/2f/f, 269.8 ± 27.72%; N1/2f/f+Cre, 161.7% ± 14.20%; **P = 0.0019, unpaired t test with Welch’s correction. Lower layer (cortical level 0.25–0.69): N1/2f/f, 222.9 ± 20.81%; N1/2f/f+Cre, 149.1 ± 8.64%; **P = 0.0033, unpaired t test with Welch’s correction. Data are expressed as mean ± SEM. N1/2f/f, Nedd4-1f/f;Nedd4-2f/f. NEX-N1/2f/f, NEX-Cre;Nedd4-1f/f;Nedd4-2f/f.
PTEN Is Neither Poly- nor Monoubiquitinated by Nedd4-1/Nedd4-2 in Developing Mammalian Neurons.
Given previous reports that Nedd4-1/Nedd4-2–dependent ubiquitination targets PTEN for proteasomal degradation in various tissues and organisms (9, 16, 17), we examined whether PTEN is a substrate of Nedd4-family E3 ligases in axon growth regulation in the mammalian brain. We first studied PTEN protein levels in developing control and Nedd4-1;Nedd4-2 double KO neurons. Despite the fact that mammalian Nedd4-1/Nedd4-2 are important for axon growth (Fig. 1), we did not detect any up-regulation of PTEN in cultured neurons at DIV8 upon Nedd4-1/Nedd4-2 deletion (Fig. 2 A and B and Fig. S3) or in brain lysates prepared from NEX-N1/2f/f mice (Fig. S4). Rather, the PTEN protein levels were significantly reduced in Nedd4-1;Nedd4-2 double KO neurons at DIV15 (Fig. 2 A and B), indicating that Nedd4-1/Nedd4-2 are dispensable for proteasomal degradation of PTEN in developing mammalian neurons. To confirm that PTEN is not polyubiquitinated by Nedd4-1/Nedd4-2 in developing neurons, we immunoprecipitated endogenous PTEN from lysates of cortices prepared from postnatal day (P)7 control and NEX-N1/2f/f mice. The ubiquitination level of PTEN was then assessed by Western blotting using anti-ubiquitin antibodies. No reduction in PTEN polyubiquitination levels was observed in NEX-N1/2f/f brains (Fig. 2 C and D), further supporting the notion that PTEN is not polyubiquitinated by Nedd4-1/Nedd4-2 in developing neurons. In view of reports that monoubiquitination by Nedd4-1 causes nuclear import of PTEN in mouse embryonic fibroblasts (8), we also compared the subcellular localization of PTEN in control and NEX-N1/2f/f neurons by immunostaining using a specific antibody for endogenous PTEN (Fig. S5A). Neither the subcellular localization nor the nuclear compartmentalization of PTEN was affected by Nedd4-1/Nedd4-2 deletion (Fig. S5 B–E), indicating that PTEN localization is not regulated by Nedd4-1/Nedd4-2–mediated monoubiquitination in mammalian developing neurons. Moreover, we assessed the phosphatase activity of immunoprecipitated PTEN with an in vitro phosphatase assay. We titrated different amounts of the anti-PTEN antibody for immunoprecipitation and measured the activity of PTEN immunoprecipitated from control or NEX-N1/2f/f mice at the nonplateau phase of the assay, where the readout of PTEN activity was not saturated (Fig. 2E). Under these assay conditions, the phosphatase activity of PTEN was unaltered in NEX-N1/2f/f brains (Fig. 2F). Our data indicate that Nedd4-family E3 ligases do not ubiquitinate PTEN, control PTEN localization, target PTEN for proteasomal degradation, or affect the phosphatase activity of PTEN in developing mammalian neurons.
Fig. 2.
Normal expression, ubiquitination, and phosphatase activity of endogenous PTEN in neurons upon deletion of Nedd4-1 and Nedd4-2. (A) Representative Western blotting results showing that upon Nedd4-1 and Nedd4-2 deletion in cultured neurons, PTEN levels were unaltered at DIV8 and down-regulated at DIV15. Primary hippocampal neurons were prepared from Nedd4-1f/f;Nedd4-2f/f mice and Cre was expressed using lentiviral infection. (B) Quantification of PTEN levels by the Western blotting shown in A. Signals for PTEN were normalized to signals for actin. *P = 0.0471, unpaired t test, n = 3 per condition. (C) No reduction of PTEN polyubiquitination levels in NEX-N1/2f/f mouse brains. (D) Quantification of PTEN polyubiquitination levels shown in C. The smear pattern (over 60 kDa) was quantified and normalized to signals of immunoprecipitated PTEN. Unpaired t test, n = 3 per genotype. (E) Endogenous PTEN was immunoprecipitated from P7 mouse brain lysates with the indicated amount of anti-PTEN antibodies and subjected to the PTEN phosphatase activity assay. Signals from the no-PTEN negative control were subtracted from all groups. Note that the readout of the assay was dependent on the PTEN amount. (F) Endogenous PTEN was immunoprecipitated with 14 ng of the anti-PTEN antibody shown in E. Normal PTEN phosphatase activity in P7 NEX-N1/2f/f brains compared with N1/2f/f brains. (Lower) Levels of immunoprecipitated PTEN (Top blot) and genotypes of animals (Middle and Bottom blots) were confirmed by Western blotting. P = 0.6859, unpaired t test, n = 3 per genotype. Data are expressed as mean ± SEM. N1/2f/f, Nedd4-1f/f;Nedd4-2f/f. NEX-N1/2f/f, NEX-Cre;Nedd4-1f/f;Nedd4-2f/f. IB, immunoblotting; IP, immunoprecipitation.
PTEN Acts as a Negative Regulator of Nedd4-1 Expression at the Translational Level.
Although we excluded the possibility that PTEN acts as a downstream target of Nedd4-1/Nedd4-2 in the regulation of axon growth, several lines of evidence indicate an inverse correlation between the expression levels of Nedd4-1 and PTEN (9, 22, 23). These findings are compatible with the notion that PTEN may act as an upstream negative regulator of Nedd4-1 or Nedd4-2 expression. To test this, we studied the levels of Nedd4-family E3 ligases in control and PTEN KO neurons. Of note, Nedd4-1 protein levels were significantly up-regulated in the absence of PTEN whereas Nedd4-2 levels were unaltered, indicating that PTEN acts as an upstream negative regulator of Nedd4-1 expression in developing neurons (Fig. 3A, Left two lanes). We further investigated the involvement of molecules acting downstream of PTEN-dependent signaling in the regulation of Nedd4-1 expression. Among those downstream signaling proteins (5, 24), we focused on mTORC1 (serine/threonine protein kinase mammalian target of rapamycin complex 1), because it was reported to control dendrite growth (25). We treated control and PTEN KO neurons with the mTORC1 inhibitor rapamycin, and found that the up-regulation of Nedd4-1 in PTEN KO neurons was reverted by rapamycin (Fig. 3 A and B), indicating that mTORC1 activity is required for PTEN-dependent regulation of Nedd4-1 levels. In wild-type neurons, rapamycin treatment caused a corresponding reduction in Nedd4-1 expression (DMSO, 1.00 ± 0.06; rapamycin, 0.53 ± 0.08; P = 0.0102, unpaired t test, n = 3 per group). We further observed an up-regulation of Nedd4-1 expression in brain lysates from PTEN conditional KO mice (GFAP-Cre;PTENf/f), indicating that the regulation of Nedd4-1 expression by PTEN is also operational in vivo (Fig. S6). Because PTEN deletion did not affect Nedd4-1 mRNA expression levels (Fig. 3C), we investigated whether PTEN regulates Nedd4-1 mRNA translation. Actively translating polysomes and silent messenger ribonucleoproteins (mRNPs) were isolated from cultured control and PTEN KO neurons using a continuous sucrose gradient. Nedd4-1 mRNA was more strongly associated with the polysomal fraction in PTEN KO neurons as compared with control cells. Similar findings were obtained for Rpl13a mRNA, as shown previously (26), whereas the polysomal association of Nedd4-2 and GAPDH mRNAs was not changed in PTEN KO neurons (Fig. 3 D and E). Because Nedd4-1 protein stability is not changed in PTEN KO neurons (Fig. 3F), increased Nedd4-1 mRNA translation is the likely cause for the observed increase in Nedd4-1 protein levels (Fig. 3 A and B). These results indicate that the PTEN-mTORC1 pathway functions upstream of Nedd4-1 to negatively regulate Nedd4-1 mRNA translation in developing mammalian neurons.
Fig. 3.
PTEN negatively regulates Nedd4-1 expression at the translational level. (A) Representative Western blotting results showing that upon deletion of PTEN, Nedd4-1 but not Nedd4-2 protein levels were up-regulated (Left two lanes) at DIV8. This up-regulation was abolished by rapamycin treatment (Right two lanes). Primary hippocampal neurons were prepared from PTENf/f mice and Cre was expressed using lentiviral infection. Reduction in phospho-S6 (p-S6) levels was used as a positive control for rapamycin treatment. (B) Quantification of Western blotting results shown in A. ***P < 0.001, one-way ANOVA and Bonferroni’s post hoc test, n = 5 per condition. (C) Real-time quantitative PCR results showing that the Nedd4-1 mRNA level in mouse hippocampal neurons is not altered in the absence of PTEN. Neurofilament H (NFH) mRNA was used as a positive control for PTEN deletion. ***P < 0.0001, unpaired t test, n = 4 per condition. (D) Representative polysome and mRNP distribution on a sucrose gradient for cultured control and PTEN KO neurons. Fractions 1–7 correspond to polysome-associated (translating) mRNAs. (E) Quantification of the relative translational efficiency of Nedd4-1, Rpl13a, Nedd4-2, and GAPDH mRNAs reported as the ratio of polysome-associated mRNAs over total mRNAs. **P = 0.004, *P = 0.018, unpaired t test, n = 3. Rpl13a was used as a positive control (26), whereas GAPDH was a negative control. (F) Neurons (DIV8) were incubated with cycloheximide (CHX; 50 μg/mL) for different durations as indicated, and the Nedd4-1 level was assessed by Western blotting. The remaining Nedd4-1 level over time showed no difference between the two groups, indicating that the stability of Nedd4-1 protein is not affected in PTEN KO neurons. Data are expressed as mean ± SEM.
Nedd4-1 Is a Major Target of mTORC1 Signaling in Neurite Development.
To investigate the physiological importance of PTEN-regulated Nedd4-1 expression in developing neurons, we studied the impact of genetic elimination of Nedd4-1 on neurite development in the PTEN KO background (Fig. 4 A–E). Consistent with a previous report (11), PTEN KO led to hypertrophy of neurites (Fig. 4 A and B). Strikingly, additional KO of Nedd4-1 in PTEN KO neurons partially rescued the neurite hypertrophy (Fig. 4 B–E), indicating that Nedd4-1 is a prominent regulator of neurite growth downstream of PTEN-dependent signaling. This rescue of neurite hypertrophy in PTEN;Nedd4-1 double KO neurons was accompanied by a rescue of the change in synapse numbers, as indicated by analyses of miniature excitatory postsynaptic currents (Fig. S7). To study whether mTORC1 is involved in the PTEN- and Nedd4-1–dependent regulation of neurite growth, we applied rapamycin to cultured control and Nedd4-1 KO neurons and quantified the neurite complexity (Fig. 4 F–K). Consistent with previous findings (25), rapamycin treatment reduced the complexity of distal neurites in control neurons (Fig. 4 F, G, J, and K). In Nedd4-1 KO neurons, on the other hand, the effect of rapamycin was not significant (Fig. 4 H–K), indicating that mTORC1 functions upstream of Nedd4-1 and plays a role in the regulation of Nedd4-1 expression to promote neurite growth. Of note, the extent of the effects of rapamycin (Fig. S8 A–F) or additional KO of Nedd4-1 (Fig. 4 A–E) in PTEN KO neurons was partial in both cases, indicating that pathways independent of mTORC1 and Nedd4-1 also contribute to the hypertrophy of neurites in PTEN KO neurons. In addition, rapamycin treatment had hardly any effect on dendrite growth in PTEN;Nedd4-1 double KO neurons (Fig. S8 G–J), further supporting the notion that Nedd4-1 operates downstream of mTORC1. Interestingly, whereas PTEN KO neurons tend to project more than one axon because of deregulation of another signaling molecule downstream of PTEN, GSK3β (12), this gain-of-function phenotype in axon acquisition was independent of Nedd4-1/Nedd4-2 deletion (Fig. S9). These results, together with our biochemical data (Fig. 3), indicate that Nedd4-1 is an important downstream effector of the PI3K/PTEN-mTORC1 pathway in the regulation of neurite growth (Fig. 5). In addition, independent regulatory programs driven by either PTEN or Nedd4-1 may interact and even converge upon a common cell biological process, such as neurite growth.
Fig. 4.
Nedd4-1 has a prominent role in neurite morphogenesis and is dependent upon mTORC1 activity. (A–C) Representative images of a control neuron (A), a PTEN KO neuron (B), and a PTEN;Nedd4-1 double KO neuron (C). (Scale bars, 50 μm.) (D) Sholl analyses for the three groups in A–C. Note that KO of Nedd4-1 partially rescues the hypertrophy of neurite structure seen in PTEN KO neurons. (E) Statistical analysis of the total number of crossing neurites obtained in the Sholl analysis shown in D. Data were analyzed by one-way ANOVA and Tukey’s post hoc test (n > 60 neurons per group). N.S., not significant. (F–I) Representative images of a control neuron treated with DMSO (F), a control neuron treated with rapamycin (G), a Nedd4-1 KO neuron treated with DMSO (H), and a Nedd4-1 KO neuron treated with rapamycin (I). (Scale bars, 50 μm.) (J) Sholl analyses for the four groups in F–I. Note that rapamycin treatment had a milder effect on the dendritic structure in Nedd4-1 KO neurons. (K) Statistical analysis of the total number of crossing neurites obtained in the Sholl analysis shown in J. Data were analyzed by one-way ANOVA and Tukey’s post hoc test (n > 60 neurons per group). Data are expressed as mean ± SEM. N1f/f, Nedd4-1f/f. NEX-N1f/f, NEX-Cre;Nedd4-1f/f. *P < 0.05, ***P < 0.001.
Fig. 5.
Model of the regulation of Nedd4-1 by the PI3K/PTEN-mTORC1 signaling pathway during neurite growth. PI3K catalyzes the phosphorylation of PtdInsP2 to generate PtdInsP3 (e.g., in response to growth factor or hormone stimulation). PTEN converts PtdInsP3 back to PtdInP2 and thus antagonizes the effect of PI3K. Elevated PtdInsP3 levels lead to the phosphorylation of AKT, which further phosphorylates GSK3β and mTORC1. The phosphorylation of GSK3β promotes axon acquisition in neurons (12). On the other hand, AKT-dependent activation of mTORC1 stimulates downstream pathways that include the protein translation machinery. Nedd4-1 is important for neurite outgrowth but dispensable for axon acquisition, and its mRNA is a prominent target of regulation by mTORC1 at the translational level. Nedd4-1 is not responsible for PTEN ubiquitination or regulation in developing mammalian neurons.
Discussion
In mammalian neurons, PTEN KO results in an enhanced dendrite arborization (11), which is opposite the phenotype of Nedd4-1 KO neurons (15). On the other hand, overexpression of PTEN causes complex phenotypic alterations, including defects in axon specification (12) and increased apoptosis (27), which are not complemented by opposite phenotypic changes upon Nedd4-1/Nedd4-2 loss (Figs. S2 C–E and S9). This partial lack of complementarity between PTEN KO and Nedd4-1;Nedd4-2 double KO phenotypes indicates that Nedd4-family E3 ligases are not upstream regulators of PTEN in developing mammalian neurons. In accord with this notion, we show here that mammalian Nedd4-1 and Nedd4-2 regulate axon growth (Fig. 1) in a manner that is not paralleled by changes in the activity, ubiquitination levels, or subcellular compartmentalization of PTEN (Fig. 2 and Fig. S5). Our data clearly indicate that alterations in PTEN levels, localization, or function cannot be involved in the regulation of axon growth by Nedd4-family E3 ligases in the mammalian brain. Moreover, only a very minor fraction of endogenous PTEN is ubiquitinated in the developing mouse brain (Fig. 2C), indicating that the ubiquitination-mediated regulation of PTEN may not be as functionally pervasive in mammalian central nervous system neurons as it appears to be in other tissues and organisms (10, 28). However, we cannot exclude the possibilities that PTEN ubiquitination by Nedd4-family E3 ligases is only functionally relevant in specific neuron types such as X. laevis retinal ganglion cells and mammalian peripheral neurons (16, 17), or that ubiquitination of PTEN occurs only in specific contexts such as neuroprotection upon ischemic stress or zinc insult (23, 29).
Beyond merely excluding a role of Nedd4-family E3 ligases in the regulation of PTEN levels, localization, or function in mammalian brain neurons, our study provides evidence for a strikingly inverse scenario, according to which PTEN antagonizes the PI3K-mTORC1 pathway to limit Nedd4-1 mRNA translation and neurite growth. Importantly, our model (Fig. 5), in which PTEN acts as an upstream negative regulator of Nedd4-1, can also account for the inverse correlation between expression levels of PTEN and Nedd4-1 found in previous studies (9, 22, 23). Further data supporting our model include a recently published study that revealed a reduction in the translational efficiency of Nedd4-1 mRNA in the presence of an mTORC1 inhibitor (26). Of note, the 5′ untranslated region (5′UTR) of Nedd4-1 (GenBank accession no. NM_010890) mRNA contains a pyrimidine-rich sequence stretch that is related to the 5′ terminal oligopyrimidine (5′TOP) motif. This 5′TOP motif is important for anchoring protein translation initiation factors to the 5′ cap structure of mTORC1 target mRNAs (26). The pyrimidine-rich sequence in the 5′UTR of Nedd4-1 mRNA may therefore play a similar role as the 5′TOP motif in starting translation of Nedd4-1 mRNA in an mTORC1-dependent manner. In view of further evidence indicating an important role of protein synthesis in neurite growth (25, 30, 31), our data are compatible with the notion that Nedd4-1 mRNA is a major target of the translational machinery in growing neurites.
Materials and Methods
A complete description of the materials and methods used in this study is provided in SI Materials and Methods.
Animals.
The Nedd4-1f/f, Nedd4-2f/f, PTENf/f, NEX-Cre, and GFAP-Cre mice were described previously (15, 32–35). All animal experiments were approved by the responsible local government [Landesamt für Verbraucherschutz und Lebensmittelsicherheit (LAVES) Niedersachsen] and conducted in compliance with German guidelines [European Parliament 2010/63/EU; comparable to National Institutes of Health (NIH) guidelines].
Expression Vectors.
The expression vector encoding the membrane-anchored variant of Venus (pCX-myrVenus; Addgene plasmid 32602) was a kind gift from A.-K. Hadjantonakis (Memorial Sloan Kettering Cancer Center, New York) (36). Information of other vectors is provided in SI Materials and Methods.
Hippocampal Culture and Cell Biology.
The primary culture of hippocampal neurons was performed based on published protocols with minor modifications (37, 38). Lentiviruses were added within 24 h after plating. Transfection of expression vectors was performed by using the calcium phosphate method at DIV1. Immunostaining of fixed neurons was carried out as described previously (38).
In Utero Electroporation and Immunohistochemistry.
In utero electroporation was performed as published (39). For immunohistochemistry, mice were perfused transcardially with PBS followed by 4% (wt/vol) paraformaldehyde/PBS. The brains were collected and postfixed in 4% paraformaldehyde/PBS at 4 °C overnight. Permeabilization and immunostaining were performed as published (38). The lengths of axons in cultured neurons were analyzed using the NeuronJ plugin (40) in ImageJ (NIH).
Mouse Brain Homogenates, Cell Lysates, and Western Blotting.
Cultured neurons were harvested at DIV8 or DIV15 by directly applying Laemmli buffer after washing the cells twice with PBS. For rapamycin treatment and Western blotting experiments (Fig. 3), DMSO or rapamycin (20 nM) was applied to the culture medium 24 h before harvesting the cells. For preparation of mouse brain homogenates, cortices from P7 mice were homogenized in 0.32 M sucrose with protease inhibitors (0.2 mM PMSF, 1 μg/mL aprotinin, 0.5 μg/mL leupeptin) and phosphatase inhibitors (10 mM NaF, 1 mM Na3VO4). Proteins were separated by SDS/PAGE and transferred to PVDF or nitrocellulose membranes for Western blotting using standard procedures.
Supplementary Material
Acknowledgments
We thank B. Hesse-Niessen, K.-P. Hellmann, I. Thanhäuser, D. Schwerdtfeger, C. Harenberg, and the staff of the animal facility at the Max Planck Institute of Experimental Medicine for technical support; D. Trono, D. Baltimore, R. Huganir, and A.-K. Hadjantonakis for reagents; and K. Fujishima, J. Stegmüller, A. Wodarz, E. Ciirdaeva, F. Benseler, M. Schwark, and V. Tarabykin for advice. This work was supported by grants from the German Research Foundation (SPP1365/KA3423/1-1 to N.B. and H.K.; SFB958/A16 to B.J.E.), the Fritz Thyssen Foundation (to H.K.), the Japan Society for the Promotion of Science (Postdoctoral Fellowship for Research Abroad; to H.K.), the Yamanouchi Foundation for Research on Metabolic Disorders (to H.K.), the Mochida Memorial Foundation for Medical and Pharmaceutical Research (to H.K.), the Max Planck Society (to N.B.), the Vlaams Instituut voor Biotechnologie (to C.B.), the Onderzoekstoelage (OT/12/089 to C.B.), NIH-National Institute of Neurological Disorders and Stroke (NS089456 to F.P.), and the HEALTH-2009-2.1.2-1 EU-FP7 “SynSys” (to N.B. and C.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400737111/-/DCSupplemental.
References
- 1.Arimura N, Kaibuchi K. Neuronal polarity: From extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8(3):194–205. doi: 10.1038/nrn2056. [DOI] [PubMed] [Google Scholar]
- 2.Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawabe H, Brose N. The role of ubiquitylation in nerve cell development. Nat Rev Neurosci. 2011;12(5):251–268. doi: 10.1038/nrn3009. [DOI] [PubMed] [Google Scholar]
- 4.Yi JJ, Ehlers MD. Emerging roles for ubiquitin and protein degradation in neuronal function. Pharmacol Rev. 2007;59(1):14–39. doi: 10.1124/pr.59.1.4. [DOI] [PubMed] [Google Scholar]
- 5.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13(5):283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 6.van Diepen MT, Eickholt BJ. Function of PTEN during the formation and maintenance of neuronal circuits in the brain. Dev Neurosci. 2008;30(1-3):59–64. doi: 10.1159/000109852. [DOI] [PubMed] [Google Scholar]
- 7.Tamguney T, Stokoe D. New insights into PTEN. J Cell Sci. 2007;120(Pt 23):4071–4079. doi: 10.1242/jcs.015230. [DOI] [PubMed] [Google Scholar]
- 8.Trotman LC, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128(1):141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128(1):129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo H, et al. E3 ubiquitin ligase Cbl-b regulates Pten via Nedd4 in T cells independently of its ubiquitin ligase activity. Cell Reports. 2012;1(5):472–482. doi: 10.1016/j.celrep.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon C-H, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50(3):377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: Critical roles of GSK-3beta and its upstream regulators. Cell. 2005;120(1):123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 13.Luikart BW, et al. Pten knockdown in vivo increases excitatory drive onto dentate granule cells. J Neurosci. 2011;31(11):4345–4354. doi: 10.1523/JNEUROSCI.0061-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amiri A, et al. Pten deletion in adult hippocampal neural stem/progenitor cells causes cellular abnormalities and alters neurogenesis. J Neurosci. 2012;32(17):5880–5890. doi: 10.1523/JNEUROSCI.5462-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawabe H, et al. Regulation of Rap2A by the ubiquitin ligase Nedd4-1 controls neurite development. Neuron. 2010;65(3):358–372. doi: 10.1016/j.neuron.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drinjakovic J, et al. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. Neuron. 2010;65(3):341–357. doi: 10.1016/j.neuron.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christie KJ, Martinez JA, Zochodne DW. Disruption of E3 ligase NEDD4 in peripheral neurons interrupts axon outgrowth: Linkage to PTEN. Mol Cell Neurosci. 2012;50(2):179–192. doi: 10.1016/j.mcn.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Fouladkou F, et al. The ubiquitin ligase Nedd4-1 is dispensable for the regulation of PTEN stability and localization. Proc Natl Acad Sci USA. 2008;105(25):8585–8590. doi: 10.1073/pnas.0803233105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Themsche C, Leblanc V, Parent S, Asselin E. X-linked inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J Biol Chem. 2009;284(31):20462–20466. doi: 10.1074/jbc.C109.009522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maddika S, et al. WWP2 is an E3 ubiquitin ligase for PTEN. Nat Cell Biol. 2011;13(6):728–733. doi: 10.1038/ncb2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed SF, et al. The chaperone-assisted E3 ligase C terminus of Hsc70-interacting protein (CHIP) targets PTEN for proteasomal degradation. J Biol Chem. 2012;287(19):15996–16006. doi: 10.1074/jbc.M111.321083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn Y, Hwang CY, Lee S-R, Kwon K-S, Lee C. The tumour suppressor PTEN mediates a negative regulation of the E3 ubiquitin-protein ligase Nedd4. Biochem J. 2008;412(2):331–338. doi: 10.1042/BJ20071403. [DOI] [PubMed] [Google Scholar]
- 23.Kwak Y-D, et al. Functional interaction of phosphatase and tensin homologue (PTEN) with the E3 ligase NEDD4-1 during neuronal response to zinc. J Biol Chem. 2010;285(13):9847–9857. doi: 10.1074/jbc.M109.091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: The path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13(3):195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 25.Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. Control of dendritic arborization by the phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci. 2005;25(49):11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thoreen CC, et al. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485(7396):109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gary DS, Mattson MP. PTEN regulates Akt kinase activity in hippocampal neurons and increases their sensitivity to glutamate and apoptosis. Neuromolecular Med. 2002;2(3):261–269. doi: 10.1385/NMM:2:3:261. [DOI] [PubMed] [Google Scholar]
- 28.Yim E-K, et al. Rak functions as a tumor suppressor by regulating PTEN protein stability and function. Cancer Cell. 2009;15(4):304–314. doi: 10.1016/j.ccr.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howitt J, et al. Ndfip1 regulates nuclear Pten import in vivo to promote neuronal survival following cerebral ischemia. J Cell Biol. 2012;196(1):29–36. doi: 10.1083/jcb.201105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crino PB, Eberwine J. Molecular characterization of the dendritic growth cone: Regulated mRNA transport and local protein synthesis. Neuron. 1996;17(6):1173–1187. doi: 10.1016/s0896-6273(00)80248-2. [DOI] [PubMed] [Google Scholar]
- 31.Kumar V, Zhang M-X, Swank MW, Kunz J, Wu G-Y. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci. 2005;25(49):11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura T, et al. Deletion of the ubiquitin ligase Nedd4L in lung epithelia causes cystic fibrosis-like disease. Proc Natl Acad Sci USA. 2011;108(8):3216–3221. doi: 10.1073/pnas.1010334108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lesche R, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32(2):148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- 34.Goebbels S, et al. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44(12):611–621. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- 35.Gregorian C, et al. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci. 2009;29(6):1874–1886. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhee JM, et al. In vivo imaging and differential localization of lipid-modified GFP-variant fusions in embryonic stem cells and mice. Genesis. 2006;44(4):202–218. doi: 10.1002/dvg.20203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126(3):397–42. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- 38.Dresbach T, et al. Functional regions of the presynaptic cytomatrix protein bassoon: Significance for synaptic targeting and cytomatrix anchoring. Mol Cell Neurosci. 2003;23(2):279–291. doi: 10.1016/s1044-7431(03)00015-0. [DOI] [PubMed] [Google Scholar]
- 39.Saito T. In vivo electroporation in the embryonic mouse central nervous system. Nat Protoc. 2006;1(3):1552–1558. doi: 10.1038/nprot.2006.276. [DOI] [PubMed] [Google Scholar]
- 40.Meijering E, et al. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 2004;58(2):167–176. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.