Significance
Bone morphogenetic proteins (BMPs) are essential signaling molecules important in embryo development and maintaining tissue function in adulthood. BMPs are regulated outside the cell by inhibitors such as chordin. The structure of chordin is unknown as extracellular proteins are generally not suitable for high-resolution methods. This study uses electron microscopy and other techniques to determine the shape of human chordin. It has a compact horseshoe-shaped structure with the terminal BMP-binding regions protruding as prongs. The spacing of these domains supports a cooperative binding arrangement with BMPs. We compared BMP binding and inhibition by chordin with two truncated chordin variants. Proteolytic cleavage has little effect on binding of BMP-4 and BMP-7 but C-terminal cleavage makes chordin a better BMP inhibitor.
Abstract
Bone morphogenetic proteins (BMPs) orchestrate key cellular events, such as proliferation and differentiation, in development and homeostasis. Extracellular antagonists, such as chordin, are essential regulators of BMP signaling. Chordin binds to BMPs blocking interaction with receptors, and cleavage by tolloid proteinases is thought to relieve this inhibition. A model has been previously proposed where chordin adopts a horseshoe-like arrangement enabling BMP binding cooperatively by terminal domains (1). Here, we present the nanoscale structure of human chordin using electron microscopy, small angle X-ray scattering, and solution-based biophysical techniques, which together show that chordin indeed has a compact horseshoe-shaped structure. Chordin variants were used to map domain locations within the chordin molecule. The terminal BMP-binding domains protrude as prongs from the main body of the chordin structure, where they are well positioned to interact with the growth factor. The spacing provided by the chordin domains supports the principle of a cooperative BMP-binding arrangement that the original model implied in which growth factors bind to both an N- and C-terminal von Willebrand factor C domain of chordin. Using binding and bioactivity assays, we compared full-length chordin with two truncated chordin variants, such as those produced by partial tolloid cleavage. Cleavage of either terminal domain has little effect on the affinity of chordin for BMP-4 and BMP-7 but C-terminal cleavage increases the efficacy of chordin as a BMP-4 inhibitor. Together these data suggest that partial tolloid cleavage is insufficient to ablate BMP inhibition and the C-terminal chordin domains play an important role in BMP regulation.
Bone morphogenetic proteins (BMPs) are members of the TGF-β superfamily of cytokines (2). Their highly diverse functions include bone and joint development, cell proliferation, differentiation, and embryonic patterning. BMP signaling is regulated by a number of extracellular antagonists, which include noggin (3), the DAN family (4), and chordin (5). During embryogenesis of vertebrates and invertebrates, antagonism between BMP and chordin/Sog is a general mechanism by which the dorsoventral axis is established (6). Chordin is secreted into the extracellular space where it binds directly to BMP-2, BMP-4, and BMP-7 and antidorsalizing morphogenetic protein (ADMP), thereby preventing interaction with their receptors (5, 7). Twisted gastrulation (Tsg), essential for the correct formation of the dorsal–ventral axis, can act as a BMP-antagonist by binding to both chordin and BMP, enhancing chordin–BMP inhibition (8).
Chordin has a modular domain architecture consisting of four von Willebrand factor C (vWC) homology domains and four chordin-specific (CHRD) domains located between the first and second vWC domains (Fig. 1A). The BMP binding and biological activities of chordin reside in the vWC domains, particularly vWC1 and -3 (1). Previous binding analysis suggests that, whereas vWC1 can bind to different BMPs with high affinity, vWC3 specifically binds to BMP-2 and BMP-4 (1), and vWC4 to BMP-7 (9). The individual vWC domains have decreased biological activity compared with full-length chordin (1), which suggests that chordin may bind BMP cooperatively via a vWC domain from each terminus. As a result, a hypothetical model (1) has been postulated where chordin adopts a horseshoe-like conformation, bringing the termini into close proximity (Fig. 1B). It is thought that the interaction with BMP occurs in a ratio of one chordin molecule to one BMP dimer (5, 9, 10). The CHRD domains, which are also found in a number of microbial proteins (11), are not thought to bind BMP but may alter the avidity of chordin for BMP by facilitating cooperative binding.
Fig. 1.
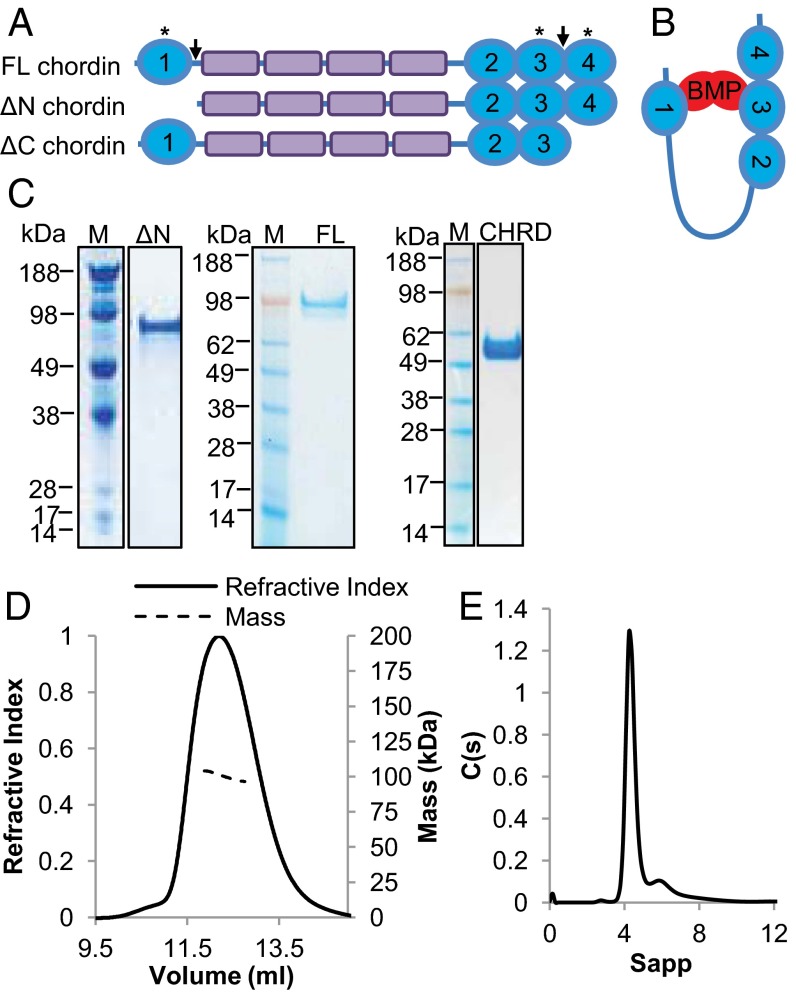
Domain structure and expression of full-length and chordin variants. (A) Schematic diagram of FL-, ΔN-, and ΔC-chordin, fragments produced following cleavage by tolloid proteinases and expressed for these studies. Cleavage sites in chordin are indicated by arrows, and asterisks indicate domains reported to bind to BMP with high affinity. (B) Horseshoe-model of chordin–BMP binding modified from ref. 1. (C) SDS/PAGE gel showing ΔN-, FL-chordin, and CHRD region following purification. (D) MALLS profile of FL-chordin showing refractive index and molecular mass against elution volume, predicting Mw of 101 kDa. (E) Velocity AUC analysis of ∆N-chordin in 250 mM NaCl showing a major peak corresponding to a monomer (87.4 kDa, S20,w 4.41, Rh 4.55 nm, and f/fo 1.52).
Members of the BMP-1/tolloid family of metalloproteinases cleave chordin at two sites, immediately downstream of vWC1 and -3 (12, 13). Tsg can also promote cleavage of chordin by tolloids and has a role in increasing the turnover of chordin fragments (14). Following tolloid cleavage, chordin is unable to antagonize BMP activity (13) but the vWC-domain–containing chordin fragments retain the ability to bind BMP (9). In some circumstances, truncation of the Drosophila homolog, Sog, can lead to gain of function (15). In zebrafish, chordin becomes a significantly more effective BMP inhibitor in vivo following cleavage of the C-terminal domain (16). However, there is significant evidence that chordin fragments have reduced biological activity in Xenopus (1, 17) and are subject to more rapid endocytotic turnover (18). Chordin interacts with cell surface proteins including crossveinless-2 (CV2) (19) and collagen IV (20). Because these interactions are mediated through the vWC domains, cleavage of chordin could give rise to altered interactions with these binding partners, which could be a means of fine tuning the localization and activity of BMP.
In contrast to the extensive studies on the function of chordin in development, the structural basis for the mechanism of BMP regulation remains unexplored. Therefore, to investigate further the molecular details of the BMP-antagonist chordin, we determined the nanostructures of human chordin, a biologically relevant chordin cleavage product and the CHRD domains, using electron microscopy, small angle X-ray scattering (SAXS), and other biophysical approaches. To determine the function of chordin fragments generated by tolloid cleavage, we used a surface plasmon-resonance–based approach and bioactivity assays. Correlation of the structural observations with binding affinity for BMP-4 and -7 provides a detailed understanding of the molecular details underpinning BMP antagonism by chordin.
Results
Hydrodynamic Properties of Chordin.
Chordin is composed of four vWC domains separated by four chordin-specific CHRD domains as shown in Fig. 1A. The tolloid cleavage sites of chordin and resultant fragments lacking vWC1 (∆N-chordin) or vWC4 (ΔC-chordin) are also shown. For structural and functional analysis full-length (FL), ∆N-, and ΔC-chordin and the CHRD region were expressed and purified (Fig. 1C and Fig. S1). The presence of N-linked glycans was detected by peptide-N-glycosidase F digestion with all predicted N-glycosylation sites residing in the CHRD region (Fig. S1 B and D). Multiangle laser light scattering (MALLS) was used to determine the molecular mass of FL-chordin (Fig. 1D). The experimentally determined mass was 101 kDa, which agrees well with that predicted for a monomer based on amino acid sequence (100.8 kDa). The radius of gyration (Rg) was 5.5 nm and hydrodynamic radius (Rh) was 4.6 nm, the ratio of which is suggestive of an elongated molecule. Sedimentation velocity analytical ultracentrifugation (AUC) was used to assess the hydrodynamic properties of ∆N-chordin. The most abundant species had a sedimentation coefficient of 4.41 S and estimated mass of 87.4 kDa (Fig. 1E), which is similar to that predicted from the primary sequence for a monomer (87 kDa). The f/fo 1.52 is also indicative of an elongated molecule.
Chordin Has a Horseshoe-Shaped Structure.
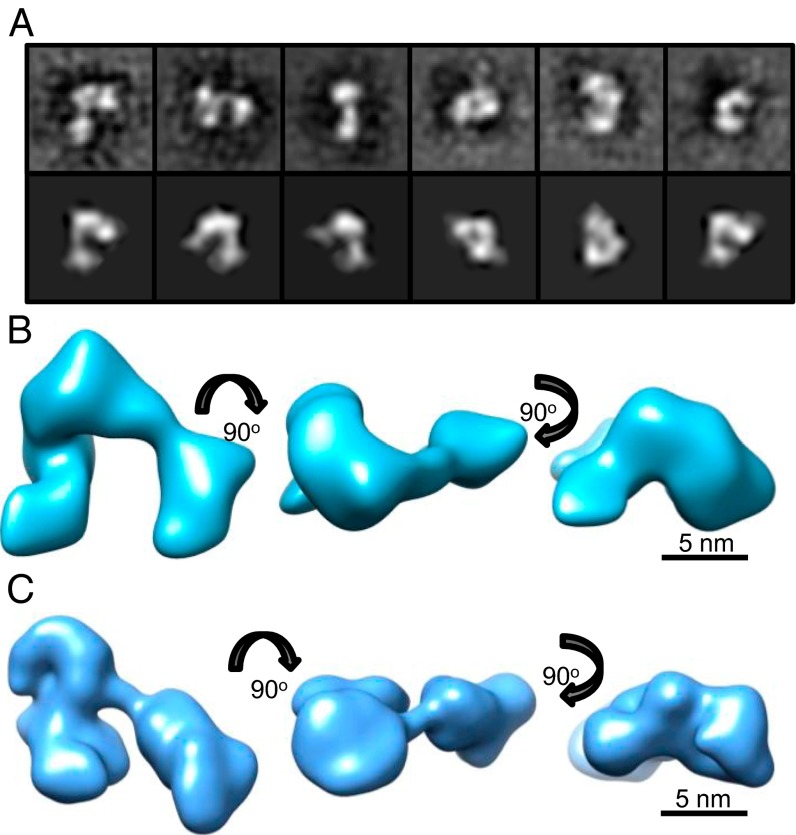
BMPs have been shown to bind to chordin via vWC1 and either vWC3 (BMP-2 (9) and -4 (1)) or vWC4 (for BMP-7) (9) placing BMP binding domains at opposite termini of chordin. This arrangement has led to the prediction that chordin forms a horseshoe-like structure placing these domains in close proximity where they may both simultaneously interact with the BMP dimer in a cooperative manner. To determine the chordin shape and arrangement of BMP binding domains, we investigated the structure of FL-chordin using single-particle transmission electron microscopy (TEM). Negatively stained chordin particles (Fig. S2) were classified using reference-free alignment (Fig. 2A) and used to calculate a 3D reconstruction to 30-Å resolution (Fig. S3). The 3D structure of chordin reveals a horseshoe-shaped conformation with dimensions 15 nm × 13 nm × 8 nm (Fig. 2B). As predicted by the original model, the structure indeed has the terminal regions in close proximity but with globular segments of density throughout the structure.
Fig. 2.
Three-dimensional EM and Solution SAXS models of FL-chordin. Three-dimensional structure of FL-chordin was generated using TEM. (A) Selected class sum images (Upper) and reprojections (Lower) that represent different views of FL-chordin are shown (box size = 27 nm). (B) Class sum images were used to generate a 3D TEM model of FL-chordin using angular reconstitution. (C) Ab initio model generated from Solution SAXS data using GASBOR shown in three orthogonal views.
Independently, we determined the 3D shape of chordin in solution using SAXS. Measurements were made at the Deutsches Elektronen Synchrotron (DESY) and Diamond Light Source (Fig. S4A). The data quality was assessed using a Guinier plot and the Rg obtained was 5.8 nm (Fig. S4B), which correlated closely with experimental MALLS data. Ab initio bead models were generated using the program GASBOR. At least 20 separate simulations were completed to determine the common structural features; the models fit the experimental data with mean discrepancy factor (x) 0.67. The averaged model had dimensions of 17.5 nm × 11 nm × 6 nm (Fig. 2C). The overall shape of the SAXS model is similar to that determined by EM and together show that chordin is more compact than expected, but they support the overall principle of cooperative binding that the horseshoe model implied.
Location of the N-Terminal vWC1 Domain.
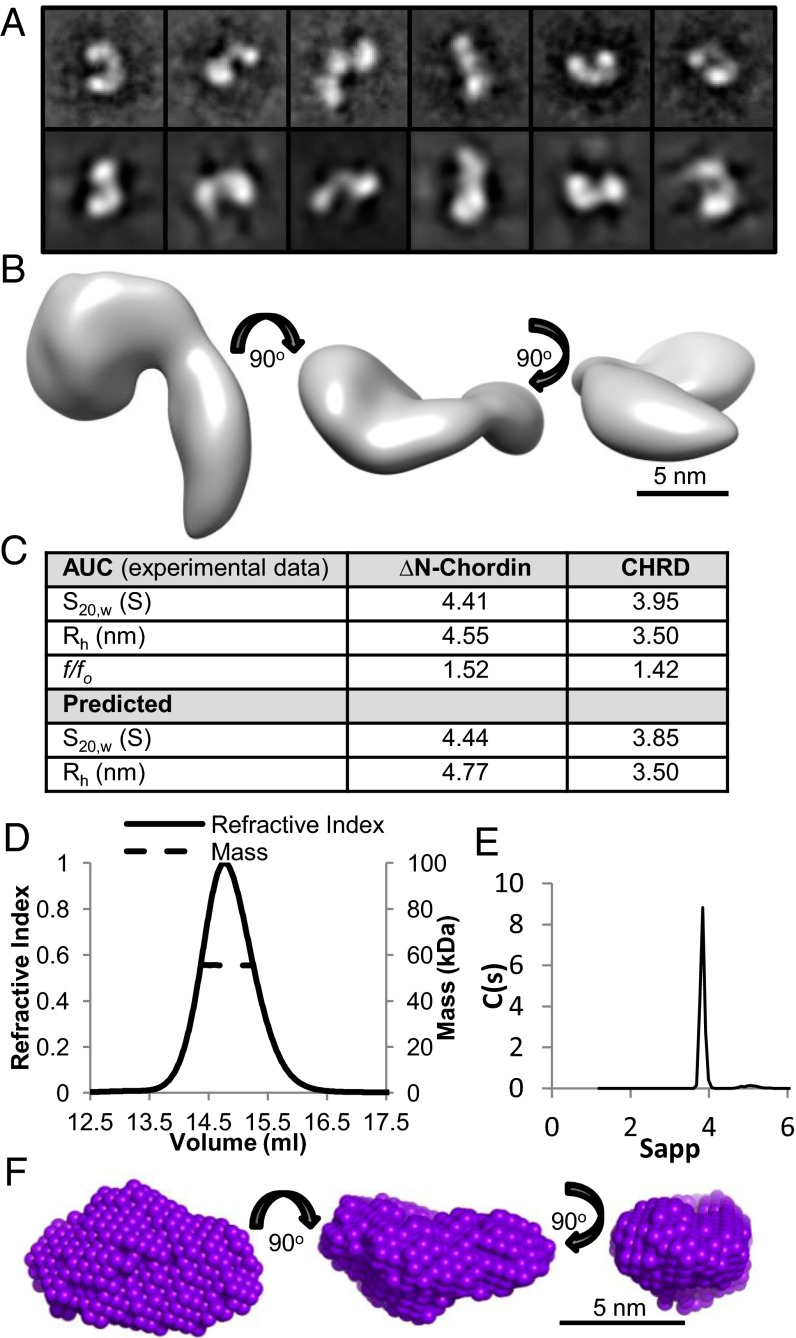
To identify where the terminal vWC domains were located in the model of FL-chordin, electron microscopy was used to analyze the ∆N-chordin variant. Negatively stained particles were classified using reference-free alignment and used to calculate a 3D reconstruction (Fig. 3A). The 3D reconstruction was similar to FL-chordin but smaller, due to the absence of the BMP binding vWC1 domain. ∆N-chordin was composed of a globular region connected to a more elongated tail region (Fig. 3B). The overall shape compared well with FL-chordin, which indicates that the loss of a domain following tolloid cleavage does not appear to give rise to major conformational rearrangement. The theoretical sedimentation properties of this model were calculated and correlated closely with experimental AUC data (Fig. 3C), giving confidence in the shape of ∆N-chordin.
Fig. 3.
EM model and SAXS models of ΔN-chordin and the CHRD region. A 3D structure of ΔN-chordin was generated using TEM. (A) Selected class sum images (Upper) and reprojections (Lower) that represent different views of ΔN-chordin are shown (box size = 27 nm). Class sum images were used to generate a 3D TEM model (B). (C) The experimentally determined hydrodynamic properties from AUC are compared with values predicted from the TEM model for ∆N-chordin using HYDROMIC (32) and the SAXS model for the CHRD region using HYDROPRO (33). (D) MALLS profile of the CHRD region showing refractive index and molecular mass against elution volume, predicting Mw of 55.5 kDa. (E) Velocity AUC analysis of the CHRD region showing a peak corresponding to a monomeric species. (F) Ab initio model generated from SAXS data using DAMMIN shown in three orthogonal views.
Shape of the CHRD Region.
The CHRD region is unique to chordin, but as yet has no defined function, and its structure is based on an uncharacterized fold. MALLS showed that this region was monomeric with experimental mass of 55.5 kDa (Fig. 3D), which agrees well with that predicted for a monomer (53.4 kDa). Sedimentation velocity AUC was used to assess the hydrodynamic properties of the CHRD region. The predominant species had sedimentation coefficient of 3.95 S and f/fo 1.42 (Fig. 3E), which suggests a more globular structure than ΔN-chordin (f/fo 1.52). SAXS data on the CHRD region was collected, the Rg obtained from the Guinier plot was 2.9 nm, and the Dmax was estimated at 9 nm using GNOM (Fig. S5). Ab initio bead models were generated using the program DAMMIN, which revealed a diamond shaped model with dimensions 9 × 5 × 4 nm (Fig. 3F). The theoretical sedimentation properties of this model correlated closely with values determined from experimental AUC data (Fig. 3C), giving further confidence in the SAXS model. This region is more globular than predicted, which suggests it is contributing to one of the globular segments within the chordin horseshoe-shaped structure.
Modeling the Chordin Structure and Chordin–BMP Complex.
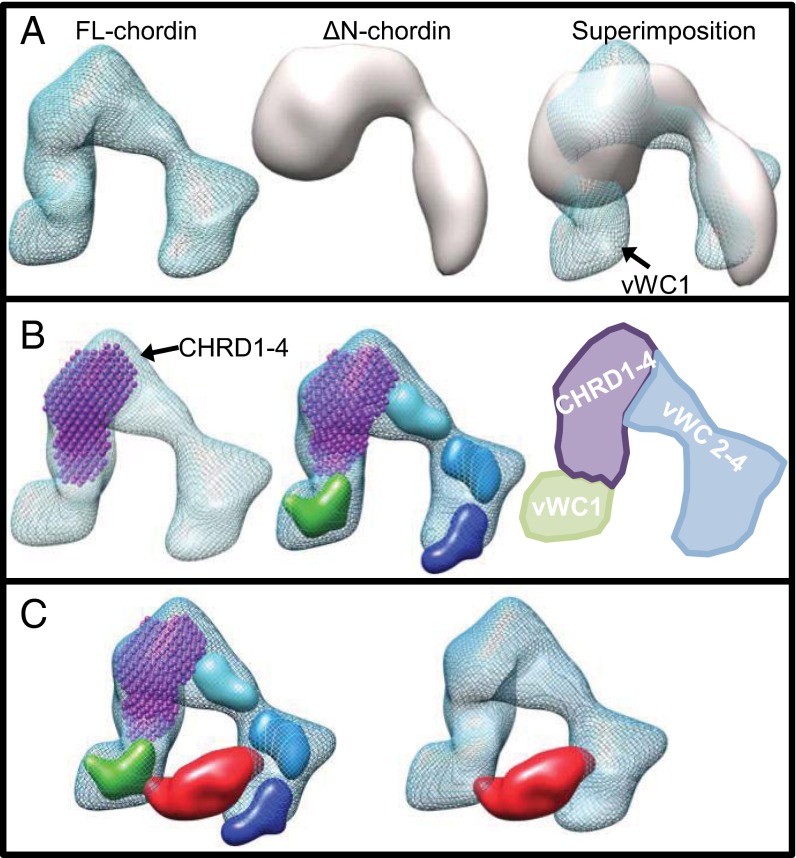
To provide an insight into the location of the chordin domains, we superimposed the EM maps for FL-chordin and ∆N-chordin. As expected, an additional region of density, the size of which agrees well with a single vWC domain, is apparent in one of the terminal regions of FL-chordin (Fig. 4A). To further dissect the domain organization of chordin, we fitted the SAXS model of the CHRD region into the FL-chordin map at a location directly adjacent to the vWC1 domain, where it fits snugly into the TEM density (Fig. 4B). The remaining additional density agrees well with that expected for the three C-terminal vWC domains.
Fig. 4.
Location of chordin domains and proposed model for a chordin–BMP complex. (A) The FL- and ΔN-chordin maps calculated from EM reconstructions are shown individually and superimposed and provide the location of the vWC1 domain. (B) The model of the CHRD region shown in purple was docked into each map and the remaining density attributed to the C-terminal vWC domains. Homology models representing vWC1 (green), vWC2 (cyan), vWC3 (blue), and vWC4 (dark blue) have been placed within the chordin electron density map to represent the likely positions of these domains. This modeling is also represented as a schematic diagram. (C) The structure of BMP-2 (PDB 3BK3) (21) rendered at 20-Å resolution and shown in red was docked into the FL-chordin structure (blue mesh) and placed interacting with the BMP-2 binding domains vWC1 and vWC3 (9).
To understand how chordin may interact with BMP, we assumed a 1:1 interaction of one chordin monomer to one BMP dimer based on BMP-2 binding data by Zhang et al. (9) and for BMP-4 and -7 shown in Table 1. These data show that the avidity of chordin for BMP does not decrease with the growth factor immobilized, suggesting that two BMP binding domains of chordin can interact with one BMP dimer. Therefore, we docked the crystal structure of the BMP-2 dimer (Protein Data Bank, PDB 3BK3) (21) into the chordin electron density map based on knowledge of the known sites of interaction of BMP-2 with chordin (9). BMP-2 interacts with vWC1 and vWC3 of chordin, which overlaps with binding sites for the type I and II BMP receptors (9), and it readily fitted between the chordin prongs (Fig. 4C).
Table 1.
Surface plasmon resonance affinity data for the interaction between chordin variants and BMP growth factors with the growth factor immobilized
| Interaction (analyte/ligand) | koff (1/s)/kon (1/M×s) | Kd, nM |
| FL-chd/BMP-4 | 0.5 × 10−4/3.9 × 105 | 0.13 ± 0.03 |
| ΔN-chdBMP-4 | 0.7 × 10−4/1.6 × 105 | 0.46 ± 0.14 |
| ΔC-chd/BMP-4 | 1.4 × 10−4/2.5 × 105 | 0.55 ± 0.20 |
| FL-chd/BMP-7 | 2.3 × 10−4/5.1 × 105 | 0.48 ± 0.16 |
| ΔN-chd/BMP-7 | 3.5 × 10−4/4.0 × 105 | 0.88 ± 0.08 |
| ΔC-chd/BMP-7 | 2.9 × 10−4/3.5 × 105 | 0.81 ± 0.14 |
Inhibition of BMP-4 and -7 by Chordin and Chordin Cleavage Products.
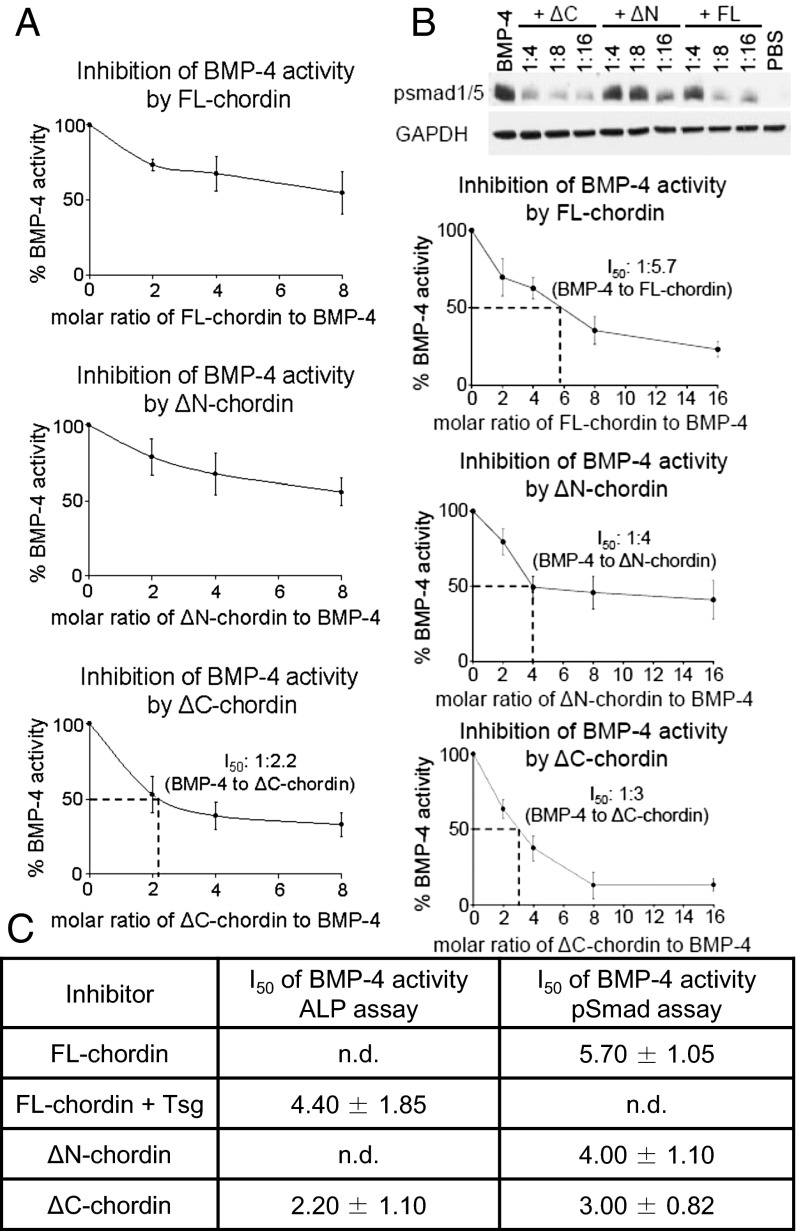
The compact nature of the chordin structure and likely cooperative mode of BMP binding suggests multiple sites of interaction with the growth factor. It is not clear how cleavage of a single vWC domain by tolloid ablates antagonism of BMP. To investigate how chordin cleavage alters the inhibitory action of chordin, we performed two different assays, inhibition of BMP-4 induced alkaline phosphate (ALP) activity (Fig. 5A), and phosphorylation of Smad-1 and -5 (Fig. 5B). In the ALP assay, FL-chordin did not quite inhibit 50% of BMP-4 activity at a eightfold molar excess but this could be enhanced with Tsg (I50 = 1:4.40 (Fig. S6). The pSmad assay indicated activity between these levels (I50 = 1:5.70) (Fig. 5B). However, chordin was a better BMP-7 antagonist; chordin reached 50% inhibition of BMP-7 activity at half the molar excess needed for BMP-4 (I50 = 1:2.72) (Fig. S7). This is consistent with a previous study where mouse FL-chordin was twice as effective in inhibiting BMP-7 than BMP-4 (22). The molar excess of chordin required to antagonize BMP signaling is comparable with the relative levels of BMP (picomolar) and chordin (nanomolar) in vivo (23).
Fig. 5.
Bioactivity of FL-chordin and variants for BMP-4. Inhibition assays of BMP-4 growth factor activity by FL-chordin, ΔN-chordin, and ΔC-chordin. Chordin variants were tested against BMP-4 growth factor (A) using the ALP assay in C2C12 cells or (B) using the pSmad assay in HEK293T cells. (C) I50 values (molar excess of chordin variant required for 50% inhibition) for the inhibitory effect of chordin and variants against BMP-4. Errors represent SD from three independent experiments.
When comparing the chordin variants, ΔN-chordin was a less effective BMP-7 inhibitor than FL-chordin, as would be anticipated following cleavage of the first vWC domain (Fig. S7); however, this was less apparent for BMP-4, where ΔN-chordin retained a similar inhibitory capacity to FL-chordin (Fig. 5). Removal of the C-terminal vWC domain in ΔC-chordin did not reduce BMP-7 inhibition (Fig. S7), and for BMP-4, it increased inhibition (Fig. 5). Together these data suggest that removal of an individual vWC domain from chordin does not give rise to a rapid loss of bioactivity and the C-terminal vWC4 domain, absent in ΔC-chordin, is not required for BMP inhibition.
Binding of Chordin and Chordin Cleavage Products to BMP Growth Factors.
To determine whether the increase in chordin activity following removal of vWC4 was due to changes in the overall affinity to BMP growth factors, we performed protein–protein interaction studies. FL-chordin and chordin variants were injected over immobilized BMP-4 and -7 growth factors and their interaction detected by surface plasmon resonance technology. Our results demonstrate that chordin cleavage products ΔN- and ΔC-chordin showed similar overall affinity to BMP-4 and -7 as FL-chordin (<1 nM) (Table 1 and Fig. S8). Binding was slightly weaker when the experiments were performed in the opposite direction (chordin variants were immobilized and BMP growth factors used as analytes) (Fig. S8) but were still high affinity interactions (∼4–8 nM). This suggests that either the BMP epitopes are more readily available when stabilized on the surface or chordin is able to bind cooperatively in solution. In all cases, the interaction between chordin and BMP-4 or -7 has low nanomolar affinity, which is similar to that previously reported for BMP-2 and -7 (46 nM with mouse FL-chordin immobilized) (9). Together, these data suggest that there is redundancy between BMP binding domains in chordin and if either terminal domain is removed, the remaining vWC domains are sufficient to maintain a high-affinity interaction with the growth factor that supports the bioactivity data.
Discussion
Here we present to our knowledge the first structural model of human chordin, a secreted BMP antagonist. Chordin–BMP models have previously been proposed based on the binding affinity of both N- and C-terminal vWC chordin domains for BMP. In these models, chordin is frequently depicted as a horseshoe-shaped molecule, which would bind a BMP dimer with both N- and C-terminal binding domains (1). Here using SAXS and TEM, we show that chordin adopts a horseshoe-shaped structure, thereby providing to our knowledge the first formal evidence in support of the predicted BMP-binding models. Further analysis of a chordin cleavage product and the CHRD region allowed us to dissect the domain organization of FL-chordin and suggest locations for the BMP binding domains. Superimposition of the ΔN- and FL-chordin EM structures showed additional density in the latter, corresponding to the location of the vWC1 domain. Subsequently, fitting the SAXS ab initio model of the CHRD region into these EM structures showed that the CHRD region occupied another lobe of density, leaving sufficient space for the C-terminal vWC domains.
Docking the structure of BMP-2 into the chordin EM map indicates that the separation of the N- and C-terminal vWC domains by the CHRD region enables BMP to interact simultaneously with more than one chordin vWC domain (for example BMP-2 with vWC1 and -3). The structure supports cooperative binding of BMP by the N- and C-terminal chordin domains. Structures of other BMP inhibitor complexes, for example CV2 (21) and noggin (24), bound to BMP-2 or BMP-7, respectively, show that receptor-binding sites are blocked in the complexes, demonstrating that CV2 and noggin act as competitive BMP inhibitors. In both cases, two BMP binding domains interact with the growth factor dimer, which we assume is also the arrangement within chordin. It is evident from our structure that the spacing provided by the CHRD region would also allow growth factors to bind in either a vWC1 and -3, or vWC1 and -4 arrangement as binding of BMP-7 may be mediated by vWC1 and -4. The shape of the CHRD region alone correlated well with the density for this region in the chordin EM map, which suggests the structure is similar, whether in the context of the entire molecule or produced alone. Although frequently depicted as an elongated spacer to separate the N- and C-terminal vWC domains, the CHRD region was more compact than expected and more globular than FL-chordin.
To understand the role the terminal vWC domains play in BMP binding and inhibition, Chordin variants corresponding to the in vivo tolloid cleavage fragments were generated for binding and activity assays. Our data suggest that removal of a single vWC domain from either the N- or C termini of chordin has little direct impact on the affinity for BMP-4 or BMP-7. Suggesting that there is redundancy between BMP binding domains in Chordin and the remaining vWC domain is sufficient to maintain the interaction with the growth factor. We expected that FL-chordin, containing two BMP binding vWC domains, would have higher bioactivity than ΔN- or ΔC-chordin (each lacking a BMP binding vWC domain). However, we found that despite there being little difference in binding affinity, ΔC-chordin had a greater capacity to inhibit BMP-4 signaling than FL-chordin. This supports a previous in vivo study (16) where increased BMP inhibitory activity by ∆C-chordin was observed in zebrafish.
However, other previous studies using individual chordin vWC domains (9) or chordin fully digested by tolloid (13) show that these chordin fragments can no longer inhibit BMPs. In our study, we are probing the effect of cleavage at either tolloid site but not both concurrently. We see that cleavage of the N-terminal vWC domain has little effect on BMP-4 inhibition but cleavage at the C-terminal site increased BMP-4 inhibition. This suggests that cleavage at both tolloid sites is required to ablate BMP inhibition by chordin, which is supported by our binding data (Table 1 and Fig. S8) and also of others that show removal of an individual vWC domain has little effect on chordin affinity for BMP.
The activity and targeting of chordin may be further modulated by a variety of extracellular ligands. Tsg promotes further cleavage of chordin fragments by tolloids and interacts with the vWC domains of chordin. The increased BMP-4 inhibition by ΔC-chordin suggests either a conformational rearrangement to enable tighter binding of the growth factor or an altered interaction with another BMP regulator following cleavage of vWC4. As the binding data suggest that the increased BMP inhibition is not directly attributable to increased binding affinity, it could occur through loss of an interaction mediated by vWC4. For example, the Drosophila chordin homolog, Sog, interacts with collagen IV to form shuttling complexes and enable long range BMP signaling (25). This interaction is mediated through vWC4, without which the growth factors may only diffuse short distances. Alternatively, following cleavage of vWC4, the remaining C-terminal domains may be more accessible to other binding partners, for example vWC2 binding to CV2 (19).
Human chordin occurs as a number of alternatively spliced variants including one lacking domains vWC3 and -4, which is expressed in a range of adult and fetal tissues and have been shown to have some biological activity in vivo (17). Generation of chordin truncation variants lacking vWC4 may represent a mechanism to modulate the activity of BMPs that plays a more pronounced role in postnatal tissues where increased production of extracellular matrix will prevent chordin from free diffusion. Tolloid levels are also lower in postnatal tissues than in development so limited tolloid cleavage could be a mechanism for fine tuning the specificity for different BMPs in postnatal homeostasis.
In summary, we show the nanoscale structure of human chordin, which although more compact than expected, supports a cooperative model of BMP binding. The chordin-specific CHRD region has a globular arrangement, providing to our knowledge the first structural data on this region. These structural data together with binding and bioactivity data suggest the existence of a fine-tuning mechanism of BMP growth factor activity using the C-terminal chordin domains.
Materials and Methods
Protein Expression and Purification.
FL-chordin was generated as described previously (26). Constructs for ΔN-chordin (residues 153–955), ΔC-chordin (residues 27–866) and the CHRD region (residues 168–652) were generated. The 6× histidine tags were incorporated at the C terminus following thrombin cleavage sites. Constructs were transfected into HEK-293 Epstein–Barr virus nuclear antigen cells as previously described (26). FL- and ΔC-chordin were harvested in the presence of 50 mM l-arginine (pH 7.4). Proteins were purified as described previously (26).
MALLS Analysis.
Samples (0.5 mL at ∼0.5 mg/mL) loaded on a Superdex200 10/300GL column (0.75 mL/min in 10 mM Tris⋅HCl pH 7.4, 150 mM NaCl) were passed through a Wyatt DAWN Heleos II EOS 18-angle laser photometer coupled to a Wyatt Optilab rEX refractive index detector and data were analyzed using Astra 6.
Sedimentation Velocity AUC.
ΔN-chordin (∼400 µg/mL) and the CHRD region (1 mg/mL) were analyzed in 10 mM Tris pH 7.4 in either 150 mM NaCl (CHRD) or 250 mM NaCl (ΔN-chordin). Studies were carried out using a Beckman XL-A ultracentrifuge as described previously (26).
TEM and Single Particle Analysis.
FL-chordin (∼10 μg/mL) or ΔN-chordin (15 µg/mL) were negatively stained as described previously (26). Data were recorded at 30,000× on a Tecnai Biotwin at 120 kV with a Gatan Orius CCD camera. Images were recorded with a 1-s exposure at defocus values 0.5–1.6 μm at 2.1 Å per pixel (Fig. S2). Single particle analysis was performed using EMAN2 (27). For FL-chordin, ∼3,300 particles were selected and for ΔN-chordin ∼2,500 particles, using a combination of manual and semiautomated picking. Following contrast transfer function correction, each dataset was subjected to 2D classification. A total of 50 projection averages for FL-chordin and 40 for ΔN-chordin were selected and used to generate an initial 3D model. This model was used as a start seed for eight rounds of iterative refinement to produce a self-consistent 3D structure. Resolution estimates were 30.2 Å (FL-chordin) and 33.6 Å (ΔN-chordin) using Fourier shell correlation with a cutoff value of 0.5 (Fig. S3). Modeling was performed using chimera (28).
SAXS Data Collection and Analysis.
Data were collected with 4 × 30 s exposures and a 2.4 m detector distance (0.008 < q < 0.54 Å−1) at the EMBL beamline ×33, DESY with concentration range 1–5 mg/mL at 10 °C. Additional data on FL-chordin were collected on I22 at Diamond Light Source with 5-m detector distance (0.005 < q < 0.25 Å−1) and wavelength 0.083 nm. For all SAXS data, the Rg and distance distribution function p(r) were evaluated with GNOM (29). Particle shapes were generated ab initio using DAMMIN or GASBOR software (30). Twenty models were combined and filtered to produce an averaged model using DAMAVER (31).
Alkaline Phosphatase Assay.
C2C12 cells were seeded into 96-well plates (Costar) at a density of 30,000 cells per well in DMEM (MediaTech) containing 2% (vol/vol) FCS. BMP stimulation was carried out in triplicates with 30 ng/mL BMP-4, or 100 ng/mL BMP-7 (both human from R&D Systems) in the presence or absence of chordin variants for 72 h. Before stimulation, proteins were dialyzed together in PBS, containing 0.01% BSA as carrier. After stimulation, cell layers were washed twice with PBS and lysed with 100 µL lysis buffer (0.1 M glycine pH 9.6, 1 mM MgCl2, 1 mM ZnCl2) containing 1% Nonidet P-40. Lysates were clarified by centrifugation at 18,800 × g. A total of 100 µL alkaline phosphatase yellow (p-nitrophenyl phosphate) liquid substrate (Sigma-Aldrich) was added to 50 µL of each lysate and incubated for 20 min at room temperature followed by measurement of activity in a TECAN infinite M1000 reader (Dynamic Instruments) at 405 nm.
Phospho-Smad Western Blot Assay.
HEKT293 cells were seeded into six-well plates at a density of 1,000,000 cells per well in DMEM. The following day, cells were stimulated for 1 h with 30 ng/mL BMP-4 or 100 ng/mL BMP-7 in the presence or absence of chordin variants. After stimulation, cell layers were incubated with lysis buffer (10 mM NaCl, 1.5 mM MgCl2, 20 mM Hepes pH 7.4, 20% glycerol, 0.1% Triton X-100, and 1 mM DTT), centrifuged at 440 × g for 5 min at 4 °C, and washed with PBS. Aliquots of the supernatant were supplemented with 1× PhosSTOP (Roche) and Western blotted. Phosphorylated Smad was detected using pooled antiphosphosmad1 (1:1,250) and smad5 (1:1,000) antibodies (Abcam). Band intensities were quantitated with ImageJ and plotted against concentration.
Supplementary Material
Acknowledgments
We thank Marge Howard (Biomolecular Analysis facility, University of Manchester), Dr. Maxim Petouckhov (beamline X33) and Dr. Marc Malfois (beamline I22) for assistance during SAXS data collection, and DESY and Diamond Light Source for beamtime. R.B.T. was supported by Biotechnology and Biological Sciences Research Council (BBSRC) Grant BB/I019286/1 and H.T. and R.B. by BBSRC studentships (to C.B.). This work was also supported by the Deutsche Forschungsgemeinschaft SFB829/Project B12 (to G.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404166111/-/DCSupplemental.
References
- 1.Larraín J, et al. BMP-binding modules in chordin: A model for signalling regulation in the extracellular space. Development. 2000;127(4):821–830. doi: 10.1242/dev.127.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rider CC, Mulloy B. Bone morphogenetic protein and growth differentiation factor cytokine families and their protein antagonists. Biochem J. 2010;429(1):1–12. doi: 10.1042/BJ20100305. [DOI] [PubMed] [Google Scholar]
- 3.Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70(5):829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- 4.Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell. 1998;1(5):673–683. doi: 10.1016/s1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
- 5.Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86(4):589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380(6569):37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- 7.Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123(6):1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross JJ, et al. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature. 2001;410(6827):479–483. doi: 10.1038/35068578. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JL, Huang Y, Qiu LY, Nickel J, Sebald W. von Willebrand factor type C domain-containing proteins regulate bone morphogenetic protein signaling through different recognition mechanisms. J Biol Chem. 2007;282(27):20002–20014. doi: 10.1074/jbc.M700456200. [DOI] [PubMed] [Google Scholar]
- 10.Oelgeschläger M, Larraín J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405(6788):757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyvönen M. CHRD, a novel domain in the BMP inhibitor chordin, is also found in microbial proteins. Trends Biochem Sci. 2003;28(9):470–473. doi: 10.1016/S0968-0004(03)00171-3. [DOI] [PubMed] [Google Scholar]
- 12.Scott IC, et al. Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev Biol. 1999;213(2):283–300. doi: 10.1006/dbio.1999.9383. [DOI] [PubMed] [Google Scholar]
- 13.Piccolo S, et al. Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91(3):407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larraín J, et al. Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development. 2001;128(22):4439–4447. doi: 10.1242/dev.128.22.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu K, et al. Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity. Development. 2000;127(10):2143–2154. doi: 10.1242/dev.127.10.2143. [DOI] [PubMed] [Google Scholar]
- 16.Xie J, Fisher S. Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms. Development. 2005;132(2):383–391. doi: 10.1242/dev.01577. [DOI] [PubMed] [Google Scholar]
- 17.Millet C, Lemaire P, Orsetti B, Guglielmi P, François V. The human chordin gene encodes several differentially expressed spliced variants with distinct BMP opposing activities. Mech Dev. 2001;106(1–2):85–96. doi: 10.1016/s0925-4773(01)00423-3. [DOI] [PubMed] [Google Scholar]
- 18.Kelley R, et al. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J Cell Biol. 2009;184(4):597–609. doi: 10.1083/jcb.200808064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JL, et al. Binding between Crossveinless-2 and Chordin von Willebrand factor type C domains promotes BMP signaling by blocking Chordin activity. PLoS ONE. 2010;5(9):e12846. doi: 10.1371/journal.pone.0012846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455(7209):72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JL, et al. Crystal structure analysis reveals how the Chordin family member crossveinless 2 blocks BMP-2 receptor binding. Dev Cell. 2008;14(5):739–750. doi: 10.1016/j.devcel.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama N, et al. A novel chordin-like BMP inhibitor, CHL2, expressed preferentially in chondrocytes of developing cartilage and osteoarthritic joint cartilage. Development. 2004;131(1):229–240. doi: 10.1242/dev.00901. [DOI] [PubMed] [Google Scholar]
- 23.Plouhinec JL, Zakin L, Moriyama Y, De Robertis EM. Chordin forms a self-organizing morphogen gradient in the extracellular space between ectoderm and mesoderm in the Xenopus embryo. Proc Natl Acad Sci USA. 2013;110(51):20372–20379. doi: 10.1073/pnas.1319745110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groppe J, et al. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature. 2002;420(6916):636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- 25.Sawala A, Sutcliffe C, Ashe HL. Multistep molecular mechanism for bone morphogenetic protein extracellular transport in the Drosophila embryo. Proc Natl Acad Sci USA. 2012;109(28):11222–11227. doi: 10.1073/pnas.1202781109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry R, et al. Role of dimerization and substrate exclusion in the regulation of bone morphogenetic protein-1 and mammalian tolloid. Proc Natl Acad Sci USA. 2009;106(21):8561–8566. doi: 10.1073/pnas.0812178106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang G, et al. EMAN2: An extensible image processing suite for electron microscopy. J Struct Biol. 2007;157(1):38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 29.Svergun DI. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J Appl Cryst. 1992;25:495–503. [Google Scholar]
- 30.Svergun DI. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys J. 1999;76(6):2879–2886. doi: 10.1016/S0006-3495(99)77443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volkov VV, Svergun DI. Uniqueness of ab initio shape determination in small-angle scattering. J Appl Cryst. 2003;36:860–864. doi: 10.1107/S0021889809000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García de la Torre J, Llorca O, Carrascosa JL, Valpuesta JM. HYDROMIC: Prediction of hydrodynamic properties of rigid macromolecular structures obtained from electron microscopy images. Eur Biophys J. 2001;30(6):457–462. doi: 10.1007/s002490100176. [DOI] [PubMed] [Google Scholar]
- 33.Ortega A, Amorós D, García de la Torre J. Prediction of hydrodynamic and other solution properties of rigid proteins from atomic- and residue-level models. Biophys J. 2011;101(4):892–898. doi: 10.1016/j.bpj.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.