Significance
Diet-induced type 2 diabetes (T2D) is becoming a worldwide epidemic. Patients with T2D fail to respond to insulin normally and have elevated blood glucose and insulin. In autoimmune diabetes, blood glucose is elevated due to uncontrolled glucagon, a hormone normally suppressed and opposed by insulin. To study the role of glucagon in T2D, rodent models of the disease were created lacking glucagon action. These animals failed to develop hyperglycemia unless glucagon action was restored or the animals were given the high concentrations of insulin typical of animals with T2D. This indicates that the unopposed glucagon action in T2D is necessary to support elevated blood glucose of diabetes. Suppressing glucagon or its action may benefit patients with T2D.
Keywords: type two diabetes, insulin resistance
Abstract
To determine the role of glucagon action in diet-induced and genetic type 2 diabetes (T2D), we studied high-fat-diet–induced obese (DIO) and leptin receptor-defective (LepR−/−) rodents with and without glucagon receptors (GcgRs). DIO and LepR−/−,GcgR+/+ mice both developed hyperinsulinemia, increased liver sterol response element binding protein 1c, and obesity. DIO GcgR+/+ mice developed mild T2D, whereas LepR−/−,GcgR+/+ mice developed severe T2D. High-fat–fed (HFF) glucagon receptor-null mice did not develop hyperinsulinemia, increased liver sterol response element binding protein 1c mRNA, or obesity. Insulin treatment of HFF GcgR−/− to simulate HFF-induced hyperinsulinemia caused obesity and mild T2D. LepR−/−,GcgR−/− did not develop hyperinsulinemia or hyperglycemia. Adenoviral delivery of GcgR to GcgR−/−,LepR−/− mice caused the severe hyperinsulinemia and hyperglycemia of LepR−/− mice to appear. Spontaneous disappearance of the GcgR transgene abolished the hyperinsulinemia and hyperglycemia. In conclusion, T2D hyperglycemia requires unsuppressible hyperglucagonemia from insulin-resistant α cells and is prevented by glucagon suppression or blockade.
The prevalence of type 2 diabetes (T2D) in the United States was 29.1 million in 2012, and 37% of adults were identified as prediabetic (1). T2D is now present on every continent (2). Despite the magnitude of this threat to world physical and fiscal health, our understanding of the pathogenic pathway is vague and is based largely on epidemiologic correlations. For example, the correlation between T2D and obesity is so high that most obese Americans can be considered prediabetic, but the precise mechanism of this relationship is unknown. Although the “lipotoxic” effects of ectopic lipids were first suggested in 1994 (3) to link diet-induced obesity to T2D and other components of the metabolic syndrome (3–11), the relationship between IR and T2D is still poorly understood. Proposed hypothetical links range from beta cell “glucotoxicity” (12) to the action of modifier genes (13) to failure of redox control (14).
It has recently been shown that glucagon receptor-null mice remain normoglycemic and nonketotic despite total insulin deficiency but that transduction of a glucagon receptor cDNA into their liver makes them severely diabetic (15, 16). This proves that, whether or not insulin action is present, suppression of glucagon action prevents hyperglycemia. It has long been known that insulin suppression of glucagon regulates alpha cell secretion (17, 18). Although the presence of hyperglucagonemia was established unequivocally in type 1 diabetes (T1D) (15, 16), direct evidence that it is essential for the hyperglycemia of T2D is lacking. However, it has long been known that glucagon is elevated in T2D (17, 19, 20) and is resistant to suppression by insulin.
Results
Role of Glucagon Action in Diet-Induced Hyperinsulinemia and Obesity.
To investigate the relationship between glucagon action and other components of diet-induced T2D, we used glucagon receptor-null (GcgR−/−) mice. First, we repeated the elegant studies of Conarello et al. (20), which had suggested that, in the absence of GcgR and hyperinsulinemia, obesity could not be induced by high-fat feeding (HFF). We placed wild-type (GcgR+/+) and GcgR−/− mice on a 60% fat diet with 10% sucrose in the drinking water. As shown in Fig. S1 A–D, after 10 wk on this diet, body fat of GcgR−/− mice did not increase significantly, and they did not become hyperinsulinemic or hyperglycemic, in contrast to the GcgR+/+ mice.
Glucagon Action on Beta Cells in Diet-Induced Hyperinsulinemia.
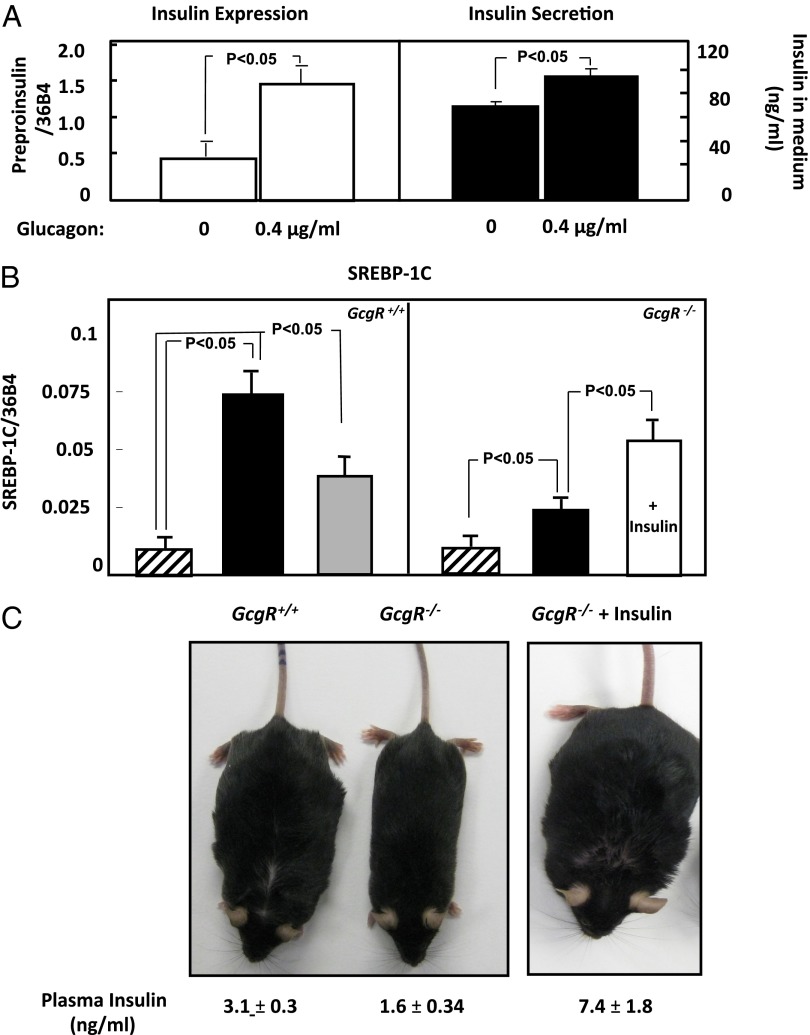
The lack of diet-induced hyperinsulinemia in GcgR−/− mice could be either the consequence of loss of a direct action of glucagon on beta cells (21), the prevention of hyperglycemia, or failure of the GcgR−/− liver to generate a betatrophin-like hepatokine (22). To obtain evidence for a direct glucagon action on beta cells, we cultured rat insulinoma Ins-1 cells in 11 mM glucose with or without 0.4 µg/mL of glucagon (Sigma) for 24 h. In the presence of glucagon, preproinsulin mRNA increased threefold and insulin release into the medium by 30% (P < 0.05; Fig. 1A). We conclude that glucagon can directly up-regulate insulin expression, but stimulation by hyperglycemia or by a hepatokine in vivo was not excluded.
Fig. 1.
(A) The effect of glucagon on preproinsulin mRNA in INS-1 cells cultured for 24 h in 0 or 0.4 µg/mL of glucagon (Left). (Right) Mean insulin concentration in the medium after 24 h incubation of INS-1 cells in the absence or presence of 0.4 µg/mL of glucagon. n = 3, and mean values ± SEM are shown. (B) Comparison of SREBP-1c expression in the liver of GcgR+/+ and GcgR−/− mice on a chow diet (striped bar) and a diet of 60% fat with sucrose supplementation in the absence (black bar) and in the presence (white bar) of treatment with exogenous insulin. The gray bar represents values from GcgR−/− mice restricted to 75% of the caloric intake of HFF GcgR+/+ mice. n = 6, and mean values ± SEM are shown. (C) The appearance of GcgR+/+ and mice fed a high-fat diet with sucrose supplementation (Left). (Right) A mouse treated with exogenous insulin. The mean plasma insulin concentration ± SEM for six mice in each group appear under the representative photograph.
Glucagon Action on Metabolic Parameters.
To understand more fully the fate of the ingested calories in the resistance of GcgR−/− mice to obesity, three mice from each group were studied in a metabolic cage. GcgR+/+ mice on a 60% high-fat diet consumed an average of 12 kcal per day, ∼1/3 higher than on a chow diet (P < 0.05). Body fat rose ∼500% from 3.5 g to 15 g at 120 d (P < 0.001). GcgR−/− mice on the high-fat diet ate 9.4 kcal per day, 25% less food than GcgR+/+ mice (P < 0.05), but their caloric intake rose only 1 kcal/d above that of GcgR−/− mice on the chow diet (Table 1). They gained only 2.5 g of body fat, only 20% of the weight gain in the GcgR+/+ controls (P < 0.001). HFF decreased physical activity of GcgR+/+ mice by ∼14%, whereas no change in physical activity was observed in GcgR−/− mice, which were half as active as GcgR+/+ mice on either diet. There were no changes in core temperature. VO2 and VCO2 were significantly reduced in GcgR−/− mice compared with GcgR+/+ mice (P < 0.05), but not by HFF (Table 2). Thus, the lack of weight gain by GcgR−/− mice on HFF compared with GcgR+/+ did not appear related to higher metabolic or physical activity.
Table 1.
Body weight, food intake, total body fat, blood glucose, and plasma insulin
| Genotype, diet | Body weight, g | Food intake, kcal/d | Total body fat, g | Blood glucose, mg/100 mL | Plasma insulin, ng/mL |
| GcgR+/+ chow | 29.3 ± 1.40 | 9.1 ± 1.0 | 3.56 ± 0.29 | 156 ± 4.5 | 1.6 ± 0.34 |
| GcgR+/+ HF | 40.0 ± 1.46 | 12.0 ± 1.0 | 15.00 ± 1.16 | 201 ± 14.2 | 3.1 ± 0.30, |
| GcgR+/+ HF, 75% | 35.0 ± 1.20 | 9.0 ± 0.1 | 13.10 ± 0.46 | 173 ± 11.3 | 3.4 ± 0.72 |
| GcgR−/− chow | 26.1 ± 1.16 | 8.4 ± 0.2 | 2.13 ± 0.25 | 116 ± 6.3 | 0.77 ± 0.1 |
| GcgR−/− HF | 29.6 ± 1.49 | 9.4 ± 0.5 | 4.50 ± 0.37 | 123 ± 9.4 | 2.1 ± 0.52 |
| GcgR−/− HF + insulin | 44.4 ± 0.33 | 10.3 ± 0.7 | 18.00 ± 0.13 | 150 ± 7.3 | 7.4 ± 1.80 |
n = 6 for each diet/treatment. Comparisons were evaluated by t test, and statistics are given in Results. HF, high fat.
Table 2.
Metabolic cage measurements and rectal temperature for GcgR mice
| Genotype, diet | VO2, ml/h/kg | VCO2, ml/h/kg | Heat, kcal/h/kg | Central movement counts | Peripheral movement counts | Rectal temperature, °C |
| GcgR+/+ chow | 4,759 ± 257* | 4,120 ± 261* | 23.3 ± 1.3 | 5,179 ± 452 | 3,831 ± 287 | 38.2 ± 0.29 |
| GcgR+/+ HF | 4,958 ± 160 | 4,315 ± 131 | 24.3 ± 0.3 | 3,477 ± 337 | 3,505 ± 548 | 37.3 ± 0.13 |
| GcgR−/− chow | 3,489 ± 346* | 2,699 ± 330* | 16.7 ± 1.7 | 2,212 ± 335 | 2,053 ± 364 | 38.1 ± 0.15 |
| GcgR−/− HF | 4,021 ± 717 | 3,306 ± 835 | 19.5 ± 3.7 | 1,717 ± 128 | 2,410 ± 390 | 37.2 ± 0.34 |
P < 0.05, comparing +/+ to −/− on the same diet for VO2 and VCO2. HF, high fat.
Role of Hyperinsulinemia in Diet-Induced Lipogenesis and Its Complications.
Given the well-established relationship between insulin and lipogenesis (23–25), failure of GcgR−/− mice to mount a hyperinsulinemic response to HFF could account for their lack of obesity. Therefore, we first compared the expression of the lipogenic transcription factor, sterol response element binding protein (SREBP1c), in the livers of GcgR−/− and GcgR+/+ mice on a chow diet and a high-fat diet (Fig. 1B). On a chow diet, SREBP1c expression in GcgR+/+ and GcgR−/− mice was the same. However, on the high-fat diet, SREBP1c mRNA rose approximately eightfold in livers of GcgR+/+ mice (P < 0.001) but only a ∼twofold difference was observed in GcgR−/− mice (P < 0.05). Thus, the up-regulation of the lipogenic transcription factor SREBP-1c in response to a high-fat diet is muted in GcgR−/− mice. In GcgR+/+ mice, a 25% reduction of HFF lowered the high-fat–induced increase in SREBP1c mRNA by half, to fourfold above that in GcgR+/+ mice on a chow diet, which was still a significant difference (P < 0.05; Fig. 1B).
In view of the role of insulin in up-regulating SREBP1c (26) and the observation that Ins1+/−, Ins2−/− mice do not become obese upon HFF (27), one would expect simulation of diet-induced hyperinsulinemia with exogenous insulin to fatten HFF GcgR−/− mice to the level of the HFF wild-type mice. Therefore, we inserted s.c. insulin pellets into GcgR−/− mice as they began the high-fat diet. After 10 wk of HFF, plasma insulin in peripheral venous blood averaged 7.4 ng/mL in insulin-treated GcgR−/− mice, compared with 3.1 ng of endogenous insulin per mL in fat-fed GcgR+/+ mice (P < 0.05; Table 1). However, because steady-state insulin levels of endogenous insulin in the portal vein average 2–3 times the peripheral arterial levels (28), it can be assumed that the livers of the GcgR−/− mice implanted with insulin pellets and those of the GcgR+/+ mice were exposed to relatively similar levels of hyperinsulinemia. As shown in Fig. 1C and Table 1, the insulin-treated HFF GcgR−/− mice became just as obese as the GcgR+/+ mice, evidence that hyperinsulinemia is an obligatory factor in the diet-induced obesity independent of its effects of suppressing glucagon. Liver SREBP1c mRNA, which is induced by insulin action (26), rose twofold in the insulin-treated GcgR−/− mice (P < 0.05; Fig. 1B). Even though the insulin levels in the insulin-treated GcgR−/− mice were 3 times higher than in the untreated GcgR−/− mice (7.4 ng/mL vs. 2.1 ng/mL, P < 0.05), their plasma glucose levels were significantly higher (150 ± 7.3 mg/dL vs. 123 ± 9.4 mg/dL, P < 0.05; Table 1). If taken literally, this would imply that the hyperinsulinemia induced IR and mild diabetes.
Role of Glucagon Action in T2D.
Because the hyperglycemia in HFF GcgR+/+ mice and in insulin-treated GcgR−/− mice was so marginal, we turned to more severe models of T2D caused by congenital deficiency of leptin signaling [both the spontaneously occurring db/db mice, engineered leptin receptor knockout leprloxTB mice (29), and spontaneously occurring Zucker Diabetic fatty (ZDFfa/fa) rats]. These leptin receptor-defective (LepR−/−) rodents become hyperinsulinemic at 10–14 d of age and identifiably obese at 21–28 d. In these genetic backgrounds, hyperglycemia typically begins between 5 and 10 wk of age. Initial hyperplasia of beta cells begins earlier and in ZDFfa/fa rats persists for ∼12 wk, after which depletion of beta cells begins, reaching a 75% loss by 4 mo. Hyperglycemia is difficult to control with insulin injections because of the marked insulin resistance. Hyperlipidemia, ectopic lipid accumulation, and evidence of lipotoxicity are apparent (30).
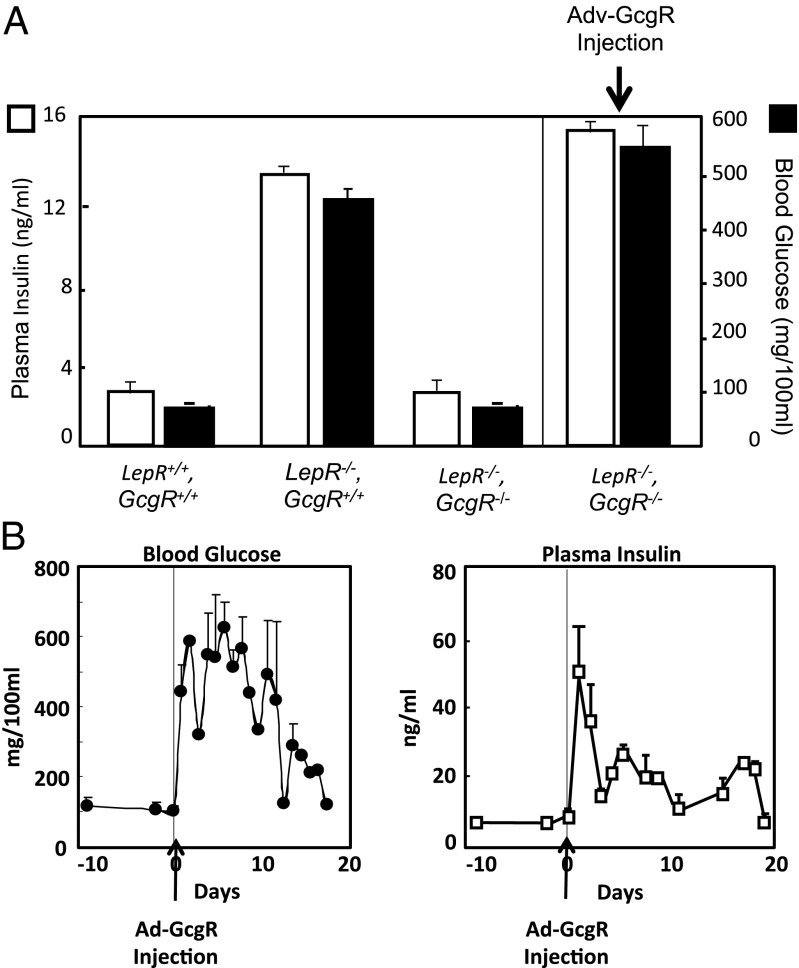
To determine if glucagon action plays a role in the pathogenesis of this form of T2D, we bred LepR,loxTB (LepR−/−) mice with GcgR−/− mice to produce mice lacking normal versions of both leptin and glucagon receptors (LepR−/−,GcgR−/−). The LepR−/−,GcgR−/− mice were obese, weighing 45 ± 1.6 g on average, but less so than LepR−/−,GcgR+/+ mice (59 ± 4.3 g on average) (P < 0.05; Table 3). In normal mice with normal insulin levels, plasma glucose concentrations were well below the diabetic level of 200 mg/dL (P < 0.05; Fig. 2A). By contrast, LepR−/−,GcgR+/+ mice were diabetic, with a mean glucose concentration of 455 mg/dL, despite extreme hyperinsulinemia. However, in LepR−/−,GcgR−/− mice, glucose and insulin levels remained in the normal range (Fig. 2A), suggesting that in severe T2D caused by leptin receptor impairment, glucagon signaling is required for hyperglycemia. Thus, lack of glucagon signaling in LepR−/− mice prevented both severe hyperglycemia and the hyperinsulinemia.
Table 3.
Body weight, food intake, blood glucose, and insulin in LepR−/−,GcgR−/− mice
| Genotype | Body weight, g | Food intake, kcal/d | Blood glucose, mg/100mL | Plasma insulin, ng/mL |
| LepR−/−,GcgR+/+ | 59.0 ± 4.3* | 22.4 ± 1.1** | 455 ± 19.2**,† | 38.1 ± 1.3** |
| LepR−/−,GcgR−/− | 45.0 ± 1.6* | 15.5 ± 1.1**,‡ | 70 ± 5.0**,‡ | 7.2 ± 1.5**,‡ |
| LepR−/−,GcgR−/− + Adv-GcgR | 48.0 ± 0.8 | 24.0 ± 1.7‡ | 391 ± 33.0‡,† | 31.0 ± 3.0‡ |
n = 6 for each diet/treatment. Body weight, food intake, blood glucose, and plasma insulin are significantly different (P < 0.05) comparing LepR−/−,GcgR−/− and LepR−/−,GcgR−/− mice. All four parameters are not significantly different comparing LepR−/−,GcgR+/+ mice to LepR−/−,GcgR−/− treated with Adv-GcgR. Within a column, symbols indicate pairwise comparisons with P < 0.05.
Fig. 2.
(A) Comparison of plasma glucose and insulin levels in GcgR+/+ (n = 6), GcgR−/− (n = 6), LepR−/−, GcgR+/+ (n = 4), and LepR−/−, GcgR−/− mice (n = 4) treated with adenovirus containing the GcgR cDNA. Blood glucose and insulin levels are significantly lower in LepR−/−,GcgR−/− mice compared with LepR−/−,GcgR+/+ mice, P < 0.05. Treatment of LepR−/−,GcgR−/− mice with Adv-GcgR significantly raised blood glucose and insulin compared with LepR−/−,GcgR−/− mice not treated with Adv, P < 0.05. Treatment with Adv-beta Gal had no effect (Table S2). (B) The time course of mean blood glucose and plasma insulin levels of the LepR−/−,GcgR−/− mice following the injection of adenovirus containing the GcgR cDNA. Errors are SEM.
To determine if the prevention of T2D in GcgR−/− mice was the result of congenital absence of GcgR, rather than a direct and immediate consequence of acute loss of glucagon signaling, we delivered GcgR by adenovirus to the liver of LepR−/−,GcgR−/− mice. The glucagon receptor mRNA appeared in the liver within 24 h and was expressed for about 7 d, after which it spontaneously disappeared. Within 24 h of the injection of adenovirus containing the GcgR cDNA into the LepR−/−,GcgR−/− mice, glucose rose fivefold to ∼500 mg/dL and insulin levels rose more than 10-fold to ∼50 ng/mL (Fig. 2B). Presumably, the insulin rise was a response to the severe hyperglycemia caused by the sudden abundance of transgenic GcgR in the liver. Thus, the hyperglycemia, which did not occur in LepR−/−,GcgR−/− mice with congenital lack of glucagon action, promptly appeared upon delivery of the GcgR transgene to their liver and persisted until spontaneous disappearance of the GcgR transgene (Fig. 2B). This signifies that the hyperglycemia of T2D, like that of T1D, cannot occur in the absence of glucose overproduction maintained by glucagon signaling in the liver. The rapid normalization of hyperglycemia after acute disappearance of GcgR mRNA suggests that acute suppression of glucagon action would be effective therapeutically.
LepR−/− mice had hyperglycemia despite insulin levels averaging 20 times the levels of wild-type mice (Fig. 2A). LepR−/−,GcgR−/− mice, by contrast, had normoglycemia and normal insulin levels, suggesting that lack of GcgR prevented both hyperglycemia and IR. When adenovirus-expressing GcgR was delivered to the liver of LepR−/−,GcgR−/− mice, hyperglycemia and hyperinsulinemia both appeared within 24 h (Fig. 2B). We had previously reported the spontaneous disappearance of the GcgR transgene in mice within 8 d (16). In these LepR−/− mice, hyperglycemia above 200 mg/dL and hyperinsulinemia above 10 ng/mL persisted for 18 d (Fig. 2B).
These results suggest that GcgR is required for the hyperglycemia of T2D. However, they do not reveal the nature of the glucose–insulin relationship—that is, whether the high insulin is caused by the hyperglycemia or if the hyperglycemia reflects IR. The fact that liver triacylglycerol (TG) levels, which averaged 400 ± 11.0 mg/g of liver weight in LepR−/− mice (n = 4), remained at 125 ± 12.4 mg/g in LepR−/−,GcgR−/− mice (n = 4), more than 12 times the level of 10 ± 0.84 mg/g in normal mice (n = 6), points to persistence of lipid-induced IR as a possible explanation for the hyperglycemia occurring despite marked hyperinsulinemia.
Role of Ceramide in Alpha Cell Insulin Resistance.
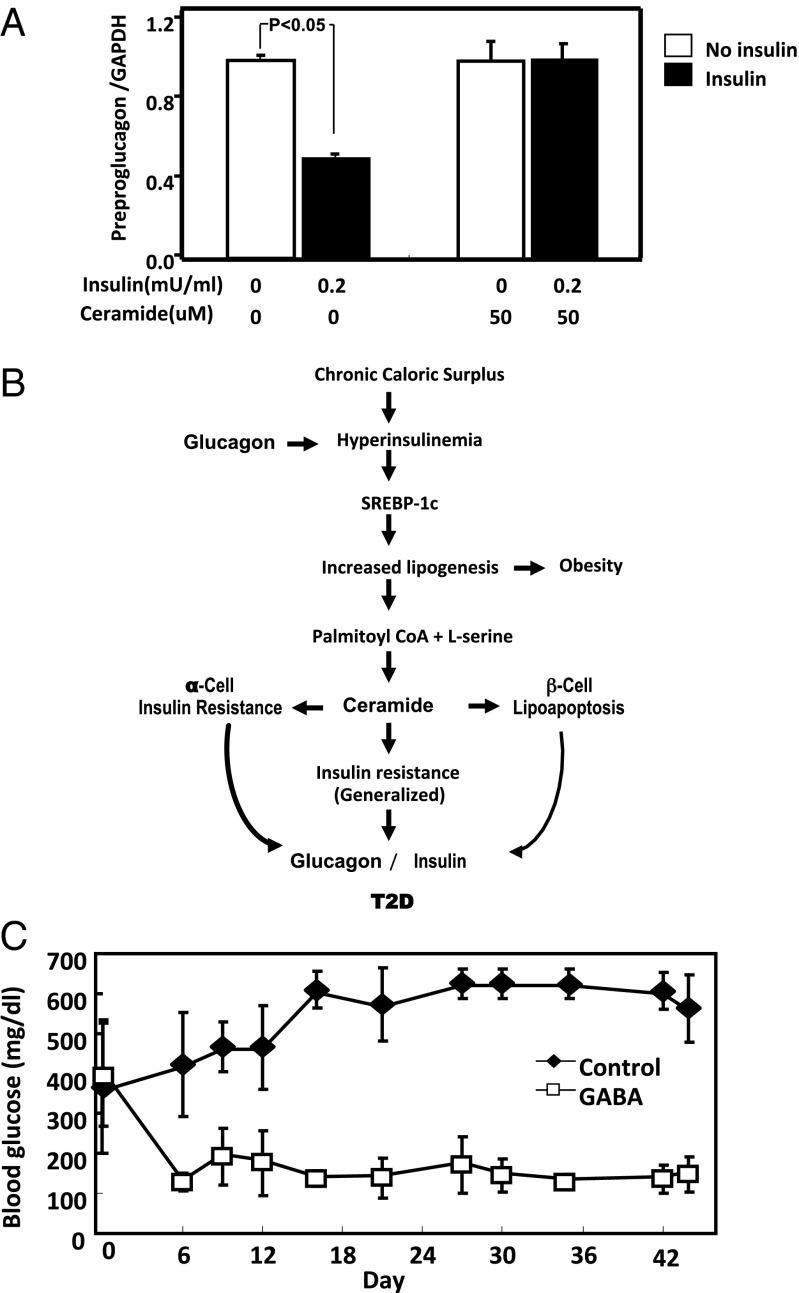
The accumulation of ceramide and other lipids has long been implicated in the pathogenesis of IR (31, 32). To determine if ceramide can mediate the resistance to insulin-mediated suppression of the hyperglucagonemia of T2D, we measured preproglucagon mRNA in cultured InR1-G9 alpha cells exposed to 200 mU of insulin with and without 50 µM ceramide. Without ceramide, insulin reduced preproglucagon mRNA by 46% (P < 0.05; Fig. 3A); with ceramide in the medium, insulin failed to lower it.
Fig. 3.
Ceramide induces insulin resistance in InR1-G9 alpha cells, and suppression of glucagon corrects the hyperglycemia in T2D. (A) InR1-G9 cells in culture were treated with insulin, ceramide, or insulin plus ceramide for 16 h, and mRNA for glucagon was measured by quantitative PCR. Values are normalized to those of the control sample and are means of triplicate measurements with SEM. The values shown are representative of two independent experiments. (B) A schema for the interaction of insulin and glucagon controlling lipogenesis under situations of caloric balance and excess is shown. (C) Blood glucose concentrations in ZDFfa/fa leptin receptor-deficient rats without (black) and with (white) oral treatment with GABA are shown. n = 6 for each test condition, and errors are SEM. Mean glucagon concentrations without treatment were 103 ± 24.4 pg/mL and with treatment were 58.7 ± 12.9 pg/mL.
We had demonstrated previously (33) that ceramide synthesis from palmitate is increased in islets isolated from rodent models of severe insulin resistance and T2D, such as db/db mice and ZDFfa/fa rats. Their islets exhibited enhanced expression of serine palmitoyl transferase, the rate-limiting enzyme in ceramide synthesis (4). In this earlier work, ceramide was implicated in the dysfunction and lipoapoptosis of beta cells in T2D. The present results suggest that ceramide excess, previously implicated in the insulin resistance in other targets (31, 32), may also cause resistance to insulin-mediated suppression of hyperglucagonemia.
The combination of ceramide-induced beta cell impairment and ceramide-induced insulin resistance of alpha cells would explain the insulin-unresponsive hyperglucagonemia in T2D patients (19). The low insulin-to-glucagon ratio would maintain hepatic overproduction of glucose during meals. The scheme offered in Fig. 3B links lipid-induced changes in islet cells to the hyperglycemia of T2D through lipotoxicity of both alpha and beta cells (4).
Therapeutic Implications of Glucagon Suppression in T2D.
The results presented here could have important therapeutic implications. If as depicted in Fig. 3B glucagon enables the diet-induced hyperinsulinemia required to generate lipogenesis, obesity, and T2D, suppression of glucagon or its action should prevent T2D. To test this, we used a known glucagon suppressor, gamma-amino butyric acid (GABA), to treat ZDFfa/fa rats, in which the phenotype of obesity, ectopic lipid deposition, IR, hyperglucagonemia, and T2D is caused by a defective LepR. Hyperglycemia was reduced to normal in the GABA-treated rats but not in the controls (Fig. 3C). These results appear to validate the pathogenic relationships depicted and imply that glucagon suppression or blockade would provide a potential defense against the pandemic of T2D.
Discussion
This study identifies a pathogenic pathway to the diet-induced diseases now plaguing Western countries. It identifies two essential pathogenic roles for glucagon in diet-induced T2D. One is the essential role of glucagon in all forms of hyperglycemia, that of maintaining a rate of hepatic glucose production exceeding the rate of glucose clearance (26, 28, 34, 35). The second, previously unsuspected role for glucagon is that of an enabler of the diet-induced hyperinsulinemia. Conarello et al. (21) reported that diet-induced hyperinsulinemia cannot occur in GcgR−/− mice, a finding that we have now confirmed. Without glucagon action, diet-enhanced lipogenesis and obesity cannot occur.
Although glucagon stimulates insulin in vivo (36), it is not clear if the effect is direct on beta cells or if it is mediated by a hepatokine such as betatrophin (22) or by the accompanying hyperglycemia. We demonstrate that Ins-1 cells exhibit increased insulin secretion and preproinsulin expression when treated with glucagon. This suggests direct paracrine glucagon signaling to beta cells, although a role for a glucagon-stimulated hepatokine such as betatrophin (22) was not excluded. These findings suggest novel strategies to combat the obesity/T2D epidemic. Using glucagon suppressors or blockers of GcgR, it should be possible to block the diet-induced hyperinsulinemia that is the sine qua non of obesity and the accompanying metabolic syndrome, in addition to simply preventing hyperglycemia by suppressing glucagon secretion.
Interference with diet-induced insulin secretion would be expected to cause diabetes. Remarkably, the lack of diet-induced obesity (DIO)-associated hyperinsulinemia in GcgR−/− mice did not cause hyperglycemia, probably because without glucagon action, glucose production by the GcgR−/− liver cannot exceed glucose clearance (35).
There are potentially useful clinical lessons to be learned from these studies. First, suppressing glucagon secretion or blocking its action can prevent diet-induced hyperinsulinemia that mediates lipogenesis and obesity. It does so without causing diabetes, which in fact it prevents. Second, the evidence that diet-induced hyperinsulinemia is a pathogenic component in the chain that ends with obesity and T2D should deter the use of intensive insulin therapy to control the hyperglycemia of obese, hyperinsulinemic T2D patients (37, 38).
Materials and Methods
Animals and HFF.
Twelve male C57BL6 wild-type GcgR+/+ and Gcgr−/− mice were housed in individual cages with constant temperature and 12 h of light alternating with 12 h darkness. Half the mice were fed a Teklad 6% (wt/wt) mouse/rat chow diet, and half of the mice were fed a 60% (wt/wt) high-fat diet (Research Diets Inc.) with 10% (wt/wt) sucrose water for 10 wk. Food intake, body weight, blood glucose, and total body fat were measured weekly. Total body fat was measured with a mq10 NMR analyzer (Bruker Biospin). All tissues were freeze-clamped, excised, frozen in liquid nitrogen immediately, and stored at −80 °C until use. Guidelines from the Institutional Animal Care and Use Committee of University of Texas Southwestern were followed.
LepR−/−,GcgR−/− Mice.
Mice lacking both the glucagon and leptin receptor were generated by crossing GcgR+/− mice with recently described mice in which a loxP flanked stop sequence was inserted to disrupt expression of the leptin receptor (leprloxTB) (39). Study animals were generated by breeding animals heterozygous for both GcgR and leprloxTB.
Insulin Treatment.
Six- to 12-wk-old Gcgr−/− mice received insulin pellet (Linshin Canada Inc.) implants and were followed for 10 wk on the high-fat diet. Body weight and blood insulin were measured before and after the 10 wk.
Adenovirus GcgR Administration.
Adv-GcgR was purchased from Vector Biolabs. Adv-GcgR or B-galactosidase was administered i.v. at a dose of 1 × 109 pfu/10 g.
Metabolic Cage Studies.
GcgR+/+ and Gcgr−/− mice, which were fed either control chow or a 60% high-fat diet (three mice in each group), were housed in metabolic cages. VCO2, VO2, peripheral and central movement, and food intake were measured.
ZDFfa/fa Rats Fed GABA.
Twelve- to 6-wk-old male ZDFfa/fa rats were divided into two groups. The experimental group was placed on 10% GABA (Sigma) with a powder chow diet, and the other group was pair-fed with the same chow diet without GABA for 6 wk. Blood glucose was measured with a glucose meter around 10:00 AM every week with a glucose meter (Bayer HealthCare LLC).
Plasma Measurements.
Blood glucose was measured in conscious animals from a hand-held glucose meter (Bayer HealthCare LLC) on tail vein blood around 10:00 AM weekly. Plasma insulin levels were measured using a rat/mouse insulin ELISA kit (Crystal Chem).
TG Content of Tissues.
Total lipids from livers were extracted, and TG content was assayed as previously described (40). Four to six livers from each treatment condition were measured.
Quantitative Real-Time PCR.
Total RNA was extracted from livers from mice and Ins-1 cells using Qiagen RNeasy kit, according to the manufacturer’s protocol. The expression of mRNA was calculated by using the standard curve method, and 36B4 RNA level was used as the invariant control. Primer sequences of genes used for quantification of mRNA by quantitative PCRs are listed in Table S1.
Culture of Hamster Pancreatic InR1-G9 Cells.
Mycoplasma-free InR1-G9 cells were cultured in RPMI medium 1640 with 10% FBS (GIBCO) and 1 mM sodium pyruvate (Invitrogen) and contained 1% of penicillin–streptomycin solution (Invitrogen). InR1-G9 cells gave the appropriate glucose-regulated glucagon secretion in the absence of insulin. To characterize the effect of ceramide directly on the alpha cell, 400,000 cells were plated per well in a 12-well tissue culture plate. The next day cells were treated with ceramide at the indicated concentrations. After 30 min, 0.2 mU insulin was added to the well for an additional 20 min before being harvested. Total RNA was extracted from the Qiagen RNeasy kit, and GAPDH was used as an internal control to normalize the variability in expression levels.
Culture of Ins-1 Cells.
Ins-1 cells were cultured in RPMI medium 1640 with 10% FBS (Gibco), 1 mM sodium pyruvate, 1 mM Hepes, 1 mM Glutamax, and 1% of peniciline–streptomycin solution (Invitrogen). One million cells were plated in a six-well plate and treated with/without glucagon (0.4 µg/mL of medium) for 24 h. Total RNA was extracted from Ins-1 cells using Qiagen RNeasy kit, and 36B4 was used as an internal control to normalize the variability in expression levels.
Statistical Analysis.
Results are presented as mean ± SEM and are compared using Student t test.
Supplementary Material
Acknowledgments
We thank Richard Hogg and Kay McCorkle for their technical assistance. This research was supported by grants from Bristol-Myers Squibb (to R.H.U.) and the Diane and Hal Brierley Distinguished Chair in Biomedical Research (to M.G.R.) and was partially conducted in laboratories renovated with support from National Institutes of Health Facilities Grant C06-RR15437.
Footnotes
Conflict of interest statement: R.H.U. and M.G.R. are founding scientists of SynAlpha Therapeutics, LLC.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409638111/-/DCSupplemental.
References
- 1.Centers for Disease Control and Prevention . National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta: US Department of Health and Human Services; 2014. [Google Scholar]
- 2.International Diabetes Foundation . IDF Diabetes Atlas. Brussels: International Diabetes Foundation; 2014. [Google Scholar]
- 3.Lee Y, et al. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: Impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci USA. 1994;91(23):10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou YT, et al. Enhanced de novo lipogenesis in the leptin-unresponsive pancreatic islets of prediabetic Zucker diabetic fatty rats: Role in the pathogenesis of lipotoxic diabetes. Diabetes. 1998;47(12):1904–1908. doi: 10.2337/diabetes.47.12.1904. [DOI] [PubMed] [Google Scholar]
- 5.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28(11):2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 7.Petersen KF, Shulman GI. New insights into the pathogenesis of insulin resistance in humans using magnetic resonance spectroscopy. Obesity (Silver Spring) 2006;14(Suppl 1):34S–40S. doi: 10.1038/oby.2006.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leichman JG, Lavis VR, Aguilar D, Wilson CR, Taegtmeyer H. The metabolic syndrome and the heart—A considered opinion. Clin Res Cardiol. 2006;95(Suppl 1):i134–i141. doi: 10.1007/s00392-006-1119-7. [DOI] [PubMed] [Google Scholar]
- 9.Morelli M, et al. Ectopic fat: The true culprit linking obesity and cardiovascular disease? Thromb Haemost. 2013;110(4):651–660. doi: 10.1160/TH13-04-0285. [DOI] [PubMed] [Google Scholar]
- 10.Borradaile NM, et al. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47(12):2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg JM. Lipotoxicity. Kidney Int. 2006;70(9):1560–1566. doi: 10.1038/sj.ki.5001834. [DOI] [PubMed] [Google Scholar]
- 12.Weir GC, et al. Towards better understanding of the contributions of overwork and glucotoxicity to the beta-cell inadequacy of type 2 diabetes. Diabetes Obes Metab. 2009;11(Suppl 4):82–90. doi: 10.1111/j.1463-1326.2009.01113.x. [DOI] [PubMed] [Google Scholar]
- 13.Tuomi T, et al. The many faces of diabetes: A disease with increasing heterogeneity. Lancet. 2014;383(9922):1084–1094. doi: 10.1016/S0140-6736(13)62219-9. [DOI] [PubMed] [Google Scholar]
- 14.Watson JD. Type 2 diabetes as a redox disease. Lancet. 2014;383(9919):841–843. doi: 10.1016/S0140-6736(13)62365-X. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60(2):391–397. doi: 10.2337/db10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee Y, et al. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proc Natl Acad Sci USA. 2012;109(37):14972–14976. doi: 10.1073/pnas.1205983109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest. 1984;74(6):2296–2299. doi: 10.1172/JCI111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawamori D, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9(4):350–361. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raskin P, Unger RH. Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. N Engl J Med. 1978;299(9):433–436. doi: 10.1056/NEJM197808312990901. [DOI] [PubMed] [Google Scholar]
- 20.Aydin I, Raskin P, Unger RH. The effect of short-term intravenous insulin administration on the glucagon response to a carbohydrate meal in adult onset and juvenile type diabetes. Diabetologia. 1977;13(6):629–636. doi: 10.1007/BF01236318. [DOI] [PubMed] [Google Scholar]
- 21.Conarello SL, et al. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia. 2007;50(1):142–150. doi: 10.1007/s00125-006-0481-3. [DOI] [PubMed] [Google Scholar]
- 22.Yi P, Park JS, Melton DA. Betatrophin: A hormone that controls pancreatic β cell proliferation. Cell. 2013;153(4):747–758. doi: 10.1016/j.cell.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001;2(4):282–286. doi: 10.1093/embo-reports/kve071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biddinger SB, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7(2):125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown MS, Goldstein JL. Selective versus total insulin resistance: A pathogenic paradox. Cell Metab. 2008;7(2):95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Shimomura I, et al. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci USA. 1999;96(24):13656–13661. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehran AE, et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012;16(6):723–737. doi: 10.1016/j.cmet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Cherrington AD. Banting lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48(5):1198–1214. doi: 10.2337/diabetes.48.5.1198. [DOI] [PubMed] [Google Scholar]
- 29.Berglund ED, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122(3):1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unger RH, Orci L. Diseases of liporegulation: New perspective on obesity and related disorders. FASEB J. 2001;15(2):312–321. doi: 10.1096/fj.00-0590. [DOI] [PubMed] [Google Scholar]
- 31.Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18(9):5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland WL, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5(3):167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Shimabukuro M, Zhou YT, Lee Y, Unger RH. Troglitazone lowers islet fat and restores beta cell function of Zucker diabetic fatty rats. J Biol Chem. 1998;273(6):3547–3550. doi: 10.1074/jbc.273.6.3547. [DOI] [PubMed] [Google Scholar]
- 34.Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic extracts in the treatment of diabetes mellitus. Can Med Assoc J. 1922;12(3):141–146. [PMC free article] [PubMed] [Google Scholar]
- 35.Cahill GF., Jr The banting memorial lecture 1971. Physiology of insulin in man. Diabetes. 1971;20(12):785–799. doi: 10.2337/diab.20.12.785. [DOI] [PubMed] [Google Scholar]
- 36.Samols E, Marri G, Marks V. Promotion of insulin secretion by glucagon. Lancet. 1965;2(7409):415–416. doi: 10.1016/s0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- 37.Unger RH. Reinventing type 2 diabetes: Pathogenesis, treatment, and prevention. JAMA. 2008;299(10):1185–1187. doi: 10.1001/jama.299.10.1185. [DOI] [PubMed] [Google Scholar]
- 38.Margolis KL, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: The ACCORD randomized trial. Diabetes Care. 2014;37(6):1721–1728. doi: 10.2337/dc13-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berglund ED, et al. Hepatic energy state is regulated by glucagon receptor signaling in mice. J Clin Invest. 2009;119(8):2412–2422. doi: 10.1172/JCI38650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.