Abstract
Stress on plants imposed by flooding of the soil and deeper submergence constitutes one of the major abiotic constraints on growth, species' distribution and agricultural productivity. Flooding stress is also a strong driver of adaptive evolution. This has resulted in a wide range of biochemical, molecular and morphological adaptations that sanction growth and reproductive success under episodic or permanently flooded conditions that are highly damaging to the majority of plant species. However, even seemingly poorly adapted species possess some short-term resilience that is important for overall success of these plants in various habitats. The papers contained in this Special Issue address these topics and emphasize molecular, biochemical and developmental processes that impact on flooding tolerance. Most of the articles are based on lectures given to the 8th Conference of the International Society for Plant Anaerobiosis (ISPA), held at the University of Western Australia, Perth, 20–24 September, 2004. Reviews and research papers are presented from the leading laboratories currently working on plant responses to flooding stress.
Keywords: Abiotic stress, adaptation, aerenchyma, anoxia, aquatic plants, hypoxia, proteome, rice, signal-transduction, submergence, transcriptome, wetland
INTRODUCTION
Soil waterlogging and submergence (collectively termed flooding) are abiotic stresses that influence species composition and productivity in numerous plant communities, world-wide. Hydrological patterns can determine the vegetation in natural and man-made wetlands, since this is dependant on ecophysiological responses of species to flooding (e.g. Voesenek et al., 2004). In rice farming, flooding regimes are manipulated (e.g. paddy rice) or are accommodated by genotype selection (e.g. deep-water rice) to secure much of the world's production of this staple crop (Grist, 1986). There have also been recent advances towards developing cultivars for lowland areas prone to short-duration flash flooding (Siangliw et al., 2003; Toojinda et al., 2003). For most other crops, excess water is a major constraint to productivity in many regions and situations (Jackson, 2004), adversely affecting grain yields (Setter and Waters, 2003) and growth of pasture species (Gibberd and Cocks, 1997; Gibberd et al., 2001).
A major constraint resulting from excess water, at least for poorly adapted species, is an inadequate supply of oxygen to submerged tissues; diffusion of oxygen through water is 104-fold slower than in air (Armstrong and Drew, 2002). In addition to the threat of oxygen deficiency, excess water also leads to other changes in the soil that influence plants; levels of the plant hormone ethylene (Smith and Russell, 1969; Jackson, 1982), and products of anaerobic metabolism by soil micro-organisms (e.g. Mn2+, Fe2+, S2−, H2S and carboxylic acids) can accumulate (Ponnamperuma, 1984; McKee and McKevlin, 1993). Moreover, when flooding results in complete submergence, and in normally submersed aquatic plants, availability to the shoots of carbon dioxide, light and oxygen typically diminish (Jackson and Ram, 2003).
Growth and development of the vast majority of vascular plant species is impeded by soil flooding, and particularly by complete submergence, both of which can result in death. However, numerous wetland species are highly productive in flood-prone areas. This is achieved by means of a combination of life-history traits (Blom, 1999) and certain key physiological adaptations and acclimations such as physical ‘escape’ from a submerged environment (Voesenek et al., 2003), avoidance of oxygen-deficiency through effective internal aeration (Jackson and Armstrong, 1999), anoxia tolerance (Gibbs and Greenway, 2003), and a capacity to prevent, or repair, oxidative damage during re-aeration (Blokhina et al., 2003).
The International Society for Plant Anaerobiosis (ISPA) represents a body of scientists interested in the mechanisms, and regulation, of acclimations and adaptations in plants to conditions during flooding. Since 1975, ISPA has organized a series of workshops and conferences, with several books and journal special issues having been published (summarized in Visser et al., 2003). The meetings and publications have been multi-disciplinary, a feature contributing to advancing knowledge on various aspects of plant life in poorly aerated environments, as well as providing insights into plant biology more generally. This mix of disciplines was again a highlight of the 8th ISPA Conference, which was held in Perth, Western Australia, from 20th to 24th September, 2004. Seventy-one delegates from 21 countries participated in the Conference, with oral and poster presentations on plants inhabiting marine, aquatic, salt marsh, wetland and terrestrial ecosystems subjected to seasonal episodes of waterlogging or submergence (including crop species and agricultural systems). The various papers dealt with processes at several levels of organization, such as ecology, ecophysiology, cell physiology, biochemistry, molecular biology and genetics. A selection of the papers by speakers at the Conference are presented in this Special Issue.
SUMMARY OF TOPICS CONTAINED IN THIS SPECIAL ISSUE
Flooding is a complex stress that imposes several often-concurrent challenges to normal plant functioning. Dominant is starvation of oxygen and carbon dioxide that is imposed by extremely slow rates of diffusion through the floodwater compared to that in air. Many papers deal directly or indirectly with the impact of oxygen deficiency at various levels of organization, ranging from the initial sensing of oxygen shortage to whole-plant processes involving escape from total inundation, and to the interaction of these basic characteristics of plants with environment to create vigorous riparian ecosystems. The Special Issue opens with a review of the processes involved in the direct and indirect sensing of low oxygen supply and the transduction of such sensing into altered patterns of gene expression (Bailey-Serres and Chang, 2005). Such mechanisms play a vital part in equipping cells that are partially deficient in oxygen with a re-ordered pattern of expressed genes that enhances tolerance to any subsequent anaerobiosis. They include the operation of signal transduction pathways mediated by calcium and reactive oxygen species. Class I haemoglobin (Hb) genes are amongst those induced by partial oxygen shortage. Igamberdiev et al. (2005) review the significance of this expression in the light of haemoglobin's high affinity for oxygen (it exceeds that of mitochondria cytochrome oxidase) and its interaction with nitric oxide (NO), a highly active gas that is generated from nitrate in increased quantities by hypoxic cells. The authors propose a novel respiratory pathway in which Hb and NO interact to regenerate energy and oxidise respiratory nucleotides. Interactions between NO, Hb and the hormone ethylene are also proposed. An extinguished oxygen supply favours a marked decrease in cytoplasmic pH that is believed to disrupt cell biochemistry to a potentially fatal extent. Felle (2005) reviews the likely sources of the protons responsible for this acidification, and also examines mechanisms for pH regulation and reasons for a loss of such regulation if anoxia is prolonged.
When flooding extends to submergence of the shoot, photosynthesis becomes severely restricted by a deficiency of external carbon dioxide and by shading. Furthermore, total submergence can interfere with flowering and pollination essential for completion of the reproductive cycle. In many aquatic and amphibious species, these debilitating effects are overcome by an oxygen-dependant, ethylene-mediated stimulation of underwater shoot elongation that encourages renewed contact with the aerial environment. Unfortunately, with the exception, perhaps, of rice, species known to adopt this strategy offer few opportunities for molecular dissection of signal sensing and transduction processes involved in the submergence escape. In contrast, the model plant species Arabidopsis thaliana poses few difficulties of this kind but, on the face of it, fails to display the developmental attributes involved in submergence escape. Intriguingly, Pierik et al. (2005) are able to circumvent the impasse by utilizing a strong similarity between the elongation responses of A. thaliana to shade and the elongation responses to submergence by amphibious plants such as Rumex palustris. Each response is thought to share several signal transduction steps that are amenable to molecular dissection using A. thaliana. At the other extreme, in terms of level of organization, Finlayson (2005) highlights the spatial and temporal complexity of a natural flood-prone environment such as that of the tropical wetlands of northern Australia. From this perspective, plant species diversity and biomass production are seen as outcomes of a spectrum of adaptations to year-round changes in availability of oxygen, water and other resources. The emerging picture defies simplistic explanations of what determines success in these complex and demanding surroundings. The article also emphasizes how little is still known about the species make-up of such intricate ecosystems and the relative tolerances of these plants to the seasonally varied environment in which they thrive.
One of the most characteristic features of plants of wetland ecosystems, and also those of drier places that possess some tolerance to flooding, is the possession of aerenchyma. This tissue type furnishes plants with an interconnected network of intercellular gas-filled spaces that permits a relatively unhindered internal diffusion or mass flow of oxygen and other gases down concentration or pressure gradients. These processes offer an oxygen-bearing lifeline to inundated organs. The oxygen is sourced in more remote parts of the plant that have direct access to the air or to well-aerated water or are photosynthesizing whilst underwater. Seago et al. (2005) describe patterns of aerenchyma development in 85 species representing 41 families of wetland plants. They conclude that the most basic process creating the intercellular spaces involves a combination of differential amounts of cell expansion and division (expansigeny). This process is shown to be typical of primitive angiosperms of the Nymphaeales and monocots of the Acorales. Aerenchyma formed by cell dissolution, or by division alone, is seen as a later evolutionary development. The forces propelling gas flow through aerenchyma are largely based on diffusion, although a mass flow component can be involved that may operate over longer distances if the aerenchyma pathway also creates a low resistance outlet. However, it has been claimed that such an outlet may not always be required for pressurised ventilation, an example of this being oxygenation of waterlogged alder roots when the stem above the waterline is illuminated. Armstrong and Armstrong (2005b) revisit this conundrum and provide strong experimental evidence that it can be explained by internal oxygen generated in the stem of alder by chlorophyll-rich photosynthesizing cells that utilize respiratory carbon dioxide. Oxygen generated by photosynthesis also makes a vital contribution to the survival of complete submergence by amphibious species. Mommer and Visser (2005) identify morphological features of leaves that promote underwater photosynthesis. These include faster uptake of dissolved carbon dioxide linked to developmental plasticity that generates thinner cuticles and leaf laminae. The importance of morphology, aerenchyma and other features of internal anatomy for the effective harvesting and internal distribution of key gases (oxygen in this case) is also made clear in a detailed quantitative study of freshwater Lobelia dortmanna and the marine seagrass Zostera marina by Sand-Jensen et al. (2005). This comparative work illustrates the benefits that can be derived from the imaginative use of oxygen-sensing microelectrodes inserted into tissue with considerable positional precision under demanding natural growing conditions. Armstrong and Armstrong (2005a) use external polarographic oxygen-sensing electrodes positioned in the rhizosphere to examine the basis of sulphide poisoning of root systems of rice, a common occurrence in paddy field rice production. They observe that sulphide induces a fast reduction in radial oxygen loss in root tips in association with a depression of elongation and water uptake. In the longer term (days), sulphide is seen to damage the roots further by inducing suberisation of the outer layers of the roots that impedes lateral root emergence. Sulphide also promotes blockage of aerenchyma and the vasculature. Radial oxygen loss from roots also features in an analysis by Kirk and Kronzucker (2005) of nitrate release from organic matter and its subsequent uptake by roots of rice. Experimental evidence and modelling, point to surprisingly vigorous nitrate generation in the oxygenated rhizosphere of flooded anaerobic soils and to a scale of nitrate uptake that rivals that of ammonium.
The analysis of patterns of gene expression that characterize oxygen-deficient cells and the mechanisms of its regulation are benefiting substantially from developments in microarray technology and from in silico scrutiny and classification of gene sequences using computer-based analysis of publicly accessible databases. Branco-Price et al. (2005) describe a genome-wide analysis of Arabidopsis thaliana seedlings exposed to 12 h of partial oxygen shortage that reveals much about the interrelations of expression pattern, level of complexing onto polysomes and rate of translation into protein. A low GC nucleotide content of 5′-untranslated regions of a small group of mRNAs is thought to confer the potential for stronger translation in hypoxic cells. The products of this translation may therefore be particularly important for short-term survival of oxygen deprivation. Gonzali et al. (2005) have developed microarrays and bioinformatics methods to describe and analyse the functional significance of changes in the global pattern of gene expression in seedlings of A. thaliana exposed to 6 h without oxygen (<10 ppm). These authors discuss how the reliability of expression data generated for about 20 000 genes can be assessed and highlight the use of appropriate software to analyse this huge output of information. The expression of 1600 genes is shown to be affected by 6 h anoxia, with many but by no means all being linked to fermentation and sucrose-related pathways. The potential of microarrays to assess the efficiency of translation and the utility of developing protein libraries based on microarray output is also explored. The power of modern informatics is also well-illustrated by Mohanty et al. (2005) who report several novel sequence motifs shared by promoter regions of anaerobically inducible genes. These are believed to be potentially important in the regulation of gene expression, notably as likely binding sites for transcription factors. Several of the motifs were previously known only for animal cells. Although modern technology is allowing an ever more global analysis of the transcriptome of oxygen-deficient tissues, there is also a pressing need to look closely at the role of individual gene products and assess their involvement in key processes known to enhance tolerance. Harada et al. (2005) provide a good example of this in their study of the remarkable ability of the aquatic monocot Potamogeton distinctus to elongate its overwintering shoot much more rapidly than normal when the supply of oxygen is totally stopped. This anaerobic growth stimulation appears to be linked to increases in sucrose synthase (SuSy), an enzyme that can contribute to the degradation of sucrose to fermentable hexose in an energy-efficient way. One of two SuSy genes is shown to be upregulated by anoxia and appears subject to hormonal regulation rather than to regulation by sugar deprivation. In a related paper, Ookawara et al. (2005) examine anoxia-promoted elongation in the aquatic species Sagittaria pygmaea. The focus here is on cell wall loosening enzymes, since faster elongation is probably predicated on an increase in their wall extensibility. The authors conclude that anoxia-enhanced accumulation of mRNA coding for two of four expansin genes found in S. pygmaea and for two of five endotransglucosylase/hydrolase genes contributes to the faster elongation by anoxic shoots. Intriguingly, a different pattern of gene expression is seen when elongation in the presence of oxygen is promoted by ethylene or carbon dioxide.
Analysis of the proteome as an adjunct to conventional biochemistry is featured in a paper by Huang et al. (2005) on the anoxia-tolerant rice coleoptile, in which both total protein content and newly synthesised proteins were analysed using mass spectrometry following separation on 2D-gels. One of the synthesized proteins is identified as pyruvate orthophosphate dikinase (PPDK) that could help to generate pyrophosphate (inorganic diphosphate, 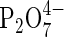 ) from ATP. Pyrophosphate is thought to be capable of substituting for scarce ATP in sucrose breakdown, in a key ATP-requiring step in glycolysis and in tonoplast proton transport. This notion receives further support from Mustroph et al. (2005) who use transgenic potato plants rendered constitutively low in pyrophosphate by an inserted Escherichia coli-derived pyrophosphatase gene. The transgenic plants are shown to be less tolerant of 4 d severe hypoxia at the roots than normal plants in association with a build-up of upstream hexose. Both these papers support the notion that pyrophosphate-mediated reactions contribute to metabolic tolerance of severe hypoxia or of anoxia. Since glycolysis followed by alcoholic fermentation is the main ATP-generating pathway for anaerobic and severely hypoxic plant cells there is obvious interest in monitoring its products. Boamfa et al. (2005) have achieved this non-destructively using highly sensitive laser-based photoacoustics to assess the output of acetaldehyde and ethanol, the penultimate and final products of the pathway. The emerging detailed kinetics produce some surprising results and lead to the proposal that the differential submergence tolerance in rice is not necessarily related to different rates of fermentation under anoxia. Instead, there seems to be a link with the rate at which ethanol is back-converted to acetaldehyde. Differential tolerance is expressed under partial oxygen deficiency when conversion of ethanol to acetaldehyde is thought to deplete hypoxic cells of potentially damaging superoxide. This detoxifying pathway appears to operate more vigorously in a submergence tolerant genotype of rice.
) from ATP. Pyrophosphate is thought to be capable of substituting for scarce ATP in sucrose breakdown, in a key ATP-requiring step in glycolysis and in tonoplast proton transport. This notion receives further support from Mustroph et al. (2005) who use transgenic potato plants rendered constitutively low in pyrophosphate by an inserted Escherichia coli-derived pyrophosphatase gene. The transgenic plants are shown to be less tolerant of 4 d severe hypoxia at the roots than normal plants in association with a build-up of upstream hexose. Both these papers support the notion that pyrophosphate-mediated reactions contribute to metabolic tolerance of severe hypoxia or of anoxia. Since glycolysis followed by alcoholic fermentation is the main ATP-generating pathway for anaerobic and severely hypoxic plant cells there is obvious interest in monitoring its products. Boamfa et al. (2005) have achieved this non-destructively using highly sensitive laser-based photoacoustics to assess the output of acetaldehyde and ethanol, the penultimate and final products of the pathway. The emerging detailed kinetics produce some surprising results and lead to the proposal that the differential submergence tolerance in rice is not necessarily related to different rates of fermentation under anoxia. Instead, there seems to be a link with the rate at which ethanol is back-converted to acetaldehyde. Differential tolerance is expressed under partial oxygen deficiency when conversion of ethanol to acetaldehyde is thought to deplete hypoxic cells of potentially damaging superoxide. This detoxifying pathway appears to operate more vigorously in a submergence tolerant genotype of rice.
CONCLUDING REMARKS
The editors of a previous Special Issue in the Annals of Botany containing papers from the 7th ISPA Conference held in 2001 noted a greater emphasis on molecular genetics and the wide-adoption of molecular techniques by scientists in this field of study (Visser et al., 2003). Accordingly, several papers in the present Special Issue highlight the rapid progress being made in understanding the molecular basis of plant acclimation and adaptation to oxygen deficiency, using genomics, proteomics, transgenic approaches and other molecular techniques. These studies have provided valuable knowledge about metabolism and signal-transduction networks underlying plant responses to oxygen-deprivation. The important contributions of the ‘model plants’ for molecular genetic studies, arabidopsis and the more flooding-tolerant rice, are highlighted. In addition, studies of ‘model’ wetland species with specialized phenotypes for various niches in flood-prone environments continue to generate advances in understanding. ‘Wetland model species’ are likely to continue to yield advances into specialized plant responses to flooding and research on these should surely continue to be encouraged. Several papers in this Special Issue highlight that much progress continues to be made in understanding the ecology of plants in flood-prone environments and in the physiology of various aspects of plant acclimation and adaptation to flooding, at cellular to whole-plant levels. Thus, although several key aspects of the physiology of plants subjected to flooding are now well described (see Introduction), papers in this Special Issue highlight the substantial gains also in this discipline that continue to be made; as examples: (1) on plant–soil interactions in the rhizosphere crucial to plant nutrition and coping with the adverse conditions in flooded soils; (2) on gas exchange and metabolism in submerged species; and (3) resolving a long-standing question in aeration of woody species. The need for additional knowledge on the ecology and physiology of wetland species (e.g. even those in world heritage wetland areas, such as in Northern Australia) is also highlighted.
A final, brief, comment on the ISPA seems appropriate. Professor Boris Vartapetian (Timiryazev Institute, Moscow, Russia) founded ISPA in 1975 and served as Inaugural President for three decades. We thank Boris for the vision and leadership that initiated this Society and ensured its successful functioning on the international stage for so many years. Boris resigned from his position in 2004. Accordingly, at its meeting held in Perth, September, 2004, the ISPA Council recognized Boris Vartatapetian's special contribution by bestowing on him the accolade of ‘Honorary President for Life’. At that time, a new president and international council were elected, and future ISPA initiatives were discussed. Details are available from the ISPA website (http://www.is-pa.org). The 9th ISPA Conference will be held during autumn, 2007, in Sendai, Japan, with Professor Kimiharu Ishizawa as chair of the local organizing committee.
Acknowledgments
The 8th Conference of the International Society for Plant Anaerobiosis was sponsored by ALCOA Australia, Annals of Botany, AusAID (Australian Agency for International Development), Australian Society of Plant Scientists, CRC for Plant-based Management of Dryland Salinity, Department of Conservation and Land Management in Western Australia, Grains Research & Development Corporation, School of Plant Biology (UWA), Faculty of Natural and Agricultural Sciences (UWA), and The University of Western Australia.
LITERATURE CITED
- Armstrong J, Armstrong W. 2005. Rice: sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Annals of Botany 96: 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong W, Armstrong J. 2005. Stem photosynthesis not pressurised ventilation is responsible for light-enhanced oxygen supply to submerged roots of alder (Alnus glutinosa). Annals of Botany 96: 591–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong W, Drew MC. 2002. Root growth and metabolism under oxygen deficiency. In: Waisel Y, Eshel A and Kafkafi U, eds. Plant roots: the hidden half, 3rd edn. New York: Marcel Dekker, 729–761. [Google Scholar]
- Bailey-Serres J, Chang R. 2005. Sensing and signalling in response to oxygen deprivation in plants and other organisms. Annals of Botany 96: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. 2003. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany 91: 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom CWPM. 1999. Adaptations to flooding stress: from plant community to molecule. Plant Biology 1: 261–273. [Google Scholar]
- Boamfa EI, Veres AH, Ram PC, Jackson MB, Reuss J, Harren FJM. 2005. Kinetics of ethanol and acetaldehyde release suggest a role for acetaldehyde production in tolerance of rice seedlings to micro-aerobic conditions. Annals of Botany 96: 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco-Price C, Kawaguchi R, Ferreira RB, Bailey-Serres J. 2005. Genome-wide analysis of transcript abundance and translation in Arabidopsis seedlings subjected to oxygen deprivation. Annals of Botany 96: 647–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle HH. 2005. pH regulation in anoxic plants. Annals of Botany 96:519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson CM. 2005. Plant ecology of Australia's tropical floodplain wetlands: a review. Annals of Botany 96: 541–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Greenway H. 2003. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology 30: 1–47. [DOI] [PubMed] [Google Scholar]
- Gibberd MR, Cocks PS. 1997. Effect of waterlogging and soil pH on the micro-distribution of naturalized annual legumes. Australian Journal of Agricultural Research 48: 223–229. [Google Scholar]
- Gibberd MR, Gray JD, Cocks PS, Colmer TD. 2001. Waterlogging tolerance among a diverse range of Trifolium accessions is related to root porosity, lateral root formation, and aerotrophic rooting. Annals of Botany 88: 579–589. [Google Scholar]
- Gonzali S, Loreti E, Novi G, Poggi A, Alpi A, Perata P. 2005. The use of microarrays to study the anaerobic response in Arabidopsis Annals of Botany 96: 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grist DH. 1986.Rice, 6th edn. New York: Longman. [Google Scholar]
- Harada T, Satoh S, Yoshioka T, Ishizawa K. 2005. Expression of sucrose synthase genes involved in enhanced elongation of pondweed (Potamogeton distinctus) turions under anoxia. Annals of Botany 96: 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Greenway H, Colmer TD, Millar AH. 2005. Protein synthesis by rice coleoptiles during prolonged anoxia: implications for glycolysis, growth and energy utilization. Annals of Botany 96: 703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igamberdiev AU, Baron K, Manac'h-Little N, Stoimenova M, Hill RD. 2005. The haemoglobin/nitric oxide cycle: involvement in flooding stress and effects on hormone signalling. Annals of Botany 96: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB. 1982. Ethylene as a growth promoting hormone under flooded conditions. In: Wareing PF, ed. Plant growth regulators 1982. London: Academic Press, 291–301. [Google Scholar]
- Jackson MB. 2004. The impact of flooding stress on plants and crops.http://www.plantstress.com/Articles/waterlogging_i/waterlog_i.htm [Google Scholar]
- Jackson MB, Armstrong W. 1999. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology 1: 274–287. [Google Scholar]
- Jackson MB, Ram PC. 2003. Physiological and molecular basis of susceptibility and tolerance of rice plants to complete submergence. Annals of Botany 91: 227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk GJD, Kronzucker HJ. 2005. The potential for nitrification and nitrate uptake in the rhizosphere of wetland plants: a modelling study. Annals of Botany 96: 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee WH, McKevlin MR. 1993. Geochemical processes and nutrient uptake by plants in hydric soils. Environmental Toxicology and Chemistry 12: 2197–2207. [Google Scholar]
- Mohanty B, Krishnan SPT, Swarup S, Bajic VB. 2005. Detection and preliminary analysis of motifs in promoters of anaerobically induced genes of different plant species. Annals of Botany 96: 669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommer L, Visser EJW. 2005. Underwater photosynthesis in flooded terrestrial plants: a matter of leaf plasticity. Annals of Botany 96: 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A, Albrecht G, Hajirezaei M, Grimm B, Biemelt S. 2005. Low levels of pyrophosphate in transgenic potato plants expressing E.coli pyrophosphatase lead to decreased vitality under oxygen deficiency. Annals of Botany 96: 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookawara R, Satoh S, Yoshioka T, Ishizawa K. 2005. Expression ofα-expansin and xyloglucan endotransglucosylase/hydrolase genes associated with shoot elongation enhanced by anoxia, ethylene and carbon dioxide in arrowhead (Sagittaria pygmaea Miq.) tubers. Annals of Botany 96: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Millenaar FF, Peeters, AJM, Voesenek LACJ. 2005. New perspectives in flooding research: the use of shade avoidance and Arabidopsis thaliana. Annals of Botany 96: 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnamperuma FN. 1984. Effects of flooding on soils. In: Kozlowski TT, ed. Flooding and plant growth. New York: Academic Press,9–45. [Google Scholar]
- Sand-Jensen K, Pedersen O, Binzer T, Borum J. 2005. Contrasting oxygen dynamics in the freshwater isoetid Lobelia dortmanna and the marine seagrass Zostera marina Annals of Botany 96: 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seago JL Jr, Marsh LC, Stevens KJ, Soukup A, Votrubová O, Enstone DE. 2005. A re-examination of the root cortex in wetland flowering plants with respect to aerenchyma. Annals of Botany 96:565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter TL, Waters I. 2003. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant and Soil 253: 1–34. [Google Scholar]
- Siangliw M, Toojinda T, Tragoonrung S, Vanavichit A. 2003. Thai Jasmine rice carrying QTLch9 (SubQTL) is submergence tolerant. Annals of Botany 91: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KA, Russell RS. 1969. Occurrence of ethylene, and its significance, in anaerobic soil. Nature 222: 769–771. [Google Scholar]
- Toojinda T, Siangliw M, Tragoonrung S, Vanavichit A. 2003. Molecular genetics of submergence tolerance in rice: QTL analysis of key traits. Annals of Botany 91: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser EJW, Voesenek LACJ, Vartapetian BB, Jackson MB. 2003. Flooding and plant growth. Annals of Botany 91: 107–109. [Google Scholar]
- Voesenek LACJ, Benschop JJ, Bou J, Cox MCH, Groeneveld HW, Millenaar FF, Vreeburg RAM, Peeters AJM. 2003. Interactions between plant hormones regulate submergence-induced shoot elongation in the flooding-tolerant dicot Rumex palustris Annals of Botany 91: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Rijnders JHGM, Peeters AJM, Van de Steeg HMV, De Kroon H. 2004. Plant hormones regulate fast shoot elongation under water: from genes to communities. Ecology 85: 16–27. [Google Scholar]


