Abstract
• Background pH regulation is the result of a complex interaction of ion transport, H+ buffering, H+-consuming and H+-producing reactions. Cells under anoxia experience an energy crisis; an early response thereof (in most tissues) is a rapid cytoplasmic acidification of roughly half a pH unit. Depending on the degree of anoxia tolerance, this pH remains relatively stable for some time, but then drops further due to an energy shortage, which, in concert with a general breakdown of transmembrane gradients, finally leads to cell death unless the plant finds access to an energy source.
• Scope In this review the much-debated origin of the initial pH change and its regulation under anoxia is discussed, as well as the problem of how tissues deal with the energy crisis and to what extent pH regulation and membrane transport from and into the vacuole and the apoplast is a part thereof.
• Conclusions It is postulated that, because a foremost goal of cells under anoxia must be energy production (having an anaerobic machinery that produces insufficient amounts of ATP), a new pH is set to ensure a proper functioning of the involved enzymes. Thus, the anoxic pH is not experienced as an error signal and is therefore not reversed to the aerobic level. Although acclimated and anoxia-tolerant tissues may display higher cytoplasmic pH than non-acclimated or anoxia-intolerant tissues, evidence for an impeded pH-regulation is missing even in the anoxia-intolerant tissues. For sufficient energy production, residual H+ pumping is vital to cope with anoxia by importing energy-rich compounds; however it is not vital for pH-regulation. Whereas the initial acidification is not due to energy shortage, subsequent uncontrolled acidosis occurring in concert with a general gradient breakdown damages the cell but may not be the primary event.
Keywords: Acclimation, anoxia tolerance, anoxia intolerance, apoplast, buffering, co-transport, ethanol, flooding, hypoxia, lactate, biophysical pH-stat, biochemical pH stat, proton transport
INTRODUCTION
In plants, the regulation of pH is a requirement that arises for a variety of reasons. The most basic reason is that water spontaneously ionizes with the consequence that protons cannot be removed entirely from a given solution. Unlike other ions, protons can be consumed or are produced in certain chemical reactions, with the result that the kind of nutrition determines to what extent protons may become a problem, or even a hazard, to the organism. The exact regulatory determinants and causalities are difficult to analyse (at a given moment) for any situation because pH influences a variety of processes in a plant cell or in its compartments, and at the same time H+ activity may be changed by the same processes. The ability to reverse a pH perturbation, as well as the extent and the velocity at which this is accomplished, defines the quality of pH regulation. However, the observation that a pH change is not reversed does not necessarily signal an inefficient pH regulation, but it could indicate a metabolic change requiring a new set-point. On the other hand, a pH transient does not always signal efficient regulation, but may indicate different kinetic processes overlapping. Although some plants can survive without oxygen for prolonged periods, anoxia always means an energy crisis that may affect the ability to regulate compartmental pH. In order to understand pH regulation of a cell within a tissue under such a critical energy situation, it is of utmost importance that the experimental conditions are clearly defined, a requirement that may sometimes be difficult to fulfil. This may be one reason why conflicting observations seem to prevent a common view on pH regulation under anoxia. Especially with respect to the terms ‘anoxia-tolerance’ vs. ‘anoxia-intolerance’ or ‘hypoxia’ vs. ‘anoxia’, the investigated tissues apparently have not always been equally well characterized or treated.
So far, most work on pH-regulation under anoxia or hypoxia has focussed on the cytoplasm and to some extent on the vacuole. Apart from the importance of other internal compartments, pH-regulation of a cell or especially a tissue may also depend on the status of the apoplast. There are two immediate ways for a cell to dispose of surplus protons, i.e. by their transport into the vacuole and by their export into the apoplast. Since the vacuole as an inner compartment can only store a limited amount of H+, the apoplast has to deal with the rest, unless H+ can be released to the environment or the entire organ is disposed of (e.g. leaves). In relation to the importance of the cell exterior during anoxia, the role of the apoplast will also be discussed in this article.
Only recently, Greenway and Gibbs (2003) published an excellent review on ‘mechanisms of anoxia tolerance in plants’ with a very thoughtful and inspiring section on pH regulation under anoxic conditions. Since their treatise already covers some essential parts of how cells and tissues deal with the anoxic energy crisis, this review will contain a number of slightly thought-provoking theses which hopefully will serve to stimulate discussion and help to modify some long-cherished opinions on pH regulation. Apart from the undisputed fact that plants under anoxia face an energy crisis, relevant literature reports cytoplasmic pH-regulation to be impaired through anoxia, leading to cellular acidosis and subsequent cell death. Therefore, the drop in pH must be prevented through different active (energy-consuming) counteractions. The proportion to which this is accomplished determines the degree of anoxia tolerance. The author does not entirely follow this interpretation: pH-regulation under anoxia follows the same principles as under normoxia with the difference that the cytoplasmic pH is shifted through the activity of enzymes that work optimally at that pH to produce energy.
Therefore, the new (anoxic) pH-level is not countered, i.e. no additional metabolic energy is fed into pH-regulation. Acidosis setting in after prolonged anoxia is not considered primarily as a consequence of an impaired pH regulation, but as the result of a general transmembrane gradient breakdown due to energy shortage.
In order to bring some definitions into a general terminological perspective, a section on principles of pH regulation—as the author sees them—will start this article.
PRINCIPLES OF pH REGULATION
There are a variety of processes and molecular characteristics that have the ability to set or change the pH on either side of a membrane. This can be through active or passive membrane transport of H+ but also of other ions, through transmembrane diffusion of weak acids or weak bases, by ion exchange or by biochemical reactions. Mostly, these processes take place simultaneously, which makes the characterization of the one or the other difficult at times.
pH regulation through membrane transport
The so-called ‘biophysical pH-stat’ (Smith and Raven, 1979) comprises all membrane transport that contributes to pH regulation in a given cellular compartment: H+ transporters such as H+ ATPases (pumps) and H+ co-transporters, translocation of weak acids and bases, and transport of so-called strong ions that accompany H+ translocation for the sake of charge compensation. H+ pumps are the only transporters that can actively deal with pH loads in the long term, which, however, finds its limits in the volumes and capacities of the apoplast and of the vacuole. All other transport is intrinsically passive (including the so-called secondary active co-transporters), and finally depends on the transmembrane energy gradient built up by the pumps. In a terrestrial plant, the slightly alkaline cytoplasm of a typical cell is sandwiched between the acidic apoplast and the likewise acidic vacuole. The vacuolar pH may vary considerably and can be as low as 2·0 (e.g. citrus fruits; Echeverria et al., 1992), but a value around 5·0 may be considered to be typical. As to the apoplast, recent measurements using microelectrodes (Hanstein and Felle, 1999; Felle et al., 2000, 2004) or fluorescent dyes (Hoffmann et al., 1992; Mühling et al., 1995; Kosegarten et al., 1999; Oja et al., 1999) show that pH 4·5–5·5 may be the typical range. This conflicts to some extent with earlier reports, in which considerably higher values were claimed, especially with those that are based on experiments with an infiltrated apoplast (Grignon and Sentenac, 1991; Husted and Schoerring, 1995).
The pump/leak principle
For the sake of simplicity the main focus will be on the plasma membrane H+ ATPase, although there are a variety of H+ pumps at the different cellular membranes. Traditionally, this pump has been considered a very central element of pH regulation as it constantly transports H+ from the cytoplasm into the aqueous apoplast (or cell wall). By exporting H+ it accomplishes two major tasks: it removes surplus H+ from the cell interior, and it generates a transmembrane energy gradient (ΔμH+/F; proton motive force) that serves to transport matter into and out of the cell. To reduce the intracellular acid load, net H+ transport out of the cell or tissue has to be the ultimate goal. Organisms may differ considerably in their ability to accomplish this. Whereas a (fresh-) water plant may find it easy to get rid of exported protons, a tissue cell within a terrestrial plant must export the protons into the apoplast, a rather thin layer with a volume comparable to that of the cytoplasm. Since this provides a poor H+ sink, a stimulated pump will rapidly acidify the apoplast, a scenario impressively proven by the use of fusicoccin, a phytotoxin that binds to the regulatory unit of the H+ ATPase (Marrè, 1979; Korthout and de Boer, 1994; Aducci et al., 1995). This will cause either thermodynamic stalling of the pump or favour H+ re-uptake into the cell and thus generate futile H+ circling. For this reason, H+ pumping into the apoplast of terrestrial plant tissues will have to be low to limit excessive acidification. In fact, membrane potentials measured in such tissues are indeed far less negative than in cells of fresh-water plants. For instance, the stimulation of the plasma membrane H+ ATPases in grass coleoptiles responding to auxin with hyperpolarization and apoplastic acidification that initiates elongation growth adds further support to this rationale (Peters and Felle, 1991). This leads to a provocative question: ‘do H+ pumps really regulate pH?’. Common sense says ‘yes’ because the pump removes surplus H+ from the cytoplasm and responds to pH-loads accordingly because protons are the transport substrate. This can be demonstrated through acid or base loading: cytoplasmic acidification (caused by a weak acid) leads to hyperpolarization of the plasma membrane, and alkalization (caused by a weak base) to depolarization. That is, an acid load will be responded to with increased H+ transport activity with the tendency to restore pH to the relevant set-point. On the other hand, a qualified ‘no’ also holds (Felle, 1991): not all eukaryotic cells possess an H+ pump; cells that operate a Na+ pump (e.g. mammalian cells) regulate their cytoplasmic pH quite well. Moreover, pump activation or deactivation does not, or at best only transiently, shift the cytoplasmic pH from its set-point.
A certain number of protons exported re-enter the cells. Being pumps, the H+ ATPases translocate much more H+ through the membrane than necessary to generate the membrane potential. This leads to a constant charge circulation, part of which are H+. Certain substrates use this as a ‘driving belt’ to be accumulated inside (H+ co-transport). This kind of H+ transport is the only physiologically meaningful H+ re-entry through the plasma membrane. H+ passage through specific channels is not likely at the plasma membrane, as it is energetically counter-productive. At pH 5, which is common for the apoplast, protons slipping unspecifically through the lipid bilayer are unlikely to either influence membrane potential or cytoplasmic pH considerably. Even assuming a relatively high proton permeability (Deamer, 1987), the factor 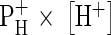 (H+-permeability × H+-activity) would not even come close to
(H+-permeability × H+-activity) would not even come close to 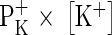 (K+-permeability × K+-concentration), because of the huge difference in free concentration, being of course on the side of K+ (Felle and Bentrup, 1977). In fact, as Fig. 1 shows, the responses of the cytoplasmic pH (pHC) to external pH changes (pHO) are relatively small and hardly exceed 0·1 pHC per pHO. Most interestingly, the same experiments carried out in the presence of cyanide (or in the absence of O2) yield even smaller responses of pHC
vs. pHO. Several questions have to be asked: are the cytoplasmic pH changes due to H+ re-entry? If yes, why are the changes at acidic external pH not much larger than those at alkaline pH; after all, pH units are logarithmic. Is this a regulatory phenomenon? If yes, the pump cannot be involved, as proven by the cyanide data (see also below). Obviously, the cytoplasmic pH responses can only partly be due to H+ import, i.e. H+ leaks are low. One explanation would be that while the transmembrane pH gradient increases the voltage gradient decreases. As such, the proton motive force will level off while pHO decreases, i.e. the actual increase in the inwardly directed driving force for H+ is only a fraction of an already high value.
(K+-permeability × K+-concentration), because of the huge difference in free concentration, being of course on the side of K+ (Felle and Bentrup, 1977). In fact, as Fig. 1 shows, the responses of the cytoplasmic pH (pHC) to external pH changes (pHO) are relatively small and hardly exceed 0·1 pHC per pHO. Most interestingly, the same experiments carried out in the presence of cyanide (or in the absence of O2) yield even smaller responses of pHC
vs. pHO. Several questions have to be asked: are the cytoplasmic pH changes due to H+ re-entry? If yes, why are the changes at acidic external pH not much larger than those at alkaline pH; after all, pH units are logarithmic. Is this a regulatory phenomenon? If yes, the pump cannot be involved, as proven by the cyanide data (see also below). Obviously, the cytoplasmic pH responses can only partly be due to H+ import, i.e. H+ leaks are low. One explanation would be that while the transmembrane pH gradient increases the voltage gradient decreases. As such, the proton motive force will level off while pHO decreases, i.e. the actual increase in the inwardly directed driving force for H+ is only a fraction of an already high value.
Fig. 1.
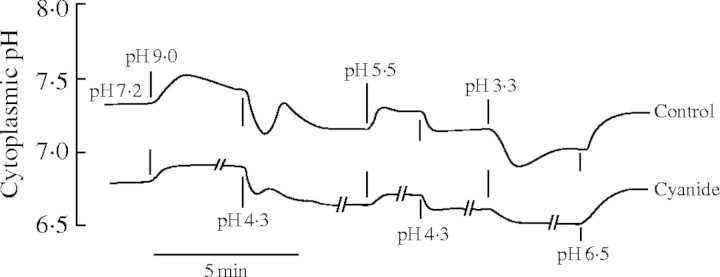
Response of cytoplasmic pH to external pH changes. Thalli of the aquatic liverwort Riccia fluitans were fixed in a Plexiglas cuvette, which was constantly perfused with a medium consisting of 1 mm KCl, 0·1 mm CaCl2 and 0·1 mm NaCl. The medium was buffered to the desired pH with 5 mm Tris and 5 mm Mes; pH 3·3 was obtained by adding HCl. The cytoplasmic pH was continuously measured with a pH-sensitive microelectrode (built and used as described in Felle, 1996) after exchanging the external solutions, as indicated. Control: normoxic conditions; cyanide: experiments carried out in the presence of 1 mm NaCN (Felle, unpublished kinetics).
The apoplast/cell wall as ion exchanger
Since cell walls contain high concentrations of uronic acids, cations are accumulated within cell walls while mobile anions are excluded. Depending on the pH, a fraction of the dissociable carboxyl groups bind protons or cations, which in the case of Ca2+ results in bridging polygalacturonic chains. When the activity of cations in the cell wall space is increased, the equilibrium is disturbed and H+ are forced from their binding sites and the aqueous phase gets more acidic: the cell wall acts like an ion exchanger (Grignon and Sentenac, 1991; Felle, 1998). Thus, the immediate pH at the plasma membrane is always a function of the cationic strength of the exterior. In a situation where this is changed, the pH of the cell wall or apoplast changes; an explicit transmembrane H+ transport is not necessary.
The strong ion difference (SID) concept
Due to the low dissociation constant of water and the requirement of charge balance, the transmembrane transport of H+ per se will not cause measurable pH changes, unless the solution is unbuffered or a so-called strong ion (cations of strong bases, anions of strong acids) moves at the same time through the membrane. This requirement has led in the past to the erroneous idea of H+/K+-ATPases or H+/K+-antiporters. In reality the K+ import (through channels) is just the charge compensation for the exported H+. In reverse, net movements of strong ions across a membrane may shift pH, i.e. K+ import into a cell would cause alkalization (Felle, 1989). The validity of this concept for a biological system has been demonstrated by Ullrich and Novacky (1990) through demonstrating that a fusicoccin-stimulated pump would change the cytoplasmic pH depending on the salts used in the medium. This issue is crucial to our understanding of pH regulation and the interested reader is referred to more basic treatises (Stewart, 1983; Good, 1988; Guern et al., 1991; Gerendas and Schurr, 1999; Greenway and Gibbs, 2003).
Cytoplasmic and apoplastic buffering
Since cytosolic buffering is based on dissociation/association of compounds already present and a priori does not require additional energy input, sudden pH perturbations can readily be absorbed to a large extent. There are some natural buffers operative at pH 6–8, e.g. phosphate compounds (pKa = 6·8–7·0), bicarbonate (pKa = 6·4), amino acids (histidine, pKa = 6·0) and several organic acids. The buffer capacity of the cytoplasm is somewhere between 20 and 100 mEq H+ per pH unit (Kurkdjan and Guern, 1989). Buffers can curb sudden pH perturbations, but get exhausted and have to be ‘recharged’, i.e. sooner or later the protons produced will have to be metabolically consumed, exported or stored elsewhere. Which of these strategies is followed depends on the plant, the tissue and of course the environment. Organic acids, being weak acids, may cross membranes quite well in their uncharged form, whereas their less membrane-permeable anions use anion channels. This has important implications for cellular H+ movements between the compartments (with different pHs) and thus for the pH-stat.
Apoplastic buffer capacity is around 5 mEq H+ per pH (Hanstein and Felle, 1999; Oja et al., 1999; Felle and Hanstein, 2002). This has profound consequences: since in many tissues the apoplastic water film is of a similar volume as the cytoplasm of the neighbouring cell, ion activities (including H+) of the apoplast may change rapidly and substantially (see H+ pumping).
The biochemical pH-stat
The biochemical pH-stat constitutes the backbone of intracellular pH regulation. It comprises a variety of H+-consuming and H+-producing reactions that are catalysed by pH-dependent enzymes, whereby some reactions contribute more than others to the pH-stat. If there is a point of intersection in the pH-activity curves of two enzymes involved in forming and removing a carboxyl group from a shared metabolite, then these two enzymes have the potential to form a pH-stat. Thus, the biochemical pH-stat consists of a set of carboxylating and decarboxylating enzymes with overlapping pH-optima in the physiological pH range. Of the models proposed, the one by Davies et al. (1974) and Davies (1986), i.e. the combination of malic enzyme and phospho-enolpyruvatecarboxylase (PEPC), has been the most predicative and popular: when the cytoplasmic pH shifts towards the acidic range, malic enzyme with its optimum in the acidic range will decarboxylate more malate resulting in a pH shift towards the alkaline range. Alternatively, when the cytoplasmic pH shifts towards the alkaline range, the PEPC, having its optimum on the alkaline side of the target pH to which the cytoplasm is to be adjusted, produces more oxalacetate, which in turn is transformed to malate by malate dehydrogenase. Since the malate produced is a strong acid, the pH shift is nullified. Although the Davies model has been challenged here and there, e.g. by pointing out that malate synthesis through PEP carboxylase and malate dehydrogenase does not produce but consumes a H+ near neutral pH (Sakano, 1998), and that malate synthesis occurs too slowly to be useful in pH regulation (Gout et al., 1993), the basic principle of a biochemical pH-stat is not under debate. Sakano (1998, 2001) presents a concise revised hypothesis of the biochemical pH-stat as an alternative model. Since this does not influence the discussion of pH regulation under anoxia, it will not be discussed here.
Is there a master pH control?
Very unlikely! With respect to our understanding of pH regulation under different conditions or within different cellular compartments, this may be a crucial question. pH regulation must be understood as the total of interactions of biochemical H+-depending processes. Their activities by consuming or producing H+ determine the actual pH set-point of a given compartment, not a separate control unit. In a situation where the basic metabolism is changed for any reason, the set of enzymes involved very likely will have other pH optima and thus will change the pH set-point in the particular compartment. All other regulators such as membrane transport may have the potential to temporarily shift the pH from its set point, but mainly serve to remove pH loads or to build up electrochemical pH gradients (pumps).
pH-REGULATION UNDER ANOXIA
In principle, there is no good reason why pH regulation under anoxia should function in a different way than it does in the presence of sufficient oxygen. However, since the anoxic pH level is generally lower, and hence a variety of biochemical reactions get activated and/or deactivated, the interplay of regulatory forces is altered as well. A major problem of pH regulation under anoxia may be the shortage of energy. Even if we do not consider the H+ pumps as directly regulating the pH, the upkeep of all transmembrane gradients depends crucially on their activity. Depending on the degree of tolerance towards anoxia the available energy either runs short or is kept at a certain level through the anaerobic catabolism. Wherever possible, such measures will, for some time, keep the most essential cellular processes working on a low, life-maintaining level. If the oxygen supply does not improve, the cellular system sooner or later will run into a non-equilibrium, with the consequence being a progressive loss of transmembrane gradients. Although this also involves pH-gradients, it is not a principal problem of pH-regulation.
The anoxic pH switch and its potential cause
A heavily discussed early phenomenon of anoxia or hypoxia is the cytoplasmic pH response. Within seconds after imposing hypoxia or anoxia to a cell, or following the application of inhibitors of oxidative phosphorylation (cyanide, oligomycin, antimycin and the like), cytoplasmic pH rapidly drops by several tenths of a pH unit (e.g. Sanders and Slayman, 1982; Roberts et al., 1984a; Fan et al., 1988; Menegus et al., 1991; Fox et al., 1995; Felle, 1996; Gout et al., 2001). There may be a slow partial recovery of pH (e.g. in rice; Menegus et al., 1991), but for as long as the suboptimal oxygen conditions last, the pH remains below the normoxic set-point. Following a period of relative stability (hours, days), the cytoplasmic pH will continue to fall, depending on the degree of tolerance towards anoxic conditions. Thus, we have to distinguish between a short-term and a long-term acidification. Whereas the latter obviously is a consequence of continuing energy shortage, the former seems an immediate consequence of the switch from aerobic to anaerobic metabolism, but can be prolonged by acclimative adaptive measures. At this point, two questions have to be asked: what causes this acidification, and why is this pH shift not reversed to the normoxic value? With respect to the first question, there are a variety of possibilities, some of which are subject to controversial discussions in the literature: (1) pump deactivation, accompanied by subsequent H+ leakage into the cytoplasm from outside or from the vacuole. (2) Anion channel activation with simultaneous cytoplasmic acidification and membrane depolarization. (3) Production of lactic acid. (4) Switch in metabolism requiring a new pH set-point.
Proton pump deactivation and subsequent H+ leakage from outside and the vacuole
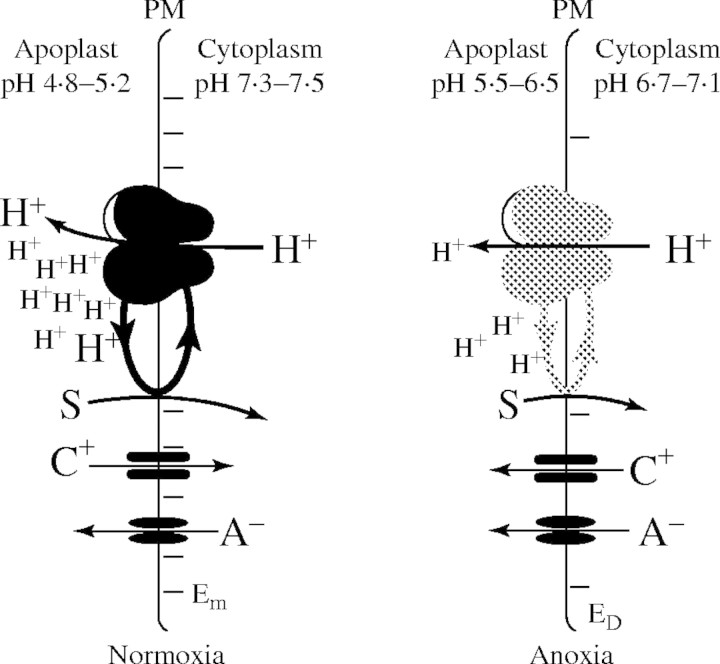
Under anoxia, H+ transporters function in the same way as in normoxia with the difference that the available energy across membranes has become scarce because of cessation of oxidative phosphorylation. H+ pumps are major consumers of ATP and thus contribute to the cytoplasmic decline in ATP very strongly. As such, following an anoxic shock, H+ pumps can work with low activity only, but may not work at all. Their contribution to pH regulation is therefore insignificant, especially under anoxia. There is a widespread opinion that after deactivating the plasma membrane H+ pump the cytoplasm acidifies because of H+ leakage into the cell. Undoubtedly, even in the depolarized state, there is a considerable inwardly directed H+ driving force. More importantly, however, is the fact that due to the pump deactivation the transmembrane H+ circulation becomes a very thin streamlet, consisting of only a small fraction of the normoxic H+ driving belt into the cells (Fig. 2). A comparison of the H+ co-transported sugars (or amino acids) in normoxia with the drastically reduced amounts measured under anoxia (about 5 %) supports above argument. H+ pumps and H+ co-transporters are a functional unit: one changes its efficiency with the other, i.e. deactivation of the pump necessarily means less H+ re-entry. This is also supported by the observation that under anoxia the response of cytoplasmic pH to changes in external pH is not increased but rather decreased (Fig. 1).
Fig. 2.
Transmembrane proton circulation under normoxia and anoxia. The plasma membrane H+ ATPase (H+ pump) not only generates the membrane potential (for which only few charges have to be translocated) but a strong H+ current. Whilst one portion of the H+ exported contributes to pH regulation, the other part of the H+ exported re-enters the cytoplasm accompanied by substrates (sugars, amino acids, anions). The efficiency of this transmembrane ‘H+-driving belt’ depends on the transport yield of the pump, but also on the availability of substrate. Under anoxia the pump works with less activity, which leads to less H+ export. As a consequence, the apoplast alkalizes, the resting potential (Em) drops close to the so-called diffusion potential (ED), and the availability of H+ for the symporters is drastically reduced. Due to the depolarized membrane, the inwardly directed driving force for cations (C+) is inverted, cations (e.g. K+) leave the cell and, according to their permeabilities, generate a diffusion potential. In a situation where the pump is shut down completely, the yield of the symporters drops further because for every charge symported, charge that would otherwise build up the diffusion potential is lost.
As across the plasma membrane, a considerable proton-motive force exists at the tonoplast, but with a strong poise on the pH gradient. The anoxic decline in energy will certainly affect trans-tonoplast ion (and H+) transport. Since for the tonoplast the same principles hold as for the plasma membrane, a deactivation of the pumps will likewise reduce the yield of the co-transporters (compare with Fig. 2). It is highly doubtful whether this reduced H+ leakage contributes considerably to the short-term cytoplasmic acidification following the onset of anoxia. As shown in Table 1, Roberts et al. (1984b) demonstrate an increase in vacuolar pH from 6·0 to 6·2, while cytoplasmic pH drops from 7·4 to 6·8. Gout et al. (2001) found only ‘minimal’ increases of vacuolar pH, i.e. a change from 5·70 to 5·74. Assuming similar buffer capacities for vacuolar sap and cytoplasm (Guern et al., 1986) and a volume ratio of 10 to 1, the changes in vacuolar pH measured by the two groups are not that small and could well be causally correlated. On the other hand, the pH changes given in the other examples in Table 1 are too far off to be brought into a reasonable range of vacuolar H+ leakage. In conclusion, it is not evident that H+ leaks from the vacuole are a main cause for the short-term acidification. In the long-term, however, H+ leaks from the vacuole and possibly from the apoplast must be considered as factors for cytoplasmic acidosis as soon as energy supplies become insufficient to maintain transmembrane gradients. Thus, the considerable vacuolar pH changes that have been measured in some studies cannot, for the above reasons, stem from H+ fluxes into the cytoplasm, but must arise from biochemical shifts within the vacuole.
Table 1.
Examples of vacuolar and cytoplasmic pH changes measured under anoxia, hypoxia or chemical anoxia (cyanide). H+ ratio denotes the ratio of the changes in free [H+] in vacuole vs. cytoplasm assuming equal buffer capacities
| Plant/tissue |
Vacuolar pH |
Cytoplasmic pH |
H+ ratio |
Reference |
|---|---|---|---|---|
| Maize root tips | 6·0 to 6·2 | 7·4 to 6·8 | 3·1 | Roberts et al., 1984b |
| Rice shoots | 5·0 to 6·1 | 7·4 to 7·1 | 236 | Menegus et al., 1991 |
| Wheat shoots | 4·6 to 5·1 | 7·4 to 6·5 | 62 | Menegus et al., 1991 |
| Maize root hairs | No significant changes | Brauer et al., 1997 | ||
| Potamogeton | 5·6 to 6·2 | 7·46 to 7·26 | 76 | Dixon, 2001; Summers et al., 2000 |
| Acer pseudoplatanus suspension cultured cells | 5·7 to 5·74 | 7·5 to 7·1 | 3·6 | Gout et al., 2001 |
Activation of anion channels
Although membrane depolarization and cytoplasmic acidification appear to occur simultaneously after the onset of anoxia, both processes are clearly not related. This can be demonstrated by separating acidification and depolarization: oligomycin (a poor inhibitor of oxidative phosphorylation) acidifies the cytoplasmic pH of Medicago root hairs by about 0·6 units (Felle, 1996). This already occurs at concentrations where cytoplasmic ATP levels are still sufficiently high to permit enough pump activity to hyperpolarize the plasma membrane. Subsequently added cyanide (a strong inhibitor of oxidative respiration) rapidly depolarized the plasma membrane without further acidification. This separation rules out activation of anion channels as a primary cause of early acidification of the cytoplasm, because the export of the organic acid anions means the export of negative charge. It adds further evidence to the notion that pump deactivation and subsequent H+ leaks cannot be the reason for the initial cytoplasmic acidification.
The lactate/ethanol hypothesis
Based on work of Davies et al. (1974), Roberts et al. (1984a) attributed the hypoxia-induced acidification in maize root tips to lactic acid formation or accumulation and argued that the stabilization of the cytoplasmic pH was due to a metabolic shift to alcoholic fermentation, mediated and pH-stimulated by pyruvate-decarboxylase, having its pH optimum in a more acid range than the lactate-dehydrogenase. This would result in a redirection of carbon flow to ethanol synthesis with no further H+ production. The hypothesis was supported by the kinetics of lactate and ethanol production, whereby the former coincided with the cytoplasmic acidification, whilst the latter started only when cytoplasmic pH had dropped to a certain level. Additional support came from the observation that a mutant, lacking the alcohol dehydrogenase, experienced severe cytoplasmic acidification and subsequent death (Roberts et al., 1984b). This view has been the basis for many valuable investigations, but it has been questioned by some workers because of several conflicting observations. Saint-Ges et al. (1991) observed a minor anoxic acidification, but lactate kept accumulating while the pH had stabilized. In rice shoots an anoxic pH drop of 0·3 to 0·4 units was accompanied by only small amounts of lactate (Menegus et al., 1989, 1991; Rivoal et al., 1989). In Catharantus roseus, Sakano et al. (1997) report an anoxic acidification of only 0·2 units that leveled off while lactic acid accumulated. It was argued that lactic acid production was not responsible for this acidification, but H+ influx from outside through ‘H+ channels’ or co-transport, a view that has been rejected in this review for reasons given above. Ratcliffe (1997, 1999) did not question the model in principle, but emphasized that it does not provide a complete understanding of pHC regulation under anoxia, because three independent analyses showed that lactate production was either insufficient, just right or too great to explain the initial drop in cytoplasmic pH. Moreover, the model assumes that lactate production is a transient process. While this is true in some species, lactate production can also be negligible, as in rice shoots (Menegus et al., 1991), or prolonged as in Limonium (Rivoal and Hanson, 1993) and in acclimated maize root tips (Xia and Saglio, 1992; Xia and Roberts, 1994). Rivoal and Hanson (1994) showed that over-expression of lactate dehydrogenase at a level 50 times higher than in control plants had no effect on the balance between lactate and ethanol production in tomato roots. Also, over-expression of pyruvate decarboxylase had no effect on the pH response. Tadege et al. (1998) argued against the Davies–Roberts hypothesis, showing that ethanolic fermentation was very differently regulated in different organs (leaf, pollen, root). They could not find significant lactate accumulation in tobacco roots in the early stages of O2 deprivation, and thus found it unlikely that a LDH/PDC pH-stat regulates ethanolic flux in tobacco. It was further argued that PDC may not be under strict pH control, because in tobacco roots and transgenic leaves there was a high PDC concentration but low ethanol flux in normoxia. This would require a very sharp pH optimum (as indeed shown in pea seed). However, in Z. mobilis PDC has a very broad pH range with activities varying less than 20 % between pH 7·5 and 6·0. Thus, if fluxes were regulated through the pH dependence of PDC, transgenic tobacco should produce ethanol during normoxia, which is not the case. It was suggested that pyruvate concentration determines the PDC activity and thereby ethanolic flux. Finally, Tadege et al. stressed again the importance of ethanolic fermentation for survival under anoxia for energy production, but rejected the idea that PDC is regulated or triggered by acidification and favoured a model in which substrate availability is the most important factor. This argument is not quite clear, because there is no doubt that acidification causes increased ethanol production as long as the PDC is pH-dependent, unless one argues that ethanol production is triggered at a certain pH. Kato-Noguchi (2000) evaluated the importance of lactate for the activation of ethanolic fermentation in lettuce roots in anoxia and found that neither activity of LDH nor lactate concentrations increased significantly in real or chemical anoxia (e.g. in the presence of cyanide), whereas the activities of ADH, PDC and subsequently the concentrations of ethanol and acetaldehyde were increased. It was argued further that ethanolic fermentation may be activated without preceding activation of lactate fermentation and may not be regulated by oxygen concentration directly. Another problem seems to be that anoxia-tolerant plants or tissues, and to some extent also acclimated plants, partly restore the pH. If there was a pH-dependent switch from lactate to ethanol synthesis, then production would switch back to lactate synthesis which should acidify the cytoplasm again, unless the reason of increased lactate production—anoxia—was gone.
Summarizing the different observations (the list above not being complete) it becomes obvious that, comparing the different plants and tissues, a ubiquitous quantitative correlation between lactate production/accumulation and cytoplasmic acidification does not exist. While the lactate/ethanol principle holds for some plants or tissues, it apparently does not do so for others. The reasons for the discrepancies may in fact be variabilities between the investigated cells, tissues or plants. Less satisfactory, different habitats or even circumstantial (experimental) imponderables may also add to the confusion. Thus, a number of the arguments raised are probably based on in vitro activity measurements from the enzymes involved, the pH-dependences of which may be quite different in vivo.
Nucleoside triphosphates
Gout et al. (2001) picked up an earlier idea (Reid et al., 1985) that proton-releasing metabolism of nucleotide-trisphosphate (NTP) was directly related to the initial cytoplasmic pH change, and that quantitatively the 0·7 pH unit acidification corresponded roughly to the liberation of protons following the hydrolysis of approx. 2·5 mm NTP. It was demonstrated that the subsequent recovery of the NPT-pool coincides well with the pH kinetics. The authors concluded from their tests that the H+ ATPase is arrested (e.g. co-transporters do not function under anoxia), but they did not think that this was due to NTP-decrease. They observed in cyanide-treated cells (200 μm) that the cytoplasmic pH was fully reset in the absence of NTP-pool recovery. The conclusion that the pump was working then seems doubtful, assuming (without evidence) that it was the pump that brought back the cytoplasmic pH. Apart from this, it would contradict their observation that co-transport did not function under anoxia. Felle (1996) found in Medicago sativa root hairs that recovery of cytoplasmic pH started well before repolarization, i.e. prior to the reactivation of the pump. Since pump activity and ATP-levels are closely related, this observation would argue against the above conclusion.
A new pH set-point under anoxia
Anoxia leads to interruption of oxidative phosphorylation and hence to an energy deficit. Undoubtedly, prolonged energy shortage will lead to further cytoplasmic acidification, simply because lack of energy means the breakdown of all transmembrane gradients sooner or later, and hence cell death. But is the initial fast acidification caused the same way? As we have seen so far, probably not. Is it an acid load that is superimposed, and will this load have to be responded to by regulatory forces or does the pH change come from within? Is it the altered metabolism, and hence the network of proton-consuming and proton-producing processes, that requires a different pH basis to work for the sake of energy production? In the instant of cutting off the respiratory chain from the rest of the metabolism, the energy flow gets redirected, and the anaerobic network starts to work with the poise on reactions that lead to a modest energy harvest, which includes the production of lactate, ethanol and alanine. Plants seem to differ in their poise from which they finally gain their energy predominantly anaerobically. Accordingly, this requires a cytoplasmic pH that suits the respective biochemical equilibria (like under normoxia). This may explain the different pH values found under anoxia that could not be brought into correlation with lactate levels. Any biochemical network functions optimally within fixed margins, which includes a certain pH. Following this rationale, it is not surprising that there is no clear and ubiquitous correlation between acidification and lactate production, because lactate concentration is only one factor. Despite its potential to acidify the cytoplasm, the H+ production through lactate fermentation probably only accompanies the metabolically initiated pH shift without being a dominant acidifying factor. There are plenty of examples where the pH set-point of a cell is changed wherever an altered metabolism requires it. Such changes in metabolism that may arise from a change in life cycle or environmental conditions are well known, especially from the animal kingdom (Table 2). The data in the table have to be read in a way that the biological event requires a certain pH, i.e. the metabolism only runs optimally when a new pH is set and kept there by pH regulation of biochemical origin. Resetting the pH would not make sense, because the respective process would then not take place.
Table 2.
Examples of cytoplasmic pH changes associated with changes in cellular metabolism and development
| Organism |
Event |
pH change* |
Reference |
|---|---|---|---|
| Lytechinus pictus | Fertilization of egg | +0·4 units | Shen and Steinhard (1978) |
| Lytechinus pictus | Mobility initiation of spermatozoon | +0·5 units | Lee et al. (1983) |
| Artemia salina | Arousal from dormancy | Transient alkalinization | Busa et al. (1982) |
| Xenopus laevis | Fertilization of egg | +0·3 units | Webb and Nuccitelli (1982) |
| Pichia pastoris | Spore germination | +1 unit | Barton et al. (1980) |
| Physarum polycephalum | Cell cycle | +0·4 units | Morizawa and Steinhardt (1982) |
pH increase is indicated by ‘+’.
A similar situation exists under anoxia when the tissue involved is suddenly transferred into a situation wherein the normal metabolism does not work and an alternative network takes over to allow at least some energy production. Since the interplay of these reactions differs from those in normoxia, a new cytosolic pH has to be set. For reasons given above, this value may slightly differ from cell to cell but does not have to be very different from the normoxic value (for example in Potamogeton; Summers et al., 2000). Thus, it may be an error to expect that cells under anoxia try to reverse the cytoplasmic acidification at all costs. Why should they do that? Any measure to reverse pH to the normoxic value would not alter the cause, i.e. the oxygen shortage, and therefore is a waste of energy. Still, as data prove, the cytoplasmic acidification following anoxia may or may not be partly transient: Xia and Roberts (1994) showed that hypoxically pretreated maize root tips held their pH longer than non-pretreated tissues; Roberts et al. (1992) showed that in 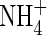 -pretreated maize root tips cytoplasmic pH slightly recovers; Fox et al. (1995) demonstrated that it depends on external pH whether cytoplasmic pH partially recovers or not: at an external pH of 4 they did not recover, whereas they did at a less-physiological pH of 10. Felle (1996) gave evidence of a constant anoxic pH, although the length of measurements was considerably shorter than all the others cited so far. Gout et al. (2001) reported that sycamore cells recovered to some extent, but did not when pretreated with glycerol. Taken together, these examples show that whether pH following anoxia is transient or not may depend on the pretreatment, but it probably also depends on how fast anoxia is imposed on the tissue. Transient kinetics may not be regulatory but can also be the result of two or more processes that overlap. Moreover, pH recovery that proceeds over hours is hardly the result of regulation, but rather reflects the adjustment from a non-equilibrium situation.
-pretreated maize root tips cytoplasmic pH slightly recovers; Fox et al. (1995) demonstrated that it depends on external pH whether cytoplasmic pH partially recovers or not: at an external pH of 4 they did not recover, whereas they did at a less-physiological pH of 10. Felle (1996) gave evidence of a constant anoxic pH, although the length of measurements was considerably shorter than all the others cited so far. Gout et al. (2001) reported that sycamore cells recovered to some extent, but did not when pretreated with glycerol. Taken together, these examples show that whether pH following anoxia is transient or not may depend on the pretreatment, but it probably also depends on how fast anoxia is imposed on the tissue. Transient kinetics may not be regulatory but can also be the result of two or more processes that overlap. Moreover, pH recovery that proceeds over hours is hardly the result of regulation, but rather reflects the adjustment from a non-equilibrium situation.
Accepting that anoxia causes a switch to a new set-point of cytoplasmic pH, any perturbation from this (new) value should be countered to bring the cytoplasmic pH back to the anoxic pH and not to the normoxic value. This requirement is indeed supported by data: Fig. 3 shows that (1) pre-acid loading does not change the new set-point (shown below), i.e. when the cytosol is acidified through a weak acid, subsequent chemical anoxia does not change the cytosolic pH by the same margin, but only to the pH of the new set point. (2) In a given plant, anoxia, cyanide, oligomycin and antimycin A all cause the same drop in cytosolic pH. Indirect support for the idea of a new pH set-point comes from the original data of Davies et al. (1974), who demonstrated that the pH optima of LDH and PDC intersected at pH 6·8.
Fig. 3.
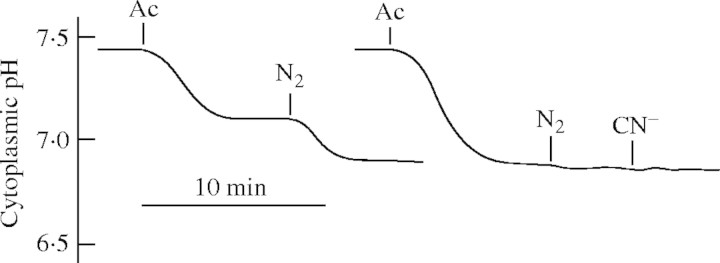
The new pH set-point under anoxia is well regulated. Cytoplasmic pH of Medicago sativa root hairs, measured with a pH-sensitive microelectrode, responding to 2 or 5 mm acetic acid (Ac) followed by N2 and 1 mm NaCN (CN−). External pH was held at 6·0 (Felle, unpublished kinetics).
Mitigation of the acid load
We have to distinguish between reversing the pH change and preventing further acidification. What can a cell under anaerobic conditions do to mitigate the acid load?
H+ pumping
Residual pumping at the plasma membrane has been detected in hypoxically acclimated maize root tips (Xia and Roberts, 1996), but the authors conceded that the H+ pump is but one, not dominant component in a complex system that regulates cytoplasmic pH (also reviewed by Guern et al., 1991) and showed that at physiologically meaningful external pH the potential contribution of the pump would be negligible or marginal. Still, the observation of residual pumping is important because it helps to fuel H+-driven co-transport of metabolizable substrates, which is essential for survival under anoxia. For instance, stored sugars can only be exploited energetically when some transport activity from the apoplast into the cytoplasm is in action. In a situation where the pump is completely shut down, the K+ dominated diffusion potential can only drive a rather limited H+ co-transport with very low yield, because with each substrate molecule co-transported the diffusion potential becomes less negative and the driving force for H+ co-transport is gradually reduced. One way out to increase the yield would be a change in stoichiometry from 1H+ per ATP hydrolysed to 2H+. This would increase the H+ turnover at half the energy cost, an option that has not been demonstrated yet.
Any H+-transporting pump responds to cytoplasmic acidification with increased activity (H+ is the transport substrate), so Ratcliffe (1997) asked the question why the H+ pumps do not respond to anoxic acidification with an increase in activity? Actually, the pumps do respond, as shown by Felle (1996), with a clear hyperpolarization responding to oligomycin. The response was measurable because the pump was not deactivated yet, and the acidification had preceded pump deactivation. But even in cases where no change in membrane potential was observed, the pump current could still have been increased, thus providing sufficient charges for co-transport. To demonstrate such stimulation, measurements of transmembrane electrical current or external pH changes would have to be done, as membrane potential measurements alone do not provide sufficient information.
At the tonoplast two H+ pumps are working in parallel. Carystinos et al. (1995), and Gibbs and Greenway (2003) suggested a switch from V-ATPase to V-PPiase when cells experience anoxia. A key argument was that with respect to free energy gains, hydrolysis of PPi is favoured to ATP when cytoplasmic pH drops as it does under anoxia. Although the actual impact on pH-regulation is hard to judge, it may be a valuable tool (at least for a time) to mitigate development of long-term cytoplasmic acidity.
Lactic acid–lactate export
As lactate is favoured by a variety of research groups to be mainly responsible for cytoplasmic acidification under anoxia, the observation of lactic acid loss from anoxic cells (Xia and Saglio, 1992; Rivoal and Hanson, 1993; Xia and Roberts, 1994) has lead to the hypothesis that export of this metabolite is an essential feature of intracellular pH regulation, as in anaerobic tissues of animals (Poole and Halestrap, 1993; Wang et al., 1996). Whether the lactate–lactic acid extrusion is indeed a real possibility for mitigating the anoxic acid load depends entirely on the apoplastic (external) pH (Fig. 4): (1) around a neutral cytoplasmic pH and an apoplastic pH of 5–6, efflux of protonated lactic acid (pKS = 3·08) appears too small to reduce any acid load, even at an assumed lactic acid concentration of 10 mm. (2) If the apoplastic pH is assumed to be around pH 7, in spite of the low [LH], LH-export will continue until [LH] equals [L−] ≈ 10 mm, which in fact should acidify the apoplast and alkalize the cytoplasm. (3) L− could leave the cells through anion channels, which could open pH-dependently. Once open, these channels will release anions rapidly into the apoplast, whereby Cl−, 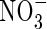 , etc. would depolarize the cells, whereas organic anions would alkalize the apoplast and slightly acidify the cytoplasm. (4) For bacteria, Michels et al. (1979) proposed a H+-symport for the excretion of metabolic end-products. Adopting this model for higher plants (Greenway and Gibbs, 2003), it would only work at an external pH of 7 or higher, as in option (2). This would apply for cells adjacent to the soil, but not so for inner cell layers because the path through the apoplast is long and has a high diffusion resistance. Thus, in the acidic apoplast of the inner cell layers excreted lactic acid would accumulate and therefore block, or at least slow down, further export. Moreover, at an external pH of 5 (e.g. Felle, 1998) such transport is up against a transmembrane H+ gradient of about 2 pH units (equivalent to 120 mV or a mass gradient of 100). In conclusion, whether lactic acid–lactate export has the potential to mitigate the acid load on the cytoplasm and thus be a means of pH regulation under anoxia remains to be shown. The use of metabolic energy for such transport appears unlikely.
, etc. would depolarize the cells, whereas organic anions would alkalize the apoplast and slightly acidify the cytoplasm. (4) For bacteria, Michels et al. (1979) proposed a H+-symport for the excretion of metabolic end-products. Adopting this model for higher plants (Greenway and Gibbs, 2003), it would only work at an external pH of 7 or higher, as in option (2). This would apply for cells adjacent to the soil, but not so for inner cell layers because the path through the apoplast is long and has a high diffusion resistance. Thus, in the acidic apoplast of the inner cell layers excreted lactic acid would accumulate and therefore block, or at least slow down, further export. Moreover, at an external pH of 5 (e.g. Felle, 1998) such transport is up against a transmembrane H+ gradient of about 2 pH units (equivalent to 120 mV or a mass gradient of 100). In conclusion, whether lactic acid–lactate export has the potential to mitigate the acid load on the cytoplasm and thus be a means of pH regulation under anoxia remains to be shown. The use of metabolic energy for such transport appears unlikely.
Fig. 4.
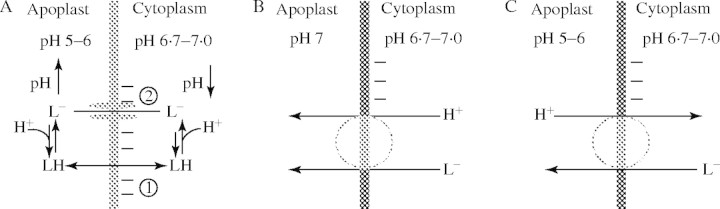
Mitigation of the anoxic acid load through lactic acid export? Several possibilities exist for how lactic acid in its protonated or anionic form may leave the cell. (A) The pathway that any weak acid would follow dependent upon its pKS and the existing transmembrane pH gradient is indicated by (1). At a cytoplasmic lactate concentration of 10 mm, a cytoplasmic pH of around 7 and an apoplastic pH between 5 and 6, too little LH would exit to mitigate the acid load. (2) L− may leave the cytoplasm through activated anion channels; however, this would not mitigate the acid load, but rather increase it because L− would be replaced from LH and thus result in an acidification. (B) H+/L− symport. At an external pH of 7 the driving force for such a transport would be solely the L− concentration difference; as there is no pH gradient, this will stop as soon as external and internal L− are about equal. (C) In a situation where the external pH is clearly below 7, the mechanism shown in (B) would not work. The driving forces would favour H+/L− antiport which, however, would rather acidify the cytoplasm.
Nitrate
Roberts et al. (1985) show that an increased nitrate supply prolongs the survival of anoxic maize root tips and, as Reggiani et al. (1985, 1993) point out, nitrate is utilized during anaerobic germination of rice. Both of these observations suggest nitrate assimilation is a significant factor for pH-regulation under anoxia and evidence in favour of this idea has been brought forward for rice coleoptiles (Fan et al., 1995, 1997). However, the role of nitrate assimilation is likely to extend beyond the simple stoichiometric consumption of H+ because the pH-dependent properties of nitrate reductase suggest that nitrate assimilation may be able to act as a biochemical pH-stat (Kaiser and Brendle-Behnisch, 1995). As it is usually expected that the nitrate reductase activity under anoxia would counteract cytoplasmic acidosis, Stoimenova et al. (2003) compared the effects of root anoxia on a tobacco wild type (WT) and a transformant lacking root nitrate reductase (LNR-H). LNR-H roots produced substantially more ethanol and lactate than those of WT, and that the anaerobic metabolism of LNR-H was more acidifying than that of WT, a conclusion based on the H+/NADH ratio. In contrast to this, NMR-pH measurements revealed the opposite, namely a greater acidification of the cytoplasm by the mutant lacking nitrate reductase. Although the ameliorating effect of nitrate on plants surviving during oxygen shortage has been documented or suggested repeatedly (Arnon, 1937; Roberts et al., 1985; Reggiani et al., 1985, 1993; Fan et al., 1988; Saglio et al., 1988; Müller et al., 1994; Kaiser and Brendle-Benisch, 1995; Ratcliffe, 1999) the idea that nitrate is a significant factor in pH-regulation under anoxia may be hampered by two problems. Firstly, it depends on the nitrate availability in vivo, and secondly, uptake of nitrate as well as reduction are extremely energy-consuming, a situation which would add to the energy problem experienced under anoxia.
Acidosis: a primary threat to the cell?
In addressing this question, compartments like the vacuole are exempt. Vacuolar saps can be very acidic. However, under normoxia acidic vacuoles do not represent a threat to the cytoplasm, nor do pH values of around 2 or lower lead to disintegration of the membrane structure and hence to leakiness; as, for example, shown for citrus fruits, whose vacuoles may have pH values as low as 2 (Echeverria et al., 1992). The steep pH gradient towards the cytoplasm can only be built up by a H+ ATPase that pumps 1H+ per 1ATP hydrolyzed, and it must be held by a rather H+-impermeable tonoplast. H+ leakage following longer periods of anoxia that is accompanied by severe cytoplasmic acidosis is thus due to membrane damage caused through lack of renewing of membrane protein or lipid constituents. Examples given in the literature (reviewed by Greenway and Gibbs, 2003) that acidosis itself is responsible for cell death are not really convincing: (1) anoxic maize tips imposed at pH 4 in citrate buffers died, while those at pH 6 did not (Xia and Roberts, 1996). This is not surprising: while at pH 6 the acid load through citrate was minimal and (calculating with a cytoplasmic buffer capacity of 50 mm per pH unit) just reduced the cytoplasmic pH by 0·2–0·3 units, the acid load of 2 mm citrate at pH 4 was fatal, because the cytoplasmic citrate concentration rose to a final 110 mm, resulting in a cytoplasmic pH of 5·8–5·9. Since weak acids not only move into the cytoplasm but into all compartments and change the pH therein, a system breakdown is not surprising! Even without anoxic conditions cells would not survive such a weak acid load at pH 4 for long. (2) Whilst  -pretreated maize root tips showing a cytoplasmic pH of 7·03 survived, non-pretreated tips (pH 6·85) died (Roberts et al., 1985). Clearly, pH 6·85 is not an acidity that would result in cell death. As will be discussed below, the reasons for survival may be based on the ameliorating effect of
-pretreated maize root tips showing a cytoplasmic pH of 7·03 survived, non-pretreated tips (pH 6·85) died (Roberts et al., 1985). Clearly, pH 6·85 is not an acidity that would result in cell death. As will be discussed below, the reasons for survival may be based on the ameliorating effect of  pretreatment. However, we have to be aware that once long-term acidosis has set in, it is self-amplifying because the enzymatic functions that could renew the energy fail more and more on a declining pH curve. It is difficult experimentally to separate energy shortage from acidosis and to relate the respective impacts on the cell of either of them, because they may occur almost simultaneously. To put energy into pH regulation to reverse acidosis would not help in this situation, but on the contrary would aggravate the energy problem. The question is, can active pH regulation mitigate the cytoplasmic acidification before long-term acidosis has set in?
pretreatment. However, we have to be aware that once long-term acidosis has set in, it is self-amplifying because the enzymatic functions that could renew the energy fail more and more on a declining pH curve. It is difficult experimentally to separate energy shortage from acidosis and to relate the respective impacts on the cell of either of them, because they may occur almost simultaneously. To put energy into pH regulation to reverse acidosis would not help in this situation, but on the contrary would aggravate the energy problem. The question is, can active pH regulation mitigate the cytoplasmic acidification before long-term acidosis has set in?
pH regulation in anoxia-tolerant and acclimated tissues
Tolerance to anoxia is relevant to wetland species, rice cultivation and transient waterlogging of agricultural crops. Since all plant cells or tissues can survive anoxia for at least several hours, the distinction between anoxia-tolerance and anoxia-intolerance appears relative (Drew, 1997). The response often depends on the experimental approach, e.g. maize root tips are intolerant towards anoxic shock but show tolerance following hypoxic pretreatment. In comparing acclimated with non-acclimated tissues or anoxia-tolerant with less tolerant tissues, typical features are that the cytoplasmic pH is either kept constant at its anoxic level for longer, the change is partly reversed, or a higher pH is generally the end result. From this apparent correlation it has been inferred that cytoplasmic acidosis is the cause of serious cell damage and sooner or later will lead to cell death or, conversely, that survival of many plants under anoxia correlates with the extent of cytoplasmic acidosis. Two examples are from Xia and Roberts (1994), who showed that excised acclimated maize root tips yielded a pH 0·25 units higher than non-acclimated root tips and survived longer, and from Menegus et al. (1991) who demonstrated that anoxia-tolerant rice shoots held their anoxic pH over many hours and even partially reversed the change, whereas anoxia-intolerant wheat shoots more and more rapidly acidified after a few hours of anoxia. Whereas anoxia-tolerance is inherent to some species and genetically manifested, acclimation appears to be a process of preparing for and getting used to the anoxic situation and in principle is a more physiological approach than anoxic shock.
Greenway and Gibbs (2003) named three crucial processes that have priority for the limited amount of energy produced under anoxia and stress, which anoxia-intolerant tissues would lack the ability to direct energy preferentially to: (1) maintenance of membrane selectivity and integrity; (2) de novo synthesis of specific proteins; and (3) effective pH-stat. With respect to the last of these, it can safely be assumed that all cells have an effective pH-stat in normoxia that allows the pH to be kept at set-points in all cellular compartments. The question is, what could make pH-regulation ineffective under anoxia? An energy deficit is the very likely answer. This leads to the question, whether energy is in fact diverted into pH-regulation and whether such a process is working more effectively in anoxia-tolerant and acclimated tissues? Apart from the requirements of activation energy that lets chemical reactions proceed, additional energy is clearly not put into the biochemical pH-stat, as the metabolic network itself creates and maintains the optimal pH. In membrane transport (the biophysical pH-stat), however, primary energy must be put into H+ export. As H+ export for the sake of pH-regulation may not be the primary intention (as pointed out above), but rather the generation of a transmembrane energy gradient (pmf) to fuel transport of matter, the only reason to invest energy into primary membrane transport would be for the sake of fuelling co-transport of energy-rich compounds (arising from stored reserves or external sources) from the apoplast into the cytoplasm. Thus, the basic question has to be asked: is anoxia-tolerance really an issue of pH-regulation? Are the different pH levels observed in anoxia-tolerant vs. intolerant tissues or in acclimated vs. non-acclimated tissues really a consequence of a pH regulation working with different efficiency? Less acidification in hypoxically pretreated tissues could arise from a different energy flow that was initiated by putting the tissues into a hypoxic situation for some time. As shown, this may cause accelerated glycolysis and more effective uptake of carbohydrates, the latter being the consequence of residual pumping that may not occur after anoxic shock. Anoxia-tolerance can be improved by the ability (1) to break down available carbohydrate reserves; (2) to form and use areas where O2 can be stored, e.g. in aerenchyma (Colmer, 2003); (3) to take up and use energy-rich compounds; and (4) to perform effective long-distance transport of photosynthesates from shoot to root. From these only (3) has a relationship to pH regulation, but certainly does not contribute to mitigation of cytoplasmic acidosis.
One feature of acclimation is of course methodically based. Sudden superimposition of anoxia (within seconds) as it is tested in the laboratory is an untypical stress for a higher plant, because in nature anoxia usually sets in relatively slowly. An anoxic shock may drive the system into a low-energy state from which it may not recover. Situations like that are known from membrane transport where certain stable energy levels exist. For instance, high salt concentrations added to cells may deactivate the plasma membrane H+ ATPase with the result being a complete depolarization to the diffusion potential, whereas a gradual increase in salt concentration will leave the membrane potential more negative and thus guarantees a better pump yield. Hence, gradually decreasing the O2 partial pressure or hypoxic pretreatment may prevent such shock and bring about better tolerance, as in fact is observed through acclimation. Another problem with anoxic shock treatment is that it may not give pH regulators the possibility to respond because the conditions are out of range. As such, it may well be that due to anoxic shock primary pumps become completely inactive (with all the associated consequences), whereas in acclimated cells co-transport works better due to residual pumping and more energy is gained. Such a scenario might have been observed by Xia and Roberts (1996) who showed that in acclimated maize root tips fusicoccin stimulated the H+ pump whereas non-acclimated tissues did not respond, very likely because the pump was inactivated.
A role for the apoplast under anoxia?
Little attention has been paid to the apoplast so far with respect to anoxia. The apoplast is an important space for storage, mineral nutrition, certain enzyme activities, stress reactions and defence responses (Sattelmacher, 2001). It consists of an aqueous phase, which (apart from the xylem) is usually a rather thin film adjacent to or within the cell walls. Whereas most transport in and out of cells has to cross this aqueous film and is influenced by its ionic milieu, in the non-aqueous part gas exchange takes place. The impact flooding has on both phases may be of importance for the degree of tolerance a tissue has towards anoxia.
Little is known of the apoplastic response to low oxygen conditions. Felle (1998) demonstrated that the apoplast of maize roots responded to cyanide (+SHAM) with an increase in pH from 5·6 to 6·2, while the membrane potential simultaneously dropped from −175 to −100 mV. Using the same inhibitor, Felle et al. (1998) showed that the root surface (root hair space) of Medicago sativa alkalized from 6·5 to 6·9. The apoplast of barley leaves responded to N2-flushing with a pH change from 5·1 to 5·3 (Felle, unpubl. res.). Although these are only a few studies, it appears that the apoplast responds to anoxic conditions with a pH increase. Since the experiments only lasted minutes, the observed pH increases may reflect changes in membrane transport; with anoxia lasting longer, the effects may be more drastic. What is the impact of such changes? Obviously, the driving force for co-transported substrates becomes smaller. However, this may not be too critical because only a fraction of it is affected, leaving the driving force still inwardly directed and the apoplast still sufficiently acidic. Although roots grow in soil pHs that may differ widely, the apoplastic pH seems to be regulated to around 5. Why is this value so important? Let's assume, quite reasonably, that the entire plant is not fully submerged and that there are still leaves that can proceed with the photosynthetic production of carbohydrates. When these are transported to the roots they can only be of use when the apoplast is functional, i.e. with a pH at which substrate transport (proton motive force) can proceed effectively with the small amount of available energy left, and at which enzymes, such as the acid invertase that splits sucrose into glucose and fructose, can work close to their pH optima. The same rationale holds for carbohydrates that are reactivated from their stores. An acidic apoplastic pH can only be maintained with a pump working at least residually. Because not many H+ are needed to acidify the apoplast this is not a problem, at least not for the inner tissue cells, the apoplasts of which are not too close to the less-controllable soil conditions. When energy levels within the cells become too low to keep up H+ export, the cells may—for a limited time—use the transmembrane cation (mainly K+) gradient to fuel some H+-symport of metabolizable compounds (Fig. 2). It has to kept in mind, however, that with every molecule imported the driving force drops.
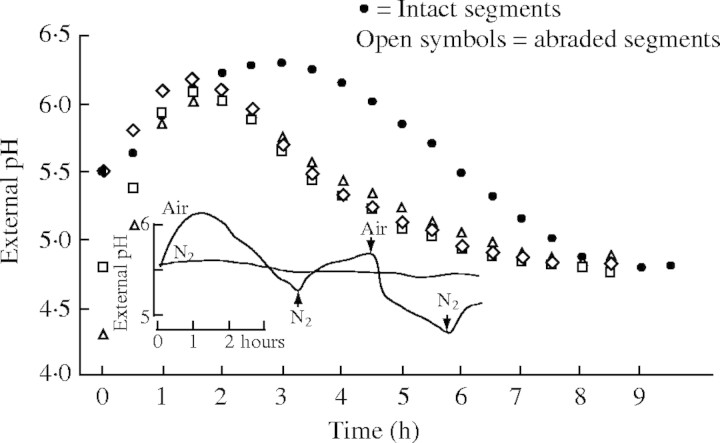
An important question is what happens to the apoplast during long-term flooding? Infiltration of the apoplast results in a rapid alteration of the apoplastic milieu. Felle and Hanstein (2002) demonstrated that the apoplast responded to an injection of picoliters of fluid into open stomata of Vicia faba leaves with a rapid increase in pH from 5·0 to 6·5. Provided that the fluid could be absorbed by the tissue, the pH recovered within an hour, but remained high in cases where larger amounts of injected fluid prevented absorption; e.g. light/dark responses were drastically quenched then. It is also evident that determinations of the apoplastic pH that were based on infiltration were over 1 pH unit less acidic than those measured non-invasively (see review of Grignon and Sentenac, 1991). Natural flooding may not lead to an infiltration or flooding of the apoplast, but it will—at least at the roots—alter the conditions within the rhizhosphere and, as a direct consequence, alter the ionic milieu of the adjacent apoplast. Whilst the outer cell layers of a root may not be affected so much, because the external sink for solutes and protons may be big enough for rapid exchange, such exchange is slow for inner cell layers. Together with the fact that the apoplast has a H+ buffer capacity in the lower millimolar range (about one-tenth of the cytoplasmic capacity), this makes the inner apoplast a poor sink for solutes such as lactic acid or H+. The relevance of this needs to be investigated in intact plant under different experimental conditions, as the apoplast is wide open in experiments in which small excised segments of roots or shoots are used. Such an approach carries with it a number of imponderables with potentially far-reaching implications for the responses to anoxia. Apart from wound reactions, which are important but not the prime issue here, the adaptation of excised tissue segments to a given solution increases the apoplastic volume immensely with the consequence that transport parameters and equilibria are altered, not to mention the direct stress. In addition, the apoplast is exposed to the test solution through the open ends on both sides of the excised segments and rapidly exchanges solutes with it. This effect has been long known as ‘aging’ and can be followed by measurements of the membrane potential, which becomes more and more negative for many hours. As shown in Fig. 5, tightly packed maize coleoptile segments (1 cm long) need about 8 h before a pH equilibrium with the medium is reached (Peters and Felle, 1991), although the solution was well aerated; as the kinetics show, this adaptation process occurs for all starting pHs! The figure also shows that the pH of the external solution rapidly increases following anoxia (N2).
Fig. 5.
Response of Avena coleoptile segments to solutions of different pH and anoxia. 1-cm segments were tightly packed in flasks containing a medium that consisted of 1 mm KCl, 0·1 mm NaCl, 0·1 mm CaCl2 and 0·5 mm Mes/Tris. The segments were aerated and the pH monitored continuously using a standard laboratory electrode at different starting pHs, as indicated. To reduce the diffusion resistance the surface of the segments was abraded. Inset: effect of anoxia (N2) on the pH response of the segments (from Peters and Felle, 1991. Journal of Plant Physiology 137: 655–661, with permission of Elsevier GMBH).
Clearly, no equilibrium is reached when the volume of the test solution is much larger than that of the segments. In this case, exported protons would not become effective in rapidly acidifying the apoplast, as would be the case in the intact plant, but instead diffuse into the medium where they are buffered off. Moreover, compounds that are transported in vivo from the apoplast into the cytoplasm would normally change their concentration, which influences their transport rates; in experimental media the external concentrations remain relatively constant, which means the cells cannot regulate the transport. It appears that not all data obtained from excised segments can be transferred to the intact plant or the field situation.
pH a signal of anoxia?
It has been discussed that pH functions as second messenger (Felle, 1989; Blatt, 1992) and signal (Wilkinson, 1999; Felle, 2001; Felle et al., 2005). In a situation where not all of the plant is affected by anoxia, it can be assumed that signals are sent from the root to the shoot. Could pH be the signal that carries the information? Microprobes, non-invasively inserted into the sub-stomatal cavity of leaves, directly and immediately detect any pH change (or of any other ion chosen as the measuring parameter) in the apoplast (e.g. Hanstein and Felle, 1999; Felle et al., 2000, 2004). Recent experiments have shown that stress in general causes apoplastic alkalinization (Felle et al., 2005); anoxic stress is no exception. Is the information ‘anoxic stress’ transferred from root to shoot via pH signal? Jackson et al. (2005) reported that in flooded tomato the xylem sap alkalized within 2 h of inundation. Although stomata closed, both ABA and conjugated ABA did not increase accordingly. Using the microprobe technique it was demonstrated that pH changes of several orders, imposed either to the root system or to the cut petiole, were not (or only minimally–about 0·1 pH per unit changed) transferred to the detecting electrode in the leaf. This makes pH an unlikely candidate as the long-distance signal of anoxia.
CONCLUDING REMARKS
Although at this stage it is common to emphasize that dealing with anoxia is mainly the management of the available energy sources, this view should always be kept in mind while investigating the problem. All cellular life depends on transmembrane gradients, which can only be built up and maintained for as long as the primary active elements (pumps) work. Depending on pool sizes and compartmental volumes, for a limited time already existing gradients may well be able to keep up some cellular functions without additional energy input. This also applies for electrochemical pH gradients. However, if these ‘batteries’ are not recharged, the cell is going to die. Electrochemical H+ gradients represent only a fraction of the transmembrane gradients, but it is a highly important one because most transport depends on it somehow, be it through voltage, ΔpH or both. Because metabolizable compounds are amongst the transported substances, the foremost task of a cell must be to keep this process working, even if it is only at a minimal level. Under anoxia transport processes are greatly impaired, leaving the H+ ATPases either reduced to low activity or worse, inactivated with no residual pumping. The ability of a cell or tissue to overcome the period of oxygen shortage depends critically on the ability to keep the transmembrane H+-driving belt moving. Although this is a priori not an issue of pH regulation, because a fraction of the exported H+ re-enter via the H+ substrate transport systems, it nevertheless serves to mitigate the acid load.
Acknowledgments
Thanks go to the organizers of the 2004 ISPA Conference, to Dr Tim Colmer for the encouragement to write this review and to Prof. Hank Greenway for inspiring discussions. The financial help provided by the Deutsche Forschungsgemeinschaft to produce some of the cited data is gratefully acknowledged.
LITERATURE CITED
- Aducci P, Marra M, Fogliano V, Fullone MR. 1995. Fusicoccin receptors: perception and translocation of the fusicoccin signal. Journal of Experimental Botany 46: 1463–1478. [Google Scholar]
- Arnon DI. 1937. Ammonium and nitrate nitrogen nutrition of barley at different seasons in relation to hydrogen-ion concentration, manganese, copper, and oxygen supply. Soil Science 44: 91–121. [Google Scholar]
- Barton JK, Den Hollander JA, Lee TM, MacLoughlin A, Shulman RG. 1980. Measurement of the internal pH of yeast spores by 31P NMR. Proceedings of the National Academy of Sciences USA 77: 2470–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR. 1992. K+ channels of stomatal guard cells: characteristics of the inward rectifier and its control by pH. Journal of General Physiology 99: 615–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer D, Uknalis J, Triana R, Shachar-Hill Y, Tu S-I. 1997. Effects of bafilomycin A1 and metabolic inhibitors on the maintenance of vacuolar acidity in maize root hair cells. Plant Physiology 113: 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busa WB, Crown JH, Matson, GB. 1982. Intracellular pH and the metabolic status of dormant and developing Artemia embryos. Archives of Biochemistry and Biophysics 216: 711–718. [DOI] [PubMed] [Google Scholar]
- Carystinos GD, MacDonald HR, Monroy AF, Dhindsa RS, Poole RJ. 1995. Vacuolar H+ -locating pyrophosphatase is induced by anoxia or chilling in seedlings of rice. Plant Physiology 108: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD. 2003. Aerenchyma and an inducible barrier to radical oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Annals of Botany 91: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DD. 1986. The fine control of cytosolic pH. Physiologia Planarum 67: 702–706. [Google Scholar]
- Davies DD, Grego S, Kenworthy P. 1974. The control of the production of lactate and ethanol by higher plants. Planta 118: 297–310. [DOI] [PubMed] [Google Scholar]
- Deamer DW. 1987. Proton permeation of lipid bilayers. Journal of Bioenergetics and Biomembranes 19: 457–479. [DOI] [PubMed] [Google Scholar]
- Dixon MH. 2001.The anaerobic metabolism of Potamogeton pectinatus L, an aquatic monocot with marked tolerance to the prolonged absence of oxygen. PhD thesis, University of Oxford, UK. [Google Scholar]
- Drew MC. 1997. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annual Reviews of Plant Physiology and Plant Molecular Biology 48: 223–250. [DOI] [PubMed] [Google Scholar]
- Echeverria E, Burns J, Felle H. 1992. Compartmentation and cellular conditions controlling sucrose breakdown in mature acid lime fruits. Phytochemistry 31: 4091–4095. [Google Scholar]
- Fan TW-M, Higashi RM, Lane AN. 1988. An in vivo 1H and 31P NMR investigation of the effect of nitrate on hypoxic metabolism in maize roots. Archives of Biochemistry and Biophysics 266: 592–606. [DOI] [PubMed] [Google Scholar]
- Fan TW-M, Higashi RM, Lane AN. 1995. Use of 15N and 13C isotope labeling and multinuclear NMR for exploring nitrate metabolism in anaerobic rice coleoptiles. Plant Physiology 108: S12. [Google Scholar]
- Fan TW-M, Higashi RM, Frenkiel TA, Lane AN. 1997. Anaerobic nitrate and ammonium metabolism in flood-tolerant rice coleoptiles. Journal of Experimental Botany 48: 1655–1666. [Google Scholar]
- Felle HH. 1989. K+/H+-antiport in Riccia fluitans: an alternative to the plasma membrane H+ pump for short-term pH regulation? Plant Science 61: 9–15. [Google Scholar]
- Felle HH. 1991. The role of the plasma membrane proton pump in short-term pH regulation in the aquatic liverwort Riccia fluitans L. Journal of Experimental Botany 42: 645–652. [Google Scholar]
- Felle HH. 1996. Control of cytoplasmic pH under anoxic conditions and its implication for plasma membrane proton transport in Medicago sativa root hairs. Journal of Experimental Botany 47: 967–973. [Google Scholar]
- Felle HH. 1998. The apoplastic pH of the Zea mays root cortex as measured with pH-sensitive microelectrodes. Journal of Experimental Botany 49: 987–995. [Google Scholar]
- Felle HH. 2001. pH: signal and messenger in plant cells. Plant Biology 3: 577–591. [Google Scholar]
- Felle HH, Bentrup FW. 1977. A study of the primary effect of the uncoupler CCCP on membrane potential and conductance in Riccia fluitans. Biochimica et Biophysica Acta 464: 179–187. [DOI] [PubMed] [Google Scholar]
- Felle HH, Hanstein S. 2002. The apoplastic pH of the sub-stomatal cavity of Vicia faba leaves and its regulation responding to different stress factors. Journal of Experimental Botany 53: 73–82. [PubMed] [Google Scholar]
- Felle HH, Hanstein S, Steinmeyer R, Hedrich, R. 2000. Dynamics of ionic activities in the apoplast of the sub-stomatal cavity of intact Vicia faba leaves during stomatal closure evoked by ABA and darkness. The Plant Journal 24: 297–304. [DOI] [PubMed] [Google Scholar]
- Felle HH, Herrmann A, Hanstein S, Hueckelhoven R, Kogel K-H. 2004. Apoplastic pH signalling in barley leaves attacked by the powdery mildew fungus Blumeria graminis f. sp. hordei. Molecular Plant-Microbe Interactions 17: 118–123. [DOI] [PubMed] [Google Scholar]
- Felle HH, Herrmann A, Hückelhoven R, Kogel K-H. 2005. Root-to-shoot signalling: apoplastic alkalinization, a general stress signal and defence factor in barley. Protoplasma (in press). [DOI] [PubMed] [Google Scholar]
- Fox GG, McCallan, Ratcliffe RG. 1995. Manipulating cytoplasmic pH under anoxia; a critical test of the role of pH in the switch from aerobic to anaerobic metabolism. Planta 195: 324–330. [Google Scholar]
- Gerendas J, Schurr U. 1999. Physicochemical aspects of ion relations and pH regulation in plants—a quantitative approach. Journal of Experimental Botany 50: 1101–1114. [Google Scholar]
- Gibbs J, Greenway H. 2003. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology 30: 1–47. [DOI] [PubMed] [Google Scholar]
- Good NE. 1988. Active transport, ion movements and pH changes. I. The chemistry of pH changes. Photosynthesis Research 19: 225–236. [DOI] [PubMed] [Google Scholar]
- Gout E, Boisson A-M, Aubert S, Douce R, Bligny R. 2001. Origin of the cytoplasmic pH changes during anaerobic stress in higher plant cells. Carbon-13 and phosphorous-31 nuclear magnetic resonance studies. Plant Physiology 125: 912–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway H, Gibbs J. 2003. Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Functional Plant Biology 30: 999–1036. [DOI] [PubMed] [Google Scholar]
- Grignon C, Sentenac H. 1991. pH and ionic conditions in the apoplast. Annual Review of Plant Physiology 42: 103–128. [Google Scholar]
- Guern J, Mathieu Y, Pean M, Pasquier C, Beloeil JC, Lallemand J. 1986. Cytoplasmic pH regulation in Acer pseudoplatanus I. A (31P)NMR description of acid-load effects. Plant Physiology 82: 840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guern J, Felle H, Mathieu Y, Kurkdijan A. 1991. Regulation of intracellular pH in plant cells. Annual Reviews of Cytology 127: 111–173. [Google Scholar]
- Hanstein S, Felle HH. 1999. The influence of atmospheric pH on the apoplastic pH of green leaves: a noninvasive approach with pH-sensitive microelectrodes. New Phytologist 143: 333–338. [Google Scholar]
- Hoffmann B, Plänker R, Mengel K. 1992. Measurement of the pH in the apoplast of sunflower leaves by means of fluorescent pH in the apoplast of sunflower leaves by means of fluorescence. Physiologia Plantarum 84: 146–153. [Google Scholar]
- Husted S, Schoerring JK. 1995. Apoplastic pH and ammonium concentration in leaves of Brassica napus L. Plant Physiology 109: 1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WM, Brendle-Behnisch E. 1995. Acid-base modulation of nitrate reductase in leaf tissues. Planta 196: 1–6. [Google Scholar]
- Kato-Noguchi H. 2000. Evaluation of the importance of lactate for the activation of ethanolic fermentation in lettuce roots in anoxia. Physiologia Plantarum 109: 28–33. [Google Scholar]
- Korthout HAAJ, de Boer AH. 1994. A fusicoccin binding protein belongs to the family of 14–3–3 brain protein homologs. The Plant Cell 6: 1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Kosegarten H, Grolig F, Esch A, Glüsenkamp K-H, Mengel K.
1999. Effects of /article/back/ref-list/ref/citation/inline-formula
 , and /article/back/ref-list/ref/citation/inline-formula
, and /article/back/ref-list/ref/citation/inline-formula
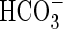 on apoplastic pH in the outer cortex of root zones of maize, as measured by the fluorescence ratio of fluorescein boronic acid. Planta
209: 444–452. [DOI] [PubMed] [Google Scholar]
on apoplastic pH in the outer cortex of root zones of maize, as measured by the fluorescence ratio of fluorescein boronic acid. Planta
209: 444–452. [DOI] [PubMed] [Google Scholar] - Kurkdjian A, Guern J. 1989. Intracellular pH: measurement and importance in cell activity. Annual Reviews of Plant Physiology and Plant Molecular Biology 40: 271–303. [Google Scholar]
- Lee HC, Johnson C, Epel D. 1983. Changes in internal pH associated with initiation of motility and acrosome reaction of sea urchin sperm. Developmental Biology 95: 31–45. [DOI] [PubMed] [Google Scholar]
- Marrè E. 1979. Fusicoccin: a tool in plant physiology. Annual Review of Plant Physiology 30: 273–288. [Google Scholar]
- Menegus F, Cattaruzza L, Chersi A, Fronza G. 1989. Differences in the anaerobic lactate-succinate production and in the changes of cell sap pH for plants with high and low resistance to anoxia. Plant Physiology 90: 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegus F, Cattaruzza L, Mattana M, Beffagna N, Ragg E. 1991. Response to anoxia in rice and wheat seedlings. Plant Physiology 95: 760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels PAM, Michels JPJ, Boonstra J, Konings WN. 1979. Generation of an electropotential proton gradient in bacteria by the excretion of metabolic end products. FEMS Letters 5: 357–364. [Google Scholar]
- Morisawa M, Steinhardt RA. 1982. Changes in intracellular pH of Physarum plasmodium during cell cycle and in response to starvation. Experimental Cellular Research 140: 341–351. [DOI] [PubMed] [Google Scholar]
- Mühling KH, Plieth C, Hansen U-P, Sattelmacher B. 1995. Apoplastic pH of intact leaves of Vicia faba as influenced by light. Journal of Experimental Botany 46: 377–382. [Google Scholar]
- Müller E, Albers BP, Janiesch P. 1994. Influence of nitrate ans ammonium nutrition on ermentation, nitrate reductase activity and adenylate energy charge of roots of Carex pseudocyperus L. and Carex sylvatica Huds. exposed to anaerobic nutrient solutions. Plant Soil 166: 221–230. [Google Scholar]
- Oja V, Savchenko G, Jakob B, Heber U. 1999. pH and buffer capacities of apoplastic cell compartments in leaves. Planta 209: 239–249. [DOI] [PubMed] [Google Scholar]
- Peters WS, Felle HH. 1991. Control of apoplastic pH in corn coleoptile segments. I. The endogenous regulation of cell wall pH. Journal of Plant Physiology 137: 655–661. [Google Scholar]
- Poole RC, Halestrap AP. 1993. Transport of lactate and other monocarboxylates across mammalian plasma membranes. American Journal of Physiology 264: C716–C782. [DOI] [PubMed] [Google Scholar]
- Ratcliffe RG. 1997.In vivo studies of the metabolic response of plant tissues to anoxia. Annals of Botany 79 (Suppl A): 39–48. [Google Scholar]
- Ratcliffe RG. 1999. Intracellular pH regulation in plants under anoxia. In: Egginton S, Taylor EW, Raven JA, eds. Regulation of acid-base status in animals and plants Society of Experimental Biology Seminar Series, Cambridge University Press, 68: 193–213. [Google Scholar]
- Reggiani R, Brambilla I, Bertani A. 1985. Effect of exogenous nitrate on anaerobic metabolism in excised rice roots II. Fermentative activity and adenylic charge. Journal of Experimental Botany 36: 1698–1704. [Google Scholar]
- Reggiani R, Mattana M, Aurisano N, Bertani A. 1993. Utilization of stored nitrate during the anaerobic germination of rice seeds. Plant and Cell Physiology 34: 379–383. [Google Scholar]
- Reid RJ, Loughman BC, Ratcliffe RG. 1985.31P NMR measurements of cytoplasmic pH changes in maize root tips. Journal of Experimental Botany 36: 889–897. [Google Scholar]
- Rivoal J, Hanson AD. 1993. Evidence for a large and sustained glycolytic flux to lactate in anoxic roots of some membranes of the halophytic genus Limonium Plant Physiology 101: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivoal J, Hanson AD. 1994. Overexpression of lactate dehydrogenase in transgenic tomato roots supports the Davies-Roberts hypothesis and points to a critical role for lactate secretion. Plant Physiology 106: 1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivoal J, Ricard B, Pradet A. 1989. Glycolytic and fermentative enzyme induction during anaerobiosis in rice seedlings. Plant Physiology and Biochemistry 27: 43–52. [Google Scholar]
- Roberts JKM, Callis J, Wemmer D, Walbot V, Jardetzky O. 1984. Mechanism of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proceedings of the National Academy of Sciences USA 81: 3379–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Callis J, Jardetzky O, Walbot V, Freeling M. 1984. Cytoplasmic acidosis as determinant of flooding intolerance in plants. Proceedings of the National Academy of Sciences USA 81: 6029–6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Andrade FH, Anderson IC. 1985. Further evidence that cytoplasmic acidosis is a determinant of flooding intolerance in plants Plant Physiology 77: 492–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Hooks MA, Miaulis AP, Edwards S, Webster C. 1992. Contribution of malate and amino acid metabolism to cytoplasmic pH regulation on hypoxic maize root tips studied using nuclear magnetic resonance spectroscopy. Plant Physiology 98: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio PH, Drew MC, Pradet A. 1988. Metabolic acclimation to anoxia induced by low (2–4 kPa partial pressure) oxygen pretreatment (hypoxia) in root tips of Zea mays. Plant Physiology 86: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Ges V, Roby C, Bligny R, Pradet A, Douce R. 1991. Kinetic studies of the variations of the cytoplasmic pH, nucleotide triphosphates (31P NMR) and lactate during normoxic and anoxic transitions in maize root tips. European Journal of Biochemistry 200: 477–482. [DOI] [PubMed] [Google Scholar]
- Sakano K, Kiyota S, Yazaki Y. 1997. Acidification and alkalinization of culture medium by Catharantus roseus cells—is anoxic production of lactate a cause of cytoplasmic acidification? Plant and Cell Physiology 38: 1053–1059. [Google Scholar]
- Sakano K. 1998. Revision of biochemical pH-stat: involvement of alternative pathway metabolisms. Plant and Cell Physiology 39: 467–473. [Google Scholar]
- Sakano K. 2001. Metabolic regulation of pH in plant cells: role of cytoplasmic pH in defence reaction and secondary metabolism. International Reviews of Cytology 206: 1–44. [DOI] [PubMed] [Google Scholar]
- Sanders D, Slayman CL. 1982. Control of intracellular pH. Predominant role of oxidative metabolism not proton transport in the eucaryotic microorganism Neurospora. Journal of General Physiology 80: 377–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattelmacher B. 2001. The apoplast and its significance for plant mineral nutrition. New Phytologist 149: 167–192. [DOI] [PubMed] [Google Scholar]
- Shen SS, Steinhardt RA. 1978. Direct measurements of intracellular pH during metabolic derepression of the sea urgin egg. Nature 272: 253–254. [DOI] [PubMed] [Google Scholar]
- Smith FA, Raven JA. 1979. Intracellular pH and its regulation. Annual Reviews of Plant Physiology 30: 289–311. [Google Scholar]
- Stewart PA. 1983. Modern quantitative acid-base chemistry. Canadian Journal of Physiology and Pharmacology 61: 1444–1461. [DOI] [PubMed] [Google Scholar]
- Stoimenova M, Libourel IGL, Ratcliffe RG, Kaiser WM. 2003. The role of nitrate in the anoxic metabolism of roots. II. Anoxic metabolism of tobacco roots with and without nitrate reductase activity. Plant and Soil 253: 155–167. [Google Scholar]
- Summers JE, Ratcliffe RG, Jackson MB. 2000. Anoxia tolerance in the aquatic monocot Potamogeton pectinatus: absence of oxygen stimulates elongation in association with an unusually large Pasteur effect. Journal of Experimental Botany 51: 1413–1422. [PubMed] [Google Scholar]
- Tadege M, Brändle R, Kuhlemeier C. 1998. Anoxia tolerance in tobacco roots: effect of overexpression of pyruvate decarboxylase. Plant Journal 14: 327–335. [Google Scholar]
- Ullrich CI, Novacki AJ. 1990. Extra- and intracellular pH and membrane potential changes induced by K+, Cl−, H2PO4- and NO3-uptake and fusicoccin in root hairs of Limnobium stoloniferum. Plant Physiology 94: 1561–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li J, Botock RM, Gilchrist DG. 1996. Apoptosis: a functional paradigm for programmed cell death induced by host-selective phytotoxin and invoked during development. Plant Cell 8: 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Nuccitelli R. 1982. Intracellular pH changes accompany the activation of development in frog eggs. Comparison of microelectrode and 31P NMR measurements. In: R Nuccitelli, DW Deamer eds. Intracellular pH: its measurement, regulation, and utilization in cellular functions. New York: Alan R. Liss, 293–324. [Google Scholar]
- Wilkinson S. 1999. pH as a stress signal. Plant Growth Regulation 29: 87–99. [Google Scholar]
- Xia J-H, Roberts JKM. 1994. Improved cytoplasmic pH regulation, increased lactate efflux, and reduced cytoplasmic lactate levels are biochemical traits expressed in root tips of whole maize seedlings acclimated to a low-oxygen environment. Plant Physiology 105: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J-H, Roberts JKM. 1996. Regulation of H+ extrusion and cytoplasmic pH in maize root tips acclimated to a low-oxygen environment. Plant Physiology 111: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J-H, Saglio PH. 1992. Lactic acid efflux as a mechanism of hypoxic acclimation of maize root tips to anoxia. Plant Physiology 100: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]