Abstract
• Background Complete submergence of Rumex palustris leads to hyponastic (upward) petiole growth followed by enhanced petiole elongation. Previous pharmacological experiments have provided insights into the signal transduction pathway leading to this combined ‘escape’ response. It will, however, be difficult to gain further knowledge using these methods. Consequently, new approaches are required.
• Scope Here we propose that different environmental signals resulting in similar phenotypes can help to understand better the submergence response. In this review, we show that both ethylene and shade induce similar growth responses in R. palustris and Arabidopsis thaliana. We illustrate how this can be exploited to unravel novel signalling components in submergence-induced elongation growth. Furthermore, we illustrate the potential of arabidopsis as a useful model in submergence research based on similarities with submergence-tolerant species such as R. palustris and the molecular opportunities it presents. This is illustrated by examples of current work exploring this concept.
• Conclusions Incorporating different model systems, such as arabidopsis and shade avoidance, into submergence research can be expected to create powerful tools to unravel signal transduction routes determining submergence tolerance.
Keywords: Rumex palustris, Arabidopsis thaliana, submergence, shade avoidance, ethylene, microarray, light, QTL, growth, elongation, differential growth, hyponasty
INTRODUCTION
Transient flooding with fresh water is a world-wide phenomenon in river floodplains and wetlands as well as other terrestrial ecosystems. It frequently results in complete submergence of plants. In general, this excess of water has a negative impact on growth and survival of most terrestrial plants, especially when flooding occurs during the growing season (Blom and Voesenek, 1996; Colmer et al., 1998). Well-adapted plants may overcome the effects of submergence by adopting an avoidance strategy in which individual leaves grow differentially to a more vertical position followed by enhanced elongation. Analysis of these processes at the molecular level will generate new insights into the complexity of plant–environment interactions and potentially help to improve submergence tolerance of agricultural crops. This review aims to highlight novel avenues of research to unravel molecular networks that are important for plant–environment interactions. We will show that, although rarely used in submergence research, experiments with Arabidopsis thaliana can be very useful. Furthermore, other signals such as shade, which are not related to submergence but that can induce phenotypically comparable responses, can help to identify signal transduction steps that determine plant responses to submergence.
SUBMERGENCE: EFFECTS AND RESPONSES
Flooding of agricultural and natural ecosystems has a dramatic impact on the growth and occurrence of terrestrial plants in these systems (Blom and Voesenek, 1996; Lenssen et al., 2004; Van Eck et al., 2004; Voesenek et al., 2004). The negative effects of flooding are strongly related to severely hampered diffusion of gases in water, which is 10 000 times slower than in air (Armstrong, 1975). As a result, the internal gas composition in plants changes, leading to oxygen and carbon dioxide shortage and thus energy deficits. Many plants, therefore, do not survive prolonged submergence (Vervuren et al., 2003; Van Eck et al., 2004). Consequently, variation in flooding tolerance among plant species strongly affects species composition and abundance in flood-prone areas (Lenssen et al., 2004; Van Eck et al., 2004). A further consequence of the selective death of plant species upon flooding stress is a marked reduction of the density of plants in flood-exposed vegetations, resulting in a reduced intensity of plant competition (Lenssen et al., 2004). This is in contrast to the rarely flooded, highly productive grasslands at higher floodplain elevations where plant densities are high and competition is fierce. Consequently, a trade-off has been predicted between competitive ability and flooding tolerance (Lenssen et al., 2004).
One way to ameliorate the effects of complete submergence on plant growth is an avoidance strategy in which plants grow out of the water and restore contact with the atmosphere. Rice is a crop species showing this adaptive response (Kende et al., 1998; Almeida et al., 2003), but species from more natural environments, including the wetland species Rumex palustris, also show this elongation response (Voesenek et al., 2004). The gaseous plant hormone ethylene accumulates in submerged plants and is the primary signal inducing shoot elongation (Musgrave et al., 1972; Kende et al., 1998; Voesenek et al., 2003a). Rather than inhibiting growth, which is the more commonly observed effect of high concentrations of ethylene (Abeles et al., 1992), elevated ethylene levels stimulate shoot elongation in these wetland plants (Voesenek et al., 2004).
Of course, this increased shoot elongation will position the leaf tips closer to and eventually above the water surface only when the orientation of the leaves is relatively close to vertical. This prerequisite is easily achieved in grass species such as rice that, by default, already possess an erect shoot morphology. In contrast, rosette species such as R. palustris have an initially horizontal orientation of the leaves, and faster elongation of leaves in this position would not result in renewed contact with the water surface. To avoid futile horizontal elongation, submergence-induced petiole extension in R. palustris is preceded by differential growth of the petiole at the abaxial side (hyponastic growth) leading to a more vertical position of these leaves (Cox et al., 2003). Only when this more upright petiole orientation is reached does vigorous overall petiole elongation begin. Thus, approximately 6 h after submergence has started, hyponastic growth brings petiole inclination close to vertical (80°) (Cox et al., 2003). In this position, petiole elongation rates achieve their maximum of more than three times that of non-submerged controls. Both this vertical leaf orientation and the high rate of petiole growth can be sustained for several days until the water surface is reached (Voesenek et al., 2003b).
REGULATION OF SUBMERGENCE-INDUCED SHOOT ELONGATION
As stated above, submergence-avoidance growth responses in R. palustris are induced by ethylene accumulation in the shoot (Voesenek et al., 1993, 2004; Peeters et al., 2002), which may, in addition, be enhanced by reduced oxygen concentrations in the submerged shoot (Voesenek et al., 1997). However, the hormonal regulation of this growth is more complex than this. Very quickly upon submergence of R. palustris, internal abscisic acid (ABA) levels drop to <20 % of normal, and this change is ethylene mediated since pre-treatment with the ethylene perception inhibitor 1-methylcyclopropene (1-MCP) prevents this decline (Benschop, 2004). Furthermore, both the production of several gibberellins (GAs) and the tissue sensitivity to GA are increased upon submergence and ethylene treatment in R. palustris (Rijnders et al., 1997; Benschop, 2004). This is significant since artificial reduction of GA biosynthesis with specific inhibitors such as paclobutrazol substantially represses the elongation response to submergence (Rijnders et al., 1997). A similar mechanism regulates fast stem elongation in deepwater rice (Métraux and Kende, 1983), where an ethylene-induced drop in ABA concentration seems to sensitize the tissue to GA (Hoffmann-Benning and Kende, 1992), positioning ABA as an intermediate between ethylene and GA (Kende et al., 1998). Interestingly, the rapid decrease of ABA observed in R. palustris does not occur in the closely related, but submergence-intolerant species, R. acetosa that lacks the enhanced petiole elongation upon submergence. However, when internal ABA levels are artificially lowered by pre-treatment with fluridone, an inhibitor of ABA biosynthesis, R. acetosa does elongate upon submergence. This suggests that the downstream steps for enhanced shoot elongation are present in this species (Benschop, 2004). Although the involvement of auxin in submergence-induced petiole elongation in R. palustris has not yet been unambiguously determined (Rijnders et al., 1996; Cox, 2004), it is likely to be a key player in differential petiole growth that reorientates leaves by means of hyponasty. Removing the leaf lamina, a putative source of auxin in a plant, or using an auxin transport inhibitor delays this submergence-induced hyponastic readjustment of leaf angle in R. palustris for several hours (Cox et al., 2004).
The submergence-induced signalling cascade is finally completed by increases in cell wall extensibility. This is believed to be the ultimate step controlling cell elongation (McQueen-Mason and Rochange, 1999). Proteins that loosen the cell wall include, among others, the so-called expansins (Cosgrove, 1998). To date 19 α-expansin genes have been isolated from R. palustris (Vriezen et al., 2000; Colmer et al., 2004). A fast upregulation of the α-expansin gene RpEXPA1 and increase of α-expansin protein of the RpEXPA1 size class have been observed during submergence or ethylene exposure in petioles of R. palustris (Vriezen et al., 2000; Vreeburg et al., 2005). This response at the gene expression level was absent in the closely related species R. acetosa that fails to elongate more quickly when submerged (Vriezen et al., 2000). A correlation between submergence-induced expansin gene expression and shoot elongation has also been shown in stems of deepwater rice (Lee and Kende, 2001).
RESPONSES TO SUBMERGENCE RESEMBLE RESPONSES TO SHADE
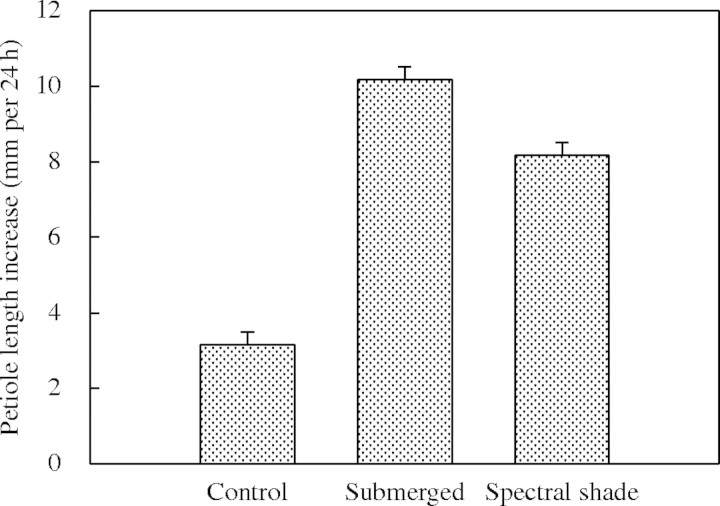
Phenotypically similar growth responses to those described for submergence can be observed when plants are shaded by proximate neighbours. When plants detect neighbours with canopy light signals, they display a suite of responses that are encompassed by the so-called shade avoidance syndrome. This includes enhanced shoot elongation and hyponastic growth (Ballaré and Scopel, 1997; Smith and Whitelam, 1997; Pierik et al., 2003). Rather than bringing the leaf tips closer to the water surface, in dense canopies these responses bring the leaves closer to the light, thereby enhancing plant performance (Pierik et al., 2003) and fitness (Schmitt et al., 1995). The similarity between submergence- and shade-induced petiole elongation is illustrated in Fig. 1. Rumex palustris shows strongly stimulated petiole elongation when put in reduced light intensity with marked changes in the spectral composition (spectral shade). This resembles a closed canopy light climate, and the elongation response is of similar magnitude to the elongation response to submergence (Fig. 1). These observations predict R. palustris to be responsive to proximate neighbours in a way that would facilitate its above-ground competitive ability. This suggests that, despite the suggested trade-off between submergence tolerance and competitive ability (Lenssen et al., 2004), R. palustris combines both characteristics. This is strengthened further by the observation that spectral shade also induces a hyponastic growth response in R. palustris, as does submergence or ethylene (Fig. 2; Table 1). Canopy shade comprises a specific reduction in the photon fluence rates of red (R) and blue light, due to specific absorption by chlorophyll. As far-red (FR) light is not absorbed by chlorophyll, the canopy light climate is characterized by a low R : FR ratio and a reduced total light intensity. Of these two components, a low R : FR ratio induces a phenotype closely similar to that given by spectral shade treatment. In contrast, spectrally neutral shade, i.e. lower intensities with no change in the R : FR ratio, induce only a very weak hyponastic response in R. palustris (Fig. 2; Table 1). In this respect, R. palustris differs from tobacco and arabidopsis, which do show pronounced hyponasty in response to spectrally neutral shade (Pierik et al., 2004b; Millenaar et al., 2005).
Fig. 1.
Petiole elongation in 27-d-old Rumex palustris plants that were exposed to control growth conditions (Cox et al., 2003), submerged or spectrally shaded [white light filtered through one layer of Fern Green filter (Lee Filters no. 122, Andover, UK), PAR = 70 µmol m−2 s−1, R : FR (655–665 nm : 725–735 nm) = 0·2]. Plants were treated for 24 h and petiole elongation was measured as the increase in length between the start and end of the experiment of the third emerged petiole. Data are means (n = 3) ± s.e.
Fig. 2.
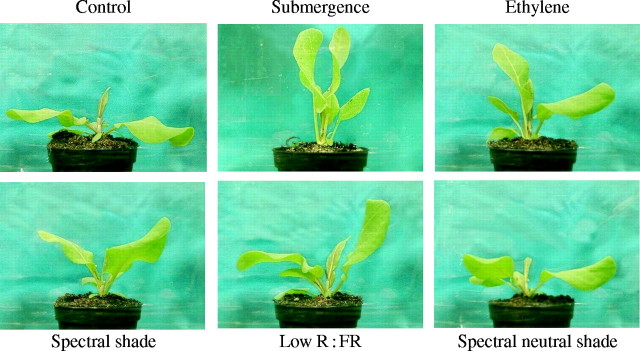
Rumex palustris plants exposed to: air (control), submergence, 3 µl L−1 ethylene (flow through at 0·4 L min−1), spectral shade, low red : far-red (R : FR) light and spectral neutral shade for 24 h. Growth conditions are described in Cox et al. (2003) and plants were 27 d old. All treatments, except neutral shade, resulted in rapid hyponastic growth of the petioles. See Table 1 for numerical details. Light conditions: control and ethylene treatment [white light: PAR = 140 µmol m−2 s−1, R : FR (655–665 nm : 725–735 nm) = 1·0], submergence treatment [PAR = 100 µmol m−2 s−1, R : FR = 1·4], spectral shade [white light filtered through one layer of Fern Green filter (Lee Filters no. 122), PAR = 70 µmol m−2 s−1, R : FR = 0·2], low R : FR [white light supplemented with far-red light (FR-emitting LEDs), PAR = 140 µmol m−2 s−1, R : FR = 0·2] and spectral neutral shade (white light filtered through neutral shade cloth, PAR = 20 µmol m−2 s−1, R : FR = 1·0).
Table 1.
Rumex palustris petiole angles (third emerged petiole of 27-d-old plants) after 24 h of a given treatment and corrected for the control angle at the same time
| Treatment |
Differential angle |
P |
|---|---|---|
| Submerged | 45·5 | <0·001 |
| Ethylene | 43·9 | <0·001 |
| Spectral shade | 25·2 | <0·001 |
| Spectral neutral shade | 7·0 | <0·05 |
| Low R : FR | 27·6 | <0·01 |
For treatment details, see Fig. 2. P-values indicate the significance of the difference between treatment and control values.
The similarity between submergence- and shade-induced growth responses, as well as the generally low light intensities during natural flooding events in river floodplains (Vervuren et al., 2003), might suggest responses to shade to be part of plant responses to submergence. However, recent experiments by Mommer et al. (2005) have already shown for photosynthetic acclimation responses that this is not the case. Furthermore, submergence-induced shoot elongation can take place when light intensities under water are relatively high (e.g. 100 µmol m−2 s−1 in Figs 1 and 2). Finally, R : FR ratios are increased under water, whereas a decrease is required to induce shade avoidance responses.
Interestingly, shade avoidance responses seem to involve hormonal interactions that bear a strong resemblance to those described for submergence avoidance. Upon detection of a reduced R : FR light ratio by the phytochrome family of photoreceptors (Smith, 2000), plant shoots are sensitized to GA (Reid et al., 1990; Weller et al., 1994; López-Juez et al., 1995) or produce more GA (Beall et al., 1996). This effect is comparable with the submergence responses of R. palustris and rice. Furthermore, ethylene production is controlled by the R : FR ratio (Finlayson et al., 1999; Pierik et al., 2004a) and was recently shown to be critical for hyponastic and stem elongation responses to neighbours (Pierik et al., 2003) and shade (Pierik et al., 2004b). Also, interactions between ethylene and GAs may occur during shade avoidance (Pierik et al., 2004a). However, it remains uncertain if ABA is also involved in low R : FR-induced shoot elongation and/or hyponastic growth in the manner discovered in submerged plants. Furthermore, the molecular basis of these hormone interactions is as yet unknown. Recent work on GA signalling in A. thaliana has identified a potential point of integration of different hormone signalling pathways. This involves the so-called DELLA proteins, which inhibit growth and are targeted for breakdown by GA signalling (Harberd, 2003). Interestingly, the stability of these proteins can also be modified by ethylene and auxin (Achard et al., 2003).
As for submergence studies, data for auxin involvement in shade avoidance are scarce. Nevertheless, regulated auxin transport has been suggested to be required for phytochrome-mediated hypocotyl elongation in arabidopsis (Morelli and Ruberti, 2000), and blue light-mediated phototropsim (Friml et al., 2002). Furthermore, the auxin-resistant axr1-12 Arabidopsis mutant has a severely attenuated hypocotyl elongation response to a low R : FR ratio (Steindler et al., 1999). These data together suggest that auxin may be involved in shade avoidance responses, comparable with the submergence avoidance response.
EXPLOITING SHADE AVOIDANCE STUDIES TO IDENTIFY COMPONENTS OF SUBMERGENCE-INDUCED SIGNAL TRANSDUCTION
Because of close phenotypical (Figs 1, 2 and Table 1) and physiological similarities between submergence- and shade-induced hyponastic and elongation responses, shade avoidance can be used as a tool to gain further insight into the regulation of submergence- and ethylene-induced elongation responses. This approach exploits the similarities in various differential screens such as cDNA subtraction and cDNA-AFLP analyses applied to shaded vs. submerged/ethylene-treated plants. This idea is based on the hypothesis that downstream parts of shade- and submergence-induced signalling cascades demonstrate a high degree of similarity. It can, therefore, be expected that when comparing pools of mRNA from submergence- and shade-treated R. palustris plants, similar downstream messengers will show up in both treatments. This is because this latter part of the cascade is likely to be shared. If this notion is valid, a cDNA-AFLP analysis should allow selection of downstream genes to be identified that are regulated in the same way in shade- and submergence-treated plants. Studying these shared genes would then significantly reduce the chance of investigating genes that are pleiotropically induced by the treatment or are related to upstream steps in the upper unshared sections of the signal transduction pathway or are unrelated to the response studied.
Upstream components of either shade- or submergence-induced signalling cascades are thus expected to be differentially expressed to a much higher or lower extent in one or other of the two treatments. These components are specifically selected for in a cDNA subtraction analysis where all mRNAs that are expressed to a similar extent in the two treatments are removed. Such carefully designed differential screens may allow identification of upstream signalling intermediates that are involved in submergence-induced shoot elongation in R. palustris. Expression of those upstream genes could then be compared in R. palustris and submergence-intolerant R. acetosa. This may ultimately provide the molecular explanation for the presence and absence of submergence-induced elongation in R. palustris and R. acetosa, respectively.
Of course, molecular analyses would be even more promising and effectively explored in species for which extensive genomic information is already available, such as rice and arabidopsis. However, little is known about potential shade avoidance in rice. Furthermore, only part of the submergence avoidance responses can be investigated since hyponastic growth is an irrelevant parameter in this species due to its inherently high leaf angles. Arabidopsis is therefore the model system of choice to investigate submergence-, ethylene- and shade-induced responses.
ARABIDOPSIS AS A SUITABLE MODEL SPECIES TO STUDY SUBMERGENCE AND SHADE AVOIDANCE
At first sight, A. thaliana would not be the ideal model species to study submergence-related responses since it is not particularly flooding tolerant (Pigliucci and Kolodynska, 2002). Yet, arabidopsis plants still show 50 % survival after approximately 1 week of complete submergence at 20 °C in the dark. Moreover, strong natural variation can be observed between different natural accessions (D. Tholen and L. A. C. J. Voesenek, unpubl. res.). Furthermore, flooding survival has been reported to be increased in plants carrying a gene containing the senescence-specific SAG12 promoter fused to an ipt gene coding for isopentenyl transferase, a rate-limiting enzyme in cytokinin biosynthesis. After 2 weeks of submergence in the light, only 6 % of the wild-type plants survived, while up to 77 % of the transgenic plants survived (Zhang et al., 2000).
One of the first responses to submergence of the submergence-tolerant species R. palustris is upward movement of the leaves. This hyponastic growth starts within 1·5 h and is saturated in approx. 6 h (Cox et al., 2003). Both submergence and ethylene are also able to induce hyponastic growth in the arabidopsis accession Columbia (Fig. 3). In both R. palustris and arabidopsis, hyponastic growth takes place over a similar time interval (Cox et al., 2004; Millenaar et al., 2005). Therefore, we hypothesize that submergence- and ethylene-induced hyponastic growth can also be studied in arabidopsis. Exploiting this could speed up research, because of the extensive molecular tools available for arabidopsis research. One such tool is the arabidopsis ‘full’ genome microarray. This makes it relatively easy to profile virtually all transcripts in a plant. To identify new genes involved in ethylene-induced hyponastic growth, microarrays can readily analyse mRNA isolated from ethylene-treated plants compared with control.
Fig. 3.
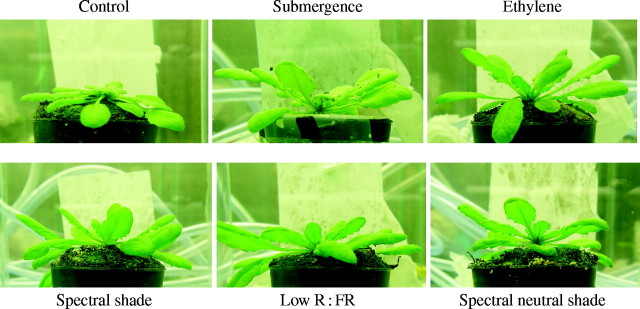
Arabidopsis thaliana Columbia plants exposed to: air (control), submergence, 3 µl L−1 ethylene (flow through at 0·4 L min−1), spectral shade, low red : far-red (R : FR) light and spectral neutral shade for 6 h. Growth conditions are described in Millenaar et al. (2005). All treatments resulted in rapid hyponastic growth of the petioles. See Table 2 for numerical details. Light conditions: control, ethylene [white light: PAR = 140 µmol m−2 s−1, R : FR (655–665 nm : 725–735 nm) = 1·0], submergence treatment [PAR = 100 µmol m−2 s−1, R : FR = 1·4], spectral shade [white light filtered through one layer of Fern Green filter (Lee Filters no. 122), PAR = 70 µmol m−2 s−1, R : FR = 0·2], low R : FR [white light supplemented with far-red light (FR-emitting LEDs), PAR = 140 µmol m−2 s−1, R : FR = 0·2] and spectral neutral shade [white light filtered through neutral shade cloth, PAR = 20 µmol m−2 s−1, R : FR = 1·0].
Another advantage of arabidopsis is the large natural variation in this species, as found for many properties including survival after soil flooding (waterlogging) (Pigliucci and Kolodynska, 2002; Kolodynska and Pigliucci, 2003) and for hyponastic responses to ethylene (Millenaar et al., 2005). The natural variation within a species can be explored, with the help of quantitative trait loci (QTLs). QTL analysis facilitates identification of genomic loci involved (either positively or negatively) in a certain trait. The basis of this technique is the presence of natural genetic variation for a given trait among accessions (natural variants) and within populations derived from crosses between accessions. The recombination present in the F2 progeny of a cross between two accessions is sufficient to identify QTLs. Usually more advanced populations, such as recombinant inbred lines (RILs; single seed descent for >8 generations of individual F2 lines), are used because they have the advantage of being homozygous and immortal. In arabidopsis, there are several populations of homozygous RILs available (Lister and Dean, 1993; Alonso-Blanco et al., 1998; El-Lithy et al., 2004). Therefore, it should be straightforward to determine loci on chromosomes that are essential for traits such as ethylene-induced hyponastic growth. Furthermore, by combining a QTL analysis with microarray data from individual RILs, it is possible to construct signal transduction pathways; a technique called genetical genomics (Jansen and Nap, 2001; Jansen 2003).
Not only does arabidopsis demonstrate hyponastic growth upon submergence and elevated ethylene levels, but it also displays this response to low light, to a reduction in the R : FR ratio or to a combination of low light intensity and reduced R : FR ratio (spectral shade) (Fig. 3; Table 2). These results are comparable with phytochrome-mediated hyponasty in R. palustris (Fig. 2). As already explained, it can be useful to use different signals which each result in the same phenotype as tools to unravel the signal transduction of hyponastic growth. The following two examples illustrate the value of this approach by making use of the molecular and genetic toolboxes available for arabidopsis.
Table 2.
Arabidopsis thaliana ‘Columbia’ petiole angles after 6 h of a given treatment and corrected for the control angle at the same time
| Treatment |
Differential angle |
P |
|---|---|---|
| Submerged | 17·3 | <0·001 |
| Ethylene | 16·0 | <0·001 |
| Spectral shade | 13·8 | <0·01 |
| Spectral neutral shade | 22·0 | <0·001 |
| Low R:FR | 14·1 | <0·001 |
For treatment details see Fig. 3. P-values indicate the significance of the difference between treatment and control values.
First, by performing a QTL analysis on both ethylene- and low-light-induced hyponastic growth, it is possible to identify which part of the signal transduction route is most likely to be shared (probably downstream steps) and which parts are specific (probably upstream steps) for either ethylene or low light. Preliminary results show that ethylene- and low-light-induced hyponastic growth share several QTLs, most probably demonstrating that ethylene and low light are switching on the same sections of a partly shared signal transduction cascade (L. B. Snoek, L. A. C. J. Voesenek and A. J. M. Peeters, unpubl. res.).
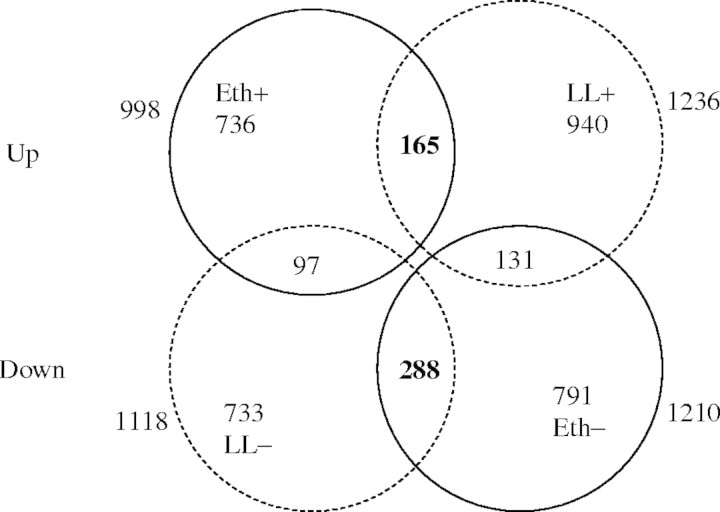
In the second example, two hyponasty-inducing signals were used to find downstream genes involved in hyponastic growth with the aid of a microarray experiment. The major disadvantage of applying only one environmental signal is that many genes will change in expression, many of which are likely to be unrelated to hyponastic growth. For example, many pathogen-related genes are also expected to be upregulated by ethylene (Schenk et al., 2000). To find genes involved in hyponastic growth more efficiently, a second microarray experiment can be conducted with the second signal, which induces much the same phenotype. By combining data sets of the two signals, many unrelated genes can be filtered out. We have performed such an experiment with low light and ethylene treatments, both of which evoke hyponastic growth in the arabidopsis accession Columbia. In this experiment, 2208 genes were changed significantly in expression by the addition of ethylene and 2354 genes by transfer to low light, giving a total of 3881 genes that were changed by either ethylene or low light, or by both of these treatments (Fig. 4). Discarding genes that were affected by only one of the two treatments allowed genes to be identified that were affected by both ethylene and low light. This reduced the number of genes from 3881 to only 453. That implies that at least 88 % of all the genes induced by ethylene or low light were probably not related to hyponastic growth or were involved in the first unshared upstream part of the cascade. In this way, genes can be selected with a greater chance of being involved in hyponastic growth. The number of candidate genes for the shared section of the pathway could be reduced further by discarding genes whose level of expression was markedly dissimilar between the two treatments. In a second refining approach, gene expression from microarrays can be calculated using several different methods (Affymetrix 2001; Li and Wong 2001a, b; Irizarry et al., 2003a, b; Zhang et al., 2003). Each of these methods will modify the apparent extent to which different sets of genes are changed by either or both treatments. Genes calculated not to be changed significantly in expression by all calculation methods can be discarded. By applying these two approaches, the number of seemingly shared genes was reduced from 453 to only 109 genes. Discarding genes that only changed marginally can decrease this number still further. Thus, when genes that changed <40 % were discarded, only 24 genes remained as candidates coding for sections of the signal transduction pathway activated by ethylene or spectral neutral shade and leading to hyponasty.
Fig. 4.
Microarray data from plants treated with 5 µl L−1 ethylene (Eth) or from plants transferred to low light (LL) conditions (decreased from 200 to 20 µmol m−2 s−1). Ethylene addition resulted in upregulation of 998 genes (Eth+) and downregulation of 1210 genes (Eth–). In low light, 1236 genes were upregulated (LL+) and 1118 genes were downregulated (LL–). There were 165 genes predicted to be upregulated by both ethylene and low light, and 288 genes predicted to be downregulated in both treatments. Growth conditions are described in Millenaar et al. (2005). Affymetrix full genome (ATH1) microarrays were used. Three biological replicates were used for each condition. Gene expression was calculated with dChip (PM model; Harvard University, MA; Li and Wong 2001a, b; http://biosun1.harvard.edu/~cli/dchip.exe), whereby the Cel files from MAS 5·0 software (Affymetrix, Santa Clara, USA; Affymetrix, 2001) are imported in dChip. A two-tailed t-test (P < 0·05) with unequal variance was used to calculate if a gene is upregulated (+) or downregulated (−). The data set is publicly available (http://affy.arabidopsis.info/narrays/experimentpage.pl?experimentid=32).
However, even when an experiment is designed this carefully, gene expression data only provide correlations with hyponastic growth and do not prove an actual involvement of a gene product in this response. To help demonstrate more certainly that any particular gene is important for the process, knock-out mutations and/or overexpression constructs may be tried. The major advantage of using arabidopsis is the existence of several knock-out and activation tagged collections, which cover virtually all the predicted genes of the genome (Weigel et al., 2000; Bouche and Bouchez, 2001; Steiner-Lange et al., 2001; Alonso et al., 2003). Therefore, it should be possible to investigate further the role of an up- or downregulated gene with the help of such mutants.
CONCLUSIONS
In summary, shade avoidance responses in physiological and molecular studies provide an excellent signalling system with which to study submergence-induced responses. Exploring such comparisons can yield novel insights into the regulatory pathways of both submergence- and shade-induced elongation growth. Furthermore, A. thaliana offers great potential for these analyses, because ethylene and low light also induce hyponastic growth in this species. Exploiting the advantages of arabidopsis for molecular work will expand submergence research by making tools, such as QTLs and genome-wide gene expression analyses, readily available.
Acknowledgments
We thank R. A. M. Welschen and T. L. Pons for their help in building the spectral shade set-up, and L. B. Snoek for his unpublished QTL data. This work was supported by a PIONIER grant (800·84·470) of the Dutch Science Foundation (NWO) to L.A.C.J.V. and by a NIELS STENSEN grant to F.F.M.
Footnotes
R. Pierik and F. F. Millenaar contributed equally to this work.
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME. 1992.Ethylene in plant biology. San Diego: Academic Press, 1–398. [Google Scholar]
- Achard P, Vriezen WH, Van der Straeten D, Harberd N. 2003. Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 15: 2816–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affymetrix 2001. Microarray Suite User Guide,Version 5. Affymetrix, http://www.affymetrix.com/support/technical/manuals.affx. [Google Scholar]
- Almeida AM, Vriezen WH, Van der Straeten D. 2003. Molecular and physiological mechanisms of flooding avoidance and tolerance in rice. Russian Journal of Plant Physiology 50: 743–751. [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, et al. 2003. Genome-wide insertional mutagenesis of Arabidopsis thaliana Science 301: 653–657. [DOI] [PubMed] [Google Scholar]
- Alonso-Blanco C, Peeters AJ, Koornneef M, Lister C, Dean C, van den Bosch N, et al. 1998. Development of an AFLP based linkage map of Ler, Col and Cvi Arabidopsis thaliana ecotypes and construction of a Ler/Cvi recombinant inbred line population. Plant Journal 14: 259–271. [DOI] [PubMed] [Google Scholar]
- Armstrong W. 1975. Waterlogged soils. In: Etherington, JR, ed. Environment and plant ecology. London: John Wiley & Sons, 181–218. [Google Scholar]
- Ballaré CL, Scopel AL. 1997. Phytochrome signalling in plant canopies: testing its population-level implications with photoreceptor mutants of Arabidopsis Functional Ecology 11: 441–450. [Google Scholar]
- Beall FD, Yeung EC, Pharis RP. 1996. Far-red light stimulates internode elongation, cell division, cell elongation, and gibberellin levels in bean. Canadian Journal of Botany 74: 743–752. [Google Scholar]
- Benschop JJ. 2004.The role of abscisic acid in ethylene-induced elongation. PhD thesis, Utrecht University, The Netherlands. [Google Scholar]
- Blom CWPM, Voesenek LACJ. 1996. Flooding: the survival strategies of plants. Trends in Ecology and Evolution 11: 290–295. [DOI] [PubMed] [Google Scholar]
- Bouche N, Bouchez D. 2001. Arabidopsis gene knockout: phenotypes wanted. Current Opinion in Plant Biology 4: 111–117. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Peeters AJM, Wagemaker CAM, Vriezen WH, Ammerlaan A, Voesenek LACJ. 2004. Expression of α-expansin genes during root acclimations to O2 deficiency in Rumex palustris Plant Molecular Biology 56: 423–437. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. 1998. Cell wall loosening by expansins. Plant Physiology 118: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MCH, Peeters AJM, Voesenek LACJ. 2005. The stimulating effects of ethylene and auxin on petiole elongation and on hyponastic curvature and independent processes in submerged Rumex palustris. Plant, Cell and Environment (in press). [DOI] [PubMed] [Google Scholar]
- Cox MCH, Benschop JJ, Vreeburg RAM, Wagemaker CAM, Moritz T, Peeters AJM, et al. 2004. The roles of ethylene, auxin, abscisic acid, and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiology 136: 2948–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MCH, Millenaar FF, de Jong van Berkel YEM, Peeters AJM, Voesenek LACJ. 2003. Plant movement. Submergence-induced petiole elongation in Rumex palustris depends on hyponastic growth. Plant Physiology 132: 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Lithy ME, Clerkx EJM, Ruys GJ, Koornneef M, Vreugdenhil D. 2004. Quantitative trait locus analysis of growth-related traits in a new Arabidopsis recombinant inbred population. Plant Physiology 135: 444–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson SA, Jung I-J, Mullet JE, Morgan PW. 1999. The mechanism of rhythmic ethylene production in Sorghum. The role of phytochrome B and simulated shading. Plant Physiology 119: 1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K. 2002. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809. [DOI] [PubMed] [Google Scholar]
- Harberd NP. 2003. Relieving DELLA restraint. Science 299: 1853–1854. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Benning S, Kende H. 1992. On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiology 99: 1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. 2003. Summaries of Affymetrix genechip probe level data. Nucleic Acids Research 31, e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. 2003. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264. [DOI] [PubMed] [Google Scholar]
- Jansen RC, Nap J-P. 2001. Genetical genomics: the added value from segregation. Trends in Genetics 17: 388–391. [DOI] [PubMed] [Google Scholar]
- Jansen RC. 2003. Studying complex biological systems using multifactorial perturbation. Nature Genetics 4: 145–151. [DOI] [PubMed] [Google Scholar]
- Kende H, Van der Knaap E, Cho H-T. 1998. Deepwater rice: a model plant to study stem elongation. Plant Physiology 118: 1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodynska A, Pigliucci M. 2003. Multivariate responses to flooding in Arabidopsis: an experimental evolutionary investigation. Functional Ecology 17: 131–140. [Google Scholar]
- Lee Y, Kende H. 2001. Expression of B-expansins is correlated with internodal elongation in deepwater rice. Plant Physiology 127: 645–654. [PMC free article] [PubMed] [Google Scholar]
- Lenssen JPM, Van de Steeg HM, de Kroon H. 2004. Does disturbance favour weak competitors? Mechanisms of altered plant abundance after flooding. Journal of Vegetation Science 15: 305–314. [Google Scholar]
- Li C, Wong WH. 2001. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proceedings of the National Academy of Sciences of the USA 98: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wong WH. 2001. Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biology 8: 0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister C, Dean C. 1993. Recombinant inbred lines for mapping RFLP and phenotypic markers in Arabidopsis thaliana Plant Journal 4: 745–750. [DOI] [PubMed] [Google Scholar]
- López-Juez E, Kobayashi M, Sakurai A, Kamiya Y, Kendrick RE. 1995. Phytochrome, gibberellins, and hypocotyl growth. Plant Physiology 107: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason S, Rochange F. 1999. Expansins in plant growth and development: an update on an emerging topic. Plant Biology 1: 19–25. [Google Scholar]
- Métraux J-P, Kende H. 1983. The role of ethylene in the growth response of submerged deep water rice. Plant Physiology 72: 441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenaar FF, Cox MCH, de Jong van Berkel YEM, Welschen RAM, Pierik R, Voesenek LACJ, Peeters AJM. 2005. Ethylene-induced differential growth of petioles in arabidopsis. Analyzing natural variation, response kinectics, and regulation. Plant Physiology 137: 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommer L, de Kroon H, Pierik R, Bögemann GM, Visser EJW. 2005. A functional comparison of acclimation to shade and submergence in two terrestrial plant species. New Phytologist, 167: 197–206. [DOI] [PubMed] [Google Scholar]
- Morelli G, Ruberti I. 2000. Shade avoidance responses. Driving auxin along lateral routes. Plant Physiology 122: 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrave A, Jackson MB, Ling E. 1972. Callitriche stem elongation is controlled by ethylene and gibberellin. Nature New Biology 238: 93–96. [Google Scholar]
- Peeters AJM, Cox MCH, Benschop JJ, Vreeburg RAM, Bou J, Voesenek LACJ. 2002. Submergence research using Rumex palustris as a model; looking back and going forward. Journal of Experimental Botany 53: 391–398. [DOI] [PubMed] [Google Scholar]
- Pierik R, Visser EJW, de Kroon H, Voesenek LACJ. 2003. Ethylene is required in tobacco to succesfully compete with proximate neighbours. Plant, Cell and Environment 26: 1229–1234. [Google Scholar]
- Pierik R, Cuppens MLC, Voesenek LACJ, Visser EJW. 2004. Interactions between ethylene and gibberellins in phytochrome-mediated shade avoidance responses in tobacco. Plant Physiology 136: 2928–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Whitelam GC, Voesenek LACJ, de Kroon H, Visser EJW. 2004. Canopy studies on ethylene-insensitive tobacco identify ethylene as a novel element in blue light and plant–plant signalling. Plant Journal 38: 310–319. [DOI] [PubMed] [Google Scholar]
- Pigliucci M, Kolodynska A. 2002. Phenotypic plasticity and integration in response to flooded conditions in natural accessions of Arabidopsis thaliana (L.) Heynh (Brassicaceae). Annals of Botany 90: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JB, Hasan O, Ross JJ. 1990. Internode length in Pisum Gibberellins and the response to far-red-rich light. Journal of Plant Physiology 137: 46–52. [Google Scholar]
- Rijnders JHGM, Barendse GWM, Blom CWPM, Voesenek LACJ. 1996. The contrasting role of auxin in submergence-induced petiole elongation in two species from frequently flooded wetlands. Physiologia Plantarum 96: 467–473. [Google Scholar]
- Rijnders JHGM, Yang YY, Kamiya Y, Takahashi N, Barendse GWM, Blom CWPM, et al. 1997. Ethylene enhances gibberellin levels and petiole sensitivity in flooding-tolerant Rumex palustris but not in flooding-intolerant R. acetosa Planta 203: 20–25. [Google Scholar]
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, et al. 2000. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proceedings of the National Academy of Sciences of the USA 97: 11655–11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J, McCormac AC, Smith H. 1995. A test of the adaptive plasticity hypothesis using transgenic and mutant plants disabled in phytochrome-mediated elongation responses to neighbors. American Naturalist 146: 937–953. [Google Scholar]
- Smith H. 2000. Phytochromes and light signal perception by plants—an emerging synthesis. Nature 407: 585–591. [DOI] [PubMed] [Google Scholar]
- Smith H, Whitelam GC. 1997. The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant, Cell and Environment 20: 840–844. [Google Scholar]
- Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, Aoyama T, et al. 1999. Shade avoidance responses are mediated by the ATHB-2 HD-Zip protein, a negative regulator of gene expression. Development 126: 4235–4245. [DOI] [PubMed] [Google Scholar]
- Steiner-Lange S, Gremse M, Kuckenberg M, Nissing E, Schächtele D, Spenrath N, et al. 2001. Efficient identification of Arabidopsis knock-out mutants using DNA-arrays of transposon flanking sequences. Plant Biology 3: 391–397. [Google Scholar]
- Van Eck W, Van de Steeg HM, Blom CWPM, de Kroon H. 2004. Is tolerance to summer flooding correlated with distribution patterns in river floodplains? A comparative study of 20 terrestrial grassland species. Oikos 107: 393–405. [Google Scholar]
- Vervuren PJA, Blom CWPM, de Kroon H. 2003. Extreme flooding events on the Rhine and survival and the distribution of riparian plant species. Journal of Ecology 91: 135–146. [Google Scholar]
- Voesenek LACJ, Banga M, Thier RH, Mudde CM, Harren FJM, Barendse GWM, et al. 1993. Submergence-induced ethylene synthesis, entrapment, and growth in two plant species with contrasting flooding resistances. Plant Physiology 103: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Vriezen WH, Smekens MJE, Huitink FHM, Bogemann GM, Blom CWPM. 1997. Ethylene sensitivity and response sensor expression in petioles of Rumex species at low O2 and high CO2 concentrations. Plant Physiology 114: 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Benschop JJ, Bou J, Cox MCH, Groeneveld HW, Millenaar FF, et al. 2003. Interactions between plant hormones regulate submergence-induced shoot elongation in the flooding-tolerant dicot Rumex palustris Annals of Botany 91: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Jackson MB, Toebes AHW, Huibers W, Vriezen WH, Colmer TD. 2003. De-submergence-induced ethylene production in Rumex palustris: regulation and ecophysiological significance. Plant Journal 33: 341–352. [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Rijnders JHGM, Peeters AJM, Van de Steeg HM, de Kroon H. 2004. Plant hormones regulate fast shoot elongation under water: from genes to community. Ecology 85: 16–27. [Google Scholar]
- Vreeburg RAM, Benschop JJ, Peeters AJM, Colmer JD, Ammerlaan AHM, Staal M, et al. 2005. Ethylene regulates expansin activity and petiole elongation by triggering fast apoplastic acidification and expansin A expression in submerged Rumex palustris. Plant Journal (in press). [DOI] [PubMed] [Google Scholar]
- Vriezen WH, de Graaf B, Mariani C, Voesenek LACJ. 2000. Submergence induces expansin gene expression in flooding-tolerant Rumex palustris and not in flooding-intolerant Rumex acetosa Planta 210: 956–963. [DOI] [PubMed] [Google Scholar]
- Weigel D, Ahn JH, Blazquez MA, Borevitz JO, Christensen SK, Fankhauser C, et al. 2000. Activation tagging in Arabidopsis. Plant Physiology 122: 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JL, Ross JJ, Reid JB. 1994. Gibberellins and phytochrome regulation of stem elongation in pea. Planta 192: 489–496. [Google Scholar]
- Zhang J, Van Toai T, Huynh L, Preiszner J. 2000. Development of flooding-tolerant Arabidopsis thaliana by autoregulated cytokinin production. Molecular Breeding 6: 135–144. [Google Scholar]
- Zhang L, Miles MF, Aldape KD. 2003. A model of molecular interactions on short oligonucleotide microarrays. Nature Biotechnology 21: 818–821. [DOI] [PubMed] [Google Scholar]