Abstract
• Aims Despite the biodiversity values of the freshwater floodplains of northern Australia being widely recognized, there has not been a concomitant investment in developing the extent of knowledge of the basic functions and ecological processes that underpin the ecological character of these habitats. This review addresses the extent of our knowledge on the plant ecology of these wetlands and covers: the relationships between the climate and the hydrological regime on the floodplain; the vegetation patterns, succession and adaptation; and primary production.
• Scope Information is available on the seasonal, but less regularly on the inter-annual, dynamics of the macrophytic vegetation and its evident inter-relationship with the extent, depth and duration of inundation by seasonal flooding. The available scientifically collected information on plant distribution and relationship with the water regime could be complemented by more attention to traditional knowledge. The productivity of the vegetation is high—the dominant wetland grass species have an annual dry weight production of 0·5–2·1 kg m−2 and the surrounding riparian (Melaleuca) trees contribute litterfall of 0·7–1·5 kg (dry weight) m−2 year−1, ∼70 % due to leaf-fall. The availability of dissolved oxygen in the water is known to vary diurnally and seasonally, at least in some habitats. The importance of seasonal differences in the availability of dissolved oxygen for the growth of micro- and macrophytic vegetation has not been investigated. The seasonal distribution and growth of plant species on a few floodplains have been investigated, and maps at scales of 1 : 10 000 to 1 : 100 000 are available for these. However, only on a few occasions have longer term analyses been conducted and long-term changes in the vegetation measured and assessed. Species lists and categorization of growth strategies and forms are available and provide a basis for further ecological investigation.
• Conclusions Despite the large investment in managing the many pressures that have degraded the ecological character of these highly valued wetlands, the fundamental ecological processes that underpin the biodiversity values have not received the same level of attention. Further information on plant growth and the environmental factors that drive seasonal and annual changes in vegetation distribution and productivity is required to assist managers in attending to changes due to increasing invasive species and changes in fire regimes.
Keywords: Aquatic plants, plant biomass, productivity, succession
INTRODUCTION
Australia's tropical floodplain wetlands are found across northern Australia, from Cape York in the east to Broome in the west. They cover an estimated 98 700 km2 (Lowry and Finlayson, 2004) and support an array of plants and animals (Finlayson et al., 1988; Finlayson and von Oertzen, 1993). The floodplains are found across an area that is broadly known as the ‘wet–dry tropics’ and which has been defined as those areas with an annual rainfall of 600–1600 mm spread over 4–7 months (see Ridpath, 1985 for an introduction to the wet–dry tropics) or taken to include the bio-geographical regions shown in Fig. 1 (Finlayson et al., 1997a). Whilst the general distribution patterns of some of the species that occur in these habitats are known (e.g. some fish and bird species), many features of plant growth and primary production have only been investigated in a cursory fashion. Far less information is available on the adaptations that the plants have to the floodplain environment.
Fig. 1.
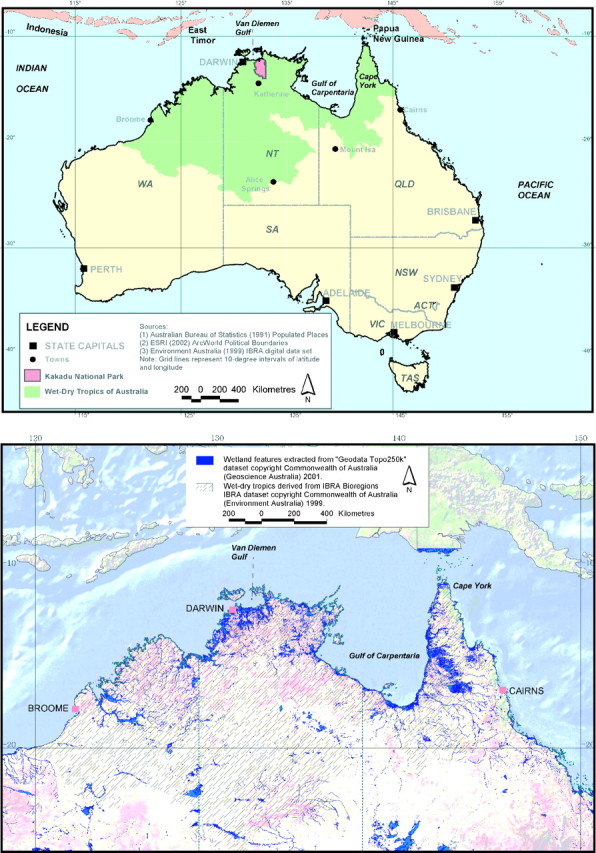
The wet–dry tropics as defined on biogeographical regions (the shaded area on the upper map) with Kakadu National Park marked (Finlayson et al., 1997a) and the distribution of wetlands (including rivers, swamps, marshes and lagoons/ponds) (from Lowry and Finlayson, 2004).
There have been many specific investigations and general reviews of the biodiversity (plant and animal taxa and habitats) and pressures on the freshwater wetlands, especially those in the northernmost part of the Northern Territory (Finlayson et al., 1988, 1991, 1997b; Finlayson and von Oertzen, 1993; Finlayson, 1995; Jonauskas, 1996; Whitehead and Chatto, 1996; Storrs and Finlayson, 1997; Cowie et al., 2000). These reviews have illustrated the extent of available information and the major gaps that exist, and invariably pointed out that the inventory base was incomplete (Finlayson et al., 1997b; Storrs and Finlayson, 1997; Spiers and Finlayson, 1999). The information gaps in the Northern Territory are in part a consequence of the extent and nature of the terrain, much of it isolated and rugged, as well as policy decisions that have biased inventory and analysis to iconic areas or areas being considered for development. As a consequence, the information base is much better where analyses have been driven by interest in economic development, such as intensive cattle feeding, recreational fishing, tourism and mining, and the advent of adverse change (see reviews by Finlayson and von Oertzen, 1993; Finlayson et al., 1997b; Storrs and Finlayson, 1997).
The analyses that have been done have often been site specific, and in many cases suffer from an absence of comparative data with other wetland landscapes, both near and further afield. The extent of knowledge about plant growth on the freshwater floodplains in the Northern Territory component of the wet–dry tropics is reviewed and covers the following: the relationships between the climate and the hydrological regime on the floodplain; the vegetation patterns, succession and adaptation; and primary production.
CLIMATE AND HYDROLOGY
The climate of the wet–dry tropics plays an important role in shaping the nature and dynamics of the region. It is generally taken to comprise two very broad seasons: the wet season, which commences late in the year (November–December) and lasts for 3–4 months, and the dry season (Taylor and Tulloch, 1985). This generalized description is shown schematically in Fig. 2. The most significant features of the wet season are thunderstorms, tropical cyclones and rain depressions. As cyclones move inland, they form rain depressions and are an important source of rain. Rainfall is also associated with monsoonal troughs, with 2–3 occurring each year, which usually produce widespread cloud and rainfall, regional convection that provides localized showers and easterly disturbances that, in some years, extend the rainy season beyond its normal limits. The dry season is characterized by south-east trade winds.
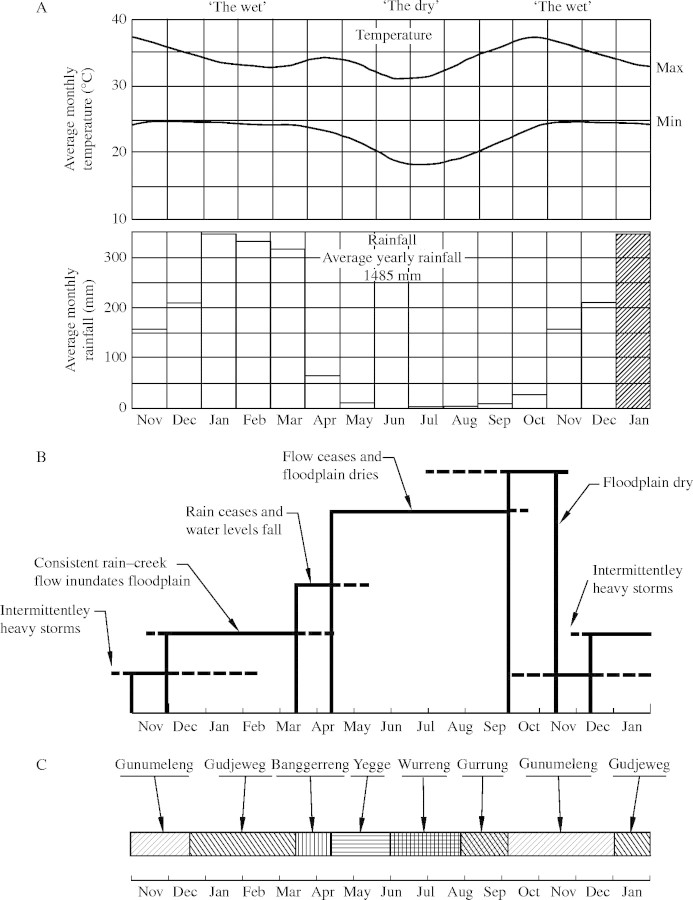
Fig. 2.
Generalized representation of: (A) the climate in Darwin in the Australian wet–dry tropics; (B) hydrological changes on the Magela floodplain in Kakadu National Park (variability is represented by dashed lines); and (C) an Aboriginal calendar from Kakadu National Park. A 14 month period is shown to illustrate the extension of seasons across the calendar year. The meteorological information was derived from Hoatson et al. (2000), hydrological information was adapted from Sanderson et al. (1983) by Finlayson et al. (1990a), and the aboriginal calendar from Ovington (1986) and Morris (1996). The aboriginal seasons are described in the following manner: Gunumeleng, pre-monsoon season; Gudjewg, monsoon season; Banggerreng, harvest time; Yegge, cool weather time; Wurreng, early dry season; Gurrung, hot dry season.
Mean monthly data for temperature, rainfall, evaporation and relative humidity recorded in Darwin (on the coast) and Katherine (approx. 300 km inland to the south) are given in Table 1. In general, temperatures are warm to hot, with more humid conditions near the coast. These data represent the general patterns, but disguise the considerable variation in timing and duration of the monsoonal rains. While very little rain falls during the dry season, the amount that does fall is more variable than during the wet season (Taylor and Tulloch, 1985).
Table 1.
Mean monthly rainfall (mm), maximum and minimum temperatures (°C), mean monthly evaporation (mm) and mean relative humidity (%) at 9 a.m. and 3 p.m. for Darwin and Katherine (300 km to the south)
| Jan |
Feb |
Mar |
Apr |
May |
Jun |
Jul |
Aug |
Sep |
Oct |
Nov |
Dec |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Darwin | ||||||||||||||||||||||||
| Rainfall | 414 | 341 | 306 | 100 | 21 | 1 | 1 | 6 | 18 | 71 | 143 | 229 | ||||||||||||
| Maximum temperature | 31·8 | 31·4 | 31·9 | 32·6 | 32·0 | 30·5 | 30·4 | 31·2 | 32·4 | 33·1 | 33·1 | 32·6 | ||||||||||||
| Minimum temperature | 24·8 | 24·6 | 24·5 | 24·0 | 22·1 | 20·0 | 19·3 | 20·6 | 23·1 | 25·0 | 25·3 | 25·3 | ||||||||||||
| Evaporation | 208 | 171 | 192 | 220 | 223 | 216 | 226 | 239 | 249 | 267 | 243 | 226 | ||||||||||||
| Humidity 9 a.m. | 82 | 83 | 83 | 75 | 67 | 62 | 63 | 67 | 71 | 71 | 73 | 77 | ||||||||||||
| Humidity 3 p.m. | 70 | 72 | 67 | 52 | 43 | 39 | 38 | 41 | 48 | 53 | 59 | 65 | ||||||||||||
| Katherine | ||||||||||||||||||||||||
| Rainfall | 233 | 214 | 163 | 33 | 6 | 2 | 1 | 1 | 6 | 30 | 87 | 194 | ||||||||||||
| Maximum temperature | 35·0 | 34·3 | 34·5 | 34·0 | 32·1 | 30·0 | 30·1 | 32·5 | 35·4 | 37·7 | 38·0 | 36·5 | ||||||||||||
| Minimum temperature | 24·0 | 23·7 | 22·9 | 20·4 | 17·1 | 14·1 | 13·2 | 15·5 | 19·6 | 23·6 | 24·7 | 24·4 | ||||||||||||
| Evaporation | 199 | 146 | 168 | 195 | 197 | 165 | 193 | 232 | 273 | 313 | 277 | 247 | ||||||||||||
| Humidity 9 a.m. | 77 | 81 | 77 | 64 | 58 | 56 | 52 | 52 | 51 | 56 | 61 | 70 | ||||||||||||
| Humidity 3 p.m. | 53 | 55 | 49 | 36 | 34 | 31 | 27 | 25 | 25 | 27 | 33 | 44 | ||||||||||||
Adapted from Storrs and Finlayson (1997).
Sources: Bureau of Meteorology, Darwin. Mean monthly evaporation data for Katherine supplied by CSIRO, Tropical Ecosystems Research Centre.
The more northern region has warm to hot temperatures all year round, while further south the temperatures are milder during the dry season (Table 1). In the wet season, warm temperatures in Darwin are accompanied by relative humidity of ∼80 %. Cloud cover is greatest during the wet season, decreasing over the interior and allowing overnight convective cooling.
Local Aboriginal people have a refined perception of the climate compared with many non-Aboriginal people in the region and recognize six seasons based on the relationship between changes in the weather and the availability of food items (Ovington, 1986; Morris, 1996). The calendar they recognize is usually presented in a circular manner, but in Fig. 2 it is presented in a linear manner and compared with the monthly meteorological data from Darwin. The patterns outlined by the Aboriginal calendar are readily identifiable from the meteorological data and illustrate the relationships that exist between plant growth and seasonal changes, although, as with the climate data, the representation of the Aboriginal calendar does not indicate the extent of the variability that occurs. The relationship between the knowledge base developed over millennia by Aboriginal people and the more recently obtained meteorological data is also shown by the generalized hydrological cycle that was developed separately from field observations at Magela Creek and floodplain in Kakadu National Park (developed by Sanderson et al., 1983, and modified by Finlayson et al., 1990a), and is also presented in Fig. 2.
The hydrological cycle has been identified as an important factor in shaping the nature of the vegetation in the freshwater wetlands (Williams, 1979; Taylor and Dunlop, 1985; Finlayson et al., 1989). Water flows on a seasonal basis, commencing early in the wet season and lasting until after the end of the rains. It consists of a series of flood events superimposed on a ‘base’ flow. At the start of the wet season, intermittent rain storms saturate the soils and as more consistent rain occurs, water collects in the creeks and thence in the large tidal rivers. Once the creeks and rivers are full, the freshwater spills across the floodplains and can cover them to a depth of several metres. The base flow in the creeks is <5 m3 s−1, with peak flows late in the wet season, reaching, and exceeding on occasions, 1000 m3 s−1. Flooding occurs once the catchment is saturated; heavy falls of rain later in the season generate more widespread flooding and discharge than equivalent flows earlier in the season. Freshwater flow in the creeks and rivers ceases within a few months of the end of the rains, and the creeks and floodplains dry out except for a few permanent swamps and billabongs (Finlayson et al., 1990a). Except for the tidally influenced channels, most creeks dry up, with a few pockets of water left in billabongs and permanent swamps. The spring tidal range in van Diemen Gulf is 5–6 m, and estuarine water can extend >100 km upstream and generally remains within the stream channel (Woodroffe et al., 1989). The groundwater level in the surrounding landscape is recharged by the wet season rains, but can fall 2–4 m during the dry season. Some creeks or river reaches are fed by springs or groundwater seeps.
VEGETATION PATTERNS
A broad-scale analysis of the floodplain vegetation across the Northern Territory was undertaken by Wilson et al. (1990), while specific published botanical studies are available for a few wetlands, such as those for the Mary River (Bach and Hoskings, 2002), the Arafura Swamp (Williams and Chudleigh, 2003) and the Magela floodplain (Finlayson et al., 1989; Finlayson, 1993). The vegetation patterns shown by these investigations are reviewed before considering information on plant growth and primary production from freshwater habitats on the Magela floodplain, located along a tributary of the East Alligator River and largely within Kakadu National Park (Finlayson and Woodroffe, 1996).
Lists of freshwater macrophytic species have been compiled (see Adams et al., 1973; Lazarides and Craven, 1980; Cowie and Finlayson, 1986; Brennan, 1993). There are many differences in these lists as a result of the collecting effort and areas covered, as well as mis-identifications and changes in the taxonomy. Cowie et al. (2000) considered this information while producing a flora of the floodplains with a key to the families of macro-algae and vascular plants. Vegetation maps at a scale of 1 : 50 000 to 1 : 100 000 have been produced for some floodplains (Finlayson et al., 1989; Bach and Hosking, 2002; Williams and Chudleigh, 2003), based on analyses of species distributions obtained largely from transect data and remotely sensed images [including photography and satellite images; see Phinn et al. (1999) for a review of the application of remote sensing to wetlands]. In many instances, these analyses have pointed out the large variability in species occurrence and dominance; Sanderson et al. (1983) determined that natural variability could undermine the usefulness of detailed mapping and analyses, and consequently resorted to less detailed (i.e. smaller scale) mapping in order to represent general and not specific changes in vegetation. Unless the issue of scale is considered in relation to the purpose (and hypothesis if a formal monitoring programme is undertaken; see Finlayson, 1994, 1996) of the sampling exercise, it is likely that the extent of natural variability could confound many analyses.
Briggs (1981) produced a generalized structural and floristic description of the vegetation associated with the seasonally inundated floodplains, including the fringing woodland and forests, and billabongs (seasonally or permanently inundated lagoons associated with the floodplain or river channels). This description was generalized and updated to accommodate the known variability and is presented below using updated taxonomic nomenclature (Cowie et al., 2000).
Paperbark swamp forest dominated by trees including Melaleuca viridiflora, Melaleuca cajaputi and Melaleuca leucadendra, and to a lesser extent Barringtonia acutangula and Pandanus spp. The forests are inundated by up to a metre of water during the wet season and are dry (lack surface water) at other times. Sedges and floating-leaved and submerged aquatic plants form an understorey during the wet.
Eleocharis sedgelands dominated by Eleocharis dulcis and Eleocharis sphacelata in association with other sedge and grass species, most notably Oryza meridionalis.
Wet grasslands containing many species including Pseudoraphis spinescens, Leersia hexandra, Echinochloa spp. and sedges, mainly Cyperus, Fimbristylis and Fuirena species.
Floating and floating-leaved herblands on the seasonally inundated plains and in amongst the paperbark forests, including Nelumbo nucifera, and Nymphaea and Nymphoides species.
Submerged and emergent herblands with floating and floating-leaved species, such as Triglochin dubium, Caldesia oligococca, Limnophila australis, Ludwigia adscendens, Utricularia spp., Eleocharis spp. and Vallisneria nana.
These generalized habitats present an overview of the wetland vegetation that is not available from more recent narrowly focused analyses. The latter have tended towards analyses of individual wetlands or floodplains and provide specific data without describing the general or characteristic patterns of seasonal and inter-annual change across the landscape. As a consequence, many recent analyses do not account sufficiently for the high natural variability between season and years or between floodplains, nor present their results within the context of the wider landscape vegetation patterns. Further, Sanderson et al. (1983) noted that an overemphasis on detailed and short-term sampling did not account sufficiently for the high natural variability. The wisdom provided by Sanderson et al. (1983) and others does not seem to have been applied in some more recent analyses that are restricted to a few years sampling and seemingly have not been based on a thorough review of previous sampling! The latter is extremely important when developing a monitoring regime that can detect change based on hypotheses that enable natural variability to be separated from anthropogenic change. Finlayson and Mitchell (1999) provide an appraisal of weaknesses in wetland monitoring, while Finlayson (1996) provides a framework that emphasizes the need to construct a valid hypothesis and undertake a pilot project to test all underlying assumptions, including dealing with natural variability.
Finlayson et al. (1989) built on the information provided by Sanderson et al. (1983) and prepared a generalized vegetation map for the Magela floodplain based on observations from 5–6 years and in particular the wet seasons when many of the aquatic plants reach their peak biomass and are easier to differentiate and map. This description is based on more comprehensive information than an earlier effort by Williams (1979) for the same floodplain, including the fringing seasonally inundated areas. It is unknown how well this description reflects the situation on other floodplains, especially given changes that have occurred on many with the removal of feral buffaloes (Bubalus bubalis) (Skeat et al., 1996). The ten communities that were identified are shown in Fig. 3 and briefly described below.
Melaleuca open forest and woodland (tree canopy cover of 10–70 %): areas dominated by one or more Melaleuca species—M. viridiflora and M. cajaputi around the edges and at the northern end of the floodplain, and M. leucadendra in back-swamps inundated for 6–8 months. The understorey varies.
Melaleuca open woodland (tree canopy cover <10 %): M. leucadendra in areas inundated for >6 months. Understorey species are usually the same as those in adjacent areas of the floodplain.
Nelumbo–Nymphoides herbland: a mixed community dominated by the water lilies N. nucifera and Nymphoides indica that occur in permanently and semi-permanently wet areas.
Orzya grassland: dominated by O. meridionalis towards the end of the wet season. In the dry season, it consists of bare ground and dead O. meridionalis stems with persistent Phyla nodiflora and L. adscendens as xerophytic forms, and P. spinescens.
Hymenachne grassland: dominated by Hymenachne acutigluma throughout the year.
Pseudoraphis grassland: dominated by the perennial emergent grass P. spinescens which has a turf-like habit during the dry season and grows up through the water during the wet season.
Hymenachne–Eleocharis grass–sedgeland: swampy areas that dry out seasonally and are dominated by H. acutigluma or Eleocharis spp., which are slower to establish.
Mixed grass–sedge–herbland: a variety of species with the dominant species depending on the topographic situation. Oryza meridionalis occurs on the drier sites, with P. spinescens in slightly wetter places, while Eleocharis spp. and H. acutigluma occur in the deeper sites. On sites that remain flooded for 10–11 months N. indica and Nymphaea macrosperma may be present.
Eleocharis sedgeland: Eleocharis spp. dominate during the wet season, but are replaced by annual herbs during the dry season;
Open-water community: permanent billabongs, flow channels and shallow waterholes contain N. macrosperma and Nymphaea pubescens and a number of submerged plant species. Floating grass mats comprised of L. hexandra, H. acutigluma and Urochloa mutica along with the herb L. adscendens occur along the banks of the billabongs.
Fig. 3.
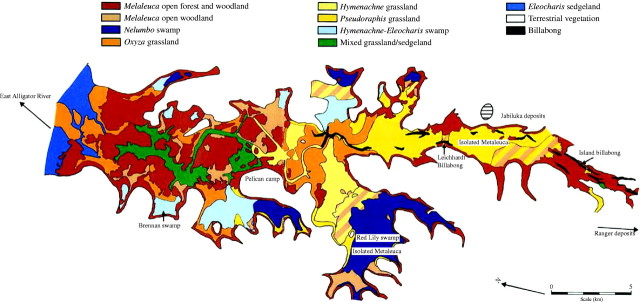
Vegetation pattern on the Magela floodplain (from Finlayson et al., 1989).
More recent analyses on the Magela have shown that these communities still predominate along with several invasive species, in particular Salvinia molesta and U. mutica, and the native species Sesbania sesbans which intermittently dominates some areas of the plain (J. Lowry, pers. comm. 2003). The reasons for the temporal changes in the occurrence of the latter are not known.
Waterholes or lagoons that retain water seasonally or permanently are a common feature of the floodplains and rivers and provide important habitats for many animal species at different times of the year (e.g. fish species; Bishop et al., 2001). Locally, these waterholes are referred to as ‘billabongs’, a term that strictly refers to oxbow lakes (Bayly and Williams, 1977) that are widely taken to include all waterholes and lagoons (Finlayson et al., 1989). Walker and Tyler (1985) identified three categories of billabongs for Magela Creek and floodplain: (a) channel billabongs or depressions in flow channels; (b) backflow billabongs located on small tributaries and initially filled by water from the main creek rather than from the tributary; and (c) floodplain billabongs that are generally remnants of deep channels on the floodplain. The floodplain and channel billabongs are, on the whole, deeper and have steeper sides than the backflow billabongs. Thus, in the former, the vegetation, with the exception of the floating aquatic plants, is restricted to a narrow belt around the edge of the billabongs, whereas the backflow billabongs are at times almost completely covered with emergent, submerged and floating-leaved plants (Finlayson et al., 1993a). The vegetation of the floodplain billabongs is greatly influenced by the adjacent plant communities on the seasonally inundated floodplain (e.g. grass mats extending across the floodplain and into the billabongs), whereas the backflow billabongs often have terrestrial vegetation in close proximity and abutting the fringing Melaleuca woodlands.
Finlayson et al. (1993a) reported a semi-quantitative analyses of vegetation dominance in three backflow billabongs (Georgetown, Coonjimba and Djalkmara) along Magela Creek and two floodplain billabongs (Leichhardt and Jabiluka) on the Magela floodplain. The three backflow billabongs had a generalized vegetation zonation consisting of: (a) fringing Melaleuca spp. woodland in seasonally inundated areas; (b) a mix of grasses and sedges in seasonally inundated areas shaded by woodland; (c) a belt of Eleocharis sp. in water that is usually <1·5 m deep during the wet season; (d) a small area of open water that is usually 1·5–2·0 m deep in the wet season; and (e) patches of water lilies and submerged plants along the boundary between the sedges and the open water.
The dominant plant species, based on ‘visual biomass’ and ‘ground’ cover, are the Melaleuca spp. trees and the geophytic, perennial Eleocharis spp. sedges. In contrast, the two floodplain billabongs had a generalized vegetation zonation comprising: (a) fringing Melaleuca spp., Pandanus aquaticus and B. acutangula trees along a levee that comprised the western bank; (b) a mix of grasses and sedges and a few trees interfacing with the floodplain grass communities along the gently sloping eastern bank; (c) a mix of grasses, herbs and sedge overlaying a floating mat of S. molesta extending from the banks towards the middle of the billabong; and (d) a discontinuous fringe of submerged plants and water lilies along the edge of the floating mat.
Over the past decade, the vegetation in the backflow billabongs has changed considerably, with an increase in abundance of Eleocharis spp., attributed to the virtual elimination of feral buffaloes (B. bubalis) from the vicinity of the billabongs. Grazing, pugging (hoof prints left in the mud) and wallowing by buffaloes previously prevented these plants from dominating or even establishing (Skeat et al., 1996).
VEGETATION HISTORY AND SUCCESSION
In recent years, there has been increased interest in determining the vegetation history of the floodplains, largely due to management interest in response to known changes and pressures resulting in adverse change (Finlayson et al., 1997a; Storrs and Finlayson, 1997). There have been a series of studies which generally support the notion of widespread vegetation changes on the wetlands over the past 8000 years or so (Chappell and Grindrod, 1985; Hope et al., 1985; Russell-Smith, 1985; Woodroffe et al., 1985; Clark and Guppy, 1988; Grindrod, 1988). Finlayson and Woodroffe (1996) provide a summary of these investigations. In brief, the stratigraphy of the deltaic–estuarine plain of the South Alligator River, and probably the other river systems in the region, indicates that the wetlands developed in three major phases: (1) the transgressive phase (8000–6000 years ago) which was characterized by marine incursion into the prior valley and terrestrial ecosystems were displaced by mangrove forests as the sea level rose; (2) the big swamp phase (6800–5300 years ago) when the sea stabilized around its present level and mangrove forests established over most of the present-day floodplains for around 6000 years; and (3) the sinuous/cuspate phase (approx. 5300 years ago) during which the river re-established a distinct channel meandering across the estuarine plains and the widespread mangroves were replaced with the grasses and sedges characteristic of the present-day freshwater wetlands.
In recent decades, the vegetation has undergone considerable change as a consequence of individual and multiple pressures, such as invasion by feral animals, especially buffalo, pigs (Sus scrofa) and, more recently, cane toads (Bufo marinus); as well as weeds, especially mimosa (Mimosa pigra), salvinia (S. molesta) and paragrass (U. mutica); changes in fire regimes; and saline intrusion (Cowie, 1996; Skeat et al., 1996; Bayliss et al., 1997; Storrs and Finlayson, 1997; Eliot et al., 1999; van Dam et al., 2002). Individual analyses and management regimes have been imposed to assess changes in vegetation as a consequence of these pressures with various monitoring programmes (e.g. for saline intrusion and weed invasion; Bach and Hosking, 2002) and management responses (e.g. Jonauskas, 1996; Finlayson et al., 1997a); however, there have been few attempts to predict changes or project future scenarios for vegetation management.
Given the importance of the water regime in determining the occurrence and distribution of plants species on the floodplains, e.g. Finlayson et al. (1989) determined that both the duration and depth of inundation greatly influenced the distribution of plant species, and attempted to explain this through an empirical model of plant succession in wetlands, developed by Van der Valk (1981), to assess changes in vegetation that occur as a result of changes in the annual hydrological pattern (Finlayson, 1990; Finlayson and Woodroffe, 1996). The model was tested using information from the general vegetation studies described above, and an analysis of the seedbank in grasslands on the Magela floodplain (Finlayson et al., 1990b). In undertaking this exercise, it was necessary to recognize that not only does the model have limitations (e.g. it does not take into account interactions between the plants), but the input information (i.e. ecological information on the many plant species involved) was limited. Despite these limitations, the model provided a framework around which to predict changes in the vegetation patterns, i.e. compare the predicted with the actual situation.
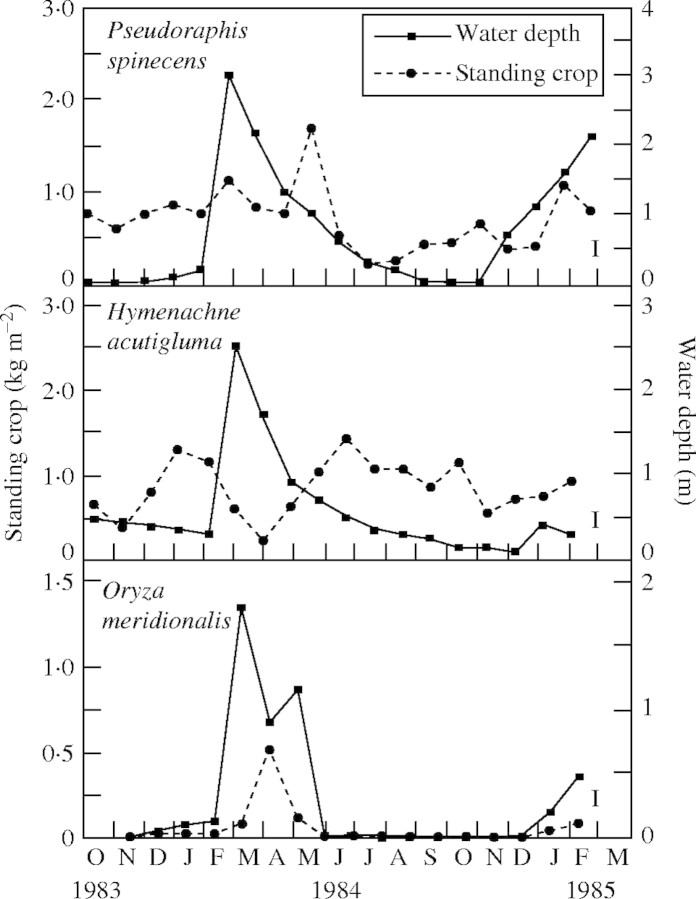
For the purposes of validating the model, the 1983–1984 hydrological cycle was considered (Finlayson, 1990; Finlayson and Woodroffe, 1996). Seasonal changes in species dominance in three widespread grass communities under these hydrological conditions are shown in Fig. 4. The species ‘successional states’ used in the model are explained in Fig. 5 (derived from van der Valk, 1981) and presented with alpha-numeric codes in parentheses in the text below.
Fig. 4.
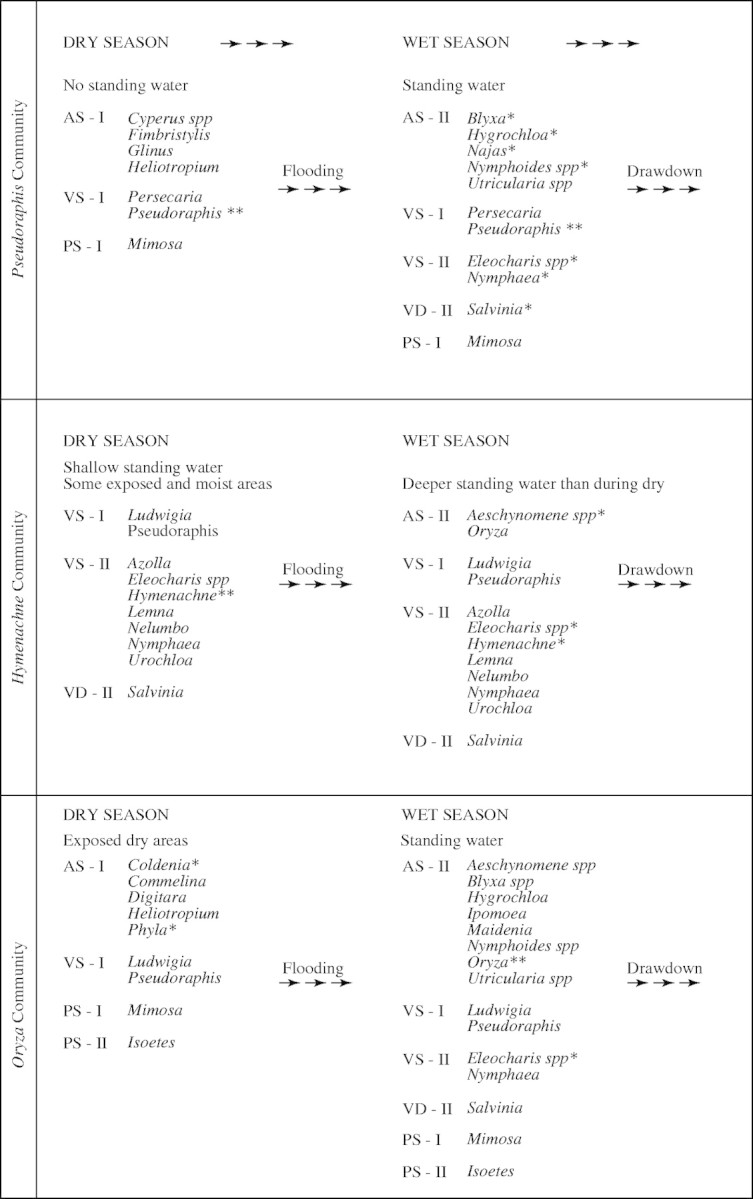
Predicted species succession due to water level fluctuations on the Magela floodplain during the 1983–1984 wet–dry cycle. The dominant species in each community is indicated by ** and the next dominant by * (A = annual; V = vegetative; P = perennial; S = short-lived propagules; D = long-lived propagules; I = propagules established in areas devoid of standing water; II = propagules established in standing water) (from Finlayson et al., 1990b).
Fig. 5.
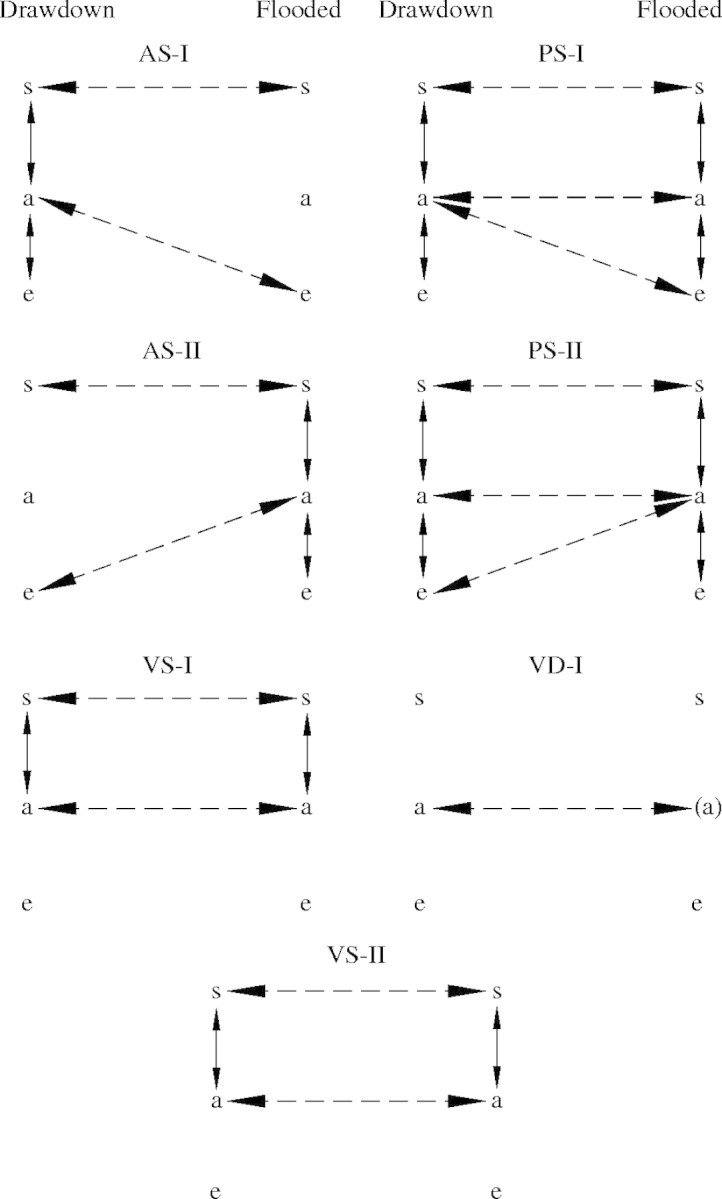
Potential species transitions between two ‘environmental situations’—increasing (i.e. flooding) and decreasing (i.e. drawdown) water periods in a wetland. Solid lines represent potential transitions within an environmental situation; dashed lines represent transitions between environmental situations. The species states are: s = present as long-lived propagules in a persistent seedbank; a = mature adults; and e = locally extinct. If establishment is dependent on dispersal from another site, adult populations are indicated in parentheses. Other abbreviations are explained in Fig. 4 (from Finlayson et al., 1990b).
The importance of water depth and period of inundation in determining these patterns was borne out by application of the model to the data from 1983–1984. In particular, the sparsely distributed annuals (AS-1) that occur during the dry season were replaced by wet season annuals (AS-II) dominated by O. meridionalis and the vegetatively reproducing Eleocharis spp. [VS-(II)]. In terms of successional changes that may occur on the floodplains, the introduced weeds M. pigra (PS-I) and S. molesta [VD-(II)] have the potential to alter both the seasonal vegetation changes and succession between years, albeit with the former more likely to affect the seasonally inundated parts of the floodplain and the latter the billabongs or few permanent swamps.
Mimosa pigra can survive throughout both the dry and wet season almost regardless of the growing conditions (Walden et al., 2004) and can rapidly spread by seed. In contrast, the vegetatively reproducing S. molesta is widespread during the wet season, but more restricted during the dry season as it does not have propagules in the sediment seedbank and can only survive in areas of permanent water. However, it does have the growth potential (Finlayson, 1984) to cover open water areas completely and change the vegetation structure and composition (e.g. the loss of wet season annuals). The ‘successional states’ of the plants can be used to determine which plants are likely to survive changes in the seasonal flooding pattern and also the likely survival of introduced species between seasons and between years from propagules stored within the wetland.
As a further example, changes in the floodplain vegetation due to the occurrence of feral animals could be predicted by using the empirical vegetation model and identifying plants that have the capability to spread under the conditions that are likely to prevail. Of particular importance has been the impact of the water buffalo which was well known to denude many seasonally inundated floodplains of much of the grass, sedge and herb cover. Whilst the presence of buffalo caused widespread changes to the floodplain vegetation, it is the changes that have occurred as a result of the removal of the buffalo that are now of interest. In particular, the popular expectation that once buffalo were removed the floodplains would re-establish a natural vegetation pattern has not been met, partly because there was no clear understanding of the nature of the natural vegetation, let alone unexpected ecological outcomes! As an example, there was an expectation that the reed Phragmites vallatoria would re-establish, having been reported by early European explorers as having dominated the plains. The veracity of these general claims has not been proved and nor has this species spread greatly since the buffalo were removed. This may not be an unexpected outcome given that the sediment on the floodplains may not have contained sufficient seeds or vegetative propagules of this species. In contrast, the perennial grass H. acutigluma has spread and now dominates large areas of the floodplain; an unexpected outcome given low correspondence between the occurrence of seeds in the substrate and the species occurrence (Finlayson et al., 1990b). In addition, the introduced grass U. mutica has continued to spread, presumably through vegetative propagules (Knerr, 1998).
The expected seasonal cycles in the main plant communities on the floodplains can be partly shown in the model diagrams (Figs 4 and 5). Any substantial changes can be noted and decisions made about the need and possibility of managing them based on the mode of distribution and reproduction. Further information on successional directions following major disturbance (e.g. the introduction of weeds or feral animals) is needed if management of this dynamically variable flora (and hence the habitat) is to be prescriptive, rather than reactive. Recent effort has focused on changes in the fire regime and the inter-relationships between native and introduced species (Knerr, 1998; Bayliss and Finlayson, 2005). Such analyses, however, have been driven more by explicit empirical concepts rather than analyses based on conceptual models and an understanding of the biology of the plant species most involved.
As an example of the complexities of plant growth on the floodplain, it is speculated that the large populations of waterbirds, especially the magpie goose (Anseranus semipalmata), have the capacity to alter the vegetation on which they depend. At times, some 1–2 million magpie geese can be present on some floodplain wetlands for feeding or nesting (Bayliss and Yeomans, 1990), with major effects on the vegetation that they consume (e.g. seeds of O. meridionalis or tubers of E. dulcis) or use for nesting (E. sphacelata). For example, does the consumption of tubers of E. dulcis by a large population of birds affect the growth of this species? Or, does damage done to the emergent stems of E. sphacelata by the geese when nesting impair transport of oxygen to submerged organs and thus impact on the growth of this important species? In some years, the extent of these sedge species has been greatly reduced—this has been attributed to prevailing hydrological conditions, but is it beyond the realm to consider that large numbers of geese in any one year may adversely affect the occurrence of these sedges in the next? These and many other biotic interactions could be investigated through empirical models and linked with knowledge from both eco-physiological analyses of plant processes and growth and hydrological analyses. Such analyses have not attracted support.
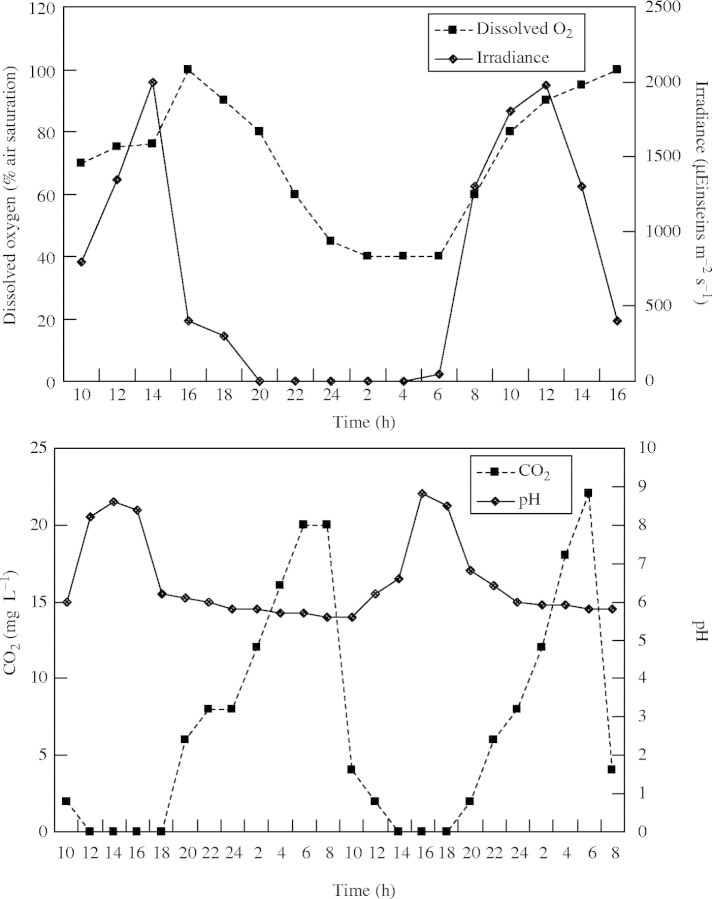
We understand that hydrological conditions greatly influence the nature of plant growth on the floodplains, but we seem blissfully unaware of how the hydrology interacts with the many other features of plant growth, e.g. nutrient and energy pathways, propagule germination and establishment. This is not a recent realization—Finlayson et al. (1984) postulated that the growth of plants in tropical lakes with low concentrations of nutrients in the water was dependent on a chemostat interaction where the rate of nutrient supply from the nutrient-enriched substrate to the water was determined by the rate of growth of the plant. This concept calls into question the simple use of nutrient concentrations as an indicator of the growth potential of at least some aquatic vegetation, noting that many plant species in these environments have particular adaptations that enable them to flourish under low incipient nutrient concentrations. Further, the water chemistry data and interpretations may be more complex than often reported. As an example, the water on the Magela floodplain is often considered to be acidic to neutral (Finlayson et al., 1990a) based on analyses that have on the whole been taken in the permanent billabongs and not across the open seasonally inundated plains. Analyses of the water quality within thick stands of submerged herbs and emergent grasses late in the wet season illustrate that not only does the dissolved O2 and CO2 vary inversely as expected (due to changes in photosynthesis and respiration rates), but also that the pH reaches alkaline values in late afternoon when the CO2 is at its lowest (Fig. 6; B. Bailey, unpubl. res. 1995). Does this make the floodplain environment acidic or alkaline? Understanding the nature of these changes and characteristics and how they affect plant growth in these environments is often done on the basis of comparisons of tissue analyses and not through detailed eco-physiological investigations of growth factors or substrate conditions, e.g. the role of flooding and anaerobiosis in plant growth has not been investigated for these species and environments. More sophisticated analyses are required.
Fig. 6.
Daily changes in water quality in mixed stands of emergent herbs and grasses on the Magela floodplain during the wet season (from B. Bailey, unpubl. res. 1995). (Dissolved oxygen units, percentage air saturation; irradiance, μEinsteins m−2 s−1; carbon dioxide, mg L−1).
GROWTH STRATEGIES, FORMS AND ADAPTATIONS
The growth forms of the 222 plant species found on the Magela floodplain across four broad habitat categories, seasonally inundated plain, seasonally inundated fringe zone, billabong and permanent swamp, were reported by Finlayson et al. (1989). The fringe zone habitat covered the edges of the floodplain and included the Melaleuca forests/woodlands. The seasonally inundated plain habitat covered the remainder of the floodplain, except for the permanently wet areas. The seasonally inundated plain and the fringe zone contained approx. 40 and 70 %, respectively, of the species, compared with 20 % in the billabongs and 10 % in the permanent swamps (Table 2). Overall, there were 139 annual species with 102 terrestrial and 37 aquatic species. Eighty-nine of the terrestrial species occurred in the fringe zone; only 27 were found on the plain which is seasonally inundated for a longer period than the fringe zone. Finalyson et al. (1989) provide a listing of growth strategy and form for each species. This included three growth strategies (annual, perennial or geophytic perennial), and two primary (terrestrial or aquatic) and seven secondary growth forms (tree, shrub, grass, sedge, vine, palm or herb). Terrestrial annuals represented a diverse group of species, with 60 of them classified as herbs, 18 as sedges and 17 as grasses. Twenty-seven of the aquatic annuals are herbs and six are shrubs. There were 68 perennial species, 50 in the fringe zone (Table 2). Thirty-four of the perennials are terrestrial, 26 aquatic, with eight others difficult to classify. There are 12 terrestrial trees including Eucalyptus spp., Pandanus spiralis, Lophostemon lactifluus and Syzygium suborbiculare. The aquatic perennial species are dominated by 12 herbs, including Hydrilla verticillata, L. adscendens, N. nucifera and N. indica, and by five grasses, including the widespread H. acutigluma and P. spinescens. There were 14 geophytic species; the more widespread include the Nymphaea and Eleocharis species.
Table 2.
Plant species found in four broad habitat areas on the Magela floodplain
| Habitat |
Total species |
Annuals |
Perennials |
Geophytic perennials |
|---|---|---|---|---|
| Permanent billabongs | 46 | 19 | 21 | 6 |
| Seasonally inundated floodplain | 94 | 57 | 29 | 8 |
| Fringe zone | 158 | 100 | 50 | 8 |
| Permanent swamps | 21 | 5 | 11 | 5 |
From Finlayson et al. (1989).
Within the broad categorization of growth strategies and forms, there are many morphological and physiological adaptations to the annual wet–dry environment with specific features that enable particular species to inhabit various niches within the cycles of dry and wet conditions. Finlayson et al. (1989) postulated that the duration of the period of inundation was a major determinant of the vegetation composition of the Magela floodplain along with the depth of water and the velocity of water flow. They further hypothesized that the pattern of vegetation variation was a function of both the flooding and drying phases of the hydrological cycle. The nature of the floodplain environment, especially the variability due to changes in the hydrological cycle, has resulted in many specific adaptations that enable the plants to establish and grow. Specific assessments of these adaptations have not been undertaken, although Cowie et al. (2000) provide a review for plant species from the freshwater floodplains in the northernmost part of the Northern Territory; some of the more obvious are presented here.
Aerenchyma, air cavities and corky tissue assist in gas exchange and buoyancy, and are common features, especially in some floating-leaved and emergent species. These features include air cavities in the leaves of Nymphoides species, and longitudinal air passages in the stems of Nymphaea and Limnophila species and in the thickened rhizomes of E. sphacelata and Lepironia articulata. The latter two species, in common with E. dulcis, have hollow, thin-walled stems with regularly spaced transverse septa. Species such as Bambusa arhemica, Paspalidium udum and Phragmites vallatoria have more or less hollow stems with partitions at the nodes, while others, such as Ipomoea aquatica, do not have partitions, although many of them develop adventitious roots on submerged nodes or other submerged parts of the stem. Many emergent species develop spongy or corky tissue in response to inundation, such as L. adscendens with inflated, white aerenchyma-filled floating roots, Aeschynomene aspera and Sphenoclea zeylandica with thickened corky aerenchyma and adventitious roots, and S. sesban with soft spongy bark on inundated stems. Many Cyperaceae have spongy aerenchymous stems often divided by longitudinal partitions. Trees on the floodplains often have modified bark structures; such as corky bark in Sesbania formosa and B. acutangula, and papery bark with internal longitudinal air passages in Melaleuca species.
The majority of dispersal mechanisms involve water, even though many parts of the floodplains are drier for a longer period than they are wet. Floating plants are transported in their entirety and typically produce small quantities of seeds or reproduce almost exclusively by vegetative means; this group includes the Lemnaceae, the ferns Azolla and Salvinia, Pistia stratiotes and the floating species of Utricularia. Fragmentation of submerged species is common, as in H. verticillata, Najas species and Ceratophyllum demersum. The grasses H. acutigluma, Panicum paludosum and P. spinescens are examples of emergent species with trailing stems that form roots at the nodes. The development of corky tissue or aerenchyma on the seed or fruit to provide floatation is common, for example, in Acanthus ilicifolius, B. acutangula and P. aquaticus. In legumes such as Cathormion umbellatum, Peltophorum pterocarpum and Aeschynomene aspera, the pod or articles of the pod are indehiscent and may be air filled in addition to having corky walls. Buoyancy in the seed or fruit in other species is achieved by chambers of various forms, while other species have seeds small enough to float by surface tension.
PRODUCTIVITY
Information on the productivity of the floodplain vegetation is available from the Magela (Finlayson, 1988, 1991), covering seasonal changes in dry weight of aquatic grasses and litterfall from Melaleuca trees. Changes in aboveground biomass (dry weight/unit area) for the dominant aquatic grasses P. spinescens, H. acutigluma and O. meridionalis were determined from samples collected during 1983–1984; the sampling was not extended over a longer period due to the danger presented by large individuals of Crocodylus porosus (Finlayson, 1991). The dry aboveground biomass of each species appears to be influenced by water depth, which in itself is a function of the rainfall and surface water flow. For two species, the increased biomass occurs at higher water levels, but not for the third, H. acutigluma (Fig. 7).
Fig. 7.
Above-ground dry biomass of three aquatic grass species (from Finlayson, 1991). The data were obtained from five quadrats (1 m2) placed in each community at approx. 4 weekly intervals throughout the sample period. The least significant difference (P = 0·05) is shown for each species.
At the start of the wet season, P. spinescens undergoes a change from a turf-like habit on a nearly dry plain to elongated culms that extend up through the water as the depth increases, reaching a maximum biomass of 1·67 ± 0·21 kg m−2 when the water level was falling, and then senescing and reverting to a turf-like appearance. Hymenachne acutigluma, unlike P. spinescens, was growing in a perennial swamp and had a semi-erect/creeping habit with short internodes, and horizontal culms anchored to the substrate by roots at the nodes. Biomass increased after the first rains in November, but then decreased from 0·78 ± 0·10 to 0·23 ± 0·03 kg m−2 following a large increase in water level. A maximum dry weight of 1·41 ± 0·10 kg m−2 was recorded at the end of the wet season when the water level was falling. Oryza meridionalis, an annual species, germinated in November after the first rains of the wet season and continued to grow as the plain filled with water, reaching a maximum biomass of, 0·51 ± 0·10 kg m−2 in April before senescing; this value represented the annual productivity (based on the maximum–minimum method of estimation). Pseudoraphis spinescens had two growth periods with dry matter production of 1·06 ± 0·23 kg m−2 (December–May) and 0·85 ± 0·03 kg m−2 (July–November). Hymenachne acutigluma similarly had two growth periods with 0·96 ± 0·26 kg m−2 (November–January) and 1·19 ± 0·12 kg m−2 (March–June). As these occurred within 1 year, the annual productivity for these two species was 1·91 and 2·09 kg m−2, respectively.
Productivity data for the widespread Melaleuca woodlands and forests on the Magela floodplain are available indirectly through an analysis of litterfall data (Finlayson, 1988; Finlayson et al., 1993b). In an intensively sampled Melaleuca forest on the Magela floodplain, the total litterfall was approx. 0·7 kg m−2 year−1, whereas at another site on the floodplain, less intensively investigated, a value of approx. 1·5 kg m−2 year−1 was recorded (Finlayson, 1988). Comparative data for Melaleuca forests are limited to a few studies of different species in wetlands in southern Australia, with annual litterfalls of 0·39–0·43 kg m−2 year−1 (Finalyson et al., 1993b). Based on an analysis of the relationship between total litterfall and latitude (Lonsdale, 1988), the value of 0·7–1·5 kg m−2 year−1 is within the range recorded for other forests at the same latitude with the higher 1·5 kg m−2 year−1 at the upper limit.
The aboveground biomass of Melaleuca species on the Magela floodplain was also investigated by Finlayson et al. (1993b) using an algorithm that related diameter at breast height (dbh) to tree height and fresh weight. The algorithm was calculated initially separately for M. viridiflora and M. cajaputi, but was combined after determining that there were no significant differences in the range of tree sizes on the experimental site or in the relationships between weight and height: log f = 3·018 (log h) – 0·941 (r = 0·929; d.f. = 27; P < 0·01) and log f = 2·266 (log dbh) – 0·502 (r = 0·984; d.f. = 27; P < 0·01), where f represents fresh weight (kg), h represents tree height and dbh was 1·3 m above the soil. This represented a weight of 260 ± 0·31 t ha−2 and an average tree weight of 775 ± 1·6 kg for M. viridiflora and 1009 ± 1·6 kg for M. cajaputi. The size classes (dbh) ranged from 11·8 to 62·0 cm dbh for M. viridiflora with a median class of 25·1–30·0 cm, and for M. cajaputi from 13·0 to 66·3 cm with a median class size of 30·1–35·0 cm. The average dbh for the two species was not significantly different, with 29·3 ± 1·0 cm for M. viridiflora and 33·5 ± 1·0 cm for M. cajaputi. The experimental site contained 294 trees ha−1, whereas other sites on the floodplain had much higher densities of 433 and 751 trees ha−1; an analysis of tree density across the floodplain was not undertaken, although Williams (1984) reported a 38 % decline in tree density on the floodplain between 1950 and 1975. A recent analysis has shown that the distribution and density of trees on at least part of the floodplain further changed to a considerable extent between 1975 and 1990 (J. Lowry pers. comm. 2003), indicating the dynamic nature of the wetland environment.
CONCLUSIONS
The vegetation of the floodplain wetlands of northern Australia has been mapped at various scales, although there are few specific and long-term analyses of the distribution and successional changes. General vegetation patterns have been identified, as have the general inter-relationships that exist between the climate cycle and plant growth. In particular, the importance of the period of inundation as well as depth of flooding has been identified. Information on morphological and physiological adaptations to the floodplain environment is largely confined to literature reviews, with few specific analyses. Given the extent of change in these environments (e.g. due to invasive species and changes in the fire regime), it is recommended that further specific investigations are undertaken to identify the importance of such adaptations especially in relation to management actions that may result in further change.
The importance of the floodplains for biodiversity is well recognized, although the interactions between the biota are also poorly investigated, especially the inter-relationships between the high primary production and the populous fauna. The recent advent of risk assessments, such as that undertaken for mimosa (Walden et al., 2004), can assist in pointing out knowledge gaps or issues critical for managing the vegetation but, on the whole, the step of linking such assessments with basic knowledge of plant growth, e.g. the many apparent adaptations to anaerobic conditions, and the condition of the substrate and inter-linked energy and nutrient pathways, has not been attempted. It is postulated that management of these valuable habitats can be vastly advanced through more attention to the features of plant growth and distribution.
Acknowledgments
The information summarized and reported in this paper is a representation of the efforts and wisdom of many people from the early 1980s until today. I thank the many earnest and valuable people who have supported the efforts of the Environmental Research Institute of the Supervising Scientist—the work was not always easy as it was a hard environment, but it was done by a dedicated and skillful team. In particular I thank Ian Cowie and Bruce Bailey for their support in the field work and for sharing their botanical knowledge.
LITERATURE CITED
- Adams LG, Byrnes N, Lazarides M. 1973.Floristics of the Alligator Rivers area. In: Alligator Rivers region environmental fact-finding study, part XI, physical features and vegetation (vol. II). Canberra, Australia: AGPS. [Google Scholar]
- Bach C, Hosking EJ. 2002.Wetland monitoring for the Mary River catchment, Northern Territory. Natural Heritage Trust Project No. 97152. Darwin, Australia: Department of Infrastructure, Planning and Environment. [Google Scholar]
- Bayliss P, Finalyson CM. 2005.Landscape-scale and multiple pressure analyses of services provided by tropical floodplains, in the Alligator Rivers Region, northern Australia. Sub-global Assessments, Millennium Ecosystem Assessment. New York: Island Press, in press. [Google Scholar]
- Bayliss P, Yeomans KM. 1990. Seasonal distribution and abundance of magpie geese, Anseranas semipalmata Latham, in the Northern Territory, and their relationship to habitat, 1983–86. Australian Wildlife Research 17: 15–38. [Google Scholar]
- Bayliss B, Brennan K, Eliot I, Finlayson C.M, Hall R, House T, Pidgeon R, Walden D, Waterman P. 1997.Vulnerability assessment of predicted climate change and sea level rise in the Alligator Rivers Region, Northern Territory Australia. Canberra, Australia: Supervising Scientist Report 123, Supervising Scientist. [Google Scholar]
- Bayly IAE, Williams WD. 1977.Inland waters and their ecology. Melbourne, Australia: Longmans. [Google Scholar]
- Bishop KA, Allen SA, Pollard DA, Cook MG. 2001.Ecological studies of the freshwater fishes of the Alligator Rivers Region, Northern Territory: autecology. Darwin, Australia: Supervising Scientist Report 145, Supervising Scientist. [Google Scholar]
- Brennan K. 1990.An annotated checklist of vascular plants of the Alligator Rivers Region, Northern Territory. Darwin, Australia: Supervising Scientist Report 109, Supervising Scientist. [Google Scholar]
- Briggs SV. 1981. Freshwater wetlands. In: Groves RH, ed. Australian vegetation. Cambridge, UK: Cambridge University Press, 335–360. [Google Scholar]
- Chappell J, Grindrod J. 1985. Pollen analysis: key to past mangrove communities and successional changes in north Australian coastal environments. In: Bardsley KN, Davie JDS, Woodroffe CD, eds. Coastal and tidal wetlands of the Australian monsoon region. Darwin, Australia: North Australian Research Unit, ANU Press, 225–236. [Google Scholar]
- Clark RL, Guppy JC. 1988. A transition from mangrove forest to freshwater wetland in the monsoon tropics of Australia. Journal of Biogeography 15: 665–684. [Google Scholar]
- Cowie ID. 1996. Weed ecology. In: Finlayson CM, von Oertzen I eds. Landscape and vegetation ecology of the Kakadu Region, Northern Australia. Dordrecht, The Netherlands: Kluwer Academic Publishers, 113–135. [Google Scholar]
- Cowie ID, Finlayson CM. 1986.Plants of the Alligator Rivers Region, Northern Territory. Canberra, Australia: Supervising Scientist for the Alligator Rivers Region Technical Memorandum 17, AGPS. [Google Scholar]
- Cowie ID, Armstrong MD, Woinarski, JCZ, Brocklehurst PS, Short PS, Dunlop CR. 2000. An overview of the floodplains. In: Cowie ID, Short PS, Osterkamp Madsen M, eds. Floodplain flora: a flora of the coastal floodplains of the Northern Territory, Australia. Canberra, Australia: ABRS, 1–33. [Google Scholar]
- Dunlop CR. 1987.Checklist of vascular plants of the Northern Territory. Darwin, Australia: Conservation Commission of the Northern Territory. [Google Scholar]
- Eliot I, Finlayson CM, Waterman P. 1999. Predicted climate change, sea level rise and wetland management in the Australian wet–dry tropics. Wetlands Ecology and Management 7: 63–81. [Google Scholar]
- Finlayson CM. 1984. Growth rates of Salvinia molesta in Lake Moondarra, Mount Isa, Australia. Aquatic Botany 18: 257–262. [Google Scholar]
- Finlayson CM. 1988. Productivity and nutrient dynamics of seasonally inundated flood plains in the Northern Territory. In: Wade-Marshall D, Loveday P, eds. North Australia: progress and prospects, vol. 2 Flood plain research. Darwin, Australia: ANU Press, 58–83. [Google Scholar]
- Finlayson CM. 1990. Plant ecology and management of an internationally important wetland in monsoonal Australia. In: Kusler JA, Day S, eds. Wetlands and river corridor management. New York: The Association of Wetland Managers, 90–98. [Google Scholar]
- Finlayson CM. 1991. Primary production and major nutrients in three grass species on a tropical flood plain in northern Australia. Aquatic Botany 41: 263–280. [Google Scholar]
- Finlayson CM. 1993. Vegetation changes and biomass on an Australian monsoonal floodplain. In: Gopal B, Hillbricht-Ilkowska A, Wetzel RG, eds. Wetlands and ecotones: studies on land–water interactions. New Delhi, India: National Institute of Ecology & International Scientific Publications, 157–172. [Google Scholar]
- Finlayson CM. 1994. Monitoring ecological change in wetlands. In: Aubrecht G, Dick G, Prentice C, eds. Monitoring ecological change in wetlands of Middle Europe. Slimbridge, UK: IWRB Special Publication 30, 163–180. [Google Scholar]
- Finlayson CM. (ed.) 1995.Wetland research in the wet–dry tropics of Australia. Workshop, Jabiru NT 22–24 March 1995. Canberra, Australia: Supervising Scientist Report 101. [Google Scholar]
- Finlayson CM. 1996. Framework for designing a monitoring programme. In: Tomas Vives P, ed. Monitoring Mediterranean wetlands: a methodological guide. Slimbrudge, UK and Lisbon, Portugal: MedWet Publication, Wetlands International, 25–34. [Google Scholar]
- Finlayson CM, Mitchell DS. 1999. Australian wetlands: the monitoring challenge. Wetlands Ecology and Management 7: 105–112. [Google Scholar]
- Finlayson CM, von Oertzen IAML. 1993. Wetlands of Australia: Northern (Tropical) Australia. In: Whigham DJ, Dykyova H, Heijny S, eds. Wetlands of the world I: inventory, ecology and management. Dordrecht, The Netherlands: Kluwer Academic Publishers, 195–243. [Google Scholar]
- Finlayson CM, Woodroffe CD. 1996. Wetland vegetation. In: Finlayson CM, von Oertzen I, eds. Landscape and vegetation ecology of the Kakadu Region, Northern Australia. Dordrecht, The Netherlands: Kluwer Academic Publishers, Geobotany 23, 81–112. [Google Scholar]
- Finlayson CM, Farrell TP, Griffiths DJ. 1984. Studies of the hydrobiology of a tropical lake in the north-western Queensland: III. Growth, chemical composition and potential for harvesting of the aquatic vegetation. Australian Journal of Marine and Freshwater Research 35: 525–536. [Google Scholar]
- Finlayson CM, Bailey BJ, Freeland WJ, Fleming M. 1988. Wetlands of the Northern Territory. In: McComb AJ, Lake PS, eds. The conservation of Australian wetlands. Sydney, Australia: Surrey Beatty and Sons, 103–116. [Google Scholar]
- Finlayson CM, Bailey BJ, Cowie ID. 1989.Macrophytic vegetation of the Magela flood plain, northern Australia. Office of the Supervising Scientist Research Report No 5. [Google Scholar]
- Finlayson CM, Bailey BJ, Cowie ID. 1990. Characteristics of a seasonally flooded freshwater system in monsoonal Australia. In: Whigham DF, Goode RE, Kvet J, eds. Wetland ecology and management—case studies. Dordrecht, The Netherlands: Kluwer Academic Publishers, 141–162. [Google Scholar]
- Finlayson CM, Cowie ID, Bailey BJ. 1990. Sediment seedbanks in grassland on the Magela Creek flood plain, northern Australia. Aquatic Botany 38: 163–176. [Google Scholar]
- Finlayson CM, Wilson B, Cowie ID. 1991. Management of freshwater monsoonal wetlands: conservation threats and issues. In: Donohue R, Phillips W, eds. Proceedings of a wetland workshop, Newcastle, Australia. Canberra, Australia: ANPWS, 109–117. [Google Scholar]
- Finlayson CM, Thompson K, von Oertzen IAML, Cowie ID. 1993. Vegetation of five Magela Creek billabongs, Alligator Rivers Region, northern Australia. Canberra, Australia: Supervising Scientist for the Alligator Rivers Region Technical Memorandum 46, AGPS. [Google Scholar]
- Finlayson, CM, Cowie ID, Bailey BJ. 1993. Litterfall in a Melaleuca forest on a seasonally inundated flood plain in tropical northern Australia. Wetlands Ecology and Management 2: 177–188. [Google Scholar]
- Finlayson CM, Storrs MJ, Lindner G. 1997. Degradation and rehabilitation of wetlands in the Alligator Rivers Region of northern Australia. Wetlands Ecology and Management 5: 19–36. [Google Scholar]
- Finlayson CM, Hall RN, Bayliss BL. 1997.Regional review of wetland management issues: wet–dry tropics of northern Australia. Canberra, Australia: LWRRDC Occasional Paper 03/97. [Google Scholar]
- Grindrod J. 1988. The palynology of Holocene mangrove and saltmarsh sediments, particularly in northern Australia. Review of Palaeobotany and Polynology 55: 229–245. [Google Scholar]
- Hoatson D, Blake D, Wygralak A, Needham S, Allen B, Miles G, Hauser P, Oswald-Jacobs I. 2000.Kakadu and Nitmiluk National Parks, Northern Territory: a guide to the rocks, landforms, plants, animals, Aboriginal culture, and human impact. Canberra: Australian Geological Survey Organisation. [Google Scholar]
- Hope G, Hughes PJ, Russell-Smith J. 1985. Geomorphological field work and the evolution of the landscape of Kakadu National Park. In: Jones R, ed. Archaelogical research in Kakadu National Park. Canberra, Australia: ANPWS Special Publication 13, 229–240. [Google Scholar]
- Jonauskas P. (ed.) 1996.Proceedings wetlands workshop: making multiple land use work. Palmerston, Australia: Department of Lands, Planning and the Environment. [Google Scholar]
- Knerr NJA. 1998.Grassland community dynamics of a freshwater tropical floodplain: invasion of Brachiaria mutica (para grass) on the Magela floodplain, Kakadu National Park. Armadale and Jabiru, Australia. Honours thesis, University of New England and Supervising Scientist Internal Report 275. [Google Scholar]
- Lazarides M, Craven LA. 1980.Report on the Kakadu National Park Flora Project—Checklist of the flora of Kakadu National Park. Canberra, Australia: CSIRO Division of Plant Industry. [Google Scholar]
- Lonsdale WM. 1988. An analysis of litterfall in forests of the world. Annals of Botany 61: 319–324. [Google Scholar]
- Lowry J, Finlayson CM. 2004.A review of spatial data sets for wetland inventory in northern Australia. Darwin, Australia: Supervising Scientist Report 178, Supervising Scientist. [Google Scholar]
- Morris I. 1996.Kakadu National Park, Australia. Archerfield, Australia: Steve Parish Publishing Pty Ltd. [Google Scholar]
- Ovington D. 1986.Kakadu: a world heritage of unsurpassed beauty. Canberra, Australia: Australian Government Publishing Service. [Google Scholar]
- Phinn S, Hess L, Finlayson CM. 1999. An assessment of the usefulness of remote sensing for wetland monitoring and inventory in Australia. In: Finlayson CM, Spiers AG, eds. Techniques for enhanced wetland inventory, assessment and monitoring. Supervising Scientist Report 147, 44–82. [Google Scholar]
- Ridpath MG. 1985. Ecology in the wet–dry tropics: how different? Proceedings of the Ecological Society of Australia 13: 3–20. [Google Scholar]
- Russell-Smith J. 1985. A record of change: studies of holocene vegetation history in the South Alligator River region, Northern Territory. Proceedings of the Ecological Society of Australia 13: 191–202. [Google Scholar]
- Sanderson NT, Koontz DV, Morley AW. 1983.The ecology of the vegetation of the Magela Creek flood plain: upper section from Oenpelli road crossing to Nankeen Billabong. Jabiru, Australia: Scientific Workshop, Environmental Protection in the Alligator Rivers Region, 33.1–33.9. [Google Scholar]
- Skeat AJ, East TJ, Corbett LK. 1996. Impact of feral water buffalo. In: Finlayson CM, Von Oertzen I, eds. Landscape and vegetation ecology of the Kakadu Region, Northern Australia. Dordrecht, The Netherlands: Kluwer Academic Publishers, 155–177. [Google Scholar]
- Spiers AG, Finlayson CM. 1999. An assessment of the extent of wetland inventory data held in Australia. In: Finlayson CM, Spiers AG, eds. Techniques for enhanced wetland inventory, assessment and monitoring. Supervising Scientist Report 147, 1–43. [Google Scholar]
- Storrs MJ, Finlayson CM. 1997.Overview of the conservation status of wetlands of the Northern Territory. Canberra, Australia: Supervising Scientist Report 116, Supervising Scientist. [Google Scholar]
- Taylor JA, Dunlop CR. 1985. Plant communities of the wet-dry tropics of Australia: the Alligator Rivers Region. Proceedings of the Ecological Society of Australia 13: 83–128. [Google Scholar]
- Taylor JA, Tulloch D. 1985. Rainfall in the wet–dry tropics: extreme events at Darwin and similarities between years during the period 1870–1983. Australian Journal of Ecology 10: 281–295. [Google Scholar]
- Van Dam RA, Walden D, Begg GW. 2002.A preliminary risk assessment of cane toads in Kakadu National Park. Darwin, Australia: Supervising Scientist Report 164, Supervising Scientist Division. [Google Scholar]
- Van der Valk AG. 1981. Succession in wetlands: a Gleasonian approach. Ecology 62: 688–696. [Google Scholar]
- Walden D, van Dam R, Finlayson M, Storrs M, Lowry J, Kriticos D. 2004.A risk assessment of the tropical wetland weed Mimosa pigra in northern Australia. Darwin, Australia: Supervising Scientist Report 177, Supervising Scientist. [Google Scholar]
- Walker TD, Tyler PA. 1985. Tropical Australia, a dynamic limnological environment. Verhandlungen Internationale Vereinigung für Theoretische und Angewandte Limnologie 22: 1727–1734. [Google Scholar]
- Whitehead PJ, Chatto R. 1996. Northern Territory. In: A directory of important wetlands in Australia. Canberra, Australia: Australian Nature Conservation Agency, 119–175. [Google Scholar]
- Williams AR. 1979. Vegetation and stream pattern as indicators of water movement on the Magela flood plain, Northern Territory. Australian Journal of Ecology 4: 239–247. [Google Scholar]
- Williams AR. 1984. Changes in Melaleuca forest density on the Magela floodplain, Northern Territory, between 1950 and 1975. Australian Journal of Ecology 9: 199–202. [Google Scholar]
- Williams D, Chudleigh I. 2003.Arafura swamp water resources study. Darwin, Australia: Department of Infrastructure, Planning and Environment. [Google Scholar]
- Wilson BA, Brocklehurst PS, Whitehead PJ. 1990.Classification, distribution and environmental relationships of coastal floodplain vegetation, Northern Territory, Australia. Palmerston, Australia: Conservation Commission of the Northern Territory, Technical Memorandum 91/2. [Google Scholar]
- Woodroffe CD, Thom BG, Chappell J. 1985. Development of widespread mangrove swamps in mid-Holocene times in northern Australia. Nature 317: 711–713. [Google Scholar]
- Woodroffe CD, Chappell J, Thom BG, Wallensky E. 1989. Depositional model of a macrotidal estuary and flood plain, South Alligator River, northern Australia. Sedimentology. 36: 737–756. [Google Scholar]