Abstract
• Background and Aims Submerged plants possess well-developed aerenchyma facilitating intra-plant gas-phase diffusion of O2 to below-ground tissues, which are usually buried in anoxic sediments. However, aquatic habitats differ in terms of O2 fluctuations in the water column and in O2 consumption of the sediment, and aquatic plants differ in aerenchymal volume and resistance to O2 diffusion through the plant and across leaf and root surfaces. The hypothesis that the freshwater isoetid Lobelia dortmanna and the marine seagrass Zostera marina should display pronounced contrasts in intra-plant O2 dynamics because of differences in morphology/anatomy, physiology and growth habitat was tested.
• Methods In order to determine the O2 dynamics and relate this to the anatomy and morphology of the two species, O2 microelectrodes were inserted in the aerenchyma of leaves and roots, the sediment pore-water, and the water column in the field. Manipulation of water column O2 in the laboratory was also carried out.
• Key Results It was found that intra-plant transport of O2 between leaf and root tips takes place more readily in L. dortmanna than in Z. marina due to shorter distances and greater cross-sections of the aerenchyma. The major exchange of O2 across roots of L. dortmanna can be accounted for by small intra-plant resistances to diffusion, larger root than leaf surfaces, and greater radial diffusive resistance of leaves than roots. In contrast, the major O2 exchange across leaves than roots of Z. marina can be accounted for by the opposite anatomical–morphological features. The larger aerenchymal volume and the smaller metabolic rates of L. dortmanna compared to Z. marina imply that turnover of O2 is slower in the aerenchyma of L. dortmanna and O2 fluctuations are more dampened following changes in irradiance. Also, O2 accumulated in the aerenchyma can theoretically support dark respiration for a few hours in L. dortmanna but for only a few minutes in Z. marina.
• Conclusions The build-up of O2 in the pore-water of L. dortmanna sediments during the day as a result of high release of photosynthetic O2 from roots and low O2 consumption of sediments means that sediment, aerenchyma and water are important O2 sources for respiration during the following night, while Z. marina relies on the water column as the sole source of O2 because its sediments are anoxic. These differences between L. dortmanna and Z. marina appear to represent a general difference between the isoetid species mainly inhabiting sediments of low reducing capacity of oligotrophic lakes and the elodeid freshwater species and marine seagrasses mainly inhabiting sediments of higher reducing capacity in more nutrient-rich habitats.
Keywords: Anaerobiosis, isoetids, oxygen microelectrodes, radial oxygen loss (ROL), root aeration, seagrasses, submerged plants
INTRODUCTION
Plants need to maintain an aerobic respiration in most, if not in all, of their tissues. Terrestrial plants face the risk of anoxia in below-ground tissues if the soil becomes water-filled because then replenishment of O2 by gas-phase diffusion from the atmosphere through the gas-filled soil pores is interrupted. The risk of anoxia is particularly prominent among totally submerged aquatic plants because the diffusion coefficient is some 104-fold lower and the solubility of O2 some 20–30-fold lower in water relative to air (Raven, 1984). Hence, the O2 pool in the water is small and can be rapidly utilized by respiratory processes, and replenishment of O2 by molecular diffusion over centimetre to metre distances is exceedingly small.
Bog plants and amphibious plants with atmospheric contact often possess a ventilated mass transport system for O2 along shoots and rhizomes (but not roots) that works efficiently over long distances in internal lacunae driven by pressure differences arising from solar heating, wind or leaf-to-air humidity gradients (Dacey, 1981; Brix et al., 1992; Armstrong et al., 1996). However, such ventilated systems cannot operate in totally submerged plants due to lack of air contact (Armstrong, 1979; Sorrell and Dromgoole, 1987). Submerged plants have to rely on O2 diffusing from an external O2 source, usually the water surrounding the leaves, and from an internal source, the photosynthetic production of O2 taking place in the leaves during daytime (Pedersen et al., 2004; Borum et al., 2005). Only during flowering do the submerged plants have air contact and an opportunity for flow-through aeration (Schuette and Klug, 1995).
In order to distribute O2 deriving from leaves and the surrounding bulk water to respiring below-ground roots and rhizomes, submerged plants have a system of internal lacunae that often occupies large proportions of the plant volume (Sand-Jensen et al., 1982; Smith et al., 1984). The most important function of these internal lacunae is to ensure rapid diffusion of O2, carbon dioxide and other gases, while other advantages include the lower respiratory rates per unit of plant volume as the lacunae are metabolically inactive, and the possibility of storage of gases between day and night and during daytime changes in incident irradiance (Sand-Jensen and Prahl, 1982). Thus, internal lacunae can to some extent, depending on their volume relative to the metabolic rate, buffer the temporal changes in O2 and carbon dioxide accompanying fluctuations in incident light (Pedersen et al., 1995).
Most submerged plants are surrounded by anoxic sediments except for a thin microsphere close to the root surfaces (e.g. Armstrong, 1964; Pedersen et al., 1998; Colmer, 2003a). Only in a few situations are the sediments likely to hold larger pools of O2. One situation is the highly permeable sandy and gravel sediments in streams and on wave-exposed shores of lakes and marine coastal waters that receive a mass transport of O2-rich water, with some of the overlying water also flowing through the interstitial pore-water of the sediments. Such a mass transport of O2 by water flow will greatly exceed the diffusive flux of O2 in stagnant water across the sediment surface and may result in relatively high O2 pools in the sediments. How common this situation might be, how effective the O2 supply via the water flow is, and how the O2 dynamics evolves have, to our knowledge, not been examined as yet.
A different situation exists for the small rosette species of the isoetid growth form inhabiting sandy sediments of oligotrophic, softwater lakes (Sculthorpe, 1967) where the release of O2 to the sediment via intra-plant transport exceeds sediment O2 consumption and results in the build-up of O2 in the sediment (Pedersen and Sand-Jensen, 1992; Pedersen et al., 1995). Lobelia dortmanna is a characteristic representative of these freshwater isoetids that is remarkable for inhabiting the most oligotrophic sediments and taking up the majority of CO2 for photosynthesis from the sediment (Wium-Andersen, 1971) and releasing virtually all produced O2 from the roots (Sand-Jensen and Prahl, 1982). In laboratory experiments with 10-cm thick blocks of natural L. dortmanna-sediments, diurnal pulses of O2 were observed in the pore-water between light and darkness, most profoundly close to root surfaces (Pedersen et al., 1995). Dynamics of O2 levels in undisturbed L. dortmanna sediments in the field have not been studied as yet. It is likely that dissolved O2 is indeed present and changes diurnally, but also that O2 levels are lower and diurnal amplitudes smaller in the natural sediments because of O2 consumption and dilution of O2 in the sediment layers below the 10-cm deep rhizosphere.
To elucidate the O2 dynamics of submerged plants and its regulation, two common temperate plants were compared, the freshwater rosette-species L. dortmanna and the marine seagrass, Zostera marina, which differ widely in morphology–anatomy, physiology, ecology and growth habitat and which should, therefore, display sharp contrasts in O2 dynamics under natural field conditions and in controlled environments. The first objective was to establish the basic contrasts regarding weight, surface area, internal gas volume and diffusion pathways in leaves, stems and roots of the two species. Obviously, the allocation of plant weight to leaves, stems and roots is essential for their relative contribution to respiratory O2 consumption, and the surface areas of leaves and roots will influence gas exchange across these two surfaces. The volume, continuity and length of internal lacunae from leaf tips to root tips will influence the effectiveness of internal gas transport. The second objective was to measure the O2 dynamics in the water, the sediment and the internal lacunae in natural undisturbed field populations of L. dortmanna and Z. marina during day and night cycles. This information should allow us to determine whether O2 pools are present in the sediment and, if so, to compare their sizes relative to O2 pools in the overlying water and in the plants. It will permit an evaluatation of how dynamic these O2 pools are and how fast they respond to fluctuations in incident irradiance over the day and to gradual changes in irradiance between day and night. The third objective was to evaluate whether critically low O2 conditions develop in the lacunae of roots or rhizomes in the dark, as O2 concentrations in the overlying water were gradually decreased through experimental manipulation. After prolonged darkness, the overlying water is the sole O2 source, as O2 levels initially in lacunae and potentially in sediments are depleted.
Species characterization and growth habitat
Lobelia dortmanna is regarded as a typical stress-selected species of low biomass confined to the coarse sediments in oligotrophic, clear-water lakes in temperate regions of Europe and North America (Moeller, 1978; Boston, 1986; Farmer and Spence, 1986). Among the freshwater plants tested, L. dortmanna has the lowest internal nutrient concentrations and the lowest rates of photosynthesis, respiration, growth and senescence (Moeller, 1978; Sand-Jensen and Søndergaard, 1978; Sand-Jensen and Prahl, 1982; Christensen and Sand-Jensen, 1998). Lobelia dortmanna penetrates to about one-third of the Secchi-depth at 40 % of surface irradiance (Nygaard, 1958; Sand-Jensen and Søndergaard, 1979) and hence it is mainly confined to shallow, wave-exposed and well-illuminated sandy bottoms of very low nutrient and organic content (Wium-Andersen and Andersen, 1972; Sand-Jensen and Søndergaard, 1979). In a classical study, Moyle (1945) reported that L. dortmanna grew best on sediments of low organic content and died when rooted in organic sediments, probably due to their more reduced state. Christensen and Sand-Jensen (1998) confirmed that enrichment of natural sandy sediments with degradable organic matter reduced sediment O2 status and plant weight and root length of L. dortmanna. O2 consumption rates of sandy L. dortmanna sediments are among the lowest measured for freshwater sediments (Sand-Jensen et al., 1982).
Lobelia dortmanna is a perennial species with a functional lifetime for leaves and roots of more than a year (Moeller, 1978; Sand-Jensen and Søndergaard, 1978) and the plant is able efficiently to retain and recycle limiting nutrients. Also, its maximum biomass per unit of vegetated area is lower than for most other freshwater species (Moeller, 1978) and together with the low metabolic rates this should dampen diurnal pulses of O2, CO2 and solutes engaged in plant metabolism (Sand-Jensen et al., 1982).
In contrast, Z. marina is the most widespread seagrass in the northern hemisphere (Hemminga and Duarte, 2000). It grows on soft bottoms in relatively protected brackish and oceanic waters at depths ranging from the surface down to more than 10 m. The lower depth limit of Z. marina corresponds approximately to the mean Secchi-depth receiving about 10–15 % of surface irradiance (Duarte, 1991; Nielsen et al., 2002), suggesting that Z. marina can survive at about 3–4 times less light than L. dortmanna. Zostera marina inhabits substrata ranging from nutrient-poor, sandy sediments of very low organic contents, resembling freshwater sediments with L. dortmanna, to nutrient-rich sediments containing high proportions of clay, silt or mud. Nutrient-rich Z. marina sediments should have O2 consumption rates markedly higher than L. dortmanna sediments. Maximum specific growth rates are typically about four times faster for Z. marina than for L. dortmanna (Sand-Jensen and Borum, 1991) and the functional life time of Z. marina leaves is <2 months during mid-summer (Sand-Jensen, 1975) compared with more than a year for L. dortmanna, as noted above. Tissue nutrient concentrations in Z. marina are highly variable depending on growth habitat (Duarte, 1990; Hemminga and Duarte, 2000) but the levels exceed those observed in L. dortmanna (Moeller, 1978; Christensen and Sand-Jensen, 1998). Maximum plant biomass and rates of photosynthesis, respiration and growth are usually much higher for Z. marina than for L. dortmanna (Sand-Jensen, 1975; Borum, 1980; Sand-Jensen and Prahl, 1982; Sand-Jensen et al., 1982; Nielsen and Sand-Jensen, 1991). These differences between the two species suggest that diurnal changes of O2 in the surrounding water and internally in the lacunae should develop much faster and have higher amplitudes for Z. marina than for L. dortmanna.
MATERIALS AND METHODS
Plant morphology
Basic morphological features of L. dortmanna and Z. marina were determined in fully grown individuals collected from shallow (<1 m), sandy sediments during summer. Data on L. dortmanna were from the oligotrophic Lake Skånes Värsjö, Sweden and extracted from Sand-Jensen and Prahl (1982). Zostera marina was collected from the eutrophic Roskilde Fjord, Denmark. These localities were also used in the studies of O2 dynamics. Porosity measurements followed the method of Jensen et al. (1969) using a pycnometer and a five-digit analytical balance at constant temperature.
O2 dynamics
For laboratory experiments, terminal rhizomes of Z. marina with five nodes were planted in pots containing homogenized sediment from the collection site and kept in large aquaria under in situ temperature and light conditions until the experiments were conducted. Likewise, cores (diameter = 12 cm) from Lake Skånes Värsjö with L. dortmanna and natural sediment were kept in aquaria prior to experiments, at conditions of light and temperature similar to those in the lake. Pots or cores were placed in water circulated by a submersible pump and O2 concentration was controlled by bubbling with mixtures of atmospheric air and N2 using a Brooks mass flow controller. Light was provided by a Tungsten halogen lamp (Schott). Oxygen concentration was continuously monitored with a Clark-type O2 minielectrode (OX-500, Unisense, Denmark). Oxygen microelectrodes (OX-25, Unisense, Denmark) were inserted into the roots, rhizomes or leaf meristem by means of micromanipulators after gently removing the sediment to expose a portion of the tissue. After insertion, the exposed tissue was again carefully covered with 2–3 cm of sediment and the set-up was left to allow re-establishment of the geo-chemical profiles around roots and rhizome. The microelectrodes were connected to an eight-channel picoammeter (PA8000, Unisense, Denmark) and the outputs were logged on a computer using an analog-to-digital converter (ADC-16, PicoTech, England). Changes in water and sediment temperatures were logged using type-K thermocouples connected to a resistance converter (TH-08, PicoTech, Denmark). During the experiment, internal O2 concentrations were measured after manipulating water column O2 concentrations (for details see Pedersen et al., 2004).
The same electrode type and data logging equipment were used for in situ measurements of internal O2 dynamics in roots, rhizomes or leaf meristems of L. dortmanna and Z. marina. The microelectrodes with micromanipulators were mounted on aluminium stands, and the electrodes were inserted into the plants. The positioning of the O2 electrodes was done by following the electrode signal during insertion until a constant O2 signal was obtained at a depth of approximately one-third of the tissue diameter (Greve et al., 2003). The diel changes in surface irradiance, water column and sediment temperature, water column O2 content and internal O2 concentrations in roots, rhizomes or leaves were recorded from the afternoon throughout the dark period until late in the morning. Sediment O2 concentrations were measured using an autonomous microprofiler (MP4, Unisense, Denmark) mounted with OX-500 minielectrodes (for details see Borum et al., 2005).
RESULTS AND DISCUSSION
Plant morphology
Fully-grown L. dortmanna plants had higher tissue longevity and held more leaves (10–17) and roots (46–77) than the numbers of leaves (3–5) and roots (20–37) of the Z. marina plants examined. Biomass allocation to long-lived rhizomes of Z. marina could be very high (63 %) and markedly exceeded the percentage allocated to the L. dortmanna stem (12 %, Fig. 1). The rhizome of Z. marina is essential for the holdfast and for long-term survival as it contains sufficient stored reserves to form a new generation of leaves if the originals are cut or damaged above the meristem (Greve et al., 2005). The ratio of leaf to root dry weight was 1·4 for the studied individuals of L. dortmanna and 3·7 for Z. marina, and the ratio of leaf to root surface area was 0·6 for L. dortmanna and 2·4 for Z. marina (Fig. 1). These figures imply that exchange of gases and solutes is directed towards the sediment in L. dortmanna and more towards the water phase in Z. marina, although surface permeability to substances and the sizes of unstirred boundary layers will also greatly influence gas and solute exchange. The difference is accentuated even further by the much wider and shorter diffusion path through leaves and roots of L. dortmanna facilitating intra-plant transport of gases compared with the path in Z. marina (Fig. 1). The richer coastal sediments should help Z. marina acquire the necessary nutrients via the roots. In both species, the weight and the surface areas allocated to leaves and roots can change with growth habitat as acclimation to different environmental conditions (Penhale and Wetzel, 1983; Christensen and Sand-Jensen, 1998). The overall differences in morphological traits between the two species will, however, persist.
Fig. 1.

Sketches of the freshwater, rosette species L. dortmanna (right) and the marine seagrass Z. marina (left). Average values are shown for percentage dry weight (W, % of plant d. wt), surface area (A, cm2 g−1 plant d. wt), gas volume (Va, cm3 g−1 plant d. wt) and cross-sectional areas of lacunae (Aa, mm2 g−1 plant d. wt) normalized to total plant dry weight. Cross-sectional areas of lacunae were determined as the summed cross-sectional area for all leaves and roots at the mid-point of their length normalized to the dry weight of the entire plant, thereby describing the cross-sectional area available for longitudinal diffusive transport of gases for a standard plant dry weight. Mean values of 11 individuals of L. dortmanna (Sand-Jensen and Prahl, 1982) and nine individuals of Z. marina. Note the 10-fold difference in scale between the two sketches.
Plant morphology and O2 diffusion and storage
The situation for individual plants, of the sizes studied, is first considered. Leaves, rhizome/stem and roots are much shorter for L. dortmanna than for Z. marina. In the studied plants, the mean path length for diffusion from leaf tip to root tip was only about 10 cm in L. dortmanna plants, but 43 cm (i.e. 4-fold longer) in Z. marina. The critical distance for the diffusive flux in the dark from the overlying water via the basal part of leaves to root tips was only 6 cm in L. dortmanna but 16 cm in Z. marina. As the porosity is some 4·2 times higher for L. dortmanna than Z. marina roots (Fig. 1), the downward resistance to diffusion through uninterrupted lacunae should be about 11-fold lower for L. dortmanna than for Z. marina. Also, diffusion through the leaf lacunae from the tip to the base can take place about eight times faster in L. dortmanna than Z. marina for a given partial pressure gradient as the studied L. dortmanna leaves had a mean length of 4·4 cm and a porosity of about 50 %, while Z. marina leaves had a mean length of 27 cm and a porosity of 38 %. The lacunae in L. dortmanna are straight, continuous channels without tissue barriers, whereas the morphological features of the lacunae in Z. marina are unknown.
The total internal gas volume was about 15 times higher per unit plant dry weight in L. dortmanna (10·5 cm3 g−1 plant d. wt) than in Z. marina (0·71 cm3 g−1 plant d. wt, Fig. 1). One reason for this profound difference is the higher porosity in leaves and roots of L. dortmanna than Z. marina, but a more important reason is the low porosity of the rhizomes of Z. marina (8 %) and their high ratio (0·58) of dry weight to fresh weight and their high percentage (63 %) of total plant dry weight in the examined population. When air volumes are normalized to the dry weight, the unit commonly used to express metabolic rates, the low porosity of the heavy rhizomes of Z. marina results in very low values compared to L. dortmanna. Because photosynthesis at saturating irradiance is typically some three times lower in L. dortmanna (1–2 mg O2 g−1 plant d. wt h−1) than in Z. marina (3–5 mg O2 g−1 plant d. wt h−1) (Sand-Jensen et al., 1982), the O2 storage capacity is much higher and the turnover time of the O2 pool in the lacunae is probably much longer in L. dortmanna than in Z. marina. At atmospheric saturation, the gas volume in L. dortmanna will contain about 3·0 mg O2 g−1 plant d. wt (at 20 °C). This pool of O2 is a relevant measure because O2 saturation typically changes about 100 % between day and night (Figs 2, 3) and the amount of O2 corresponds to the photosynthetic production over 1·5–3·0 h. Assuming a respiration rate of 30 % of the photosynthetic rate, the O2 pool in the lacunae can theoretically sustain respiratory O2 consumption for a period of 5–10 h. These simple calculations using gas volumes and metabolic rates suggest that internal O2 dynamics are much dampened in L. dortmanna. In contrast, the 15-fold smaller gas volume in Z. marina can hold only 0·2 mg O2 g−1 plant d. wt at atmospheric saturation, corresponding to the photosynthetic O2 production for only 2–4 min and the respiratory O2 consumption for only 8–13 min. Thus, the storage capacity for O2 in the lacunae of Z. marina is insignificant both during light and darkness and the main role of lacunae is to serve as an intra-plant transport route for gases and to ensure buoyancy of the leaves.
Fig. 2.
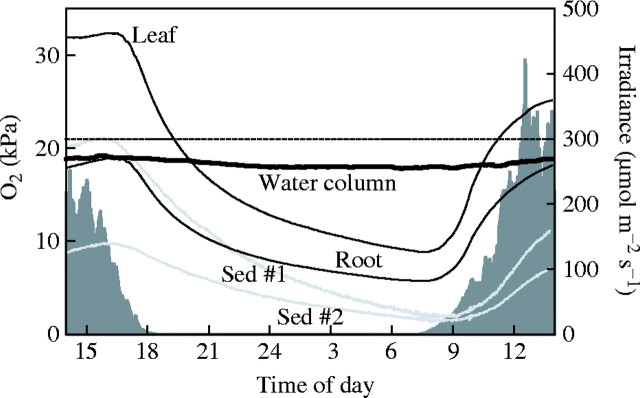
In situ diurnal variations of irradiance and O2 dynamics in L. dortmanna populations inhabiting sandy sediment in shallow water (50 cm) of the oligotrophic Lake Skånes Värsjö. Oxygen concentrations were measured by microelectrodes located in water, gas-filled lacunae in leaves and roots, and in the sediment at 40 mm depth at two different positions (#1 and #2). The dashed horizontal line shows the air-saturated value.
Fig. 3.

In situ diurnal variations of irradiance and O2 dynamics in Z. marina populations inhabiting shallow coastal sediments (150 cm) of eutrophic Roskilde Fjord. Oxygen concentrations were measured in the water column and in the leaf meristem of three different plants. The dashed horizontal line shows the air-saturated value.
The situation for field populations is now considered. Maximum biomass during summer can reach about 90 g m−2 d. wt for L. dortmanna and 400 g m−2 d. wt for Z. marina (see legend to Table 1). Assuming the same allocation between leaves, stem/rhizome and roots as in Fig. 1, the approximate leaf surface area will be 2·2 m2 for L. dortmanna and 4·7 m2 for Z. marina and the root surface area will be 3·6 m2 for L. dortmanna and 2·0 m2 for Z. marina. Applying the measured weight and root number of the studied plants, the dense L. dortmanna populations will contain about 28 000 roots per m2 and the mean distance between them will be about 5 mm. The Z. marina populations will have approximately half the number of roots per m2 and a mean distance between roots of about 8 mm. So, in dense plant populations, most of the sediment in the root zone will be in close contact with roots and the sediment can be strongly influenced by exchange of gases and solutes with the roots. Essentially there are about the same densities of roots in dense populations of L. dortmanna and Z. marina, so differences in sediment O2 concentrations are caused by higher root O2 release and smaller sediment O2 consumption of the L. dortmanna plant–sediment assemblage relative to Z. marina. Moreover, the porosity of the sandy L. dortmanna sediments is often only about half (40–45 %) of that in the more organic Z. marina sediments, so for a given O2 exchange concentrations would tend to change more in L. dortmanna sediments.
Table 1.
Surface area, internal gas volume and O2 pools at atmospheric saturation at 20 °C in dense populations of 90 g m−2 d. wt in L. dortmanna and 400 g m−2 d. wt in Z. marina
| Surface area (m2 m−2 of sediment) |
|||||
|---|---|---|---|---|---|
| Species |
Leaves |
Roots |
Gas volume (cm3 m−2) |
O2 pool (mg O2 m−2) |
|
| Lobelia dortmanna | 2·2 | 3·6 | 945 | 265 | |
| Zostera marina | 4·7 | 2·0 | 284 | 80 | |
Background values from Fig. 1.
Further, the high internal gas volume in L. dortmanna populations contributes significantly to the O2 dynamics. Lobelia dortmanna populations weighing 90 g m−2 d. wt hold about 950 cm3 of lacunal gas m−2 and about 265 mg O2 m−2 at atmospheric saturation (Table 1). This amount is equivalent to the O2 pool in 29 L of air-saturated freshwater at 20 °C, or in the volume of a 2·9-cm deep water column above 1 m2 of sediment, or in the pore-water of a 6·5-cm thick sandy sediment with a typical porosity of 45 % (Pedersen et al., 1995; Christensen and Sand-Jensen, 1998). Oxygen pools within L. dortmanna populations and in the rhizosphere are thus of the same magnitude at atmospheric saturation, while O2 pools in the overlying water are higher once water depths exceed 3 cm. These evaluations suggest that as long as the O2 pool in the water remains high and is accessible for the plants, this source should be very important to ameliorate O2 stress in the plants.
Internal O2 pools in the aerenchyma are more than 3-fold smaller in dense populations of Z. marina than in L. dortmanna (Table 1) and can not provide an important storage pool for O2 consumption in the dark. Obviously, large O2 pools in the water are needed to meet the respiratory demand. Maximum rates of photosynthesis in Z. marina populations reach about 1000 mg O2 m−2 h−1 (K. Sand-Jensen, unpubl. res.), which corresponds to the O2 pool held in 13-cm deep seawater at 20 °C at air saturation. Thus, profound diurnal changes in O2 concentrations occur within dense seagrass beds in shallow waters (Greve et al., 2003; Borum et al., 2004).
Gas exchange across leaves and roots
In L. dortmanna, the low resistance to downward gas diffusion from leaves to roots provided by wide diffusion paths and the high surface areas of roots relative to leaves (Fig. 1) are morphological keys to understanding the extensive exchange of O2 and other gases between the roots and the sediment. The 2–3 times greater O2 permeability per unit surface area of roots compared with leaves (Sand-Jensen and Prahl, 1982) facilitates root exchange even further. In contrast, Z. marina has a much greater resistance to downward gas diffusion and greater leaf than root surfaces, facilitating leaf exchange with the water column. Surface permeability of leaves, rhizomes and roots of Z. marina has not been measured directly but observations of radial O2 loss to anoxic agars using dyes or microelectrodes indicate that this release is insignificant along the rhizome and along most of the length of roots except for the apical 5 mm (T. M. Greve, pers. comm.).
Previous experiments in split-chambers have revealed that O2 release to O2-depleted water surrounding the roots is only 1 % of the total release by photosynthesis in the light in Z. marina, but over 97 % of the total release in L. dortmanna in accordance with the structural differences between the two species (Fig. 1). Respiration in the rhizome and roots of Z. marina consumes the majority of the downward O2 flux in the light (seven times more than root release); whereas respiration of L. dortmanna roots consumes relatively little (14 times less than root release) of the downward O2 flux, resulting in a substantial O2 flux to the sediment. Repetition of these experiments with Z. marina yielded essentially the same result although root release was somewhat higher (10 %, Caffrey and Kemp, 1991). The percentage of O2 respired relative to that released by the roots will increase with the size and the respiratory demand of the root system for a downward diffusion path of given length and dimension, and it will also increase with greater resistance to radial O2 loss to the sediment relative to downward diffusion (Caffrey and Kemp, 1991). This pattern confirms measurements of high permeability to radial diffusion of O2 from L. dortmanna roots (Sand-Jensen and Prahl, 1982) and supports the observations of low radial diffusion along most of the length of rhizome-roots of Z. marina. In order to ensure longitudinal transport of O2 from the leaves to the meristematic region at the tip of Z. marina roots surrounded by anoxic and highly reducing sediments, it is essential that radial loss is small at the basal and distal regions (Armstrong, 1971; Colmer, 2003b). This structural feature not only permits Z. marina to use the majority of the downward O2 flux for below-ground respiration but presumably also impedes the entry of sediment gases (mostly sulphide) or other potential phytotoxins produced under anoxic, reducing conditions.
The protection against potentially harmful sediment substances is not perfect, however. Once O2 disappears from the lacunae close to the meristem and in the rhizome of Z. marina on highly-reducing sediments, gaseous sulphide enters the aerenchyma and reaches relatively high concentrations exceeding those considered to be toxic to normal cell metabolism (Pedersen et al., 2004). Sulphide poisoning of the meristem has been implicated as a major cause of Z. marina dieback on reducing sediments during hot summers and episodes of O2 depletion in the overlying water (Greve et al., 2003).
In contrast, L. dortmanna needs high root permeability to ensure ready CO2 supply from the sediment to plant photosynthesis (Sand-Jensen and Prahl, 1982). However, this should also leave L. dortmanna susceptible to anoxic and highly reducing sediments as internal O2 is readily lost by radial O2 diffusion over the whole root surface (Armstrong, 1971; Colmer, 2003b) and potentially harmful sediment substances can more easily invade and harm the tissue (Pedersen et al., 2004). This scenario may explain why L. dortmanna—being the aquatic species with the least resistance to intra-plant and radial gas transport and with an almost 100 % release of photosynthetic O2 over the root surfaces—is so susceptible to anoxia and reducing conditions in the sediment (Moyle, 1945; Christensen and Sand-Jensen, 1998).
Diurnal O2 changes in natural field populations
When measured in the field, there were several distinct differences in diel O2 dynamics in the lacunae of L. dortmanna and Z. marina and in the water and sediments surrounding them (Figs 2, 3). Oxygen concentrations in the water column remained almost constant in L. dortmanna populations as a consequence of low metabolic rates in plants, sediment and water, whereas O2 fluctuations were much more pronounced in the water surrounding Z. marina populations as a result of higher metabolic rates. Oxygen was present throughout the day and night at 40 mm depth in the pore-water of L. dortmanna sediments, while the sediment of Z. marina sediments was always anoxic. Oxygen was present in the lacunae of both species, but changes were slow and gradual within L. dortmanna as a result of large lacunae and low metabolic rates per unit biomass, while they were fast within Z. marina as a result of small lacunae and high metabolic rates.
The overall changes in O2 concentrations in the lacunae of leaves and roots of L. dortmanna followed the gradual diurnal changes between day and night, and sudden changes in incident irradiance did not influence the internal O2 concentrations substantially, because the turnover time of the O2 pool in the lacunae is long (i.e. one to a few hours, Fig. 2). Oxygen concentrations in the leaf lacunae of L. dortmanna ranged between 32 kPa (approx. 150 % of that in air) late in the afternoon and about 10 kPa (approx. 50 % of that in air) late in the night. Oxygen concentrations were lower in the root lacunae, ranging from 6 to 19 kPa (approx. 20–90 % of that in air) but otherwise followed the diurnal pattern in the leaf lacunae, which is anticipated because of the uninterrupted gas-filled lacunae running from leaves to roots. Oxygen concentrations measured at two locations at 40 mm depth in the sediment followed the same dampened diurnal pattern but differed in amplitude. One of the microelectrodes, most likely located very close to a root, varied from 21 to 3 kPa (approx. 100–10 % of air-saturation), while the other microelectrode, presumably located further away from the roots, varied from 10 to 3 kPa (approx. 50–10 % of air-saturation). The exact O2 concentration will obviously depend on time and location with respect to sediment depth and distance to roots. Thus, while the O2 flux occurs from the roots to the sediment in the light, the O2 flux during the dark could take place in either direction depending on position in the sediment and time during the night (i.e. the O2 gradient). Also, because the most intensive O2 consumption takes place in the root wall and in the microbial community of bacteria, fungi and protozoa associated with the root surface, the O2 flux can simultaneously be directed from the root lacunae, and from the sediment pore-water, towards the root walls and the microbial surface communities (see Armstrong et al., 2000 for a thorough discussion on O2 diffusion pathways in roots of hydrophytes].
In the Z. marina population, O2 concentrations in the water varied from about 26 to 15 kPa (approx. 125–80 % of that in air, Fig. 3). Oxygen concentrations were measured in three individuals in the aerenchyma close to the leaf meristem located at the base of the leaves and at the apex of the rhizome, which is usually buried in the sediment. The diurnal patterns followed each other very closely in the three individuals. The O2 concentration reached a maximum during the day between 30 and 38 kPa (approx. 150–180 % of that in air) and a minimum late at night that was close to 4 kPa (20 % of that in air). Sudden changes in daytime irradiance resulted in profound and swift changes in the internal O2 concentrations which, as already emphasized, was anticipated because of the small storage capacity in the lacunae relative to metabolic demands and, therefore, short turnover times (i.e. minutes) of O2 within the lacunae.
The field studies of L. dortmanna populations showed small diurnal changes in O2 concentrations in the water column, while O2 concentrations were much more variable in the lacunae and in the sediment. In the light, lacunal O2 in L. dortmanna changed over the day in overall accordance with the incident irradiance (Fig. 4A). This apparent relationship is, however, partly artificial. Had the sky been clear before noon and overcast after noon, the relationship would be less clear, because high irradiances before noon would then have been associated with lower O2 concentrations in the lacunae and low irradiances in the afternoon with higher O2 as this builds up across the light period. Measurements in the dark also demonstrate the lack of relationships in this non-steady state system (Fig. 4B). Oxygen concentrations in the lacunae vary greatly despite very small changes in the water column, and both are the result of gradual reduction over time in the dark due to respiratory processes and declining sediment O2 concentrations. Therefore, it is not possible to estimate by extrapolation the O2 concentration in the water resulting in anoxia in the lacunae. To reach such an estimate requires controlled variation in water column O2 concentrations over long periods during which sediments are devoid of O2 (see next section).
Fig. 4.
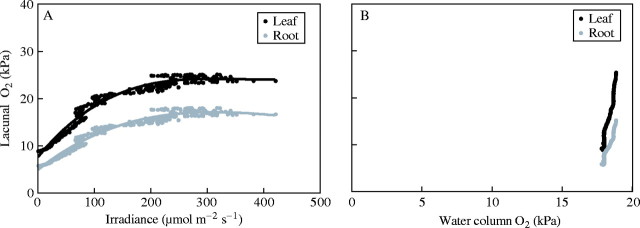
Oxygen concentrations in leaf meristem of L. dortmanna (same data as in Fig. 2). (A) Measured during the morning as a function of irradiance; and (B) measured during the night as a function of O2 concentrations in the overlying water column.
By contrast, field studies of Z. marina populations revealed that O2 concentrations measured in the lacunae close to the meristem were closely related to changes in irradiance and the relationship resembled a classical PI curve (Fig. 5A). In the dark, the internal O2 concentration was linearly related to the O2 concentration in the water serving as the sole O2 source (Fig. 5B). Extrapolating the internal O2 concentration to zero suggested that the meristem, in this example, would turn anoxic at 10 kPa (approx. 45 % air saturation in the water). While the linear relationship between O2 concentrations in the water and in the meristem during darkness has been observed previously for Z. marina, the O2 concentration in the water resulting in anoxic meristems varies considerably and can be as low as 3 kPa (Pedersen et al., 2004) depending on the balance between supply rates and consumption rates, which are influenced by water movement, temperature, sediment type and allocation between above- and below-ground biomass (Greve et al., 2003; Larsen, 2004).
Fig. 5.
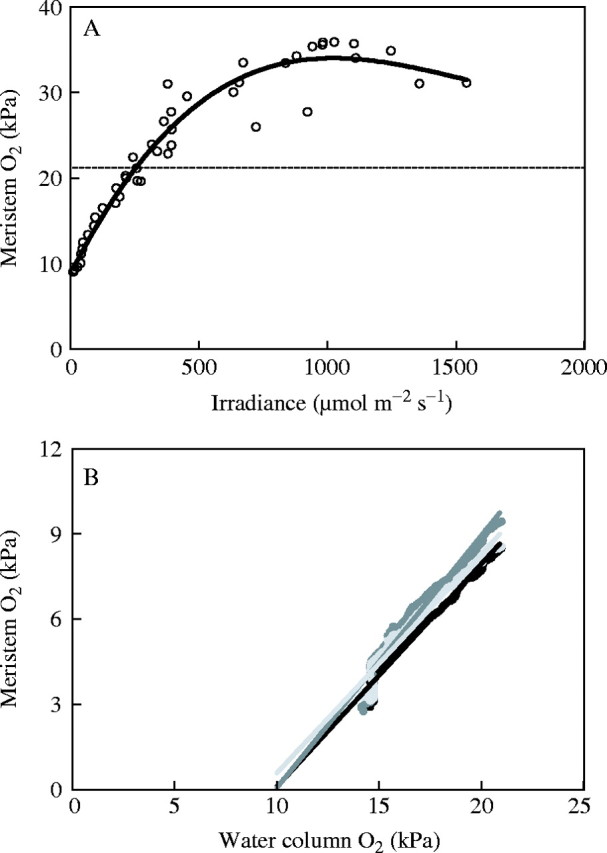
Oxygen concentrations in leaf meristem of Z. marina (same data as in Fig. 3). (A) Measured during the afternoon as a function of irradiance. The dashed horizontal line shows the air-saturated value. (B) Measured during the night as a function of oxygen concentrations in the overlying water (data are shown for different individual plants). The straight lines are linear extrapolations to zero oxygen in the meristem.
Critical O2 concentrations in the dark
In L. dortmanna populations in the dark, O2 pools in the lacunae and in the pore-water are important sources of respiratory O2 consumption, together with the O2 pool in the water. With decreasing redox potential in the sediment, the pool of O2 in the sediment shrinks and the importance of O2 in the water column increases. In Z. marina populations, the water column is the only O2 source in the dark and water motions within the canopy are an important regulator of the O2 flux into leaves and the subsequent downward diffusion to roots (Larsen, 2004).
To evaluate these aspects, the O2 concentrations were varied in well-stirred water overlying vegetated sediments and the internal O2 concentrations were measured in the plants. In L. dortmanna transferred from light to darkness, but kept at 21 kPa in the water for 14 h, the O2 concentration in the root lacunae declined from 28 to 4 kPa (approx. 140–20 % of that in air) and had not yet reached steady state (Fig. 6). The O2 concentration at 40 mm depth in the sediment declined from about 8 kPa (approx. 40 % air-saturation) to zero within 5 h. When O2 was stripped from the water column, O2 decreased to below detection in the root lacunae within 2 h. In another L. dortmanna plant (data not shown), O2 only dropped to 8 kPa (approx. 40 % of that in air) in the root after 14 h exposure to air-saturated water in the dark, and this larger internal O2 pool lasted for 4 h after the water phase turned anoxic. When O2 concentrations in the water were reduced stepwise over 3 h intervals, O2 concentrations in the root lacunae declined in concert with the falling external O2 concentration (Fig. 7). Steady state was not reached in the root lacunae after 3 h, however, and O2 was still present in the root lacunae, although at very low concentrations (about 0·8 kPa, approx. 4 % of that in air), at 4 kPa (approx. 20 % air-saturation) in the overlying water. These trace amounts of O2 in the root lacunae may eventually decline to zero after more than 3 h.
Fig. 6.
Oxygen concentrations measured in water, gas-filled lacunae of a root and a leaf, and at 20 mm depth in the sediment of a L. dortmanna population from Skånes Värsjö transferred to the laboratory and exposed to an initial shift from light to darkness, maintained in air-saturated water for 14 h, and finally exposed to deoxygenated water in the dark for an additional 4 h.
Fig. 7.
Oxygen concentrations measured in water and in the gas-filled lacunae of a root of L. dortmanna exposed to a stepwise reduction of O2 concentrations in the water column in the dark.
These experiments with L. dortmanna have three important implications. Firstly, internal O2 concentrations change gradually and very slowly in the dark, as also observed in situ (Fig. 2). Secondly, air-saturated water can maintain O2 at low levels in the root lacunae in the sandy sediment tested here. Thirdly, when sediments are anoxic, O2 concentrations in the root lacunae follow O2 concentrations in the overlying water in the dark, demonstrating its importance as regulator of internal O2 levels. The relationship between external and internal O2 concentrations is difficult to establish, however, because steady state conditions are not achieved during a normal night period. Results of artificially prolonged dark periods are difficult to interpret because they are likely to deplete respiratory substrates and thereby reduce O2 consumption and increase internal concentrations relative to those anticipated if O2 consumption rates had remained constant.
The same type of experiment was performed with Z. marina with microelectrodes inserted into the meristem and in internodes 3 and 4 located further down the rhizome (Fig. 8). As anticipated, O2 concentrations declined gradually along the length of this diffusion path and responded to stepwise reductions of O2 concentrations in the water column with gradually longer time lags of 6·3, 9·2 and 9·5 min (inset of Fig. 8). These time lags are in accordance with O2 transport by diffusion through the laminary boundary layer surrounding the leaves and through the lacunae down the leaves and rhizome. Mass transport does not seem to be involved. The internal O2 concentrations in the meristem and rhizome of Z. marina reached steady state levels with a time lag of 0·5–1 h relative to that in the water, in accordance with the swifter responses to fluctuating irradiances of Z. marina than L. dortmanna (Figs 2, 4).
Fig. 8.
Oxygen concentrations measured in water and in leaf meristem, internode 3 and internode 4 of Z. marina exposed to a stepwise reduction of O2 concentrations in the water column in the dark. The inset is a close-up of the O2 dynamics and the time delay of O2 reduction in the aerenchyma following a sudden reduction of O2 concentration in the water column. The time delay was calculated by linear extrapolation back to the initial O2 concentration.
These differences between L. dortmanna and Z. marina appear to represent a general difference between the isoetid species mainly inhabiting sediments of high redox potential and the elodeid freshwater species and marine seagrasses mainly inhabiting sediments of low redox potential (Smith et al., 1988; Smits et al., 1990). For the isoetid species to acquire CO2 for photosynthesis via the roots from the sediment, they need adaptations ensuring ready gas exchange across the root surfaces and internally between roots and leaves (Søndergaard and Sand-Jensen, 1979; Richardson et al., 1984). As emphasized, this ready gas exchange will create an O2 problem as the reducing capacity of the sediments increases, whereas in nutrient-poor sediments with high redox potential the ready release of oxygen by the roots of isoetid species helps the acquisition of limiting nutrients through symbiosis with aerobic mycorrhiza fungi (Søndergaard and Laegaard, 1977; Wigand et al., 1998; Nielsen et al., 2004). What the main selective forces have been during the evolution of isoetid growth forms from terrestrial ancestors of many plant families can never be established, but it has led to considerable similarity between different species as well as some differences regarding light requirements and tolerance to reducing sediments that have not been fully established as yet.
There are additional physiological differences between L. dortmanna and Z. marina and between isoetids and seagrasses and other aquatic plants as collective groups that are probably important for the tolerance to anoxia and reducing sediment conditions, but these have not been further explored here. Although O2 may be present at low concentrations in the lacunae of roots and rhizomes/stems, anoxic conditions may, nevertheless, develop in meristematic and vascular tissues of high metabolism and low porosity, resulting in less efficient ATP production and inhibition of solute transport and growth. This effect should be most prominent in the dark and under severe O2 depletion in the water (Sorrell et al., 2004). The capacity for anaerobic respiration and ethanol production in the roots appears to be low among isoetid species, both relative to aerobic respiration of the isoetids and to anaerobic respiration of nymphaeid macrophytes (Smits et al., 1990). There is an untested community ecological dimension to the tolerance of isoetids to reducing sediments as well. The plants are not passive components but actively influence the sediment environment (Christensen and Sørensen, 1986; Pedersen et al., 1995). For example, sediments inhabited by isoetids have greater O2 penetration, higher redox potentials and higher 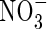 concentrations than bare sediments (Wium-Andersen and Andersen, 1972; Christensen and Sørensen, 1984) and larger plants and higher densities of L. dortmanna also generate higher O2 pools in the surrounding sediment (Pedersen et al., 1995). Therefore, in the colonization of bare sediments from seeds, a high density of seedlings may be important for collective survival, as many seedlings produce more O2 and can better maintain oxidized conditions. Moreover, during eutrophication and associated higher sedimentation rates of organic matter, dense populations may have greater chances of maintaining an acceptable O2 and redox status of the sediment (Tessenow and Baynes, 1978). Thus, healthy isoetid populations of high density and high seed production may be more capable of buffering anthropogenic impacts.
concentrations than bare sediments (Wium-Andersen and Andersen, 1972; Christensen and Sørensen, 1984) and larger plants and higher densities of L. dortmanna also generate higher O2 pools in the surrounding sediment (Pedersen et al., 1995). Therefore, in the colonization of bare sediments from seeds, a high density of seedlings may be important for collective survival, as many seedlings produce more O2 and can better maintain oxidized conditions. Moreover, during eutrophication and associated higher sedimentation rates of organic matter, dense populations may have greater chances of maintaining an acceptable O2 and redox status of the sediment (Tessenow and Baynes, 1978). Thus, healthy isoetid populations of high density and high seed production may be more capable of buffering anthropogenic impacts.
CONCLUSIONS
The main features of O2 dynamics in L. dortmanna and Z. marina were predictable from basic morphological and anatomical traits. Therefore, it is beneficial routinely to establish morphological and anatomical features, and to a greater detail than has usually been done, in studies of O2 transport in plants. Intra-plant transport of O2 and other gases between leaf and root tips takes place more readily in L. dortmanna than in Z. marina due to shorter distances and greater cross-sections of uninterrupted gas-filled lacunae. The major exchange of O2 across roots of L. dortmanna and leaves of Z. marina can be accounted for by the smaller intra-plant resistance to diffusion, larger root than leaf surfaces and greater radial diffusive resistance of leaves than roots of L. dortmanna, while the opposite is the case for Z. marina. The susceptibility of L. dortmanna to anoxia caused by low O2 concentrations in the water column and reducing sediments is perhaps due to the absence of a barrier to radial O2 loss in its roots and, therefore, a low resistance to radial diffusion in roots compared to leaves, while a barrier to radial O2 loss is present in rhizomes and roots of Z. marina, apart from the 5 mm root tips. Downward O2 diffusion in roots of Z. marina to the root tip, where it may support aerobic meristematic cell growth, is promoted by the barrier to radial O2 loss.
The larger lacunae and the lower metabolic rates of L. dortmanna compared with Z. marina imply that turnover of O2 is slower in the gas-filled lacunae of L. dortmanna and O2 fluctuations are more dampened following changes in irradiance compared to Z. marina. Also, O2 accumulated in the lacunae can theoretically support dark respiration for hours in L. dortmanna but only for minutes in Z. marina. The build-up of O2 in the pore-water of L. dortmanna sediments means that sediment, lacunae and water are important O2 sources of dark-time respiration, while Z. marina relies on the water column as the sole source of O2 because its sediments are anoxic.
LITERATURE CITED
- Armstrong J, Armstrong W, Beckett PM, Halder JE, Lythe S, Holt R, Sinclair A. 1996. Pathways of aeration and the mechanisms and beneficial effects of humidity- and venture-induced convections in Phragmites australis (Cav. ) Trin. Ex Steud. Aquatic Botany 54: 177–198. [Google Scholar]
- Armstrong W. 1964. Oxygen diffusion from roots of some British bog plants. Nature 206: 801–802. [Google Scholar]
- Armstrong W. 1971. Radial oxygen losses from intact rice roots as affected by distance from the apex, respiration, and waterlogging. Physiologia Plantarum 25: 192–197. [Google Scholar]
- Armstrong W. 1979. Aeration in higher plants. Advances in Botanical Research 7: 225–332. [Google Scholar]
- Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM. 2000. Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phrargmites australis Annals of Botany 86: 687–703. [Google Scholar]
- Borum J. 1980. Biomasse- og produktionsforhold hos Ålegræs (Zostera marina L.) og det tilknyttede epifytsamfund. MSc Thesis, Freshwater Biological Laboratory, University of Copenhagen, Denmark. [Google Scholar]
- Borum J, Pedersen O, Greve T, Frankovich TA, Zieman JC, Fourqurean JW, Madden CJ. 2005. The potential role of plant oxygen and sulphide dynamics in die-off events of the tropical seagrass, Thalassia testudinum. Journal of Ecology, in press. [Google Scholar]
- Boston HL. 1986. A discussion of the adaptations for carbon acquisition in relation to the growth strategy of aquatic isoetids. Aquatic Botany 26: 258–270. [Google Scholar]
- Brix H, Sorrell BK, Orr PT. 1992. Internal pressurization and convective gas flow in some emergent freshwater macrophytes. Limnology and Oceanography 37: 1420–1433. [Google Scholar]
- Caffrey JM, Kemp WM. 1991. Seasonal and spatial patterns of oxygen production and root-rhizome release in Potamogeton perfoliatus L. and Zostera marina L. Aquatic Botany 40: 41–65. [Google Scholar]
- Christensen KK, Sand-Jensen K. 1998. Precipitated iron and manganese plaques restrict root uptake of phosphorus in Lobelia dortmanna. Canadian Journal of Botany 76: 2158–2163. [Google Scholar]
- Christensen PB, Sørensen J. 1986. Temporal variation of denitrification activity in plant-covered sediment from Lake Hampen, Denmark. Applied Environmental Microbiology 51: 1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD. 2003. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment 26: 17–36. [Google Scholar]
- Colmer TD. 2003. Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water Rice (Oryza sativa L.). Annals of Botany 91: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey JWH. 1981. Internal winds in water lilies: an adaptation to life in anaerobic sediments. Science 210: 1017–1019. [DOI] [PubMed] [Google Scholar]
- Duarte CM. 1990. Seagrass nutrient content. Marine Ecology Progress Series 67: 201–207. [Google Scholar]
- Duarte CM. 1991. Seagrass depth limits. Aquatic Botany 40: 363–373. [Google Scholar]
- Farmer AM. 1987. Flowering, germination and zonation of the submerged aquatic plant Lobelia dortmanna L. Journal of Ecology 75: 1065–1076. [Google Scholar]
- Farmer AM, Spence DHN. 1986. The growth strategies and distribution of isoetids in Scottish freshwater lochs. Aquatic Botany 26: 247–258. [Google Scholar]
- Greve TM, Borum J, Pedersen O. 2003. Meristematic oxygen variability in eelgrass (Z. marina L.). Limnology and Oceanography 48: 210–216. [Google Scholar]
- Greve TM, Krause-Jensen D, Rasmussen MM, Christensen PB. 2005. Means of rapid eelgrass (Zostera marina L.) recolonization in former die-back areas. Aquatic Botany 82: 143–156. [Google Scholar]
- Hemminga MA, Duarte CM. 2000.Seagrass ecology. Cambridge: Cambridge University Press. [Google Scholar]
- Jensen CR, Luxmoore SD, Van Gundy SD, Stolzy JB. 1969. Root air-space measurements by a pycnometer method. Agronomy Journal 61: 474–475. [Google Scholar]
- Larsen P. 2004. Strømhastighedens indflydelse på grænselagstykkelse og interne iltforhold hos ålegræs (Z. marina L.). MSc Thesis, Freshwater Biological Laboratory, University of Copenhagen, Denmark. [Google Scholar]
- Moyle JB. 1945. Some chemical factors influencing the distribution of aquatic plants in Minnesota. American Midland Naturalist 34: 402–420. [Google Scholar]
- Moeller RE. 1978. Seasonal changes in biomass, tissue chemistry and net production of the evergreen hydrophyte Lobelia dortmanna Canadian Journal of Botany 56: 1425–1433. [Google Scholar]
- Nielsen KB, Kjøller R, Olsson PA, Schweiger PF, Andersen FØ, Rosendahl S. 2004. Colonisation and molecular diversity of arbucular mycorrhizal fungi in the aquatic plants Littorella uniflora and Lobelia dortmanna in southern Sweden. Mycological Research 108: 616–625. [DOI] [PubMed] [Google Scholar]
- Nielsen SL, Sand-Jensen K. 1991. Variation in growth rates of submerged rooted macrophytes. Aquatic Botany 39: 109–120. [Google Scholar]
- Nielsen SL, Sand-Jensen K, Borum J, Geertz-Hansen O. 2002. Depth colonization of eelgrass (Z. marina L.) and macroalgae as determined by water transparency in Danish coastal waters. Estuaries 25: 1025–1032. [Google Scholar]
- Nygaard G. 1958. On the productivity of the bottom vegetation in the lake Grane Langsø. Internationale Vereinigung für theoretische und angewandte Limnologie, Verhandlungen 13: 144–155. [Google Scholar]
- Pedersen O, Sand-Jensen K. 1992. Adaptations of submerged Lobelia dortmanna to aerial life form: morphology, carbon sources and oxygen dynamics. Oikos 65: 89–96. [Google Scholar]
- Pedersen O, Sand-Jensen K, Revsbech NP. 1995. Diel pulses of O2 and CO2 in sandy lake sediments inhabited by Lobelia dortmanna Ecology 76: 1536–1545. [Google Scholar]
- Pedersen O, Borum J, Duarte CM, Fortes MD. 1998. Oxygen dynamics in the rhizosphere of Cymodocea rotundata Marine Ecology Progress Series 169: 283–288. [Google Scholar]
- Pedersen O, Binzer T, Borum J. 2004. Sulphide intrusion in eelgrass (Zostera marina L.). Plant, Cell and Environment 27: 595–602. [Google Scholar]
- Penhale PA, Wetzel RG. 1983. Structural and functional adaptations of eelgrass (Zostera marina L.) to the anaerobic sediment environment. Canadian Journal of Botany 61: 1421–1428. [Google Scholar]
- Raven JA. 1984.Energetics and transport in aquatic plants. New York: Alan Liss. [Google Scholar]
- Raven JA, Scrimgeour CM. 1997. The influence of anoxia on plants of saline habitats with special reference to the sulphur cycle. Annals of Botany 79: (Suppl. A) 79–86. [Google Scholar]
- Richardson K, Griffiths H, Reed ML, Raven JA, Griffiths NM. 1984. Inorganic carbon assimilation in the isoetids, Isoetes lacustris L. and Lobelia dortmanna L. Oecologia 61: 115–121. [DOI] [PubMed] [Google Scholar]
- Risgaard-Petersen N, Jensen K. 1997. Nitrification and denitrification in the rhizosphere of the aquatic macrophyte Lobelia dortmanna L. Limnology and Oceanography 42: 529–537. [Google Scholar]
- Sand-Jensen K. 1975. Biomass, net production and growth dynamics in an eelgrass population (Zostera marina L.) in Vellerup Vig, Denmark. Ophelia 14: 185–201. [Google Scholar]
- Sand-Jensen K, Borum J. 1991. Interactions among phytoplankton, periphyton and macrophytes in temperate freshwaters and estuaries. Aquatic Botany 41: 137–175. [Google Scholar]
- Sand-Jensen K, Prahl C. 1982. Oxygen exchange with the lacunae and across leaves and roots of the submerged vascular macrophyte, Lobelia dortmanna L. New Phytologist 91: 103–120. [Google Scholar]
- Sand-Jensen K, Søndergaard M. 1978. Growth and production of isoetids in oligotrophic lake Kalgaard, Denmark. Internationale Vereinigung für Theoretische und Angewandte Limnologie, Verhandlungen 20: 659–666. [Google Scholar]
- Sand-Jensen K, Søndergaard M. 1979. Distribution and quantitative development of aquatic macrophytes in relation to sediment characteristics in oligotrophic Lake Kalgaard, Denmark. Freshwater Biology 9: 1–11. [Google Scholar]
- Sand-Jensen K, Prahl C, Stokholm H. 1982. Oxygen release from roots of submerged aquatic macrophytes. Oikos 38: 349–354. [Google Scholar]
- Schuette JL, Klug MJ. 1995. Evidence for mass flow in flowering individuals of the submersed vascular plant Myriophyllum heterophyllum. Plant Physiology 108: 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculthorpe CD. 1967.The biology of aquatic vascular plants. London: Edward Arnold. [Google Scholar]
- Smith RD, Pregnall AM, Alberte RS. 1988. Effects of anaerobiosis on root metabolism of the seagrass Zostera marina L. Marine Biology 98: 131–141. [Google Scholar]
- Smits AJM, Laan P, Their RH, van der Velde G. 1990. Root aerenchyma, oxygen leakage patterns and alcoholic fermentation ability of the roots of some nymphaeid and isoetid macrophytes in relation to sediment type of their habitat. Aquatic Botany 38: 3–17. [Google Scholar]
- Sorrell BK. 2004. Regulation of root anaerobiosis and carbon translocation by light and root aeration in Isoetes alpinus Plant, Cell and Environment 27: 1102–1111. [Google Scholar]
- Sorrell BK, Dromgoole FI. 1987. Oxygen transport in the submerged freshwater macrophytes Egeria densa Planch. I. Oxygen production, storage and release. Aquatic Botany 28: 63–80. [Google Scholar]
- Søndergaard M, Laegaard S. 1977. Vesicular-arbuscular mycorrhizae in some aquatic vascular plants. Nature 268: 232–233. [Google Scholar]
- Søndergaard M, Sand-Jensen K. 1978. Total autotrophic production in oligotrophic Lake Kalgaard, Denmark. Internationale Vereinigung für Theoretische und Angewandte Limnologie, Verhandlungen 20: 667–673. [Google Scholar]
- Søndergaard M, Sand-Jensen K. 1979. Carbon uptake by leaves and roots of Littorella uniflora (L.) Aschers. Aquatic Botany 6: 1–12. [Google Scholar]
- Tessenow U, Baynes Y. 1978. Redoxchemische Einflüsse von Isoetes lacustris L. im Littoralsediment des Feldsees (Hochschwarzwald). Archiv für Hydrobiologie 82: 20–48. [Google Scholar]
- Wigand C, Andersen FØ, Christensen KK, Holmer M, Jensen HS. 1998. Endomycorrhizae along a biogeochemical gradient. Limnology and Oceanography 43: 508–515. [Google Scholar]
- Wium-Andersen S. 1971. Photosynthetic uptake of free CO2 by the roots of Lobelia dortmanna. Physiologia Plantarum 25: 245–248. [Google Scholar]
- Wium-Andersen S, Andersen JM. 1972. The influence of vegetation on the redox profile of the sediment of Grane Langsø, a Danish Lobelia lake. Limnology and Oceanography 17: 948–952. [Google Scholar]





