Abstract
• Background and Aims Akagare and Akiochi are diseases of rice associated with sulfide toxicity. This study investigates the possibility that rice reacts to sulfide by producing impermeable barriers in roots.
• Methods Root systems of rice, Oryza sativa cv. Norin 36, were subjected to short-term exposure to 0·174 mm sulfide (5·6 ppm) in stagnant solution. Root growth was monitored; root permeability was investigated in terms of polarographic determinations of oxygen efflux from fine laterals and the apices of adventitious roots, water uptake, anatomy and permeability to Fe2+ using potassium ferricyanide.
• Key Results Both types of root responded rapidly to the sulfide with immediate cessation of growth, decreased radial oxygen loss (ROL) to the rhizospheres and reduced water uptake. Profiles of ROL measured from apex to basal regions of adventitious roots indicated that more intense barriers to ROL than normal were formed around the apices. Absorption of Fe2+ appeared to be impeded in sulfide-treated roots. In adventitious roots, deposition of lipid material (suberisation) and thickenings of walls within the superficial cell layers were obvious within a week after lifting the treatment and could prevent the emergence of laterals and commonly result in their upward longitudinal growth within the cortex. Death of laterals sometimes occurred prior to emergence; emergent laterals eventually died. In adventitious roots, blockages formed within the vascular and aeration systems in response to the sulfide.
• Conclusions In both adventitious and lateral roots, sulfide-induced cell wall suberization and thickening of the superficial layers were correlated with reduced permeability to O2, water and Fe2+. This study sheds light on some of the symptoms of diseases such as Akiochi. The results correlate with the authors' previous findings on the effects on roots of sulfide and lower organic acids in Phragmites and of acetic acid in rice.
Keywords: Rice, Oryza sativa, roots, sulfide toxicity, barrier, permeability, water uptake, anatomy, rhizosphere, radial oxygen loss, suberization, growth
INTRODUCTION
‘Straighthead’, Akagare (‘summer decline’) and Akiochi (‘autumn decline’) diseases of rice have long been associated with sulfide toxicity in soils. The latter disease, for example, involves rotting of roots, bronzing of leaves, poor growth at the reproductive phase and poor yield (e.g. Vámos, 1959; Baba and Iwata, 1963; Takijima, 1965). Symptoms also include reduced root respiration and nutrient uptake especially of K, Mn, Mg and Si (Baba and Iwata, 1963; Mitsui, 1965; Tanaka et al., 1968; Allam and Hollis, 1972; Joshi et al., 1975), and a reduced ability to oxidize iron in the rhizosphere (Takijima, 1964). Allam (1971) reported sulfide inhibitions of catalase, peroxidase, ascorbic acid oxidase, polyphenol oxidase and terminal cytochrome oxidase, which influence the oxidative capacity of rice roots.
The maintenance of a low resistance pathway of aerenchyma and intercellular spaces, which facilitates gaseous exchange between the atmosphere and underground organs, has long been regarded as an essential feature for the survival of emergent macrophytes, such as rice, in anaerobic flooded soils (e.g. Van Raalte, 1940; Barber et al., 1962; Armstrong, 1964, 1979; John et al., 1974; Sorrell et al., 2000). Moreover, radial oxygen loss (ROL) from root to the rhizosphere is considered to be essential for the detoxification of phytotoxins such as Fe2+, Mn2+, H2S, S2−, HS− and organic acids by direct oxidation or by the agencies of oxidizing aerobic microorganisms maintained in the rhizosphere regions (e.g. Armstrong, 1970; Mendelssohn et al., 1988; Trolldenier, 1988; Begg et al., 1994; Revsbech et al., 1999). In this way, the vulnerable regions of roots, that are permeable for the uptake of water and nutrients, are protected from toxin damage. Moreover, ROL has been found to be an important factor in nutrient acquisition (e.g. Kirk and Bajita, 1995; Saleque and Kirk, 1995; Kirk, 2003).
The factors influencing levels of sulfide accumulation in soils are many and their inter-relationships complex, involving, for example, degrees of sulfate availability and its reduction, soil temperature, redox potential, pH, organic matter content, CO2 and bicarbonate accumulation, and sulfide ion immobilization, for example by Fe2+ (e.g. Ponnamperuma, 1965; Connell and Patrick, 1968; Hollis et al., 1975). It has been claimed that warm, flooded soils that are rich in organic matter and/or 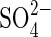 , and those of low Fe content are particularly prone to produce diseased plants (e.g. Baba et al., 1965).
, and those of low Fe content are particularly prone to produce diseased plants (e.g. Baba et al., 1965).
Sulfate-reducing bacteria, e.g. Desulfovibrio desulfuricans, produce sulfide, require anaerobic conditions and function at pH 5·5–9; they do not tolerate highly acid conditions (Starkey, 1966). Hydrogen sulfide (H2S) produced in the reduction is readily soluble and above pH 7 dissociates to S2− and HS−. All three reduced S species are highly toxic to plants.
The overall reduction reaction, catalysed by sulfate-reducing bacteria, where ‘CH2O’ represents organic matter oxidized in the process of reduction, is:
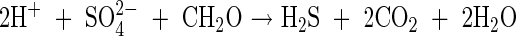 |
The sulfide equilibria are:
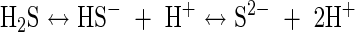 |
Low concentrations of Fe2+ in the soil may be inadequate to detoxify the sulfide by deposition of FeS. However, Bloomfield and Pruden (1962) estimated that even in Fe2+-rich soils, only half of the sulfide may exist as FeS, the rest being in the form of H2S. Also, Hollis et al. (1975) reported that sulfide diseases of rice occurred on iron-excess soils in the US Gulf Coast region, and that <1 ppm (0·03 mm) sulfide could be toxic to rice at pH 5–6. Submerged soils that are rich in sulfates are likely to produce H2S. Sulfide concentrations in soils may also be linked to the type of clay present (Allam et al., 1972): kaolinite does not sorb reduced-S species, but montmorillonite does.
Sulfide is toxic because it combines with the iron of cytochromes and other iron-containing compounds in cells. In species other than rice, sulfide has also been identified as a respiratory toxin. Mendelssohn and McKee (1988) have demonstrated that in Spartina alternifolia, high soil sulfide levels can have an inhibitory effect on aerobic respiration but less effect on ADH activity; Fürtig et al. (1996) showed inhibition of aerobic respiration in Phragmites. Sulfide is known to be as effective as cyanide as a respiratory toxin, inhibiting cytochrome oxidase in eukaryotic cells at concentrations of 1–10 µm (Fenchel and Finlay, 1995; Raven and Scrimgeour, 1997). Sulfide has also been cited as an inhibitor of photosynthesis in Spartina (Pezeshki et al., 1988), reducing it to one-sixth of normal at only 0·001 mm (0·034 ppm) sulfide in stirred suspensions.
We have found previously that the common reed, Phragmites australis, responded to 1·4 mm sulfide, 45 ppm (Armstrong et al., 1996a) and to the low molecular weight organic acids at toxic undissociated concentrations of 0·35–0·42 mm (Armstrong and Armstrong, 1999, 2001) in terms of (a) cessation of root growth and inhibition of lateral root emergence, (b) the induction of blockages within the internal gas space system, (c) lignification and suberization in the normally permeable parts of the root system such as the superficial layers of fine laterals and of the apical regions of adventitious roots, and (d) blockages within the vascular system. We also reported that rice responded similarly to 1·05 mm toxic, undissociated acetic acid, but here there was less cell wall lignification than in Phragmites. It was shown that ROL from adventitious root apices was greatly decreased in rice by acetic acid (1·5 mm as a single dose) and in Phragmites by cocktails of low molecular weight organic acids (Armstrong and Armstrong, 2001). We concluded that the roots responded to these phytotoxins by inducing barriers in the vulnerable, permeable parts, with the result that less toxin is absorbed. Moreover, Colmer et al. (1998) and Colmer (2003a) have demonstrated that in rice, a barrier to root ROL is induced in response to anoxia. Although it has long been known that, especially under waterlogged conditions, a barrier to ROL normally develops in adventitious roots sub-apically, and increases in strength with the distance from the apex (e.g. Armstrong, 1971), there recently has been renewed interest in the nature, causes and effects of such barriers (Colmer et al., 1998; Armstrong and Armstrong, 1999, 2001; Armstrong et al., 2000; Visser et al., 2000; McDonald et al., 2002; Colmer, 2003a, b).
In so far as we are aware, there is comparatively little documentation for rice regarding the effects of sulfide on its anatomy or on ROL from root to rhizosphere. In this study, we tested the hypothesis that a comparatively low level of sulfide, 0·174 mm (5·6 ppm) applied in stagnant culture medium, would produce similar responses in roots of rice (cv. Norin 36) that we had reported previously with acetic acid, and for roots of Phragmites in relation to both sulfide and organic acid toxicities (Armstrong et al., 1996a; Armstrong and Armstrong, 1999, 2001).
MATERIALS AND METHODS
Plant material
Seeds of rice, ‘Norin 36’, were germinated in the UK in summer on moist tissue in shallow trays covered in polythene in a propagating frame, with natural light. Germination occurred in about a week and the trays were then transferred to the open bench in a glasshouse for 3–4 weeks until the shoots were approx. 6–8 cm high. The seedlings were then transplanted into buckets (volume = 15 L) containing moist John Innes compost No. 2; the water table was gradually raised over 2 weeks, until the soil was flooded to a depth of 2 cm. Conditions were: air temperature = 16 °C (minimum) and 29 °C (maximun), with natural light, and an 18 h day length.
Treatments
When the shoots were approx. 20–30 cm tall, plants were separated into individual tillers; the roots were trimmed back to approx. 20–30 mm, the root systems washed free of soil and each plant was transferred to a black polythene-covered glass tube (height = 400 mm; diameter = 50 mm) containing 25 % Yoshida nutrient solution (Yoshida et al., 1976) in 0·05 % (w/v) deoxygenated agar to resume root and shoot growth, in a growth room. The plants were secured in the tubes with the shoots emergent and their bases submerged under approx. 40 mm of culture solution; they were arranged randomly in line, parallel to a bank of lights, and their positions changed on alternate days to ensure that, as far as possible, they experienced identical external conditions. The shoots received continuous lighting from the side; PAR = 80–100 µmol m−2 s−1, air temperature = 18 °C; the medium was changed every 2 days. Plants were used for experiments when the longest roots were approx. 130–150 mm, about 2 weeks after transplanting.
For control and sulfide treatments, which lasted 1–2 d, plants were transferred, unless otherwise stated, into deoxygenated agar (controls) or, for the sulfide treatment, into deoxygenated agar containing 0·174 mm (5·6 ppm) sulfide, added as sodium sulfide (Na2S·9H2O); the sulfide concentration had previously been confirmed using a dropping mercury sulfide electrode.
Root growth
Daily growth increments on 34 adventitious roots (original length approx. 40–65 mm) of 16 plants (two roots per plant), grown in 25% Yoshida medium, were measured on the 6 d preceding treatment. The plants were then divided into two groups of eight plants each. The controls were transferred to fresh deoxygenated agar, while the other group was subjected to the sulfide treatment as described above. The treatment period lasted 2 d. The plants were then transferred back into deoxygenated Yoshida medium. Growth increments were measured during the treatment period and during 4 d after this. Growth increments were recorded by marking the positions of the tips of the selected roots on the glass container and measuring the distances between marks using digital callipers (RS Components Ltd, Corby, UK).
For each treatment, lateral root growth and emergence were observed on (a) young adventitious roots (original length = 60 mm), (b) more mature roots (original length = 100–120 mm) where laterals had emerged and were maximally 3–4 mm long, and (c) on new adventitious roots produced post-treatment. On day 18, post-treatment, lateral root frequencies and lengths for those laterals produced post-treatment were measured using an Olympus SZ40 dissecting microscope. During weeks 1–4, post-treatment, the plants were also examined for evidence of lateral root death using acridine orange, as described in the anatomical methods.
Radial oxygen loss (ROL) from roots
Plants were placed with their roots in rectangular Perspex vessels (80 × 80 × 200 mm) covered in black polythene. Each vessel was fitted with a rubber bung to accommodate the shoot base and electrodes and to minimize re-oxygenation of the medium by the atmosphere. Also, deoxygenated 0·05 % (w/v) agar medium was added by siphoning to minimize re-oxygenation.
ROL was measured using cylindrical or coiled wire Pt cathodes in conjunction with Ag–AgCl anodes, after the method of Armstrong and Wright (1975). Some measurements were made with the roots in sulfide medium; it was therefore necessary to confirm that the electrodes were unaffected by this medium. This was done using ‘artificial’ Si-rubber roots after Armstrong (1979).
Adventitious roots
Measurements of ROL were made using sleeving cylindrical Pt cathodes (length = 5 mm; i.d. = 2·25 mm) either (a) positioned sub-apically, 3 mm from the apex, or (b) used to measure profiles of O2 flux along the roots. (a) For apical ROL measurements, the root systems were first placed in freshly deoxygenated agar containing 7 mm KCl for approx. 2 h; for the sulfide treatment, this was then replaced by deoxygenated agar containing 0·174 mm sulfide and 7 mm KCl. Three plants were used per treatment and at least two roots were examined per plant; n = 6. (b) For ROL profiles along the roots (apex to base), there were two types of sulfide treatment: (1) 36 h in sulfide medium and then into deoxygenated agar containing 7 mm KCl for ROL measurements, or (2) 24 h in sulfide medium initially, but the roots were maintained in this medium (containing 7 mm KCl) for the ROL measurements. ROL profiles were taken from 3–6 mm sub-apically to the points at which laterals emerged. Four plants were used for each control and sulfide treatment, and measurements were made on at least two roots per plant.
Fine lateral roots
ROL from lateral roots was detected by one of two types of cathode each used in conjunction with an Ag–AgCl anode. (a) Bare Pt wire cathode (length = 50 mm; diameter = 0·37 mm) loosely coiled around the adventitious root. Four measurements were made around basal laterals whose lengths were approx. 3–4·5 mm and two measurements were made around more apical, immature laterals whose lengths were approx. 1–2 mm; n = 8. (b) Wide cylindrical Pt cathode (l = 10 mm; i.d. = 4 mm) which fitted around basal laterals; n = 3. Electrodes were also inserted in the media in positions remote from the roots to measure background O2 diffusion rates. All root systems were first placed in freshly deoxygenated control medium for 800 min (approx. 13 h); this was then replaced by sulfide medium as above; ROL measurements were made on the roots in this medium.
Test for presence of oxygen within adventitious roots
We have found previously (unpubl. res.) that penetration of rice roots with 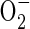 microelectrodes is difficult. Evidence for the presence of O2 within the roots was obtained using a cylindrical cathode positioned around the cut end of the root from which the apical 15–20 mm had been removed using a razor blade. This was useful for confirming that reduced ROL in sulfide treatments was a function of reduced root permeability and not due to a lack of O2 within the root.
microelectrodes is difficult. Evidence for the presence of O2 within the roots was obtained using a cylindrical cathode positioned around the cut end of the root from which the apical 15–20 mm had been removed using a razor blade. This was useful for confirming that reduced ROL in sulfide treatments was a function of reduced root permeability and not due to a lack of O2 within the root.
Test for ROL from sulfide-treated roots
Where ROL from roots from the sulfide was almost zero, it was useful to see if there was still internal gas transport and if impermeability to ROL was complete by attempting to measure ROL with the shoots first in air, then O2 and finally in air again. The same was done with control plants.
Water uptake
Water uptake was estimated from the decreases in the liquid level on a daily basis. After each 24 h period, the liquid levels were readjusted to the originals by adding deoxygenated agar. Control readings on similar tubes of liquid without plants were taken into account.
Measurements were taken during 3 d with the plants in nutrient solution without sulfide. They were then placed in sulfide medium for 2 d and then transferred back to fresh Yoshida solution; water uptake was again measured as before for 3 d; n = 9.
Uptake of Fe2+ by roots
This was a preliminary experiment to try to detect barriers to ion uptake within adventitious roots. Roots were excised from controls and plants whose roots had been in the sulfide medium for 2 d and then replaced in nutrient medium for 2 weeks. The roots (length = 90–120 mm) with basal laterals were washed in distilled water, their cut ends sealed with lanolin and placed for 1·2 h in deoxygenated agar containing 25% Yoshida medium minus the phosphate and enriched with ferrous sulfate (FeSO4·7H2O) to give 2 mm Fe2+ (De Rufz de Lavison, 1910). The roots were washed and sectioned transversely at positions 0·5, 2·5 and 6 cm from the apex and in the basal regions. The sections were placed immediately in fresh 1 % (w/v) 8 mm potassium ferricyanide solution [K4Fe(CN)6·3H2O] plus 0·5 % HCl, to precipitate the iron absorbed by the roots as ‘Prussian blue’, ferric ferrocyanide [Fe4(Fe(CN6)3] (Pearse, 1968). Roots of similar lengths from each treatment were compared.
Anatomy
Transverse sections were made of selected adventitious roots and laterals and of rhizomes from plants used in the ROL measurements and from the growth experiment, from both control and sulfide treatments during treatment and between days 1 and 28 post-treatment, when the plants had been replaced in Yoshida medium. Sections were stained with phloroglucinol and concentrated hydrochloric acid to detect lignification (confirmed with aniline hydrochloride) or in Gram's iodine (Gurr, 1965). Some sections were left unstained. Specimens were photographed either in white light using an Olympus BX40 photomicroscope or, using a fluorescence attachment, in blue light, unstained sections only, for autofluorescence of lipid materials; lipids were confirmed using Sudan IV (Gurr, 1965). For indications of cell viability, transverse sections and whole lateral roots were stained in 0·01 % acridine orange and examined for greenish fluorescence of nuclei and cytoplasm, which indicates cell viability (Gurr, 1965).
RESULTS
Growth
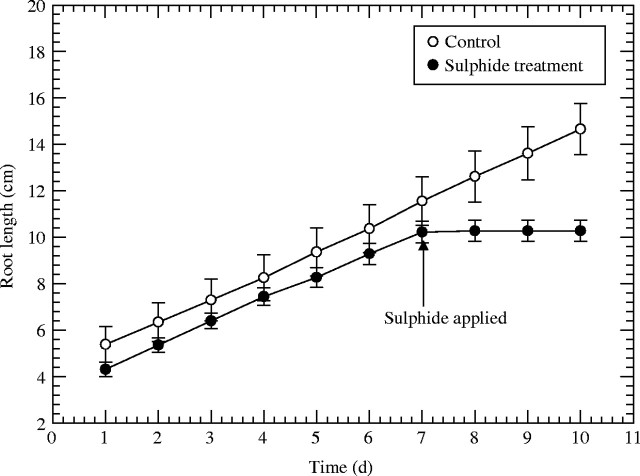
Adventitious roots from the control treatment continued to grow, whereas those treated with sulfide for 2 d stopped growing virtually immediately (Fig. 1) and generally did not resume growth when transferred back into Yoshida medium. Production and growth in length of lateral roots were observed in the controls, but the sulfide treatment curtailed lateral root growth immediately and these laterals did not grow again when the treatment was lifted (data not shown). The emergence of laterals from the sulfide treatment was <10 % and lengths of laterals were <9 % of those of the controls (Table 1). Consequently, it was obvious that the sulfide treatment had greatly affected the production of lateral roots in terms of area. For a 1 cm length of adventitious root from the sulfide treatment, the surface area of laterals was only approx. 0·36 mm2, whereas the value for the control was 43 mm2, a decrease in lateral root area of approx. 99 %. However, within 2–3 weeks of the sulfide treatment, new adventitious roots with normal laterals developed from the rhizomes and shoot bases.
Fig. 1.
Rice: effects of 0·174 mm sulfide (5·6 ppm) on adventitious root elongation. Treatment time in sulfide medium was 2 d; n = 17; means±s.e.
Table 1.
Effect of 2 d of 0.174 mm sulfide on numbers of emergent laterals and lateral root growth 18 d after treatment
| Sulfide |
Post-sulfide* |
Control |
|
|---|---|---|---|
| No. of emergent laterals per cm length of root† | 2·98 ± 0·64a | 33·53 ± 2·3b | 32 ± 1·5b |
| Length of laterals (mm)† | 0·35 ± 0·24x | 3·44 ± 0·45y | 3·9 ± 0·31y |
Means ± s.d., n = 12.
Treatment time was 2 d.
New adventitious roots produced post-sulfide.
Laterals produced post-treatment period.
Significant differences (Tukey test: P < 0·05) between treatments are indicated by letters.
With these short-term treatments with comparatively low concentrations of sulfide, root death was largely confined to the fine laterals and in a few cases to the adventitious root apices. In all cases, the plants recovered with renewed root production; sometimes, especially where root density was high, adventitious root apices resumed growth and production of laterals.
Radial oxygen loss from roots
Testing the cathodes on artificial roots showed that the sulfide media had no effect on the readings for O2 flux over the typical periods of the experiments (data not shown). This was relevant to experiments where the ROL measurements were taken first in control and then in sulfide media.
When apices of sulfide-treated roots were excised, O2 flux from the cut ends was considerably higher than from the intact apex, indicating that the observed decreases in ROL (see below) had not been due to a lack of O2 in the roots. Also for the sulfide treatment, with the shoot systems in O2 rather than air, apical ROL rapidly increased and then decreased when the O2 supply was switched off and the air supply was resumed (results not shown). This too indicated internal gas space continuity and some permeability of the root to O2 efflux. (The effects were similar with control roots.)
Adventitious root apices: radial oxygen loss versus time
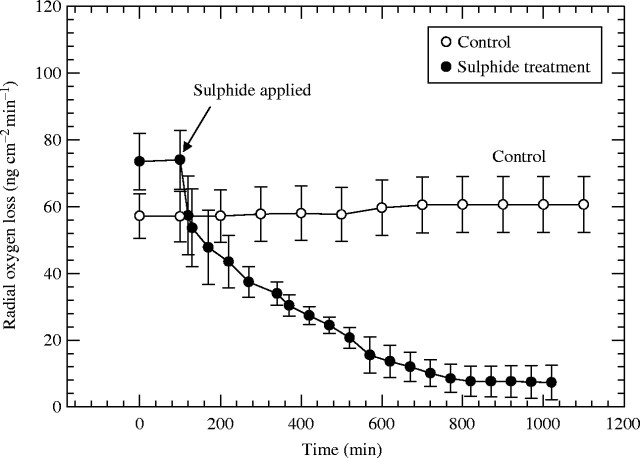
Values of ROL for control roots remained stable at approx. 60 ng O2 cm−2 min−1, whereas the sulfide treatment produced a rapid decrease in ROL from approx. 74 to approx. 8 ng O2 cm−2 min−1 within 12 h (Fig. 2).
Fig. 2.
Rice: effect of 0·174 mM (5·6 ppm) sulfide on radial oxygen loss (ROL) from adventitious root apices. Root length = 90–110 mm; n = 6; means±s.e.
Adventitious roots: profiles of radial oxygen loss along roots
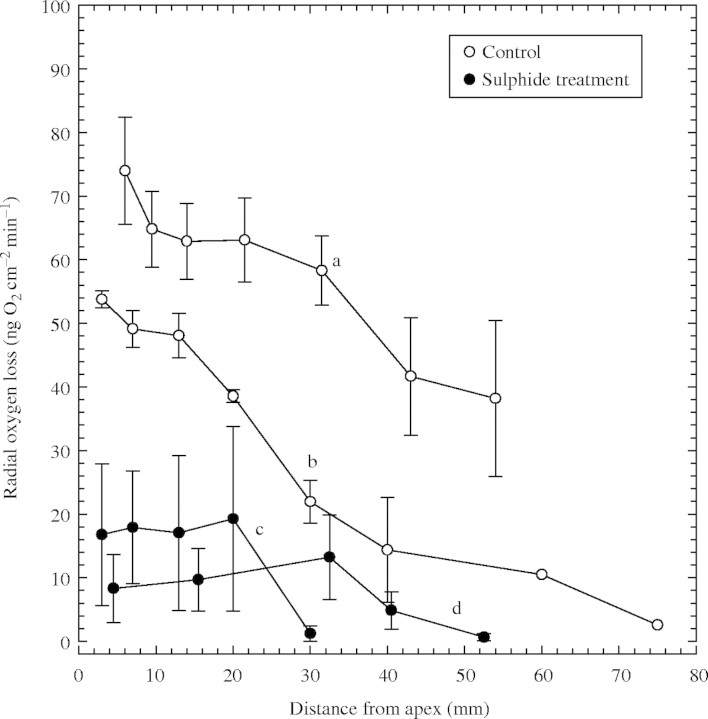
The patterns of ROL profiles along control roots were typical for rice (Fig. 3) with the highest values in these experiments being 54–74 ng O2 cm−2 min−1 from around the apical 3–6 mm and decreasing to 13·5–41 ng O2 cm−2 min−1 at approx. 45 mm from the apex. In some cases, the emergence of laterals prevented measurements further towards the base (Fig. 3a). In the region below this where the laterals had not emerged, ROL values sometimes increased slightly, almost certainly due to ‘oxygen-permeable windows’ within the thickened hypodermal layers opposite the developing laterals (see section on anatomy). Where lateral emergence began further from the apex (Fig. 3b), ROL values fell to almost 0 ng O2 cm−2 min−1 at about 80 mm from the apex. In contrast, for the sulfide treatments, apical ROL values were considerably lower, at most only approx. 30 % of those of the controls; moreover, zero values were reached only 25–45 mm from the apices (Fig. 3c and d). For the sulfide treatment, results were similar whether ROL measurements were made in control medium (Fig. 3c) or in the sulfide medium (Fig. 3d).
Fig. 3.
Rice: effects of 0·174 mm sulfide (5·6 ppm) on profiles of radial oxygen loss (ROL) along adventitious roots. Root length = 90–120 mm. Treatment time in sulfide medium was 1·5 d only prior to ROL measurements (c) and 1 d prior to and during ROL measurements (d); n = 4 for each plot; means±s.e. Controls a relate to c, and controls b relate to d.
Lateral roots: radial oxygen loss versus time
With the Pt cathodes coiled around basal laterals (Fig. 4A, upper plots), O2 fluxes were higher (1·7–2 µA) before the roots were exposed to sulfide. However, O2 flux declined immediately after the application of sulfide and in 12 h had fallen by between 55 and 100 %. Even for laterals near the adventitious root apices (Fig. 4A, lowest plot) where initial O2 fluxes were comparatively low, there was still a measurable reaction to sulfide. Equilibration with the cylindrical Pt cathodes (Fig. 4B) was slower after the sulfide treatment, presumably because of some degree of O2 accumulation within the electrode and a slower renewal of sulfide at the root surfaces from the bulk medium. The renewal of the sulfide medium after 1000 min caused further decreases in ROL.
Fig. 4.
Rice: effect of 0·174 mM (5·6 ppm) sulfide on radial oxygen loss (ROL) from fine lateral roots; adventitious root length approx. 90–130 mm; n = 4–6; means±s.e. (A) Using Pt wire cathodes coiled around laterals; circles and triangles for basal laterals (length = approx. 3·6 mm), squares for more apically situated laterals (length = approx. 2 mm). (B) Using cylindrical Pt cathodes around clusters of laterals (length = pprox. 3·6 mm).
Water uptake
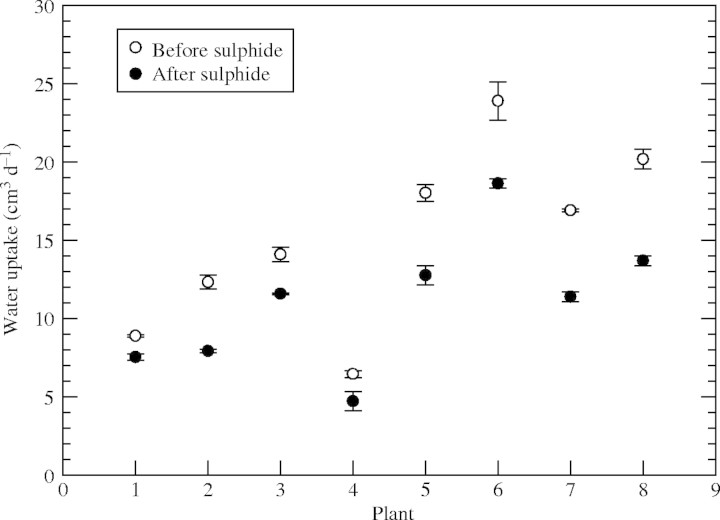
The 2 d sulfide treatment caused reductions in water uptake of 9–25 % (Fig. 5). All plants showed some reduction in water uptake. Over the same time period, untreated plants showed no reductions in water uptake; rather it tended to increase slightly (results not presented).
Fig. 5.
Rice: effect of 0·174 mm (5·6 ppm) sulfide on water uptake by root systems; maximum shoot height = approx. 30 cm; maximum root length = approx. 20 cm. Daily water uptake was measured on 3 d preceding sulfide treatment (open circles), and on 3 d after a 2 d 0·174 mm sulfide treatment (filled circles).
Anatomy
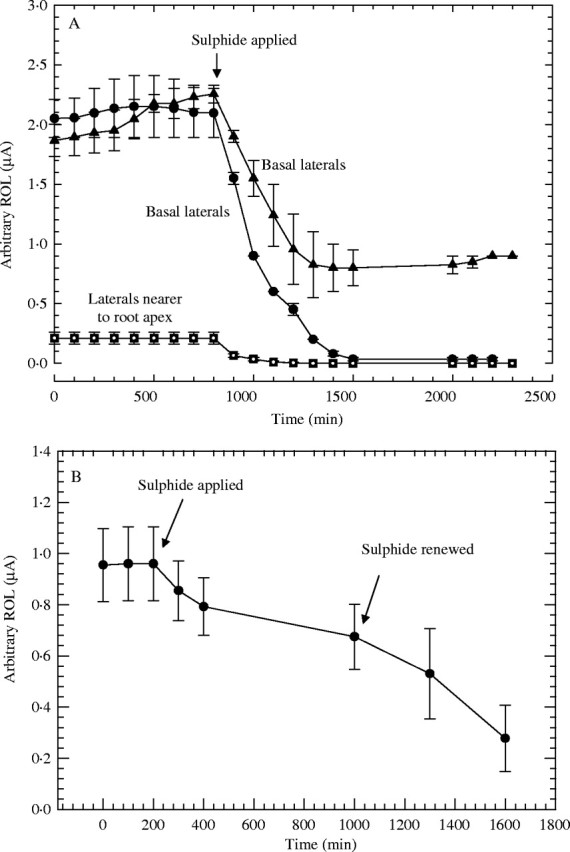
Even within 1–2 d of starting the sulfide treatment, slightly more autofluorescence in blue light around apical regions of sulfide-treated adventitious roots than in the controls was detectable, but this was more obvious within 4–6 d of treatment (not shown). At this stage, cell walls of the hypodermal and exodermal layers and sometimes the epidermis in the apical 6 mm of roots from the sulfide treatment showed yellow autofluorescence; there was comparatively little fluorescence in the controls. This indicated that the sulfide-treated roots had developed some kind of lipid-based cell wall thickening, and this was confirmed with Sudan IV. After 2–3 weeks, strong lignification was present in the endodermis and central stele, and blockages in the phloem, xylem and cortical intercellular spaces were common; those of the protoxylem often became lignified (Fig. 6A and B). Apical regions of control roots showed no lignifications or blockages. After 2–3 weeks post-treatment, hypodermal, exodermal (Fig. 7A) and stelar lignifications were far more extreme in the more basal regions of sulfide-treated roots than in the controls.
Fig. 6.
Rice: effects of 2 d in 0·174 mm sulfide on root anatomy, 2 weeks after lifting of treatment. Fresh hand-cut transverse sections of adventitious roots; (A), (B) and (C) stained with phloroglucinol and concentrated hydrochloric acid to show lignification (red); (D), (E) and (F) viewed with blue light to show yellow autofluorescence of lipid material in cell walls. (A) Sulfide treatment, 15 mm from the apex showing heavy lignification of stele, vascular blockages, brown occlusions in cortical intercellular spaces and slight lignification of exodermis. None of these blockages or lignifications was seen in the controls. Bar = 50 µm. (B) High power of stele similar to (A), showing lignified blockages of protoxylem (red) and blocked phloem (brown). Bar = 50 µm. (C) Sulfide treatment 30 mm from the apex, showing a lateral root which had died prior to emergence. Bar = 50 µm. (No such death of laterals was seen in the controls.) (D) Sulfide treatment, 40 mm from the apex showing emergent lateral root swollen and fluorescing within the adventitious root cortex; control laterals did not become swollen or fluoresce. Bar = 50 µm. (E) Control, 30 mm from the apex, with lateral root prior to emergence; note that opposite the lateral is a ‘window’ in the hypodermis and exodermis with virtually no thickening or autofluorescence (cf. F). Bar = 50 µm. (F) Sulfide treatment, 30 mm from the apex, with lateral root, showing thickened exodermis and strong autofluorescence of adventitious root exo/hypodermis opposite the lateral, and of the edge of the lateral (cf. E). Bar = 50 µm.
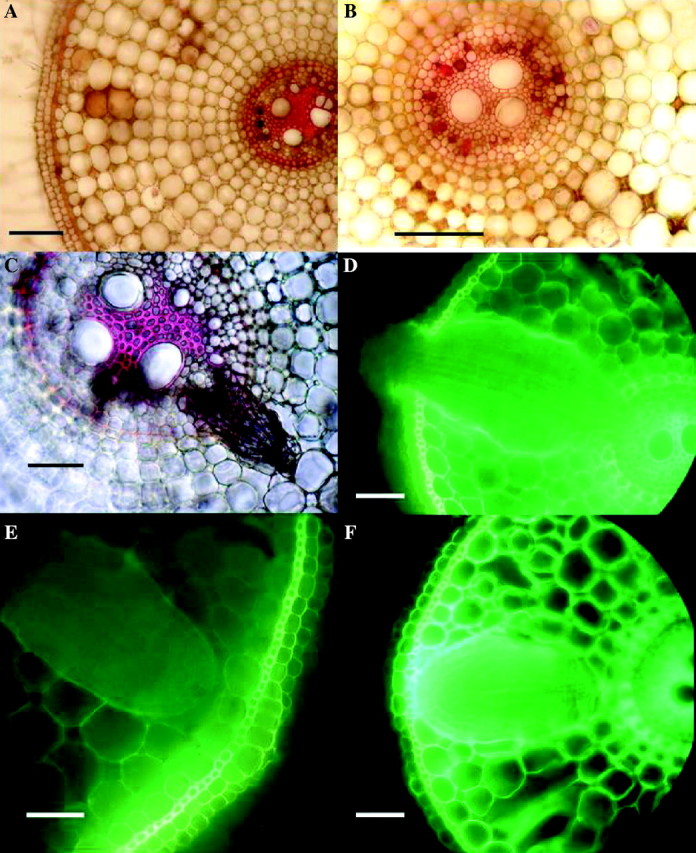
Fig. 7.
Rice: effects of 2 d in 0·174 mm sulfide on lateral root growth, 2 weeks after lifting of treatment. Fresh hand-cut transverse sections of adventitious roots (length = 100–120 mm); (A) stained with phloroglucinol and concentrated hydrochloric acid to show lignification (red); (B–E) unstained. (A) Approx. 45 mm from the apex, showing strongly thickened and lignified exodermis and lateral root growing through the cortex. (Control roots had only slightly lignified exodermis.) Bar = 50 µm. (B) Approx 40 mm from the apex, showing a single lateral growing through adventitious root cortex; note that the lateral has enlarged cortical cells and gas spaces and hypodermal differentiation. Bar = 50 µm. (C), (D) and (E) are serial sections of one root from the base towards the apex, taken 90–95 mm from the apex; (C) is near the tip of the lateral, while (E) is near the basal part which connects with the stele of the adventitious root. The laterals were therefore growing upwards through the adventitious root cortex. Bars = 50 µm. (No control roots were found with laterals growing within the parent root cortex; here laterals emerged normally.)
At distances of 20–80 mm from the apex of adventitious roots, fine laterals at various stages of development were visible. In control roots, the hypodermal regions opposite developing laterals appeared as ‘windows’ with virtually no fluorescence and relatively unthickened exodermis (Fig. 6E), presumably to allow for their emergence; the rest of the hypodermal layers fluoresced and the exodermis had thickened by this stage. However, it was obvious in the sulfide-treated roots that after 2–4 weeks these hypodermal regions opposite developing laterals had become thickened and fluoresced in blue light (Fig. 6F); moreover, the parts of the emergent laterals embedded in the adventitious root cortex became swollen and their outer cell walls fluoresced (Fig. 6D). In sections taken 2–4 d after the sulfide treatment, brown discolorations of the ‘windows’ were visible and sometimes the apices of the developing laterals were affected. This indicated some penetration of the ‘window’ by the toxin. Moreover, there was evidence of lateral root death prior to emergence (Fig. 6C), presumably due to transmission of the sulfide via a ‘window’ or the stele. Non-emergent laterals were commonly found to have grown within the adventitious root cortex (Fig. 7A and B). Those that had become swollen (Fig. 7B) often had enlarged cortical gas spaces, differentiated hypodermal layers and strongly lignified steles. An interesting characteristic of these laterals was that they had grown upwards through the adventitious root cortex (Fig. 7C, D and E). None of these features was observed in the controls, which produced normal emergent laterals and no premature death. Autofluorescence of cell walls in blue light was confirmed to be indicative of lipid deposition/suberization; this was confirmed with Sudan IV (not shown).
Emergent laterals that had been subjected to sulfide were commonly dead within a week, as detected by the lack of green fluorescence with acridine orange, in contrast to control laterals which gave a positive reaction (not shown). The sulfide-treated emergent laterals commonly showed increased thickening and fluorescence of the walls of the hypodermis and the layer within this compared with the controls, indicating that they had reacted to the sulfide. Four weeks after the sulfide treatment, there were signs of vascular blockages in the rhizome in some plants.
Uptake of Fe2+
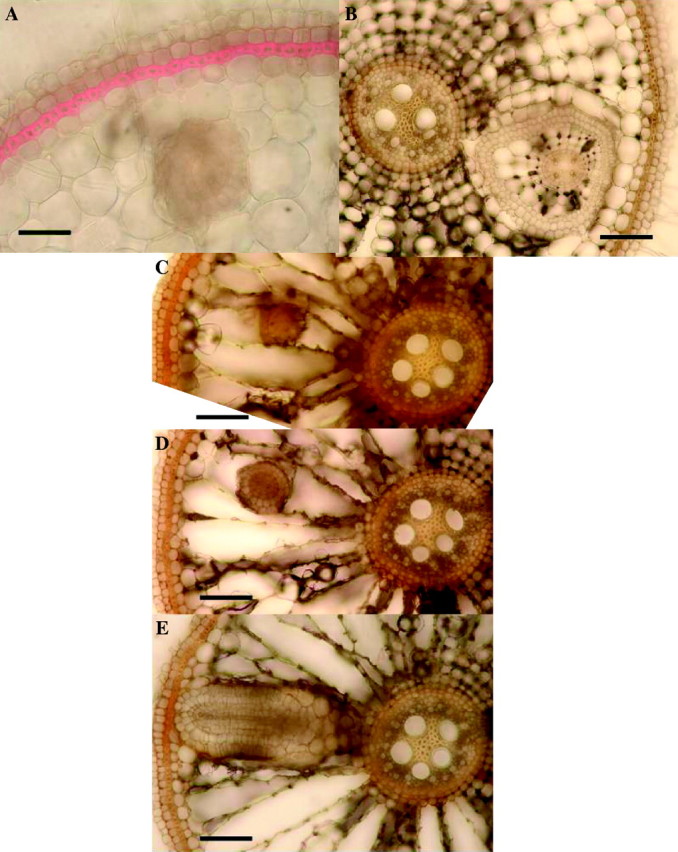
Transverse sections of roots indicated that there was greater permeability and absorption of iron in the control roots than those from the sulfide treatment. In controls, there was greater penetration of the stele throughout the root than in the sulfide treatment (cf. Fig. 8A and B). In controls, lateral roots allowed penetration of Fe2+ into the stele, but this was not so obvious in the sulfide treatment, where a barrier appeared to form around the point of lateral emergence. In the sub-apical regions, it seemed that the sulfide-treated adventitious roots formed a barrier to Fe2+ absorption in the hypodermis, between the epidermis and the exodermis; this effect was far less obvious in the controls (cf. Fig. 8C and D); it appeared that the thickened outer tangential wall of this layer could be a barrier. High power views of the outer layers of more basal regions clarified this wall thickening (Fig. 8G); it was also seen to fluoresce (Fig. 6F). However, there was also evidence that the outer tangential epidermal walls might form a barrier especially in the most apical regions (Fig. 8F) and that the thickened exodermal layer which became suberized and lignified could also form a barrier more basally.
Fig. 8.
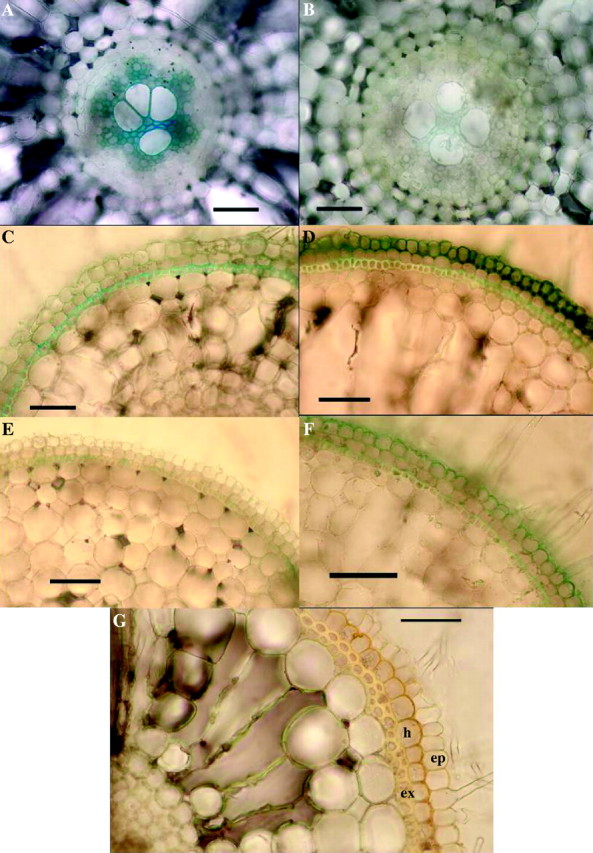
Rice: evidence for sulfide-induced barrier to Fe2+ absorption in adventitious roots. (A–F) Effects of 2 d of 0·174 mm sulfide on Fe2+ absorption, 2 weeks after lifting of treatment. Excised roots (length = 100–120 mm); iron indicated as Prussian blue precipitate of Fe4(Fe(CN)6)3. (A) Control, root base: appreciable iron present in the stele. Bar = 50 µm. (B) Sulfide treatment, root base: comparatively little iron present in the stele (cf. A). Bar = 50 µm. (C) Control, 25 mm from the apex: hypodermal layers do not appear to be great barriers to Fe2+ (cf. D). Bar = 50 µm. (D) Sulfide treatment, 25 mm from the apex: Fe2+ accumulated in the epidermis and hypodermal layer appears to be the barrier to Fe2+ (cf. C and G). Bar = 50 µm. (E) Control, 5 mm from the apex: hypodermal layers apparently formed little barrier to Fe2+ (cf. F). Bar = 50 µm. (F) Sulfide treatment, 5 mm from the apex: Fe2+ accumulated in epidermis; the hypodermal layer appears to be the barrier to Fe2+ (cf. D, E and G). Bar = 50 µm. (G) Transverse section of the base of a root stained in iodine from sulfide treatment, but which had not been in an Fe2+ absorption experiment. Note the thickening of outer tangential and radial walls of the hypodermal layer which appear to form a barrier (cf. D and F). Bar = 50 µm. Ep = epidermis; h = hypodermis; ex = exodermis.
Fe2+ penetrated the ‘windows’ opposite developing laterals in the controls; however, in the sulfide treatment where the windows were suberized and thickened, there was far less penetration.
DISCUSSION
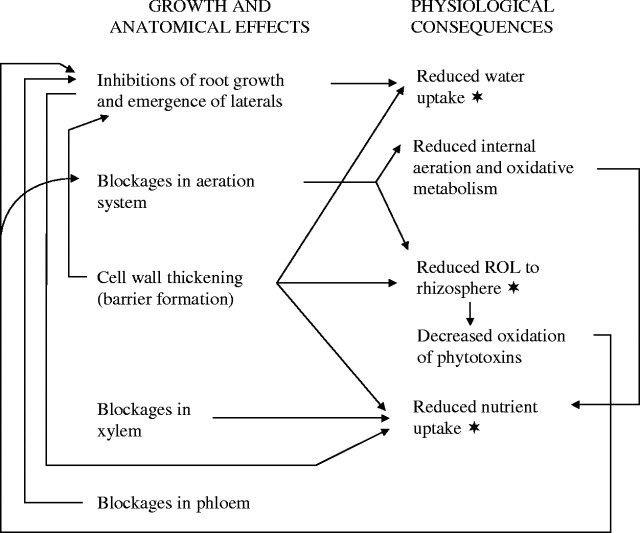
This is, to our knowledge, the first documentation of sulfide inducing barriers to root ROL, blockages in the internal aeration and vascular systems and inhibiting lateral root emergence in rice. The results correlate with previous findings on the toxic effects of sulfide on Phragmites and of organic acids on rice and Phragmites (Armstrong et al., 1996a, b; Armstrong and Armstrong, 1999, 2001). The influence of sulfide on anatomy and root growth can be correlated with a number of physiological effects (Fig. 9) some of which were observed in this study and/or previously documented as symptoms of sulfide-induced diseases of rice. The variety, Norin 36, has been cited as being comparatively susceptible to Akagare, a disease associated with sulfide toxicity. Nevertheless, despite the highly toxic nature of sulfide, the dosage in this study was sufficiently low and brief, and in a stagnant rather than stirred medium, to allow the plants to react to the toxin and to recover in terms of new adventitious and lateral root production when the treatment was lifted. Sulfide inhibited the growth of adventitious roots (Fig. 1) and laterals, and prevented lateral emergence (Figs 6F and 7); it also caused the death of non-emergent (Fig. 6C) and emergent lateral roots, and occasionally of some adventitious root apices.
Fig. 9.
Rice: scheme for inter-relationships between growth and anatomical symptoms induced by sulfide and observed and predicted physiological effects (the asterisk indicated the physiological effects observed in this study).
Adventitious and fine lateral roots of rice responded to sulfide almost immediately in terms of reduced ROL to the rhizosphere (Figs 2–4). From the anatomical evidence, it seems reasonable to associate this with suberized cell walls, thickening and perhaps with some lignification of the surface cell layers and, in the case of adventitious roots, with blockages within the apical cortical gas space system (Fig. 6A and B). Blockages in the non-aerenchymatous gas space system of the rhizome were also found. We previously have recorded callus occluding aerenchyma channels in rhizomes and root bases: in rice in response to acetic acid and in Phragmites in response to sulfide and organic acids. In the present study, however, callus was not observed; this may well have been due to the comparatively low dosage and short-term treatment periods.
Sulfide-induced barriers to root ROL will in turn decrease the thickness of oxidized rhizospheres and reduce the plant's ability to oxidize phytotoxins in the rhizosphere such as sulfide, organic acids, Fe2+ and Mn2+. This could account for the decreased ability of rice roots affected by Akiochi (another disease linked to sulfide) to oxidize iron in the rhizosphere (e.g. Takijima, 1964). Pedersen et al. (2004) found that in Zostera, sulfide intrusion did not occur when the concentration of O2 in rhizomes was >35 % of air saturation, even with sediment sulfide concentrations in excess of 1 mm. This indicated the protective effect of sulfide oxidation in the rhizosphere due to root ROL. However, in darkness, with the plant under O2 stress, sulfide intrusion occurred. Reduced thicknesses of oxidized rhizospheres could also result in decreased uptake of certain nutrients such as P, since ROL is linked to the mobilization and availability of P (Saleque and Kirk, 1995). Adventitious roots of rice can often be seen growing in parallel groups in soil, and the oxidized rhizospheres, especially of laterals, tend to overlap (see Fig. 10; included for reference). Here there must be greater protection from phytotoxins such as sulfide and Fe2+ because the zone of oxidation around the root is extended and the potential flux of phytotoxins into the root correspondingly decreased. In this study, it was noted that adventitious root tips were more prone to being killed by the sulfide when the roots were comparatively sparse and the apices isolated. As previously mentioned, sulfide is a well known inhibitor of oxidative metabolism in rice and other species, and this inhibition could be exacerbated by sulfide-induced blockages in the underground internal gas space system.
Fig. 10.

Rice roots growing in flooded soil containing reduced (colourless) methylene blue (1 g MeB in approx. 1 L of soil). Reduction of dye was due to soil microorganism activity. Note overlapping oxidized rhizospheres (blue) and adventitious roots growing in parallel groups. Bar = 9 mm; after S. H. F. W. Justin and W. Armstrong, unpublished.
Normally, there are unthickened, non-suberized ‘windows’ in the adventitious root exo/hypodermis opposite developing laterals (Fig. 6E) to allow for their eventual emergence (Justin and Armstrong, 1987; Armstrong, 1992; Votrubová and Pecháčková, 1996; Soukup et al., 2002). The exodermal thickening and suberization of these ‘windows’ (Fig. 6F) were also sulfide-induced responses, and appeared to prevent lateral emergence. Moreover, the laterals tended to grow upwards through the adventitious root cortex (Fig. 7). The latter response could have been a positive ‘aero-tropism’ towards the better aerated basal parts and/or a negative ‘chemo-tropism’ away from the sulfide which entered the apical part of the root. We previously have found these responses to be typical of Phragmites roots to sulfide and various organic acids and of rice roots to acetic acid (Armstrong et al., 1996a; Armstrong and Armstrong, 2001).
The suberization and thickenings noted in normally permeable regions of the root system, together with xylem blockages, stunting of adventitious roots and the serious reductions, up to 99 %, in lateral root surface area, would be expected to reduce the water and nutrient uptake in plants subjected to sulfide toxicity. There was evidence of both of these effects in the experiments on water and Fe2+ uptake (Figs 5 and 8, respectively). In connection with the decreased permeability to Fe2+ induced by the sulfide, it is interesting that Soukup et al. (2002) found similar patterns of iron penetration in Phragmites roots (not subjected to toxins) as in our controls, in relation to cell wall thickenings/suberin deposits and the presence of laterals.
This study has incidentally highlighted the importance of fine laterals in terms of their contribution to root surface area; in relatively young control roots, production of 4 mm long laterals more than doubled the area of the root in the lateral root zone. For the adventitious root as a whole, the total area of the laterals was more than seven times that of the O2-permeable apical region. Kirk (2003) has mathematically modelled both internal aeration and nutrient acquisition in rice and highlighted the importance of the fine laterals. He concluded that ‘a system of coarse, aerenchymatous primary roots with gas-impermeable walls, conducting O2 to short, fine gas-permeable laterals, provides the greatest absorbing surface per unit of aerated root mass’. In Kirk's model, laterals were 7 mm long (compared with approx. 4 mm in the present study) and at approx. 1·75 times the density. One may conclude that in such roots, laterals could triple the root area in the lateral zone and, for a root as a whole, the laterals could have >13 times the area of the permeable apical region. It is therefore to be expected that damage to these laterals must have serious consequences for the well-being of the plant in terms of water uptake and the acquisition of nutrients.
Rice roots appear to respond to sulfide almost immediately in terms of anatomical effects, which we associate with reduced permeability to O2. Nevertheless, it was obvious that even at comparatively low and brief doseage, some toxin entered the stele and in some cases reached the rhizome. From this and previous work, it also seems reasonable to infer that plant roots react to soil-borne toxins such as sulfide and organic acids by producing barriers in their most vulnerable, absorptive regions, namely the fine laterals and the apices of adventitious roots, and this decreases or halts further ingress of the toxin. Vascular blockages also develop, which presumeably prevent further spreading of the toxin within the plant. Similarly, with such a potentially gaseous toxin as sulfide, it is not surprising that blockages within the aeration system can develop. Pedersen et al. (2004) found that during darkness, gaseous sulfide can enter the Zostera plant and spread through the aerenchyma by gas phase diffusion. In the present study, we suspect that suberization/lipid deposits, induced by the sulfide in hypodermal and/or exoermal cell walls, and sometimes in the epidermis, may be effective barriers in reducing ROL and the uptake of Fe2+ and possibly water. However, the heavily lignified and suberized endodermis and the xylem blockages induced by sulfide probably also contributed in decreasing Fe2+ and water absorption. We have not observed lignification without some suberization in any of our studies, but it is difficult at this stage to judge whether lignification per se can contribute to the efficiency of barriers. Lignification is commonly cited as a defence reaction to fungal attack (e.g. Friend, 1981; Asada and Matsumoto, 1987). Soukup (pers. comm. 2000) has found that vascular and intercellular blockages, induced in roots of Phragmites by wounding, contain polysaccharide gums; this and our previous work also presents strong evidence of lipid/suberin and sometimes lignin-type components. Recently, De Simone et al. (2003) stated that suberization and not lignification formed the apoplastic barriers to ROL and water transport in some Amazonian trees.
Although the barriers and blockages described here may be viewed as primarily beneficial and protective against the ingress and spreading of sulfide within the plant, our study has indicated that they may have detrimental consequences in terms of the temporary curtailment of water and nutrient uptake. Blockages within the aeration and vascular systems of the rhizome could be particularly detrimental as their influence could be pervasive. Moreover, the root growth inhibition, lateral root death and barriers to water and nutrient uptake could result in the necessity for renewed growth of root systems and resulting weakening of the plant. Although rice appears to tolerate and recover from relatively low concentrations of sulfide (and acetic acid, as previously found), one could envisage how repeated or prolonged exposure and/or higher concentrations than those used here could eventually have devastating consequences. In addition to an exacerbation of the effects recorded here, one could expect the sulfide to reach the shoot system and cause inhibition of photosynthesis and premature senescence. The latter was found when Phragmites was exposed to a higher doseage of sulfide than that used in this study (Armstrong et al., 1996a).
Acknowledgments
We thank Mr Vic Swetez of the University of Hull's Botanic Gardens for cultivating the rice plants, and Dr Tim Colmer, University of Western Australia, Professor Guy Kirk of Cranfield University UK and Dr Eric Visser, University of Njimegen, The Netherlands, for helpful comments on the manuscript.
LITERATURE CITED
- Allam AI. 1971.Soluble sulphides in rice fields and their in vitro effects on rice seedlings. PhD Dissertation, Louisiana State University, USA. [Google Scholar]
- Allam AI, Hollis JP. 1972. Sulphide inhibition of oxidases in rice roots. Phytopathology 62: 634–639. [Google Scholar]
- Allam AI, Pitts G, Hollis JP. 1972. Sulphide determination in soils with an ion-selective electrode. Soil Science 114: 457–467. [Google Scholar]
- Armstrong J. 1992.Pathways and mechanisms of aeration in Phragmites australis. PhD thesis, University of Hull, UK. [Google Scholar]
- Armstrong J, Armstrong W. 1999.Phragmites die-back: toxic effects of propionic, butyric and caproic acids in relation to pH. New Phytologist 142: 201–218. [Google Scholar]
- Armstrong J, Armstrong W. 2001. Rice and Phragmites: effects of organic acids on growth, root permeability, and radial oxygen loss to the rhizosphere. American Journal of Botany 88: 1359–1370. [PubMed] [Google Scholar]
- Armstrong J, Afreen-Zobayed F, Armstrong W. 1996.Phragmites die-back: sulphide- and acetic acid-induced bud and root death, lignifications, and blockages with the aeration and vascular systems. New Phytologist 134: 601–614. [DOI] [PubMed] [Google Scholar]
- Armstrong J, Armstrong W, Van der Putten WH. 1996.Phragmites die-back: bud and root death, blockages within the aeration and vascular systems and the possible role of phytotoxins. New Phytologist 133: 399–414. [DOI] [PubMed] [Google Scholar]
- Armstrong W. 1964. Oxygen diffusion from the roots of some British bog plants. Nature 204: 801–802.14235692 [Google Scholar]
- Armstrong W. 1970. Rhizosphere oxidation in rice and other species; a mathematical model based on the oxygen flux component. Physiologia Plantarum 23: 623–630. [Google Scholar]
- Armstrong W. 1971. Radial oxygen losses from intact rice roots as affected by distance from the apex, respiration and waterlogging. Physiologia Plantarum 25: 192–197. [Google Scholar]
- Armstrong W. 1979. Aeration in higher plants. In: Woolhouse H.W.W., ed. Advances in botanical research Vol. 7. New York: Academic Press, 225–332. [Google Scholar]
- Armstrong W, Wright E. 1975. The theoretical basis for the manipulation of flux data obtained by the cylindrical platinum electrode technique. Physiologia Plantarum 35: 21–26. [Google Scholar]
- Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM. 2000. Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis Annals of Botany 86: 687–703. [Google Scholar]
- Asada Y, Matsumoto J. 1987. Induction of disease resistance in plants by a lignification-inducing factor. In: Nishimura, S., Vance, C.P. and Doke, N., eds. Molecular determinations of plant diseases. Tokyo, Japan, Science Society of Japan Press, 223–231. [Google Scholar]
- Baba I, Inada K, Takijima K. 1965. Mineral nutrition and the occurrence of physiological diseases. In: The mineral nutrition of the rice plant. Baltimore, MD, Johns Hopkins University Press, 295–326. [Google Scholar]
- Baba I, Iwata I. 1963.Akiochi disease of rice plants. In: Matsubayashi M, Ito R, Nomoto T et al, eds. Theory and practice of growing rice. Fuji Publishing Co. Ltd, Tokyo, Japan, 149–158. [Google Scholar]
- Barber DA, Ebert M, Evans NTS. 1962. The movement of 15O through barley and rice plants. Journal of Experimental Botany 13: 397–403. [Google Scholar]
- Begg CBM, Kirk GJD, MacKenzie AF, Neue HU. 1994. Root-induced iron oxidation and pH changes in the lowland rice rhizosphere. New Phytologist 128: 469–477. [DOI] [PubMed] [Google Scholar]
- Bloomfeld CJ, Pruden G. 1962. Report for Rothamsted Experimental Station for 1962: 65. [Google Scholar]
- Colmer TD, Gibberd MR, Wiengeera A, Tinh TK. 1998. The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. Journal of Experimental Botany 49: 1431–1436. [Google Scholar]
- Colmer TD. 2003. Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Annals of Botany 91: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD. 2003. Long-distance transport of gases in plants. Plant, Cell and Environment 26: 17–36. [Google Scholar]
- Connell WE, Patrick WH. 1968. Sulfate reduction in soil: effects of redox potential and pH. Science 159: 86–87. [PubMed] [Google Scholar]
- De Rufz de Lavison M. 1910. Du mode de pénétration de quelues sels dans la plante vivante. Role de l'endoderme. Revue Généralé De Botanique 22: 225–240. [Google Scholar]
- De Simone O, Haase K, Müller E, Junk WJ, Hartmann K, Schreiber L, Schmidt W. 2003. Apoplastic barriers and oxygen transport properties of hypodermal cell walls in roots from four Amazonian tree species. Plant Physiology 132: 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenchel T, Finlay BJ. 1995.Ecology and evolution in anoxic worlds. Oxford: Oxford University Press. [Google Scholar]
- Friend J. 1981. Plant phenolics, lignification and plant disease. Progress in Phytochemistry 7: 197–261. [Google Scholar]
- Fürtig K, Ruegsegger A, Brunhold C, Brändle R. 1996. Sulphide utilization and injuries in hypoxic roots and rhizomes of common reed (Phragmites australis). Folia Geobotanica et Phytotaxonomica 31: 143–151. [Google Scholar]
- Gurr E. 1965.The rational use of dyes in biology. London: Leonard Hill. [Google Scholar]
- Hollis JP, Allam AI, Pitts G, Joshi MM, Ibrahim IKA. 1975. Sulphide diseases of rice on iron-excess soils. Acta Phytopathologica Academiae Scientiarum Hungaricae 10: 329–341. [Google Scholar]
- John CD, Limpinuntana V, Greenway H. 1974. Adaptation of rice to anaerobiosis. Australian Journal of Plant Physiology 1: 513–520. [Google Scholar]
- Joshi MM, Ibrahim IKA, Hollis JP. 1975. Hydrogen sulphide: effects on the physiology of rice plants and relation to straighthead disease. Phytopathology 65: 1165–1170. [Google Scholar]
- Justin SHFW, Armstrong W. 1987. The anatomical characteristics of roots and plant response to soil flooding. New Phytologist 105: 465–495. [Google Scholar]
- Kirk GJD, Bajita JB. 1995. Root-induced iron oxidation, pH changes and zinc solubilization in the rhizosphere of lowland rice. New Phytologist 131: 129–137. [DOI] [PubMed] [Google Scholar]
- Kirk GJD. 2003. Rice root properties for internal aeration and efficient nutrient acquisition in submerged soil. New Phytologist 159: 185–194. [DOI] [PubMed] [Google Scholar]
- McDonald MP, Galwey NW, Colmer TD. 2001. Similarity and diversity in adventitious root anatomy as related to root aeration among a range of wetland and dryland grass species. Plant, Cell and Environment 25: 441–451. [Google Scholar]
- Mendelssohn IA, McKee KL. 1988.Spartina alterniflora die-back in Louisiana: time-course investigation of soil water-logging effects. Journal of Ecology 76: 509–521. [Google Scholar]
- Mendelssohn IA, McKee KL, Hester M. 1988. Re-examination of pore water sulfide concentrations and redox potentials near the aerial roots of Rhizophora mangle and Avicennia germinans American Journal of Botany 75: 1352–1359. [Google Scholar]
- Mitsui S. 1965. Dynamic aspects of nutrient uptake. In: The mineral nutrition of the rice plant: Tanaka, A., ed. Baltimore, MD: Johns Hopkins University Press, 53–62. [Google Scholar]
- Pearse AG. 1968.Histochemistry (theoretical and applied). London: J & A Churchill Ltd. [Google Scholar]
- Pedersen O, Binzer T, Borum J. 2004. Sulphide intrusion in eelgrass (Zostera marina L.). Plant, Cell and Environment 27: 595–602. [Google Scholar]
- Pezeshki SR, Pan SZ, Delaune RD, Patrick WH Jr. 1988. Sulfide-induced toxicity: inhibition of carbon assimilation in Spartina alterniflora. Photosynthetica 22: 437–442. [Google Scholar]
- Ponnamperuma FN. 1965. Dynamic aspects of flooded soils and the nutrition of the rice plant. In: Tanaka, A., ed. The mineral nutrition of the rice plant. Baltimore, MD: Johns Hopkins University Press, 295–328. [Google Scholar]
- Raven JA, Scrimgeour CM. 1997. The influence of anoxia on plants of saline habitats with special reference to the sulphur cycle. Annals of Botany 79 (Supplement A): 79–86. [Google Scholar]
- Revsbech NP, Pedersen O, Reichart W, Briones A. 1999. Microsensor analysis of oxygen and pH in the rice rhizosphere under field and laboratory conditions. Biology and Fertility of Soils 29: 379–385. [Google Scholar]
- Saleque MA, Kirk GJD. 1995. Root-induced solubilization of phosphate in the rhizosphere of lowland rice. New Phytologist 129: 325–336. [DOI] [PubMed] [Google Scholar]
- Sorrell BK, Mendelssohn IA, McKee KL, Woods RA. 2000. Ecophysiology of wetland plant roots: a modelling comparison of aeration in relation to species distribution. Annals of Botany 86: 675–686. [Google Scholar]
- Soukup A, Votrubova O, Cízková H. 2002. Development of anatomical structure of roots of Phragmites australis New Phytologist 153: 277–287. [Google Scholar]
- Starkey RL. 1966. Oxidation and reduction of sulphur compounds in soils. Soil Science 101: 297–306. [Google Scholar]
- Takijima Y. 1964. Studies on the mechanism of root damage of rice plants in the peat paddy fields. Soil Science and Plant Nutrition 11: 20–27. [Google Scholar]
- Takijima Y. 1965. Studies on the mechanism of root damage of rice plants in the paddy fields (Part 1). Root damage and growth inhibitory substances found in the peaty and peat soil. Soil Science and Plant Nutrition 10: 1–8. [Google Scholar]
- Tanaka A, Ranjit P, Mulleriyawa RP, Yasu T. 1968. Possibility of hydrogen sulphide induced iron toxicity of the rice plant. Soil science and Plant Nutrition 14: 1–6. [Google Scholar]
- Trolldenier G. 1988. Visualization of oxidizing power of rice roots and of possible participation of bacteria in iron deposition. Zeitschrift für Pflanzenernährung und Bodenkunde 151: 117–121. [Google Scholar]
- Vámos R. 1959. ‘Brusone’ disease of rice in Hungary. Plant and Soil XI: 65–7. [Google Scholar]
- Van Raalte MH. 1940. On the oxygen supply of rice roots. Annales de Jardin Botanique Buitenzorg 50: 99–113. [Google Scholar]
- Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ. 2000. Changes in growth, porosity and radial oxygen loss from roots of selected mono- and dicotyledonous wetland species with contrasting aerenchyma types. Plant, Cell and Environment 20: 1237–1245. [Google Scholar]
- Votrubová O, Pecháčková A. 1996. Effect of nitrogen over-supply on root structure of common reed. Folia Geobotanica et Phytotaxonomica 31: 119–125. [Google Scholar]
- Yoshida S, Foorno DA, Cock JH, Gomez KA. 1976.Laboratory manual for physiological studies of rice, 3rd edn. Los Banos, Philippines: International Rice Research Institute. [Google Scholar]