Abstract
• Background and Aims Anoxia-tolerant plant tissues synthesize a number of proteins during anoxia, in addition to the ‘classical anaerobic proteins’ involved in glycolysis and fermentation. The present study used a model system of rice coleoptile tips to elucidate patterns of protein synthesis in this anoxia-tolerant plant tissue.
• Methods Coleoptile tips 7–11 mm long were excised from intact seedlings exposed to anoxia, or excised from hypoxically pre-treated seedlings and then exposed to anoxia for 72 h. Total proteins or 35S-labelled proteins were extracted, separated using two-dimensional isoelectric focusing/SDS–polyacrylamide gel electrophoresis and analysed using mass spectrometry.
• Key Results The coleoptile tips excised after intact seedlings had been exposed to anoxia for 72 h had a similar proteome to tips that were first excised and then exposed to anoxia. After 72 h anoxia, Bowman–Birk trypsin inhibitors and a glycine-rich RNA-binding protein decreased in abundance, whereas a nucleoside diphosphate kinase and several proteins with unknown functions were strongly enhanced. Using [35S]methionine as label, proteins synthesized at high levels in anoxia, and also in aeration, included a nucleoside diphosphate kinase, a glycine-rich RNA-binding protein, a putative elicitor-inducible protein and a putative actin-depolymerizing factor. Proteins synthesized predominately in anoxia included a pyruvate orthophosphate dikinase (PPDK), alcohol dehydrogenase 1 and 2, fructose 1,6-bisphosphate aldolase and a protein of unknown function.
• Conclusion The induction of PPDK in anoxic rice coleoptiles might, in combination with pyruvate kinase (PK), enable operation of a ‘substrate cycle’ producing PPi from ATP. Production of PPi would (a) direct energy to crucial transport processes across the tonoplast (i.e. the H+-PPiase); (b) be required for sucrose hydrolysis via sucrose synthase; and (c) enable acceleration of glycolysis, via pyrophosphate:fructose 6-phosphate 1-phosphotransferase (PFP) acting in parallel with phosphofructokinase (PFK), thus enhancing ATP production in anoxic rice coleoptiles; ATP production would need to be increased if there was a substantial requirement for PPi.
Keywords: Anoxia, coleoptile, ethanol production, glycolysis, protein synthesis, proteomics, pyruvate orthophosphate dikinase, pyrophosphate, Oryza sativa
INTRODUCTION
Rice coleoptile tips were used as an anoxia-tolerant model system in the present investigation of proteins synthesized during acclimation to anoxia. Information on anoxia tolerance in rice coleoptiles is now substantial (Ricard et al., 1994; Gibbs and Greenway, 2003; Greenway and Gibbs, 2003), and the draft genome sequence is available (Goff et al., 2002; Yu et al., 2002), providing an ideal framework for the present experiments on the proteome. The present system consists of 7–11 mm long tips of rice coleoptiles, which are hypoxically pre-treated, excised and then healed before being exposed to anoxia in the presence of exogenous glucose (Huang et al., 2003). Tips with 50 mm exogenous glucose survive anoxia for at least 120 h (Huang, 2003).
Plants acclimate to anaerobiosis by switching from oxidative to fermentative catabolism, yet an energy crisis persists since, even in species with high rates of fermentative catabolism, ATP production is at most 37 % of that under aerated conditions (Gibbs and Greenway, 2003). As a consequence of anoxia, net protein synthesis rates decrease well below those in aerated tissues, even in the anoxia-tolerant tissues investigated so far, such as rice coleoptiles and embryos (Alpi and Beevers, 1983; Pradet et al., 1985), and shoots of Sagittaria pygmaea (Ishizawa et al., 1999). In primary roots of maize exposed to anoxia, synthesis of aerobic proteins ceased and a specific set of approx. 20 polypeptides (anaerobic polypeptides, ANPs) were synthesized (Sachs et al., 1980). Most of the ANPs so far identified are enzymes involved in sugar metabolism, glycolysis and fermentation (Sachs et al., 1996). Several ANPs identified in maize such as alcohol dehydrogenase (ADH), pyruvate decarboxylase (PDC), sucrose synthase (SS) and glyceraldehyde phosphate dehydrogenase (GAPDH) (Sachs et al., 1996) have also been demonstrated in rice (Ricard et al., 1994).
Anoxic rice embryos and coleoptiles contain many more newly synthesized polypeptides than the classical ANPs (Mocquot et al., 1981; Pradet et al., 1985 for embryos; Ricard and Pradet, 1989 for coleoptiles). In contrast, only the ‘classical ANPs’ were synthesized in roots of 2-d-old rice seedlings (Ricard and Pradet, 1989), but since these rice roots were ‘anoxically shocked’ it remains possible that the difference from the coleoptiles is less pronounced than indicated by the results. Nevertheless, a well studied anoxia-tolerant system, 5 mm root tips of hypoxically pre-treated intact maize seedlings, also showed synthesis of far fewer proteins, other than the ‘classical ANPs’ (Chang et al., 2000), than in rice embryos or coleoptiles. Whether this is a species or a plant organ difference cannot be ascertained, since maize shoots were not studied by Chang et al. (2000); these shoots are in any case much less tolerant of anoxia than maize roots (Andrews et al., 1994a).
The relatively large numbers of proteins synthesized in anoxic rice embryos and coleoptiles is also consistent with responses to anoxia in shoots of two aquatic plant species (Ishizawa et al., 1999). The larger numbers of proteins, in addition to the ‘classical ANPs’, might therefore be associated with growth in anoxia, as occurred in rice (Alpi and Beevers, 1983) and in the two aquatic species (Ishizawa et al., 1999). Complementary to the synthesis of many proteins in anoxic rice coleoptiles, there was also appreciable incorporation of UTP in RNA, yet net RNA synthesis had ceased (Aspart et al., 1983).
Information on the identity of proteins synthesized during anoxia, other than the well-known ANPs first described by Sachs et al. (1980), is still limited. The recent release of a draft sequence of the rice genome (Goff et al., 2002; Yu et al., 2002) and advances in techniques of identification of proteins using mass spectrometry (Chang et al., 2000) now make it possible to identify some of the proteins, in addition to the ‘classical ANPs’, expressed during anoxia in rice. The objectives of the present study were: (a) to compare the protein patterns in intact and excised tips (top 7–11 mm) of rice coleoptiles prior to, and during 72 h anoxia; (b) to investigate differences in patterns of de novo protein synthesis in excised tips of rice coleoptiles using 4 h [35S]methionine labelling during aeration and different periods in anoxia; and (c) to elucidate consequences of reductions in energy production on protein synthesis in these anoxic coleoptile tips, using 1 and 50 mm glucose to manipulate ethanol production and hence ATP production. Use of coleoptile tips (i.e. approximately the top half of the coleoptile) avoids sampling enclosed leaves, thus providing a uniform, anoxia-tolerant tissue. Protein synthesis in anoxic coleoptiles, not including leaf tissues, has only been reported once before (Mujer et al., 1993), using coleoptiles emerged from rice seeds germinated under anoxia. In other investigations of the proteome of rice coleoptiles, ‘coleoptiles’ were excised from 60-h-old seedlings germinated in air (Breviario et al., 1994), so these would have included leaf tissues. Moreover, the shoot tissues used in earlier studies of rice were suddenly transferred to anoxia (Ricard and Pradet, 1989; Breviario et al., 1994; Huq and Hodge, 1999), giving a risk of ‘anoxic shock’. In the present experiments, many more of the newly synthesized proteins were identified than in previous studies, enabling us to develop a new hypothesis regarding anaerobic catabolism in rice coleoptiles.
MATERIALS AND METHODS
Preparation of the seedlings
Dehulled seeds of rice (Oryza sativa L. ‘Amaroo’, which is very tolerant to anoxia as shown by Huang et al., 2003) were surface sterilized with acidic HgCl2 [0·1 % (w/v) in 0·1 % HCl] for 3 min and then washed thoroughly with de-ionized water. The composition of the culture solution (mm) used was: Ca2+ 0·5; 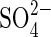 0·3; MES 0·5. The pH was adjusted to 6·5 using Ca(OH)2. Culture vessels were surface sterilized with 2 % (v/v) sodium hypochlorite overnight and washed with distilled water. Solutions were autoclaved (before adding MES, glucose and carbenicillin) and all procedures were performed in a lamina flow hood to minimize bacterial contamination. All experiments were conducted in the dark at 30 °C.
0·3; MES 0·5. The pH was adjusted to 6·5 using Ca(OH)2. Culture vessels were surface sterilized with 2 % (v/v) sodium hypochlorite overnight and washed with distilled water. Solutions were autoclaved (before adding MES, glucose and carbenicillin) and all procedures were performed in a lamina flow hood to minimize bacterial contamination. All experiments were conducted in the dark at 30 °C.
Aerated, hypoxic and anoxic treatments
Intact coleoptiles
After 48 h in aeration, a portion of the rice seedlings remained in aerated solution for another 24 h before sampling the 7–11 mm tips of coleoptiles (these tips do not include leaves). Another portion of the rice seedlings were in hypoxia (0·028 mm O2) for 16 h and then exposed to anoxia for 72 h. There was no continued aeration treatment beyond the first 72 h after germination, since the coleoptiles senesce in air; the accompanying physiological changes have best been shown by a decrease in the energy charge from 0·7 to 0·43 between 2 and 7 d after germination in air, while values in aerated conditions became lower than those in anoxia at 3 d after germination (Ishizawa et al., 1999). The tips were sampled prior to and after anoxic treatment. All tips were sampled and frozen with liquid N2 and stored at −70 °C before extraction.
Excised coleoptiles
After 48 h in aeration and 16 h of hypoxic treatment (0·028 mm O2), tips of coleoptiles (top 7–11 mm) were excised and then transferred to Thunberg tubes containing 10 ml of culture solution (plus 20 mm glucose and 10 g m−3 carbenicillin). Thunberg tubes were as described by Zhang et al. (1992). Tips were the top half of the coleoptile of each seedling; thus excluding leaf tissues which are inside the bottom half of the coleoptiles. The excised tips were incubated for 5 h in hypoxic solution (0·028 mm O2) to promote ‘healing’. This procedure is required since the coleoptiles lose K+ for the first 2 h after excision; after 5 h of healing, vigorous net K+ uptake had resumed (Colmer et al., 2001). After this hypoxic incubation, a portion of tips were frozen in liquid N2 and stored at −70 °C. Another portion of the tips were incubated in 10 mL of anoxic nutrient solution (composition given below) supplemented with 10 g m−3 carbenicillin and 50 or 1 mm glucose. The solutions were refreshed every 24 h with N2-pre-flushed nutrient solution. The O2 concentration in the gas space of the Thunberg tubes was less than the detection limit of 0·01 % as analysed by gas chromatography (KOR-75, GL Science, Tokyo, Japan). After 72 h in anoxia, the solutions were washed with 10 mL of anoxic solution three times for 3 min. The tips were then immediately frozen in liquid N2 and stored at −70 °C.
The composition of the nutrient solution was macronutrients (mm) K+ 0·25; Ca2+ 0·625; Mg2+ 0·1; 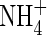 0·10;
0·10; 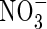 0·20;
0·20; 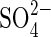 0·44; [
0·44; [ ] 0·10; and micronutrients (μm) Cl− 250;
] 0·10; and micronutrients (μm) Cl− 250; 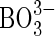 6·25; Mn2+ 0·5; Zn2+ 0·5; Cu2+ 0·125;
6·25; Mn2+ 0·5; Zn2+ 0·5; Cu2+ 0·125; 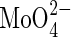 0·125; Ni2+ 0·25; Fe-EDTA 12·5. The solution also contained MES at 0·5 mm; and the pH was adjusted to 6·5 using Ca(OH)2 (this Ca2+ is included in the concentration given above).
0·125; Ni2+ 0·25; Fe-EDTA 12·5. The solution also contained MES at 0·5 mm; and the pH was adjusted to 6·5 using Ca(OH)2 (this Ca2+ is included in the concentration given above).
Ethanol production
For ethanol production, the solutions in Thunberg tubes were refreshed with anoxic solution and collected during 0–4, 20–24 and 68–72 h in anoxia. Vials containing water placed in an ice bath were connected to the outlet from each Thunberg tube, in order to trap ethanol. All samples were stored at −20 °C. The recovery of ethanol from Thunberg tubes and trap vials after 4 h of flushing with N2 gas was 98%. Ethanol in the medium and trap solution was assayed using an enzymatic method (Beutler, 1984). A cuvette (1 mL) contained 100 mm glycylglycine buffer at pH 9·0, 300 mm KCl, 1·7 mm NAD+ and 0·3 U of ADH. The reaction was started by addition of 90 U of ADH and monitored at 340 m.
Investigating de novo protein synthesis in excised coleoptile tips using [35S]methionine
Aerated and anoxic treatment and labelling with [35S]methionine
After 5 h of healing, a portion of the coleoptile tips (0·15 g) were exposed to 3 mL of anoxic nutrient solution with 50 or 1 mm glucose containing 20 µCi (0·74 MBq) of [35S]methionine (ICN Co. catalogue 51001, Costa Mesa, CA, USA) for 4 h. The initial concentration of methionine in the external solution was 35·2 µm. For aerated control, coleoptiles tips were exposed to 3 ml of aerated nutrient solution at 50 mm exogenous glucose with the same amount of [35S]methionine for 4 h of labelling. Other portions of the tips were exposed to 10 mL of nutrient solution with 50 or 1 mm glucose for 20 and 68 h. For the longer time period, the solutions were refreshed every 24 h. After 20 and 68 h of anoxic treatment, the solution in each tube was removed, coleoptile tips were rinsed with 10 mL of anoxic solution three times (3 × 3 min), and then 3 mL of anoxic nutrient solution containing 20 µCi (0·74 MBq) of [35S]methionine was added to each tube. After labelling for 4 h, the coleoptiles were washed three times (each wash was 3 min) using anoxic nutrient solution. The fresh weight of each sample was recorded and then samples were immediately frozen with liquid N2 and stored at −70 °C.
Determination of the uptake and protein incorporation of [35S]methionine by coleoptile tips
The uptake of [35S]methionine by coleoptile tips was determined by counting of the 35S in the nutrient solution. Aliquots of 50 µL of the incubation solution were added to 3 mL of Stracient scintillation fluid (Catalogue no. 6013248, Packard BioScience, Meriden, CT, USA). Frozen tips were ground and extracted as described in ‘Protein extraction for electrophoresis’. Total 35S tissue content was measured in 10 µL sub-samples of tissue homogenate added to 3 mL of scintillation fluid. Proteins were resuspended in 400 µL of sample buffer as described below. A 20 µL aliquot of resuspended solution was added to 3 mL of scintillation fluid. The amounts of 35S were determined using a liquid scintillation analyser (Model: Tri-Crab 1500, Parkard, Meriden, CT, USA).
Protein extraction for electrophoresis
Frozen tips (0·15–0·3 g) were ground using a mortar and pestle with 1 mL of extraction buffer [Tris–HCl 125 mm, SDS 7 % (w/v), mercaptoethanol 10 % (v/v), pH 7·0] and centrifuged at 10 000 g for 5 min. The supernatants were collected. To 400 µL of supernatant, 1·6 ml of methanol, 400 µL of chloroform and 1 ml of distilled water were added and vortexed. The mixtures were again centrifuged at 10 000 g for 5 min. After discarding the upper aqueous phase, 1 mL of methanol was added and samples were centrifuged at 9000 g for 10 min. After discarding the supernatant, the protein pellets were air-dried and then stored at −70 °C.
Separation of proteins by 2D IEF/SDS–PAGE
The protein pellets were re-suspended in 400 µL of sample buffer [6 m urea, 2 m thiourea, 2 % (v/v) CHAPS, 2·0 % (v/v) immobilized pH gradient (IPG) buffer (pH 3–10 non-linear, Amersham Pharmacia Biotech, Uppsala, Sweden), 2·0 % (w/v) n-decyl-N,N-dimethyl-3-ammonio-1-propanesulfonate]. After determining the protein concentrations in the sample buffer using the Bradford method (Bradford, 1976), 300–350 µL of buffer solution (containing 300 or 600 µg of protein) was loaded onto a dry IPG strip (180 mm, non-linear pH 3–10 gradient, Amersham Pharmacia Biotech, Uppsala, Sweden) hydrated overnight and separated according to the manufacturer's instructions for a total of 35 kVh. IPG dry strips were equilibrated for 20 min in 4 mL of 0·05 m Tris–HCl (pH 6·8), 6 m urea, 4 % (w/v) SDS, 20 % (v/v) glycerol, 10% (v/v) mercapoethanol. Second dimension SDS–polyacrylamide gels (180 × 200 × 1·0 mm) comprised a 12 % (w/v) polyacrylamide gel. Equilibrated IPG strips were placed on top of the gel and overlaid with 0·5 % (w/v) agarose in running buffer. The gels were run for 5 h at a constant current of 25 mA in SDS–PAGE running buffer. Gels were then stained in colloidal Coomassie [17 % (w/v) ammonium sulfate, 34 % (v/v) methanol, 3 % (v/v) phosphoric acid and 0·1 % (w/v) Coomassie brilliant blue G-250]. After overnight staining, the gels were washed with 0·5 % (v/v) phosphoric acid and then scanned using a flatbed scanner. The [35S]methionine-labelled gels were exposed to Fujifilm imaging plates (Model: Bas-IP MS 2040, Fuji Photo Film Co., Kanagawa, Japan) for 3 d. The imaging plates were then scanned using an image plate reader (Model: FujiFilm Bas-2500, Fuji Photo Film Co. Kanagawa, Japan). The density of selected protein spots was analysed by ImageMaster 2D Elite software (Version 3·01, Amersham Pharmacia Biotech, Uppsala, Sweden).
Identification of proteins using mass spectrometry
Spots for identification were cut from 2D isoelectrofocusing (IEF)/SDS–polyacrylamide gels, and stored in a sealed microtitre plate at −20 °C. The proteins in the microtitre plate were de-stained with 50 % acetonitrile in 25 mm NH4HCO3, twice for 45 min. Then the gel slices were dried at 50 °C for 20 min. For sequencing analysis, proteins were digested overnight with 12·5 µg mL−1 trypsin in 25 mm NH4HCO3 at 37 °C. An equal volume of 100 % methanol plus 0·2 % (v/v) formic acid was added to each gel slice. After 15 min incubation, the supernatant containing peptides was used for mass spectrometric analysis. Samples of 10 µL were loaded directly into an ElectroSpray inlet of a QStar Pulsar MS/MS system (Applied Biosystems, Foster City, CA, USA) utilizing an inline Agilent 1100 capillary LC system at a flow rate of 2 µL min−1. The eluted peptide mixture was analysed by tandem mass spectrometric sequencing with an automated MS-to-MS/MS switching protocol. The collision-induced dissociation (CID) for peptide sequencing by MS/MS was performed with N2 gas. Mass spectra were analysed against the TIGR rice protein set with BioAnalyst and ProID (Applied Biosystems, Foster City, CA, USA) and against NCBI rice entries at Mascot (www.matrixscience.com).
RESULTS
Ethanol production
Rates of ethanol production by excised rice coleoptile tips were measured to assess the rates of energy production. This is feasible since in rice coleoptiles energy produced during anoxia lasting more than approx. 2 h is nearly entirely via glycolysis linked to ethanol production, as shown by Menegus and colleagues. First, the ethanol/CO2 ratio was 0·95 (Menegus et al., 1991). Secondly, 1H nuclear magnetic resonance (NMR) spectroscopy showed there were no substantial amounts of end-products of anaerobic catabolism other than ethanol (Menegus et al., 1988). This pattern is consistent with that for many other species at least after the first 3–4 h of anoxia (ap Rees et al., 1987; Ricard et al., 1994). In the present study, rates of ethanol production were measured for three 4 h periods (0–4, 20–24 and 68–72 h) during which incorporation of [35S]methionine was evaluated. At 50 mm exogenous glucose, ethanol production during the first 4 h was as high as 9·4 µmol g−1 f. wt h−1, decreased by only 19 % between 20 and 24 h of anoxia and was then maintained at a stable level even after 68 h in anoxia (Table 1). At 1 mm exogenous glucose, however, rates of ethanol production by excised coleoptiles decreased with time in anoxia and, after 68 h in anoxia, had decreased to only 34 % of the rate during the initial 4 h (Table 1).
Table 1.
Rates of ethanol production in excised tips (7–11 mm) of rice coleoptiles at 50 or 1 mm glucose during the periods when [35S]methionine incorporation was measured in anoxia for 72 h
| Time in anoxia (h) |
Glucose (mm) |
Rates of ethanol production (μmol g−1 f. wt. h−1) |
|---|---|---|
| 0–4 | 1 | 7·3 ± 1·4 |
| 50 | 9·4 ± 1·6 | |
| 20–24 | 1 | 4·6 ± 1·2 |
| 50 | 7·6 ± 0·3 | |
| 68–72 | 1 | 2·5 ± 0·2 |
| 50 | 7·9 ± 0·6 |
Seedlings were germinated and grown for 2 d in aerated solution (0·25 mm O2), then pre-treated with 0·028 mm O2 for 16 h prior to excision of the tips. Excised tips were ‘healed’ for 5 h in hypoxia (0·028 mm O2) prior to treatments. Data given are means of three replicates ± s.e.m.
Change of protein patterns during prolonged anoxia
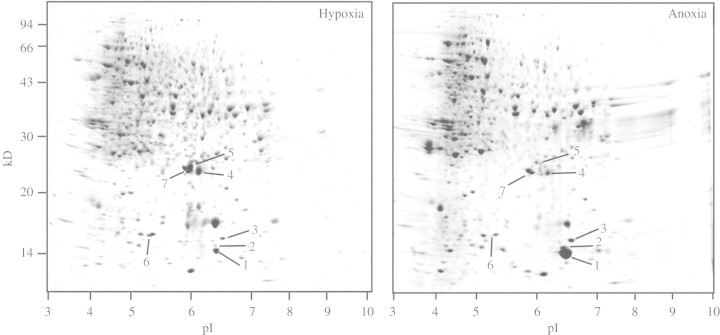
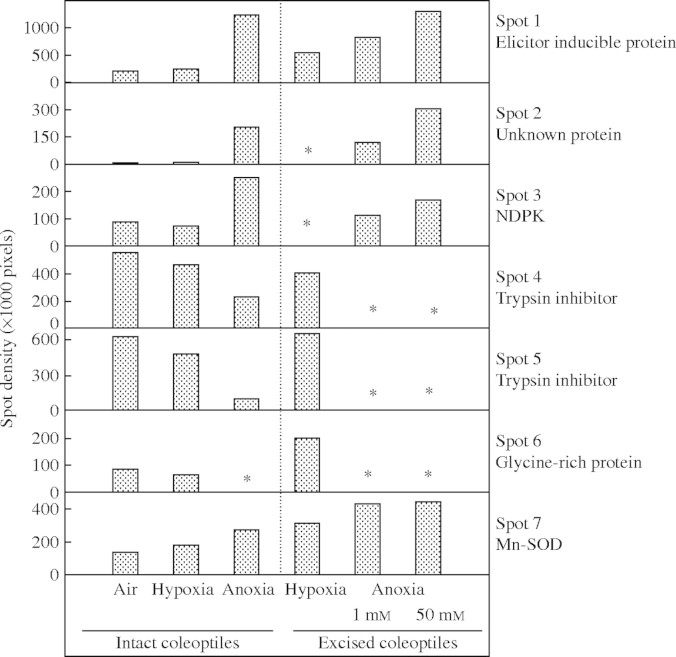
Overall, the patterns of proteins extracted from tips of coleoptiles excised from aerated and 16 h hypoxically pre-treated seedlings were similar (supplementary Fig. 1A, B; all supplementary figures can be seen at Annals of Botany online, http://aob.oxfordjournals.org). After 72 h anoxia, in intact rice seedlings the patterns of protein abundance in 7–11 mm tips of coleoptiles had changed, notably through changes in the abundances of several proteins with molecular masses in the 14–25 kDA range (Fig. 1). After 72 h anoxia, spots 1, 2 and 3 were enhanced by 6-, 20- and 3-fold respectively, while spots 4 and 5 were reduced by 2- and 5-fold, and spot 6 had disappeared (Figs 1 and 2). Similar changes in patterns of protein abundance were observed when excised 7–11 mm coleoptile tips were exposed to anoxia, although there were some quantitative differences to the changes in tips of coleoptiles from intact seedlings (Fig. 2 and supplementary Fig. 1). In excised coleoptile tips, there were only slight differences in patterns of protein abundance between those at 1 or 50 mm exogenous glucose, even after 72 h in anoxia (Fig. 2 and supplementary Fig. 1).
Fig. 1.
Patterns of proteins in 7–11 mm tips of coleoptile dissected from intact rice seedlings before and after 72 h in anoxia. Data are Coomassie blue G-250-stained coleoptile proteins (600 µg per gel) separated by two-dimensional IEF/SDS–PAGE. Seedlings were germinated and grown for 48 h in aerated solution (0·25 mm O2), then pre-treated with hypoxia (0·028 mm O2) for 16 h prior to 72 h anoxia. Coleoptile tips contained no leaf tissues. A gel showing the pattern of proteins for aerated coleoptiles can be seen in supplementary Fig. 1. It is similar to that shown above for coleoptiles treated hypoxically for 16 h.
Fig. 2.
Changes of spot density in gels for protein separation of samples from rice coleoptile tips, after 72 h anoxia. Protein was extracted from coleoptile tips (top 7–11 mm), either dissected after intact seedlings had been exposed to anoxia, or from tips excised and healed before anoxia was imposed, and then supplied with either 1 or 50 mm exogenous glucose. Data are derived from supplementary Fig. 1. An asterisk indicates that the spot could not be analysed due to low abundance.
For the 72 h treatment, no aerated comparisons were taken, in view of senescence of coleoptiles when in air (cf. Materials and methods). However, it is reassuring that patterns of protein levels in excised coleoptiles were very similar to those in coleoptiles of intact seedlings. Furthermore, in the anoxic coleoptiles with 50 mm glucose, the pattern of proteins synthesized at 68–72 h remained similar to that during the first 4 h of anoxia, and did not show up additional proteins. Rigorous proof that the 72 h results are not confounded with development, associated with time, might be possible for the excised tips, which, in contrast to coleoptiles of intact seedlings, show net protein synthesis during 72 h of aerated treatment (Huang et al., 2003), but, in contrast to coleoptile tips in anoxia, those in aerated solution turned a brownish colour by 72 h after excision. Hence, it perhaps could be more rewarding to elucidate changes in proteins synthesized after returning the 72 h anoxia-exposed tips to air.
Spots changing in abundance were excised, digested with trypsin in-gel and identified by tandem mass spectrometry. Spots 4 and 5, which were reduced in abundance during anoxia, were identified as Bowman–Birk trypsin inhibitors (Table 2). These results are consistent with the decrease in the measured activities of trypsin inhibitors in coleoptiles after 3-d-old rice seedlings were submerged for 2 d in stagnant, 8 cm deep distilled water (Lee and Lin, 1995). The plant trypsin inhibitors might regulate the proteolytic activity of proteases (Tashiro and Maki, 1978), so that decreased levels of these trypsin inhibitors in anoxic coleoptile tips may therefore contribute to the degradation of specific proteins not required during anoxia. This would be an energy-efficient way to synthesize new proteins by recycling amino acids rather than employing energy-consuming processes such as ion transport, assimilation of nitrogen and synthesis of new amino acids. Interestingly, these trypsin inhibitors can also have other functions as monodehydroascorbate reductases and dehydroascorbate reductases in etiolated mung bean seedlings (Hou et al., 2000) and roots of sweet potato (Hou and Lin, 1997), and thus might help in protection of cells against free radicals, by regeneration of ascorbate. It is then plausible that such proteins were degraded in rice coleoptiles exposed to prolonged anoxia due to the lack of reactive oxygen species (ROS); however, the mechanism of such a selective degradation is unknown. If these proteins do contribute to the efficacy of the oxidative protection system, the degradation during anoxia could have implications upon re-entry of O2; although, as shown below, another components of the oxidative protection system, namely superoxide dismutase (SOD), was increased between 1·4- and 2·1-fold during anoxia (Table 2).
Table 2.
Identification of two-dimensional separated protein spots from rice coleoptile tips (top 7–11 mm) that differed in abundance between anoxic and aerated treatment
| Spot no. |
Gene indices |
Description |
MP |
Cov |
Score |
Match (MM/pI) |
Gel (MM/pI) mol. wt/PI |
Fold change (72 h anoxia/aerated) |
|---|---|---|---|---|---|---|---|---|
| 1 | 20279443 | Elicitor-inducible protein | 9 | 38% | 266 | 17 509/8·42 | 14 kDA/6·5 | 3, 2·4, 5·2, 2·4 |
| 2 | 13129480 | Unknown protein | 16 | 37% | 248 | 17 552/7·66 | 15 kDA/6·5 | 4·6, 3·8, >10, >10 |
| 3 | 585551 | Nucleoside diphosphate kinase (NDPK) | 21 | 57% | 474 | 16 851/6·30 | 16 kDA/6·6 | 1·3, 1·8, 3·4, >10 |
| 4 | 7619799 or 14090367 | Bowman–Birk trypsin inhibitor | 5 | 10% | 108 | 27 766/5·36 | 23 kDA/6·2 | 0·3, 0·5, <0·1 |
| 5 | 14090367 or 7619799 | Bowman–Birk trypsin inhibitor | 6 | 23% | 82 | 27 716/5·36 | 24 kDA/6·0 | 0·2, 0·4, <0·1 |
| 6 | 2331131 or 2293480 | Glycine-rich protein | 11 | 19% | 148 | 15 862/7·79 | 16kDA/5·4 | 0·1, 0·26, <0·1, <0·1 |
| 7 | 7433348 | Mn-superoxide dismutase (Mn-SOD) | 14 | 72% | 557 | 24 982/6·50 | 24 kDA/6·0 | 2·1, 1·5, 1·4 |
| 8 | 3550549 | Pyruvate orthophosphate dikinase (PPDK) | 4 | 5% | 87 | 96 504/5·42 | 96 kDA/5·2 | >10, 3·2, 3·8, 5·5 |
| 9 | 6979319 | Alcohol dehydrogenase 2 (ADH2) | 3 | 13% | 137 | 41 180/6·04 | 42 kDA/6·3 | – |
| 10 | 113360 | Alcohol dehydrogenase 1 (ADH1) | 5 | 14% | 140 | 40 826/6·32 | 42 kDA/6·5 | – |
| 11 | 7436606 | Fructose 1,6-bisphosphate aldolase | 4 | 16% | 82 | 38 799/8·35 | 33 kDA/6·4 | – |
| 12 | 2293480 or 1221131 | Glycine-rich protein | 4 | 24% | 121 | 16 534/8·97 | 16 kDA/5·4 | 0·3, 0·8, 0·9, 0·55 |
| 13 | 29124123 | Actin-depolymerizing factor | 14 | 65% | 242 | 15 936/5·72 | 16 kDA/5·2 | 1·2, 0·8, 1·5, 0·8 |
Tips were either dissected from intact seedlings which had been exposed to anoxia, or excised and healed before anoxia was imposed with 50 mm glucose. Numbered spots were derived from gels in Figs 1 and 3. MS/MS spectra derived from trypsinated peptides of proteins were matched at Mascot against a translated NCBI database (P < 0·05). MP = number of peptides matched; MM = molecular mass from gel or predicted from matched gene product sequence; pI = isoelectric point from gel or predicted from matched gene product sequence; Cov = percentage coverage of the matched sequence with the matched peptides; Score = Mascot match score, protein score >50 (P < 0·05). Fold change is the ratio of spot intensity following 72 h anoxia treatment in 50 mm glucose with the spot intensity in aerated treatment (n = 3 or 4 from Figs 1 and 3 and other independent experiments (gels not shown). Spots 9–11 could only be detected as [35S]methionine-labelled proteins from Fig. 3.
Spot 6, that decreased in abundance or even disappeared during anoxia, was identified as a specific glycine-rich protein (gi 2331131 or gi 2293480). Sequence similarity searches of the amino acid sequences of those two proteins against the NCBI protein database showed that both contain an RNA recognition motif domain, a feature of a variety of RNA-binding proteins. Degradation of specific glycine-rich proteins may be a factor in the regulation of protein synthesis if these play roles in selective binding to, or translation of, mRNA. Fennoy et al. (1998) reported that mRNAs that encode certain anaerobic proteins are efficiently loaded onto ribosomes in O2-deprived maize roots, while those encoding aerobic proteins are poorly loaded.
Spot 7 increased approx. 1·5- to 2-fold in abundance after 72 h in anoxia (Figs 1 and 2), and was identified as Mn-dependent SOD (gi 7433348) (Table 2). High SOD activity might be an essential component of a system to protect against oxidative damage, during the first couple of hours after re-aeration. Increased activity of SOD during anoxia has been documented in the anoxia-tolerant rhizomes of Iris pseudacorus (Monk et al., 1987). Another protein, pyruvate orthophosphate dikinase (PPDK) (spot 8), was not initially detected by Coomassie blue staining, but was located by [35S]methionine incorporation (see next section), and when Coomassie-stained gels were re-analysed, PPDK was found to have increased 3- to 10-fold during 72 h anoxia.
Several proteins with low molecular masses (14–25 kDA) were enhanced, or newly synthesized, during 72 h anoxia, including: spot 1, a putative elicitor-inducible protein (gi 20279443); spot 2, a protein of unknown function (gi 13129480); and spot 3, a nucleoside diphosphate kinase (NDPK; gi 585551) (Table 2). Similarly, anoxia caused strong induction of a group of 13–16 kDA proteins in coleoptiles of rice seedlings raised in air for 60 h, and then excised, healed and exposed to anoxia for 2 h (Breviario et al., 1994), but these were not identified. In addition, anoxia imposed on 14-d-old rice plants caused upregulation of a mRNA predicted to encode an approx.14 kDA protein; the mRNA abundance peaked at 3 h anoxia and then remained at higher levels than in aerated tissues for 72 h anoxia, but the putative protein was not identified (Huq and Hodge, 1999). In contrast, no such proteins with low molecular masses were induced in ‘anoxically shocked’, primary roots of maize (Sachs et al., 1996) or ‘hypoxically pre-treated’, maize root tips (Chang et al., 2000).
Uptake of [35S]methionine and its incorporation into protein
[35S]Methionine was supplied to coleoptile tips to investigate which proteins were synthesized during three time periods (each lasting 4 h) during 72 h anoxia. Only 15–17% of the supplied radioactivity remained in the external solution at the end of each 4 h period. Incorporation of absorbed 35S into soluble proteins in anoxic coleoptile tips was between 53 and 65% of that in aerated tips (data not shown).
Differences in patterns of [35S]methionine-labelled proteins between coleoptile tips in aeration and in anoxia
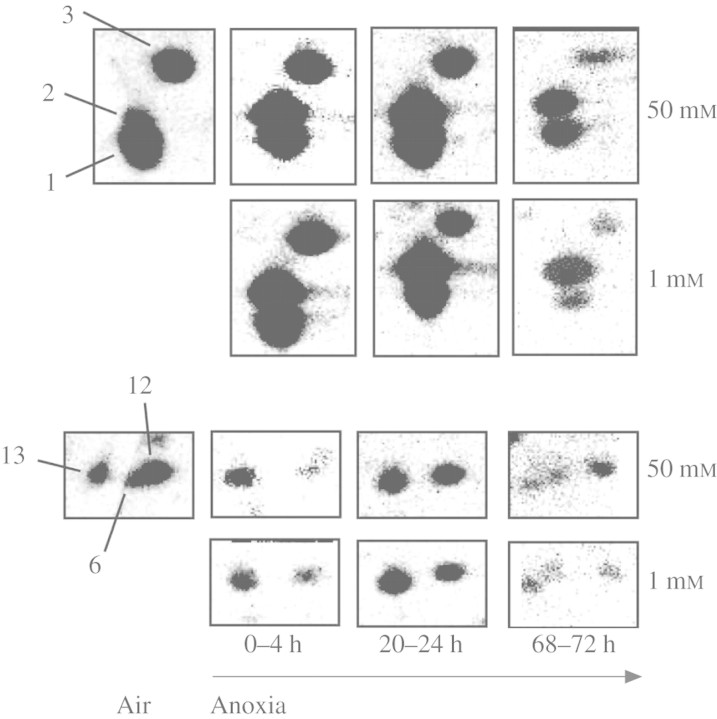
The patterns of de novo protein synthesis in excised coleoptile tips differed between aeration and anoxia. The synthesis of proteins with molecular mass from 28 to 66 kDA and pI from 4 to 7 dominated in aerated coleoptile tips (Fig. 3). The synthesis of proteins with molecular mass from 14 to 22 kDA and pI from 4 to 8, and from 33 to 43 kDA and pI from 7 to 8, was strongly enhanced in anoxia (Fig. 3). Furthermore, several proteins with molecular mass of >94 kD and pI 5–6 were also induced in anoxia (Fig. 3 and supplementary Fig. 2). Proteins in coleoptile tips that were expressed differently in anoxia and aeration, which are noted as spots 1–3, 6 and 8–13 on Fig. 3, were cut from the gels for identification (Fig. 3).
Fig. 3.
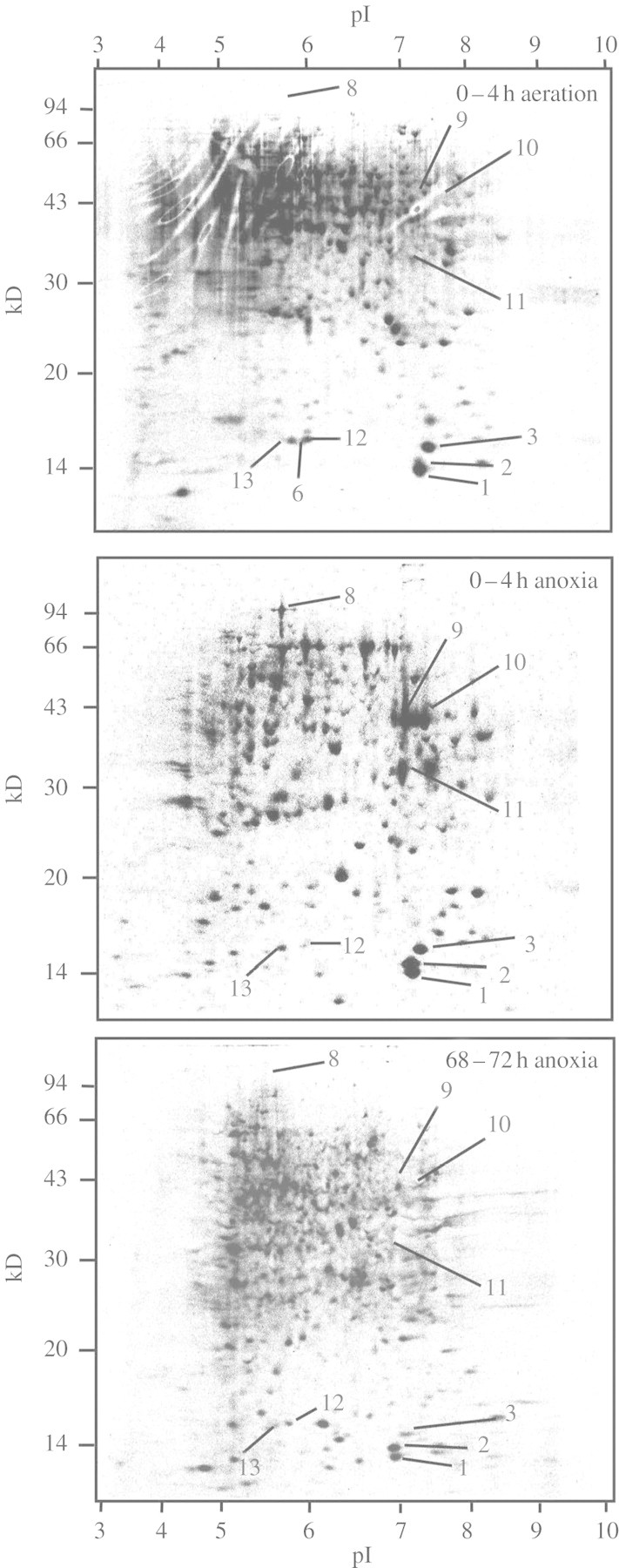
Patterns of de novo protein synthesis labelled with [35S]methonine in excised tips of rice coleoptiles in aeration or anoxia. Data are proteins (300 µg per gel) separated by two-dimensional IEF/SDS–PAGE and exposed for 3 d to a phospho-imaging plate. Seedlings were grown as described in Fig. 1. Coleoptile tips (top 7–11 mm) were excised and ‘healed’ for 5 h in hypoxia (0·028 mm O2) prior to exposure to 0–4 h in aeration or anoxia, and 68–72 h in anoxia; in all cases with 50 mm exogenous glucose.
A glycine-rich protein (gi 2331131 or gi 2293480, spot 6) was synthesized in aeration but not in anoxia (Figs 3 and 4). This is consistent with the disappearance of this spot under anoxia noted in Figs 1 and 2. The putative elicitor-inducible protein (gi 20279443, spot 1) and the NDPK (gi 585551, spot 3) identified in Fig. 1 as increasing during anoxia were labelled during both aeration and anoxia when exogenous [35S]methionine was supplied (Figs 3 and 4). Similarly, the newly identified glycine-rich protein (gi 2293480 or gi 2331131, spot 12) and a putative actin-depolymerizing factor (gi 29124123, spot 13) were also synthesized in both anoxia and aeration. Actin-depolymerizing factor is present in a family of essential eukaryotic actin regulatory proteins; these proteins enhance the turnover rate of actin and interact with actin monomers as well as actin filaments (Maciver, 1998). It is not clear what function this protein may play in anoxic rice coleoptiles. Its synthesis could be related to continuation of coleoptile cell expansion in anoxia, since actin-depolymerizing factor promotes the dis-aggregation of actin filaments that regulate cell shape (Maciver, 1998).
Fig. 4.
Changes in abundance of de novo synthesis of selected proteins (spots 1, 2, 3 and spots 6, 12, 13 from Fig. 3) in excised tips of rice coleoptiles in aeration or anoxia. These regions of the gels are shown for samples extracted from coleoptile tips (top 7–11 mm) after 4 h labelling periods at three time points after anoxia was imposed, with 1 or 50 mm exogenous glucose.
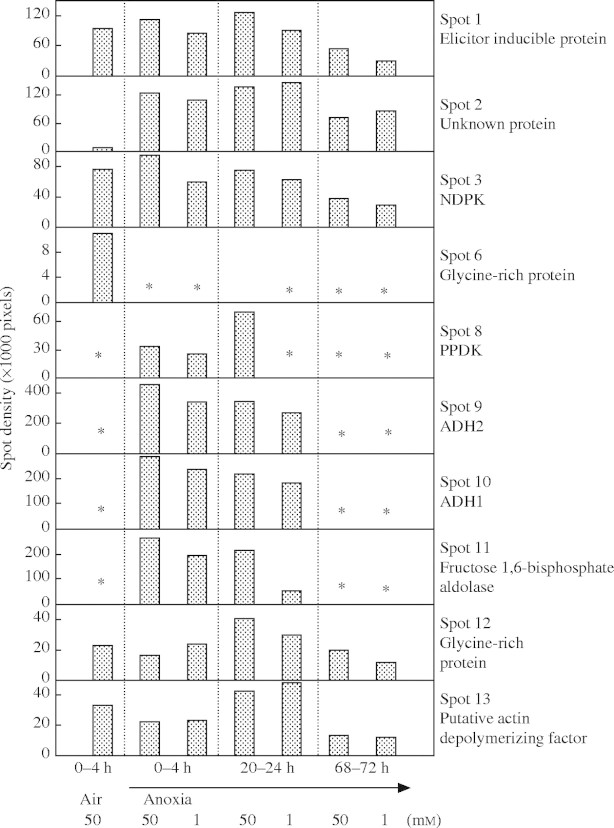
The [35S]methionine labelling showed several proteins to be predominantly synthesized in anoxia. This set included a protein of unknown function (gi 13129480, spot 2 in Fig. 5), that also increased markedly in abundance during anoxia (Figs 1 and 2), PPDK (gi 3550549, spot 8), ADH2 (gi 6979319, spot 9), ADH1 (gi 113360, spot 10) and fructose-bisphosphate aldolase (gi 7436606, spot 11) (Fig. 3 and supplementary Fig. 2). For most of these proteins, synthesis during anoxia was substantial during 0–4 and 20–24 h, but was much less by 68–72 h (Fig. 5). Presumably, synthesis of those proteins during the early period of anoxia resulted in levels sufficient for further growth and/or cell maintenance in prolonged anoxia. Other [35S]methionine-labelled proteins predominantly expressed in anoxia (Fig. 3) could not be identified because of low abundance in the gels or difficulties in distinguishing the spots between aerated and anoxic samples.
Fig. 5.
Changes of spot density of de novo protein synthesis labelled with [35S]methionine in excised tips of rice coleoptiles in aeration or anoxia. Samples were extracted from coleoptile tips (top 7–11 mm) after 4 h labelling periods at three times after anoxia was imposed, with 1 or 50 mm exogenous glucose. Data are derived from supplementary Fig 2. An asterisk indicates that a spot could not be analysed due to low abundance. Phospho-intensity measurements were made in the linear range from the Bas-2500 imaging plates.
Subsequent re-analysis of Coomassie-stained gels such as those shown in Fig. 1, and of independent experiments with aerated and anoxic treatments with 50 mm glucose (gels not shown), showed that PPDK (spot 8) increased 3- to 10-fold during 72 h of anoxia (Table 2), so the high de novo synthesis detected by [35S]methionine labelling underscored a substantial increase in abundance of this protein in anoxic coleoptile tips. With 1 mm glucose, the low [35S]methionine incorporation into PPDK (Fig. 5) was matched by little increase in Coomassie-stained PPDK spots during 72 h of anoxia (data not shown).
Exogenous glucose-dependent changes of [35S]methionine-labelled protein patterns with time in anoxia
There was no difference in overall pattern of protein synthesis between coleoptile tips with 50 or 1 mm exogenous glucose during the first 4 h in anoxia (supplementary Fig. 2), so there was no immediate effect on protein synthesis of external glucose (including the possible side effect of the increase in external osmotic pressure of 0·12 MPa). After 20 h in anoxia, the overall pattern of protein synthesis for coleoptile tips at 50 mm exogenous glucose was similar to that between 0 and 4 h in anoxia (supplementary Fig. 2). For the coleoptile tips at 1 mm exogenous glucose, there was no synthesis of PPDK (spot 8) between 20 and 24 h of anoxia (Fig. 5), while synthesis of fructose-bisphosphate adolase (spot 11) was decreased 3·7-fold (Fig. 5). Between 68 and 72 h of anoxia, synthesis of the elicitor-inducible protein (spot 1), NDPK (spot 3), the glycine-rich protein (spot 12) and a putative actin-polymerizing factor (spot 13) was reduced by 1·3- to 2·5-fold in tips with 1 mm exogenous glucose, compared with tips with 50 mm exogenous glucose (Figs 4 and 5).
The synthesis of the specific protein with unknown function (spot 2) was least affected by time in anoxia and glucose supply (Figs 4 and 5). It was synthesized at 60% of the initial rate throughout 72 h in anoxia even in coleoptile tips with 1 mm exogenous glucose (Fig. 5). Interestingly, the unknown protein (spot 2) shares 89% amino acid sequence identity with the putative elicitor-inducible protein (spot 1) (Table 2). Given the lack of known functions for these proteins, further investigations, such as genetic manipulation, are required to elucidate whether these proteins play a role in tolerance of rice coleoptiles to anoxia.
DISCUSSION
Rice coleoptile tips synthesized many proteins during anoxia. Substantial protein synthesis in coleoptile tips over 72 h anoxia was indicated by the large suite of proteins labelled during the 4 h periods of [35S]methionine supply. Such data are consistent with other results for rice embryos and coleoptiles (Mocquot et al., 1981; Ricard and Pradet, 1989), and for shoots of the anoxia-tolerant S. pygmaea (Ishizawa et al., 1999). Some further support for substantial protein synthesis comes from the percentage incorporation of the absorbed [35S]methionine in all these investigations, which was usually >50 % of the percentage incorporation in aerated conditions. However, reasonable estimates of the rates of protein synthesis cannot be made from our experiments since nearly all the supplied [35S]methionine was absorbed. In an earlier study in which substantial [35S]methionine remained available in the medium, rates of incorporation in anoxic rice coleoptiles were estimated at 50 % of those in aerated conditions (Breviario et al., 1994). Furthermore, in anoxic rice coleoptiles, endogenous methionine was 50 % higher than in air (Fan et al., 1997). Such high endogenous levels would tend to depress the percentage incorporation of [35S]methionine under anoxia; particularly since, in our experiment, the supplied methionine would only have provided 10–15 % of the endogenous content. So, a strong case exists for substantial de novo protein synthesis in anoxic rice coleoptiles.
This more complex pattern of protein synthesis during anoxia in rice coleoptiles (present study) is very different from the response to anoxia in maize roots: (a) most enzymes synthesized in ‘anoxically shocked’ maize roots are involved in sugar metabolism, glycolysis and fermentation (Sachs et al., 1996); and (b) several proteins additional to the set identified by Sachs et al. (1996) were synthesized during anoxia in intact 5 mm root tips of maize given a hypoxic pre-treatment prior to anoxia; however, the number of these proteins labelled diminished between 0–4 and 9–13 h of anoxia (Chang et al., 2000). In contrast, in the rice coleoptile tips (present study), proteins in addition to the ‘classical ANPs’ were still synthesized after 20–24 and 68–72 h anoxia. The basis for these differences in proteins synthesized by rice coleoptiles and maize root tips during anoxia is not clear. There is no substantial difference in cytosolic pH in rice coleoptiles and hypoxically pre-treated maize root tips (Greenway and Gibbs, 2003). Furthermore, adenylate energy charge (AEC) was also similar in these two tissues after 24 h anoxia; AEC was 0·78–0·85 in hypoxically pre-treated maize root tips (Saglio et al., 1988) and 0·75–0·8 in rice (embryos in Mocquot et al., 1981; Pradet et al., 1985; coleoptiles in Ricard and Pradet, 1989).
As part of our study, we manipulated ethanol production rates (Table 1), and hence ATP supply, in the coleoptile tips by feeding low (1 mm) or high (50 mm) exogenous glucose. Our objective was to determine whether a hierarchy exists amongst the range of proteins synthesized in anoxia as the energy crisis becomes more severe. After 68–72 h anoxia, ethanol production by tips with 1 mm exogenous glucose was only 32 % of the rate in those with 50 mm glucose (Table 1). So, it was surprising that a large range of proteins were still synthesized in the coleoptile tips at 1 mm exogenous glucose. Notable, however, in tips with only 1 mm glucose, was the apparent cessation of synthesis of PPDK and the reduced synthesis of fructose 1,6-bisphosphate aldolase (at 20–24 h), and modest reductions also in 35S labelling of several proteins by 68–72 h anoxia (Figs 4 and 5). These lower rates of synthesis of specific proteins in tips with 1 mm glucose, compared with those with 50 mm, might be symptomatic for the development of injury which occurred when anoxia at 1 mm glucose was continued, as indicated by losses of K+ to the medium, which commenced between 96 and 120 h of anoxia (Huang et al., 2005).
Several of the proteins enhanced, or newly synthesized, during anoxia reflect alterations of the glycolytic pathway, for example ADH (spots 9 and 10) and fructose 1,6-bisphosphate aldolase (spot 11). The expression of ADH1 and ADH2 in anoxic rice coleoptiles in the present study (Figs 3 and 5) was consistent with previous reports of two ADH polypeptides being induced in whole coleoptiles and roots after anoxia was imposed on 2-d-old intact rice seedlings for 24 h (Ricard and Pradet, 1989). The activity of fructose 1,6-bisphosphate aldolase in coleoptiles increased 2-fold when 2-d-old intact rice seedlings were exposed to anoxia (Rivoal et al., 1989). Expression of fructose 1,6-bisphosphate aldolase was also found in anoxic shoots of four anoxia-tolerant Echinochloa species, but not in two intolerant species (Mujer et al., 1993). In both ‘anoxically shocked’ and hypoxic pre-treated maize seedlings, ald1 mRNA encoding fructose 1,6-bisphosphate aldolase was induced in roots and shoots when exposed to anoxia (Andrews et al., 1994b), but the activity of fructose 1,6-bisphosphate aldolase did not increase in anoxic primary roots of maize (Kelley and Freeling, 1994). The cessation of synthesis of ADH and fructose 1,6-bisphosphate aldolase in coleoptile tips by 68 h in anoxia (present study) is consistent with the transient nature of increases in mRNA of ADH and fructose 1,6-bisphosphate aldolase in leaves and rhizomes of the marsh species, Acorus calamus; after peaking in the first 3 d, these transcripts had dropped to very low levels by 56 d anoxia (Bucher and Kuhlemeier, 1993).
NDPK (spot 3) was expressed both in aeration and in anoxia (Fig. 4), but accumulated 2- to 3-fold after 72 h in anoxia compared with the initial level (Figs 1 and 2). Maximum catalytic activities of NDPK in shoots of rice seedlings were similar at 4 d, but 30 % higher after 8 d in anoxia, compared with levels in aerated seedlings (Guglielminetti et al., 1995). NDPK is a ubiquitous enzyme, which catalyses the transfer of the terminal phosphate group of nucleoside triphosphates (NTPs) to NDPs (Parks and Agarwal, 1973). The reactions catalysed by NDPK provide NTPs for nucleic acid synthesis, CTP for lipid synthesis, UTP for polysaccharide synthesis and GTP for protein elongation, signal transduction and microtubule polymerization. UTP is essential for cell wall synthesis (Stitt, 1998). Consequently, when NDPK activity was lowered in rice by an antisense mRNA approach, this resulted in dwarfism after 60 d in air, and reduced the length of submerged coleoptiles (Pan et al., 2000). Alternatively, NDPK may be required to synthesize ATP from UTP produced when sucrose is hydrolysed via SS and UDP-glucose pyrophosphorylase (Guglielminetti et al., 1995). The activity of SS increases during acclimation to anoxia in rice seedlings (Ricard et al., 1991; Guglielminetti et al., 1995) and in intact coleoptiles is probably receiving sucrose via the phloem. In contrast, in excised coleoptile tips fed glucose, this role would presumably not exist, since sucrose increased in excised coleoptiles fed glucose during anoxia (Colmer et al., 2001). The stimulation of NDPK during anoxia might also play a role in signal transduction in rice coleoptiles by producing GTP for the activation of GTP-binding proteins, as suggested in studies of the slime mould Dictyostelium (Bominaar et al., 1993).
The remainder of our Discussion focuses on one of the newly identified proteins synthesized during anoxia in the coleoptile tips of rice, PPDK (spot 8). The 35S labelling experiment showed substantial de novo synthesis of PPDK during anoxia (Fig. 4) and its total abundance also increased during anoxia (Table 2). These findings confirm and expand the observations that PPDK was induced in roots, sheaths and shoots of rice seedlings during submergence and anoxia (Moons et al., 1998). That PPDK is involved in the anoxic response was also indicated by a decrease of 65% in maximum catalytic activity after the tissues were returned after 72 h anoxia to air for 12 h (Moons et al., 1998).
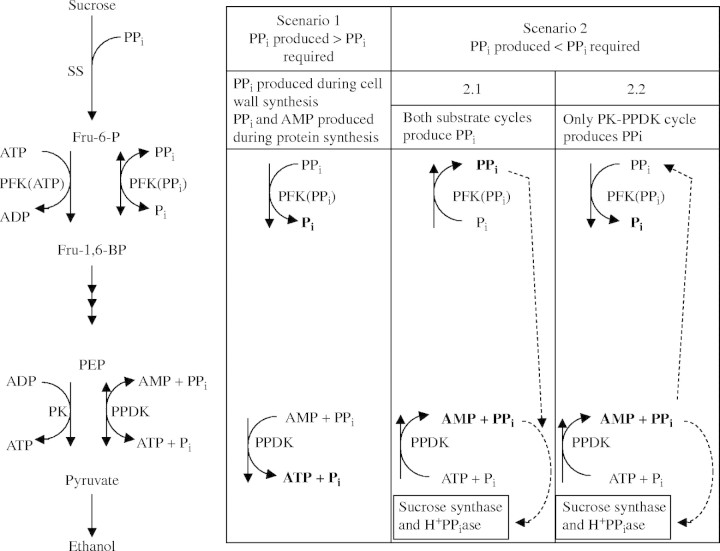
PPDK catalyses the reversible reaction: pyruvate + ATP + Pi ↔ PEP + AMP + PPi + 2H+ (Fig. 6). Thus, PPDK can convert PPi to ATP, or vice versa. PPi is required for SS and the H+-PPiase at the tonoplast (reviewed by Greenway and Gibbs, 2003). The alternative source of PPi would be via the reaction catalysed by PFP (pyrophosphate:fructose 6-phosphate 1-phosphotransferase) (Fig. 6); its maximum catalytic activity was increased by 3-fold in whole 3-d-old rice seedlings exposed to anoxia (Mertens et al., 1990) and by 6- to 7-fold in cultured anoxic rice cells (Mohanty et al., 1993).
Fig. 6.
Possible role of pyruvate orthophosphate dikinase (PPDK) in rice coleoptiles in adaptation to anoxia. PPi could be produced or consumed by PPDK or pyrophosphate:fructose 6-phosphate 1-phosphotransferase (PFP). Two scenarios are possible; either PPi produced during polymer synthesis exceeded PPi required, or vice versa. Possible roles of PPDK and PFP during these two scenarios are shown. Scenario 1. PPi produced > PPi required. PPi produced during cell wall synthesis and protein synthesis allows a large glycolytic flux and economizes on energy spent during cell wall synthesis and amino acid activation. Scenario 2. PPi produced < PPi required, then the first possibility, scenario 2·1, is that both PPDK and PFP synthesize PPi for (a) sucrose conversion starting with sucrose synthase (SS) via UDP glucose pyrophosphorylase and for (b) tonoplast energization (via H+-PPiase). However, phosphofructokinase (PFK) would then have to catalyse the combination of the glycolytic flux and the flux of the substrate cycle flowing from PFK via F-1,6-bisP back to PFK, and in rice coleoptiles this may be well be above the capacity of PFK (see text and Noguchi, 2002). Hence we favor scenario 2·2: PPi would be synthesized by PPDK in a substrate cycle with pyruvate kinase (PK), while some of the PPi produced is used by PFP, allowing a large glycolytic flux to produce additional ATP for PPi synthesis in the PPDK substrate cycle.
The functions of PPDK and PFP are now discussed further in detail, considering two possible scenarios for PPi production and consumption (Fig. 6).
Scenario 1: production of PPi in polymer synthesis exceeds PPi requirements under anoxia (PPi produced > PPi required). This scenario was proposed for anoxic bacteria, which would produce substantial PPi during protein synthesis and lack pyruvate kinase (Plaxton, 1996). This scenario is not discussed further since we consider it is unlikely to apply to anoxic rice coleoptiles.
Scenario 2: PPi requirements exceed PPi production during polymer synthesis under anoxia (PPi produced < PPi required). In rice coleoptiles, there are strong indications that there will be a need for more PPi than that which is produced during polymer synthesis. First, PPi would be required for the tonoplast H+-PPiase, which increased during anoxia to 12 times the level of the vacuolar H+-ATPase (Carystinos et al., 1995). A switch from the V-ATPase to the H+-PPiase during an energy crisis might be crucial to direct energy flow to the tonoplast, thus contributing to mitigation of cytosolic acidosis (Greenway and Gibbs, 2003). The detailed arguments to support this notion are given by Greenway and Gibbs (2003). A key piece of evidence is the failure of the inhibitor of the V-ATPase, bafilomycin A1, to increase the pHvac in anoxic maize cells, though this increase occurred in aerobic cells (Brauer et al., 1997). Secondly, PPi is required for UPD-glucose pyrophophorylase participating in sucrose conversion to hexoses, starting with SS (Huber and Akazawa, 1986), which would improve efficiency of energy production (Gibbs and Greenway, 2003). This function of PPi would be expressed in the coleoptiles of intact seedlings, which will receive sucrose from the caryopsis. The hypothesis that more PPi is required than produced during polymer synthesis in anoxic plant cells needs further elucidation; here we address the question of how the required PPi would be produced.
As stated earlier, PPi could theoretically be produced by both PFP and PPDK (scenario 2·1 in Fig. 6). PFP would act in concert with PFK in the ‘PFK–PFP substrate cycle’ (Fig. 6; Stitt, 1998). However, PFK almost certainly has a high control coefficient, i.e. a rather low maximum catalytic activity in vivo. First, contrary to several other glycolytic enzymes, the maximum catalytic activity of PFK did not increase or even decreased in anoxic maize root tips (Bailey-Serres et al., 1988; Bouny and Saglio, 1996). Secondly, in rice coleoptiles, the assessed in vivo activity of PFK was close to the observed rate of ethanol production (Gibbs et al., 2000), or even lower (Noguchi, 2002). So, in anoxic rice coleoptiles, the substrate cycle PFK–PFP is unlikely to convert ATP to PPi. Instead, we hypothesize that the combined fluxes through PFK and PFP will allow a fast glycolytic flux and hence ATP synthesis, providing among others the ATP required for PPi synthesis in a PK–PPDK substrate cycle (scenario 2·2 in Fig. 6).
The model presented in Fig. 6 needs further testing. Levels of PPi in cultured rice cells increased under anoxia, as did levels of ATP (Mohanty et al., 1993). Additionally, PPi was even maintained in anoxia-intolerant pea roots (Dancer and ap Rees, 1989). Such observations merely show there must be substantial production of PPi in anoxic cells, since, as stated earlier, there would be considerable consumption of PPi, at least in anoxic rice cells due to the activity of SS and the tonoplast H+-PPiase. The key metabolic evidence required is establishment of the directions of the reactions catalysed by PPDK and PFP. These directions might depend on the metabolic state of the cell, and such experimentation is not trivial (see Stitt, 1998 for PFP).
Summing up, a substantial production of PPi by the substrate cycle PK–PPDK would allow PFP to operate in the direction of glycolysis, this could bypass the rate-limiting step in glycolysis catalysed by PFK (scenario 2·2 in Fig. 6), thus enhancing rates of glycolysis and hence ATP formation, while resulting in a net conversion of ATP to PPi.
CONCLUSIONS
The pattern of protein synthesis in anoxic rice coleoptile tips (present study) is very different from that in ‘anoxically shocked’ primary roots of maize in which most enzymes induced are involved in glycolysis and fermentation (Sachs et al., 1996). After hypoxic acclimation, 5 mm root tips of intact maize seedlings synthesized some proteins additional to the set identified by Sachs et al. (1996) but only for the first 4 h of anoxia, and not later (Chang et al., 2000). The polypeptides, in addition to those involved in glycolysis, identified in the present study might contribute to the extreme anoxia tolerance of rice coleoptiles, and to their growth during anoxia. The notable induction of PPDK observed here, coupled to the well characterized increase in PFP (Mertens et al., 1990; Mohanty et al., 1993), provides a proposed new mechanism in anoxic rice coleoptiles to provide PPi; PPDK in concert with PK in a substrate cycle would produce PPi, while the consequent increased demand for ATP would be met by a combination of PFK and PFP enhancing the rate of glycolysis (scenario 2·2 in Fig. 6). Further experiments are required to evaluate this hypothesis.
SUPPLEMENTARY INFORMATION
Supplementary Fig. 1 shows the patterns of proteins in intact and excised rice coleoptile tips before and after 72 h in anoxia. Supplementary Fig. 2 shows changes in pattern of de novo protein synthesis labelled with [35S]methonine in excised rice coleoptile tips in aeration or anoxia. Both figures can be seen at Annals of Botany online (http://aob.oxfordjournals.org).
Acknowledgments
Dr Joshua Heazlewood is thanked for expertise and advice in MS/MS identifications, and Professor Pierdomenico Perata for his helpful feedback on a draft of this manuscript. S.H. is grateful to the University of Western Australia for a University Postgraduate Award and International Postgraduate Fee Waiver Scholarship. A.H.M. is an Australian Research Council QEII Research Fellow and is grateful for grants from the Australian Research Council Discovery Programme that helped fund this research.
LITERATURE CITED
- Alpi A, Beevers H. 1983. Effects of O2 concentration on rice seedlings. Plant Physiology 71: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DL, Drew MC, Johnson JR, Cobb BG. 1994. The response of maize seedlings of different ages to hypoxia and anoxic stress. Changes in induction of ADH1 mRNA, ADH activity and survival of anoxia. Plant Physiology 105: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DL, MacAlpine DM, Johnson JR, Kelley PM, Cobb BG, Drew MC. 1994. Differential induction of mRNAs for the glycolytic and ethanolic fermentative pathways by hypoxia and anoxia in maize seedlings. Plant Physiology 106: 1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspart L, Got A, Delseny M, Mocquot B, Pradet A. 1983. Adaptation of ribonucleic acid metabolism to anoxia in rice embryos. Plant Physiology 72: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Kloeckener-Gruissem B, Freeling M. 1988. Genetic and molecular approaches to the study of the anaerobic response and tissue specific gene expression in maize. Plant, Cell and Environment 11: 351–357. [Google Scholar]
- Beutler HO. 1984. Ethanol. In: Bergmeier HU, ed. Methods of enzymatic analysis Vol VI. Metabolites I: carbohydrates. Weinheim, Basel: Verlag Chemie, 598–606. [Google Scholar]
- Bominaar AA, Molijn AC, Pestel M, Veron M, Van Haastert PJM. 1993. Activation of G-proteins by receptor-stimulated nucleoside diphosphate kinase in Dictyostelium EMBO Journal 12: 2275–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouny JM, Saglio PH. 1996. Glycolytic flux and hexokinase activities in anoxic maize root tips acclimated by hypoxic pretreatment. Plant Physiology 111: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Brauer D, Uknalis J, Triana R, Shachar-Hill Y, Tu SI. 1997. Effects of Bafilomycin A1 and metabolic inhibitors on the maintenance of vacuolar acidity in maize root hair cells. Plant Physiology 113: 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breviario D, Giani S, Morello L, Coraggio I. 1994. Anaerobiosis-mediated early transcriptional and translational responses in rice (Oryza sativa L.) coleoptiles and roots. Plant, Cell and Environment 17: 925–934. [Google Scholar]
- Bucher M, Kuhlemeier C. 1993. Long-term anoxia tolerance: multi-level regulation of gene expression in the amphibious plant Acorus calamus L. Plant Physiology 103: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carystinos GD, MacDonald HR, Monroy AF, Dhindsa RS, Poole RJ. 1995. Vacuolar H+-translocating pyrophosphatase is induced by anoxia or chilling in seedlings of rice. Plant Physiology 108: 641–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WWP, Huang L, Shen M, Webster C, Burlingame AL, Roberts JKM. 2000. Patterns of protein synthesis and tolerance of anoxia in root tips of maize seedlings acclimated to a low-oxygen environment, and identification of proteins by mass spectrometry. Plant Physiology 122: 295–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD, Huang S, Greenway H. 2001. Evidence for down-regulation of ethanolic fermentation and K+ effluxs in the coleoptiles of rice seedlings during prolonged anoxia. Journal of Experimental Botany 52: 1507–1517. [DOI] [PubMed] [Google Scholar]
- Dancer JE, ap Rees T. 1989. Effects of 2,4-dinitrophenol and anoxia on the inorganic-pyrophosphate content of the spadix of Arum maculatum and the root apices of Pisum sativum Planta 178: 421–424. [DOI] [PubMed] [Google Scholar]
- Fan TWM, Higashi RM, Frenkiel TA, Lane AM. 1997. Anaerobic nitrate and ammonium metabolism in flood-tolerant rice coleoptiles. Journal of Experimental Botany 48: 1655–1666. [Google Scholar]
- Fennoy SL, Nong T, Bailey-Serres J. 1998. Transcriptional and post-transcriptional processes regulate gene expression in oxygen-deprived roots of maize. Plant Journal 15: 727–735. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Greenway H. 2003. Mechanism of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology 30: 1–47. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Morrell S, Valdez A, Setter TL, Greenway H. 2000. Regulation of alcoholic fermentation in coleoptiles of two rice cultivars differing in tolerance to anoxia. Journal of Experimental Botany 51: 785–796. [PubMed] [Google Scholar]
- Goff SA, Ricke D, Lan T, Presting G, Wang R, Dunn M, et al. 2002. A draft sequence of the rice genome (Oryza sativa L. spp. Japonica). Science 296: 92–100. [DOI] [PubMed] [Google Scholar]
- Greenway H, Gibbs J. 2003. Mechanism of anoxia tolerance in plants. II. Maintenance requirements for energy and energy consuming processes. Functional Plant Biology 30: 999–1036. [DOI] [PubMed] [Google Scholar]
- Guglielminetti L, Perata P, Alpi A. 1995. Effect of anoxia on carbohydrate metabolism in rice seedlings. Plant Physiology 108: 735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W-C, Lin Y-H. 1997. Dehydroascorbate reductase and monodehydroascorbate reductase activities of trypsin inhibitors, the major sweet potato (Ipomoea batatas [L] Lam) root storage protein. Plant Science 128: 151–158. [Google Scholar]
- Hou W-C, Wang Y-T, Lin Y-H, Hsiao L-J, Chen T-E, Wang C-W, Dai H. 2000. A complex containing both trypsin inhibitor and dehydroascorbate reductase activities isolated from mitochondria of etiolated mung bean (Vigna radiata L. (Wilczek) cv. Tainan No. 5) seedlings. Journal of Experimental Botany 51: 713–719. [PubMed] [Google Scholar]
- Huang S, Ishizawa K, Greenway H, Colmer TD. 2005. Manipulation of ethanol production in anoxic rice coleoptiles by exogenous glucose determines rate of ion fluxes and provides estimates of energy requirements for cell maintenance during anoxia. Journal of Experimental Botany (in press). [DOI] [PubMed] [Google Scholar]
- Huang S, Greenway H, Colmer TD. 2003. Anoxia tolerance in rice seedlings: exogenous glucose improves growth of an anoxia-‘intolerant’, but not of a -‘tolerant’, genotype. Journal of Experimental Botany 54: 2363–2373. [DOI] [PubMed] [Google Scholar]
- Huber SC, Akazawa T. 1986. A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiology 81: 1008–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq E, Hodge TK. 1999. An anaerobically inducible early (aie) gene family from rice. Plant Molecular Biology 40: 591–601. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Murakami S, Kawakami Y, Kuramochi H. 1999. Growth and energy status of arrowhead tubers, pondweed turions and rice seedlings under anoxic conditions. Plant, Cell and Environment 22: 505–514. [Google Scholar]
- Kelley PM, Freeling M. 1994. Anaerobic expression of maize fructose-1,6-diphosphate aldolase. Journal of Biological Chemistry 259: 14180–14183. [PubMed] [Google Scholar]
- Lee TM, Lin YH. 1995. Trypsin inhibitor and trypsin-like protease activity in air- or submergence-grown rice (Oryza sativa L.) coleoptiles. Plant Science 106: 43–45. [Google Scholar]
- Maciver SK. 1998. How ADF/cofilin depolymerizes actin filaments. Current Opinion on Cell Biology 10: 140–144. [DOI] [PubMed] [Google Scholar]
- Menegus F, Cattaruzza L, Chersi A, Selva A, Fronza G. 1988. Production and organ distribution of succinate in rice seedlings during anoxia. Physiologia Plantarum 74: 444–449. [Google Scholar]
- Menegus F, Cattaruzza L, Mattana M, Beffagna N, Ragg E. 1991. Response to anoxia in rice and wheat seedlings. Changes in the pH of intracellular compartments, glucose-6-phosphate level and metabolic rate. Plant Physiology 95: 760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens E, Larondelle Y, Hers HG. 1990. Induction of pyrophosphate:fructose 6-phosphate 1-phosphotransferase by anoxia in rice seedlings. Plant Physiology 93: 584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocquot B, Prat C, Mouches C, Pradet A. 1981. Effect of anoxia on energy charge and protein synthesis in rice embryo. Plant Physiology 68: 636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty B, Wilson PM, ap Rees T. 1993. Effects of anoxia on growth and carbohydrate metabolism in suspension cultures of soybean and rice. Phytochemistry 34: 75–82. [Google Scholar]
- Monk LS, Fagerstedt KV, Crawford RMM. 1987. Superoxide dismutase as an anaerobic polypeptide. A key factor in recovery from oxygen deprivation in Iris pseudacorus Plant Physiology 85: 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons A, Valcke R, Montagu MV. 1998. Low oxygen stress and water deficit induce cytosolic pyruvate orthophosphate dikinase (PPDK) expression in roots of rice, a C3 plant. Plant Journal 15: 89–98. [DOI] [PubMed] [Google Scholar]
- Mujer CV, Rumpho ME, Lin JJ, Kennedy RA. 1993. Constitutive and inducible aerobic and anaerobic stress proteins in the Echinochloa complex and rice. Plant Physiology 101: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi HK. 2002. The catalytic direction of pyrophosphate:fructose 6-phosphate 1-phosphotransferase in rice coleoptiles in anoxia. Physiologia Plantarum 116: 345–350. [Google Scholar]
- Pan L, Kawai M, Yano M, Uchimiya H. 2000. Nucleoside diphosphate kinase required for coleoptile elongation in rice. Plant Physiology 122: 447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks RE Jr, Agarwal RP. 1973. Nucleoside diphosphokinases group transfer part A. In: Boyer PD, ed. The enzymes, Vol. VIII. New York: Academic Press, 307–333. [Google Scholar]
- Plaxton WC. 1996. The organization and regulation of plant glycolysis. Annual Review of Plant Physiology and Plant Molecular Biology 47: 185–214. [DOI] [PubMed] [Google Scholar]
- Pradet A, Mocquot B, Raymond P, Morisset C, Aspart L, Delseny M. 1985. Energy metabolism and synthesis of nucleic acids and proteins under anoxic stress. In: Key JL, Kosuge T, eds. Cellular and molecular biology of plant stress. New York: Alan R. Liss, 227–245. [Google Scholar]
- ap Rees T, Jenkin LET, Smith AM, Wilson PM. 1987. The metabolism of flood tolerant plants. In: Crawford RMM, ed. Plant life in aquatic and amphibious habitats. Oxford: Blackwell Scientific, 227–238. [Google Scholar]
- Ricard B, Pradet A. 1989. Anaerobic protein synthesis in different organs of germinating rice seeds. Plant Physiology and Biochemistry 27: 761–768. [Google Scholar]
- Ricard B, Rivoal J, Spiteri A, Pradet A. 1991. Anaerobic stress induced the transcription and translation of sucrose synthase in rice. Plant Physiology 95: 669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard B, Couee I, Raymond P, Saglio PH, Saint-Ges V, Pradet A. 1994. Plant metabolism under hypoxia and anoxia. Plant Physiology and Biochemistry 32: 1–10. [Google Scholar]
- Rivoal J, Ricard B, Pradet A. 1989. Glycolytic and fermentative enzyme induction during anaerobiosis in rice seedlings. Plant Physiology and Biochemistry 27: 43–52. [Google Scholar]
- Sachs MM, Freeling M, Okimoto R. 1980. The anaerobic proteins of maize. Cell 20: 761–767. [DOI] [PubMed] [Google Scholar]
- Sachs MM, Subbaiah CC, Saab IN. 1996. Anaerobic gene expression and flooding tolerance in maize. Journal of Experimental Botany 47: 1–15. [Google Scholar]
- Saglio PH, Drew MC, Pradet A. 1988. Metabolic acclimation to anoxia induced by low (2–4 kPa partial pressure) oxygen pretreatment (hypoxia) in root tips of Zea mays Plant Physiology 86: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. 1998. Pyrophosphate as an energy donor in the cytosol of plant cell: an enigmatic alternative to ATP. Botanic Acta 111: 167–175. [Google Scholar]
- Tashiro M, Maki Z. 1978. Partial purification and some properties of a trypsin inhibitor from rice bran. Agricultural and Biological Chemistry 42: 1119–1124. [Google Scholar]
- Yu J, Hu S, Wang J, Wong G, Li S, Liu B, et al. 2002. A draft sequence of the rice genome (Oryza sativa L. ssp. Indica). Science 296: 79–92. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lauchli A, Greenway H. 1992. Effect of anoxia on solute loss from beetroot storage tissue. Journal of Experimental Botany 43: 897–905. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.