Abstract
• Background and Aims The aim of this study was to investigate the importance of pyrophosphate (PPi) for plant metabolism and survival under low oxygen stress. Responses of roots of wild-type potato plants were compared with roots of transgenic plants containing decreased amounts of PPi as a result of the constitutive expression of Escherichia coli pyrophosphatase in the cytosol.
• Methods For the experiments, roots of young wild-type and transgenic potato plants growing in nutrient solution were flushed for 4 d with nitrogen, and subsequently metabolite contents as well as enzyme activities of the glycolytic pathway were determined.
• Key Results and Conclusions In roots of transgenic plants containing 40 % less PPi, UDPglucose accumulated while the concentrations of hexose-6-phosphate, other glycolytic intermediates and ATP were decreased, leading to a growth retardation in aerated conditions. Apart from metabolic alterations, the activity of sucrose synthase was increased to a lower extent in the transgenic line than in wild type during hypoxia. These data suggest that sucrose cleavage was inhibited due to PPi deficiency already under aerated conditions, which has severe consequences for plant vitality under low oxygen. This is indicated by a reduction in the glycolytic activity, lower ATP levels and an impaired ability to resume growth after 4 d of hypoxia. Interestingly, the phosphorylation of fructose-6-phosphate via PPi-dependent phosphofructokinase was not altered in roots of transgenic plants. Nevertheless, our data provide some evidence for the importance of PPi to maintain plant growth and metabolism under oxygen deprivation.
Keywords: Fermentation, glycolysis, hypoxia, pyrophosphate, Solanum tuberosum, sucrose synthase
INTRODUCTION
Plants are aerobic organisms that rely on oxygen for development and metabolism. However, plants occasionally experience low oxygen concentration due to environmental stress such as soil flooding or low oxygen diffusion in dense or bulky tissues. Although some species have evolved physiological and morphological adaptations enabling them to cope with prolonged oxygen deprivation occurring in their natural habitats, most plants cannot survive longer periods of low oxygen, which causes tissue injury and yield loss (Perata and Alpi, 1993; Crawford and Braendle, 1996; Drew, 1997; Schlueter and Crawford, 2001).
Oxygen deprivation, either partial (hypoxia) or complete (anoxia), restricts ATP production through oxidative phosphorylation and, consequently, inhibits energy-dependent processes. Plants respond with the induction of fermentative enzymes to allow production of ATP and regeneration of NAD. Nevertheless, the efficiency of ATP formation by the fermentative pathway is much less than during oxidative phosphorylation. Recent studies indicate that plants respond differentially to hypoxia and anoxia. While glycolysis was found to be accelerated under anoxia leading to rapid carbohydrate starvation (Drew, 1997; Vartapetian and Jackson, 1997; Mustroph and Albrecht, 2003; Albrecht et al., 2004), respiration, glycolysis and Krebs cycle might be downregulated during hypoxia (Albrecht and Wiedenroth, 1994; Biemelt et al., 1999; Geigenberger et al., 2000; Albrecht et al., 2004).
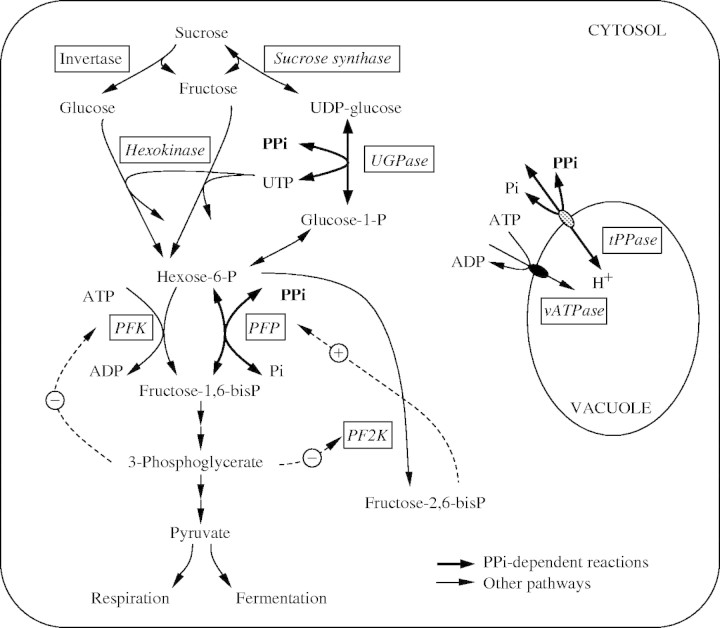
Complementary to the downregulation of ATP-consuming processes, plants may utilize pyrophosphate (PPi) as an alternative energy donor during ATP deficiency (Stitt, 1998). PPi is produced by a wide range of biosynthetic reactions and is usually present in high amounts in the cytosol (Weiner et al., 1987). The theoretical energy release by the cleavage of PPi was estimated to be about one-half of that of ATP hydrolysis (Weiner et al., 1987). In contrast to the strong decline in the amount of ATP, PPi content was found to be relatively stable under hypoxic conditions (Dancer and ap Rees, 1989; Mohanty et al., 1993; Geigenberger et al., 2000). Three metabolic processes can be catalysed in parallel by ATP- and PPi-utilizing enzymes, and PPi-driven reactions may substitute those requiring ATP to support metabolic and cellular function under low oxygen (Stitt, 1998), as summarized in the scheme (Fig. 1).
Fig. 1.
PPi-dependent processes in plant cells (indicated as bold arrows). PFK, ATP-dependent phosphofructokinase; PFP, PPi-dependent phosphofructokinase; tPPase, tonoplastic pyrophosphatase; PF2K, phosphofructose-2-kinase; UGPase, UDPGlc pyrophosphorylase; vATPase, vacuolar ATP-dependent H+ translocase.
First, plants possess two pathways for sucrose breakdown, which differ in their energy demand. Sucrose cleavage by invertase and subsequent metabolism via hexokinases requires two molecules of ATP, whereas sucrose mobilization via sucrose synthase (SuSy) and UDPglucose-pyrophosphorylase (UGPase) consumes only 1 mol PPi mol−1 sucrose. Interestingly, under oxygen deficiency, SuSy activity was found to be increased in several plant species, whereas invertase activity declined (Guglielminetti et al., 1995; Perata et al., 1996, 1997; Biemelt et al., 1999; Mustroph and Albrecht, 2003). SuSy belongs to a group of anaerobically induced proteins (Chourey et al., 1991; Ricard et al., 1991; Sachs et al., 1996). Using double mutants of SuSy, Ricard et al. (1998) pointed to a crucial role for SuSy in maintaining glycolysis in oxygen-deprived maize roots. However, antisense inhibition of SuSy gene expression did not alter glycolysis in potato plants under oxygen deficiency but strongly impaired the ability to recover (Biemelt et al., 1999).
Secondly, beside the ATP-dependent phosphorylation of fructose-6-phosphate (Fru6P) by phosphofructokinase (PFK), the reaction can also be catalysed by the PPi-dependent pyrophosphate-fructose-6-phosphate-phosphotransferase (PFP) to form fructose-1,6-bisphosphate (Fru1, 6BP; Fig. 1). The latter reaction is reversible, and the enzyme is strongly activated by fructose-2,6-bisphosphate (Fru2,6BP) (Stitt, 1990; Plaxton, 1996). PFP activity was shown to be induced in anoxic rice seedlings (Mertens et al., 1990), rice coleoptiles (Gibbs et al., 2000; Kato-Noguchi, 2002) and rice suspension cells (Mohanty et al., 1993). In addition, increased PFP activity was also measured during Pi deficiency (Theodorou et al., 1992, 1996). The increase in PFP activity was paralleled by an accumulation of Fru2,6BP during Pi deficiency (Theodoru and Plaxton, 1996) and low oxygen stress (Mertens et al., 1990; Kato-Noguchi and Watada, 1996).
Thirdly, PPi can serve as an alternative energy donor for proton pumping across the tonoplast, which can be driven by the tonoplastic H+ pyrophosphatase (PPase) in addition to the proton-pumping V-ATPase (Stitt, 1998). Indeed, Carystinos et al. (1995) found a clear increase in tonoplastic PPase activity and protein amount in anoxic rice seedlings, supporting this hypothesis.
Direct evidence for the importance of PPi for plant growth and metabolism was provided by Sonnewald (1992) who transformed tobacco and potato plants with inorganic PPase from Escherichia coli in order to reduce the cytosolic PPi content. The resulting plants contained less PPi and were strongly affected in carbohydrate metabolism and allocation (Jelitto et al., 1992; Sonnewald, 1992). The authors suggested that sucrose mobilization via SuSy and UGPase represents one key step at which PPi is required. Consistently, removal of PPi favours sucrose synthesis in source leaves and inhibits sucrose degradation in sink tissues. In further studies, it could also be demonstrated that long-distance transport of sucrose seems to be particularly dependent on cytosolic PPi (Lerchl et al., 1995; Geigenberger et al., 1996).
The aim of the present study was to investigate the importance of PPi to sustain glycolytic metabolism and cellular function under oxygen deficiency stress. Therefore, we made use of transgenic potato plants expressing a cytosolic PPase from E. coli under control of the chimeric STLS1/35S cauliflower mosaic virus (CaMV) promoter (UPPa II) (Hajirezaei and Sonnewald, 1999). The detectable amount of PPase protein was much higher in these lines compared with potato plants expressing the E. coli PPase under control of the unmodified 35S CaMV promoter (UPPa I) (Jelitto et al., 1992; Sonnewald, 1992). In UPPa II lines, the impact on tuber metabolism was characterized in detail, indicating impaired sucrose loading into the phloem and utilization, causing an inhibition of tuber sprouting (Hajirezaei and Sonnewald, 1999). In our studies, we mainly focused on changes in root metabolism during oxygen deficiency, since mainly roots experience this stress during flooding under field conditions.
MATERIALS AND METHODS
Transgenic plants, plant growth and treatment
Potato plants (Solanum tuberosum L. ‘Desiree’) were maintained and propagated on Murashige and Skoog medium containing 2 % sucrose (Murashige and Skoog, 1962) at 22 °C under a 16 h light/8 h dark regime. Transgenic potato plants expressing an E. coli inorganic PPase under the control of the chimeric STLS1/35S CaMV promoter (UPPa II) were described in Hajirezaei and Sonnewald (1999). For our analysis, we selected the transgenic line UPPa II-2.
Plants were transferred to vessels containing 5 L of aerated half-strength Knop nutrient solution (Biemelt et al., 1999) and cultivated under a 16 h light/8 h dark regime at 22 °C/19 °C and 350 µmol m−2 s−1 PAR in a growth chamber. The nutrient solution of control plants was continuously flushed with air. Plants were subjected to hypoxia by flushing the nutrient solution with nitrogen for 4 d. Shoots remained in air to allow restricted oxygen transport to the roots, as described earlier (Mustroph and Albrecht, 2003; Albrecht et al., 2004). Roots of wild-type and transgenic plants from both treatments were harvested after 96 h, rinsed briefly in water, weighed and immediately frozen in liquid nitrogen (a process taking in total 30–120 s).
The ability to resume growth was assessed by re-aeration of plants. Growth rates were determined by measuring the increase in fresh weight of roots and shoots.
Northern blot analysis
Total RNA was extracted from roots as described by Logemann et al. (1987). For northern blot analysis, 20–30 µg of total RNA were separated on 1·5 % formaldehyde-containing agarose gels and transferred onto a nylon membrane (GeneScreen, NEN, Boston, MA, USA) by capillary blotting overnight.
The membranes were pre-hybridized and hybridized at 65 °C. As a probe, the E. coli PPase was excised from the binary construct using the EcoRI cleavage site and labelled with [32P]dCTP by means of the High Prime Kit (Roche, Basel, Switzerland). After stringent washing, radioactive membranes were exposed to X-Ray film (Kodak) overnight at −70 °C.
Metabolite measurements
To determine concentrations of ATP, PPi, pyruvate, phosphoenolpyruvate (PEP), 3-phosphoglycerate (3PGA), UDP-glucose (UDPGlc), glucose-6-phosphate (Glc6P) and Fru6P, 200 mg of root material was ground to a fine powder in liquid nitrogen and was extracted in trichloroacetic acid as described by Weiner et al. (1987). For Fru1,6BP, Fru2,6BP and glucose-1-phosphate (Glc1P), 200 mg of root material was extracted in 0·5 mL of 50 mm NaOH containing 0·1 % Triton X-100 and neutralized with 20 mm HEPES containing 1 m acetic acid (van Schaftingen, 1984). Soluble sugars and starch were extracted and measured as described by Mustroph and Albrecht (2003).
Most metabolite concentrations were determined by enzyme-linked reduction of NAD or oxidation of NADH, monitored at 340 nm using a dual-wavelength spectrophotometer (Sigma-ZWS II). UDPGlc, Glc6P, Glc1P, Fru6P, Fru1,6BP and 3PGA were measured as described by Stitt et al. (1984). ATP was assayed according to Stitt (1982). Pyruvate and PEP were determined as described in Hatzfeld (1989), and PPi as in Weiner et al. (1987). The reliability of extraction and assay methods for potato roots was shown previously by Biemelt et al. (1999).
Fru2,6BP measurement was carried out using ion chromatography coupled to mass spectrometry, consisting of a Dionex HPLC system (Dionex; Idstein, Germany) with an MSQ mass detector (Dionex) as described in detail by Chen et al. (2005). The recovery was between 90 and 110 %.
Enzyme activities
Samples of 200 mg of root tissue were ground to a fine powder in liquid nitrogen and extracted in 50 mm HEPES-KOH, pH 6·8 containing 5 mm Mg acetate, 5 mm β-mercaptoethanol, 15 % (v/v) glycerine, 1 mm EDTA, 1 mm EGTA, 5 mm dithiothreitol (DTT) and 0·1 mm Pefabloc proteinase inhibitor (Boehringer Mannheim, Germany), essentially as described by Biemelt et al. (1999). The homogenate was centrifuged at 13 000 g at 4 °C for 15 min. The resulting supernatant was used for spectrophotometric determination of the activities of the different enzymes at 340 nm.
Activities of acid and alkaline invertase (EC 3.2.1.26), SuSy (EC 2.4.1.13) fructokinase (EC 2.7.1.1.), glucokinase (EC 2.7.1.2.), UGPase (EC 2.7.7.9), phosphoglucomutase (PGM, EC 5.4.2.2), ATP-dependent PFK (EC 2.7.1.11), PPi-dependent PFP (EC 2.7.1.90), aldolase (EC 4.1.2.13), enolase (EC 4.2.1.11), alcohol dehydrogenase (ADH, EC 1.1.1.1), pyruvate decarboxylase (PDC, EC 4.1.1.1) and lactate dehydrogenase (LDH, EC 1.1.1.27) were measured as described by Mustroph and Albrecht (2003). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, EC 1.2.1.12) was measured according to Biemelt et al. (1999).
To measure PPase activity, 10 µL of protein extract was incubated with 190 µL of 50 mm Tris–HCl (pH 8·0) containing 10 mm MgCl2 and 1·3 mm Na4PPi for 10 min at 25 °C. Released Pi was determined in a colorimetric reaction containing 0·1 m ascorbate, 4 mm ammonium molybdate and 1·2 n H2SO4 according to Chen et al. (1956). Colour development was for 2 h at 37 °C, and subsequently extinction was measured at 820 nm. Amounts of Pi were calculated by means of a standard curve.
In vivo measurement of respiration and glycolytic activity under aerated conditions
CO2 production was measured manometrically with a Warburg respirometer to monitor the glycolytic activity of excised roots under aerated conditions. Warburg flasks were filled with 5 mL of water and 200 mg of freshly harvested roots. Additionally, flasks either contained 300 μL of 20 % KOH in a separate chamber for CO2 absorption in order to measure O2 uptake or contained water to measure CO2 release. Decarboxylation and CO2 production are considered to be a measure for the activity of the glycolytic pathway.
Flasks without roots were used to correct the obtained values for temperature and air pressure changes. Measurements were performed in air for 1 h at 21 °C (while shaking). Calculations were carried out according to Umbreit et al. (1951).
In vivo production of ethanol in excised roots
For determination of the glycolytic activity under anaerobic conditions, the fermentation rate was measured in a closed system, since most of the produced ethanol diffuses from the roots into the surrounding medium (Bertani et al., 1980). Fresh root material of aerated and hypoxic-treated plants was carefully rinsed with water, dried with filter paper and placed into 2 mL vessels filled with nitrogen-flushed water. Vessels were carefully closed avoiding any air bubbles. After shaking the samples for 2 h at 23 °C in the dark, root material and solution were separated and frozen rapidly in liquid nitrogen, and ethanol content was detected in the solution as described by Albrecht et al. (2004).
RESULTS
Changes in root primary metabolism due to decreased PPi concentration
In order to elucidate the impact of a reduced concentration of cytosolic PPi on root metabolism in response to oxygen deficiency, we used transgenic potato plants in which the concentration of cytosolic PPi was reduced by expressing the E. coli PPase under control of the chimeric STLS1/35S CaMV promoter (Hajirezaei and Sonnewald, 1999). For our experiments, we have chosen the line UPPa II-2 with a decreased concentration of PPi due to a strong increase in PPase activity as previously shown in potato tubers (Hajirezaei and Sonnewald, 1999). Since the experiments in the earlier study were only performed with tubers, we initially confirmed that the E. coli PPase is also expressed in roots.
Northern blot analysis revealed a strong accumulation of E. coli PPase mRNA in roots of the transgenic line UPPa II-2 (Fig. 2B). The expression of E. coli PPase was paralleled by a 3·6 times higher PPase activity (Fig. 2C) as compared with the wild type. In agreement with the results obtained for tubers (Hajirezaei and Sonnewald, 1999), PPi concentration was 40 % lower in roots of the transgenic line compared with the wild type (Table 1).
Fig. 2.
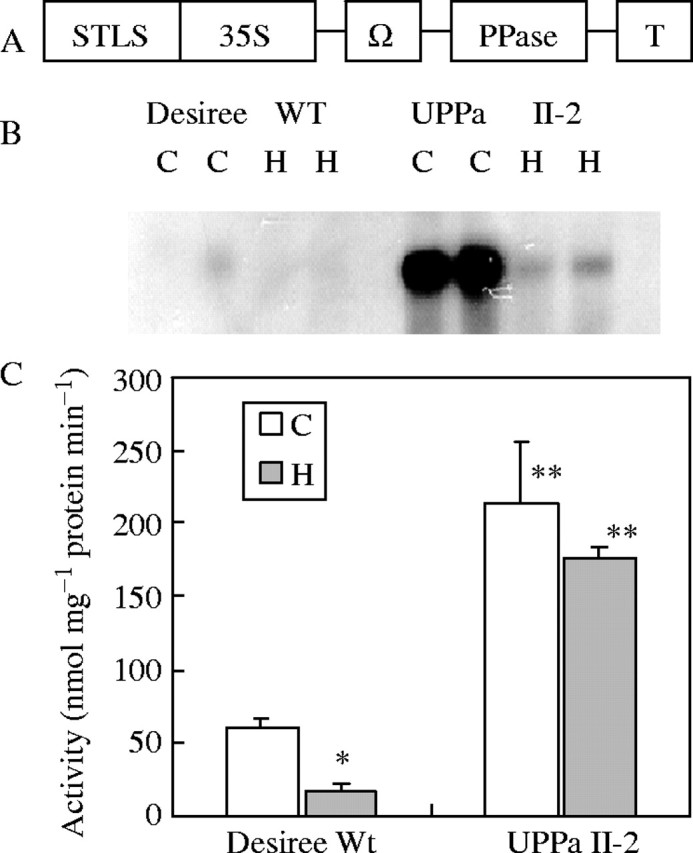
Expression of E. coli PPase in roots of transgenic potato plants (‘Desiree’). (A) Schematic structure of the construct used for transformation of potato plants. STLS, leaf-stem-specific promoter; 35S, 35S CaMV promoter; Ω, TMV-U1 translational enhancer; T, octopine synthase terminator. (B) Northern blot analysis of roots of E. coli PPase-expressing plants (UPPa II-2) as well as of wild-type plants (Desiree WT) under aerated conditions, C, and after 4 d of hypoxic treatment, H, with two replicates each. (C) Enzymatic activity of soluble PPase in potato root extracts after 4 d of hypoxia, H, compared with aerated conditions, C. Activity values are means of at least 8–12 samples ± s.e. from four independent experiments. Asterisks show significant changes between aeration and hypoxia (*) and between wild-type and transgenic plants (**) at the 5 % level (t-test).
Table 1.
Concentration of soluble sugars (μmol g FW−1), starch (μmol hexose equivalents g FW−1) and glycolytic intermediates (nmol g FW−1) in roots of wild-type (Desiree WT) and transgenic potato plants (UPPa II-2) following 4 d of hypoxia (H) compared with aerated controls (C)
| Desiree WT |
UPPa II-2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C |
H |
C |
% of WT |
H |
% of WT |
|||||
| PPi | 82·09 ± 5·65 | 99·93 ± 14·41 | 49·75 ± 4·63 | 61** | 50·72 ± 13·02 | 51** | ||||
| ATP | 104·35 ± 13·60 | 53·01 ± 5·78* | 66·65 ± 8·91 | 64** | 41·05 ± 7·10* | 77 | ||||
| Glucose (μmol) | 1·72 ± 0·22 | 11·20 ± 0·77* | 1·55 ± 0·18 | 90 | 12·13 ± 1·08* | 108 | ||||
| Fructose (μmol) | 2·50 ± 0·28 | 14·55 ± 0·82* | 2·08 ± 0·21 | 83** | 13·63 ± 0·93* | 94 | ||||
| Sucrose (μmol) | 3·91 ± 0·31 | 8·88 ± 0·48* | 4·28 ± 0·42 | 110 | 13·60 ± 0·93* | 153** | ||||
| Starch (μmol) | 5·55 ± 0·51 | 9·64 ± 1·02* | 6·25 ± 0·97 | 113 | 10·21 ± 1·68* | 106 | ||||
| Sucrose/hexose | 0·93 | 0·34 | 1·18 | 0·53 | ||||||
| UDPGlc | 48·54 ± 4·75 | 67·76 ± 6·90* | 104·67 ± 9·37 | 216** | 97·78 ± 15·03 | 144** | ||||
| Glc1P | 45·69 ± 10·44 | 26·51 ± 4·80* | 63·07 ± 11·09 | 138** | 45·61 ± 11·05 | 172** | ||||
| Glc6P | 163·61 ± 16·40 | 168·25 ± 20·63 | 97·86 ± 15·25 | 60** | 78·91 ± 8·96* | 47** | ||||
| Fru6P | 33·52 ± 2·49 | 34·52 ± 2·36 | 16·15 ± 1·41 | 48** | 15·70 ± 1·37 | 45** | ||||
| Fru1,6BP | 7·82 ± 1·06 | 6·42 ± 0·74 | 3·92 ± 0·86 | 50** | 2·74 ± 0·55 | 43** | ||||
| 3PGA | 47·75 ± 7·57 | 40·41 ± 6·95 | 10·42 ± 1·30 | 22** | 17·02 ± 2·69* | 42** | ||||
| PEP | 8·73 ± 1·94 | 6·66 ± 1·47 | 5·27 ± 0·72 | 60 | 6·02 ± 0·87 | 90 | ||||
| Pyruvate | 7·70 ± 1·28 | 10·63 ± 0·61* | 11·33 ± 2·53 | 147 | 11·41 ± 3·30 | 107 | ||||
| Fru6P/3PGA | 0·70 | 0·85 | 1·55 | 0·92 | ||||||
| Fru2,6BP (pmol) | 210·97 ± 18·22 | 157·70 ± 6·58* | 295·76 ± 8·43 | 140** | 155·88 ± 18·89* | 99 | ||||
Asterisks show significant changes between aeration and hypoxia (*) and between wild-type and transgenic plants (**) at the 5 % level (t-test). Results are means of 6–12 samples ± SE from four independent experiments.
To investigate the impact of reduced concentration of PPi on root primary metabolism, the concentration of sugars, hexose phosphates and glycolytic intermediates as well as the activities of several glycolytic enzymes were determined in roots of transgenic potato plants and compared with wild-type plants (Tables 1 and 2).
Table 2.
Enzyme activities (nmol mg prot−1 min−1) in roots of wild-type (Desiree WT) and transgenic potato plants (UPPa II-2) following 4 d of hypoxia (H) compared with aerated controls (C)
| Desiree WT |
UPPa II-2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C |
H |
C |
% of WT |
H |
% of WT |
|||||
| PPase | 60 ± 7 | 17 ± 5* | 214 ± 42 | 356** | 177 ± 6 | 1031** | ||||
| SuSy | 28 ± 3 | 73 ± 4* | 27 ± 3 | 98 | 57 ± 5* | 77** | ||||
| Alkaline invertase | 60 ± 4 | 46 ± 9 | 56 ± 4 | 95 | 41 ± 4 | 88 | ||||
| Acid invertase | 117 ± 32 | 38 ± 5* | 118 ± 26 | 101 | 45 ± 8* | 121 | ||||
| UGPase | 1964 ± 285 | 1514 ± 103* | 2098 ± 262 | 107 | 1589 ± 140* | 105 | ||||
| PGM | 845 ± 29 | 667 ± 47* | 847 ± 53 | 100 | 699 ± 60* | 105 | ||||
| Fructokinase | 50 ± 6 | 18 ± 6* | 56 ± 10 | 111 | 15 ± 6* | 84 | ||||
| Glucokinase | 53 ± 11 | 46 ± 4 | 33 ± 4 | 63 | 25 ± 5 | 55** | ||||
| PFK | 153 ± 19 | 184 ± 8 | 156 ± 13 | 102 | 190 ± 18 | 103 | ||||
| PFP | 224 ± 25 | 185 ± 10 | 215 ± 23 | 96 | 186 ± 15 | 100 | ||||
| Aldolase | 110 ± 9 | 97 ± 9 | 114 ± 10 | 103 | 83 ± 9 | 85** | ||||
| Enolase | 324 ± 56 | 375 ± 45 | 311 ± 48 | 96 | 352 ± 36 | 94 | ||||
| ADH | 121 ± 16 | 3770 ± 491* | 129 ± 36 | 106 | 3378 ± 303* | 90 | ||||
| PDC | 1·7 ± 1·1 | 10·8 ± 1·5* | 0·5 ± 0·3 | 31 | 13·1 ± 1·6* | 121 | ||||
| LDH | 36 ± 9 | 80 ± 14* | 44 ± 9 | 120 | 82 ± 13* | 103 | ||||
Asterisks show significant changes between aeration and hypoxia (*) and between wild-type and transgenic plants (**) at the 5 % level (t-test). Results are means of at least 8–12 samples ± SE from four independent experiments.
Under aerated conditions, hexose concentration was lower in roots of transgenic plants, whereas the amounts of sucrose and starch were not significantly altered (Table 1). The UPPa II-2 line showed increases in UDPGlc and Glc1P, which were 2·0- and 1·4-fold, respectively. The amounts of Glc6P and Fru6P were reduced in UPPa II-2 plants down to 60 and 48 % of the wild-type level, respectively. The clear decreases in the amounts of the glycolytic intermediates Fru1,6BP and 3PGA were not paralleled by changes in concentrations of PEP and pyruvate (Table 1). While in UPPa II-2 tubers an ∼10-fold accumulation of Fru2,6BP was observed (Hajirezaei and Sonnewald, 1999), only a small increase (40%) was found in roots of the transgenic plants compared with the wild type (Table 1). Interestingly, the ATP concentration was diminished in transgenic roots to 60 % of the wild-type level (Table 1). These metabolic changes observed indicate a reduced flux through glycolysis causing a lack of energy in the UPPa II-2 line. Accordingly, growth of the transgenic lines was found to be inhibited compared with the wild type under aerated conditions, as shown by the lower increase in fresh weight monitored over a 4 d period (Table 3).
Table 3.
Impact of hypoxic treatment on root and shoot growth of potato plants
| Desiree WT |
UPPa II-2 |
|||||
|---|---|---|---|---|---|---|
| Shoot |
Root |
Shoot |
Root |
|||
| 0 d | 0·97 ± 0·16 | 0·39 ± 0·08 | 0·52 ± 0·07** | 0·26 ± 0·05 | ||
| 4 d aeration | 2·43 ± 0·34 | 1·01 ± 0·16 | 1·05 ± 0·16** | 0·54 ± 0·08** | ||
| Increase during 4 d | 1·46 | 0·62 | 0·54 | 0·28 | ||
| 4 d hypoxia | 1·51 ± 0·20* | 0·42 ± 0·05* | 0·82 ± 0·08** | 0·29 ± 0·04* | ||
| Increase during 4 d | 0·54 | 0·03 | 0·30 | 0·03 | ||
| 8 d aeration | 4·20 ± 0·26 | 1·71 ± 0·13 | 2·19 ± 0·24** | 1·24 ± 0·16** | ||
| Increase during 4 d | 1·77 | 0·70 | 1·14 | 0·69 | ||
| 4 d hypoxia + 4 d re-aeration | 2·81 ± 0·38* | 1·30 ± 0·20 | 1·33 ± 0·11*/** | 0·69 ± 0·06*/** | ||
| Increase during 4 d | 1·31 | 0·88 | 0·51 | 0·40 | ||
Three-week-old wild-type (Desiree WT) and transgenic potato plants (UPPa II-2) either were subjected for 4 d to hypoxia or were continuously aerated. Subsequently, hypoxia-treated plants were re-aerated for a further 4 d. The fresh weight (g) of shoots and roots was determined at the start of the experiment (0 d) and after 4 and 8 d. We choose out of three, one representative experiment, and show the mean ± SE of 8–10 individual values. Asterisks show significant changes between aeration and hypoxia (*) and between wild-type and transgenic plants (**) at the 5 % level (t-test).
Although an increase in UDPGlc was observed in the transgenic line, the activities of SuSy and UGPase did not differ significantly between wild-type and UPPa II-2 plants (Table 2). The activity of glucokinase, however, showed a 40 % decrease in the transgenic line as compared with the wild type. Activities of the other glycolytic enzymes investigated were not altered in the transgenic line compared with the wild type (Table 3).
Influence of oxygen deficiency stress on primary metabolism in roots of plants with reduced cytosolic PPi
Growth and metabolite concentrations
To evaluate the metabolic consequences of oxygen deficiency in roots of potato plants with reduced PPi levels, plants were hypoxically treated for 4 d by flushing the nutrient solution with nitrogen. This treatment caused a growth retardation in both wild type and transgenic plants (Table 3). Roots were much more affected than shoots (Table 3). The retardation of root growth was accompanied by a strong accumulation of soluble sugars in roots of wild type as well as of UPPa II-2 (Table 1). Whereas hexose levels increased to similar levels in wild-type and transgenic plants, sucrose accumulation was more pronounced in the transgenic line. This resulted in a 30 % higher sucrose/hexose ratio compared with the wild type. In addition, starch concentration increased by ∼50 % in both the wild type and transgenic line (Table 1).
Hypoxic treatment of wild-type plants resulted in a 40 % increase of UDPGlc, whereas there was no significant change in the transgenic line. Nevertheless, the total amount of UPDGlc was also higher under low oxygen than in wild-type plants (Table 1). The level of Glc1P decreased in wild-type and in UPPa II-2 roots, but remained on a higher level in the transgenic line. The amounts of Glc6P, Fru6P and Fru1,6BP were not significantly altered due to hypoxic treatment; however, the absolute levels in the UPPa II-2 plants were only half of those of wild-type plants. While the amount of 3PGA was stable in wild-type plants, there was an increase by about 60 % in transgenic plants in response to oxygen deficiency. Nevertheless, wild-type plants still contained twice as much 3PGA as the transgenic plants. The concentrations of PEP and pyruvate remained nearly unaltered in UPPa II-2 plants under hypoxic conditions, whereas in wild type the pyruvate concentration increased. Surprisingly, Fru2,6BP concentration decreased in both types of plants by ∼40 % as compared with aerated controls (Table 1).
The PPi concentration in wild-type plants did not change significantly during the hypoxic stress (Table 1), even though a decrease in PPase activity to one-third of the aerated control level was observed (Table 2). The PPi concentration of UPPa II-2 being reduced to about half of the wild-type level also remained stable in aerated and hypoxically stressed roots (Table 1). As a consequence of the inhibited mitochondrial respiration caused by a lack of oxygen, both wild type and UPPa II-2 exhibited a lower ATP concentration after 4 d of hypoxia, with a stronger decline in wild-type plants (47 %) than in transgenic plants (23 %). However, the total ATP level was 15 % lower in roots of hypoxia-stressed transgenic plants than in wild-type plants (Table 1).
The observed differences described above in levels of several metabolites between wild-type plants and UPPa II-2 plants in response to hypoxic stress suggest that the glycolytic pathway seems to be more severely impaired during oxygen deficiency in roots low in PPi. To test this hypothesis in more detail, enzyme activities and the in vivo glycolytic rate were investigated.
Enzyme activities
A strong induction of fermentative enzyme activities (ADH, PDC and LDH) was observed for both wild-type and UPPa II-2 plants (Table 2). While ADH and PDC activity increased 30 and 10 times, respectively, LDH activity was only doubled. The PPi-dependent sucrolytic pathway via SuSy was proposed to be the predominating one under hypoxia because of its ATP-saving mode of sucrose breakdown (Perata et al., 1996, 1997). In fact, SuSy activity was enhanced 2·7 times in wild-type plants and 2·1-fold in transgenic plants (Table 2) after 4 d of hypoxia compared with aerated controls. In contrast, activities of acid as well as of alkaline invertase decreased during hypoxia, by 65 and 25 % in wild-type and UPPa II-2 plants, respectively. PGM and UGPase activities showed a tendency to decrease by ∼20 % during hypoxia, but their activities seem not to be limiting since total activities were very high.
Most of the glycolytic enzymes did not show significant changes in response to hypoxic treatment in wild-type or in transgenic plants (Table 2). Only fructokinase showed a 60 % lower activity under hypoxia in both types of plants compared with during aeration, and glucokinase activity was reduced by 10 % in wild-type and 25 % in UPPa II-2 plants. Hypoxic treatment did not lead to clear changes in the activities of the ATP-dependent PFK and the PPi-dependent PFP either in wild-type or in transgenic plants (Table 2).
Glycolytic activity
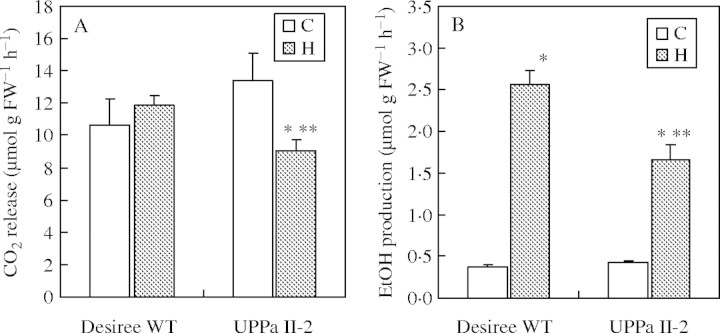
CO2 production from aerobic roots was determined using a Warburg respirometer in order to estimate whether or not a reduced concentration of cytosolic PPi has an impact on glycolytic activity. CO2 formation did not differ in aerated roots of transgenic and wild-type plants (Fig. 3A). In wild-type plants, CO2 production by hypoxically pre-treated roots was similar to that by the aerated ones. In contrast, CO2 production by transgenic roots was 40 % less after hypoxic stress, compared with non-stressed controls (Fig. 3A).
Fig. 3.
Glycolytic rates in excised roots of wild-type (Desiree WT) and transgenic potato plants (UPPa II-2) following 4 d of hypoxia (H) compared with aerated controls (C). (A) The CO2 release [μmol g FW−1 h−1] was measured with a Warburg apparatus under aerated conditions as described in Materials and Methods. (B) Ethanol production rate was measured enzymatically during anoxic incubation of excised roots (for 2 h). Values are the means ± s.e. of at least three independent experiments with three samples each. Asterisks show significant changes between aeration and hypoxia (*) and between wild-type and transgenic plants (**) at the 5 % level (t-test).
In order to measure the glycolytic activity under oxygen deficiency, the amount of ethanol produced was determined in a closed anoxic system as described in Materials and Methods. Ethanol formation was very low in roots of both wild-type and UPPa II-2 plants grown under aerated conditions prior to transfer into anoxia for the measurements (Fig. 3B). In accordance with the strong induction of ADH and PDC activities (Table 2), ethanol production was increased after 4 d of root hypoxia (Fig. 3B). In wild type, there was a 6·8-fold increase in ethanol production, but only a 3·9-fold increase in the hypoxia-stressed transgenic roots, which is significantly lower than in wild type (Fig. 3B). This was despite higher amounts of soluble sugars in the transgenic plants (Table 2), indicating an impaired utilization of substrates via glycolysis, which is, however, consistent with lower ATP levels in roots of UPPa II-2 plants compared with the wild type.
Growth during the re-aeration phase
As a consequence of the introduced PPase activity, the glycolytic activity in roots of hypoxia-treated transgenic plants seemed to be reduced under aerated and oxygen-deficient conditions indicated by measurements of metabolites, gas exchange and ethanol formation (Table 1, Fig. 3). To test whether the ability to recover after hypoxia was altered in UPPa II-2 plants, plants were re-aerated for 4 d, and the increase in fresh weight was determined (Table 1). Roots of wild-type plants increased in fresh weight as high as unstressed roots. In contrast, shoot growth was still inhibited by ∼40 % during the 4 d re-aeration period (Table 3). The fresh weight increase in roots and shoots of transgenic plants transferred from hypoxic to aerated conditions was clearly reduced compared with continuously aerated control plants, and was lower than in wild-type plants (Table 3). This indicates that the ability to resume growth upon re-aeration was impaired in UPPa II-2 plants.
DISCUSSION
It has been postulated by Stitt (1998), that PPi could be used as an alternative energy source in plants under ATP-limiting conditions (e.g. under oxygen deficiency). To investigate the importance of PPi for maintaining plant metabolism and growth under low oxygen availability, we analysed changes in transgenic potato plants with decreased levels of cytosolic PPi in response to root hypoxia and compared them with the wild-type plants.
Low levels of PPi negatively affect sucrose mobilization
Expression of PPase from E. coli in the cytosol was shown to result in dramatic alterations in carbohydrate metabolism and allocation in transgenic tobacco and potato plants (Jelitto et al., 1992; Sonnewald, 1992). The transgenic plants were characterized by an increased ratio between soluble sugars and starch, pointing towards a shift of photoassimilate distribution (Sonnewald, 1992). Whereas transgenic tobacco plants exhibited stunted growth, potato plants expressing the PPase gene under control of either the unmodified CaMV 35S (UPPa I) or of the chimeric STLS1/CaMV 35S promoter (UPPa II) were less affected, showing only a slightly reduced plant height (Sonnewald, 1992; Hajirezaei and Sonnewald, 1999). Apart from the changes in carbohydrate metabolism in leaves, a perturbation in sink metabolism was also shown in potato tubers (Jelitto et al., 1992; Hajirezaei and Sonnewald, 1999). The authors showed that starch accumulation in transgenic tubers was inhibited even though sucrose concentration increased, which indicated that sucrose mobilization was impaired.
In our study, we were interested in metabolic changes occurring in roots. To investigate these, we used a hydroponic system. In aerated conditions (nutrient solution flushed with air), growth of shoots and roots of the UPPa II-2 was retarded compared with wild-type plants. Similar to the changes observed in tubers, the most dramatic metabolic effect of reduced PPi concentration in potato roots was observed in sucrose cleavage. Overexpression of E. coli PPase in roots caused an increased UDPGlc concentration, whereas the contents of Glc6P and Fru6P were diminished (Table 1). Similar changes have been described for potato tubers containing less PPi (Jelitto et al., 1992; Geigenberger et al., 1998; Hajirezaei and Sonnewald, 1999). However, they also detected an increased concentration of sucrose as well as a higher SuSy activity (Geigenberger et al., 1998), which was not found for roots in the present experiments (Tables 1 and 2). However, as in tubers, the amounts of glycolytic intermediates (Fru1,6BP and 3PGA) were decreased in roots of the transgenic line. These data imply an inhibition of sucrose breakdown via SuSy due to PPi deficiency, and consequently less substrates being available to fuel glycolysis. This eventually could be the cause for lower formation of ATP and reduced plant growth.
During low oxygen stress, oxidative phosphorylation ceases, with severe consequences for plant growth and metabolism (recently reviewed by Gibbs and Greenway, 2003). However, PPi content remained stable during this time (Table 1). This result is in accordance with previous reports, also showing no changes in PPi level in response to oxygen deficiency (Dancer and ap Rees, 1989; Mohanty et al., 1993; Geigenberger et al., 2000). Despite the strong induction of ethanolic fermentation, carbohydrates accumulated in both wild-type and transgenic potato plants in response to hypoxia, which indicates that the supply of carbohydrates is not limiting metabolism under these conditions (Table 1). The observed accumulation of soluble sugars was also described for other hypoxically treated plants (Atwell et al., 1985; Setter et al., 1987; Albrecht et al., 1993; Biemelt et al., 1999; Mustroph and Albrecht, 2003) and is thought to be brought about by a reduced growth rate and a lower rate of consumption of sugars, whereas the photosynthesis rate remains stable (Mustroph and Albrecht, 2003).
Roots with lower PPi concentration, however, showed during hypoxia a stronger accumulation of sucrose than of hexoses compared with wild-type roots (Table 1). Together with the increased UDPGlc concentration as well as the decrease in the hexose-6-phosphate pool, the low level of glycolytic intermediates and the more dramatic decrease in ATP level, the data suggest that sucrose breakdown via the PPi-dependent pathway is more severely inhibited in the UPPa II-2 plants during hypoxic stress than in wild-type plants. An additional hint for that comes from the lower induction of SuSy activity in the transgenic line (Table 2). The importance of SuSy for maintaining metabolism under oxygen deprivation was shown using maize mutants (Ricard et al., 1998).
The significant increase in UDPGlc forces us to consider how PPi metabolism interacts with specific reactions using UDPGlc as substrate, such as the synthesis of cellulose and other cell wall components. Recently, it was demonstrated that the hypoxically induced increase in SuSy activity in wheat roots was associated with an increased cellulose content leading to a strengthening of the cell wall (Albrecht and Mustroph, 2003). However, in the UPPa II-2 plants, no significant increase in cellulose content could be detected (data not shown) although the UDPGlc concentration was already increased under aerated conditions and remained high during hypoxia. It seems that the structural strengthening of roots by cellulose deposition is a feature of more flooding-tolerant plants, e.g. wheat and barley (Albrecht and Mustroph, 2003), and may not occur in susceptible plants such as potato.
No clear impact of low PPi level on phosphofructokinase activities
While over-expression of E. coli PPase impeded sucrose cleavage in the roots, especially during conditions of low oxygen availability, the activity of enzymes involved in the phosphorylation of Fru6P were not significantly altered in aerated roots of transgenic plants compared with wild-type plants (Table 2). Our result is in contrast to earlier studies with tubers of UPPa I plants revealing that activities of PFP and PFK were induced compared with the wild type (Geigenberger et al., 1998). The induction of these enzymes was thought to partly compensate for the lack of PPi for the phosphorylation of Fru6P (Geigenberger et al., 1998). Additionally, previous results also showed a large accumulation of Fru2,6BP in UPPa I as well as in UPPa II lines, which is a potent activator of PFP (Jelitto et al., 1992; Hajirezaei and Sonnewald, 1999). In our experiments, we only observed a slightly higher concentration of Fru2,6BP in roots of aerated transgenic plants compared with the wild type (Table 1). This increase is most likely to be a consequence of the decreased 3PGA concentration leading to an activation of phosphofructo-2-kinase (Stitt, 1990) and indicates a further adaptation mechanism of plants to compensate for PPi deficiency, because Fru2,6BP may activate the glycolytic reaction of PFP.
The PPi-deficient roots contained a higher Fru6P/3PGA ratio compared with the wild type under aerated conditions (Table 1). This finding could be an indication that PFP is active in the gluconeogenetic direction in the aerated PPi-deficient roots. Thus, plants could use a PFK/PFP cycle to generate PPi from ATP (Geigenberger et al. 1998). Thereby, Fru1,6BP would be formed by PFK being the substrate for PFP to generate PPi. A hint for this hypothesis is the finding that UPPa II-2 roots contained less ATP than the wild type (Table 1). One possible function of this cycle could be the generation of PPi used for sucrose mobilization via SuSy and UGPase. A comparable mechanism was suggested in maize plants (Costa dos Santos et al., 2003). Here the authors provided evidence for the gluconeogenetic direction of PFP to produce PPi used by the tonoplast PPase. During hypoxia, however, the proposed mechanism is apparently inhibited by severely decreased ATP amounts.
The activities of PFK and PFP were only slightly changed in response to hypoxic stress in wild-type and transgenic potato plants, providing no evidence for the hypothesis that the PPi-dependent formation of Fru1,6BP is the preferred route under oxygen deficiency. This result is consistent with our previous study (Biemelt et al., 1999), but contrasts with results obtained with shoots of anoxia-tolerant rice seedlings showing an enormous induction of PFP during low oxygen stress (Mertens et al., 1990; Mohanty et al., 1993; Gibbs et al., 2000; Kato-Noguchi, 2002). While in rice, PFP activation was accompanied by an increase of Fru2,6BP, in our study the concentration was even lower under hypoxia. Thus, one might speculate that rice plants have evolved strategies allowing them to cope with low oxygen stress by using the energy-saving mode of action of PFP for hexose phosphate phosphorylation. In general, plants seem to be able tightly to regulate the phosphorylation of Fru6P. This assumption is supported by PFP antisense potato plants showing no clear effects on plant primary metabolism and growth, although PFP activity was drastically diminished (Hajirezaei et al., 1994).
Glycolysis and growth are severely affected by PPi deficiency under low oxygen stress
Our results provide some evidence that sucrose cleavage was inhibited in the transgenic potato line, and consequently concentrations of many glycolytic intermediates decreased probably due to lower flow through glycolysis (Table 1). However, CO2 release was not affected in aerated UPPa II-2 roots (Fig. 3), although ATP formation and root growth were inhibited compared with the wild type (Table 3). Thus, it seems that the plants can compensate for the low PPi level under aerated conditions by decelerating plant growth.
During oxygen deficiency stress, the transgenic plants were no longer able to maintain the glycolytic activity on a wild-type level (Fig. 3). The amount of CO2 released and ethanol produced was less in roots of the transgenic plants than for the wild type, reflecting a lower glycolytic activity under anoxia and also re-aeration. Nevertheless, anoxic incubation resulted in a strongly induced rate of ethanol production compared with aerated roots, which is also reflected by a strong increase in the activities of PDC and ADH (Table 2). The induction of the ethanolic fermentative pathway is a common response to low oxygen (Drew et al., 1997; Gibbs and Greenway, 2003). LDH activity, however, was only doubled in response to hypoxia (Table 2). It has been shown that for the majority of plant species, lactic acid fermentation is only active in the early phase of plant response to oxygen deficiency stress, whereas ethanolic fermentation prevails during long-lasting hypoxic periods (Muench et al., 1993; Greenway and Gibbs, 2003).
Hypoxic treatment resulted in a retardation of root growth due to a lack of energy in both transgenic and wild-type plants, as was also shown previously for potato and other plants (Biemelt et al., 1999; Mustroph and Albrecht, 2003). In plants expressing an E. coli PPase, however, the combination of both PPi deficiency and low oxygen stress resulted in a strongly impaired ability to resume growth during re-aeration following hypoxic treatment (Table 3). It seems that the slow down of glycolysis as a consequence of PPi and ATP shortage cannot be compensated by the plants. These results emphasize the importance of PPi for maintaining plant growth and metabolism under oxygen deficiency and for re-activation of metabolic function during the post-hypoxic phase.
CONCLUSIONS
Taking all the data together, it could be demonstrated that PPi has an important function in maintaining glycolysis, especially during the initial step of sucrose cleavage. Inhibition of the SuSy pathway by decreased cytosolic PPi negatively affected primary metabolism under aeration, but had a more severe effect on plant metabolism under low oxygen conditions. In our study, we could not detect changes in activity of the PPi-dependent PFP in the transgenic line, neither under aerated nor under hypoxic conditions. Thus, it still remains to be elucidated whether or not PFP contributes to adaptation of plants to low oxygen.
Although our experiments provide considerable evidence for the importance of PPi metabolism during low oxygen stress, the role of PPi in anaerobic conditions still requires critical examination, for example by using genetically modified plants with lower activity of cytosolic PPase in order to evaluate if a higher PPi concentration can contribute to higher tolerance to oxygen deficiency.
Acknowledgments
We wish to thank Uwe Sonnewald for proving the transgenic plants, for his support and the stimulating discussions.
Footnotes
Present address: Friedrich-Alexander Universität Erlangen, Lehrstuhl für Biochemie, Institut für Mikrobiologie, Biochemie und Genetik, Staudtstrasse 5, D-91058 Erlangen, Germany
LITERATURE CITED
- Albrecht G, Mustroph A. 2003. Localization of sucrose synthase in wheat roots: increased in situ activity of sucrose synthase correlates with cell wall thickening by cellulose deposition under hypoxia. Planta 217: 252–260. [DOI] [PubMed] [Google Scholar]
- Albrecht G, Wiedenroth EM. 1994. Is long-term hypoxia met by the Pasteur effect in roots of wheat seedlings? Proceedings of the Royal Society of Edinburgh 102b: 407–412. [Google Scholar]
- Albrecht G, Kammerer S, Praznik W, Wiedenroth EM. 1993. Fructan content of wheat seedlings (Triticum aestivum) under hypoxia and following re-aeration. New Phytologist 123: 471–476. [DOI] [PubMed] [Google Scholar]
- Albrecht G, Mustroph A, Fox TC. 2004. Sugar and fructan accumulation during metabolic adjustment between respiration and fermentation under low oxygen conditions in wheat roots. Physiologia Plantarum 120: 93–105. [DOI] [PubMed] [Google Scholar]
- Atwell BJ, Thomson CJ, Greenway H, Ward G. 1985. A study of the impaired growth of roots of Zea mays seedlings at low oxygen concentration. Plant, Cell and Environment 8: 179–188. [Google Scholar]
- Bertani A, Brambilla I, Menegus F. 1980. Effect of anaerobiosis on rice seedlings: growth, metabolic rate, and fate of fermentation products. Journal of Experimental Botany 31: 325–331. [Google Scholar]
- Biemelt S, Hajirezaei MR, Melzer M, Albrecht G, Sonnewald U. 1999. Sucrose synthase activity does not restrict glycolysis in roots of transgenic potato plants under hypoxic conditions. Planta 210: 41–49. [DOI] [PubMed] [Google Scholar]
- Carystinos GD, Heather MR, Monroy AF, Rajinder DS, Poole RJ. 1995. Vacuolar H+-translocating pyrophosphatase is induced by anoxia or chilling in seedlings of rice. Plant Physiology 108: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS, Toribara TY, Warner H. 1956. Microdetermination of phosphorus. Analytical Chemistry 28: 1756–1758. [Google Scholar]
- Chen S, Hajirezaei M, Peisker M, Tschiersch H, Sonnewald U, Börnke F. 2005. Decreased sucrose-6-phosphate phosphatase level in transgenic tobacco inhibits photosynthesis, alters carbohydrate partitioning and reduces growth. Planta 221: 479–492. [DOI] [PubMed] [Google Scholar]
- Chourey PS, Taliercio EW, Kane EJ. 1991. Tissue-specific expression and anaerobically induced posttranscriptional modulation of sucrose synthase genes in Sorghum bicolor M. Plant Physiology 96: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa dos Santos A, da Silva WS, de Meis L, Galina A. 2003. Proton transport in maize tonoplasts supported by fructose-1,6-bisphosphate cleavage. Pyrophosphate-dependent phosphofructokinase as a pyrophosphate-regenerating system. Plant Physiology 133: 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford RMM, Braendle R. 1996. Oxygen deprivation stress in a changing environment. Journal of Experimental Botany 47: 145–159. [Google Scholar]
- Dancer JE, ap Rees T. 1989. Effects of 2,4-dinitrophenol and anoxia on the inorganic pyrophosphate content of the spadix of Arum maculatum and the root apices of Pisum sativum Planta 178: 421–424. [DOI] [PubMed] [Google Scholar]
- Drew MC. 1997. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annual Review of Plant Physiology and Plant Molecular Biology 48: 223–250. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Lerchl J, Stitt M, Sonnewald U. 1996. Phloem-specific expression of pyrophosphatase inhibits long-distance transport of carbohydrates and amino acids in tobacco plants. Plant, Cell and Environment 19: 43–55. [Google Scholar]
- Geigenberger P, Hajirezaei M, Geiger M, Deiting U, Sonnewald U, Stitt M. 1998. Overexpression of pyrophosphatase leads to increased sucrose degradation and starch synthesis, increased activities of enzymes for sucrose–starch interconversions, and increased levels of nucleotides in growing potato tubers. Planta 205: 428–437. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Fernie AR, Gibon Y, Christ M, Stitt M. 2000. Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biological Chemistry 381: 723–740. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Greenway H. 2003. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology 30: 1–47. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Morrell S, Valdez A, Setter TL, Greenway H. 2000. Regulation of alcoholic fermentation in coleoptiles of two rice cultivars differing in tolerance to anoxia. Journal of Experimental Botany 51: 785–796. [PubMed] [Google Scholar]
- Greenway H, Gibbs J. 2003. Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Functional Plant Biology 30: 999–1036. [DOI] [PubMed] [Google Scholar]
- Guglielminetti L, Perata P, Alpi A. 1995. Effect of anoxia on carbohydrate metabolism in rice seedlings. Plant Physiology 108: 735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajirezaei M, Sonnewald U. 1999. Inhibition of potato tuber sprouting: low levels of cytosolic pyrophosphate lead to non-sprouting tubers harvested from transgenic potato plants. Potato Research 42: 353–372. [Google Scholar]
- Hajirezaei M, Sonnewald U, Viola R, Carlise S, Dennis D, Stitt M. 1994. Transgenic potato plants with strongly decreased expression of pyrophosphate:fructose-6-phosphate phosphotransferase show no visible phenotype and only minor changes in metabolic fluxes in their tubers. Planta 192: 16–33. [Google Scholar]
- Hatzfeld WD, Dancer JE, Stitt M. 1989. Direct evidence that pyrophosphate:fructose 6-phosphate phosphotransferase can act as a glycolytic enzyme in plants. FEBS Letters 254: 215–218. [Google Scholar]
- Jelitto T, Sonnewald U, Willmitzer L, Hajirezaei M, Stitt M. 1992. Inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing E. coli pyrophosphatase in their cytosol. Planta 188: 238–244. [DOI] [PubMed] [Google Scholar]
- Kato-Noguchi H. 2002. The catalytic direction of pyrophosphate:fructose 6-phosphate 1-phosphotransferase in rice coleoptiles in anoxia. Physiologia Plantarum 116: 345–350. [Google Scholar]
- Kato-Noguchi H, Watada AE. 1996. Low-oxygen atmosphere increases fructose-2,6-bisphosphatase in fresh-cut carrots. Journal of the American Society of Horticultural Science 121: 307–309. [Google Scholar]
- Lerchl J, Geigenberger P, Stitt M, Sonnewald U. 1995. Impaired photoassimilate partitioning caused by phloem-specific removal of pyrophosphate can be complemented by a phloem-specific cytosolic yeast-derived invertase in transgenic plants. Plant Cell 7: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L. 1987. Improved method for the isolation of RNA from plant tissues. Analytical Biochemistry 163: 16–20. [DOI] [PubMed] [Google Scholar]
- Mertens E, Laroundelle Y, Hers H-G. 1990. Induction of pyrophosphate:fructose 6-phosphate 1-phosphotransferase by anoxia in rice seedlings. Plant Physiology 93: 584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty B, Wilson PM, apRees T. 1993. Effects of anoxia on growth and carbohydrate metabolism in suspension cultures of soybean and rice. Phytochemistry 34: 75–82. [Google Scholar]
- Muench DG, Archibold OW, Good AG. 1993. Hypoxic metabolism in wild rice (Zizania palustris): enzyme-induction and metabolite production. Plant Physiology 99: 165–171. [Google Scholar]
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio-essays with tobacco tissue cultures. Physiologia Plantarum 15: 473–497. [Google Scholar]
- Mustroph A, Albrecht G. 2003. Tolerance of crop plants to oxygen deficiency stress: fermentative activity and photosynthetic capacity of entire seedlings under hypoxia and anoxia. Physiologia Plantarum 117: 508–520. [DOI] [PubMed] [Google Scholar]
- Perata P, Alpi A. 1993. Plant responses to anaerobiosis. Plant Science 93: 1–17. [Google Scholar]
- Perata P, Guglielminetti L, Alpi A. 1996. Anaerobic carbohydrate metabolism in wheat and barley, two anoxia-intolerant cereal seeds. Journal of Experimental Botany 47: 999–1006. [Google Scholar]
- Perata P, Guglielminetti L, Alpi A. 1997. Mobilization of endosperm reserves in cereal seeds under anoxia. Annals of Botany 79: 40–56. [Google Scholar]
- Plaxton WC. 1996. The organization and regulation of plant glycolysis. Annual Review of Plant Physiology and Plant Molecular Biology 48: 185–214. [DOI] [PubMed] [Google Scholar]
- Ricard B, Rivoal J, Spiteri A, Pradet A. 1991. Anaerobic stress induces the transcription of sucrose synthase in rice. Plant Physiology 95: 669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard B, Van Toai T, Chourey P, Saglio P. 1998. Evidence for the critical role of sucrose synthase for anoxic tolerance of maize roots using a double mutant. Plant Physiology 116: 1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs MM, Subbaiah CC, Sabb IN. 1996. Anaerobic gene expression and flooding tolerance in maize. Journal of Experimental Botany 47: 1–15. [Google Scholar]
- van Schaftingen E. 1984.d-Fructose 2,6-bisphosphate. In: Bergmeyer HU, ed. Methods of enzymatic analysis, Vol. 6. Weinheim: Verlag Chemie, 335–341. [Google Scholar]
- Schlueter U, Crawford RMM. 2001. Long-term anoxia tolerance in leaves of Acorus calamus L. and Iris pseudacorus L. Journal of Experimental Botany 52: 2213–2225. [DOI] [PubMed] [Google Scholar]
- Setter TL, Kupkanchanakul T, Kupkanchanakul K, Bhekasut P, Wiengweera A, Greenway H. 1987. Concentrations of CO2 and O2 in floodwater and in internodal lacunae of floating rice growing at 1–2 meter water depths. Plant, Cell and Environment 10: 767–776. [Google Scholar]
- Sonnewald U. 1992. Expression of E. coli inorganic pyrophosphatase in transgenic plants alters photoassimilate partitioning in leaves. Plant Journal 2: 571–581. [PubMed] [Google Scholar]
- Stitt M. 1982. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts (Triticum aestivum). Plant Physiology 70: 971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. 1990. Fructose-2,6-bisphosphate as a regulatory molecule in plants. Annual Review of Plant Physiology and Plant Molecular Biology 41: 153–185. [Google Scholar]
- Stitt M. 1998. Pyrophosphate as an energy donor in the cytosol of plant cells: an enigmatic alternative to ATP. Botanica Acta 111: 167–175. [Google Scholar]
- Stitt M, Cseke C, Buchanan BB. 1984. Regulation of fructose 2,6-bisphosphate concentration in spinach leaves. European Journal of Biochemistry 143: 89–93. [DOI] [PubMed] [Google Scholar]
- Theodorou ME, Plaxton WC. 1996. Purification and characterization of pyrophosphate-dependent phosphofructokinase from phosphate-starved Brassica nigra suspension cells. Plant Physiology 112: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorou ME, Cornel FA, Duff SM, Plaxton WC. 1992. Phosphate starvation-inducible synthesis of the alpha-subunit of the pyrophosphate-dependent phosphofructokinase in black mustard suspension cells. Journal of Biological Chemistry 267: 21901–21905. [PubMed] [Google Scholar]
- Umbreit WW, Burris RH, Stauffer JW. 1951.Manometric techniques and tissue metabolism. Burgess Publishing Co., Minneapolis, Minnesota. [Google Scholar]
- Vartapetian BB, Jackson MB. 1997. Plant adaptions to anaerobic stress. Annals of Botany 79: 3–20. [Google Scholar]
- Weiner H, Stitt M, Heldt HW. 1987. Subcellular compartmentalization of pyrophosphate and alkaline pyrophosphatase in leaves. Biochimica and Biophysica Acta 893: 13–21. [Google Scholar]