Abstract
• Background and Aims This paper examines the basis of the greater tolerance of an indica rice cultivar FR13A to complete submergence compared with relatively intolerant japonica rice CT6241. We study whether this superior tolerance is related to its greater tolerance to O2 shortage and to an ability to run a more favourable rate of alcoholic fermentation during and after O2 deprivation.
• Methods Fermentation products were analysed using sensitive laser-based photoacoustics at high time resolution to establish patterns and rates of ethanol and acetaldehyde emission by intact rice seedlings exposed to micro-aerobic (0·05–0·5 % O2) or zero O2 supply, and also during their return to air. Oxygen and CO2 emission or uptake was also quantified.
• Key Results In the dark, no acetaldehyde and ethanol emission was observed until external O2 concentration in a gas phase decreased to ≤0·3 % O2. The ethanol production rate was maximal in 0 % O2, similar in both cultivars and gradually diminished with increasing O2 concentration. Lag time for induction of fermentation increased with O2 up to 0·3 % and was shorter in CT6241. Light strongly suppressed fermentation. In contrast to that of ethanol, emission of acetaldehyde in the dark under micro-aerobic conditions (≤0·15 % O2 gas phase) exceeded that under anaerobiosis, was maximal in 0·05 % O2 and was greater in FR13A than in CT6241. A drop in acetaldehyde emission to about half its value immediately followed a switch to anaerobic conditions after 6·5 h treatment under 0·05 % O2, while ethanol release showed a further increase. A large peak in acetaldehyde emission immediately followed the return of seedlings to air after treatment with ≤0·15 % O2. The emission from FR13A was up to three times larger than from CT6241.
• Conclusions Tolerance to submergence in FR13A appears not to be connected to its rate of ethanol production during anaerobiosis, but to the increased acetaldehyde output during and after experiencing micro-aerobic conditions (0·05–0·15 % O2). Extra acetaldehyde production from ethanol may be a consequence of diversion of the reactive oxygen species away from the damaging lipid peroxidation pathway.
Keywords: Rice, Oryza sativa, anaerobiosis, alcoholic fermentation, hypoxia, post-anoxia, acetaldehyde, ethanol, carbon dioxide, oxygen, lipid peroxidation, stress, trace gas detection
INTRODUCTION
During complete submergence, limited gas diffusion and low light intensity in floodwater are considered to be the most important factors causing injury and fatality to rice seedlings (Setter et al., 1997). Under these conditions, O2 shortage may impose an ‘energy crisis’ (Greenway et al., 1996), as respiration switches to less efficient energy-providing alcoholic fermentation in response to slow inward diffusion and low and sometimes near-anaerobic levels of dissolved O2 (Setter et al., 1987; Ito et al., 1999). Other factors that contribute to submergence injury of rice plants include loss of carbohydrate reserves (Ram et al., 2002) and lack of internal O2 formation resulting from inhibition of photosynthesis caused by a lack of light and CO2. In accordance with this, illumination is known to enhance tolerance in rice (e.g. Boamfa et al., 2003), with irradiances as low as 20 µmol m−2 s−1 (Vervuren et al., 2003) being effective in this way. Similarly, submergence in the morning when carbohydrate reserves are already low is especially damaging (Ram et al., 2002; Santosa, 2002). The cultivar FR13A is known to tolerate complete submergence better than most others. This resilience may, in part, be the result of a downregulation of energy consumption during submergence (e.g. avoidance of elongation) and of a suppression of leaf senescence underwater (Jackson and Ram, 2003). Thus, in addition to differences in energy provision and availability of respiratory substrates, more prudent use of available energy may also distinguish submergence-tolerant from submergence-susceptible genotypes. However, the causes of submergence injury and the tolerance mechanism of FR13A are not yet fully understood.
Currently, it is unclear whether a faster or slower rate of fermentation leads to longer survival in the complete absence of O2. It is also not clear if damage from anoxia is actually the main cause of submergence injury or if the contrasting tolerance of certain cultivars of rice such as FR13A and CT6241 (Jackson and Ram, 2002) depends on differences in anoxia tolerance. These doubts spring from observations that CT6241 and other susceptible lines remain more intolerant to submergence than FR13A even when the submergence water contains O2 (Jackson et al., 1987; Ram et al., 1999). Furthermore, using a post-anoxic burst of acetaldehyde production as a marker for the presence of anoxic tissue, Boamfa et al. (2003) found no evidence of anoxic tissue in either cultivar under submergence treatments with initially O2-containing water, though leaves were damaged. Nevertheless, Ellis and Setter (1999) have demonstrated that a superior tolerance of anaerobic conditions by FR13A compared with CT6241 does exist, thus generating the need to re-examine the fermentation kinetics of these lines under different levels of O2 availability.
Our main aim was to examine if inherently different rates of fermentation during anaerobiosis characterize submergence-tolerant and -intolerant rice plants. We were also interested in identifying any differences in the lowest external O2 concentration at which fermentation begins. A third aim was to assess the possible importance of partial O2 shortage since this is a characteristic of submergence conditions in which differential sensitivities of FR13A and CT6241 are strongly expressed (Jackson et al., 1987; Ram et al., 1999). It is often stated that plants deprived of O2 become susceptible to oxidation damage, especially when re-exposed to air (Blokhina et al., 2003). Accordingly, we also tried to explore this possibility in our experiments. For our analyses of trace gases, we used a highly sensitive laser-based trace gas detector to compare alcoholic fermentation rates of FR13A and CT6241 seedlings exposed to O2-free gas phase conditions and to the micro-aerobic (0·05–0·5 % O2) surroundings more typical of submerged conditions. Post-stress acetaldehyde emission was also compared for the two genotypes to mark the severity of preceding O2-deficient conditions and appraise post-stress biochemistry. Acetaldehyde production has a double significance. On the one hand, it serves as the precursor of ethanol in alcoholic fermentation, while it can also be generated as a product of H2O2 removal via catalase action involving ethanol (Monk et al., 1987). This enhances the value of acetaldehyde as a diagnostic probe.
MATERIALS AND METHODS
Plant material, germination and plant culture
Seeds of the submergence-tolerant Oryza sativa L. ‘FR13A’, an indica rice, and ‘CT6241’, a susceptible line of japonica rice, were kindly supplied by Dr S. Sarkarung, IRRI Thailand Office, Bangkok, Thailand. Germination and plant culture were performed as described by Boamfa et al. (2003). Rice seeds were surface sterilized with 1 % w/v sodium hypochlorite solution for 10 min, washed under running tap water for 5 min and placed in 110 mm diameter glass Petri dishes lined with filter paper wetted with 15 mL of tap water. The Petri dishes were placed in the dark at 30 °C and 65 % relative humidity. Sprouted seedlings with 1 cm long coleoptiles were transferred to culture trays (30 × 20 × 15 mm) filled with black Lacqtene low-density polyethylene grains and nutrient solution (Yoshida, 1976), pH 5·0. The pH of the solution was adjusted regularly and the nutrient solution was replaced on every fourth day. The culture trays were kept aerated with air flowing through perforated silicone tubing placed across the bottom of the tray. The plants were grown under a 12 h light/12 h dark regime of 28/22 °C (PPFD: 500 µmol m−2 s−1, Philips SON-T Agro400 source) and a relative humidity of 60–65 %. Typical shoot : root ratios (fresh weight) at the start of experiments were 1·72 ± 0·06 (FR13A) and 1·37 ± 0·04 (CT6241).
On-line detection of acetaldehyde, ethanol and carbon dioxide
Acetaldehyde and ethanol concentrations down to the nL L−1 level (0·1 nL L−1 for acetaldehyde and 3 nL L−1 for ethanol), released by young FR13A and CT6241 rice seedlings into the gas flowing by, were measured with a laser-based photoacoustic trace gas detector (Bijnen et al., 1996). The photoacoustic signals correspond to release rates, in the case of ethanol possibly influenced by tissue storage of the produced gas. This possible storage effect for ethanol is not relevant for the comparison of the signals from the two different cultivars because in both cases the tissue response can be assumed to be equal. The system used in the present study was similar to that described in Boamfa et al. (2003) and was equipped with three detection cells capable of monitoring three independent samples simultaneously. The residency time of gas in the photoacoustic detector was approx. 40 s using flow rates of 2–5 L h−l.
Carbon dioxide and O2 were also monitored in real time, simultaneously with acetaldehyde and ethanol, using a commercial CO2 infrared analyser (URAS 14, Hartmann & Braun, Frankfurt, Germany; detection limit: 1 µL L−1 CO2) in which an electrochemical O2 sensor (detection limit: 0·01 % O2) was incorporated.
Trace gas measurement procedure
Anaerobic, micro-aerobic (0·05–0·5 % O2) and aerobic gas phase conditions were imposed on 14-d-old seedlings of FR13A and CT6241 rice genotypes, using gas flows comprising air, N2 and mixtures of air with N2 as appropriate. For each measurement, batches of three plants per cuvette were used to generate more easily detectable amounts of gas and to minimize effects of differences between individual plants. The fresh weight of plants was measured just before each experiment and was approx. 0·35 g per seedling for FR13A and 0·2 g per seedling for CT6241. The seedlings were placed in a glass cuvette (300 mL) with the roots in 25 mL of full strength nutrient solution. The inlet to the cuvette allowed various gas flow treatments (air, N2 or gas containing low O2 concentrations flowing at 2 L h−1). The outlet flow was divided into two. One gas line was connected to the laser-based detector and the other to the CO2 and O2 analysers. In this way, we monitored on-line and simultaneously the trace gases relevant to fermentation. For the micro-aerobic treatments, flows of air and N2 were mixed in different proportions using mass flow controllers to obtain the desired concentrations of O2. At the end of the measurement, plants were transferred back to the culture trays for recovery and 7 d later were scored for survival and injury. Anaerobic, micro-aerobic and aerobic experiments in the dark were performed at approx. 22 °C. When treatments were made in the light, the contents of the cuvette were unavoidably warmed by the lamps to approx. 27 °C, while maintaining an irradiance of 500 µmol m−2 s−1. Every experiment was repeated four or five times.
Statistical analysis
Student t-test (P < 0·05) was used to compare FR13A and CT6241 emissions of acetaldehyde, ethanol and CO2 for each treatment and to determine the significance of differences between means at various time points.
RESULTS
Anaerobic responses
Emission of acetaldehyde and ethanol was recorded from intact seedlings of the submergence-tolerant FR13A and submergence-susceptible CT6241 rice genotypes exposed to an O2-free external gas (N2) for 1, 2, 4, 6, 8, 10 and 14 h in the dark, and for 2 and 12 h in the light (500 µmol m−2 s−1). Carbon dioxide emissions were recorded after 4 and 14 h without O2. After switching from air to N2 gas flow, O2 in the cuvette declined from 21 to <0·05 % within 15 min. Each on-line analysis was repeated on four or five different occasions. Production rates at the end of the anaerobic periods are shown in Table 1. In the dark, as little as 1 h without O2 increased the output of ethanol and acetaldehyde, with formation of the latter already at its maximum at about this time. In contrast, ethanol output rose steadily over 8 h before stabilizing. Ethanol emission always exceeded acetaldehyde production 10- to 20-fold, with overall rates of production being similar in both cultivars. Illumination suppressed ethanol and acetaldehyde output strongly. Under light, ethanol emission after 12 h anaerobic treatment was only approx. 20 % of the rate in darkness for FR13A and 30 % for CT6241. Thus, light decreased alcoholic fermentation by 70–80 % when the seedlings were exposed to an external O2 free gas phase. Anaerobic conditions decreased CO2 production within 30 min and, overall, by approximately two-thirds in the dark. In the light, there was no CO2 output in an N2 atmosphere, indicating photosynthetic fixation of most if not all respiratory CO2.
Table 1.
Effect of up to 14 h anaerobic treatment in the dark or in the light on rates of acetaldehyde, ethanol and CO2 production by batches of three 14-d-old FR13A and CT6241 rice seedlings, measured at the end of the anaerobic period
| Production rates at the end of anaerobiosis (μL h−1g−1 f. wt) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetaldehyde |
Ethanol |
CO2 |
||||||||||
| Duration of anaerobiosis |
FR13A |
CT6241 |
FR13A |
CT6241 |
FR13A |
CT6241 |
||||||
| Dark | ||||||||||||
| 0 h | 0·040 ± 0·013a | 0·05 ± 0·01a | 0·27 ± 0·06i | 0·35 ± 0·05i | 300 ± 30q | 285 ± 10q | ||||||
| 1 h | 1·10 ± 0·05b | 1·45 ± 0·07B | 2·40 ± 0·04j | 2·8 ± 0·3j | ||||||||
| 2 h | 1·00 ± 0·08c | 2·00 ± 0·03C | 20·9 ± 0·2k | 11·3 ± 1·0K | ||||||||
| 4 h | 0·95 ± 0·06d | 0·90 ± 0·11d | 17·0 ± 1·3l | 15·5 ± 1·8l | 120 ± 15r | 95 ± 7r | ||||||
| 6 h | 0·55 ± 0·02e | 0·52 ± 0·04e | 26 ± 2m | 22 ± 3m | ||||||||
| 8 h | 0·90 ± 0·13f | 0·70 ± 0·13f | 55 ± 5n | 55 ± 6n | ||||||||
| 10 h | 1·07 ± 0·07g | 1·7 ± 0·3G | 43 ± 4o | 47 ± 1o | ||||||||
| 14 h | 0·90 ± 0·13h | 1·4 ± 0·3h | 40 ± 9p | 39 ± 6p | 100 ± 15s | 90 ± 5s | ||||||
| Light (500 µmol m−2 s−1) | ||||||||||||
| 2 h | 0·075 ± 0·005x | 0·19 ± 0·02X | 2·20 ± 0·13t | 2·5 ± 0·2t | Below detection | Below detection | ||||||
| 12 h | 0·050 ± 0·013y | 0·10 ± 0·02y | 8·0 ± 0·9u | 11 ± 1U | Below detection | Below detection | ||||||
All values are means with standard errors of 4–5 individual experiments. For each volatile at each time point, means for the two cultivars showing similar superscript letters are not significantly different at P < 0·05 (Student's t-test).
The data for FR13A were already presented in Table 2 of an earlier paper (Boamfa et al., 2003).
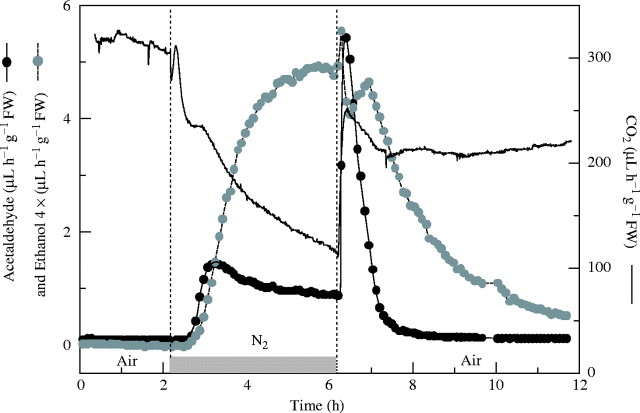
Figure 1 presents detailed kinetics of the alcoholic fermentation products, acetaldehyde, ethanol and CO2, emitted by CT6241 exposed to 4 h anaerobic treatment followed by a 4 h re-aeration period. A single measurement was selected out of four or five independent experiments for this illustration. Acetaldehyde emission began within 0·5 h of imposing anaerobiosis, followed 10 min later by ethanol, irrespective of the illumination conditions (dark or light). Acetaldehyde output increased quickly under O2-free conditions in the dark, and reached a maximum in both genotypes (1·1 µL h−1 g−1 f. wt for FR13A after 1 h of treatment, and 2 µL h−1 g−1 f. wt for CT6241) after 2 h. In contrast, CO2 output declined within 0·5 h of starting the O2-free treatment, and within 1 h CO2 output decreased by 30 % and after 14 h by 60 %. Aerobic CO2 production was similar for both genotypes, approx. 300 µL h−1 g−1 f. wt. Figure 1 and Table 2 reveal marked changes in acetaldehyde and ethanol formation when air is re-admitted after 1–14 h without O2. The basic effect was a strong decrease in ethanol emission beginning almost immediately, accompanied by a marked temporary upsurge in acetaldehyde production peaking only 10 min after the re-introduction of O2. The post-anoxic acetaldehyde production was similar for the two genotypes. Subsequently, the acetaldehyde emission rate decreased, reaching the initial aerobic rate approx. 2·5 h after the re-introduction of air. When the anaerobic treatment was lengthened to >6 h, the integral value and the peak height of the post-anoxic acetaldehyde outburst increased and was no longer characterized by a clear defined peak in output. Instead, production remained elevated for periods extending beyond the period of monitoring (Boamfa et al., 2003). Under illumination, the post-anoxic acetaldehyde outburst was much smaller compared with dark conditions. For example, it was 6-fold slower after 2 h of anaerobiosis and 3-fold slower after 12 h of anaerobiosis, for each genotype (Table 2).
Fig. 1.
Effect of 4 h anaerobic treatment and subsequent relief from anaerobiosis on patterns of ethanol (grey circles), acetaldehyde (black circles) and CO2 (black line) emissions from single batches of three 14-d-old CT6241 rice seedlings. At t = 0 h, plants were placed in air in the dark. At t = 2·1 h, they were given an anaerobic treatment for 4 h (light grey bar) followed by a 4 h re-aeration period. One representative measurement selected from four or five independent experiments is shown.
Table 2.
Effect of up to 14 h anaerobic treatment in the dark or in the light on the increase of acetaldehyde and CO2 production by batches of three 14-d-old FR13A and CT6241 rice plants, measured during the recovery in air
| Post-anaerobiosis upsurge (μL h−1 g−1 f wt.) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Duration of anaerobiosis |
Acetaldehyde |
CO2 |
||||||
| FR13A |
CT6241 |
FR13A |
CT6241 |
|||||
| Dark | ||||||||
| 1 h | 0·5 ± 0·02a | 1·6 ± 0·03A | ||||||
| 2 h | 2·5 ± 0·5b | 3·2 ± 0·13b | ||||||
| 4 h | 1·4 ± 0·3c | 4·7 ± 0·7C | 51 ± 0·9h | 145 ± 13H | ||||
| 6 h | 2·0 ± 0·2d | 2·0 ± 0·8d | ||||||
| 8 h | 3·5 ± 0·4e | 4·3 ± 0·5e | ||||||
| 10 h | 3·3 ± 0·8f | 3·0 ± 0·27f | ||||||
| 14 h | 4·6 ± 0·8g | 2·3 ± 0·6G | 21 ± 2·0i | 25 ± 1·0i | ||||
| Light (500 µmol m−2 s−1) | ||||||||
| 2 h | 0·25 ± 0·05x | 0·4 ± 0·2x | ||||||
| 12 h | 1·4 ± 0·7y | 1·0 ± 0·6y | ||||||
The post-anaerobic increase in acetaldehyde and CO2 was calculated as the difference between the post-anaerobic peak values and the production rates at the end of anaerobic treatment. All values are means with standard errors of 4–5 individual experiments. For each volatile at each time point, means for the two cultivars showing similar superscript letters are not significantly different at P < 0·05 (Student's t-test).
Simultaneously with the post-anoxic release of acetaldehyde, an upsurge in CO2 production was observed after an anaerobic dark treatment (Fig. 1). The peak in CO2 output corresponds to that for acetaldehyde (10 min after re-exposure to air) and was similar in size for both genotypes. The post-anoxic outburst of CO2 is calculated as the difference between the maximum value in post-anoxia and the CO2 emission rate at the end of anaerobic treatment. After 4 h without O2, post-anoxic CO2 output peaked at 51 µL h−1 g−1 f. wt for FR13A and 145 µL h−1 g−1 f. wt for CT6241, while after 14 h of nitrogen treatment, the values were 21 µL h−1 g−1 f. wt for FR13A and 25 µL h−1 g−1 f. wt for CT6241 (Table 2).
Micro-aerobic responses in the dark
Completely submerged rice plants are thought normally to experience anoxia only in extreme conditions, for example during the night, especially in roots, in the day when the water is very turbid, or after extended periods of flooding. Mostly, floodwater is not completely anaerobic and contains some O2, and yet FR13A still demonstrates more tolerance than CT6241 and other susceptible lines such as CT6241 and IR42 (Jackson et al., 1987; Ram et al., 1999). In field conditions, it was found that the submerging water could even be super-oxygenated but remained damaging (Ram et al., 1999, 2002). Since no clear differences in the fermentation rates between FR13A and CT6241 under gas phase anaerobiosis were found, we investigated whether the tolerance to submergence in rice is better correlated with the fermentation outputs when seedlings are exposed to the partially O2-deficient conditions that are more prevalent in the rice fields.
Accordingly, seedlings of submergence-tolerant FR13A and submergence-susceptible CT6241 were exposed for 8 h to mixtures of air and N2 gas containing low O2 concentrations ranging from 0·05 to 21 %. These experiments were performed in the dark to avoid interference from O2 produced by photosynthesis. An important issue was to identify if the external O2 concentration at which fermentation starts was different in each genotype. However, no such difference was seen and in both cultivars no fermentation took place until O2 in the gas flow was decreased to 0·3 % O2 or below, during an 8 h exposure (Table 3). This indicates how little external O2, in a gas phase, is needed to prevent anaerobic fermentation by any part of the rice seedlings. The lag phase for acetaldehyde and ethanol emissions shortened from >6 h in 0·3 % O2 to <1 h in zero O2 (Fig. 2) and was always longer in FR13A than CT6241. For example, when exposed to 0·05 % O2, fermentation by FR13A commenced after a delay of approx. 2·5 h as against only 1 h in CT6241.
Table 3.
Effect of 8 h anaerobic, micro-aerobic and aerobic treatment in the dark, on the rates of production for acetaldehyde, ethanol and CO2 by batches of three 14-d-old FR13A and CT6241 rice plants, measured at the end of the treatment
| Acetaldehyde (μL h−1 g−1 f. wt) |
Ethanol (μL h−1 g−1 f. wt) |
CO2 (μL h−1 g−1 f. wt) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| O2 (%) |
FR13A |
CT6241 |
FR13A |
CT6241 |
FR13A |
CT6241 |
|||
| 0·00 | 0·90 ± 0·13a | 0·52 ± 0·04A | 55 ± 6j | 55 ± 5j | 115 ± 16t | 93 ± 20t | |||
| 0·05 | 5·5 ± 1·0b | 3·40 ± 0·15B | 47 ± 9k | 46 ± 2k | 150 ± 40u | 193 ± 2u | |||
| 0·10 | 4·4 ± 0·4c | 3·1 ± 0·5c | 31 ± 2l | 31 ± 4l | 100 ± 7v | 154 ± 13V | |||
| 0·15 | 2·60 ± 0·16d | 1·60 ± 0·10D | 9·8 ± 1·9m | 14·0 ± 0·7M | 124 ± 8x | 163 ± 11X | |||
| 0·20 | 0·77 ± 0·12e | 0·64 ± 0·10e | 3·2 ± 0·3N | 6·0 ± 1·3n | 140 ± 6y | 149 ± 8y | |||
| 0·25 | 0·80 ± 0·14f | 0·33 ± 0·09F | 5·8 ± 0·1o | 4·5 ° 0·8o | 115 ± 9z | 180 ± 40z | |||
| 0·30 | 0·35 ± 0·05g | 0·14 ± 0·05G | 1·43 ± 0·06p | 2·30 ± 0·08P | 138 ± 11w | 156 ± 9w | |||
| 0·50 | 0·10 ± 0·02h | 0·10 ± 0·02h | 0·86 ± 0·15r | 0·9 ± 0·1r | 117 ± 8q | 102 ± 10q | |||
| 20·9 | 0·14 ± 0·04i | 0·10 ± 0·02i | 0·75 ± 0·04s | 0·75 ± 0·04s | 140 ± 20a | 201 ± 6A | |||
All values are means with standard errors of 4–5 individual experiments. For each volatile at each O2 concentration, means for the two cultivars showing different superscript letters are significantly different at P < 0·05 (Student's t-test).
Fig. 2.
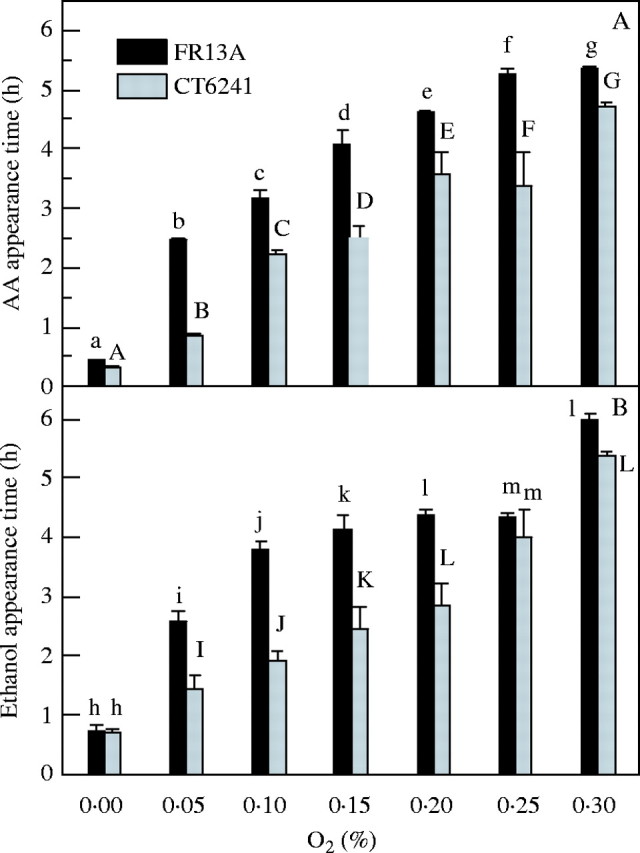
Effect of 8 h anaerobic (0 % O2) and micro-aerobic treatment (0.05–0.3 % O2) in the dark, on the appearance time (in hours) of acetaldehyde (A) and ethanol (B) in batches of three 14-d-old FR13A (black bars) and CT6241 (grey bars) rice seedlings. All values are means with standard errors of 4–5 individual experiments. Means for each cultivar at each O2 concentration that show different superscript letters are significantly different at P < 0.05 (Student's t-test).
The dynamic behaviour of acetaldehyde release during micro-aerobic treatments between 0·05 and 0·15 % O2 differed markedly from that of ethanol (Fig. 3). While ethanol formation decreased with increasing input of O2, acetaldehyde output rose strongly in 0·05–0·15 % O2 and came to exceed those seen in anaerobic conditions. Thus, after 8 h in 0·05 % O2, acetaldehyde production rates were six times the anaerobic rate in FR13A and 4·6 times the anaerobic rate in CT6241 (Table 3). Acetaldehyde formation in 0·1 and 0·15 % O2 was also larger than the anaerobic rate. Only when O2 was raised to >0·2 % did acetaldehyde release decrease to anaerobic levels. No acetaldehyde emission above background was observed for concentrations >0·3 % O2.
Fig. 3.
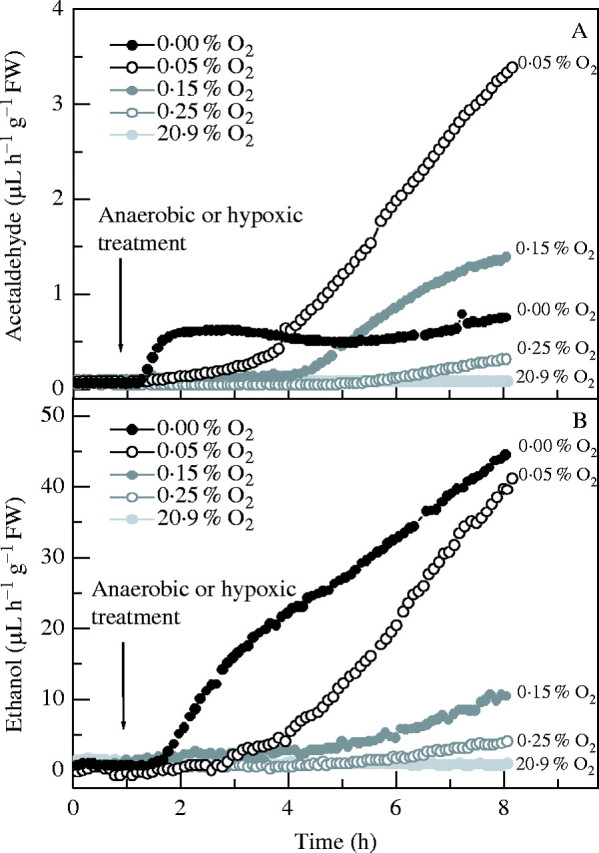
Effect of 8 h aerobic (closed light grey circles), anaerobic (closed black circles) and micro-aerobic treatments (0.05 %, open black circles; 0.15 %, closed grey circles; 0.25 % O2, open grey circles), in the dark, on patterns of acetaldehyde (A) and ethanol (B) emissions from single batches of three 14-d-old CT6241 rice seedlings. For each O2 concentration treatment, one representative measurement selected from four or five independent experiments is shown.
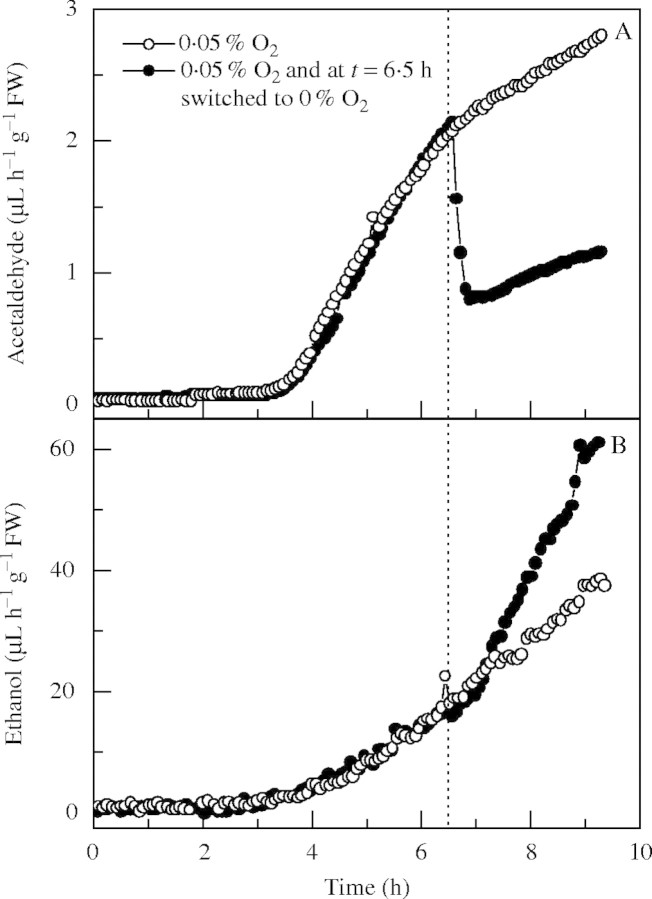
For further clarification, FR13A seedlings were first exposed in the dark to a gas flow containing 0·05 % O2, but after 6·5 h a flow of O2-free gas (N2) replaced it (Fig. 4). At the time of the switch from 0·05 % O2 to 0 % O2, acetaldehyde production immediately dropped from 2 µL h−1 g−1 f. wt to a stable 1 µL h−1 g−1 f. wt while ethanol release started to rise steeply. During this experiment, CO2 output showed an upsurge just after the switch to pure N2 (data not shown). This result shows that a complete absence of O2 immediately represses micro-aerobic acetaldehyde production.
Fig. 4.
Effect of 6.5 h of 0.05 % O2 micro-aerobic treatment in the dark, followed by exposure to N2 (filled circles), on patterns of acetaldehyde (A) and ethanol (B) emission from single batches of three 14-d-old FR13A rice seedlings. Micro-aerobic treatments started at t = 0 h by flushing the cuvettes with a 2 L h−1 gas flow (mixture of air and N2). At t = 6.5 h, the plants were exposed to a flow of N2 (filled circles). A control measurement with seedlings exposed for 9 h to 0.05 % O2 is also shown (open circles).
Post-micro-aerobic responses
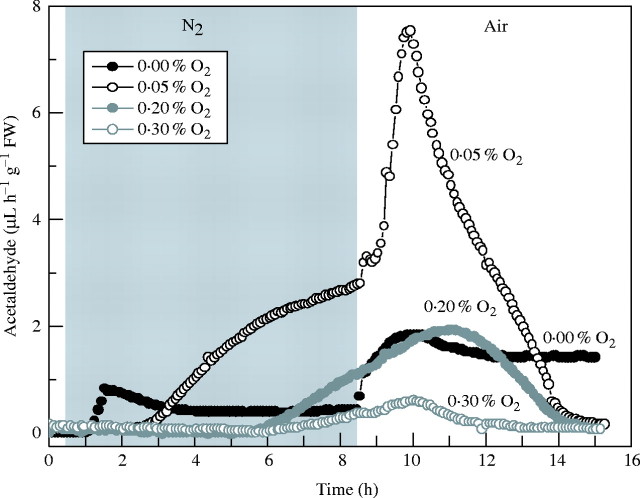
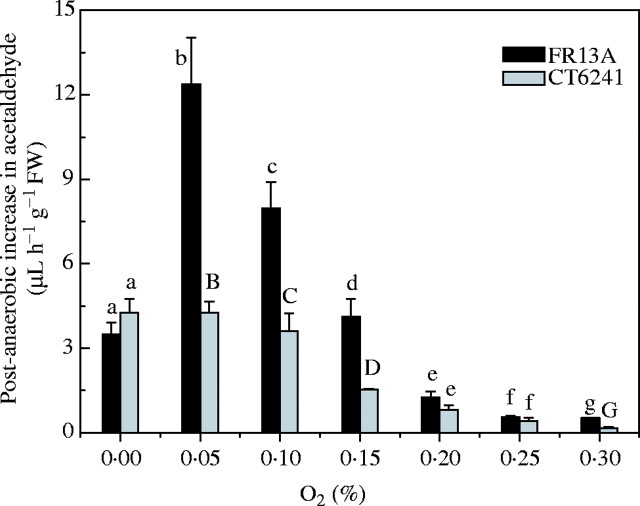
As with anoxically pre-treated seedlings, re-introduction of air after 8 h micro-aerobic pre-treatment resulted in a temporary upsurge of acetaldehyde emission, exemplified in Fig. 5 by FR13A. However, its magnitude was much greater after exposure to 0·1 or 0·05 % O2 than after anaerobiosis. Only when O2 levels were raised further to 0·2 or 0·3 % did acetaldehyde output fall to below anaerobic levels (Fig. 6). The size of the post-micro-aerobic acetaldehyde peak was much greater for FR13A compared with CT6241. For example, a maximum rate of 4·2 µL h−1 g−1 f. wt was recorded for CT6241, after exposure to 8 h of 0·05 % O2, while FR13A presented a sharper peak, with a maximum of 12·4 µL h−1 g−1 f. wt (Fig. 6).
Fig. 5.
Effect of 8 h anaerobic (filled black circles) and micro-aerobic treatments (0.05 % O2, open black circles; 0.2 % O2, filled grey circles; 0.3 % O2, open grey circles) and subsequent recovery in air, in the dark, on patterns of acetaldehyde emission from single batches of three 14-d-old FR13A rice seedlings. Anaerobic and micro-aerobic treatments started at t = 0.5 h, by flushing the cuvettes with a 2 L h−1 gas flow (N2 or a mixture of air and N2). After 8 h, the plants were returned to a flow of air (2 L h−1). For each O2 concentration, one representative measurement selected from four or five independent experiments is shown.
Fig. 6.
Effect of 8 h anaerobic (0 % O2) and micro-aerobic treatment (0.05–0.3 % O2) in the dark on the increase in acetaldehyde production by batches of three 14-d-old FR13A (black bars) and CT6241 (grey bars) rice seedlings, during recovery in air. The post-anaerobic increase in acetaldehyde was calculated as the difference between the post-anaerobic peak values and the production rates at the end of anaerobic treatment. All values are means with standard errors of 4–5 individual experiments. Means for each cultivar at each O2 concentration that show different superscript letters are significantly different at P < 0.05 (Student's t-test).
Plant survival rates and leaf injury were investigated 7 d after anaerobic and micro-aerobic treatments. In all the cases, survival was 100 % for both rice genotypes, but leaves showed different levels of injury. After 8 h anaerobic treatment, the area of leaf damage (foliar dehydration and necrosis) was approx. 20 % for FR13A and 35 % for CT6241. Leaf damage was less after micro-aerobic treatments, with CT6241 always suffering approx. 15 % more leaf damage on an area basis than FR13A.
DISCUSSION
In our previous work (Boamfa et al., 2003), we used the post-anoxic burst of acetaldehyde as a diagnostic marker of the existence of anoxic tissue prior to re-exposure to air. We noted then that submergence in initially O2-containing water could damage rice seedlings in the absence of any significant acetaldehyde and ethanol signal at de-submergence. In particular, no post-submergence peak of acetaldehyde was observed. The leaf damage was about twice as large for CT6241 as for FR13A. This indicated that submergence injury and the difference in susceptibility between FR13A and CT6241 are not necessarily linked to effects of anoxia. The absence of a post-stress acetaldehyde peak may indicate that internal O2 concentrations did not fall below the value needed to initiate fermentation under water. However, the occurrence of damage under these presumably non-anoxic conditions left open the question of the non-fermentative origin of this damage. These earlier results (Boamfa et al., 2003) did not help to resolve the basis of the difference in submergence tolerance between the two cultivars nor did they explain how non-anaerobic submergence damage could arise. Further work was clearly needed to confirm the findings and examine the alternatives. Accordingly, we exposed rice seedlings to a wider range of O2-deficient gas phase conditions while monitoring their impact on fermentation by measuring the output of ethanol, acetaldehyde and CO2. These treatments more realistically represented the extent of O2 deprivation during submergence in the field.
Both cultivars showed remarkably similar responses to the complete absence of O2. Evidence of fermentation was seen within 1 h of imposing these conditions in the dark, with an earlier plateau for acetaldehyde that was released in much smaller amounts than was ethanol. Since, for technical reasons, it took approx. 30 min for all O2 to be removed from the plants' surroundings, the fermentation reaction time may actually be shorter than 30 min. The rise in acetaldehyde preceded that of ethanol by approx. 10 min, confirming our earlier findings. Light strongly suppressed these emissions but did not eliminate them entirely, indicating that photosynthetic O2 was unable to oxygenate the entire seedling. Fermentation was associated with a large depression of CO2 output. However, all these detailed features of anaerobic performance were similar in FR13A and CT6241. This confirmed that the greater submergence tolerance of FR13A does not necessarily depend on a differential fermentation response to anoxia, and that submergence damage to rice is not necessarily a question of a response to anoxia. However, the possibility remained that differences in submergence tolerance between FR13A and CT6241 may have something to do with responses to partial O2 shortage.
Small increases in O2 concentration above zero had effects on ethanol emission very similar to illuminating the plant, i.e. they suppressed fermentation. As external O2 was increased, the onset of fermentation was increasingly delayed and ethanol emissions decreased (Fig. 3B), yielding 85 % of the anaerobic production rate at 0·05 % O2, and only 3·6 % at 0·3 % external O2. Thus, O2 concentrations below 0·3 % are required in the gas phase to initiate alcoholic fermentation and, judging from the CO2 output data, to inhibit normal aerobic respiration via the Krebs' cycle. Although under micro-aerobic conditions ≤0·3 % O2 CT6241 began fermenting >1 h earlier than FR13A (Fig. 2), the actual rates of ethanol production of the two genotypes were very similar, after 8 h (Table 3). In contrast, the picture obtained for acetaldehyde was very different from that for ethanol. Surprisingly, in micro-aerobic conditions ≤0·2 % O2, acetaldehyde emission was much stronger than from seedlings exposed to an O2-free environment. At the same time, ethanol production slowed considerably (Table 3). During these micro-aerobic conditions, a steady rise of acetaldehyde was observed until at least the end of the 8 h treatment (Fig. 3A). The almost instantaneous inhibition by imposing anaerobic environment indicates that fermentation is not the direct source of the acetaldehyde. A further strong enhancement of acetaldehyde formation was seen to take place when micro-aerobic seedlings were returned to air. This post-micro-aerobic effect is reminiscent of that seen when plants are re-aerated after anoxia, but occurs on a much larger scale. This effect was particularly marked after re-aerating plants given 0·05 % O2 but was also evident after giving 0·1 % O2. This appears to be the first report that small amounts of O2 promote acetaldehyde formation to rates substantially above those of anoxic tissue both during the exposure and after a return to air. The apparent divergence of ethanol and acetaldehyde under these treatments suggests that a non-fermentative pathway for acetaldehyde formation is involved.
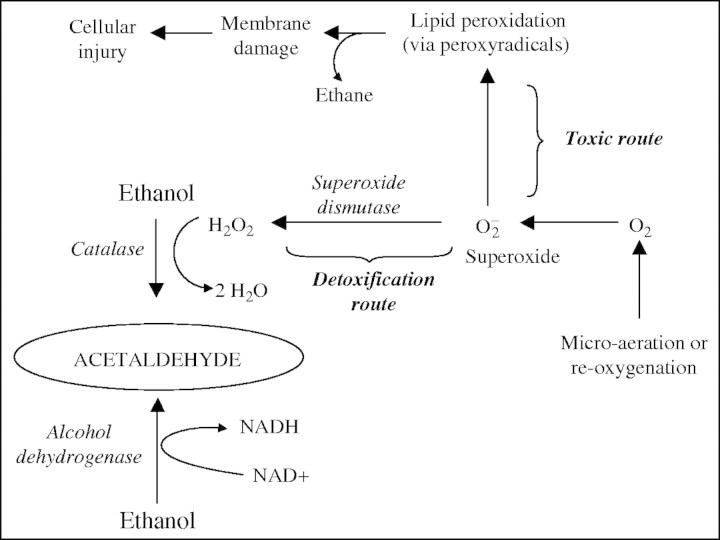
An immediate upsurge in acetaldehyde release after re-exposure of anaerobic rice seedlings to air has been reported before (Boamfa et al., 2003). It is also a feature of post-anaerobic tissue of other species (Cossins, 1978; Monk et al., 1987; Zuckermann et al., 1997) and is likely to be the outcome of the oxidation of ethanol to acetaldehyde. This is achievable either by NAD+-dependent back-conversion of ethanol to acetaldehyde, catalysed by alcohol dehydrogenase, or by an H2O2-dependent oxidation of ethanol that is promoted by catalase, an enzyme that can increase in activity under these circumstances (Garnczarska et al., 2004). The H2O2 is thought to have superoxide radicals (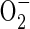 ) as its source. These originate from the incoming O2 probably in mitochondria, which produce
) as its source. These originate from the incoming O2 probably in mitochondria, which produce 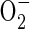 via NADH and reduced ubiquinone (Møller, 2001). The superoxide is then susceptible to conversion to H2O2 by the activity of superoxide dismutase (Monk et al., 1987). Zuckermann et al. (1997) suggested that insufficient NAD+ would be available to alcohol dehydrogenase during the first minutes after re-exposure of rice plants to air to support increased formation of acetaldehyde. The alternative catalase-based reaction using H2O2 to oxidize ethanol is therefore the more likely route for acetaldehyde synthesis. Indirect experimental evidence that reactive oxygen species (ROS), the putative source of the H2O2, are present in post-anaerobic tissues of rice has come from results showing a post-anaerobic release of ethane (C2H6) by seedlings grown under the same conditions used in the present experiments (Santosa, 2002). Supporting evidence is also available from the electron spin resonance studies of Thongbai and Goodman (2000). Ethane is a marker for lipid peroxidation of polyunsaturated fatty acids by free radicals such as superoxide
via NADH and reduced ubiquinone (Møller, 2001). The superoxide is then susceptible to conversion to H2O2 by the activity of superoxide dismutase (Monk et al., 1987). Zuckermann et al. (1997) suggested that insufficient NAD+ would be available to alcohol dehydrogenase during the first minutes after re-exposure of rice plants to air to support increased formation of acetaldehyde. The alternative catalase-based reaction using H2O2 to oxidize ethanol is therefore the more likely route for acetaldehyde synthesis. Indirect experimental evidence that reactive oxygen species (ROS), the putative source of the H2O2, are present in post-anaerobic tissues of rice has come from results showing a post-anaerobic release of ethane (C2H6) by seedlings grown under the same conditions used in the present experiments (Santosa, 2002). Supporting evidence is also available from the electron spin resonance studies of Thongbai and Goodman (2000). Ethane is a marker for lipid peroxidation of polyunsaturated fatty acids by free radicals such as superoxide 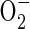 (Halliwell and Gutteridge, 1989).
(Halliwell and Gutteridge, 1989). 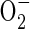 is normally kept under control by detoxifying scavengers but here is thought to damage membranes as a result of the lack of detoxifying systems that a shortage of O2 brings about (Pfister-Sieber and Braendle, 1994).
is normally kept under control by detoxifying scavengers but here is thought to damage membranes as a result of the lack of detoxifying systems that a shortage of O2 brings about (Pfister-Sieber and Braendle, 1994).
We envisage that the faster acetaldehyde production during and after micro-aerobic treatments of rice seedlings has a similar origin to that occurring after anoxia, but on a larger scale. We also envisage that the difference in its extent may help to explain the marked tolerance of FR13A to submergence in water where some O2 is present. The essence of the link lies in the coincidence between the greater tolerance of FR13A, its faster rate of acetaldehyde formation compared with CT6241 (Fig. 6) and its slower rate of ethane emission (Santosa, 2002) under similar conditions. The synchronous outbursts of acetaldehyde and ethane are both connected to peroxidation, with ethane as a product of lipid peroxidation and acetaldehyde as a product of H2O2 removal by catalase action on ethanol. Faster removal of H2O2 (high acetaldehyde production) could then result in a larger fraction of potentially harmful superoxide radicals being detoxified, thus decreasing the amount of peroxidation of polyunsaturated fatty acids. The resulting lessening of membrane damage could then explain the slower ethane production and greater resilience of FR13A to submergence. The scheme is summarized in Fig. 7. We recognize that this novel suggestion is based entirely on correlative evidence. However, the scheme receives support from our finding that as soon as generation of ROS is halted by imposing anoxia on to micro-aerobic plants, acetaldehyde production decreases quickly (presumably because the supply of H2O2 is halted) and by the observation that the least damaged plants (FR13A) produce the most acetaldehyde during and after micro-aerobic exposure. Future work could include (a) intensifying simultaneous analyses of ethane and acetaldehyde; (b) investigating the impact of chemicals that interfere with alcohol dehydrogenase (e.g. 4-methylpyrazole hydrochloride or tetraethylthiuram disulfide) or catalase (e.g. 3-amino-1, 2, 4-triazole) on post-micro-aerobic acetaldehyde and enzyme-mediated ethane production and on the differential sensitivities of FR13A and CT6241 cultivars; and (c) examining the phenomenon in Arabidopsis thaliana where a wide range of mutants and transformants interfering with key steps in fermentation and in the production or disposal of free radicals is available (Mittler et al. 2004) to test our hypothesis further.
Fig. 7.
Scheme depicting the proposed pathways that convert ethanol to acetaldehyde during and after micro-aerobic exposure of rice seedlings. Faster running of the pathway involving conversion via catalase action is thought to benefit submergence-tolerant FR13A by utilizing more H2O2; this, in turn, diverts superoxide radicals away from damaging lipid peroxidation. This peroxidation releases ethane (C2H6), with emissions of this gas being smaller in FR13A compared with the less-tolerant cultivar CT6241.
In summary, fermentation of rice seedlings is initiated within 30 min once external O2 supply falls below 0·3 %, in the gas phase. Detailed comparisons of the kinetics of ethanol, acetaldehyde and CO2 in anaerobic seedlings reveal no marked difference in anoxia response, at least during the first several hours, between a submergence-tolerant line (FR13A) and a more susceptible one (CT6241). However, these cultivars differ in their reactions to micro-aerobic conditions (notably 0·05 % O2) where both during and after a micro-aerobic episode, acetaldehyde production rises strongly while ethanol production decreases. The effect is more pronounced in submergence-tolerant FR13A and is linked to less lipid membrane peroxidation as revealed by reports of slower ethane efflux. We suggest that less lipid damage in FR13A is an outcome of diverting more ROS away from membrane attack and into enhanced production of less harmful H2O2 that serves as a substrate in the conversion of ethanol to acetaldehyde.
Acknowledgments
This work was part of the European Union INCO-DC programme (project ERB3514-PL95-0708) ‘Rice for Life’.
LITERATURE CITED
- Bijnen FGC, Harren FJM, Hackstein JHP, Reuss J.1996. Intracavity CO laser photoacoustic trace gas detection: cyclic CH4, H2O and CO2 emission by cockroaches and scarab beetles. Applied Optics 35: 5357–5368. [DOI] [PubMed] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstadt KV. 2003. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany 91: 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boamfa EI, Ram PC, Jackson MB, Reuss J, Harren FJM. 2003. Dynamic aspects of alcoholic fermentation of rice seedlings in response to anaerobiosis and to complete submergence: relationship to submergence tolerance. Annals of Botany 91: 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins EA. 1978. Ethanol metabolism in plants. In: Hook DD, Crawford RMM, eds. Plant life in anaerobic environments. Ann Arbor, MI: Ann Arbor Science Publishers, 169–202. [Google Scholar]
- Ellis MH, Setter TL. 1999. Hypoxia-induces anoxia tolerance in completely submerged rice seedlings. Journal of Plant Physiology 154: 219–230. [Google Scholar]
- Garnczarska M, Bednarski W, Morkunas I. 2004. Re-aeration-induced oxidative stress and antioxidative defenses in hypoxically pretreated lupine roots. Journal of Plant Physiology 161: 415–422. [DOI] [PubMed] [Google Scholar]
- Greenway H, Setter TL. 1996. Is there anaerobic metabolism in submerged rice plants? A viewpoint. In: Singh VP, Singh RK, Singh BB and Zeigler RS eds. Physiology of stress tolerance in rice. Manila, Philippines: Narendra Deva University of Agriculture & Technology & International Rice Research Institute, 11–30. [Google Scholar]
- Halliwell B, Gutteridge JMC. 1989.Free radicals in biology and medicine. Oxford: Clarendon Press, 188–276. [Google Scholar]
- Ito O, Ella E, Kawano N. 1999. Physiological basis of submergence tolerance in rainfed lowland rice ecosystem. Field Crops Research 64: 75–90. [Google Scholar]
- Jackson MB, Ram PC. 2002. Physiological and molecular basis of susceptibility or tolerance of rice plants to complete submergence. Annals of Botany 91: 227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Waters I, Setter T, Greenway H. 1987. Injury to rice plants by complete submergence; a contribution by ethylene (ethane). Journal of Experimental Botany 38: 1826–1838. [Google Scholar]
- Møller IM. 2001. Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annual Review of Plant Physiology and Plant Molecular Biology 52: 561–591. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. 2004. Reactive oxygen gene network of plants. Trends in Plant Sceince 9: 490–498. [DOI] [PubMed] [Google Scholar]
- Monk LS, Braendle R, Crawford RMM. 1987. Catalase activity and post-anoxic injury in monocotyledonous species. Journal of Experimental Botany 38: 233–246. [Google Scholar]
- Pfister-Sieber M, Braendle R. 1994. Aspects of plant behavior under anoxia and post-anoxia. Proceedings of the Royal Society of Edinburgh B 102: 313–324. [Google Scholar]
- Ram PC, Singh AK, Singh BB, Singh VK, Singh HP, Setter TL, Singh VP, Singh RK. 1999. Environmental characterization of floodwater in Eastern India: relevance to submergence tolerance of lowland rice. Experimental Agriculture 35: 141–152. [Google Scholar]
- Ram PC, Singh BB, Singh AK, Ram P, Singh PN, Singh HP, et al. 2002. Physiological basis of submergence tolerance in rainfed lowland rice: prospects for germ plasm improvement through marker aided breeding. Field Crops Research 76: 131–152. [Google Scholar]
- Santosa E. 2002.Oxidative stress and pathogenic attack in plants, studied by laser based photoacoustic trace gas detection PhD Thesis, University of Nijmegen, The Netherlands (http://webdoc.ubn.kun.nl/mono/s/santosa_i/oxidstanp.pdf). [Google Scholar]
- Setter TL, Kupkanchanakul T, Kupkanchanakul K, Bhekasut P, Wiengweera A, Greenway H. 1987. Concentrations of CO2 and O2 in floodwater and in internodal lacunae of floating rice growing at 1–2 metre depths. Plant, Cell and Environment 10: 767–776. [Google Scholar]
- Setter TL, Ellis M, Laureles EV, Ella ES, Senadhira D, Mishra SB, Sarkarung S, Datta S. 1997. Physiology and genetics of submergence tolerance in rice. Annals of Botany 79: 67–77. [Google Scholar]
- Thongbai P, Goodman BA. 2000. Free radical generation and post-anoxic injury in rice grown in an iron-toxic soil. Journal of Plant Nutrition 23: 1887–1900. [Google Scholar]
- Vervuren PJA, Blom CWPM, de Kroon H. 2003. Extreme flooding events on the Rhine and the survival and distribution or riparian plant species. Journal of Ecology 91: 135–146. [Google Scholar]
- Yoshida S, Fordo DA, Cock JH, Gomez K. 1976.Laboratorymanual for physiological studies of rice. 3rd edn. Los Banos: International Rice Research Institute, 43–48. [Google Scholar]
- Zuckermann H, Harren FJM, Reuss J, Parker DH. 1997. Dynamics of acetaldehyde production during anoxia and post-anoxia in red bell pepper studied by photoacoustic techniques. Plant Physiology 113: 925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]