Abstract
• Aims The molecular mechanisms that correlate with gravity perception and signal transduction in the tip of angiosperm primary roots are discussed.
• Scope Gravity provides a cue for downward orientation of plant roots, allowing anchorage of the plant and uptake of the water and nutrients needed for growth and development. Root gravitropism involves a succession of physiological steps: gravity perception and signal transduction (mainly mediated by the columella cells of the root cap); signal transmission to the elongation zone; and curvature response. Interesting new insights into gravity perception and signal transduction within the root tip have accumulated recently by use of a wide range of experimental approaches in physiology, biochemistry, genetics, genomics, proteomics and cell biology. The data suggest a network of signal transduction pathways leading to a lateral redistribution of auxin across the root cap and a possible involvement of cytokinin in initial phases of gravicurvature.
• Conclusion These new discoveries illustrate the complexity of a highly redundant gravity-signalling process in roots, and help to elucidate the global mechanisms that govern auxin transport and morphogenetic regulation in roots.
Keywords: Root gravitropism, gravity signal transduction, root cap, elongation zone, amyloplast, statolith, auxin transport, cytokinin
INTRODUCTION
Gravitropism is a process that dictates plant organs' growth along a specific vector relative to gravity. It ensures that roots will grow down into the soil, where they take up water and nutrients, whereas shoots will grow upwards, into the air, where they can photosynthesize, reproduce and disperse seed (Fig. 1A). In crop plants, gravitropism contributes to optimal utilization of available resources, and permits plants prostrated by wind and rain to straighten up, allowing mechanical harvesting while avoiding product damage by soil moisture or pathogens. Conversely, the gravitropic response can be deleterious to horticultural production. For instance, cut flowers will develop unwanted stem bends if positioned horizontally during transport.
Fig.1.
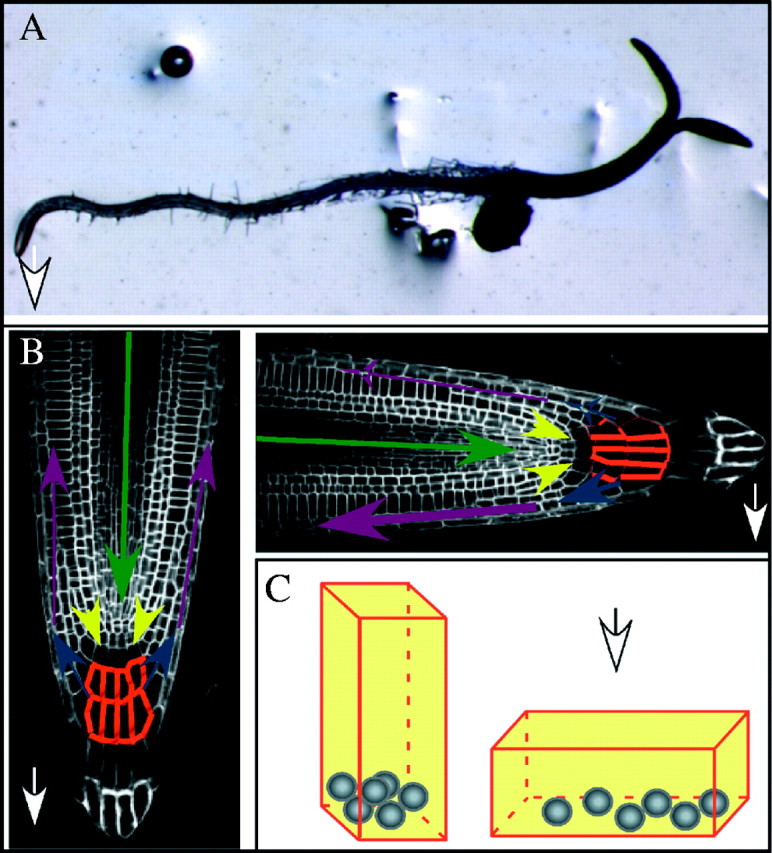
Gravitropism directs downward root growth in Arabidopsis thaliana. (A) A 4-d-old arabidopsis seedling grown on vertical agar-based medium in the light was positioned horizontally (gravistimulated) and allowed to respond. After 3.5 h, the root has initiated a downward gravitropic curvature (arrowhead), whereas the hypocotyl is curving upward. (B) The fountain model of auxin transport in a 5-d-old arabidopsis root tip, stained with propidium iodine to mark the cells. Arrows indicate the direction of auxin transport in different tissues. The arrows' width indicates the relative intensity of transport, and their colour represents the combination of PIN proteins expressed in these tissues (Blilou et al., 2005): green = PIN1, 3, 7; yellow = PIN1, 2, 4; blue = PIN1, 3, 4, 7; purple = PIN1, 2. The red mark delineating the central columella cells of the cap represents the distribution of PIN3 at the plasma membrane of these cells. PIN3 is symmetrically distributed in vertical roots (left panel), but accumulates quickly in the lower membrane upon gravistimulation (right panel). (C) The columella cells of the root cap perceive gravity through the sedimentation of amyloplasts. A columella cell is schematized before (left) and after (right) gravistimulation. Grey circles represent amyloplasts. In all panels the gravity vector is directed downward (white arrows).
If a plant organ is to grow at a defined angle from gravity, it will have to sense its orientation within the gravity field. Perception of a change in organ orientation (gravistimulation) by gravity-perceiving cells (statocytes) results in the formation of a biochemical signal that will have to be transmitted to a site of that organ where a curvature response can develop. Although a substantial amount of work has been carried out on all phases of gravitropism in many susceptible organs, the focus of this review is on the current state of knowledge of the molecular mechanisms that govern gravity signal transduction in the primary root of angiosperms. This system is considered as a model in the analysis of gravitropism because the physical separation existing in this organ between the primary sites of gravity perception (root cap columella) and curvature response (elongation zone; see below) facilitates the analysis of successive phases of the process.
ROOT GRAVITROPISM INVOLVES GROWTH RESPONSES TO LATERAL AUXIN GRADIENTS
The Cholodny–Went theory proposes that gravistimulation promotes the lateral transport of auxin across gravistimulated organs, with accumulation at the bottom (reviewed in Blancaflor and Masson, 2003). The consequence of such redistribution is an upward curvature of the shoot and a downward curvature of the root. Hence, it is reasonable to hypothesize that gravity signal transduction controls specific aspects of auxin transport across the tip, and it is necessary first to enunciate current models explaining auxin transport in the root before describing its contribution to root gravitropism.
The fountain model of auxin transport in roots postulates that auxin is transported through the vasculature into the root cap (reviewed in Wolverton et al., 2002a). There, auxin is redistributed laterally to peripheral tissues and transported back to the elongation zone where it inhibits cellular elongation (Fig. 1B). Polar auxin transport through cell files is mediated by a combination of auxin influx- and efflux-facilitators. In arabidopsis roots, AUX1 and other members of the LAX family of putative transmembrane transporters facilitate auxin influx within transporting cells (Bennett et al., 1996). Members of several families of transmembrane proteins appear to modulate auxin efflux, including members of the PIN family of auxin efflux facilitators, ABC transporters and putative potassium channels such as TRH1 (Noh et al., 2003; Vicente-Agullo et al., 2004; Blilou et al., 2005). Together, these proteins form a complex and redundant network of putative transport facilitators that contribute to the regulation of root morphogenesis and growth behaviour (Blilou et al., 2005).
Highly relevant to early phases of gravity signal transduction is the observation that PIN3, typically localized symmetrically along the plasma membrane of statocytes in layers S2 and S3 of the root cap columella, relocalizes quickly to lower membranes upon gravistimulation (Friml et al., 2002). This gravity-induced redistribution of PIN3 across the tip occurs within 2 min of gravistimulation, and is believed to contribute to the establishment of a lateral auxin gradient across the cap (Fig. 1B). Asymmetric distribution of PIN3 in gravistimulated statocytes remains visible for at least 20 min, and appears to be a direct consequence of gravity signal transduction. Mutations in PIN3 were reported to affect hypocotyl gravitropism and phototropism, organ growth and apical hook formation (Friml et al., 2002). However, the root gravitropic defect also reported in this study is very subtle or non-existent under most growth conditions, suggesting that the contribution of PIN3 to lateral auxin transport across the root cap is redundant in arabidopsis (B. R. Harrison and P. H. Masson, unpubl. res.).
PIN3 appears to cycle between the plasma membrane and endomembranes via vesicle trafficking along actin microfilaments (Friml et al., 2002), a process that may be affected by gravity signal transduction. Accordingly, gravistimulation would modulate PIN3 trafficking, targeting it preferentially to lower membranes. Before this is considered to be a possibility, however, the mechanisms that govern gravity perception by root statocytes need to be described.
HOW CAN A PLANT ORGAN PERCEIVE GRAVITY?
The starch-statolith hypothesis postulates that gravity perception in plants is mediated by the sedimentation or pressure/tension exerted by starch-filled plastids (statoliths) within the gravity-perceiving columella cells of the cap in roots and the endodermal starch sheath cells in shoots (reviewed in Blancaflor and Masson, 2003; Fig. 1C). This model is supported by the observation that genetic or physiological depletion of starch in these cells results in altered gravitropism (Sack, 1991; Kiss et al., 1997; Tsugeki and Fedoroff, 1999). Also, lateral mobilization of amyloplasts within statocytes of vertically orientated root tips with high-gradient magnetic fields promotes organ curvature in a direction predicted from this model in a starch-dependent manner (Kuznetsov and Hasenstein, 1996).
How amyloplast sedimentation is sensed within the root statocytes remains unsolved, however. It is possible that sedimenting statoliths might contact receptors embedded in sensitive membranes on the side of the statocyte, thereby triggering gravity signalling within the cell (Braun, 2002). In vertically growing roots, such receptors could be associated with the cortical endoplasmic reticulum (ER), with a specialized nodular ER found only in root cap columella cells (Yoder et al., 2001), or with the plasma membrane. For instance, PIN3 activity or recycling between plasma membrane and endosome could be the target for such a regulatory mechanism (Friml et al., 2002). However, this statolith–receptor interaction model is inconsistent with observations, which suggest that amyloplast sedimentation per se may not be needed for gravity perception. For instance, starch-deficient mutants lacking amyloplast sedimentation still display some gravitropic response (Caspar and Pickard, 1989). Also, it is possible to trigger a productive signal transduction response by subjection of seedlings to a succession of very short gravitropic stimuli that are insufficient to promote amyloplast sedimentation (reviewed in Sack, 1991; Perbal et al., 2002).
Alternatively, changes in the distribution of pressure or tension exerted by amyloplasts on resisting structures within the statocytes, such as the ER and/or the actin cytoskeleton, might be sufficient either to promote the opening of mechano-sensitive ion channels [thereby triggering gravitropic signalling (Sievers et al., 1984, 1989; Volkmann and Baluška, 1999; Yoder et al., 2001)] or to redirect PIN3 cycling to the plasma membrane (Friml et al., 2002). It should, however, be noted that direct connections between amyloplasts and ER are not needed for gravitropism (Wendt et al., 1987), and that the disruption of statocytes' actin microfilaments with pharmacological agents such as cytochalasin D results in an enhancement of gravitropism, rather than its inhibition (Hou et al., 2004). Hence, actin microfilaments inhibit root gravitropism, thereby ensuring that the lateral asymmetry dictated by gravistimulation is eliminated at the end of a response (Hou et al., 2004).
A purely speculative alternative to the models described above might involve stroma-filled tubules (stromules) that connect plastids to each other, to other organelles and/or to the plasma membrane in many plant cell types (Kwok and Hanson, 2004). If stromules exist in root cap statocytes, the connections they might establish could be sensitive to amyloplast sedimentation, facilitating transduction of gravity signals within these cells. Unfortunately, this model remains untested as in vivo amyloplast morphology has yet to be carefully established in live root cap statocytes. The use of stroma-targeted GFP reporters (Kwok and Hanson, 2004) will undoubtedly help to resolve this interesting question.
Thus, to date, there is no good candidate for a receptor to the gravity-induced forces exerted by amyloplasts on sensitive intracellular structures. However, one cannot exclude the possibility that several pathways act in concert to perceive gravity in roots (Barlow, 1995; Guan et al., 2003; LaMotte and Pickard, 2004). Under this assumption, experiments that disrupt only one of these pathways would not completely obliterate gravitropism. Notably, despite wide acceptance of the starch-statolith hypothesis, other models have also been suggested. The hydrostatic pressure model proposes that statocytes detect the total weight exerted by the protoplast on its cell wall (Staves et al., 1997). Perception of the force ratio at the top versus bottom of the cell could allow correction of background information from isotropic turgor pressure. Mechano-sensitive ion channels would be activated upon gravistimulation, triggering a signalling cascade within the statocytes (Staves et al., 1997). Experimental support for the hydrostatic pressure model has been reported in studies of gravity-modulated cytoplasmic streaming in large internodal cells of Chara, and in gravity perception by rice root statocytes where amyloplasts might simply serve as ballasts (Staves et al., 1997).
In addition, a century's-worth of evidence supports the existence of a secondary mechanism of gravity perception in roots (reviewed in Wolverton et al., 2002a, b; LaMotte and Pickard, 2004). A new technology called ROTATO has recently been used to investigate this system. ROTATO maintains specific regions of the root tip at defined angles from gravity while recording curvature development over time. Using it, researchers detected a site of gravity perception within the distal elongation zone (DEZ) of roots, and estimated that this site contributes approx. 20 % of their total gravity perception capacity (Wolverton et al., 2002b).
How could such a secondary mechanism of gravity perception contribute to root gravitropism? It is possible that this system provides necessary redundancy to the main system, perhaps by regulating the activity of auxin transporters along the DEZ. It is interesting to note that the expression domain of the AGR1/PIN2 auxin efflux facilitator overlaps with the secondary gravity perception site identified in the DEZ using ROTATO. Because AGR1/PIN2-mediated basipetal auxin transport along the root tip appears regulated by protein kinases and phosphatases (Shin et al., 2005), it is possible that auxin flow might be regulated by gravity-mediated asymmetric control of AGR1/PIN2 activity across the DEZ. More work is needed to evaluate the validity of this model.
Recently, a physiological analysis of primary root gravitropism in light-grown maize seedlings (Zea mays ‘Silver Queen’) offered an alternative explanation for redundant gravity perception mechanisms (LaMotte and Pickard, 2004). In this cultivar, the primary root grows vertically downwards, but will not undergo complete tip curvature upon reorientation. Instead, root growth will resume along a vector that is neither vertical nor horizontal, a process called plagiogravitropism (LaMotte and Pickard, 2004). The final vector of plagiogravitropic root growth may not be dictated by a new gravitropic set point angle (Firn and Digby, 1997), distinct from the vertical, but rather reflect ‘graded orthotropism’, a process that couples the gravity-induced curvature response to a facilitative mechanism that requires gravity perception, but does not impart directional information. The vectorial receptor system would not promote tip curvature if the facilitative system were not activated. Furthermore, a curvature response to gravistimulation would end before reaching the vertical if the facilitative system became inactive over time. Graded orthotropism was proposed to involve two gravity receptors, although neither the nature of these receptors nor their association with the root cap or DEZ sensing centres was addressed (LaMotte and Pickard, 2004).
COULD MECHANOSENSITIVE ION CHANNELS CONSTITUTE THE ELUSIVE GRAVITY RECEPTORS IN PLANTS?
Most of the gravity perception mechanisms discussed above postulate the activation of mechano-sensitive ion channels as a first step in gravity signal transduction. However, the molecular identity of these channels remains a mystery. Several mechano-sensitive ion channels have recently been shown to function in a variety of physiological processes in bacteria, yeast and animals (Barritt and Rychkov, 2005). While plant genomes do not appear to contain close relatives of the transient receptor potential (TRP) channels found to mediate mechano-perception in several animal cell types and in yeast (Barritt and Rychkov, 2005), arabidopsis genome annotations recently revealed the existence of ten proteins sharing a functional PF00924 domain with bacterial mechano-sensitive channels (http://www.sanger.ac.uk/cgi-bin/Pfam/getacc?PF00924). Some of these genes appear to be highly expressed in the root tip, and expression of one of them is regulated by gravistimulation (Kimbrough et al., 2004). These putative channels may function as mechano-receptors in the root tip. Reverse genetic strategies, widely used in arabidopsis, should be useful for addressing their potential role in gravity-, touch- and/or osmo-perception.
INORGANIC IONS MAY FUNCTION AS GRAVITY SIGNAL TRANSDUCERS
Several inorganic ions have been postulated to play significant roles in gravity signal transduction within the root cap statocytes. For instance, experiments tracking cytosolic and apoplastic pH changes documented a dramatic alkalinization of the statocytes' cytoplasm within seconds of a gravistimulus, accompanied by an acidification of the apoplast. The gravity-induced alkalinization of root cap statocytes appears necessary for full graviresponsiveness, may be related to the plasma membrane hyperpolarization observed in root cap statocytes within the same time frame, and could result from activation by gravistimulation of plasma membrane or vacuolar-type proton ATPases (Scott and Allen, 1999; Fasano et al., 2001).
How could cytosolic pH changes transduce gravity signals in root cap statocytes? Unlike Ca2+, protons diffuse easily within the cytoplasm. Gravity-induced cytoplasmic alkalinization may thus play a global regulatory role within the cell, rather than regulating localized cellular changes or lateral polarization. Proposed roles include favouring auxin transport, or regulating the gating of ion channels (Fasano et al., 2002).
Ca2+ has long been postulated to function as a second messenger in gravity signal transduction within the root cap statocytes. In agreement with this model, the levels of inositol 1,4,5-trisphosphate (IP3), a second messenger known to activate Ca2+ release into the cytosol from intracellular stores, fluctuate early in response to gravistimulation on both sides of maize and oat pulvini, before eventually stabilizing with increased concentration at the lower flank (Perera et al., 2001). Similar changes in IP3 levels may also occur in the root tip, as arabidopsis seedlings over-expressing a human gene encoding inositol polyphosphate 5-phosphatase as a way to enhance IP3 turnover also display altered kinetics of root gravitropism (Salinas-Mondragon et al., 2005).
It is interesting that both Ca2+ and multiple intermediates and products of the phosphatidylinositol pathway have been implicated in the control of vesicular trafficking in animal systems (Reddy, 2001; Wenk and De Camilli, 2004), and may function likewise in plants (Stevenson et al., 2000; Reddy, 2001). Therefore, these signalling molecules may provide the necessary information to dictate the re-localization of PIN3 and, possibly, of other auxin transport facilitators in root cap statocytes upon gravistimulation.
Unfortunately, most experiments aimed at detecting cytosolic Ca2+ transients in the statocytes upon gravistimulation have failed to date (Legue et al., 1997). However, localized changes in cytosolic Ca2+ levels, below detection, could still occur and be functionally relevant in root statocytes, if one considers the high concentration of calmodulin present in those cells (Sinclair and Trewavas, 1997). Consistent with this note, recent experiments taking advantage of the transgenic aequorin Ca2+-reporter system, which emits light in a Ca2+-dependent manner, allowed the detection of light transients derived from groups of seedlings subjected to gravistimulation (Plieth and Trewavas, 2002). These transients are sensitive to polar auxin transport inhibitors, and can be enhanced by exogenous auxin, suggesting they are a consequence, direct or indirect, of auxin stimulation (Plieth and Trewavas, 2002). Unfortunately, these experiments did not identify the source of these gravity-induced light signals.
Touch-stimulated Ca2+ pulses have been reported in multiple plant cell types (Knight et al., 1991), and recent data from the Gilroy laboratory suggest that touch stimulation of peripheral- or tip-cap cells transiently inhibits root gravitropism. This inhibition is accompanied by a decrease in amyloplast sedimentation rates (Massa and Gilroy, 2003) and an inhibition of gravity-induced cytoplasmic alkalinization within the statocytes. These alterations of gravity signalling within the statocytes appear to be the consequence of a Ca2+ wave that originates within the peripheral cap cells upon mechano-stimulation (Gilroy et al., 2004). Hence, potential Ca2+ responses to gravistimulation in the statocytes would have to be highly compartmentalized to avoid similar interference with cytoplasmic alkalinization and gravitropic signalling. More work is needed to solve this interesting conundrum.
J-DOMAIN PROTEINS ALSO CONTRIBUTE TO GRAVITY SIGNAL TRANSDUCTION WITHIN THE ROOT STATOCYTES
Two proteins have been directly implicated as gravity signal transducers in arabidopsis root tips: ARG1/RHG and ARL2. These two paralogous proteins [i.e. encoded by genes that derive from a common ancestral gene through duplication(s) followed by sequence divergence] appear to function in the same genetic pathway leading to root and hypocotyl gravitropism (Guan et al., 2003). They contain a J domain at their N-terminus, a coiled coil domain at the C-terminal half, and a hydrophobic linker domain in the middle. The coiled coil domain shares similarity with various proteins known to bind microtubules or actin microfilaments (Boonsirichai et al., 2003). However, there is no good experimental evidence to support ARG1 interaction with the cytoskeleton.
J-domain proteins interact with dnaK/HSP70 proteins, thereby contributing to the folding, trafficking, multimerization, targeting or activation of specific substrates. Interestingly, ARG1 is a peripheral membrane protein that associates with various components of the vesicular trafficking pathway (Boonsirichai et al., 2003). Its expression in root and/or hypocotyl statocytes is necessary for full gravitropic responsiveness of these organs. Furthermore, arg1 knockouts are unable to respond to gravistimulation by cytoplasmic alkalinization of root statocytes, and by asymmetric activation of expression of the auxin-responsive DR5-GUS reporter in lateral cap cells upon gravistimulation (Boonsirichai et al., 2003). Altogether, the data suggest that ARG1 and ARL2 function in the same pathway to modulate the trafficking and/or activity of membrane proteins that mediate lateral auxin transport across the cap, such as PIN3 (Friml et al., 2002). Because auxin appears to accumulate to higher levels in a larger number of cap cells in vertically orientated arg1 and arl2 seedlings than in wild type, it is hypothesized that these J-domain proteins affect a general process needed for lateral auxin transport across the cap, and that the corresponding gravitropic defect in mutant seedlings results from an inability of the mutant transport machinery to respond to gravistimulation (Boonsirichai et al., 2003). This interpretation is in line with recent observations suggesting that arl2 and arg1 function in genetic pathways that are distinct from the pgm pathway (phosphoglucomutase, involved in starch biosynthesis; Guan et al., 2003).
COULD AN ANALYSIS OF GRAVITY-INDUCED CHANGES IN ROOT TIP TRANSCRIPTOME IDENTIFY NEW GRAVITY SIGNAL TRANSDUCERS?
While ionic responses to gravistimulation appear to precede other forms of physiological outputs in the root tip, changes in transcription profiles are surprisingly close behind. Indeed, oligonucleotide-based microarray analysis of gene expression in arabidopsis root tips allowed the identification of genes whose transcript abundance varies early in response to gravity- and mechano-stimulation (Kimbrough et al., 2004). Whereas most differentially expressed genes display transcriptional responses to both stimuli, a smaller group responds specifically to either gravity- or mechano-stimulation. The 65 genes whose transcript abundance increases during the first hour of gravistimulation encode proteins that belong to a variety of functional groups, including transcription factors, transporters, wall-modifying enzymes and proteins involved in other stress responses, providing a rich resource of potential gravity signal transducers or effectors.
Expression of these gravity-regulated genes follows different temporal profiles. Interestingly, five of the 65 root-tip gravity-regulated genes appear to be highly responsive to gravistimulation, displaying fast increases in transcript abundance within 1–2 min (Kimbrough et al., 2004). Subsequent analysis using transgenic IP3-depleted plants indicates that gravity-induced expression of these five genes is IP3-dependent, whereas that of other gravity-responsive genes, including most of those previously shown to be hormone-responsive, is not. Hence, at least two signalling pathways may regulate gene expression in the root tip upon gravistimulation (Kimbrough et al., 2004; Salinas-Mondragon et al., 2005). Future analysis of the molecular mechanisms that allow the IP3-dependent pathway to modulate steady-state transcript levels over such short periods of time should be insightful for our understanding of gravity signal transduction within the root tip.
One of the five early gravi-responding genes is predicted to encode an isoform of S-adenosyl-l-methionine : carboxyl methyltransferase, an enzyme that transfers methyl groups from S-adenosyl-l-methionine (AdoMet; the donor substrate) on to an unknown acceptor molecule (Kimbrough et al., 2004). AdoMet is generated by the AdoMet biochemical cycle, which provides substrates for the biosynthesis of ethylene and polyamines, and donates methyl groups in metabolic reactions involving substrates as diverse as IAA, cytokinins, jasmonate, salicylic acid, pectins, lignins, etc. (Schoor and Moffatt, 2004). Interestingly, several enzymes associated with this pathway are up-regulated in the root tip upon gravistimulation (L.-S. Young, N. Murthy and P. H. Masson, unpubl. res.). One of them, adenosine kinase (ADK), which salvages adenosine, a feedback-inhibitory product of the AdoMet pathway (Schoor and Moffatt, 2004), is needed for wild-type root sensitivity to gravistimulation, root cap morphogenesis and proper PIN3 distribution within the root statocytes (Young et al., unpubl. res.). Together, the data are compatible with an important contribution of ADK, the AdoMet cycle and derived regulatory compounds in the control of gravity signal transduction in roots.
COULD PHYTOHORMONES OTHER THAN AUXIN CONTRIBUTE TO GRAVITY SIGNAL TRANSMISSION IN ROOTS?
While a large body of evidence supports a role for auxin in root gravitropism, it should be cautioned that the initial phase of gravitropic curvature, which involves an activation of cell elongation on the upper flank of the DEZ, might occur independently of an auxin gradient (reviewed in Wolverton et al., 2002a). Although the secondary gravity-perceiving site that has been localized to this region of the root might contribute to this initial phase of the response (Wolverton et al., 2002a), it is also possible that different growth regulators might control it. Recent work using a cytokinin-sensitive ARR5–GUS reporter construct demonstrates differential reporter expression on opposite flanks of gravistimulated root caps, with strong activation in the lower lateral cap cells (Aloni et al., 2004). This response precedes the gravity-induced asymmetric activation of the auxin-sensitive DR5-GUS reporter, and application of exogenous cytokinin on one side of vertical roots promotes a curvature in the direction of cytokinin application (Aloni et al., 2004). Hence, a lateral cytokinin gradient might be generated across the root cap upon gravistimulation, and transmitted to the DEZ where it might contribute to the initial curvature phase of the response (Aloni et al., 2004).
The mechanisms necessary for establishment of this cytokinin gradient across the root tip remain unclear, however. Indeed, the IPT5 gene, encoding an isopentenyltransferase that contributes to cytokinin biosynthesis in arabidopsis root tips, is highly responsive to exogenous auxin (Miyawaki et al., 2004), suggesting that differential activation of cytokinin biosynthesis across gravistimulated caps might contribute to gradient formation. A careful analysis of cytokinin biosynthesis and redistribution across the tip upon gravistimulation should allow evaluation of the role of cytokinins in the early phases of gravicurvature. Also, an analysis of gravity-induced cytokinin redistribution across the root tip should be carried out in mutants affected in early phases of gravity signal transduction (pgm, arg1, arl2 and adk) so that this response can be functionally positioned within the known pathways.
CONCLUSIONS
The recent advances in genetic and genomic analysis of gravitropism described in this review have converged with classical physiological observations to suggest that multiple pathways may modulate gravity signal transduction within the root tip. The primary mechanism of gravity perception, located in the root cap, appears to be accompanied by secondary machinery whose location within the DEZ overlaps with the site of initial, auxin-gradient-independent curvature response. Genetic analysis of double mutants indicates that arg1 and arl2 function in a genetic pathway that is distinct from the pgm pathway. Also, genomic analyses of transcriptional responses to gravistimulation suggest the existence of IP3-dependent and -independent regulatory pathways in roots. The relatedness between independently identified pathways is under investigation through various collaborative efforts between multiple laboratories around the world. Importantly, their discovery brings exciting new tools and opportunities in the field. Side-by-side utilization of these tools and strategies, along with an increasingly large number of high-resolution approaches to in vivo analyses of cell biological processes, should allow the dissection of each pathway, an identification of the regulatory network connecting parallel pathways, and an improved understanding of the mechanisms that integrate these pathways with other environmental responses. This information, bound to come from basic research, is badly needed by programmes aimed at engineering plants that respond better or less efficiently to gravistimulation for utilization in agriculture, or that better respond to alternative directional cues for utilization in long space-exploration missions. Fascinating times, indeed!
Acknowledgments
We thank Heike Winter Sederoff for useful comments on this manuscript. Research in the Masson laboratory is supported by grants from NASA, NSF and UW–Madison USDA-HATCH. R.M.P. is supported by a post-doctoral fellowship from NIH. We apologize to our many colleagues whose work we could not discuss owing to space limitations. This is manuscript no. 3626 of the Laboratory of Genetics, University of Wisconsin–Madison.
LITERATURE CITED
- Aloni R, Langhans M, Aloni E, Ullrich C. 2004. Role of cytokinin in the regulation of root gravitropism. Planta 220: 177–182. [DOI] [PubMed] [Google Scholar]
- Barlow P. 1995. Gravity perception in plants—a multiplicity of systems derived by evolution. Plant, Cell and Environment 18: 951–962. [DOI] [PubMed] [Google Scholar]
- Barritt G, Rychkov G. 2005. TRP as mechanosensitive channels. Nature Cell Biology 7: 105–107. [DOI] [PubMed] [Google Scholar]
- Bennett M, Marchant A, Green H, May S, Ward S, Millner P, et al. 1996.Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273: 948–950. [DOI] [PubMed] [Google Scholar]
- Blancaflor E, Masson PH. 2003. Plant gravitropism. Unraveling the ups and downs of a complex process. Plant Physiology 133: 1677–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, et al. 2005. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44. [DOI] [PubMed] [Google Scholar]
- Boonsirichai K, Sedbrook J, Chen R, Gilroy S, Masson PH. 2003. ARG1 is a peripheral membrane protein that modulates gravity-induced cytoplasmic alkalinisation and lateral auxin transport in plant statocytes. Plant Cell 15: 2612–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M. 2002. Gravity perception requires statoliths settled on specific plasma membrane areas in characean rhizoids and protonemata. Protoplasma 219: 150–159. [DOI] [PubMed] [Google Scholar]
- Caspar T, Pickard B. 1989. Gravitropism in a starchless mutant of Arabidopsis: implications for the starch-statolith theory of gravity sensing. Planta 177: 185–197. [PubMed] [Google Scholar]
- Fasano J, Swanson S, Blancaflor E, Dowd P, Kao T, Gilroy S. 2001. Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13: 907–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano J, Massa G, Gilroy S. 2002. Ionic signaling in plant responses to gravity and touch. Journal of Plant Growth Regulation 21: 71–88. [DOI] [PubMed] [Google Scholar]
- Firn RD, Digby J. 1997. Solving the puzzle of gravitropism: has a lost piece been found? Planta 203: S159–S163. [DOI] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K. 2002. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis Nature 415: 806–809. [DOI] [PubMed] [Google Scholar]
- Gilroy S, Swanson S, Richter G, Bibikova T, Monshausen G. 2004. Ca2+-dependent signaling in touch and gravity sensing in the root cap. Abstract 104, ASGSB Annual Meeting 2004, New York, NY. [Google Scholar]
- Guan C, Rosen E, Boonsirichai K, Poff K, Masson PH. 2003. The ARG1-LIKE2 (ARL2) gene of Arabidopsis thaliana functions in a gravity signal transduction pathway that is genetically distinct from the PGM pathway. Plant Physiology 133: 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G, Kramer V, Wang Y, Chen R, Perbal G, Gilroy S, et al. 2004. The promotion of gravitropism in Arabidopsis roots upon actin disruption is coupled with the extended alkalinisation of the columella cytoplasm and a persistent lateral auxin gradient. The Plant Journal 39: 113–125. [DOI] [PubMed] [Google Scholar]
- Kimbrough J, Salinas-Mondragon R, Boss W, Brown C, Winter Sederoff H. 2004. The fast and transient transcriptional network of gravity and mechanical stimulation in the Arabidopsis root apex. Plant Physiology 136: 2790–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss, J, Guisinger M, Miller A, Stackhouse K. 1997. Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis Plant Cell Physiology 38: 518–525. [DOI] [PubMed] [Google Scholar]
- Knight M, Campbell AK, Smith SM, Trewavas AJ. 1991. Transgenic plant aequorin reports the effects of touch, cold-shock and elicitors on cytoplasmic calcium. Nature 352: 524–526. [DOI] [PubMed] [Google Scholar]
- Kuznetsov O, Hasenstein K. 1996. Intracellular magnetophoresis of amyloplasts and induction of root curvature. Planta 198: 87–94. [DOI] [PubMed] [Google Scholar]
- Kwok E, Hanson M. 2004. Stromules and the dynamic nature of plastid morphology. Journal of Microscopy 214: 124–137. [DOI] [PubMed] [Google Scholar]
- LaMotte C, Pickard B. 2004. Control of gravitropic orientation. II. Dual receptor model for gravitropism. Functional Plant Biology 31: 109–120. [DOI] [PubMed] [Google Scholar]
- Legue V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. 1997. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiology 114: 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa G, Gilroy S. 2003. Touch modulates gravity sensing to regulate the growth of primary roots of Arabidopsis thaliana The Plant Journal 33: 435–445. [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Matsumoto-Kitano M, Kakimoto T. 2004. Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. The Plant Journal 37: 128–138. [DOI] [PubMed] [Google Scholar]
- Noh B, Bandyopadhyay A, Peer W, Spalding E, Murphy A. 2003. Enhanced gravi- and phototropism in plant mdr mutants mislocalising the auxin efflux protein PIN1. Nature 423: 999–1002. [DOI] [PubMed] [Google Scholar]
- Perbal G, Jeune B, Lefranc A, Carnero-Diaz E, Driss-Ecole D. 2002. The dose–response curve of the gravitropic reaction: a re-analysis. Physiologia Plantarum 114: 336–342. [DOI] [PubMed] [Google Scholar]
- Perera I, Heilmann I, Chang S, Boss W, Kaufman P. 2001. A role for inositol 1,4,5-trisphosphate in gravitropic signaling and the retention of cold-perceived gravistimulation of oat shoot pulvini. Plant Physiology 125: 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plieth C, Trewavas A. 2002. Reorientation of seedlings in the Earth's gravitational field induces cytosolic calcium transients. Plant Physiology 129: 786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A. 2001. Molecular motors and their functions in plants. International Reviews in Cytology 204: 97–178. [DOI] [PubMed] [Google Scholar]
- Sack F. 1991. Plant gravity sensing. International Reviews in Cytology 127: 193–252. [DOI] [PubMed] [Google Scholar]
- Salinas-Mondragon R, Brogan A, Ward N, Perera I, Boss W, Brown CS, Winter Sederoff H. 2005. Gravity and light: integrating transcriptional regulation in roots. Gravitational and Space Biology 18: 121–122. [PubMed] [Google Scholar]
- Schoor S, Moffatt B. 2004. Applying high-throughput techniques in the study of adenosine kinase in plant metabolism and development. Frontiers in Bioscience 9: 1771–1781. [DOI] [PubMed] [Google Scholar]
- Scott A, Allen N. 1999. Changes in cytosolic pH within Arabidposis root columella cells play a key role in the early signaling pathway for root gravitropism. Plant Physiology 121: 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Shin H-S, Guo Z, Blancaflor E, Masson P, Chen R. 2005. Complex regulation of Arabidopsis AGR1/PIN2-mediated root gravitropic response and basipetal auxin transport by cantharidin-sensitive protein phosphatases. The Plant Journal 42: 188–200. [DOI] [PubMed] [Google Scholar]
- Sievers A, Behrens H, Buckhout T, Gradmann D. 1984. Can a Ca2+ pump in the endoplasmic reticulum of the Lepidium root be the trigger for rapid changes in membrane potential after gravistimulation? Journal of Plant Physiology 114: 195–200. [DOI] [PubMed] [Google Scholar]
- Sievers A, Kruse S, Kuo-Huang L, Wendt M. 1989. Statoliths and microfilaments in plant cells. Planta 179: 275–278. [DOI] [PubMed] [Google Scholar]
- Sinclair W, Trewavas A. 1997. Calcium in gravitropism: a re-examination. Planta 203: S85–S90. [DOI] [PubMed] [Google Scholar]
- Staves M, Wayne R, Leopold A. 1997. The effect of external medium on the gravitropic curvature of rice (Oryza sativa, Poaceae) roots. American Journal of Botany 84: 1522–1529. [PubMed] [Google Scholar]
- Stevenson J, Perera I, Heilmann I, Persson S, Boss W. 2000. Inositol signaling and plant growth. Trends in Plant Science 5: 252–258. [DOI] [PubMed] [Google Scholar]
- Tsugeki R, Fedoroff NV. 1999. Genetic ablation of root cap cells in Arabidopsis Proceeding of the National Academy of Sciences of the USA 96: 12941–12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Agullo F, Rigas S, Desbrosses G, Dolan L, Hatzopoulos P, Grabov A. 2004. Potassium carrier TRH1 is required for auxin transport in Arabidopsis roots. The Plant Journal 40: 523–535. [DOI] [PubMed] [Google Scholar]
- Volkmann D, Baluška F. 1999. Actin cytoskeleton in plants: from transport networks to signaling networks. Microscope Research Techology 47: 135–154. [DOI] [PubMed] [Google Scholar]
- Wendt M, Kuo-Huang L, Sievers A. 1987. Gravitropic bending of cress roots without contact between amyloplasts and complexes of endoplasmic reticulum. Planta 172: 321–329. [DOI] [PubMed] [Google Scholar]
- Wenk M, De Camilli P. 2004. Protein–lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proceedings of the National Academy of Sciences of the USA 101: 8262–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolverton C, Ishikawa H, Evans M. 2002. The kinetics of root gravitropism: dual motor and sensors. Journal of Plant Growth Regulation 21: 102–112. [DOI] [PubMed] [Google Scholar]
- Wolverton C, Mullen J, Ishikawa H, Evans M. 2002. Root gravitropism in response to a signal originating outside of the cap. Planta 215: 153–157. [DOI] [PubMed] [Google Scholar]
- Yoder T, Zheng H, Todd P, Staehelin L. 2001. Amyloplast sedimentation dynamics in maize columella cells support a new model for the gravity-sensing apparatus of roots. Plant Physiology 125: 1045–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]


