Abstract
• Background and Aims Flooding stress leads to a significant reduction in transcription and translation of genes involved in basal metabolism of plants. However, specific genes are noted to be up-regulated in this response. With the aim of isolating genes that might be specifically involved in flooding stress-tolerance mechanism(s), two subtractive cDNA libraries for the flooding-stress-tolerant rice genotype FR13A have been constructed, namely the single and double subtraction libraries (SSL and DSL, respectively).
• Methods To construct the SSL, mRNAs present in the unstressed control FR13A roots were subtracted from the mRNA pool present in low O2-stressed roots of FR13A rice seedlings. The DSL was constructed from mRNAs isolated from the roots of low O2-stressed FR13A rice seedlings from which pools of low-O2-stress up-regulated mRNAs from Pusa Basmati 1 and constitutively expressed mRNAs from FR13A roots were subtracted.
• Results In all, 400 and 606 cDNA clones were obtained from the SSL and DSL, respectively. Global transcript profiling by reverse northern analysis revealed that a large number of clones from these libraries were up-regulated by anaerobic stress. Importantly, selective up-regulated clones showed characteristic cultivar- and tissue-specific expression profiles. Sequencing and annotation of the up-regulated clones revealed that specific signal proteins, hexose transporters, ion channel transporters, RNA-binding proteins and transcription factor proteins possibly play important roles in the response of rice to flooding stress. Also a significant number of novel cDNA clones was noted in these libraries.
• Conclusions It appears that cellular functions such as signalling, sugar and ion transport and transcript stability play an important role in conferring higher flooding tolerance in the FR13A rice type.
Keywords: Low O2, flooding stress, FR13A, Pusa Basmati 1, rice, subtraction library
INTRODUCTION
Field-grown plants are exposed to a magnitude of environmental stresses during their life cycle. O2 deficit is most frequently the consequence of flooding stress (the terms flooding stress, submergence stress, anaerobic stress and O2 deficit/deprivation stress are used interchangeably henceforth). Rice is one of the major food crops of the world. According to Widawsky and O'Toole (1990), submergence stress ranks as the third important constraint to rice production (after drought and weeds). Rice can tolerate partial submergence as paddy rice or deepwater rice. However, it suffers damage when totally submerged for relatively longer intervals. A huge wealth of literature is available on the physiological and biochemical changes associated with flooding-stress response in rice (Das and Uchimiya, 2002; Geigenberger, 2003; Greenway and Gibbs, 2003a, b).
Plants respond to low O2-stress treatment through specific alterations in gene expression. The regulation of gene expression under low O2 conditions is noted to be at both transcriptional (Sachs et al., 1980; Breviario et al., 1994) and translational levels (Sachs et al., 1980; Bailey-Serres and Freeling, 1990). The proteins specifically up-regulated upon anaerobic stress are referred to as anaerobic proteins (ANPs), which mainly comprise metabolic pathway enzymes. However, comprehensive understanding of the genetic components that underlie plant stress responses requires in-depth information on gene expression changes at the global level. Klok et al. (2002) carried out global transcriptional profiling upon hypoxia stress in arabidopsis using the oligonucleotide array method. This group showed that genes involved in sugar metabolism, cell wall extension, signalling components and transcription regulation play principal role(s) in hypoxia stress.
The aim of the study was to isolate genes associated with flooding tolerance in rice. To achieve this objective, a PCR-based hybridization procedure was followed to construct subtraction cDNA libraries using a flood-tolerant (FR13A) and a flood-sensitive (PB1) rice genotype. While PB1 and FR13A rice types show great deal of contrast in their flooding response, there is an issue how genetically close or far these contrasting types are for developing an effective screen to isolate exclusively the flooding stress tolerance-related genes using the hybridization experiment. Owing to the fact that there is a significant synteny in different cereal genomes (Devos and Gale, 2000; Shimamoto and Kyozuka, 2002) as well as within the rice genome, as shown for anaerobically inducible early gene in FR13A and IR54 rice types (Huq and Hodges, 1999), it is surmised that subtraction library screening is possible because these rice types are not too diverge in terms of their genetic constitution. The subtraction cDNA library approach used in this study has several advantages: (a) it is PCR based so rare transcripts can be amplified as well; (b) it is enriched for differentially expressed transcripts; and (c) it yields cDNA fragments that can be directly cloned in various vectors.
Two subtraction libraries were made in this study, namely Single Subtraction Library (SSL) and Double Subtraction Library (DSL). A total of 1006 cDNAs from these two subtractive cDNA libraries (400 clones from SSL and 606 clones from DSL) were isolated. Preliminary screening for the transcript expression characteristics corresponding to these clones were examined using macro-array-based reverse northern approach. This study reports that the anoxia stress-related gene expression response in rice is mostly made up of proteins associated with transcriptional regulation, transportation, signal transduction and carbon and nitrogen metabolism.
MATERIALS AND METHODS
Growth conditions
Pusa Basmati 1 (PB1) and FR13A rice seeds were procured from the Indian Agricultural Research Institute, New Delhi, India and Rice Research Station, Orissa, India, respectively. These seeds were initially washed in water and a mild detergent and then given 70 % ethanol treatment for 45 s for surface sterilization. Finally, the seeds were washed with sterile water five or six times before placing them for germination on cotton pads. These seeds were germinated under completely dark conditions for 2 d at 28 ± 2 °C. Seedlings were grown further for 7 d under 14 h light/10 h dark cycle (100–125 µmol m−2 s−1) with 70–75 % relative humidity maintained in a growth chamber at 28 ± 2 °C (Conviron Inc.).
Stress treatments
For the O2-deprivation treatment, 9-d-old seedlings were transferred to an air-tight water-filled container bubbled with highly purified (approx. 99 % pure) N2 gas (obtained from SMS Multitech Ltd). The seedlings were subjected to O2-deprivation stress for 4 h. O2-deprivation stress treatments were carried out in the dark at 28 ± 2 °C to minimize any interference from photosynthetically produced O2. For a control, a set of seedlings of the same age growing under aerated conditions was placed in the dark for 4 h before being frozen in liquid nitrogen.
Subtractive cDNA libraries construction
Subtractive cDNA libraries were prepared as described by Sahi et al. (2003). For construction of these libraries, total RNA isolated from the root tissues of FR13A and PB1 were taken. Chen et al. (2002) have earlier observed that the root region expresses higher levels of stress-related transcription factor genes. According to Jackson (2002), the flooding stress signal is perceived initially by the roots and the signal is subsequently transferred to the shoots. Considering these arguments, the root tissues were employed specifically for the construction of the subtraction libraries. SSL was constructed using the mRNA pool from FR13A low O2-stressed roots as the tester and the mRNA population from FR13A control roots as the driver. For DSL construction, driver system was composed of mRNA species pooled from PB1 low O2-stressed roots and FR13A roots under control conditions while the tester remained the same, i.e. FR13A low O2-stressed roots.
For construction of libraries, total RNA from root tissues of driver and tester samples was isolated using guanidine thiocyanate according to Chomczynski and Sacchi (1987). mRNA was enriched from the total RNA population by using the PolyATract mRNA isolation kit (Promega Inc.). First-strand cDNA synthesis was initiated from the enriched mRNA of the tester and driver tissues using different poly (dT)-linker primers (with the sequence 5′-TATAGATCTGCGGCCGCAAGCTTTTTTTTT-3′ for the tester tissues and 5′-GTAATACGACTCACTATAGGGTTTTTTTTT-3′ for the driver tissues) and M-MLV Reverse Transcriptase (Promega Inc.).
The double-stranded mRNA–cDNA hybrids were converted to a single-stranded cDNA population by digestion of mRNA with RNaseH. First strand cDNA was purified using Qiaquick columns (Qiagen) and specific oligonucleotides (with sequences 5′-GCTAGCATATGGGCCCGAATTCC-3′ for the tester and 5′-CCCTTTAGTGAGGGTTAATTTC-3′ for the driver) were ligated at the 3′ end of the respective cDNA populations using T4 RNA ligase (Roche). The first-strand cDNA of the driver was amplified using excess of biotin-labelled forward primer (corresponding to the respective ligated oligonucleotide) and a reverse primer (corresponding to the respective linker-primer). The PCR conditions were as follows: 94 °C for 3 min; for 20 cycles at 94 °C for 1 min, 50 °C for 1 min and 72 °C for 4 min; and at 72 °C for 10 min. The double-stranded cDNA of the driver was heat denatured at 94°C for 5 min and immediately the biotin-labelled upper strand was captured using streptavidin paramagnetic particles (SPMPs; Roche). The captured upper strand was hybridized with the first-strand cDNA population of the tester system for 16 h at 65 °C. The tester–driver hybrids and excess of driver were separated from the unhybridized tester cDNA using SPMPs. Tester cDNAs that did not hybridize with driver were amplified using primers specific to the respective ligated oligonucleotide and linker primers (with sequence 5′-CGATCGTATACCCGGGCTTAAGG-3′ and 5′-TATAGATCTGCGGCCGCAAGC-3′, respectively). The PCR was performed as follows: at 94 °C for 3 min; for 30 cycles at 94 °C for 1 min, 55 °C for 1 min and 72 °C for 4 min; and at 72 °C for 10 min. PCR product was purified using Qiaquick column (Qiagen) and digested with ApaI and NotI (the sites for these enzymes were present in the ligated oligonucleotide and the linker primer, respectively) and cloned in ApaI/NotI-digested pBC SK(−) (Stratagene) using T4 DNA ligase (Roche). The ligation mix was transformed into competent XLIB-MRF' cells.
Reverse northern blotting
For reverse northern analysis, plasmid DNA isolated from the cDNA clones was probed with the radiolabelled total cDNA pool made using mRNA isolated from driver and tester tissues. Plasmid DNA was isolated from the clones as per the procedure of Birnboim and Dolly (1979). One hundred and fifty micrograms of plasmid DNA was dot blotted on nylon membrane using a 96-well dot blotter (Bio Rad). Each DNA sample was loaded in duplicate and these replicate blots were probed with cDNA corresponding to driver and tester populations. mRNA was isolated according to PolyATract mRNA isolation kit (Promega Inc.) and first-strand cDNA was synthesized using poly (dT) primers and M-MLV reverse transcriptase, according to the instructions of the suppliers (Amersham Pharmacia Biotech). Using the first-strand cDNA, the radiolabelled second cDNA strand was synthesized employing the random labelling kit as per the instructions of the suppliers (Amersham Pharmacia Biotech). The washing and developing treatments to the reverse northern blots were given together to minimize handling errors.
Isolation of total RNA and northern hybridization
Total RNA from rice root and shoot tissues was extracted as mentioned above. Ten micrograms of the RNA was run on 1·2 % formaldehyde-denaturing agarose gel and blotted to nylon membrane. For probing the membranes with specific probes, the cloned cDNA sequence was amplified using universal T3 and T7 primers. The amplified product was purified and radiolabelled probes were made using random labelling kits from Amersham Pharmacia Biotech.
Sequencing of clones
DNA sequencing was carried out manually by Sanger's dideoxy method of chain termination, using Thermosequenase kit (USB) as per the instructions provided by the suppliers. Specific clones were sequenced using commercial facilities available at Microsynth GmBH (Switzerland) and Macrogen Inc. (Korea). Specific (T3 or M13) primers were used for sequencing of clones from one end or both the ends.
Analysis of the sequences and homology search
The nucleotide and protein sequences were analysed using the respective DNA analysis softwares such as DNASTAR. Nucleotide and the deduced amino acid sequences were searched for their homology with the various previously existing sequences in the NCBI database (EST, Genome and Protein database) using the BlastN and BlastP programs (Altschul et al., 1997). Domain searches in the protein sequences were done using EXPASY, PFAM and SMART protein analysis softwares (www.expasy.org; www.sanger.ac.uk/software/Pfam/; http://smart.embl-heidelberg.de/).
RESULTS
The two subtraction libraries constructed in this study, namely SSL and DSL, comprised 400 and 606 clones in pBC SK vector, respectively. These clones were examined for their transcript expression profile by reverse northern and conventional northern hybridization methods.
Differential expression of genes identified by subtractive hybridization
cDNA clones obtained from the SSL and DSL were screened for identification of the differentially expressed clones by reverse northern hybridization, using the cDNA pools representing driver and tester tissues (under control and low O2-stress conditions) as probes. This screen identified a large number of cDNA clones that showup-regulation in the flooding-tolerant FR13A rice under anaerobic stress conditions (as shown using a representative blot in Fig. 1).
Fig. 1.
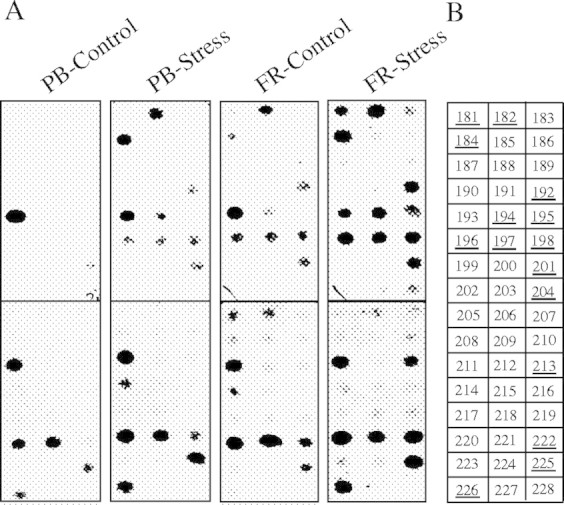
Representative reverse northern blot showing hybridization analysis of cDNA clones obtained from the two subtraction libraries. One hundred and fifty nanograms of plasmid DNA of various cDNA clones were blotted to nylon membranes. The membranes were probed with radiolabelled cDNA made from mRNA isolated from driver and tester populations (roots of PB1 and FR13A rice types, respectively) under aerated and 4-h low O2-stressed conditions (see text for details). (A) Hybridization results obtained using four different probes as indicated on the top of each blot; (B) details of the clones tested in this blot (see Appendix 1 for the details of these clones).
Sequence analysis of ESTs
293 clones, selected for differential expression as described above, were sequenced using T3 or M13 universal primers. The nucleotide sequences of the ESTs thus obtained were searched for the homologous sequences in various databanks and putative functions were assigned to the genes. The unique nucleotide sequences obtained in the present study were submitted to the EMBL databank. Appendix 1 presents a representative list of the clones identified, with the accession numbers obtained from EMBL databank and the putative functions predicted from the similarity in sequence or presence of domains in the sequence. In Appendix 1, the clones having no significant similarity in the database or for which no annotation has been done are categorized in ‘unknown function’ group. The clones for which accession numbers procured in this study were also named in the Osfsp (1–113) series depicting Oryza sativa flooding-stress-associated protein (see Appendix 1).
Transcript expression profile analysis
The transcript expression profile of selective clones was further examined by northern blot hybridization. RNA isolated from the root and shoot tissues of PB1 and FR13A rice under normoxic and low O2-stressed conditions were taken for the analysis. The plasmid DNA isolated from the specific clones was used to synthesize radiolabelled probes. Representative northern blots are shown in Fig. 2.
Fig. 2.
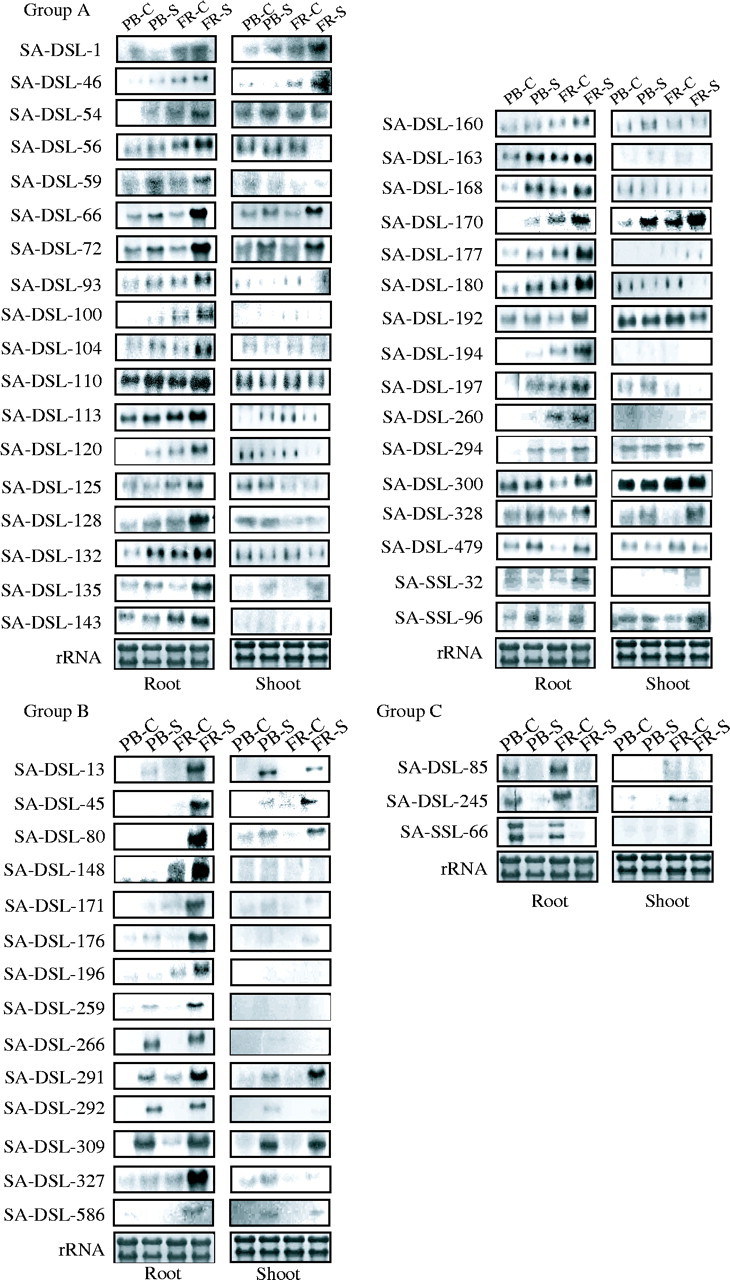
Representative northern blot showing hybridization analysis of cDNA clones obtained from double subtraction and single subtraction libraries. Ten micrograms of total RNA isolated from PB1 and FR13A root and shoot tissues was size-fractionated in 1·2 % formaldehyde-denaturing agarose gel and blotted on to nylon membrane. cDNA sequences inserted in various plasmid clones were radiolabelled and employed as probes. Equal loading of total RNA in different lanes is reflected by comparable intensities of methylene blue-stained bands of rRNA on nylon membrane as shown in the lowermost panels. Group A represents transcripts up-regulated upon low O2 stress, Group B represents transcripts specifically present upon low O2 stress and Group C represents transcripts down-regulated upon low O2 stress. Control: RNA isolated from rice tissues under aerated conditions; Stress: RNA isolated from 4 h low O2-stressed tissues (see text for details). PB, Pusa Basmati1; FR, FR13A.
Transcript expression profile analysis in root tissues of PB1 and FR13A, shows that the clones can be broadly classified into the following three main groups: (A) in which transcript was present at basal level in unstressed control seedlings but enhanced expression was seen upon anoxia stress (as represented by SA-DSL-1, 46, 54, 56, 59, 66, 72, 93, 100, 104, 110, 113, 120, 125, 128, 132, 135, 143, 151, 160, 163, 168, 170, 177, 180, 192, 194, 197, 260, 294, 300, 328 and 479 and SA-SSL-24, 32 and 96 clones in Fig. 2); (B) in which the transcript presence was seen specifically upon stress and was barely detectable in control conditions (as represented by SA-DSL-13, 45, 80, 148, 171, 176, 259, 266, 291, 292, 309, 327 and 586 clones in Fig. 2); (C) in some clones (as represented by SA-DSL-85, 245 and SA-SSL-66), transcript expression level was high under control conditions but was reduced to barely detectable levels under anoxia stress (Fig. 2).
On the basis of cultivar-specific expression analysis, the clones can be clustered into following groups: (A) in which transcript was specifically induced upon anoxia stress in FR13A rice type but negligible amount of transcript was noted in PB1 rice (as represented by clones SA-DSL-13, 45, 46, 80, 100, 148, 170, 171, 176, 194, 196 and 586 in Fig. 2; and (B) in which transcript was noted at enhanced levels in FR13A but low levels were also detected in PB1 rice upon stress (as represented by SA-DSL-56, 66, 72, 93, 104, 110, 113, 128, 132, 135, 143, 163, 168, 177, 192, 294, 300 and 479 clones in Fig. 2).
On comparing organ-related transcript expression profiles of the clones, variable patterns were observed. Many transcripts showed stress-specific induction in roots as well as in shoots of both FR13A and PB1 rice types, such as SA-DSL-1, 13, 45, 46, 66, 72, 80, 170, 291, 309, 328 and 586 (Fig. 2). In another set, clones showed a high level of transcript expression in roots while negligible expression was notable in shoots like SA-DSL-59, 85, 93, 100, 104, 143, 148, 163, 171, 176, 177, 194, 259, 260 and 266 (Fig. 2). In yet another set, clones corresponding to SA-DSL-110, 132, 160 and 192 showed transcript induction in roots upon anoxia stress while shoots showed constitutive expression (Fig. 2). The transcript level representing clones SA-DSL-56, 125, 128 and 180 was enhanced upon stress in roots while it declined in shoots (Fig. 2).
DISCUSSION
Global gene expression analysis has provided valuable insights into metabolic perturbations and readjustments in plants associated with different abiotic stresses such as salt, low and high temperatures, drought, etc. (Cheong et al., 2002; Provart et al., 2003; Rabbani et al., 2003). As co-ordinated regulation of multiple genes appears an important factor in long-term flooding-stress response of plant systems (Andrews et al., 1994; Minhas and Grover, 1999; Dennis et al., 2000), a similar approach was followed in this study for understanding how gene expression changes might differ in flooding-tolerant FR13A rice as compared with the flooding-sensitive PB1 rice type. cDNA libraries were constructed with the aim of identifying genes/proteins that play roles in flooding stress tolerance.
Macro-array-based reverse northern blot analysis was employed for the screening of the cDNA clones. Based on reverse northern analysis, specific clones showing a markedly different hybridization profile under low O2 stress were further examined for their nucleotide sequence as well as for their transcript expression characteristics by conventional northern blot hybridization. Appendix 1 shows putative functions of selected clones obtained in this study based on the sequence homology to the proteins in databank. The clones that showed significant up-regulation in this study were those encoding for various transporter proteins, transcription factors, proteins involved in RNA binding and stability and proteins involved in carbon and nitrogen metabolism. Importantly, some overlapping was noted between the stress cDNA collection of hypoxia stress-regulated genes from micro-array analysis in arabidopsis (Klok et al., 2002) and this study. The proteins identified to be in common in these two studies (rice and arabidopsis) included AP2-EREBF transcription factor, aminotransferase protein, ABC transporters. However, several differences were also noted in expression profiles in these two studies. For instance, clones representing glucose transporters, formate tetrahydrofolate synthase and NADH-ubiquinone oxidoreductase noted in this study were not observed in arabidopsis. On the other hand, clones representing cell wall extension enzymes and transcription factors like WRKY transcription factor noticed in arabidopsis were not observed in this study with rice. Using the suppression subtraction hybridization method, Caturla et al. (2002) identified several genes associated with submergence in adventitious root primodia of Sesbania. Some common proteins identified in the present study and that of Caturla et al. (2002) include pyrophosphatases, aminotransferase and NADH-ubiquinone oxidoreductase. Caturla et al. (2002) also identified reverse transcriptase, protein kinase and ubiquitin extension protein that were not identified in the present screen. The differences in gene expression noted amongst the above reports may be attributable to the different plant systems used. There could also be certain technical reasons behind transcript differences such as the ability of subtraction procedure versus the micro-array chip to identify stress-induced genes expressed differentially in different cultivars. The stringency of the differential expression criterion used in selecting clones in this study could also be an important factor. The total number of ESTs used in arabidopsis work was only 3500 (Klok et al., 2002), which is an under-representation of the EST database of this model weed plant. The total number of ESTs identified for rice and arabidopsis are 284 779 and 322 651, respectively (as per the dbEST release 102204, dated 22 Oct. 2004). The work on identification of stress-associated ESTs is further hampered by the fact that most of the ESTs reported so far in the database are derived from cDNA libraries made from plants grown under normal conditions (Schenk et al., 2000; Desikan et al., 2001), hence, ESTs representing stress-induced transcripts are likely to be under-represented in them. On the other hand, gene expression changes noted to be common in these three studies may represent highly conserved elements in stress response.
One hundred and thirteen clones identified from the two subtraction libraries in this work (referred to as the Osfsp series in Appendix 1) can be classified into seven functional categories based on the predicted functions. These are signalling, transcription factors, carbon and nitrogen metabolism including electron transport chain, RNA binding and recognition, transport, antioxidative pathway and a category of clones with unknown functions (Fig. 3). The largest set of genes (10·7 %) was assigned to C and N metabolism, while genes involved in signal transduction and antioxidative pathway constituted the smallest group, comprising each of 0·89 % of the genes. Genes involved in transportation formed the second (8·9 %) largest group. Genes involved in RNA binding and recognition and transcription regulation constituted 8 and 2·68 % of the stress cDNA clones collection, respectively. A large proportion of the clones (approx. 67·9 %) present in the libraries in this study either did not share homology with any known protein or the function of the respective protein is not yet annotated (Fig. 3). Major categories of genes obtained in the course of this work are as follows.
Fig. 3.
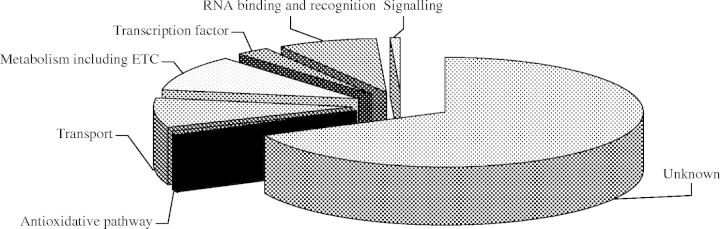
Pie diagram representing the distribution of clones obtained from the two subtraction libraries (DSL and SSL) into seven functional categories based on sequence homology. The seven functional categories identified are metabolism including electron transport chain (10·7 %), RNA binding and recognition (representing 8·0 % of clones), transport (8·9 %), transcription factor (2·68 %), antioxidative pathway (0·89 %) and signalling (0·89 %). The clones with no defined functional roles are categorized in the ‘unknown’ category (representing 67·9 % of the clones). Percentages are based on clones ascribed an accession number in the Appendix. ETC, electron transport chain.
Transcription factor genes
Two transcription factors, ethylene responsive element binding factor (EREBP-type) and homeobox leucine zipper (Hb-ZIP) identified in this study were observed to be up-regulated upon hypoxia stress in arabidopsis (Klok et al., 2002). There are indications that arabidopsis homeobox leucine zipper element is induced by water deficit and abscisic acid (ABA) (Soderman et al., 1996; Lee and Chun, 1998). It seems that regulation of expression of these genes may be governed under multiple stresses. In other words, these transcription factors may thus play a role in the co-ordinated regulation of different abiotic stress regulons.
Transporter genes
Several cDNA clones coding for transporter proteins were identified in this work. Transporter proteins comprise a large group that includes monosaccharide transporter (MST), vacuolar H+-translocating pyrophosphatase (V-ppase), and ATP-binding cassette (ABC) transporter proteins. ABC transporters are members of a large family of active transport proteins energized directly by ATP hydrolysis (Theodoulou, 2000; Sanchez-Fernandez et al., 2001). ABC transporter proteins have been assigned a role in salt and drought stress (Klein et al., 2004; Lee et al., 2004). Recently hypoxia stress was noted to markedly induce gene expression of an ABC transporter (Ospdr9), within 2 h of submergence (Moons, 2003). The transcript corresponding to ABC transporter gene was observed to be induced by low O2 stress (as represented by clone SA-DSL-586 in Fig. 2) in this study as well.
MSTs are energy-dependent H+ symporters, whose expression is cell specific and developmentally regulated (Bick et al., 1998). MSTs are considered to have a possible role in sugar sensing (Smeekens and Rook, 1997). There is ample evidence that several biotic and abiotic stress factors influence the expression of MSTs (Truernit et al., 1996; Delrot et al., 2000; Stadler et al., 2003). Recently, glucose transporters in mammals have been implicated in adaptive and survival response to hypoxic stress (Zhang et al., 2003). Several cDNA clones in this study were identified as MSTs. Transcript profiling of these clones showed variable anoxia inducibility patterns (clones SA-DSL-72, 80, 176 and 320 in Fig. 2). This could be attributed to the fact that clones in this study might represent different members of MSTs (different isoforms of MST1 and 3) that have characteristically variable expression patterns. Maathuis et al. (2003) have reported that most plant membrane transporters are encoded by multigene families whose members show over-lapping expression patterns.
Vacuolar H+ translocating pyrophosphatase (V-ppase) is another type of transporter identified in the present study. This enzyme utilizes energy released upon hydrolysis of pyrophosphate to translocate protons from cytosol to vacuolar lumen against the electrochemical gradient, which generates the proton motive force (PMF) across the membrane. This PMF is used as a driving force for secondary active and passive transport processes (Baltscheffsky et al., 1999; Maeshima, 2000; Drozdowicz and Rea, 2001). V-ppase proteins have been reported to be induced upon anoxia and chilling stress (Carystinos et al., 1995). V-ppase has also been reported to be induced upon salt- and drought-stress conditions in barley (Kasai et al., 1998; Fukuda et al., 2004). It is suggested that this induction may be important for conserving high energy-yielding ATP under stressful conditions or to prevent the cytotoxic effects arising due to release of vacuolar material to cytoplasm under stress. In the present study, V-ppase was distinctly induced upon 4 h of anoxia stress specifically in root tissues of the FR13A rice type (clone SA-DSL-13, Fig. 2).
Glycine-rich proteins (GRPs) are a class of simple structured proteins, often consisting of repetitive amino acid patterns with high glycine content. The glycine-rich region in these proteins is proposed to be involved in protein–protein interactions. In plants, the expression of genes encoding GRPs is developmentally regulated, and also induced by physical, chemical and biological factors such as wounding, virus infection, circadian rhythm, temperature, salinity, drought, flooding, light, salicylic acid, ABA and ethylene (Sachetto-Martins et al., 2000). RNA-binding GRPs are postulated to play an important role in post-transcriptional regulation of gene expression, including RNA processing (Gomez et al., 1988). Klok et al. (2002) noted that GRP transcript is induced upon anoxia stress in arabidopsis. de Oliveira et al. (1990) have shown differential expression of five arabidopsis cell wall-associated GRPs and have found that Atgrp5 transcript remarkably increased upon flooding stress. Glycine-rich RNA binding (Osgrp 1 and 5) in this study was noted to be induced upon anoxia stress (clones SA-DSL-46, 56, 93, 113, 168, 177, 194 and 196 in Fig. 2). All GRP clones in this study showed root specificity in transcript expression. Variable pattern of the Osgrp transcript profile seen in this study can be due the variations in the nucleotide sequences of these clones.
Metabolic pathways
Selective clones in this study were noted to be associated with C and N metabolism. Specifically, cDNA encoding aminotransferases and tetrahydrofolate synthase were isolated from this study. Tetrahydrofolate synthase plays a role in reactions involved in several major cellular processes, including the synthesis of purines, amino acid metabolism, mitochondrial and chloroplastic protein biogenesis and methionine synthesis. It is hypothesized that this enzyme might play a role in the transition from heterotrophic to photoautotrophic growth in plants (Jabrin et al., 2003). The transcript of this enzyme was found to be down-regulated under anoxia stress in this study (clone SA-DSL-85, 245 in Fig. 2). A clone representing aminotransferase was also identified in the present study. Aminotransferase was earlier reported to be induced upon anaerobic stress in barley roots and pondweed turions (Muench and Good, 1994; Sato et al., 2002). Though precise function of alanine accumulation under anoxia is unknown, it is speculated that alanine under anaerobic conditions might serve as a storage form of pyruvate, controlling supply of pyruvate to lactate dehydrogenase and pyruvate decarboxylase, and hence flux to lactate and ethanol (Good and Crosby, 1989). Greenway and Gibbs (2003a) speculated alanine as a possible end-product of anaerobic sugar catabolism.
A cDNA clone encoding the mitochondrial electron transport chain component NADH-ubiquinone oxidoreductase was isolated in the present study. Based on mitochondrial proteome analysis, it is reported that this protein is an integral constituent of anoxic rice samples (Millar et al., 2004). It is suggested that this protein could be implicated in providing tolerance to post-anoxic shock by keeping near-functional mitochondria. Anoxia-induced up-regulation of NADH-ubiquinone oxidoreductase enzyme has been reported in turtle and rat heart (Cai and Storey, 1996; Maklashina et al., 2002).
Antioxidative pathway
A clone encoding metallothionein (MT) protein was obtained in this study. English and Storey (2003) showed that transcript of MT is induced in marine gastropods within 1 h of anoxia. This group suggested that up-regulation of this protein under anoxia could serve a function in antioxidative defence. Li et al. (2004) reported that MT overexpression leads to protection of islets from hypoxia during islets transplantation. There is not much published work in plants on the role of MTs in anaerobic stress as yet.
In summary, the comparative global transcript profiling in rice plants undertaken in this study suggests that proteins associated with cell signalling, sugar and ion transport and transcript stability play important role in conferring higher flooding tolerance in the FR13A rice type. The sensitivity of the subtraction hybridization technique allowed the identification of factors involved in transcription regulation and signal transduction components. The clones obtained from this work will need to be further characterized using simpler model systems like yeast and Escherichia coli or higher systems, particularly gene knockouts (Thornycroft et al., 2001) or through ectopic over-expression of genes using a transgenics approach. Future work on above theme should definitely enhance our understanding of the genetic basis of the flooding stress tolerance mechanism(s) and provide newer directions for raising transgenic plants tolerant to flooding stress.
Appendix 1.
Details of clones obtained from SSL (single subtraction library) and DSL (double subtraction library) with their accession numbers and putative functions predicted according to the homologous sequences present in various databanks
| Clone no. |
Clone name |
Accession no. |
Similarity in the databank |
Functional category |
|---|---|---|---|---|
| SA-SSL-1 | Osfsp67 | AJ767024 | cDNA available but no annotation | Unknown |
| SA-SSL-2 | Osfsp68 | AJ767025 | No significant homology | Unknown |
| SA-SSL-3 | Monosaccharide transporter AK103047 | Transport | ||
| SA-SSL-4 | Monosaccharide transporter AK103047 | Transport | ||
| SA-SSL-5 | Monosaccharide transporter AK103047 | Transport | ||
| SA-SSL-6 | Monosaccharide transporter AK099079 | Transport | ||
| SA-SSL-7 | Monosaccharide transporter AK099079 | Transport | ||
| SA-SSL-8 | Monosaccharide transporter AK103047 | Transport | ||
| SA-SSL-9 | Osfsp66 | AJ767026 | Novel, similar to genomic DNA | Unknown |
| SA-SSL-10 | Osfsp70 | AJ767027 | Novel, similar to genomic DNA | Unknown |
| SA-SSL-11 | Osfsp71 | AJ767028 | Novel, similar to cDNA AK061581 | Unknown |
| SA-SSL-12 | Osfsp72 | AJ767029 | No significant homology | Unknown |
| SA-SSL-13 | Osfsp73 | AJ767030 | Similar to Hordeum metallothionein-like protein | Antioxidative pathway |
| SA-SSL-14 | Similar to glycine-rich RNA-binding protein 5 AK302060 | RNA binding and recognition | ||
| SA-SSL-15 | Monosaccharide transporter AK099079 | Transport | ||
| SA-SSL-16 | Osfsp74 | AJ767031 | Novel, no significant homology | Unknown |
| SA-SSL-17 | Osfsp75 | AJ767032 | Novel, no significant homology | Unknown |
| SA-SSL-18 | Osfsp76 | AJ767033 | Novel, no significant homology | Unknown |
| SA-SSL-19 | Similar to glycine-rich RNA-binding protein AK119238 | RNA binding and recognition | ||
| SA-SSL-20 | Osfsp77 | AJ767034 | No significant homology | Unknown |
| SA-SSL-21 | Osfsp78 | AJ767035 | No significant homology | Unknown |
| SA-SSL-22 | Osfsp79 | AJ767036 | Very little homology to Reiske protein | Metabolism including electron transport chain components |
| SA-SSL-23 | Osfsp80 | AJ767037 | No significant homology | Unknown |
| SA-SSL-24 | Osfsp81 | AJ767038 | Similar to Chlamydomonas NADH-ubiquinone oxidoreductase | Metabolism including electron transport chain components |
| SA-SSL-25 | Osfsp82 | AJ767039 | No significant homology | Unknown |
| SA-SSL-26 | Osfsp83 | AJ767040 | No significant homology | Unknown |
| SA-SSL-27 | Similar to glycine-rich RNA-binding protein AK119238 | RNA binding and recognition | ||
| SA-SSL-28 | Similar to glycine-rich RNA-binding protein 5 AK302060 | RNA binding and recognition | ||
| SA-SSL-29 | Osfsp84 | AJ767041 | Very little homology to Reiske protein | Metabolism including electron transport chain components |
| SA-SSL-30 | Osfsp85 | AJ767042 | No significant homology | Unknown |
| SA-SSL-32 | Osfsp86 | AJ767043 | Very little homology to Reiske protein | Metabolism including electron transport chain components |
| SA-SSL-33 | Similar to glycine-rich RNA-binding protein 5 AK302060 | RNA binding and recognition | ||
| SA-SSL-34 | Similar to glycine-rich RNA-binding protein 5 AK302060 | RNA binding and recognition | ||
| SA-SSL-35 | Similar to glycine-rich RNA-binding protein AK119238 | RNA binding and recognition | ||
| SA-SSL-36 | Osfsp87 | AJ767044 | No significant homology | Unknown |
| SA-SSL-37 | Similar to glycine-rich RNA-binding protein 5 AK302060 | RNA binding and recognition | ||
| SA-SSL-38 | Osfsp88 | AJ767045 | Very little homology to Reiske protein | Metabolism including electron transport chain components |
| SA-SSL-39 | Osfsp89 | AJ767046 | cDNA available but no annotation AK062472 | Unknown |
| SA-SSL-40 | Osfsp90 | AJ767047 | No significant homology | Unknown |
| SA-SSL-41 | Monosaccharide transporter 3 | Transport | ||
| SA-SSL-42 | Osfsp91 | AJ767048 | No significant homology | Unknown |
| SA-SSL-43 | Similar to glycine-rich RNA-binding protein AK 119242 | RNA binding and recognition | ||
| SA-SSL-44 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-SSL-46 | Osfsp92 | AJ767049 | No significant homology | Unknown |
| SA-SSL-49 | Osfsp93 | AJ767050 | No significant homology | Unknown |
| SA-SSL-50 | Osfsp94 | AJ767051 | No significant homology | Unknown |
| SA-SSL-51 | Similar to glycine-rich RNA-binding protein AK 119242 | RNA binding and recognition | ||
| SA-SSL-52 | Osfsp95 | AJ767052 | No significant homology | Unknown |
| SA-SSL-53 | Osfsp96 | AJ767053 | No significant homology | Unknown |
| SA-SSL-55 | Osfsp97 | AJ781008 | No significant homology | Unknown |
| SA-SSL-56 | Similar to glycine-rich RNA-binding protein AK 119242 | RNA binding and recognition | ||
| SA-SSL-57 | Osfsp98 | AJ781009 | Similar to cDNA AK061581 | Unknown |
| SA-SSL-58 | Osfsp99 | AJ781010 | No significant homology | Unknown |
| SA-SSL-59 | Monosaccharide transporter 3 | Transport | ||
| SA-SSL-60 | Osfsp100 | AJ781011 | No significant homology | Unknown |
| SA-SSL-61 | No significant homology | Unknown | ||
| SA-SSL-62 | Similar to glycine-rich RNA-binding protein AK 119242 | RNA binding and recognition | ||
| SA-SSL-63 | Similar to glycine-rich RNA-binding protein AK 119242 | RNA binding and recognition | ||
| SA-SSL-64 | Monosaccharide transporter 3 | Transport | ||
| SA-SSL-65 | Osfsp101 | AJ781012 | No significant homology | Unknown |
| SA-SSL-66 | Osfsp102 | AJ781013 | Similar to Zea mays NOD26 aquaglyceroporins | Transport |
| SA-SSL-67 | Similar to glycine-rich RNA-binding protein 5 AK302060 | RNA binding and recognition | ||
| SA-SSL-68 | Osfsp103 | AJ781014 | No significant similarity | Unknown |
| SA-SSL-69 | Similar to glycine-rich RNA-binding protein AK 119242 | RNA binding and recognition | ||
| SA-SSL-70 | Very little homology to Reiske Fe-S protein | Metabolism including electron transport chain components | ||
| SA-SSL-71 | Similar to glycine-rich RNA-binding protein AK 119242 | RNA binding and recognition | ||
| SA-SSL-72 | Osfsp104, Osfsp105 | AJ781015, AJ781016 | No significant similarity | Unknown |
| SA-SSL-73 | Similar to glycine-rich RNA-binding protein AK 119242 | RNA binding and recognition | ||
| SA-SSL-74 | Very little homology to Reiske Fe-S protein | Metabolism including electron transport chain components | ||
| SA-SSL-75 | Very little homology to Reiske Fe-S protein | Metabolism including electron transport chain components | ||
| SA-SSL-76 | Monosaccharide transporter 3 | Transport | ||
| SA-SSL-77 | Glycine-rich RNA-binding protein 5 AK302060 | RNA binding and recognition | ||
| SA-SSL-78 | Osfsp106 | AJ781017 | No significant similarity | Unknown |
| SA-SSL-79 | Osfsp107 | AJ781018 | No significant similarity | Unknown |
| SA-SSL-80 | Osfsp108 | AJ781019 | No significant similarity | Unknown |
| SA-SSL-81 | Monosaccharide transporter 3 | Transport | ||
| SA-SSL-82 | Osfsp109 | AJ781020 | Very little homology to Reiske Fe-S protein | Metabolism including electron transport chain components |
| SA-SSL-83 | Osfsp110 | AJ781021 | Similar to membrane type II serine protease | Metabolism including electron transport chain components |
| SA-SSL-84 | Similar to glycine-rich RNA-binding protein 5 AK302060 | RNA binding and recognition | ||
| SA-SSL-86 | Monosaccharide transporter 3 | Transport | ||
| SA-SSL-88 | Osfsp111 | AJ781022 | Very little homology to Reiske Fe-S protein | Metabolism including electron transport chain components |
| SA-SSL-90 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-SSL-91 | Osfsp112 | AJ781023 | Very little similarity to Reiske Fe-S protein | Metabolism including electron transport chain components |
| SA-SSL-92 | Glycine-rich RNA-binding protein 5 AK302060 | RNA binding and recognition | ||
| SA-SSL-93 | Monosaccharide transporter 3 | Transport | ||
| SA-SSL-96 | Osfsp113 | AJ781024 | Deduced protein with a ring finger domain | Putative Transcription factor |
| SA-DSL-1 | Osfsp11 | AJ550894 | No significant similarity found | Unknown |
| SA-DSL-2 | Osfsp44 | AJ634708 | Identical to clone AK062472 | Unknown |
| SA-DSL-3 | Osfsp9 | AJ550647 | No significant similarity found | Unknown |
| SA-DSL-6 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-8 | Monosaccharide transporter 3 | Transport | ||
| SA-DSL-9 | Monosaccharide transporter 3 | Transport | ||
| SA-DSL-10 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-11 | Monosaccharide transporter 3 | Transport | ||
| SA-DSL-13 | Osfsp10 | AJ550648 | New rice pyrophosphatase | Transport |
| SA-DSL-14 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-15 | Osfsp7 | AJ550645 | No significant similarity found | Unknown |
| SA-DSL-16 | Osfsp8 | AJ550646 | No significant similarity found | Unknown |
| SA-DSL-18 | Osfsp45 | AJ634709 | Homology with genomic DNA | Unknown |
| SA-DSL-19 | Osfsp46 | AJ634710 | No significant similarity found | Unknown |
| SA-DSL-20 | Osfsp12 | AJ550895 | No significant similarity found | Unknown |
| SA-DSL-22 | Osfsp47 | AJ634711 | Monosaccharide transporter 3 variant | Transport |
| SA-DSL-24 | Osfsp48 | AJ634712 | No significant similarity found | Unknown |
| SA-DSL-26 | Monosaccharide transporter | Transport | ||
| SA-DSL-28 | Osfsp49 | AJ634713 | Glycine-rich RNA-binding protein 1 variant | RNA binding and recognition |
| SA-DSL-30 | Osfsp2 | AJ550640 | No significant similarity found | Unknown |
| SA-DSL-32 | Monosaccharide transporter | Transport | ||
| SA-DSL-34 | Osfsp13 | AJ550896 | No significant similarity found | Unknown |
| SA-DSL-36 | Osfsp1 | AJ550161 | No significant similarity found | Unknown |
| SA-DSL-46 | Putative glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-49 | Osfsp41 | AJ631211 | Similarity to homeobox leucine zipper protein | Transcription factor |
| SA-DSL-50 | Osfsp50a | AJ634714 | Glycine-rich RNA-binding protein 1 variant AK070016 | RNA binding and recognition |
| SA-DSL-54 | Osfsp50b | AJ634715 | Similarity to drought-inducible EST | Unknown |
| SA-DSL-55 | Glycine-rich RNA-binding protein 1 AK119242 | RNA binding and recognition | ||
| SA-DSL-56 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-59 | Monosaccharide transporter | Transport | ||
| SA-DSL-62 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-64 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-66 | Monosaccharide transporter 3 | Transport | ||
| SA-DSL-69 | Osfsp16 | AJ608756 | Function not annotated | Unknown |
| SA-DSL-70 | Osfsp51 | AJ634716 | No significant similarity found | Unknown |
| SA-DSL-72 | Putative glucose transport protein variant (XM_476381) | Transport | ||
| SA-DSL-74 | Monosaccharide transporter 3 | Transport | ||
| SA-DSL-76 | Osfsp14 | AJ557262 | No significant similarity found | Unknown |
| SA-DSL-80 | Osfsp21 | AJ609507 | Monosaccharide transporter | Transport |
| SA-DSL-81 | Osfsp15 | AJ557263 | No significant similarity found | Unknown |
| SA-DSL-82 | Osfsp22 | AJ609508 | No significant similarity found | Unknown |
| SA-DSL-83 | Osfsp23 | AJ609509 | No significant similarity found | Unknown |
| SA-DSL-85 | Osfsp17 | AJ608757 | (Novel) 10-tetra hydro folate synthase | Metabolism including electron transport chain components |
| SA-DSL-87 | Monosaccharide transporter 3 | Transport | ||
| SA-DSL-88 | Osfsp18 | AJ608758 | No significant similarity found | Unknown |
| SA-DSL-93 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-94 | Monosaccharide transporter 3 | Transport | ||
| SA-DSL-98 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-99 | Osfsp25 | AJ609511 | No significant similarity found | Unknown |
| SA-DSL-100 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-101 | Osfsp19 | AJ608759 | Ser Ther protein phosphatase | Signalling |
| SA-DSL-103 | Osfsp52 | AJ634717 | Monosaccharide transporter variant | Transport |
| SA-DSL-104 | Osfsp20 | AJ608760 | No significant similarity found | Unknown |
| SA-DSL-107 | Osfsp53 | AJ634718 | Ethylene-responsive binding factor3 | Transcription factor |
| SA-DSL-108 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-109 | Osfsp3 | AJ550641 | No significant similarity found | Unknown |
| SA-DSL-110 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-113 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-115 | Osfsp4 | AJ550642 | No significant similarity found | Unknown |
| SA-DSL-116 | Function not annotated | Unknown | ||
| SA-DSL-118 | Monosaccharide transporter 3 | Transport | ||
| SA-DSL-119 | Osfsp5 | AJ550643 | No significant similarity found | Unknown |
| SA-DSL-120 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-125 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-127 | Osfsp6 | AJ550644 | No significant similarity found | Unknown |
| SA-DSL-128 | Monosaccharide transporter | Transport | ||
| SA-DSL-130 | Similar to chilling inducible protein | Unknown | ||
| SA-DSL-135 | Putative glucose transport protein variant | Transport | ||
| SA-DSL-137 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-143 | Osfsp42 | AJ631212 | Function not annotated | Unknown |
| SA-DSL-147 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-148 | Monosaccharide transporter | Transport | ||
| SA-DSL-149 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-151 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-158 | Osfsp54 | AJ634719 | No significant similarity found | Unknown |
| SA-DSL-160 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-162 | Monosaccharide transporter | Transport | ||
| SA-DSL-163 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-165 | Osfsp55 | AJ634720 | No significant similarity found | Unknown |
| SA-DSL-166 | Osfsp56 | AJ634722 | No significant similarity found | Unknown |
| SA-DSL-168 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-170 | Monosaccharide transporter | Transport | ||
| SA-DSL-171 | Monosaccharide transporter | Transport | ||
| SA-DSL-172 | No significant similarity found | Unknown | ||
| SA-DSL-173 | No significant similarity found | Unknown | ||
| SA-DSL-174 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-175 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-176 | Putative glucose transport protein | Transport | ||
| SA-DSL-177 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-178 | No significant similarity found | Unknown | ||
| SA-DSL-180 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-181 | Monosaccharide transporter | Transport | ||
| SA-DSL-182 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-184 | Vacuolar pyrophosphatase | Transport | ||
| SA-DSL-192 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-194 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-195 | Novel no significant homology | Unknown | ||
| SA-DSL-196 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-197 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-198 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-204 | Monosaccharide transporter 3 | Transport | ||
| SA-DSL-206 | Osfsp43 | AJ631213 | No significant similarity found | Unknown |
| SA-DSL-213 | Monosaccharide transporter | Transport | ||
| SA-DSL-220 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-222 | Osfsp38 | AJ629331 | Monosaccharide transporter | Transport |
| SA-DSL-225 | Osfsp39 | AJ629332 | Glycine-rich RNA-binding protein | RNA binding and recognition |
| SA-DSL-226 | Osfsp40 | AJ629333 | Glycine-rich RNA-binding protein | RNA binding and recognition |
| SA-DSL-229 | Homology to vacuolar pyrophosphatase protein | Transport | ||
| SA-DSL-232 | Osfsp57 | AJ634723 | No significant similarity found | Unknown |
| SA-DSL-233 | Glycine-rich RNA-binding protein 1 | RNA binding and recognition | ||
| SA-DSL-234 | Monosaccharide transporter | Transport | ||
| SA-DSL-235 | Osfsp26 | AJ629319 | No significant similarity found | Unknown |
| SA-DSL-242 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-243 | Monosaccharide transporter | Transport | ||
| SA-DSL-244 | Osfsp58 | AJ634724 | No significant homology | Unknown |
| SA-DSL-245 | Osfsp59 | AJ634725 | Similarity to maize formate tetrahyrofolate ligase | Metabolism including electron transport chain components |
| SA-DSL-247 | Osfsp60 | AJ634726 | No significant similarity found | Unknown |
| SA-DSL-248 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-249 | Monosaccharide transporter | Transport | ||
| SA-DSL-250 | Osfsp27 | AJ629320 | Some homology to wheat drought-stress library EST | Unknown |
| SA-DSL-251 | Monosaccharide transporter | Transport | ||
| SA-DSL-252 | Monosaccharide transporter | Transport | ||
| SA-DSL-255 | Vacuolar pyrophosphatase | Transport | ||
| SA-DSL-256 | Monosaccharide transporter | Transport | ||
| SA-DSL-259 | Monosaccharide transporter | Transport | ||
| SA-DSL-260 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-261 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-263 | Osfsp28 | AJ629321 | Not annotated RNA recognition motif | RNA binding and recognition |
| SA-DSL-265 | Monosaccharide transporter | Transport | ||
| SA-DSL-266 | Osfsp29 | AJ629322 | No significant similarity found | Unknown |
| SA-DSL-271 | Monosaccharide transporter | Transport | ||
| SA-DSL-288 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-290 | Monosaccharide transporter 3 | Transport | ||
| SA-DSL-291 | Putative glucose transport protein variant (XM_476381) | Transport | ||
| SA-DSL-292 | Vacuolar pyrophosphatase | Transport | ||
| SA-DSL-294 | Monosaccharide transporter | Transport | ||
| SA-DSL-298 | Glycine-rich RNA-binding protein 5 | RNA binding and recognition | ||
| SA-DSL-300 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-301 | Glycine-rich RNA-binding protein 1 | RNA binding and recognition | ||
| SA-DSL-303 | Osfsp30 | AJ629323 | Similarity to clone AK070704 with an RNA recognition motif | RNA binding and recognition |
| SA-DSL-309 | Vacuolar pyrophosphatase | Transport | ||
| SA-DSL-317 | Glycine-rich RNA-binding protein 1 | RNA binding and recognition | ||
| SA-DSL-320 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-327 | Putative glucose transport protein variant | Transport | ||
| SA-DSL-340 | Monosaccharide transporter | Transport | ||
| SA-DSL-342 | Osfsp31 | AJ629324 | Similar to EST AJ420714 | Unknown |
| SA-DSL-346 | Monosaccharide transporter | Transport | ||
| SA-DSL-347 | Monosaccharide transporter | Transport | ||
| SA-DSL-349 | Monosaccharide transporter | Transport | ||
| SA-DSL-358 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-359 | Pyrophosphatase | Transport | ||
| SA-DSL-361 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-365 | Monosaccharide transporter | Transport | ||
| SA-DSL-371 | Monosaccharide transporter | Transport | ||
| SA-DSL-373 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-376 | Monosaccharide transporter | Transport | ||
| SA-DSL-377 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-379 | Monosaccharide transporter | Transport | ||
| SA-DSL-382 | Monosaccharide transporter | Transport | ||
| SA-DSL-383 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-388 | Monosaccharide transporter | Transport | ||
| SA-DSL-391 | Monosaccharide transporter | Transport | ||
| SA-DSL-393 | Osfsp62 | AJ635351 | Glycine-rich RNA-binding protein 1 | RNA binding and recognition |
| SA-DSL-394 | Osfsp32 | AJ629325 | Similar to drought-stress library EST clone NF026B07PL | Unknown |
| SA-DSL-396 | Osfsp33 | AJ629326 | AK066311, similar to Nicotiana mRNA for U2 snRNP auxiliary factor RNA recognition motif | RNA binding and recognition |
| SA-DSL-397 | Monosaccharide transporter | Transport | ||
| SA-DSL-398 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-401 | Osfsp61 | AJ635350 | Putative aminotransferase protein | Metabolism including electron transport chain components |
| SA-DSL-402 | Osfsp34 | AJ629327 | Putative protein tyrosine phosphatase and RNA-binding domain | RNA binding and recognition |
| SA-DSL-403 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-406 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-408 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-411 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-412 | Monosaccharide transporter | Transport | ||
| SA-DSL-414 | Vacuolar pyrophosphatase | Transport | ||
| SA-DSL-429 | Osfsp63 | AJ635352 | Monosaccharide transporter 3 variant | Transport |
| SA-DSL-430 | Monosaccharide transporter 3 variant | Transport | ||
| SA-DSL-431 | Glycine-rich RNA-binding protein 1 | RNA binding and recognition | ||
| SA-DSL-432 | Osfsp64 | AJ635353 | Monosaccharide transporter 3 variant | Transport |
| SA-DSL-433 | Glycine-rich RNA-binding protein 1 variant | RNA binding and recognition | ||
| SA-DSL-434 | Osfsp65 | AJ635354 | Monosaccharide transporter 3 variant | Transport |
| SA-DSL-470 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-472 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-473 | Vacuolar pyrophosphatase | Transport | ||
| SA-DSL-477 | Monosaccharide transporter | Transport | ||
| SA-DSL-480 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-481 | Vacuolar pyrophosphatase | Transport | ||
| SA-DSL-485 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-488 | Osfsp35 | AJ629328 | No significant similarity found | Unknown |
| SA-DSL-489 | Glycine-rich RNA-binding protein 1 | RNA binding and recognition | ||
| SA-DSL-491 | Monosaccharide transporter 3 variant | Transport | ||
| SA-DSL-498 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-499 | Monosaccharide transporter | Transport | ||
| SA-DSL-502 | Osfsp66 | AJ704820 | Function not annotated | Unknown |
| SA-DSL-512 | Vacuolar pyrophosphatase | Transport | ||
| SA-DSL-549 | Monosaccaride transporter 3 variant | Transport | ||
| SA-DSL-552 | Monosaccharide transporter 3 variant AK103047 | Transport | ||
| SA-DSL-560 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-562 | Vacuolar pyrophosphatase | Transport | ||
| SA-DSL-565 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-566 | Osfsp36 | AJ629329 | No significant similarity found | Unknown |
| SA-DSL-569 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-574 | Vacuolar pyrophosphatase | Transport | ||
| SA-DSL-575 | Monosaccharide transporter | Transport | ||
| SA-DSL-579 | Vacuolar pyrophosphatase | Transport | ||
| SA-DSL-581 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-586 | Osfsp37 | AJ629330 | Not annotated, putative ABC transporter | Transport |
| SA-DSL-594 | Glycine-rich RNA-binding protein | RNA binding and recognition | ||
| SA-DSL-595 | Monosaccharide transporter | Transport | ||
| SA-DSL-596 | Monosaccharide transporter | Transport | ||
| SA-DSL-601 | Monosaccharide transporter | Transport |
Supplementary Material
Acknowledgments
We thank members of the Department of Biotechnology (DBT), Government of India, for their kind support. S.A. is thankful to the Council of Scientific and Industrial Research (CSIR), Government of India, for a fellowship grant.
LITERATURE CITED
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DL, MacAlpine DM, Johnson JR, Kelley PM, Cobb BG, Drew MC. 1994. Differential induction of mRNAs for the glycolytic and ethanolic fermentative pathways by hypoxia and anoxia in maize seedlings. Plant Physiology 106: 1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Freeling M. 1990. Hypoxic stress induced changes in ribosomes of maize seedling roots. Plant Physiology 94: 1237–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltscheffsky M, Schultz A, Baltscheffsky H. 1999. H+-PPases: a tightly membrane-bound family. FEBS Letters 457: 527–533. [DOI] [PubMed] [Google Scholar]
- Bick JA, Neelam A, Smith E, Nelson SJ, Hall JL, Williams LE. 1998. Expression analysis of a sucrose carrier in the germinating seedling of Ricinus communis Plant Molecular Biology 38: 425–435. [DOI] [PubMed] [Google Scholar]
- Birnboim HC, Dolly J. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Research 7: 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breviario D, Giani S, Morello L, Coraggio I. 1994. Anaerobiosis mediated early transcriptional and translational responses in rice (Oryza sativa L.) coleoptiles and roots. Plant, Cell and Environment 17: 925–934. [Google Scholar]
- Cai Q, Storey KB. 1996. Anoxia-induced gene expression in turtle heart: up-regulation of mitochondrial genes for NADH-ubiquinone oxidoreductase subunit 5 and cytochrome c oxidase subunit 1. European Journal of Biochemistry 241: 83–92. [DOI] [PubMed] [Google Scholar]
- Carystinos GD, MacDonald HR, Monroy AF, Dhindsa RS, Poole RJ. 1995. Vacuolar H(+)-translocating pyrophosphatase is induced by anoxia or chilling in seedlings of rice. Plant Physiology 108: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caturla M, Chaparro C, Schroeyers K, Holsters M. 2002. Suppression subtractive hybridization to enrich low-abundance and submergence enhanced transcripts of adventitious root primodia of Sesbania rostrata Plant Science 162: 915–921. [Google Scholar]
- Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T, et al. 2002. Expression profile matrix of Arabidopsis Transcription Factor genes suggests their putative functions in response to environmental stresses. The Plant Cell 14: 559–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong YH, Chang HS, Gupta R, Wang X, Zhu T, Luan S. 2002. Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiology 129: 661–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry 162: 156–159. [DOI] [PubMed] [Google Scholar]
- Das A, Uchimiya H. 2002. Oxygen stress and adaptation of a semi-aquatic plant: rice (Oryza sativa). Journal of Plant Research 115: 315–320. [DOI] [PubMed] [Google Scholar]
- Delrot S, Atanassova R, Maurousset L. 2000. Regulation of sugar, amino acid and peptide plant membrane transporters. Biochimica et Biophysica Acta 1465: 281–306. [DOI] [PubMed] [Google Scholar]
- Dennis ES, Dolferus R, Ellis M, Rahman M, Wu Y, Hoeren FU, et al. 2000. Molecular strategies for improving waterlogging tolerance in plants. Journal of Experimental Botany 51: 89–97. [PubMed] [Google Scholar]
- Desikan R, A-H-Mackerness S, Hancock JT, Neill SJ. 2001. Regulation of Arabidopsis transcriptome by oxidative stress. Plant Physiology 127: 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos KM, Gale MD. 2000. Genome relationships: the grass model in current research. The Plant Cell 12: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozdowicz YM, Rea PA. 2001. Vacuolar H(+) pyrophosphatases: from the evolutionary backwaters into the mainstream. Trends in Plant Sciences 6: 206–211. [DOI] [PubMed] [Google Scholar]
- English TE, Storey KB. 2003. Freezing and anoxia stresses induce expression of metallothionein in the foot muscle and hepatopancreas of the marine gastropod Littorina littorea Journal of Experimental Biology 206: 2517–2524. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Chiba K, Maeda M, Nakamura A, Maeshima M, Tanaka Y. 2004. Effect of salt and osmotic stresses on the expression of genes for the vacuolar H+-pyrophosphatase, H+-ATPase subunit A, and Na+/H+ antiporter from barley. Journal of Experimental Botany 55: 585–594. [DOI] [PubMed] [Google Scholar]
- Geigenberger P. 2003. Response of plant metabolism to too little oxygen. Current Opinion in Plant Biology 6: 247–256. [DOI] [PubMed] [Google Scholar]
- Gomez J, Sanchez-Martinez D, Stiefel V, Rigau J, Puigdomenech P, Pages M. 1988. A gene induced by the plant hormone abscisic acid in response to water stress encodes a glycine-rich protein. Nature 334: 262–264. [DOI] [PubMed] [Google Scholar]
- Good AG, Crosby WL. 1989. Anaerobic induction of alanine aminotransferase in barley root tissue. Plant Physiology 90: 1305–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway H, Gibbs J. 2003. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology 30: 1–47. [DOI] [PubMed] [Google Scholar]
- Greenway H, Gibbs J. 2003. Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Functional Plant Biology 30: 999–1036. [DOI] [PubMed] [Google Scholar]
- Huq E, Hodges TK. 1999. An anaerobically inducible early (aie) gene family from rice. Plant Molecular Biology 40: 591–601. [DOI] [PubMed] [Google Scholar]
- Jabrin S, Ravanel S, Gambonnet B, Douce R, Rebeille F. 2003. One-carbon metabolism in plants. Regulation of tetrahydrofolate synthesis during germination and seedling development. Plant Physiology 131: 1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB. 2002. Long-distance signalling from roots to shoots assessed: the flooding story. Journal of Experimental Botany 53: 175–181. [DOI] [PubMed] [Google Scholar]
- Kasai M, Nakamura T, Kudo N, Sato H, Maeshima M, Sawada S. 1998. The activity of the root vacuolar H(+)-pyrophosphatase in rye plants grown under conditions deficient in mineral nutrients. Plant Cell Physiology 39: 890–894. [DOI] [PubMed] [Google Scholar]
- Klein M, Geisler M, Suh SJ, Kolukisaoglu HU, Azevedo L, Plaza S, et al. 2004. Disruption of AtMRP4, a guard cell plasma membrane ABCC-type ABC transporter, leads to deregulation of stomatal opening and increased drought susceptibility. The Plant Journal 39: 219–236. [DOI] [PubMed] [Google Scholar]
- Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, et al. 2002. Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. The Plant Cell 14: 2481–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EK, Kwon M, Ko JH, Yi H, Hwang MG, Chang S, et al. 2004. Binding of sulphonylurea by AtMRP5, an Arabidopsis multidrug resistance-related protein that functions in salt tolerance. Plant Physiology 134: 528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Chun JY. 1998. A new homeodomain-leucine zipper gene from Arabidopsis thaliana induced by water stress and abscisic acid treatment. Plant Molecular Biology 37: 377–384. [DOI] [PubMed] [Google Scholar]
- Li X, Chen H, Epstein PN. 2004. Metallothionein protects islets from hypoxia and extends islet graft survival by scavenging most kinds of reactive oxygen species. Journal of Biological Chemistry 279: 765–771. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Filatov V, Herzyk P, Krijger GC, Axelsen KB, Chen S, et al. 2003. Transcriptome analysis of root transporters reveals participation of multiple gene families in the reponse to cation stress. The Plant Journal 35: 675–692. [DOI] [PubMed] [Google Scholar]
- Maeshima M. 2000. Vacuolar H(+)-pyrophosphatase. Biochimica et Biophysica Acta 1465: 37–51. [DOI] [PubMed] [Google Scholar]
- Maklashina E, Sher Y, Zhou HZ, Gray MO, Karliner JS, Cecchini G. 2002. Effect of anoxia/reperfusion on the reversible active/de-active transition of NADH-ubiquinone oxidoreductase (complex I) in rat heart. Biochimica et Biophysica Acta 1556: 6–12. [DOI] [PubMed] [Google Scholar]
- Millar AH, Trend AE, Heazlewood JL. 2004. Changes in the mitochondrial proteome during the anoxia to air transition in rice focus around cytochrome-containing respiratory complexes. Journal of Biological Chemistry 279: 39471–39478. [DOI] [PubMed] [Google Scholar]
- Minhas D, Grover A. 1999. Towards developing transgenic rice plants tolerant to flooding stress. Proceedings of Indian National Science Academy B65: 33–50. [Google Scholar]
- Moons A. 2003. Ospdr9, which encodes a PDR-type ABC transporter, is induced by heavy metals, hypoxic stress and redox perurbations in rice roots. FEBS Letters 553: 370–376. [DOI] [PubMed] [Google Scholar]
- Muench DG, Good AG. 1994. Hypoxically inducible barley alanine aminotransferase: cDNA cloning and expression analysis. Plant Molecular Biology 24: 417–427. [DOI] [PubMed] [Google Scholar]
- de Oliveira DE, Seurinck J, Inze D, Van Montagu M, Botterman J. 1990. Differential expression of five Arabidopsis genes encoding glycine rich proteins. The Plant Cell 2: 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provart NJ, Gil P, Chen W, Han B, Chang HS, Wang X, et al. 2003. Gene expression phenotypes of Arabidopsis associated with sensitivity to low temperatures. Plant Physiology 132: 893–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, et al. 2003. Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiology 133: 1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachetto-Martins G, Franco LO, Felix D, de Oliveira D. 2000. Plant glycine rich proteins: a family or just proteins with a common motif? Biochimica et Biophysica Acta 1492: 1–14. [DOI] [PubMed] [Google Scholar]
- Sachs MM, Freeling M, Okimoto R. 1980. The anaerobic proteins of maize. Cell 20: 761–767. [DOI] [PubMed] [Google Scholar]
- Sahi C, Agarwal M, Reddy MK, Sopory SK, Grover A. 2003. Isolation and expression analysis of salt stress-associated ESTs from contrasting rice cultivars using a PCR-based subtraction method. Theoretical and Applied Genetics 106: 620–628. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez R, Davies TGE, Coleman JOD, Rea P. 2001. The Arabidopsis thaliana ABC protein superfamily: a complete inventory. Journal of Biological Chemistry 276: 30231–30244. [DOI] [PubMed] [Google Scholar]
- Sato T, Harada T, Ishizawa K. 2002. Stimulation of glycolysis in anaerobic elongation of pondweed (Potamogeton distinctus) turions. Journal of Experimental Botany 53: 1847–1856. [DOI] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, et al. 2000. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proceedings of National Academy of Sciences of the USA 97: 11655–11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto K, Kyozuka J. 2002. Rice as a model of comparative genomics of plants. Annual Review of Plant Physiology and Plant Molecular Biology 53: 399–419. [DOI] [PubMed] [Google Scholar]
- Smeekens S, Rook F. 1997. Sugar sensing and sugar-mediated signal transduction in plants. Plant Physiology 115: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderman E, Mattsson J, Engstrom P. 1996. The Arabidopsis homeobox gene ATHB-7 is induced by water deficit and by abscisic acid. The Plant Journal 10: 375–381. [DOI] [PubMed] [Google Scholar]
- Stadler R, Buttner M, Ache P, Hedrich R, Ivashikina N, Melzer M, et al. 2003. Diurnal and light-regulated expression of AtSTP1 in guard cells of Arabidopsis. Plant Physiology 133: 528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoulou FL. 2000. Plant ABC transporters. Biochimica et Biophysica Acta 1465: 79–103. [DOI] [PubMed] [Google Scholar]
- Thorneycroft D, Sherson SM, Smith SM. 2001. Using gene knock-outs to investigate plant metabolism. Journal of Experimental Botany 52: 1593–1601. [PubMed] [Google Scholar]
- Truernit E, Schmid J, Epple P, Illg J, Sauer N. 1996. The sink-specific and stress regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors and pathogen challenge. The Plant Cell 8: 2169–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widawsky DA, O'Toole JC. 1990.Prioritizing rice biotechnology research agenda for Eastern India. New York, NY: The Rockefeller Foundation. [Google Scholar]
- Zhang Z, Wu RS, Mok HO, Wang Y, Poon WW, Cheng SH, Kong RY. 2003. Isolation, characterization and expression analysis of a hypoxia-responsive glucose transporter gene from grass carp, Ctenopharyngodon idellus European Journal of Biochemistry 270: 3010–3017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


