Abstract
• Background and Aims Free-flowing surface exudates at the stigmatic (wet versus dry stigma) and adaxial epidermis at the site of angiospermy in carpels of Chloranthaceous species have been proposed to comprise a continuous extracellular matrix (ECM) operating in pollen tube transmission to the ovary. The aim of this research was to establish the spatial distribution and histo/immunochemical composition of the ECM involved in pollen tube growth in Sarcandra glabra and Chloranthus japonicus (Chloranthaceae).
• Methods Following confirmation of the pollen tube pathway, the histo/immunochemical make-up of the ECM was determined with histochemistry on fresh tissue to detect cuticle, esterase, proteins, pectins, and lipids and immunolocalization at the level of the TEM on sections from cryofixed/freeze-substituted tissue to detect molecules recognized by antibodies to homogalacturonans (JIM7, 5), arabinogalactan-proteins (JIM13) and cysteine-rich adhesion (SCA).
• Key Results Pollen germinability is low in both species. When grains germinate, they do so on an ECM comprised of an esterase-positive cuticle proper (dry versus wet stigma). Pollen tubes do not track the surface ECM of stigma or adaxial epidermal cells at the site of angiospermy. Instead, tubes grow between stigmatic cells and subsequently along the inner tangential walls of the stigmatic and adaxial carpel cells at the site of angiospermy. Pollen tubes enter the ovary locule at the base of the funiculus. The stigmatic ECM is distinct by virtue of the presence of anti-JIM5 aggregates, lipids, and a protein recognized by anti-SCA.
• Conclusions The Chloranthaceae joins a growing number of basal angiosperm taxa whereby pollen tubes germinate on a dry versus wet stigma to subsequently grow intercellularly en route to the ovary thereby challenging traditional views that the archetype pollen tube pathway was composed of the surface of stigma and adaxial epidermal cells covered with a free-flowing exudate.
Keywords: Chloranthaceae, dry stigma, transmitting tissue, extracellular matrix, homogalacturonans, lipids, anti-SCA
INTRODUCTION
The Chloranthaceae, a basal angiosperm taxon, is composed of approx. 65–75 extant species and four extant genera, Ascarina, Chloranthus, Hedyosmum and Sarcandra (Zhang and Renner, 2003; Eklund et al., 2004). The systematic position of Chloranthaceae within the basal angiosperms has long been uncertain (Eklund et al., 2004), a situation still not resolved because recent molecular phylogenies place the family in various positions above the ‘ANITA’ grade, a paraphyletic group of seven taxa now situated at the base of the angiosperm phylogeny (Qiu et al., 1999; Mathews and Donoghue, 2000;Soltis et al., 2000; Sun et al., 2002; Zanis et al., 2002; Friis et al., 2003). The Chloranthaceae is a key taxon because it has played an important and long-standing role in the discussion on the origins and early evolution of reproduction in flowering plants due to the development of primitively simple flowers with a fossil record that extends back to the Early Cretaceous (Crane et al., 1989; Herendeen et al., 1993; Friis et al., 1994, 1999; Eklund et al., 1997).
The gynoecium of the perianthless flowers of Chloranthaceae shares many features found in other members of basally rooted angiosperms (Endress and Igersheim, 2000). The carpel is ascidate and lacks a style. As well, carpel closure (angiospermy) occurs by production of a secretion in the absence of post-genital fusion (Endress and Igersheim, 2000). Exudates at the epidermal surface at the site of angiospermy appear continuous with those at the surface of epidermal stigmatic cells and the ovary locule (Endress, 1986, 1994; Endress and Igersheim, 2000). Thus, in addition to playing a role in carpel closure, secretions at the site of angiospermy have been proposed to function in transmission of the pollen tube from the region of pollen capture, hydration, and germination at the stigma to the ovary with pollen tube growth occurring along the surface of the adaxial carpel epidermis (Edwards, 1920; Endress and Igersheim, 2000). Although structural studies have provided insight into the distribution and potential role of epidermal surface exudates in carpel closure, histo/immunochemical information on these secretions is absent for the taxon as is data confirming their role in pollen tube transmission from the stigma to the ovary. In angiosperms, the network of molecules involved in pollen tube growth situated external to the plasma membrane form a specialized transmitting tissue extracellular matrix (ECM; Lord, 2003). Some ECM molecules, important in compatible pollen tube growth in transmitting tissue of other angiosperms, include calcium, pectins, lipids, arabinogalactans/arabinogalactan-proteins (AGs/AGPs) as well as other proteins (Wu et al., 2000; Lord and Russell, 2002; Khosravi et al., 2003; Kim et al., 2003) to include cysteine-rich adhesion (SCA), a lipid-like transfer protein recently described from lily (Mollet et al., 2000; Park et al., 2000; Park and Lord, 2003).
The purpose of this study was to examine the ECM produced by the gynoecium of species from two of the sister-groups within the Chloranthaceae with bisexual flowers, Sarcandra glabra and Chloranthus japonicus. Two basic questions were addressed: (1) Does the ECM at the stigma and site of angiospermy form a continuous pathway for pollen tube growth to the ovary locule whereby pollen tubes grow solely along the surface of the carpel epidermis? (2) What is the histo/immunochemical nature and organization of the ECM at the stigma and other transmitting tissues?
MATERIALS AND METHODS
Plant material
Plants of Sarcandra glabra and Chloranthus japonicus were grown in the University of Toronto greenhouse facility at day/night temperatures of 25 ± 3 °C/22 ± 2 °C, respectively, with an average light intensity of 300 µm−2 s−1. Plants were watered daily and fertilized once a week with a 20 : 20 : 20 NPK mix. Flowers were pollinated with both cross- and self-pollen at stigma receptivity, the timing of which has been described by Tosaki et al. (2001). For each species, four or five plants were used for pollinations. Pollinations were conducted over a 3-month period of flowering.
Pollen tube pathway
To confirm the pathway of pollen tube growth, and hence distribution of transmitting tissue ECM, 17–24 flowers per plant were pollinated with cross- and self-pollen and harvested at 24 and 48 h and prepared for aniline-blue fluorescence microscopy as described by Martin (1959). Pollinated gynoecia of each species were also prepared for serial sectioning of resin embedded samples for observation at the light microscope level 24 and 48 h (n = 14 flowers per plant per harvest time) after cross-pollination and 3 h and 5 d following cross- and self-pollination (n = 5 flowers per plant per pollination treatment) as described by Pontieri and Sage (1999). Serial sections were stained with toluidine blue (O'Brien et al., 1964).
Transmitting tissue ECM composition
Histochemical features of the unpollinated gynoecial ECM were characterized on both whole mounts and free-hand sections of fresh carpels (n = 17–40 carpels per histochemical stain per species) at stigma receptivity using the following stains: (a) 0·01 % auramine-O in 0·05 m TRIS/HCl buffer at pH 7·2 observed under UV light for lipids and cutin (Heslop-Harrison and Shivanna, 1977); (b) nile red in 75 % glycerol for neutral lipids (Greenspan et al., 1985); (c) 0·001 % 8-anilinonapthalene-1-sulphonic acid (8-ANS) observed under UV for proteins (Mattsson et al., 1974; Fulcher and Wong, 1980); and (d) indoxyl acetate in 0·1 m TRIS/HCl buffer at pH 7·0, in 0·1 m potassium ferrocyanide and potassium ferricyanide for detection of nonspecific esterase activity of the stigma pellicle (Dejong et al., 1967). Controls for nonspecific esterase activity were run by omitting the substrate. Controls for remaining histochemical reagents were run by omitting the stain.
The presence or absence and spatial distribution of molecules recognized by antibodies to the high methyl-esterified homogalacturonans (JIM7), low methyl-esterified homogalacturonans (JIM5), an arabinogalactan-protein (JIM13), and the cysteine-rich adhesion from lily (SCA; Park et al., 2000) was determined using immunolocalization at the level of the transmission electron microscope (TEM). Unpollinated flowers in the female phase of ontogeny at the time of stigma receptivity were cryofixed, freeze-substituted, and embedded as described by Lam et al. (2001). Serial sections (70 nm thick) of six to nine carpels per plant per species were collected on formvar-coated grids and incubated with monoclonal antibodies to JIM7, JIM5 and JIM13 (PlantProbes, Leeds, UK), and polyclonal antibodies to SCA (Elizabeth Lord, UC Riverside, CA, USA). Primary and secondary (anti-rat IgG-gold conjugate 18 nm for JIMs; anti-rabbit IgG-gold conjugate 18 nm for SCA; Jackson Immunoresearch, West Grove, PA, USA) antibody dilutions were 1 : 50 and 1 : 20 respectively. Incubation times in 1° and 2° antibodies were 2 and 1 h, respectively, for JIMs and 6 and 1 h, respectively, for SCA. Controls were run by omitting 1° antibody. To determine antibody specificity and distribution, localization events of anti-JIMs and anti-SCA were quantified on an area basis by determining the number of gold particles µm−2 using Image-Pro Plus (Media Cybernetiks, Silver Springs, MD, USA). Density values for each antibody were determined at the surface, between, and base (tangential wall) of stigmatic epidermal cells, as well as epidermal cells at the abaxial carpel surface and adaxial surface at the site of angiospermy, to determine if transmitting tissue ECM was unique in composition. First, all density values at each epidermal location for each antibody per carpel were contrasted using one-way ANOVA (Sigma Stat 2.03). No differences were observed between carpels, therefore all values per carpel per antibody were pooled for each location at each respective epidermal position. Density values per antibody were then contrasted between the stigma and abaxial epidermis and stigma and adaxial epidermis at the site of angiospermy at the surface, between and base of each respective epidermal cell. Images were captured on the Phillips 201 TEM equipped with an Advantage HR Camera System (Advanced Microscopy Techniques Crop, Danvers, MA, USA).
General structural features
Sections from cryofix/freeze-substituted female phase carpels prepared for TEM were also used to determine general cytoplasmic features of cells comprising the transmitting tissue and the associated ECM. As well, cryofix/freeze-substituted gynoecia were prepared for scanning electron microscopy (SEM) as described by Thien et al. (2003).
RESULTS
Observations on the pollen tube pathway and composition of the gynoecial ECM were the same for both Sarcandra glabra and Chloranthus japonicus. Therefore, only results for S. glabra will be presented.
Pollen tube pathway
Tubes from germinating pollen first grow downward between stigmatic epidermal cells (Fig. 1A and B) and subsequently intercellularly towards the carpel margin at the site of angiospermy within an expanded ECM at the base of stigmatic epidermal cells (Fig. 1A and B). Upon reaching the base of epidermal cells at the site of angiospermy, pollen tubes change direction and again grow downward, but still intercellulary within an expanded ECM of the inner tangential wall of the epidermal cells along the site of angiospermy towards the ovary locule (Fig. 1B and C). Pollen tubes then exit the site of intercellular growth at the base of the funiculus where they continue to grow within the ovary locule along the adaxial carpel surface (Fig. 1D) to finally enter the ovule micropyle (Fig. 1E). Pollen tubes penetrate the ovule micropyle by 24 h post-pollination at which time the embryo sac contains two polar nuclei, numerous antipodal cells, an egg cell (Fig. 1E) and two synergids as noted previously by Yoshida (1957, 1960) as cited by Davis (1966).
Fig. 1.
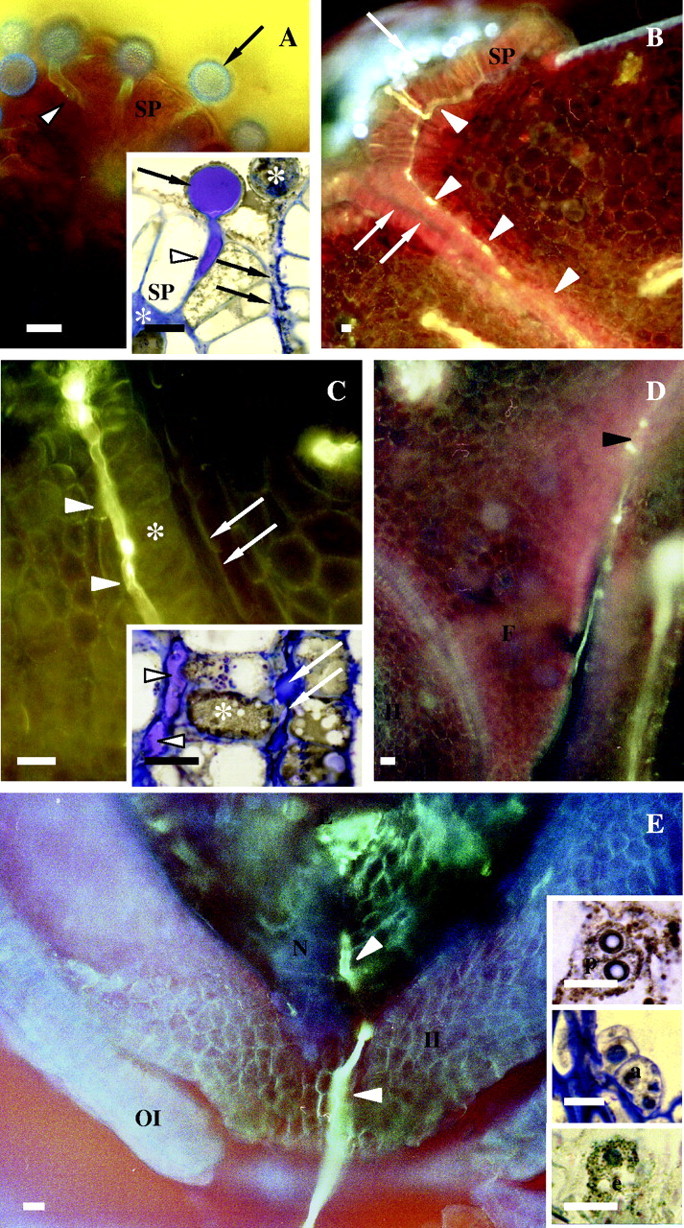
Fluorescence and light micrographs illustrating pollen tube growth in Sarcandra glabra. Single arrows label pollen grains. White arrowheads denote pollen tubes. Double arrows mark epidermal adaxial surface at site of angiospermy. (A) Pollen tube growth between stigmatic papillae. Six-pronged and five-pronged asterisks label expanded ECM and ungerminated pollen grain, respectively. Whole-mount, aniline blue fluorescence and cryo-fixed, 1·5-µm resin-embedded section, toluidine blue (insert). (B) Intercellular pollen tube growth at base of stigmatic epidermal cells and adaxial epidermal cells at the site of angiospermy. Free-hand section, aniline blue fluorescence. (C) Intercellular pollen tube growth at base of adaxial epidermal cells at the site of angiospermy. Asterisk marks papillate adaxial epidermal cells. Free-hand section, aniline blue fluorescence and cryo-fixed, 1·5-µm resin-embedded section, toluidine blue (insert). (D) Pollen tube exiting intercellular growth at base of funiculus (black arrowhead) and entering site of extracellular growth in ovary locule. Free-hand section, aniline blue fluorescence. (E) Pollen tube growth within ovule micropyle and nucellus. Embryo sacs at the time of pollen tube entrance into the ovule micropyle contain two unfused polar nuclei (upper inset), numerous antipodals (middle inset), an egg cell (low inset) and two synergids (not shown). Free-hand section, aniline blue fluorescence, and cryo-fixed, 1·5-µm resin-embedded section, toluidine blue (inserts). Scale bars = 20 µm. a, Antipidal cells; e, egg cell; E, embryo sac; F, funiculus; II, inner integument; N, nucellus; p, polar nuclei; SP, stigmatic papilla.
The aforementioned patterns of pollen tube growth were observed in only 20 flowers of over 100 cross-pollinated flowers per species and three of over 100 self-pollinated flowers per species. In general, pollen tube growth was observed in two or three flowers per plant for every 20 flowers cross-pollinated per plant and one of every 15 flowers per plant receiving self-pollen. Within flowers with germinating pollen, the percentage of cross-pollen grains germinating was approx. 7 % with only 1 % of germinated grains reaching the ovule micropyle. The percentage of self-pollen germination was <1 %. Germinating pollen grains that do not reach the micropyle cease growth within the expanded ECM. Inspection of serially sectioned pollinated carpels indicates that approx. 89 % of grains contain cytoplasm, a vegetative nucleus and nucleated generative cell.
Transmitting tissue ECM composition
The ECM of the stigmatic epidermal cells is composed of an outermost region histochemically positive for esterase activity (Fig. 2A and B), 8-ANS (Fig. 2C), nile red (Fig. 2D) and auramine-O (Fig. 2E). In each instance, staining is continuous over stigmatic epidermal cells with localized regions of increased staining resembling droplets in the case of nile red (Fig. 2D) and auramine-O (Fig. 2E) that vary in size. Droplets positive for auramine-O (Fig. 2E) and nile-red are also present in the expanded ECM at the base of stigmatic epidermal cells. Similar osmiophilic droplets are also observed in sections of cryofixed tissue (Fig. 2F) indicating that droplets are not artefacts of fresh tissue preparation.
Fig. 2.
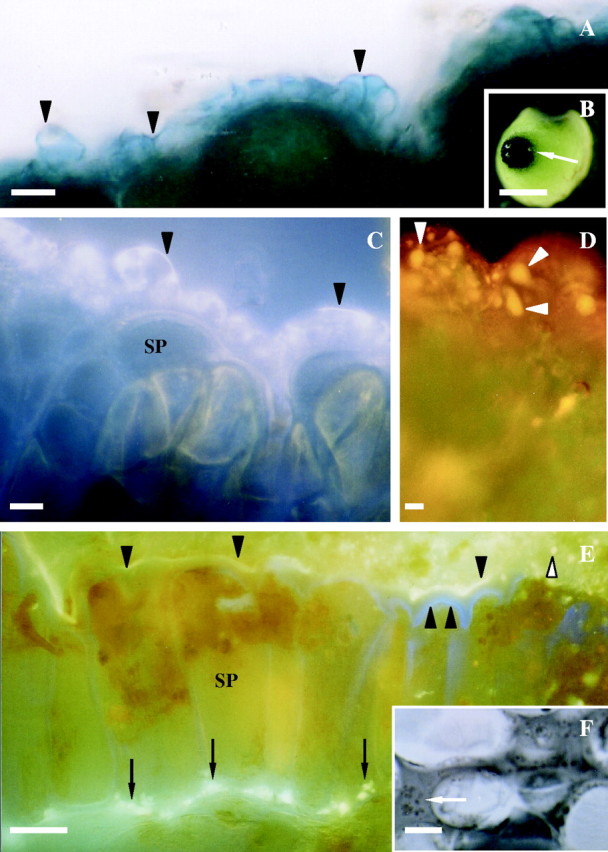
Histochemistry of stigmatic region of Sarcandra glabra at receptivity. (A) Arrowheads mark dark blue-positive esterase at cuticle surface of stigmatic epidermal cells. Free-hand section of fresh tissue. (B) Low magnification view of esterase-positive stigma (arrow). (C) 8-ANS-positive fluorescence (arrowheads) indicating protein distribution at the cuticle proper and cuticular layer of the stigmatic epidermal cell. Fresh tissue whole mount. (D) Orange nile-red-positive fluorescence demonstrating presence of neutral lipids (arrowheads) throughout the cuticle proper and cuticular layer of stigmatic epidermal ECM. Fresh tissue whole mount. (E) Auramine-O-positive yellow-green fluorescence (arrowheads) showing presence of culticle. Arrows denote lipids in expanded ECM between stigmatic epidermal cells and adjacent cells where pollen tubes will grow. Double arrowhead marks primary wall. White arrowhead notes auramine-O-positive droplet. Free-hand section of fresh tissue. (F) Arrow marks osmiophilic-positive droplets in expanded ECM between stigmatic epidermal cells and subtending cells where pollen tubes will grow. Cryofix, serial section. Scale bars: B = 7 mm; A, C–F = 10 µm. SP, Stigmatic papilla.
The ECM of the stigma epidermal cells is structurally and immunologically heterogeneous at the level of the SEM and TEM (Figs 3–5). The primary wall of the surface of stigmatic epidermal cells is immunopositive for JIM7 (Fig. 3C and G) and JIM5 (Fig. 3E). Anti-JIM7 is dispersed equally throughout the primary wall (Fig. 3C), whereas anti-JIM5 is present primarily towards the outer periphery of the primary wall (Fig. 3E). A continuous, thinly lamellate, outer region that varies in thickness (Fig. 3C and E) overlies large expanded peg-like areas of ECM that extend from the primary wall and localize with anti-JIM7 (Fig. 3C and G). Pockets of anti-JIM5 aggregates (Fig. 3E), as well as spherical areas with an absence of any immunopositive compounds (Fig. 3G), are embedded within this anti-JIM7-rich ECM. The spherical immunonegative areas are interpreted to be the equivalent of the auramine-O- and nile-red-positive regions described above. The expanded peg-like regions of ECM that localize with anti-JIM7 and anti-JIM5 are divided by channels (Fig. 3G) that are 0·06 ± 0·004 µm (n = 48) in diameter. Anti-JIM7 is also dispersed throughout the primary wall between adjoining stigmatic cells (Fig. 3H), whereas anti-JIM5 is localized exclusively within the middle lamella (Fig. 3I). The expanded ECM where pollen tubes will grow intercellularly at the base of stigmatic epidermal cells is immunopositive for JIM7 and JIM5, although JIM5 is evenly dispersed throughout and not present in localized pockets as observed in the ECM at the surface of stigmatic epidermal cells. Anti-JIM13 localizes to the plasma membrane/primary wall boundary at all locations of stigmatic epidermal cells (Fig. 4A and B).
Fig. 3.
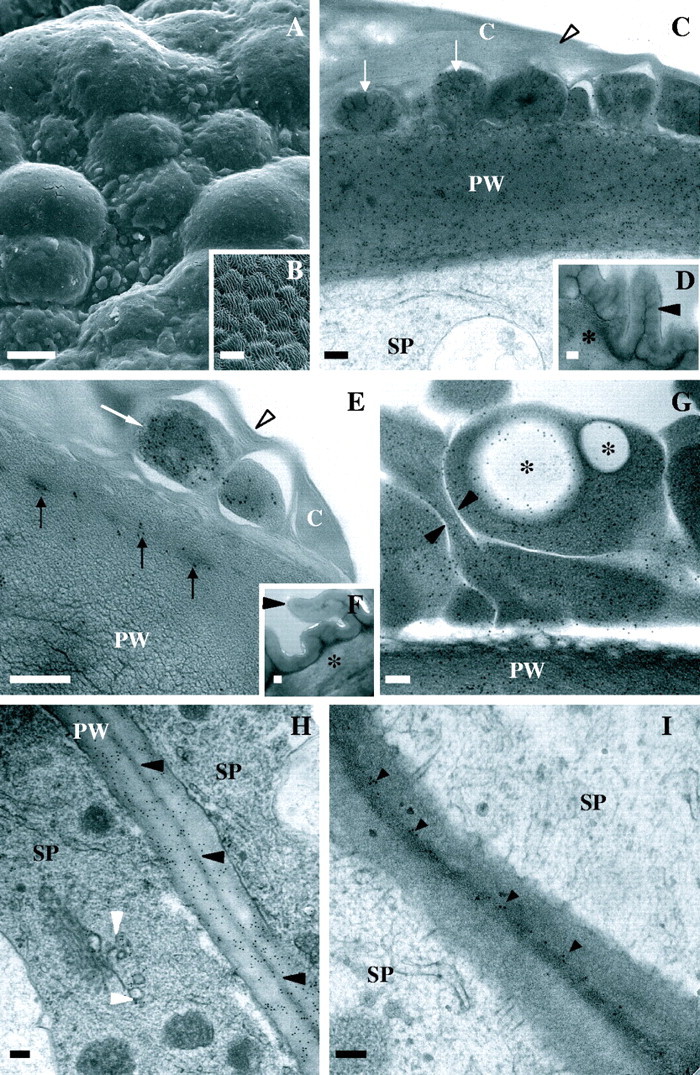
Scanning and transmission electron micrographs of stigmatic and abaxial epidermal cells of Sarcandra glabra. (C–I) Immunolocalization of anti-JIM7 or JIM5 (18 nm gold particles). (A) Heterogenous surface topology of stigmatic ECM. (B) Ridged surface topology of abaxial epidermis. (C) Anti-JIM7 in cuticular layer (arrows) and subtending primary wall of stigmatic epidermal cells. Arrowhead marks thinly lamellate cuticle proper. (D) Anti-JIM7 in primary wall of abaxial epidermal cell. Arrowhead and asterisk mark thick cuticle and primary wall, respectively. (E) Ant-JIM in cuticular layer (arrows) and primary wall of stigmatic epidermal cells. Arowhead denotes thinly lamellate cuticle. (F) Anti-JIM5 in primary wall of abaxial epidermal cell. Arrowhead and asterisk mark thick cuticle and primary wall, respectively. (G) Anti-JIM7 in cuticular layer arrows and primary wall of stigmatic epidermal cell. Note large spherical immunonegative spherical regions (asterisks) which are likely to correspond to nile-red- and auramino-O-positive regions. Arrowheads mark channels separating homogalacturonan-rich areas within the cuticular layer. (H) Anti-JIM7 between stigmatic epidermal cells. Black arrowheads mark middle lamella. White arrowheads denote gold particles in Golgi body-associated vesicles. (I) Anti-JIM5 in middle lamella (arrowheads) between stigmatic epidermal cells. Scale bars: A and B = 10 µm; C–I = 200 nm. C, Cuticle; PW, primary wall; SP, stigmatic papilla.
Fig. 4.
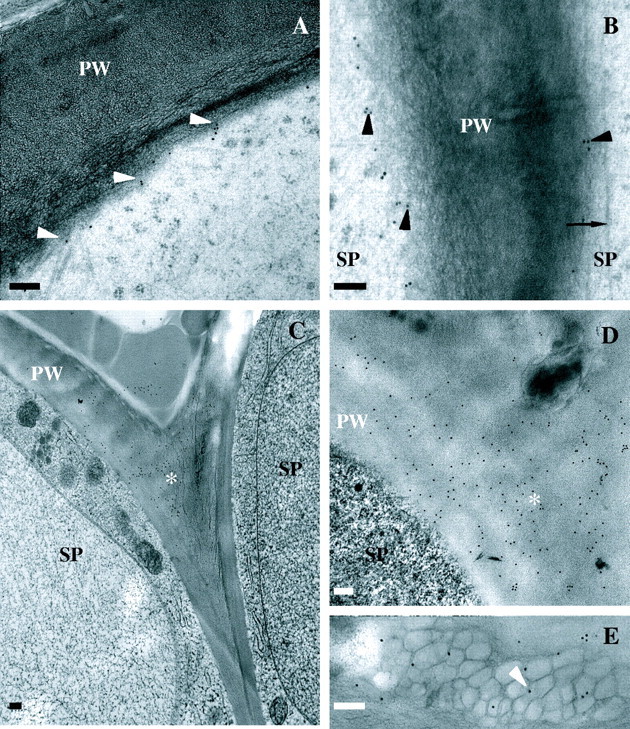
Immunolocalization of JIM13 (18 nm gold particles; A and B) and SCA (18 nm gold particles; C–E) in stigmatic epidermal cell ECM. (A) Localization at plasma membrane/primary wall boundary (arrowheads) at surface of stigmatic epidermal cells. (B) Localization at plasma membrane/primary wall boundary (arrowheads) between stigmatic epidermal cells. Arrow marks microtubule. (C and D) Localization in primary wall at notch (asterisk) between stigmatic epidermal cells. (E) Localization in expanded ECM in association with putative auramine-O/nile red-positive droplets (arrowhead). Scale bars = 200 nm; PW, primary wall; SP, stigmatic papilla.
Fig. 5.
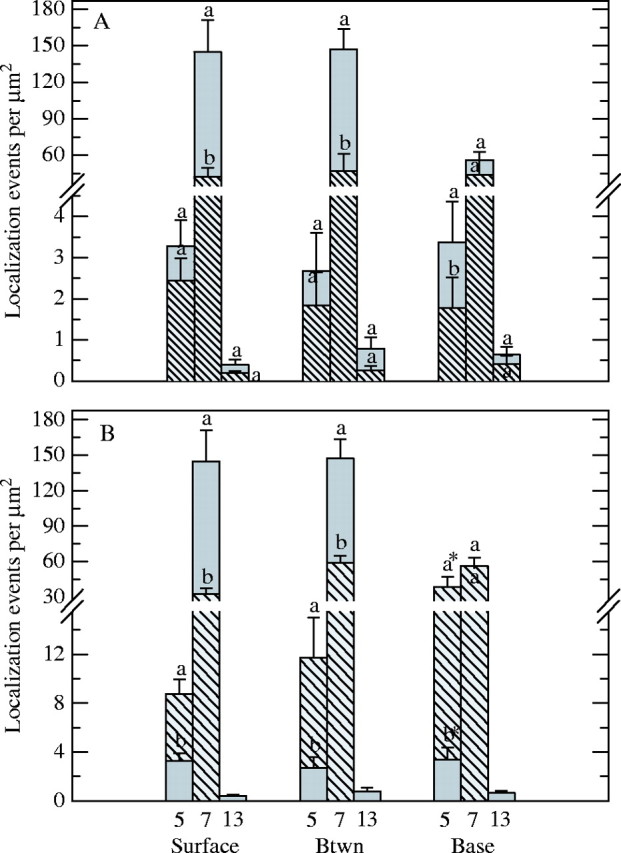
Density of anti-JIM7, anti-JIM5 and anti-JIM13 localization in the ECM at the surface, between (Btwn) and base of stigmatic (A and B, solid bars), abaxial (A, hatched bars) and adaxial (B, hatched bars) epidermal cells of Sarcandra glabra. Values labelled with a different letter at each position for each antibody are significantly different from one another at P ≤ 0·05. Error bars represent 95 % confidence interval. Note brake in y-axis. Asterisk labelling hatched bar for anti-JIM5 localization (B) represents density values for ECM at base of papillate versus tabular cells on opposing side of site of angiospermy where anti-JIM5 localization was only 4× that of stigmatic epidermal cells (data not shown).
The ECM associated with the adaxial carpel epidermal cells at the site of angiospermy and abaxial epidermal carpel cells is different at the histo/immunochemical level from the ECM of stigmatic epidermal cells at stigma receptivity. The ECM at both sites is not histochemically positive for esterase (Fig. 2B), 8-ANS, or nile red, but is positive for auramine-O except for an absence of droplets. An undulating, thick cuticle covers a primary wall of the surface of abaxial epidermal cells (Fig. 3B, D and F). The primary wall is immunopositive for anti-JIM7 (Fig. 3D) and anti-JIM5 (Fig. 3F), both of which are evenly distributed, and is quantitatively distinct from that of stigmatic epidermal cells with respect to the density of anti-JIM7 (Fig. 5A). The ECM between the abaxial epidermal cells is also quantitatively distinct from that of the stigma with respect to anti-JIM7 density, whereas the ECM at the base of adaxial epidermal cells is significantly different for anti-JIM5 (Fig. 5A). The ECM at the base of the abaxial epidermal cells is not expanded as observed at the stigma. The ECM associated with the surface of the adaxial epidermis at the site of angiospermy is ultrastructurally similar to that of the stigmatic epidermal cells except for an absence of anti-JIM5 aggregates and spherical immunonegative regions. The surface ECM of adaxial epidermal cells that are continuous with the funiculus is associated with papillate epidermal cells (Fig. 1C) and is separated from the ECM produced by the epidermis on the opposite side of angiospermy by the cuticle. Epidermal cells of the opposite side at the site of angiospermy are tabular (Fig. 1C) and the ECM is approx. 3× as wide as that of the opposing papillate cells. The ECM at the surface, between, and base of the adaxial epidermal cells contains significantly higher immunodensity of anti-JIM5 versus that quantified for stigmatic epidermal cells (Fig. 5B). Anti-JIM13 localization is absent at the site of angiospermy. No non-specific binding was present on the Spurr's resin outside of the sectioned tissue in the presence and absence of 1° antibodies.
Anti-SCA localization is restricted to the stigmatic epidermal surface and expanded ECM at the base of the stigmatic epidermal cells (Fig. 4C–E) versus the ECM of abaxial and adaxial epidermal cells at the site of angiospermy. Although present within the ECM of the surface of stigmatic epidermal cells (x = 7·7 ± 1·8 gold particles µm−2; n = 28) and expanded ECM (x = 9·6 ± 0·9 gold particles µm−2; n = 31), the density of anti-SCA is significantly highest (P ≤ 0·05) in the notch between stigmatic cells (Fig. 4C and D; x = 18·4 ± 2·5 gold particles µm−2;n = 28). Non-specific binding was only present in cells with cryofixation damage and, therefore, these areas were excluded from analysis.
The protoplast of stigmatic cells contains an extensive endomembrane system composed of rough endoplasmic reticulum, Golgi bodies, vesicles, and tubular-vesicular endosomal-like arrays (Fig. 6). Cortical microtubule arrays are abundant and vesicles and the tubular-vesicular endosomal-like arrays are frequently tethered to the microtubules (Fig. 6B and C). Golgi body-derived vesicles as well as vesicles associated with the plasma membrane are immunopositive for anti-JIM7 (Fig. 6D–F) and anti-SCA (Fig. 6G).
Fig. 6.
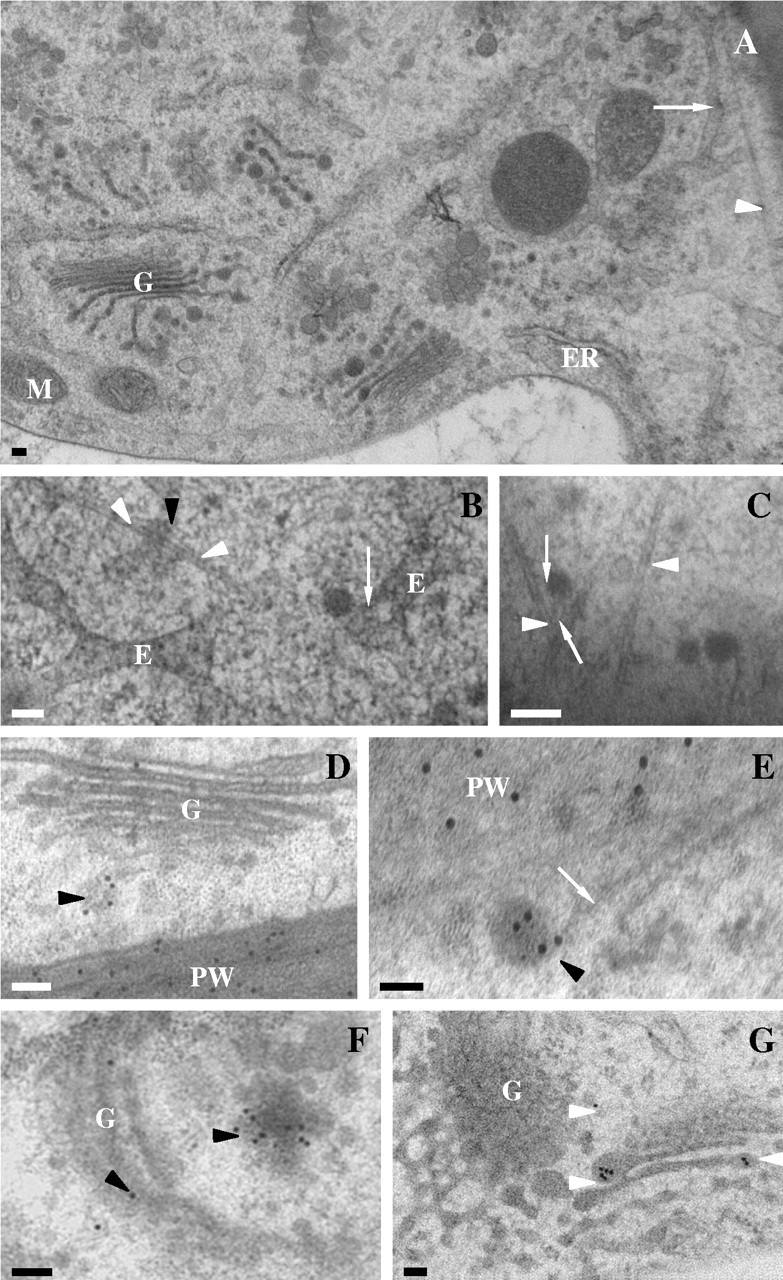
Stigmatic epidermal cell ultrastructure in Sarcandra glabra. (A) Low magnification illustrating extensive endomembrane system. Arrow denotes tubular-vesicular endosome-like array. Arrowhead marks microtubule. (B) Tubular-vesicular endosomal-like array with tethered vesicles (arrow) and microtubule (arrowheads). (C) Vesicles tethered (arrows) to microtubules (arrowheads). (D–F) Immunolocalization of JIM7 (18-nm gold particles) to Golgi body-associated vesicle (black arrowheads). White arrow marks microtubule. (G) Immunolocalization of SCA (18-nm gold particles) to Golgi body-associated vesicles (arrowheads). Scale bars = 100 nm. E, Tubular-vesicular endosome-like array; ER, endoplasmic reticulum; G, Golgi body; PW, primary wall.
DISCUSSION
The present study provides significant data on the spatial features of pollen tube growth within transmitting tissue as well as ultrastructure and composition of transmitting tissue and associated ECM in carpels of Sarcandra glabra and Chloranthus japonicus, two species of the basal angiosperm taxon, Chloranthaceae. Characterization of pollen tube growth determined that, even though the ECM of the stigma epidermal cells is continuous with the surface ECM of the adaxial carpel epidermis at the site of angiospermy, pollen tubes do not track the epidermal surface ECM en route to the ovary. Rather, pollen tubes penetrate the ECM between adjacent stigmatic epidermal cells, grow intercellularly in an expanded ECM that extends along the tangential walls of stigmatic epidermal cells and subsequently along the tangential walls of adaxial epidermal cells at the site of angiospermy. Pollen tubes exit this ‘solid’ transmitting tissue at the entrance of the ovary locule at the base of the funiculus. Significantly, intercellular pollen tube growth at the stigmatic surface occurs even if pollen grains land on the stigma ECM at the entrance to the site of angiospermy. Evidence demonstrates that, although the ECM of the stigmatic surface of each species is heterogeneous in thickness and composition, containing high and low methyl-esterified homogalacturonans, lipids, and proteins, to include an arabinogalactan-protein recognized by anti-JIM13 and a protein recognized by anti-SCA, these components do not comprise a free-flowing secretion but they are discretely contained and organized below a cuticle with associated esterase activity at the time of stigma receptivity. The stigmatic ECM is distinct from that at the site of angiospermy and abaxial epidermis with respect to the collective presence of anti-JIM5 aggregates, lipids, and a protein recognized by anti-SCA. Unexpectedly, confirmation of the pollen tube pathway with fluorescence and light microscopy also revealed that a very low number of cross-pollen grains germinate and virtually no self-pollen grains germinate in either species. We begin the discussion by placing the structural and histo/immunochemical results on the stigma in S. glabra and C. japonicus in the context of observations on the stigma of other angiosperms, to include previous conclusions regarding the nature of the stigmatic epidermal cells of Chloranthaceae. We then discuss the functional significance of the presence of low methyl-esterified homogalacturonan aggregates, a protein recognized by anti-SCA, and lipids for pollen tube growth within the stigmatic transmitting tissue ECM. The low rates of pollen germination have implications for the breeding system of these two species. Thus, the present observations on pollen germinability are also compared with previous studies on the breeding system of numerous species within the taxon. Finally, because the Chloranthaceae have many features that may have been common to ancestral angiosperms, the above results are placed in the context of current hypotheses regarding the evolution of angiosperm transmitting tissue and pollen–carpel interactions therein.
Stigmatic ECM
The stigma of angiosperms has been classified into dry and wet types, with some families containing species of both types (Heslop-Harrison and Shivanna, 1977). The ECM of both dry and wet type stigmas are characterized by the presence of a protein layer with esterase activity (pellicle; Heslop-Harrison and Heslop-Harrison, 1970; Mattsson et al., 1974) that is associated with a cuticle (Heslop-Harrison and Shivanna, 1977). However, in the dry type, the pellicle and cuticle remain intact in the functional phase, whereas they become discontinuous in the wet type (Heslop-Harrison and Shivanna, 1977; Shivanna and Sastri, 1981). Functionally, the dry stigma is posited to exert more control over pollen adhesion, recognition and hydration than the wet stigma (Dickinson, 1995; Zinkl et al., 1999).
The present study on the stigma ECM of both S. glabra and C. japonicus provides clear evidence that the stigma is of the dry type. At the time of stigma receptivity, the outermost portion of the stigmatic epidermal cell ECM is composed of a continuous esterase-positive pellicle associated with a structurally continuous lamellate region that corresponds ultrastructurally to the cuticle proper of epidermal cells from leaves and dry stigmas of many species (Jeffree, 1996). This outermost region of the ECM, while exhibiting a positive reaction for both esterase activity and protein, also fluoresces a yellow-green in the presence of auramine-O indicative of a cuticle (Heslop-Harrison and Shivanna, 1977). At the ultrastructural level, the cuticle proper of S. glabra and C. japonicus stigmas is subtended by expanded pegs of a pectin-rich matrix that extend from the adjacent cellulosic/pectinaceous primary wall. This region of the ECM with the pectin-rich pegs corresponds to a cuticular layer in models of the plant cuticle (Jeffree, 1996) and also occurs in the dry stigmas of monocots and eudicots (Heslop-Harrison and Heslop-Harrison, 1982), and to a lesser degree in Amborella trichopoda (Thien et al., 2003) and Illicium floridanum (Koehl, 2002), two species belonging to the ‘ANITA’ grade placed at the base of the angiosperm phylogeny (Mathews and Donoghue, 2000; Qiu et al., 2000; Soltis et al., 2000; Sun et al., 2002; Zanis et al., 2002).
To date, classification of stigma type of species within the Chloranthaceae has been contradictory. The stigmas of Ascarina and Chloranthus have been reported to be either of the dry (Heslop-Harrison and Shivanna, 1977; Endress, 1986) or the wet type (Endress, 1986), and the stigmas of Hedyosmum and Sarcandra have been ascribed as the dry and wet type, respectively (Endress, 1986; Endress and Igershiem, 2000). Pectins, while assuming numerous functions in the plant cell (Carpita and Gibeaut, 1993; Knox, 1999), operate within the ECM to maintain a highly hydrated state. A hydrated ECM of the stigmatic papilla of S. glabra and C. japonicus may result in a ‘glistening’ of the cells which upon inspection could easily lead to the interpretation that the stigma is of the wet type.
Functional significance of transmitting tissue ECM composition
The ECM of transmitting tissue in the stigmatic region versus the ECM of the adaxial epidermal cells at the site of angiospermy of S. glabra and C. japonicus carpels is composed of a suite of components previously demonstrated to operate in the biology of pollen tube growth in other angiosperms (Lord and Russell, 2002). High and low methyl-esterified homogalacturonans are common components of the angiosperm transmitting tissue ECM (Vennigerholz, 1992; Jauh and Lord, 1996; Wu et al., 2000; Lenartowska et al., 2001; Khosravi et al., 2003). Homogalacturonans, synthesized and secreted as highly methyl-esterified forms by the plant cell Golgi apparatus, are de-esterified within the cell wall by a pectin methylesterase giving rise to low methyl-esterified homogalacturonans (Zhang and Staehelin, 1992). Low methyl-esterified homogalacturonans strongly bind Ca+2 (Carpita and Gibeaut, 1993). Recently, Lenartowska et al. (2001) suggested that low methyl-esterified homogalacturonans within the transmitting tissue may act as a Ca+2 reservoir to be released following a pollination-induced degradation of the homogalactoronans. The Ca+2, an ion essential for pollen tube growth (Taylor and Hepler, 1997), is then functionally available. The localization of anti-JIM7 versus anti-JIM5 to Golgi body-associated vesicles and vesicles adjacent the plasma membrane, and the presence of anti-JIM5 towards the periphery of the primary cell wall at the surface of stigmatic epidermal cells of S. glabra and C. japonicus indicates that processes of secretion and subsequent de-esterification of the highly methyl-esterified homogalacturonans in these species is spatially similar to what has been reported for other plant cells. Significantly, homogalacturonan de-esterification within the cuticular layer of both S. glabra and C. japonicus leads to a punctuate distribution of concentrated regions of anti-JIM5 throughout the outer stigma surface at the point of immediate pollen contact. A functional role for these low methyl-esterified homogalacturonan regions as a Ca+2 reservoir to support pollen growth at the stigma or elsewhere in the carpel in either of these Chloranthaceous species remains to be determined.
Both low methyl-esterified homogalacturonans and SCA within the transmitting tissue ECM have been implicated in pollen adhesion and pollen tube guidance (Lord, 2003). Specifically, SCA, has recently been shown to operate in combination with a low-esterified homoglacturonan that is recognized by monoclonal antibodies to JIM5 in pollen tube adhesion in the lily style (Mollet et al., 2000). As well, the unbound form of SCA also appears to function in combination with the protein chemocyanin at the stigma as a chemotropic (Kim et al., 2003) versus adhesion-mediated (haptotactic) guidance molecule (Lord, 2003). Localization of the polyclonal anti-SCA to the transmitting tissue ECM where pollen tube growth will occur at the stigma of S. glabra and C. japonicus suggests that an SCA-like molecule may be present in transmitting tissue ECM of these species. Interestingly, although anti-SCA localizes to both the ECM at the epidermal surface and expanded ECM at the base of stigmatic epidermal cells, anti-SCA localization is highest at the stigmatic surface in the notch between adjacent stigmatic cells where pollen tubes grow downward following germination. The high density of anti-SCA in this notch region does not correspond with a similar high density distribution of anti-JIM5, although both anti-SCA and anti-JIM5 exhibited the same diffuse distribution in the expanded ECM at the base of stigmatic epidermal cells. An important issue for the biology of pollen tube growth at the stigmatic epidermal cells of these Chloranthaceous species will be to determine whether or not either compound acts alone or together as proposed for lily in chemotactic/haptotactic growth. As well, since anti-SCA is absent within the expanded ECM of adaxial epidermal cells at the site of angiospermy prior to pollination, and pollination can stimulate changes in transmitting tissue ECM (Sedgley and Scholefield, 1980; Arbeloa and Herrero, 1987; Lenartowska et al., 2001; Koehl, 2002), it will be valuable to determine if SCA is secreted to the expanded ECM at the base of adaxial epidermal cells following pollination where it could be functionally important for chemotactic/haptotactic pollen tube growth.
Lipids within the angiosperm transmitting tissue ECM are known to play an important role in pollen hydration, adhesion, penetration of the stigma cuticle by pollen tubes, and subsequent pollen tube growth (Lush et al., 1998; Zinkl et al., 1999; Wolters-Arts et al., 2002). Therefore, it is noteworthy that, within the carpel of S. glabra and C. japonicus, lipids are confined to (a) the ECM of the surface of stigmatic epidermal cells where pollen grains adhere and germinate, and (b) the base of the stigmatic cells in the expanded ECM where pollen tubes change direction to grow towards the site of angiospermy. Although differential localization of lipids, as well as anti-SCA, to the ECM where pollen tubes grow at the stigma may implicate a similar role for these molecules in pollen tube growth in S. glabra and C. japonicus as observed in lily (SCA) and other angiosperms (lipids), the family of lipid transfer-like proteins to which SCA belongs (Mollet et al., 2000) and lipids in the plant ECM may serve numerous functions to include cuticle synthesis and anti-microbial activity (Kader, 1997).
Pollen compatibility in S. glabra and C. japonicus
Numerous studies on the breeding systems of various species within the Chloranthaceae have been conducted (von Balthazar and Endress, 1999; Yi-Bo and Zhen-Yu, 1999; Tosaki et al., 2001). Within the genera that develop bisexual flowers, agamospermy appears to be present in Sarcandra chloranthoides (von Balthazar and Endress, 1999) as well as Chloranthus serratus and C. fortunei (Yi-Bo and Zhen-Yu, 1999). Sarcandra glabra exhibits self-compatibility (SC; von Balthazar and Endress, 1999; Tosaki et al., 2001), and C. spicatus appears to be SI (von Balthazar and Endress, 1999).
Results from the present study demonstrating low pollen germinability following cross- and self-pollination have significant implications for the breeding system of S. glabra and C. japonicus. On the one hand, low pollen germinability indicates that pollen viability is very limited, even though a high percentage of pollen grains contain cytoplasm, a vegetative nucleus, and nucleated generative cell. On the other hand, low pollen germinability provides tentative evidence for the presence of a leaky stigmatic SI in S. glabra and C. japonicus which, in the case of S. glabra, is in contrast to conclusions from previous studies (von Balthazar and Endress, 1999; Tosaki et al., 2001). Conflicting results from the present study with those from other studies on the presence of SI versus SC in S. glabra may be attributed to a number of factors. First of all, the beginning of fruit set, used as an indicator of successful fruit/seed production, has lead to the conclusion of the presence of SC in S. glabra (von Balthazar and Endress, 1999). Such conclusions can be erroneous because carpel/ovule enlargement may result merely from a pollen-borne stimulus (O'Neill, 1997) and can occur even in the presence of stigmatic SI (Pontieri and Sage, 1999). Self-fruit set has also been interpreted to indicate the presence of SC in S. glabra (von Balthazar and Endress, 1999; Tosaki et al., 2001). However, self-fruit/seed set can occur in the presence of a leaky SI system, whereby the strength of SI may be variously expressed amongst individuals within a population, as well as between populations, and can be influenced by the types of S-alleles and their genetic backgrounds, mutations at S-alleles, temperature, and humidity conditions and floral age (de Nettancourt, 1977; Barrett, 1988; Stephenson et al., 2000; Sage et al., 2001). Leaky SI may provide for fruit set following self-pollination in Chloranthaceous species because only one pollen tube would be required to effect fertilization within the one ovule/carpel. Thus, in the absence of published data from the studies on the breeding system of species within the Chloranthaceae on (a) how many flowers per plant and plants per population were examined, (b) self- versus cross-pollen tube growth within treatments across a population, (c) the distribution of the results per plant within a population, (d) and environmental conditions at the time of pollination, all conclusions regarding the presence of agamospermy, SI or SC in any species of Chloranthaceae are inconclusive. Given the importance of SI in the evolution of the breeding systems of the Chloranthaceae and angiosperms as a whole (see below), more rigorous studies are required to provide definitive evidence of the presence or absence of the phenomenon in any of the species with bisexual flowers in the Chloranthaceae.
CONCLUSIONS
The well-resolved picture of basal angiosperm relationships has been noted to provide new opportunities to examine a suite of reproductive characters potentially present in early angiosperms through comparative work on extant basal taxa (Friis et al., 2000; Endress, 2001). It has been hypothesized that the ancestral carpel lacked a style and transmitting tissue was composed of the surface of a wet stigma that was continuous with the surface of the secretory epidermal cells at the site of angiospermy (Endress and Igersheim, 2000). The dry stigma has been hypothesized to have evolved in phylogenetically younger taxa and has been viewed as more advantageous because of the tighter control such a stigma exerts over pollen capture, adhesion, recognition, hydration, and germination (Dickinson, 1995). Moreover, it has been proposed that the functional role of some ECM components integral to pollen capture, retention, recognition, hydration and germination (e.g. lipids) have, over time, been transferred from wet stigma ECM to the pollen ECM (Dickinson, 1995) during the transition from a wet to dry stigma. When considering SI evolution, researchers have noted that basal extant taxa exhibit SI due to the presence of low self-seed production, and have concluded that SI was present in the early origins of the group and played an important role in early angiosperm success (Whitehouse, 1950; Bernhardt and Thien, 1987; Bell, 1995). In one scenario, the adaxial epidermal surface at the site of angiospermy has been predicted to be the site of SI in the early angiosperms (Endress and Igersheim, 2000). Self recognition and rejection at a dry type stigma has been posited to have evolved later in the history of the group. Others have observed that basal extant taxa exhibit some self-seed set, and have proposed that that the first angiosperms were therefore self-compatible (Weller et al., 1995).
The Chloranthaceae is the fifth basal angiosperm taxon posited to have species with wet stigmas that have subsequently been demonstrated to be dry with limited accumulations of lipids confined under the cuticle at stigma receptivity (Saururaceae, Pontieri and Sage, 1999; Illiciaceae, Koehl, 2002; Trimeniaceae, Bernhardt et al., 2003; Amborellaceae, Thien et al., 2003). As well, if SI can be demonstrated to be present in the Chloranthaceae, it would represent the third basal angiosperm taxon to exhibit SI whereby self-recognition and rejection occurs at a dry stigma. Stigmatic SI has been reported in Trimeniaceae (Bernhardt et al., 2003) and Saururaceae (Pontieri and Sage, 1999). Collectively, these data call into question the ancestral state of the stigma as ‘wet’ and provide the possibility that the dry stigma, with highly selective regulation over pollen adhesion, self recognition and rejection, hydration, and germination may have been present during the early history of the group.
Along similar lines, the Chloranthaceae joins an increasing number of basal angiosperm taxa, to include those at the base of the tree, whereby pollen tubes do not grow along the adaxial epidermis at the site of angiospermy following germination on the stigma, but rather grow intercellularly in ground tissue below epidermal stigma cells prior to extracellular growth within the ovary (Nymphaeaceae, Orban and Bouharmount, 1995; Winteraceae, Sage et al., 1998; Saururaceae, Pontieri and Sage, 1999; Amborellaceae, Thien et al., 2003; Trimeniaceae, Bernhardt et al., 2003; Winteraceae, Sage and Sampson, 2003). These results are significant because previous reconstructions of the ancestral site of transmitting tissue distribution based on the presence of exudates on the epidermal surface at the site of angiospermy have viewed this region as the site of pollen tube growth (Endress and Igersheim, 2000). Intercellular growth of pollen tubes following germination is likely to require a different suite of physiological parameters versus those needed to grow extracellularly along the epidermal surface (Bell, 1995). And, while Lloyd and Wells (1992) suggest that carpel closure during the early history of angiosperms would have protected pollen/pollen tubes that were growing on the carpel surface from foraging insects, intercellular growth of pollen tubes following germination versus growth along the epidermal surface would have provided protection prior to carpel closure.
Acknowledgments
This research was funded by a grant from the Natural Sciences and Engineering Research Council of Canada to T.L.S. The authors thank Paul Knox for anti-JIM7, 5 and 13, Elizabeth Lord for anti-SCA and Kathy Sault for technical assistance.
LITERATURE CITED
- Arbeloa A, Herrero M. 1987. The significance of the obturator in the control of pollen tube entry into the ovary in peach (Prunus persica). Annals of Botany 60: 681–686. [Google Scholar]
- Barrett SCH. 1988. The evolution, maintenance, and loss of self-incompatibility systems. In: Lovett Doust J, Lovett Doust L, eds. Plant reproductive ecology patterns and strategies. Oxford: Oxford University Press, 98–124. [Google Scholar]
- Bell PR. 1995. Incompatibility in flowering plants: adaptation of an ancient response. The Plant Cell 7: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt P, Thien L. 1987. Self-isolation and insect pollination in the primitive angiosperms: new evaluations of older hypotheses. Plant Systematics and Evolution 156: 159–176. [Google Scholar]
- Bernhardt P, Sage TL, Weston P, Azuma H, Lam M, Thien LB, et al. 2003. The pollination of Trimenia moorei (Trimeniaceae): floral volatiles, insect/wind pollen vectors, and stigmatic self-incompatibility in a basal angiosperm. Annals of Botany 92: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. 1993. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. The Plant Journal 3: 1–30. [DOI] [PubMed] [Google Scholar]
- Crane PR, Friis EM, Pedersen KR. 1989. Reproductive structure and function in Cretaceous Chloranthaceae. Plant Systematics and Evolution 165: 211–226. [Google Scholar]
- Davis GL. 1966.Systematic embryology of the angiosperms. New York, NY: John Wiley. [Google Scholar]
- Dickinson H. 1995. Dry stigmas, water and self-incompatibility in Brassica. Sexual Plant Reproduction 8: 1–10. [Google Scholar]
- de Nettancourt D. 1977.Incompatibility in angiosperms. Berlin: Springer-Verlag. [Google Scholar]
- Dejong DW, Jansen EF, Olsen AC. 1967. Oxidoreductive and hydrolytic enzyme patterns in plant suspension culture cells: local and time relationships. Experimental Cell Research 47: 139–156. [DOI] [PubMed] [Google Scholar]
- Edwards JG. 1920. Flower and seed of Hedyosmum nutans Botanical Gazette 6: 409–424. [Google Scholar]
- Eklund H, Friis EM, Pererson KR. 1997. Chloranthaceous floral structures from the Late Cretaceous of swede. Plant Systematics and Evolution 207: 13–42. [Google Scholar]
- Eklund H, Doyle JA, Herendeen PS. 2004. Morphological phylogenetic analysis of living and fossil Chloranthaceae. International Journal of Plant Sciences 165: 107–153. [Google Scholar]
- Endress PK. 1986. Reproductive structures and phylogenetic significance of extant primitive angiosperm. Plant Systematics and Evolution 152: 1–28. [Google Scholar]
- Endress PK. 1994. Floral structure and evolution of primitive angiosperm: recent advances. Plant Systematics and Evolution 192: 79–97. [Google Scholar]
- Endress PK. 2001. The flowers in extant basal angiosperms and inferences on ancestral flowers. International Journal of Plant Sciences 162: 1111–1140. [Google Scholar]
- Endress PK, Igersheim A. 2000. Gynoecium structure and evolution in basal angiosperms. International Journal of Plant Sciences 161: S211–S223. [DOI] [PubMed] [Google Scholar]
- Friis EM, Pedersen KR, Crane PR. 1994. Angiosperm floral structures from the Early Cretaceous of Portugal. Plant Systematics and Evolution 192: 31–49. [Google Scholar]
- Friis EM, Pedersen KR, Crane PR. 1999. Early angiosperm diversification: the diversity of pollen associated with angiosperm reproductive structures in Early Cretaceous floras from Portugal. Annals of the Missouri Botanical Garden 86: 259–296. [Google Scholar]
- Friis EM, Pedersen KR, Crane PR. 2000. Reproductive structure and organization of basal angiosperms from the early Cretaceous (Barremian or Aptian) of western Portugal. International Journal of Plant Sciences 161: S169–S182. [DOI] [PubMed] [Google Scholar]
- Friis EM, Doyle JA, Endress PK, Leng Q. 2003. Archaefructus—angiosperm precursor or specialized early angiosperm? Trends in Plants Science 8: 369–373. [DOI] [PubMed] [Google Scholar]
- Fulcher RG, Wong SI. 1980. Inside cereals—a fluorescence microchemical view. In: Inglett GE, Munck L, eds. Cereals for food and beverages. Academic Press: New York, 1–25. [Google Scholar]
- Greenspan P, Meyer EP, Flower SD. 1985. Nile red: a selective fluorescent stain for intracellular lipid droplets. Journal of Cell Biology 100: 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herendeen PS, Crepet WL, Nixon KC. 1993.Chloranthus-like stamens from the Upper Cretaceous of New Jersey. American Journal of Botany 80: 865–871. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. 1970. Evaluation of pollen viability by enzymatically induced fluorescence: intracellular hydrolysis of fluorescein diacetate. Stain Technology 45: 115–120. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. 1982. The specialized cuticles of the receptive surfaces of angiosperm stigmas. In: Cutler DF, Alvin KL, Price CE, eds. The plant cuticle, Linnean Society Symposium No. 10. London: Academic Press, 99–120. [Google Scholar]
- Heslop-Harrison Y, Shivanna KR. 1977. The receptive surface of the angiosperm stigma. Annals of Botany 41: 1233–1258. [Google Scholar]
- Jauh G-Y, Lord EM. 1996. Localization of pectins and arabinogalactan-proteins in lily (Lilium longiflorum L.) pollen tube and style, and their possible roles in pollination. Planta 199: 251–261. [Google Scholar]
- Jeffree CE. 1996. Structure and ontogeny of plant cuticles. In: Kersteins G, ed. Plant cuticles, an integrated and functional approach. Oxford: Bios Scientific Publishers, 33–82. [Google Scholar]
- Kader JK. 1997. Lipid-transfer proteins: a puzzling family of plant proteins. Trends in Plant Sciences 2: 66–70. [Google Scholar]
- Khosravi D, Joulaie R, Shore JS. 2003. Immunocytochemical distribution of polygalacturonase and pectins in styles of distylous and homostylous Turneraceae. Sexual Plant Reproduction 16: 179–190. [Google Scholar]
- Kim S, Mollet J, Dong J, Zhang K, Park S, Lord EM. 2003. Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proceedings of the National Academy of Sciences of the USA 100: 16125–16130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JP. 1999. Intriguing, complex and everywhere: Getting to grips with arabinogalactan proteins. Trends in Plant Science 4: 123–125. [Google Scholar]
- Koehl V. 2002.Functional reproductive biology of Illicium floridanum (Illiciaceae). MSc Thesis, University of Toronto. [Google Scholar]
- Lam BCH, Sage TL, Bianchi F, Blumwald E. 2001. Role of SH3 domain-containing proteins in clathrin-mediated vesicle trafficking in Arabidopsis. The Plant Cell 13: 2499–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowska M, Rodriguez-Garcia MI, Bednarska E. 2001. Immunochemical localization of esterified and unesterified pectins in unpollinated and pollinated styles of Petunia hybrida Hort. Planta 213: 182–191. [DOI] [PubMed] [Google Scholar]
- Lloyd DG, Wells MS. 1992. Reproductive biology of a primitive angiosperm, Pseudowintera colorata (Winteraceae), and the evolution of pollination systems in the Anthophyta. Plant Systematics and Evolution 181: 77–95. [Google Scholar]
- Lord EM. 2003. Adhesion and guidance in compatible pollination. Journal of Experimental Biology 54: 47–54. [DOI] [PubMed] [Google Scholar]
- Lord EM, Russell SD. 2002. The mechanisms of pollination and fertilization in plants. Annual Reviews of Cell Developmental Biology 18: 81–105. [DOI] [PubMed] [Google Scholar]
- Lush WM, Grieser F, Wolters-Arts M. 1998. Directional guidance of Nicotiana alata pollen tubes in vitro and on the stigma. Plant Physiology 118: 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FM. 1959. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technology 34: 436–437. [DOI] [PubMed] [Google Scholar]
- Mathews S, Donoghue MJ. 2000. Basal angiosperm phylogeny inferred from duplicate phytochromes A and C. International Journal of Plant Science 161: S41–S55. [DOI] [PubMed] [Google Scholar]
- Mattsson O, Knox RB, Heslop-Harrison J, Heslop-Harrison Y. 1974. Protein pellicle of stigmatic papillae as a probable recognition site in incompatibility reactions. Nature 247: 298–300. [Google Scholar]
- Mollet JC, Park SY, Nothnagel EA, Lord EM. 2000. A lily stylar pectin is necessary for pollen tube adhesion to an in vitro stylar matrix. The Plant Cell 12: 1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban I, Bouharmount J. 1995. Reproductive biology of Nymphaea capensis Thunb. Var. zanzibarensis (Casp.) Verdc (Nymphaeaceae). Botanical Journal of the Linnean Society 119: 35–43. [Google Scholar]
- O'Brien TP, Feder N, McCully ME. 1964. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59: 367–373. [Google Scholar]
- O'Neill S. 1997. Pollination regulation of flower development. Annual Review of Plant Physiology and Plant Molecular Biology 48: 547–574. [DOI] [PubMed] [Google Scholar]
- Park S-Y, Jauh G-Y, Mollet JC, Eckard KJ, Nothnagel EA, Walling LL, et al. 2000. A lipid transfer-like protein is necessary for lily pollen tube adhesion to an in vitro stylar matrix. The Plant Cell 12: 151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-Y, Lord EM. 2003. Expression studies of SCA in lily and confirmation of its role in pollen tube adhesion. Plant Molecular Biology 51: 183–189. [DOI] [PubMed] [Google Scholar]
- Pontieri V, Sage TL. 1999. Evidence for stigmatic self-incompatability, pollination induced ovule enlargement, and transmitting exudates in the paleoherb, Saururus cernuus L. (Saururaceae). Annals of Botany 84: 507–519. [Google Scholar]
- Qiu YL, Lee J, Bernasconi-Quadroni F, Soltis DE, Soltis PS, Zanis M, et al. 1999. The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. Nature 402: 404–407. [DOI] [PubMed] [Google Scholar]
- Sage TL, Sampson FB. 2003. Evidence for ovarian self-incompatibility as a cause of self-sterility in the relictual woody angiosperm, Pseudowintera axillaris (Winteraceae). Annals of Botany 91: 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage TL, Sampson FB, Bayliss P, Gordon MG, Heij EG. 1998. Self-sterility in the Winteraceae. In: Owens SJ, Rudall PJ, eds. Reproductive biology in systematics, conservation and economic botany, Kew: Royal Botanic Gardens, 317–328. [Google Scholar]
- Sage TL, Griffin SR, Pontieri V, Drobac P, Cole WW, Barrett SCH. 2001. Stigmatic self-incompatibility and mating pattern in Trillium grandiflorum and Trillium erectum (Melanthiaceae). Annals of Botany 88: 829–841. [Google Scholar]
- Sedgley M, Scholefield PB. 1980. Stigma secretion in the watermelon before and after pollination. Botanical Gazette 141: 428–434. [Google Scholar]
- Shivanna KR, Sastri DC. 1981. Stigma-surface esterase activity and stigma receptivity in some taxa characterized by wet stigmas. Annals of Botany 47: 53–64. [Google Scholar]
- Soltis DE, Soltis PS, Chase MW, Mort ME, Albach DC, Zanis M, et al. 2000. Angiosperm phylogeny inferred from a combined data set of 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society 133: 381–461. [Google Scholar]
- Stephenson AG, Good SV, Vogler DW. 2000. Interrelationships among inbreeding depression, plasticity in the self-incompatibility system, and the breeding system of Campanula rapunculoides L. (Campanulaceae). Annals of Botany 85: 211–219. [Google Scholar]
- Sun G, Ji Q, Dilcher DL, Zheng S, Nixon KC, Wang X. 2002. Archaefructaceae, a new basal angiosperm family. Science 296: 899–904. [DOI] [PubMed] [Google Scholar]
- Taylor LP, Hepler PK. 1997. Pollen germination and tube growth. Annual Review of Plant Physiology and Plant Molecular Biology 48: 461–491. [DOI] [PubMed] [Google Scholar]
- Thien LB, Sage TL, Jaffre T, Bernhardt P, Pontieri V, Weston P, et al. 2003. The population structure and floral biology of Amborella trichopoda (Amborellaceae). Annals of the Missouri Botanical Garden. 90: 466–490. [Google Scholar]
- Tosaki Y, Renner SS, Takahashi H. 2001. Pollination of Sarcandra glabra (Chloranthaceae) in natural populations in Japan. Journal of Plant Research 114: 423–427. [Google Scholar]
- Vennigerholz F. 1992. The transmitting tissue in Brugmansia suaveolens: immunocytochemical localization of pectin in the style. Protoplasma 171: 117–122. [Google Scholar]
- von Balthazar M, Endress PK. 1999. Floral bract function, flowering process and breeding systems of Sarcandra and Chloranthus (Chloranthaceae). Plant Systematics and Evolution 218: 161–178. [Google Scholar]
- Weller SG, Donoghue MJ, Charlesworth D. 1995. The evolution of self-incompatibility in flowering plants: a phylogenetic approach. In: Hoch PD, Stephenson AG, eds. Experimental and molecular approaches to plant biosystematics. St Louis: Missouri Botanical Garden, 355–382. [Google Scholar]
- Whitehouse HLK. 1950. Multiple-allelomorph incompatibility of pollen and style in the evolution of angiosperms. Annals of Botany 14: 198–216. [Google Scholar]
- Wolters-Arts M, Weerd van der L, Aelst van AC, Weerd van der J, As van H, Mariani C. 2002. Water-conducting properties of lipids during pollen hydration. Plant, Cell and Environment 25: 513–519. [Google Scholar]
- Wu H, Wong E, Ogdahl J, Cheung AY. 2000. A pollen tube growth-promoting arabinogalactan protein from Nicotiana alata is similar to the tobacco TTS protein. The Plant Journal 22: 165–176. [DOI] [PubMed] [Google Scholar]
- Yi-Bo L, Zhen-Yu L. 1999. Pollination ecology of Chloranthus serratus (Thunb.) Roem. Et Schult. and Ch. Fortunei (A. Gray) Solms-Laub. (Chloranthaceae). Annals of Botany 83: 489–499. [Google Scholar]
- Yoshida O. 1957. Embryologische Studien fiber die Ordnung Piperales. I. Embryologie von Chloranthus japonicus Sueb. Journal of the College of Arts and Science, Chiba University 2: 172–178. [Google Scholar]
- Yoshida O. 1960. Embryologische Studien fiber die Ordnung Piperales. III. Embryologie von Sarcandra glabra Nakai. Journal of the College of Arts and Science, Chiba University 3: 55–60. [Google Scholar]
- Zanis MJ, Soltis DE, Soltis PS, Mathews S, Donoghue MJ. 2002. The root of the angiosperms revisited. Proceedings of the National Academy of Sciences of the USA 99: 6848–6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GF, Staehelin LA. 1992. Functional compartmentation of the Golgi apparatus of plant cells: immunocytochemical analysis of high-pressure frozen- and freeze-substituted sycamore maple suspension culture cells. Plant Physiology 99: 1070–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LB, Renner SS. 2003. The deepest splits in Chloranthaceae as resolved by chloroplast sequences. International Journal of Plant Science 164 (Suppl. 5): S383–S392. [Google Scholar]
- Zinkl GM, Zwiebel BI, David GG, Preuss D. 1999. Pollen-stigma adhesion in Arabidopsis: a species-specific interaction mediated by lipophilic molecules in the pollen exine. Development 126: 543. [DOI] [PubMed] [Google Scholar]


