Abstract
• Background and Aims This study examined the effect of plant traits and environmental factors on pollinator visitation in the winter-flowering Helleborus foetidus (Ranunculaceae) in three distant regions in the Iberian Peninsula.
• Methods Geographical variation in floral visitor assemblage, plant traits and environmental factors were analysed during the flowering season.
• Key Results Differences were found in all plant traits measured (number of open flowers, flower size, number of stamens per flower, and number of nectaries) both within and among regions, and differences among regions in all the environmental factors considered (air temperature, exposure to sunlight, canopy cover, and distance to the nearest neighbour). Differences were also found among regions in the probability that plants would be visited by pollinators.
• Conclusions The results show that, although floral display (i.e. number of open flowers on a plant on a given day) consistently explained among-plant differences in visitation rate in all regions, visitation rate was not significantly affected by any other biological or environmental variable. In Helleborus foetidus, then, ‘how’ the plant is would seem to be more important than ‘where’ is it.
Keywords: Environmental factors, floral display, Helleborus foetidus, plant traits, pollinator visitation, winter flowering
INTRODUCTION
Recent studies on the evolutionary ecology of plant–pollinator interactions are based on the premise that variation in plant traits influences reproductive success by affecting such interactions (e.g. Schemske and Horvitz, 1989; Campbell et al., 1991; Herrera, 1993; Mitchell, 1993; O'Connell and Johnston, 1998; Guitián et al., 1999; Maad, 2000). A considerable number of studies have shown that individual variation in flower and/or inflorescence traits frequently translates into individual fitness differences, as a result of effects on pollinator behaviour, visitation frequency and/or pollinator spectrum composition (e.g. Waser and Price, 1981; Klinkhamer et al., 1989; Robertson and Wyatt, 1990; Cresswell and Galen, 1991; Conner and Rush, 1996; Gómez, 2000; Philipp and Hansen, 2000; Gigord et al., 2001). Plant characteristics influencing pollinator visitation rate include those related to floral design (e.g. flower colour, size or number), and the spatial and temporal arrangement of flowers (see references in Thompson, 2001; Mitchell et al., 2004). However, other studies have shown that individual differences in flower or inflorescence traits are either inconsequential or quantitatively negligible in explaining individual fitness differences (Herrera, 1996).
Two categories of factors may account for the latter observations: (1) variations in flower or inflorescence traits may have little or no influence on pollination success (e.g. Andersson, 1994; Wilson, 1995; Wilson and Thomson, 1996; Herrera, 2001); (2) even if pollinators discriminate among individual plants in response to floral phenotypes, eventual fitness differences may be blurred by factors unrelated to the plant–pollinator interaction (e.g. post-pollination events on developing fruits; Herrera, 1993, 2000a). But site-specific effects may be as, or even more, important than plant phenotypic characteristics as determinants of plant–pollinator interactions. O'Connell and Johnston (1998), for example, found that microhabitat characteristics (e.g. presence of ericaceous shrubs or open canopy) had a larger effect on pollination success in the orchid Cypripedium acaule than floral traits. In the Mediterranean shrub Lavandula latifolia, Herrera (1995a) found that individual variation in pollinator composition depended on the sunlight regime associated with each plant's location, rather than on intrinsic plant characteristics. Site-specific effects of this kind (see also Laverty, 1992; Niesenbaum, 1994), whereby ‘where the plant is’ may be more important than ‘how the plant is’, in explaining individual differences in plants' reproductive performance, are probably quite common among forest understorey plants (Herrera, 1995a, and references therein), but few studies have directly addressed such effects.
The aim of this paper is to evaluate the importance of intrinsic factors (i.e. plant- and flower-related phenotypic traits) and extrinsic factors (i.e. site- and environment-related factors) in explaining individual differences in pollinator visitation in the winter-flowering herb Helleborus foetidus (Ranunculaceae). To explore whether patterns remained consistent among regions, the study was conducting at three widely separated areas of the species range in the Iberian Peninsula. Helleborus foetidus is well suited to investigate the relative importance of intrinsic and extrinsic factors as determinants of pollination-related individual differences in reproductive success. The species flowers in winter in different habitat types Thus, unfavourable weather frequently limits pollinator activity; further, differences in environment between H. foetidus growing sites could in turn affect the foraging behaviour and visitation rates of insect pollinators (e.g. Beattie, 1971; Herrera, 1995b). Specifically, this study aims to assess (a) whether floral traits and environmental factors vary among the study regions, and (b) whether such variations account for differences in pollinator visitation rates.
MATERIALS AND METHODS
Study system and sites
Helleborus foetidus L. is a perennial herb widely distributed in western, central and south-western Europe (Werner and Ebel, 1994). In Spain it may be found in clearings, forest edges and understorey of mixed forests. Every season, plants produce one to several inflorescences (range 1–9) with 25–100 flowers each. Flowers are apocarpous (no. of carpels range 1–5), with the stigmas borne at the end of a long style. The anthers (range 25–60) start to dehisce when flowers are 6–8 d old, producing pollen for approx. 2 weeks. The floral nectaries are hidden inside a globose corolla, and produce abundant nectar (Herrera and Soriguer, 1983; Vesprini et al., 1999). Functionally, flowers are hermaphroditic, protogynous and self-compatible, but substantial seed production requires insect pollination. The extremely long-lived flowers (up to 20 d; Vesprini et al., 1999) are mainly pollinated by bumblebees (Herrera et al., 2001).
This study was conducted in 1998 in three mountain regions in Spain (Caurel, Cazorla and Mágina, Fig. 1; see also Herrera et al., 2001, 2002). The closest regions (Cazorla and Mágina) were 50 km apart; the most distant regions (Caurel and Cazorla) were 850 km away. In each region two populations were selected and 192 plants were tagged overall (Caurel, n = 31 and n = 33 in each population; Cazorla, n = 34 and n = 34; Mágina, n = 30 and n = 30). Plants grew isolated or in groups of three to five individuals, scattered throughout the study areas. In Mágina, H. foetidus is the only flowering plant during most of the study period. In Cazorla it coexists with Daphne laureola (Thymeleaceae), and in Caurel with Primula acaulis subsp. acaulis (Primulaceae).
Fig. 1.
Location of the study regions in the Iberian Peninsula: 1, Caurel, 2, Cazorla, 3, Mágina.
Pollinator censuses
Geographical variation in floral visitor assemblage, abundances, and preferences for tagged plants, were assessed by censuses conducted in all regions during the flowering season (late February to late April). Between 20–25 censuses (3 min each) were conducted for each plant (3262 censuses overall). In each census the taxonomic identity of all floral visitors, and the number of flowers visited on the focal plant, were noted. A detailed description of the procedure is given elsewhere (Herrera et al., 2001).
Plant traits
To investigate possible relationships between visitation rates and plant traits, several variables related to floral advertisement and reward were estimated. As a measure of the plants' flowering pattern, the numbers of open, fully functional flowers (NOF), irrespective of whether they might be in the female or male phase, were recorded every day, before censuses were started and for each plant. At the end of the flowering season, plant size (PLSZ) was estimated as the total number of flowers produced by each plant. Five fully functional flowers, in the early male stage, were collected randomly from each plant. Flowers were preserved in a 2·5 : 2·5 : 95 % formaldehyde–acetic acid–ethanol (FAA) solution. From these, corolla length (CorLen; ±0·01 mm; measured with a digital caliper), number of stamens (NStams) and number of nectaries (NNect) were estimated. NOF and CorLen may be related to pollinators' perception of floral display, thus to the decision to visit or reject a plant. NStams and NNect describe the amount of reward that visitors may find once they probe a flower. Overall, 820 flowers were collected.
Environmental factors
To characterize the plants' light environment, hemispheric photographs of the forest canopy were taken above each tagged plant in all regions, using a Nikon SLR camera with a Nikkor fisheye 16-mm lens, placed horizontally and oriented north–south by each plant. All photographs were taken under similar light conditions (i.e. uniformly overcast sky, late in the afternoon; Steege, 1996). Photographs were scanned and then analysed with WinPhot 5.0 (Steege, 1996; available at http://www.bio.uu.nl/~boev/staff/personal/htsteege/winphot/wp_index.htm) for estimation of direct light, diffuse light, total light and percentage vegetation cover (%Cover) above the plant. In addition, the sunlight regime of each plant (Sun) was characterized before each census, by scoring plants according to the following scale: if the plant was under direct sunlight during the census it was scored 1; if it was partially shaded it was scored 0·5; if it was in full shade it was scored 0. The average value of the scores for all censuses of a given plant provides a global measure of that plant's sunlight regime. Finally, temperature (Temp) in the immediate vicinity of each plant was recorded before each census, and the distance between each tagged plant and its closest flowering conspecific (Dist) was also noted, in order to assess whether the location of a plant relative to other plants affected the likelihood of its visitation.
Data analyses
A preliminary analysis indicated that none of the variables considered showed significant variation among populations within each region, so the data were pooled within each region. One of the populations in Mágina was excluded due to the very low pollinator visitation rate (six visits in 626 censuses).
Among-region variability in plant traits and environmental factors was analysed using generalized linear mixed models (GLIMMIX; SAS Institute, 1996), one for each variable tested, according to their error distribution and using the default link function. Variables replicated within plants (i.e. obtained from censuses or flowers) were nested within plants, and these within region. These included all floral traits, Temp and Sun. In the case of variables obtained ‘per plant’ (i.e. no within-plant replicates), plants were nested within regions. These were Dist and %Cover. Variations among regions in pollinator abundance were tested by fitting a generalized linear model (GENMOD; SAS Institute, 1996). All visitors recorded during censuses were grouped into five classes: Andrena spp., Anthophora spp., Apis mellifera, Bombus spp. and other. Region, pollinator class and their interaction were considered as factors. The response variable was modelled as binomial (ratio between the abundance of each pollinator group and the overall pollinator abundance in each region). Variations among regions in pollinator visitation rates were tested in a similar way, treating each 3-min census as a sampling unit. A binomial response variable (whether the plant was visited or not during each census) was used as the estimator of visitation rates, because the large number of zero values recorded in some regions (see Results) precluded the use of direct counts.
To investigate the relationships between visitation probability and plants' biotic and environmental traits, individual plants were considered as study subjects; thus data obtained from censuses or flowers were averaged for each plant, while measures taken per plant were used as such. The dependent variable was modelled as binomial (ratio between the number of censuses yielding at least one visit and the total number of censuses on that plant), thus a generalized linear model (multiple regression; GENMOD) was used. As predictors for intrinsic traits the following were used: mean number of open flowers (NOF), mean corolla length (CorLen), mean number of stamens per flower (NStams), and mean number of nectaries per flower (NNect). To select predictors among extrinsic traits the following procedure was used. Information obtained from photographs mainly account for the environmental light conditions around each study plant. A principal component analysis (not shown here) was previously carried out on the variables obtained from hemispherical photographs (separately for each region), to identify orthogonal factors that might reduce the number of original variables. In all cases, the analyses revealed the existence of a single axis accounting for most of the variance. Thus, to simplify the interpretation of the results, only %Cover was retained as predictor for the environmental variables extracted from hemispherical photographs, given its straightforward interpretation in the context of this study. Further, mean air temperature (Temp) and average sunlight regime (Sun), both accounting for the environmental conditions suitable for pollinators, and distance to the closest flowering neighbour (Dist), were also included. A quadratic term for Temp was also considered, to detect possible non-linear relationships.
RESULTS
Variation in plant size, floral display and biotic traits
All floral traits showed significant among-region variation (Table 1A). Plants in Mágina had the largest flowers and highest stamen number, while nectary number was highest in Caurel (Table 1B). Significant variations were also observed both within and among plants, but within-plants variation was greater than among-plants variation. Further, significant among-region variation in plant size (PLSZ, total number of flowers produced by each plant over the season; Table 1A) was also found. Plants in Mágina were larger than plants in Caurel and Cazorla (Table 1B). Similarly, significant among-region variation in floral display (number of flowers open on a census day, NOF) was found. Again, plants in Mágina had significantly higher floral display than plants in Caurel and Cazorla (Tables 1A and 1B).
Table 1A.
Results of the generalized linear mixed models testing the differences in floral traits among study regions
| PLSZ |
NOF |
Clen |
NNectars |
NStams |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fixed factors | χ2 | P | χ2 | P | χ2 | P | χ2 | P | χ2 | P | |||||
| Among regions | 23·26 | 0·001 | 68·89 | 0·001 | 31·03 | 0·001 | 60·05 | 0·001 | 158·97 | 0·001 | |||||
| Random factors | Z | P | Z | P | Z | P | Z | P | Z | P | |||||
| Among plants | 9·62 | 0·001 | 9·59 | 0·001 | 5·50 | 0·001 | 4·82 | 0·001 | 5·33 | 0·001 | |||||
| Within plants | – | – | – | – | 19·00 | 0·001 | 19·09 | 0·001 | 19·01 | 0·001 | |||||
Individual plants were nested within region, and, where appropriate, individual flowers were nested within plants.
Table 1B.
Plant means (±1 s.e.) of floral traits among regions
| Caurel (n = 64) |
Cazorla (n = 67) |
Mágina (n = 30) |
|
|---|---|---|---|
| PLSZ | 31·37 ± 1·56 | 30·89 ± 2·67 | 83·58 ± 7·14 |
| NOF | 9·41 ± 0·51 | 4·64 ± 0·39 | 31·66 ± 2·90 |
| CLen | 15·38 ± 0·12 | 15·43 ± 0·12 | 16·41 ± 0·15 |
| NNectars | 5·69 ± 0·09 | 4·89 ± 0·04 | 5·09 ± 0·09 |
| NStams | 35·10 ± 0·40 | 41·31 ± 0·49 | 43·68 ± 0·62 |
See Materials and methods for a description of the variables.
n, the number of plants used in the analyses.
Variation in environmental traits
All environmental variables showed among-region variation (Table 2A). Significant variations were also observed, both within plants (Temp and Sun) and among plants (all variables). Again, for Temp and Sun, the within-plants variation (i.e. the day-to-day pattern of differences among censuses) was more marked than the among-plants variation. Overall, Cazorla was the coldest region (Table 2B). Plants in Cazorla were also the least exposed to direct sunlight. Mágina was the warmest region and had the most sunlight-exposed plants.
Table 2A.
Results of the generalized linear mixed models testing the differences in environmental traits among study regions
| Temp |
Sun |
Dist |
%Cover |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fixed factors | χ2 | P | χ2 | P | χ2 | P | χ2 | P | ||||
| Among regions | 928·23 | 0·001 | 43·22 | 0·02 | 16·80 | 0·001 | 160·78 | 0·02 | ||||
| Random factors | Z | P | Z | P | Z | P | Z | P | ||||
| Among plants | 2·59 | 0·005 | 5·43 | 0·001 | 9·59 | 0·001 | 9·70 | 0·001 | ||||
| Within plants | 30·39 | 0·001 | 28·24 | 0·001 | – | – | – | – | ||||
Individual plants were nested within region, and, where appropriate, individual flowers were nested within plants.
Table 2B.
Plant means (±1 s.e.) of environmental traits among regions
| Caurel (n = 64) |
Cazorla (n = 67) |
Mágina (n = 30) |
|
|---|---|---|---|
| Temp. | 15·77 ± 0·24 | 8·61 ± 0·08 | 13·69 ± 0·21 |
| Sun | 0·31 ± 0·03 | 0·17 ± 0·02 | 0·52 ± 0·04 |
| Dist | 228·59 ± 25·55 | 211·97 ± 22·03 | 113·58 ± 13·61 |
| %Cover | 38·16 ± 1·72 | 60·13 ± 1·21 | 25·81 ± 2·75 |
See Materials and methods for a description of the variables.
n, the number of plants used in the analyses.
Plant traits and environmental factors in relation to pollinator visitation rates
Overall pollinator abundance differed among regions (Region: χ2 = 121·78, P < 0·001), and the relative abundance of the different pollinator groups also differed among regions (Region × PollClass effect: χ2 = 89·76, P < 0·001). Thus, Bombus species accounted for 85·79 % of visits in Cazorla, 61·26 % in Caurel and 50·77 % in Mágina (Table 3). Significant differences were found among regions in the probability that a plant was visited (Region: χ2 = 7·64, P < 0·02). Plants were more likely to be visited in Caurel (11·64 % of the censuses yielded at least one visit) than in Cazorla (6·46 %) and Mágina (2·10 %). Some plants in Caurel and Cazorla were visited regularly (i.e. showed consistently higher visitation rates); in Mágina, however, no such pattern was observed. Overall, visitation rates were rather low, even in the regions where pollinators were more abundant.
Table 3.
Number of visits and relative abundance of the different pollinator taxa in the censuses in each of the three regions studied
| Caurel (985) |
Cazorla (1065) |
Mágina (569) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pollinator species |
n |
% |
n |
% |
n |
% |
|||
| Andrena spp. | 7 | 1·83 | 10 | 5·08 | 9 | 13·85 | |||
| Anthophora acervorum | 91 | 23·82 | 11 | 5·58 | 8 | 12·31 | |||
| Apis mellifera | 45 | 11·78 | 0 | 0 | 0 | 0 | |||
| Bombus pascuorum | 28 | 7·33 | 0 | 0 | 0 | 0 | |||
| Bombus pratorum | 201 | 52·62 | 72 | 36·55 | 0 | 0 | |||
| Bombus terrestris | 5 | 1·31 | 97 | 49·24 | 33 | 50·77 | |||
| Others | 5 | 1·31 | 7 | 3·55 | 15 | 23·07 | |||
| Total | 382 | 197 | 65 | ||||||
Values in brackets are numbers of censuses.
Table 4 shows the results of the regression models of plant and environmental traits on the likelihood that a plant was visited. Plants with a larger floral display (i.e. higher NOF) tended to receive more visits than those with a smaller display (parameter estimates±standard error: Caurel, 0·09 ± 0·04; Cazorla, 0·14 ± 0·05; Mágina, 0·02 ± 0·01; Fig. 2). None of the other plant or environmental traits was a consistently significant determinant of likelihood of visitation.
Table 4.
Results of the generalized linear models testing the relationship between floral and environmental traits and the probability that the plants were visited, in each study region
| Caurel (n = 64) |
Cazorla (n = 67) |
Mágina (n = 30) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| χ2 |
P |
χ2 |
P |
χ2 |
P |
|||||||
| Floral traits | ||||||||||||
| NOF | 7·18 | 0·007 | 6·92 | 0·009 | 9·07 | 0·003 | ||||||
| Clen | 0·65 | 0·42 | 0·10 | 0·75 | 0·01 | 0·95 | ||||||
| Nnectars | 0·08 | 0·78 | 3·12 | 0·08 | 0·15 | 0·70 | ||||||
| Nstams | 0·01 | 0·94 | 0·33 | 0·57 | 2·50 | 0·11 | ||||||
| Environmental traits | ||||||||||||
| Temp | 0·43 | 0·51 | 0·17 | 0·68 | 2·46 | 0·12 | ||||||
| (Temp)2 | 0·31 | 0·56 | 0·09 | 0·77 | 2·63 | 0·10 | ||||||
| Sun | 0·33 | 0·04 | 0·10 | 0·75 | 0·08 | 0·78 | ||||||
| Dist | 1·12 | 0·29 | 0·08 | 0·78 | 11·64 | 0·001 | ||||||
| %Cover | 0·22 | 0·64 | 0·01 | 0·91 | 2·74 | 0·10 | ||||||
Dependent variable was modelled as binomial (no. censuses with at least one visit/total no. of censuses). See Materials and methods for a description of the variables.
n, the number of plants used in the analyses.
Fig. 2.
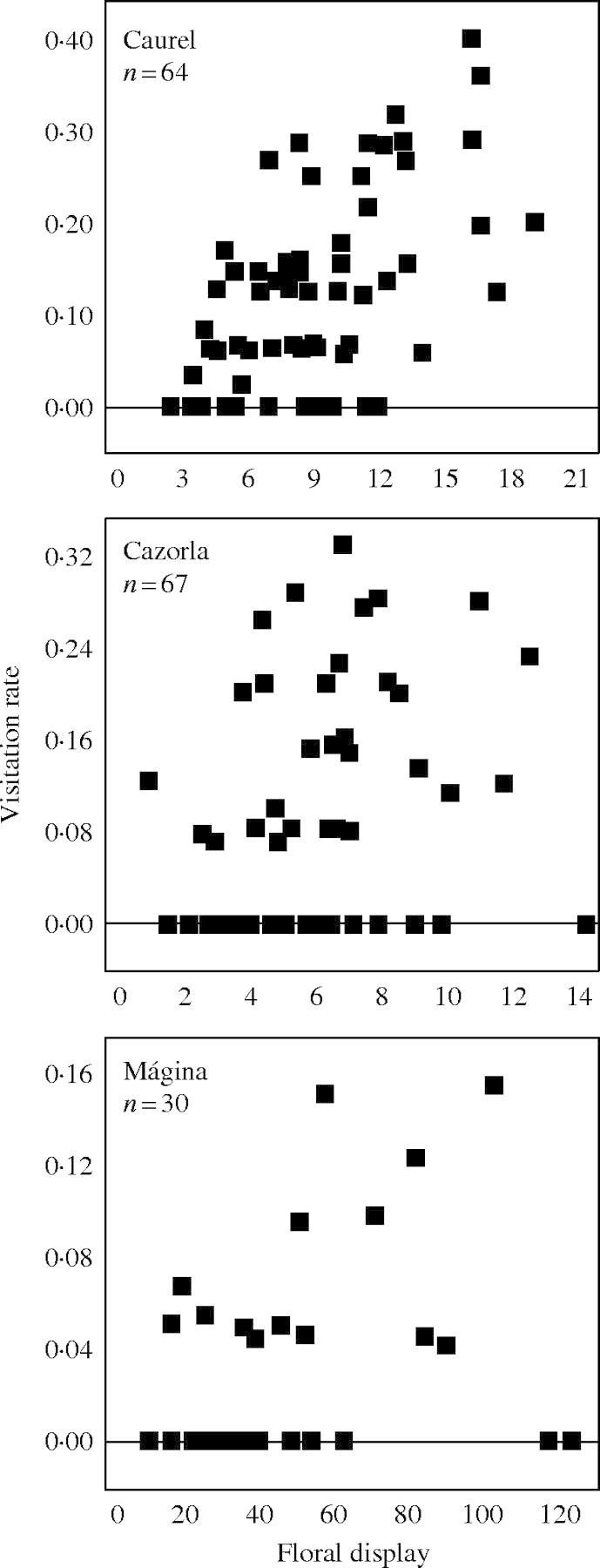
Scatter plots of visitation rates (number of censuses with visits/total number of censuses) versus mean floral display (averaged for all censuses from each region) in the three study areas. n, the number of plants considered in each region.
DISCUSSION
The results show significant variation: (a) in all plant traits within plants, among plants and among regions, confirming the findings by Herrera et al. (2001, 2002) for a wider range of H. foetidus populations throughout the Iberian Peninsula; (b) in all environmental factors within plants, among plants and among regions (see Herrera, 1995a, and references therein); and (c) in pollinator abundance and in the likelihood that a plant will be visited among regions, as found by many other authors (see Herrera, 1988, 1995a; Horvitz and Schemske, 1990; Eckhart, 1992; Waser et al., 1996). Finally, the analyses show that floral display (i.e. number of open flowers on a plant) was the only one of the factors considered that had a consistent significant effect on pollinator visit likelihood in all the study regions.
Considering the relative importance of biotic versus abiotic factors as determinants for a successful pollination, the influence of the latter may be presumably more important in plants flowering during periods of adverse weather (e.g. Herrera, 1995a; Aizen, 2003), as has been observed, both at a macro- and micro-climatic level, for some winter-flowering herbs (see Herrera, 1995b, and references therein). However, there are examples of winter- or early spring-flowering plant species that show high natural, although slightly pollen-limited, fruit set (see Herrera, 2002), and it has been documented, for several early-blooming species, that the influence of abiotic factors is not relevant when compared with that of biotic traits related to pollinator attraction (e.g. floral display or flower duration; see Motten, 1986; Totland and Matthews, 1998; Herrera, 2002). In the present study, only floral display had a significant effect on pollinator visits in all the study regions. Although flower size (corolla length) and potential reward (number of stamens and nectaries) may be potentially important traits for explaining differences between plants in visitation rates (e.g. Young and Stanton, 1990; Eckhart, 1991; Burd, 1995; Conner and Rush, 1996; Totland et al., 1998), none of them were significant predictors in the present study (see also Totland and Matthews, 1998).
Numerous authors have found that pollinator attraction is affected by floral display, whether defined as plant size, number of flowers per inflorescence, or in terms of the spatiotemporal pattern of presentation (Cruzan et al., 1988; Eckhart, 1991; de Jong and Klinkhamer, 1994). The present results agree with these findings, suggesting that pollinators of H. foetidus select plants with a larger floral display, and are consistent with the view that, in winter-flowering species in areas with adverse climatic conditions, a better floral display improves attractiveness to pollinators (see references in Totland and Matthews, 1998). The way and extent to which different pollinators may respond to variations in floral display has recently been the subject of considerable attention (see Thompson, 2001). In the case of H. foetidus the effect of floral display on visit rate was consistent among regions, suggesting that the different pollinators respond in a similar ways to variations in floral display.
None of the environmental factors considered were significant predictors of visitation rates, despite the variability found in these factors at the spatial scale analysed. The importance of sunlight to visitors has been suggested, as it may modify their selection preferences and ability to manipulate certain plants depending on location (e.g. Beattie, 1971; Chazdon, 1988; Herrera, 1997). Furthermore, the irradiance mosaic may influence not only the plants' likelihood of being visited, but also the taxonomic composition of the visitor assemblage (Herrera, 1997), thus affecting plant fitness in demographic and evolutionary terms (Herrera, 2000b). Finally, related variables, such as air temperature or plant cover, may also impose restrictions on flower visitors (e.g. Murcia, 1990; Herrera, 1995a; Totland, 2001). Nevertheless, no consistent effects of any of these factors on visitation rates in any region were detected. Similarly, visitation rates were not affected by the spatial distribution of plants, which may also affect the likelihood of successful pollination (e.g. Sih and Baltus, 1987; Sowig, 1989; Laverty, 1992; Karban, 1997; O'Connell and Johnston, 1998; but see Jennersten and Nilsson, 1993).
Pollinator preferences according to biotic and abiotic traits
The lack of effects of the factors analysed, other than floral display, on pollinator visitation likelihood might be attributable to (a) a lack of variability in the factors analysed at the spatial scale considered here, which is obviously not the case (see Results and Herrera et al., 2002) or (b) variability in these factors at a level below that detectable by pollinators. In this sense, pollination services throughout the range of H. foetidus are mainly provided by bumble bees (Bombus spp.; Proctor and Yeo, 1973; Vesprini et al., 1999), namely B. terrestris and B. pratorum in the populations studied. Bumble bees can be considered as effective pollinators over a wide range of floral phenotypes and environmental conditions (e.g. Mayfield et al., 2001), and their thermoregulatory abilities facilitate their role as pollinators both for plants flowering in winter (like H. foetidus) and plants flowering in summer (e.g. Lavandula latifolia; Herrera, 1995a), when weather conditions impose limitations on other insect groups in the Mediterranean area. Thus, if variability in plant traits and environmental conditions falls within the preferences and abilities of bumble bees, and if the scattered distribution of plants in the study populations is able to neutralize any directional behaviour of the bees (see e.g. Cresswell, 2000), a selection by the main pollinators of certain plants or floral phenotypes, or a limitation due to environmental conditions would not necessarily be expected. Nevertheless, the present results indicate that pollinators of H. foetidus select plants primarily in view of their floral display (number of open flowers on the plant on a given day), which suggests that there may be selection for greater within-plant flowering synchrony. In H. foetidus, then, ‘how’ the plant is would seem to be more important than where it is.
Helleborus foetidus is self-compatible, thus pollinators visiting plants with a large floral display may visit several flowers sequentially, presumably favouring geitonogamous self-pollination. Additionally, changes in flight distance in response to floral displays may influence mating patterns within populations (see Mitchell et al., 2004, and references therein). From a different perspective, it has been predicted (see Cruzan et al., 1988; Mitchell, 1993) that selection for floral traits that increase attractiveness for pollinators will act primarily through male function, i.e. pollen donation (see Willson, 1979; Queller, 1997). As a result, it is difficult to predict the extent to which differences in the rate of visits to H. foetidus plants will translate into fitness differences. Further, geographical variation captured in this study involves differences between areas with a Mediterranean (Cazorla and Mágina) and Atlantic climate (Caurel), with contrasting conditions. Although such climatic differences do not influence the flowering phenology of the study species (H. foetidus starts flowering roughly at the same time in all study regions; pers. obs.), their contribution may modulate in a different way the pollination processes considered. Thus, a more complete understanding of the consequences of environmental factors and plant traits for reproductive success in H. foetidus will require additional exploration of the extent to which variation in environmental factors may lead to among-plant variation in vegetative characters influencing plant fitness.
Acknowledgments
We thank B. García, J. L. Garrido and A. Manzaneda for field assistance; M. Carrión, A. Prieto, and R. Requerey for technical assistance; and Ø. Totland, J. Cresswell and J. D. Thompson for comments on the manuscript. This study was supported by grant PB96-0856 (Spanish Ministerio de Educación y Cultura). During the preparation of the manuscript J.G. received funding from the ‘Fundación Ramón Areces’ and A.M.S.-L. was supported by grant BOS2003-00292 (Spanish Ministerio de Ciencia y Tecnología).
LITERATURE CITED
- Aizen MA. 2003. Influences of animal pollination and seed dispersal on winter-flowering in a temperate mistletoe. Ecology 84: 2613–2627. [Google Scholar]
- Andersson S. 1994. Floral stability, pollination efficiency, and experimental manipulation of the corolla phenotype in Nemophila menziesii (Hydrophyllaceae). American Journal of Botany 81: 1397–1402. [Google Scholar]
- Beattie AJ. 1971. Itinerant pollinators in a forest. Madroño 21: 120–124. [Google Scholar]
- Burd M. 1995. Pollinator behavioural responses to reward size in Lobelia deckenii: no escape from pollen limitation of seed set. Journal of Ecology 3: 865–872. [Google Scholar]
- Campbell DR, Waser NM, Price MV, Lynch EA, Mitchell RJ. 1991. Components of phenotypic selection: pollen export and flower corolla width in Ipomopsis aggregata Evolution 45: 1458–1467. [DOI] [PubMed] [Google Scholar]
- Chazdon RL. 1988. Sunflecks and their importance to forest understorey plants. Advances in Ecological Research 18: 1–63. [Google Scholar]
- Conner JK, Rush S. 1996. Effects of flower size and number on pollinator visitation to wild radish, Raphanus raphanistrum Oecologia 105: 509–516. [DOI] [PubMed] [Google Scholar]
- Cresswell JE. 2000. A comparison of bumblebees' movements in uniform and aggregated distributions of their forage plant. Ecological Entomology 25: 19–25. [Google Scholar]
- Cresswell JE, Galen C. 1991. Frequency-dependent selection and adaptive surfaces for floral character combinations: the pollination of Polemonium viscosum American Naturalist 138: 1342–1353. [Google Scholar]
- Cruzan MB, Neal PR, Wilson MF. 1988. Floral display in Phyla incisa: consequences for male and female reproductive success. Evolution 42: 505–515. [DOI] [PubMed] [Google Scholar]
- de Jong TJ, Klinkhamer PGL. 1994. Plant size and reproductive success through female and male function. Journal of Ecology 82: 399–402.2 [Google Scholar]
- Eckhart VM. 1991. The effects of floral display on pollinator visitation vary among populations of Phacelia linearis (Hydrophillaceae). Evolutionary Ecology 5: 370–384. [Google Scholar]
- Eckhart VM. 1992. Spatio-temporal variation in abundance and variation in foraging behavior of the pollinators of gynodioecious Phacelia linearis (Hydrophyllaceae). Oikos 64: 573–586. [Google Scholar]
- Gigord LDB, Macnair MR, Smithson A. 2001. Negative frequency-dependent selection maintains a dramatic flower color polymorphism in the rewardless orchid Dactylorhiza sambucina (L.). Proceedings of the National Academy of Sciences of the USA 98: 6253–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez JM. 2000. Phenotypic selection and response to selection in Lobularia maritima: importance of direct and correlational components of natural selection. Journal of Evolutionary Biology 13: 689–699. [Google Scholar]
- Guitián J, Guitián P, Medrano M, Sánchez JM. 1999. Variation in floral morphology and individual fecundity in Erythronium dens-canis (Liliaceae). Ecography 22: 708–714. [Google Scholar]
- Herrera CM. 1988. Variation in mutualisms: the spatio-temporal mosaic of a pollinator assemblage. Biological Journal of the Linnean Society 35: 95–125. [Google Scholar]
- Herrera CM. 1993. Selection on floral morphology and environmental determinants of fecundity in a hawk moth-pollinated violet. Ecological Monographs 63: 251–275. [Google Scholar]
- Herrera CM. 1995. Microclimate and individual variation in pollinators: flowering plants are more than their flowers. Ecology 76: 1516–1524. [Google Scholar]
- Herrera CM. 1995. Floral biology, microclimate, and pollination by ectothermic bees in an early-blooming herb. Ecology 76: 218–228. [Google Scholar]
- Herrera CM. 1996. Floral traits and plant adaptation to insect pollinators: a devil's advocate approach. In: Lloyd, DG, Barrett, SCH, eds. Floral biology—studies on floral evolution in animal-pollinated plants. New York, NY: Chapman & Hall, 65–87. [Google Scholar]
- Herrera CM. 1997. Thermal biology and foraging responses of insect pollinators to the forest floor irradiance mosaic. Oikos 78: 601–611. [Google Scholar]
- Herrera CM. 2000. Measuring the effects of pollinators and herbivores: evidence for non-additivity in a perennial herb. Ecology 81: 2170–2176. [Google Scholar]
- Herrera CM. 2000. Flower-to-seedling consequences of different pollination regimes in an insect-pollinated shrub. Ecology 81: 15–29. [Google Scholar]
- Herrera CM. 2001. Deconstructing a floral phenotype: do pollinators select for corolla integration in Lavandula latifolia? Journal of Evolutionary Biology 14: 574–584. [Google Scholar]
- Herrera CM. 2002. Censusing natural microgametophyte populations: variable spatial mosaics and extreme fine-graininess in winter-flowering Helleborus foetidus (Ranunculaceae). American Journal of Botany 89: 1570–1578. [DOI] [PubMed] [Google Scholar]
- Herrera CM, Soriguer RC. 1983. Intra- and inter-floral heterogeneity of nectar production in Helleborus foetidus L. (Ranunculaceae). Botanical Journal of the Linnean Society 86: 253–260. [Google Scholar]
- Herrera CM, Sánchez-Lafuente AM, Medrano M, Guitián J, Cerdá X, Rey PJ. 2001. Geographical variation in autonomous self-pollination levels unrelated to pollinator service in Helleborus foetidus (Ranunculaceae). American Journal of Botany 88: 1025–1032. [PubMed] [Google Scholar]
- Herrera CM, Cerdá X, García MB, Guitián J, Medrano M, Rey PJ, et al. 2002. Floral integration, phenotypic covariance structure and pollinator variation in bumblebee-pollinated Helleborus foetidus Journal of Evolutionary Biology 15: 108–121. [Google Scholar]
- Horvitz CC, Schemske DW. 1990. Spatiotemporal variation in insect mutualisms of a Neotropical herb. Ecology 71: 1085–1097. [Google Scholar]
- Jennersten O, Nilsson SG. 1993. Insect flower visitation frequency and seed production in relation to patch size of Viscaria vulgaris (Caryophyllaceae). Oikos 68: 283–292. [Google Scholar]
- Karban R. 1997. Neighbourhood affects a plant's risk of herbivory and subsequent success. Ecological Entomology 22: 433–439. [Google Scholar]
- Klinkhamer PGL, de Jong TJ, De Bruyn GJ. 1989. Plant size and pollinator visitation in Cynoglossum officinale Oikos 54: 201–204. [Google Scholar]
- Laverty TM. 1992. Plant interactions for pollinator visits: a test of the magnet species effect. Oecologia 89: 502–508. [DOI] [PubMed] [Google Scholar]
- Maad J. 2000. Phenotypic selection in hawkmoth-pollinated Platanthera bifolia: targets and fitness surfaces. Evolution 54: 112–123. [DOI] [PubMed] [Google Scholar]
- Mayfield MM, Waser NM, Price MV. 2001. Exploring the ‘most effective pollinator principle’ with complex flowers: bumblebees and Ipomopsis aggregata Annals of Botany 88: 591–596. [Google Scholar]
- Mitchell RJ. 1993. Adaptive significance of Ipomopsis aggregata nectar production: observation and experiment in the field. Evolution 47: 25–35. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Karron JD, Holmquist KG, Bell JM. 2004. The influence of Mimulus ringens floral display size on pollinator visitation patterns. Functional Ecology 18: 116–124. [Google Scholar]
- Motten AF. 1986. Pollination ecology of the spring wildflower community of a temperate deciduous forest. Ecological Monographs 56: 21–42. [Google Scholar]
- Murcia C. 1990. Effect of floral morphology and temperature on pollen receipt and removal in Ipomoea trichocarpa Ecology 71: 1098–1109. [Google Scholar]
- Niesenbaum RA. 1994. Spatial and temporal variation in pollen tube numbers in Lindera benzoin (Spicebush). Canadian Journal of Botany 72: 268–271. [Google Scholar]
- O'Connell LM, Johnston MO. 1998. Male and female pollination success in a deceptive orchid: a selection study. Ecology 79: 1246–1260. [Google Scholar]
- Philipp M, Hansen T. 2000. The influence of plant and corolla size on pollen deposition and seed set in Geranium sanguineum (Geraniaceae). Nordic Journal of Botany 20: 129–140. [Google Scholar]
- Proctor M, Yeo P. 1973. The pollination of flowers. London: Collins. [Google Scholar]
- Queller DC. 1997. Pollen removal, paternity, and the male function of flowers. American Naturalist 149: 585–595. [Google Scholar]
- Robertson JL, Wyatt R. 1990. Evidence for pollination ecotypes in the yellow-fringed orchid, Platanthera ciliaris Evolution 44: 121–133. [DOI] [PubMed] [Google Scholar]
- SAS Institute. 1996.SAS/STAT software: changes and enhancements through Release 6·11. Cary, NC: SAS Institute. [Google Scholar]
- Schemske DW, Horvitz CC. 1989. Temporal variation in selection on a floral character. Evolution 43: 461–465. [DOI] [PubMed] [Google Scholar]
- Sih A, Baltus MS. 1987. Patch size, pollinator behavior and pollinator limitation in Catnip. Ecology 68: 1679–1690. [DOI] [PubMed] [Google Scholar]
- Sowig P. 1989. Effects of flowering plant's patch size on species composition of pollinator communities, foraging strategies, and resource partitioning in bumble bees (Hymenoptera: Apidae). Oecologia 78: 550–558. [DOI] [PubMed] [Google Scholar]
- Steege HT. 1996.WinPhot 5: a programme to analyze vegetation indices, light and light quality from hemispherical photographs. Tropenbos Guyana Reports 95-2, Tropenbos Guyana Programme, Georgetown, Guyana. [Google Scholar]
- Thompson JD. 2001. How do visitation patterns vary among pollinators in relation to floral display and floral design in a generalist pollination system? Oecologia 126: 386–394. [DOI] [PubMed] [Google Scholar]
- Totland Ø. 2001. Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82: 2233–2244. [Google Scholar]
- Totland Ø, Matthews I. 1998. Determinants of pollinator activity and flower preference in the early spring blooming Crocus vernus Acta Oecologica 19: 155–165. [Google Scholar]
- Totland Ø, Andersen HL, Bjelland T, Dahl V, Eide W, Houge S, et al. 1998. Variation in pollen limitation among plants and phenotypic selection on floral traits in an early-spring flowering herb. Oikos 82: 491–501. [Google Scholar]
- Vesprini JL, Nepi M, Pacini E. 1999. Nectary structure, nectar secretion patterns and nectar composition in two Helleborus species. Plant Biology 1: 560–568. [Google Scholar]
- Waser NM, Price MV. 1981. Pollinator choice and stabilizing selection for flower color in Delphinium nelsonii Evolution 35: 376–390. [DOI] [PubMed] [Google Scholar]
- Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. 1996. Generalization in pollination systems, and why it matters. Ecology 77: 1043–1060. [Google Scholar]
- Werner K, Ebel F. 1994. Zur Lebensgeschichte der Gattung Helleborus L. (Ranunculaceae). Flora 189: 97–130. [Google Scholar]
- Willson MF. 1979. Sexual selection in plants. American Naturalist 113: 777–790. [Google Scholar]
- Wilson P. 1995. Selection for pollination success and the mechanical fit of Impatiens flowers around bumblebee bodies. Biological Journal of the Linnean Society 55: 355–383. [Google Scholar]
- Wilson P, Thomson JD. 1996. How do flowers diverge? In: Lloyd, DG, Barrett, SCH, eds. Floral biology—studies on floral evolution in animal-pollinated plants. New York, NY: Chapman & Hall, 88–111. [Google Scholar]
- Young HJ, Stanton ML. 1990. Influences of floral variation on pollen removal and seed production in wild radish. Ecology 71: 536–547. [Google Scholar]