Abstract
• Background and Aims Root axes elongate slowly and swell radially under mechanical impedance. However, temporal and spatial changes to impeded root apices have only been described qualitatively. This paper aims (a) to quantify morphological changes to root apices and (b) assess whether these changes pre-dispose young root tissues to hypoxia.
• Methods Lupin (Lupinus angustifolius) seedlings were grown into coarse sand that was pressurized through a diaphragm to generate mechanical impedance on growing root axes. In situ observations yielded growth rates and root response to hypoxia. Roots were then removed to assess morphology, cell lengths and local growth velocities. Oxygen uptake into excised segments was measured.
• Key Results An applied pressure of 15 kPa slowed root extension by 75 % after 10–20 h while the same axes thickened by about 50 %. The most terminal 2–3 mm of axes did not respond morphologically to impedance, in spite of the slower flux of cells out of this region. The basal boundary of root extension encroached to within 4 mm of the apex (cf. 10 mm in unimpeded roots), while radial swelling extended 10 mm behind the apex in impeded roots. Oxygen demand by segments of these short, thick, impeded roots was significantly different from segments of unimpeded roots when the zones of elongation in each treatment were compared. Specifically, impeded roots consumed O2 faster and O2 consumption was more likely to be O2-limited over a substantial proportion of the elongation zone, making these roots more susceptible to O2 deficit. Impeded roots used more O2 per unit growth (measured as either unit of elongation or unit of volumetric expansion) than unimpeded roots. Extension of impeded roots in situ was O2-limited at sub-atmospheric O2 levels (21 % O2), while unimpeded roots were only limited below 11 % O2.
• Conclusions The shift in the zone of extension towards the apex in impeded roots coincided with greater vulnerability to hypoxia even after soil was removed. Roots still encased in impeded soil are likely to suffer from marked O2 deficits.
Keywords: Lupinus angustifolius, lupin, mechanical impedance, hypoxia, root, apex, oxygen
INTRODUCTION
The mechanism by which roots penetrate impeded matrices has been studied extensively since the early experiments of Pfeffer (1893) on maize and broad bean roots opposed by gypsum blocks. Early analyses of the forces that roots exert produced many estimates of axial pressures well above 1 MPa (Barley and Greacen, 1967; Taylor and Ratliff, 1969) but growth pressures in the range 0·5–0·6 MPa are more credible (Clark et al., 2003). Radial pressures exerted by roots on the surrounding soil are also believed to be a critical feature in penetration of hard soils (Abdalla et al., 1969; Richards and Greacen, 1986). In spite of an extensive literature on root–soil interactions (Cockroft et al., 1969; Whiteley et al., 1981; Bengough et al., 1997), there is scant information on the biological processes that underpin adaptations to mechanical impedance. Eavis (1969) made measurements on roots of peas in hard soils, showing that cell production and expansion was perturbed in response to the matrix. Atwell and Newsome (1990), Clark et al. (1996) and Croser et al. (1999, 2000) further addressed the connection between water relations and the generation of root force but conclusive evidence as to what other cellular processes limit root growth in hard soils in situ is still not available. For example, the allusions to O2 limitations in impeded root axes in early studies (Gill and Miller, 1956; Eavis, 1972) lead to the hypothesis that root morphological changes might indirectly perturb ATP regeneration by inhibiting respiration. A further hypothesis that ethylene mediates growth of impeded roots is unresolved (Moss et al., 1988; Sarquis et al., 1991) but deserves attention particularly as ethylene-induced swelling could exacerbate the effects of hypoxia reported in this paper. Better knowledge of the precise location of radial swelling of root axes in relation to inhibition of elongation rates should offer clues as to the regulation of cell growth in impeded conditions.
Narrow-leaved lupin (Lupinus angustifolius) is a species that appears to penetrate hard soils with greater efficacy than most agricultural species tested (Materechera et al., 1991). Lupin was chosen as a good candidate to study root expansion rates and O2 demand in a mechanically impeded sand because it offers a substantial primary root axis (approx. 1 mm diameter) and grows quickly through hard soils. Materechera et al. (1991) ascribed this rapid growth to the capacity of lupin roots to swell radially. In the pressure cell used in the present study (similar to that used by Goss and Scott Russell, 1980), root growth was monitored closely as mechanical impedance was varied in situ, while roots could be removed rapidly for other cell and physiological measurements. Quantifying dynamic properties such as respiration and water relations in impeded roots in a steady state has always been problematic (Atwell and Newsome, 1990; Croser et al., 2000). In this study the compromise was to estimate O2 uptake rates into excised root segments and infer that these reflected rates in situ. However, in the growth studies, O2 was varied at will in the pressure chambers, allowing the response of root growth to hypoxia to be confirmed and the hypothesis tested that mechanical impedance leads directly to changes in morphology which inhibit respiration and growth of the root tip.
The aims were to measure (a) precise axial and radial growth rates of mechanically impeded roots; (b) the response of mechanically impeded roots to hypoxia; and (c) the effect of morphological changes induced by mechanical impedance on O2 uptake characteristics by these roots.
MATERIALS AND METHODS
Growth conditions
Seeds of Lupinus angustifolius L. ‘Danja’ were rinsed in 2·5 % sodium hypochlorite solution for 5 min, followed by deionized water and placed on tissue dampened with 1 mm CaSO4 to germinate in the dark at 20 °C. After radicle emergence 24 h later, seedlings were placed in the growth apparatus (Fig. 1). One emerging radicle was inserted into each ≈3-mm opening at the top of the diaphragm apparatus, sealed off with dampened cotton wool and allowed to grow down the layer of soil against the transparent Perspex face (inclined 15° to the vertical). There were ten plants per apparatus, which was kept in darkness at 20 ± 0·5 °C or illuminated during measurements with green light (wavelength 530 nm). Shoots were maintained at 100 % relative humidity. The cotyledons were retained by plants throughout the experiments with no sign of dehydration: only in the longest experiments did first leaves emerge. No lateral roots emerged during experiments; roots were grown for <7 d in all cases.
Fig. 1.
The apparatus in which seedlings were grown and roots were pressurized. For further details of use, see Materials and Methods.
The soil medium used was crushed quartz, sieved between 1·18 and 2·5 mm2 mesh sizes. The soil medium was poured loosely into the apparatus and drained readily, thus maintaining large pores and a calculated Ψ of approx. −2 kPa near the bottom. Drainage was maintained by suction through sintered glass inlets (see Fig. 1). Prior to inserting seedlings, the apparatus was filled with nutrient solution and immediately allowed to drain freely. After this, 3 mL of nutrient solution was applied daily at the soil surface near each seedling. Nutrient solution pH was maintained within the range 6·3–6·5 during experiments. Observation of soil pores showed continuous air spaces adjacent to the Perspex face, except at the extreme bottom where water saturated the pore space; experiments were discontinued when roots approached the bottom of the apparatus.
Mechanical impedance
Mechanical impedance was applied to roots via the soil, by applying air pressure to a gas-tight rubber diaphragm at the back of the soil chamber (Fig. 1). Mechanical impedance in all experiments was achieved by an applied pressure of 15 kPa, while the controls had no applied pressure. Application of 15 kPa pressure did not produce any evidence of increased soil packing relative to the controls; pore sizes measured by Vernier microscope were similar across pressure treatments (diameter 0·40 ± 0·03 mm). The pressure was increased from 0 to 15 kPa over a period of at least 2 min.
Where possible, the diaphragm was not accompanied by a rigid backing plate in control treatments because this induced low levels of impedance. In cases where pressure was to be applied or relieved during the experiment, the backing plate had to be left in place during the control period. Independent estimates showed that effects of this plate on root growth disappeared once roots were >30 mm long.
Gas supply
Air was flushed into the sand matrix through fine holes (0·5 mm diameter) in the Perspex wall of the apparatus (Fig. 1). Root extension rates were the same when humidified air was supplied at 60 mL min−1 or soil was left open to the atmosphere. Both techniques were employed in various experiments.
The shoots were contained in 340-mL boxes, each covering five plants. Air was supplied to the shoots by either venting the volume within the boxes to the atmosphere, while keeping each box humid with moistened cotton wool, or supplying pre-humidified air at a rate of 60 mL min−1.
Root measurements
Root tip displacement was calculated using measurements of longitudinal (Lo) and lateral (La) displacement by a travelling Vernier microscope mounted parallel to the Perspex wall. Total displacement (d) was assumed to be in a straight line within any time interval, calculated using the Pythagoras Theorem thus:
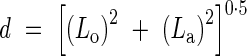 |
Root extension rate (r) of the radicles was calculated from root tip displacement (d) and elapsed time (t):
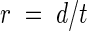 |
Radii along extracted roots were measured under a light microscope with a calibrated eye-piece graticule and distance from the apex recorded.
Transverse sections of roots
Roots grown in impeded and loose soil were washed free of soil then fixed in 4 % paraformaldehyde (in 0·1 m phosphate buffer). Roots were then dehydrated in a graded ethanol series and embedded in LR White resin. Sections 1 µm thick were cut, stained with Toluidine Blue (0·1 % w/v) then mounted on slides for imaging. Sections were taken at prescribed displacements from the root apex as described in the legend to the light micrographs.
Root epidermal cell length measurement
Radicles grown as previously described were extracted from the soil and, within 5 min, washed with deionized water to remove adhering sand before being stained for 1 min with 1 % Toluidine Blue. Epidermal cell lengths were measured under a light microscope using a calibrated eye-piece graticule. Cells that had not yet emerged from underneath the root cap were observed by cutting longitudinal hand-sections through the apex and then stained for 1 min with 1 % Toluidine Blue. Each recorded cell length was a mean of 10–30 cells per root for those cells distal to the root cap and of four to eight cells per root for those underneath the root cap. The distance from the root apex (i.e. the farthest extremity of the root cap) was recorded.
Nine roots that had been extracted 3–6 d after germination were measured from each treatment. In this way, only cells formed after a uniform growth rate had been established were measured. The distribution of cell lengths along the mature axis did not vary significantly with time at this age and local root extension rates were approximately constant. This enabled calculation of the local velocity of growth (vz) (Scott et al., 1968; Silk and Erickson, 1979) where:
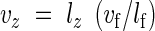 |
where z is distance from the root apex (mm), lz is epidermal cell length (mm) at z, vf is the final measured longitudinal extension rate of the root (mm h−1) and lf is the mature (final) epidermal cell length (mm).
The ratio vf/lf is an expression of the rate of addition of cells to a cell file (cells h−1). The local velocity of growth (vz) was expressed as a function of the distance from the root apex.
Establishment of impedance conditions
Reduction in growth rate with pressure
Four diaphragm apparatuses were initially kept at 0 kPa and root extension rates recorded. Pressure (15 kPa) was applied to two of the apparatuses when roots were ≈30 mm deep into the soil (0 h), while no pressure was applied to the other apparatuses for the duration of the experiment. Root extension immediately following pressure application was monitored frequently. It was generally observed that roots responded to applied pressure more rapidly if they had first become established in unpressurized medium for 36 h. Thus this initial period of establishment was used in all experiments.
Recovery of growth rate
No pressure was applied to two diaphragm apparatuses and root extension rates were measured. Pressure (15 kPa) was applied to two of the apparatuses when roots were ≈30 mm deep into the matrix (termed 0 h) then replaced by 0 kPa after 47 h in one apparatus (recovery treatment). For the duration of the experiment, 15 kPa was maintained in the other apparatus (impeded treatment).
Oxygen measurements
Root growth and critical O2 pressure of extension
The root environment was isolated from the shoots by sealing plants with a beeswax–lanolin mixture into the pressure apparatus at the root–shoot junction, thus allowing the gaseous environment of the roots and shoots to be varied independently. Plants were initially supplied with air to both shoot and root environments. Then, in separate experiments, the concentration of O2 around the roots was altered, while the shoots were aerated and isolated from the root environment. Altered O2 treatments were applied with a gas stream at a rate of 60 mL min−1 per apparatus and allowed to continue for at least 24 h. Root extension rates were measured in control plants supplied continuously with 21 % O2 (air) and a range of other O2 concentrations after extension rates had stabilized. Preliminary experiments not reported here showed that by 24 h the new O2 regime was established, as indicated by stable root extension rates.
Concentrations of O2 from 0–30 % in the soil atmosphere were used to determine that concentration at which root extension was first inhibited by O2 deficits (termed the critical O2 pressure of extension, or COPe).
Oxygen consumption and critical O2 pressure of respiration
Plants were grown as described previously. Roots subjected to 0 kPa and 15 kPa pressure were extracted from the soil and washed in deionized water to remove adhering sand.
Roots were immediately cut into sections of three separate series depending upon the experiment: series I, 0–2, 2–5, 5–10, 20–25 mm; series II, 0–1, 1–3, 3–5, 5–7, 20–25 mm; series III, 0–1, 1–2, 2–3, 3–4 mm. All measurements are quoted in millimetres from the root apex. Root sections from eight to ten plants were placed in 1·5 mL of nutrient solution in the cuvette of a Clark O2 electrode. Nutrient solution was saturated with air prior to addition of the root segments. The cuvette was sealed and O2 consumption was calculated from the rate of change in O2 concentration in the cuvette. Rates of O2 consumption by root tips were measured for 30–90 min during which maximal O2 consumption rate was stable, suggesting no time-dependent loss of respiratory activity. The rate of stirring within the cuvette was raised to a point where O2 consumption did not respond, thus minimizing boundary layer effects on O2 uptake rates. Contents of the cuvette were maintained at 20 °C.
The sections closest to the root apex (i.e. 0–1 or 0–2 mm) were sometimes placed in nutrient solution equilibrated with an O2 concentration greater than 0·284 mm (the O2 concentration in equilibrium with air at 20 °C). Concentrations exceeding 0·35 mm often resulted in a decreased rate of O2 consumption compared with rates observed up to 0·35 mm O2. Plotting O2 consumption rate against O2 concentration in the cuvette shows a break of slope at the point where O2 consumption becomes limited by O2 diffusion to the tissue—this value was termed the critical O2 pressure of respiration (COPr). COPr values and the maximal O2 consumption were recorded.
Curve fitting
The experiments were carried out in four pressure chambers, each containing ten plants. Thus each observation was the result of ten replicate roots, except in cases such as Fig. 2 where 20 roots gave rise to each observation. Standard errors of means are quoted in the figures. Anatomical observations were made on four replicate roots of which one representative example is shown. Logistic curves were fitted to the root local velocity (vz), O2 consumption and critical O2 pressure data using non-linear regressions in the statistics package Genstat Version 6·1 (Genstat, 2002). Logistic curves were fitted due to the excellent fit to the data (P < 0·001 in all cases) and were of the form:
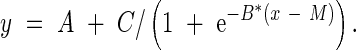 |
Recent criticism of the use of logistic curves in representing root cell elongation suggests that in precise measurement of individual roots that growth can be separated into three zones, with abrupt changes in local velocity at the boundaries—rapid increase at the boundary of division/elongation and rapid decrease at the boundary of elongation/maturation. However, it was considered that the gradual changes in oxygen consumption observed at these boundaries justified the fitting of logistic curves, especially as observations were means of a number of roots (van der Weele et al., 2003).
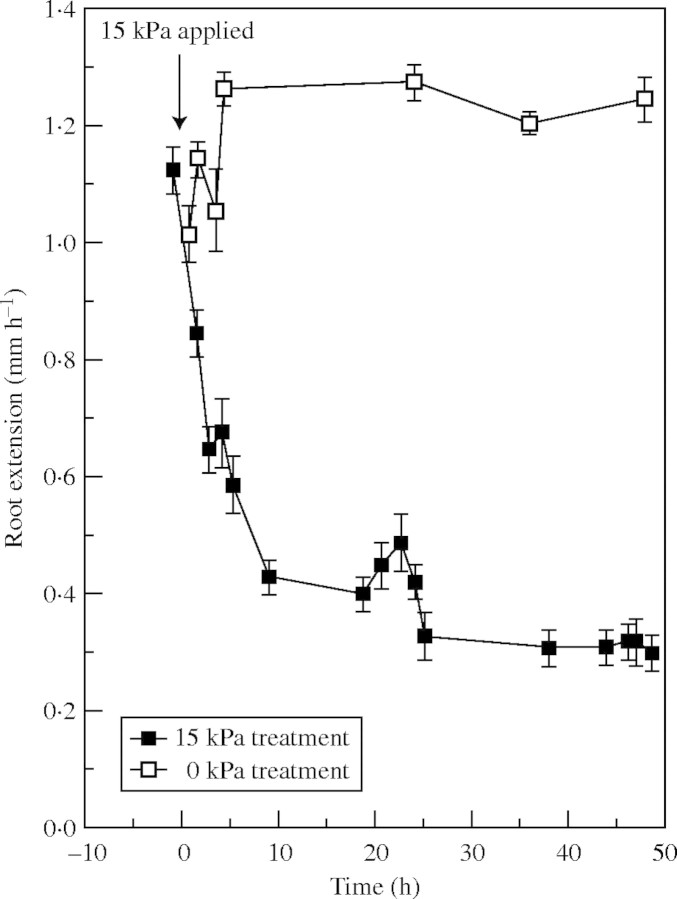
Fig. 2.
Rate of extension of lupin roots in the first 47 h after coarse sand was pressurized to 15 kPa or left unpressurized (0 kPa). Observations are means ± s.e.
RESULTS
Root response to impedance
Figure 2 demonstrates a rapid but not instantaneous decrease in elongation of primary roots of lupin when pressure was applied to entire axes. Rates of elongation declined from 1·1–1·3 mm h−1 to approx. 0·4 mm h−1 within <10 h and settled to approx. 0·3 mm h−1 after the first day. Experiments not reported here showed that an equivalent decrease in root elongation was achieved by 30 mm depth, whether roots were observed as they grew into a pressurized matrix or pressure (15 kPa) was applied to the matrix 36 h after roots entered it.
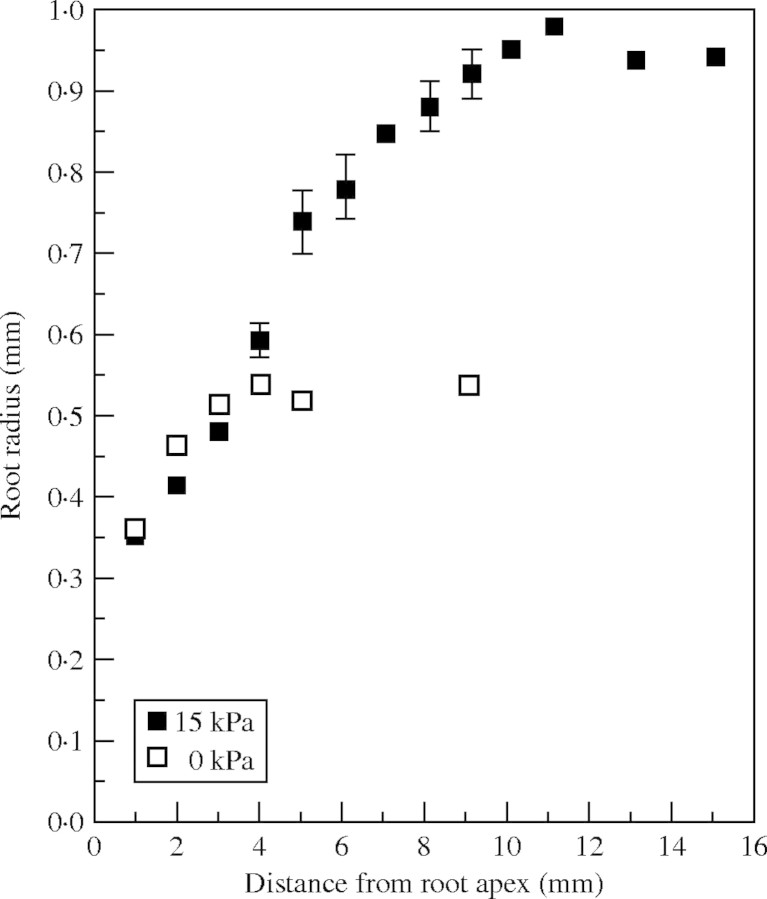
While elongation of roots was restricted by mechanical impedance, radial expansion was typically enhanced in basal regions of the apex (Fig. 3). Segments of root >10 mm basal to the apex were about twice as thick when impeded and all tissues >4 mm from the apex swelled to some degree when impeded. The most distal reaches of the zone of elongation did not swell in response to impedance. Roots were extracted for measurement 47 h after impedance was applied.
Fig. 3.
Local radii of root axes grown in coarse sand unpressurized (0 kPa) and pressurized to 15 kPa for 47 h. Observations are means ± s.e.
The manner in which roots thickened radially in response to mechanical impedance is shown in Fig. 4. Root axes growing normally in unimpeded matrices (A and B) were approx. 50 % thicker as cells were displaced from the apex, while impeded roots (C and D) swelled specifically in response to impedance. The increase in radial dimensions of cortical cells and conservation of the volume of stele is clear from these sections. There is no indication of an increase in the number of cell files as roots swelled, rather that individual cells became larger.
Fig. 4.
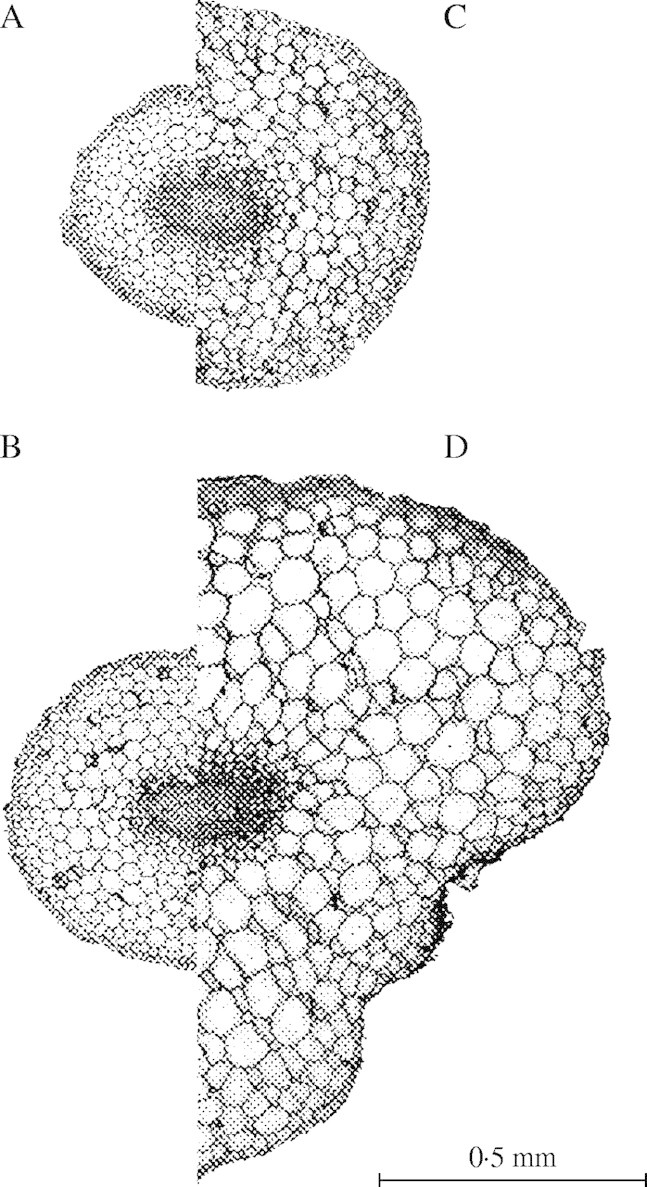
Transverse root sections taken either 4–5 mm (top pair) or 10–11 mm (bottom pair) from the root apex. Sections A and B are from unimpeded roots (0 kPa) and sections C and D from impeded roots (15 kPa). Impeded roots were pressurized to 15 kPa for 47 h.
Recovery from impedance
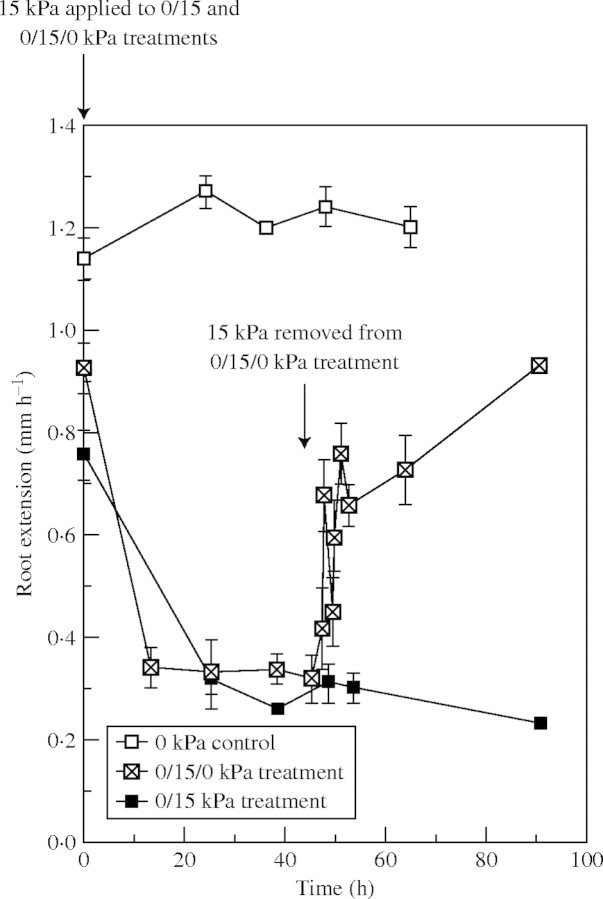
Roots elongated faster once mechanical impedance was relieved (Fig. 5): the extension rate rose rapidly within 1 h to 0·70 mm h−1 and then increased more gradually. The initial surge in growth was repeatable in duplicate experiments. Recovery of elongation, when compared with roots growing without restriction, was approx. 50 % within 3 h and finally reached approx. 75 % 2 d later. By this time (>90 h after impedance was initially imposed), elongation rates of roots that had earlier been mechanically impeded were at least three times faster than in roots that remained impeded (approx. 0·9 mm h−1 vs. 0·25 mm h−1).
Fig. 5.
Extension of lupin roots that have grown in coarse sand that were either unpressurized (0 kPa) or pressurized to 15 kPa for 47 h. Half the roots that were growing under pressure were returned to unpressurized conditions for a further 43 h. Observations are means ± s.e.
Effect of oxygen concentration on root extension
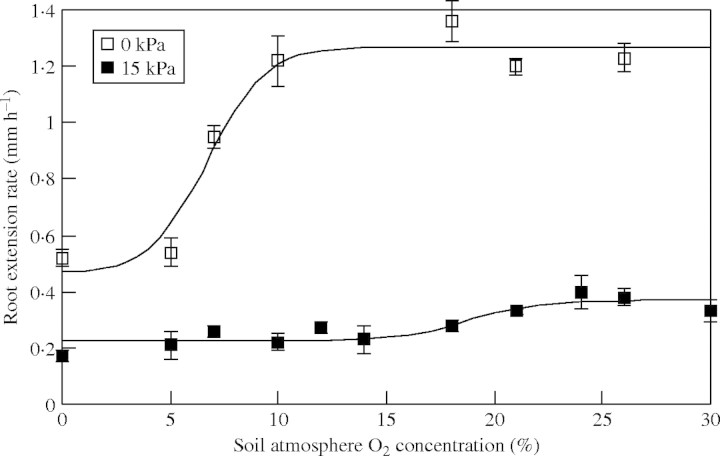
The fitted curves in Fig. 6 show a distinct and large difference between the O2 concentration at which root elongation was slowed (i.e. COPe): impeded roots had a COPe of ≈21 % O2 compared with a COPe of ≈11 % in unimpeded roots. COPe values were defined as the O2 concentration at which 95 % of the maximum elongation rate was reached.
Fig. 6.
Rates of root extension of unimpeded (0 kPa) or impeded (15 kPa) roots at O2 concentrations ranging from 0 to 30 % in the root environment. Observations are means ± s.e. The non-linear regression of logistic curves fitted was highly significant (P < 0·001); the two fitted logistic curves (for 0 kPa and 15 kPa roots, respectively) were significantly different (P < 0·001) and explained 97 % of the variance.
Cell biology of impeded roots
Epidermal cell lengths were measured in the distal 20 mm of unimpeded and impeded root axes so as to include the zones of elongation and differentiation (data not presented). Mechanical impedance did not affect epidermal cell length significantly in the terminal 4 mm of axes where cell division and the early stages of cell elongation occur. However, epidermal cells in mechanically impeded roots were up to 50 % shorter in zones basal to the terminal 4 mm, a trend that persisted through the elongation zone into the zone of differentiation where cell growth had ceased. Final length of epidermal cells was 40 ± 2 µm for impeded roots compared with 95 ± 4 µm in the unimpeded roots.
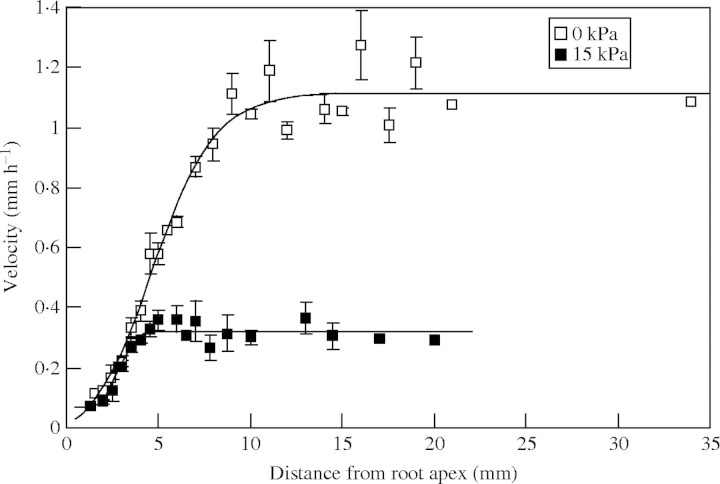
Local velocities of root elongation, relative to apices, were calculated from the measured epidermal cell lengths and root extension rates (Fig. 7). Velocities at each position along root axes revealed that mechanical impedance (15 kPa applied pressure) slowed root elongation at all points 3–10 mm from the root apices, corresponding approximately to the zone of cell expansion in normal lupin roots. Another element of the slower rates of root elongation in mechanically impeded roots is the significantly shorter zone of elongation compared with roots that grew without restriction. The fitted logistic curves to the velocity data (Fig. 7) indicate that 95 % of maximum velocity (and hence the asymptotic rise in epidermal cell length that is used to calculate velocity) was reached 3·95 mm from the apex in impeded roots and 9·70 mm in the unimpeded roots. That is, even though cells expanded until they were approx. 10 mm from the apex in unimpeded roots, elongation of cells in impeded roots ceased just 4 mm from the apex. Figure 7 reveals that while roots in an impeded medium did not elongate in the region 4–10 mm from the apex, they still expanded (Fig. 3), demonstrating radial swelling in cells that had ceased to elongate. Root hairs initiated immediately basal to the position of maximum epidermal cell length, i.e. in impeded roots root hairs initiated 4 mm from the apex and in unimpeded roots at 10 mm from the apex.
Fig. 7.
Local longitudinal velocities throughout the distal zone of lupin roots, relative to the root apex. Roots were either unpressurized (0 kPa) or pressurized to 15 kPa for 47 h. Terminal velocities observed at points further than 10 mm from the root apex are thus equivalent to the elongation rate of the root axis. Observations are means ± s.e. The non-linear regression of logistic curves fitted was highly significant (P < 0·001); the two fitted logistic curves (for 0 kPa and 15 kPa roots, respectively) were significantly different (P < 0·001) and explained 98 % of the variance.
Oxygen consumption by impeded roots
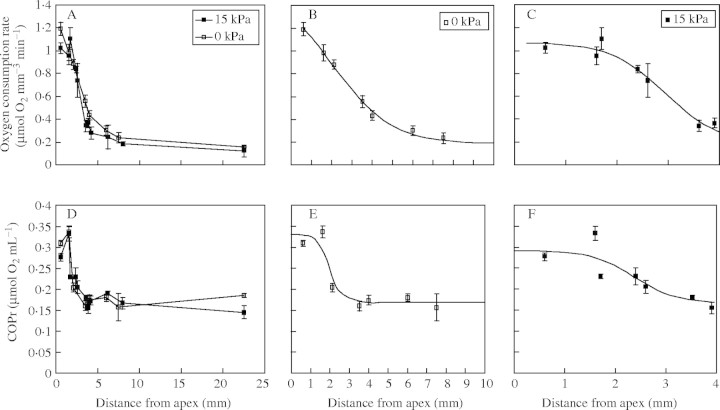
Oxygen consumption rates on a volume basis in the apical 2 mm of roots were about five-fold above the basal rate measured in mature root axes (Fig. 8A). Rates of O2 consumption decreased rapidly in tissues basal to the apex in all roots, when plotted against absolute distance from the apex; there was no obvious effect of impedance on this decline. The lower rates in fully expanded tissue were similar in both impeded and unimpeded roots. When logistic curves were fitted to the data in a similar fashion to those in Fig. 7, the result was highly significant (P < 0·001), the two curves accounting for 90 and 98 % of the variation. Detail of the logistic curves is shown in Fig. 8B and C, representing the respective zones of cell elongation in unimpeded and impeded roots. The shapes of the curves in Fig. 8B and C reflect the differences in O2 demand between treatments: O2 consumption was faster over the zone of elongation in impeded roots than in unimpeded roots: the two curves being significantly different (P < 0·001) from each other.
Fig. 8.
(A) Local O2 consumption rate (µmol mm−3 min−1) in the terminal 22 mm of unimpeded or impeded roots and (D) COPr values of roots over the same region. (B) and (E) show data from the unimpeded roots while (C) and (F) show data from impeded roots. (B) and (E) present data for the 10-mm-long elongating zone of unimpeded roots, while those in (C) and (F) present data for the 4-mm-long elongating zone of impeded roots. (B) and (C) are derived from (A) with fitted curves included, while (E) and (F) are derived from (D) (note symbols within the figures). The non-linear regressions of logistic curves fitted to (B), (C), (E) and (F) were highly significant (P < 0·001): in each case the fitted logistic curves were significant (P < 0·001) and explained between 85 and 98 % of the variance. Observations are means ± s.e. Straight lines connecting points in (A) and (D) are for ease of distinguishing treatment type.
The pattern of O2 consumption was also seen in COPr, which declined basally (Fig. 8D). Logistic curves were again fitted to the data and were highly significant (P < 0·001), accounting for 85 and 89 % of the variation. The detail of the curves is shown for unimpeded and impeded roots in Fig. 8 (E and F, respectively).
The fitted curve in Fig. 8E indicates that in unimpeded roots COPr was at a minimum for the basal 60 % of the zone of elongation (≈4–10 mm from the apex). However, in the impeded roots COPr decreased basally throughout the zone of elongation (Fig. 8F). Data in Fig. 7 from unimpeded roots show that the 4- to 10-mm zone, where COPr was minimal, accounted for 68 % of total elongation. Thus only 32 % of elongation in unimpeded roots occurred in tissues with elevated COPr, whereas all the elongating cells in impeded roots had a COPr above that in mature tissues.
The COPr of the terminal 2 mm of both root types was close to the O2 concentration at full aeration. Hence, O2 supply was compromised by even the mildest hypoxic conditions in the most distal tissues of all roots.
The O2 demand to sustain growth was calculated as follows. Root elongation rates were derived from the mean of all root extension experiments conducted at atmospheric O2 concentration (21 % O2). Local longitudinal velocities (Fig. 7) and root radii (Fig. 3) were used to calculate rates of volume expansion in these roots. Oxygen consumption of the terminal 10 mm of roots was estimated from measured O2 uptake rates by segments (Fig. 8A). The oxygen required to sustain root growth was then calculated (Table 1). Oxygen demand for elongation compared only elongating zones (0–10 mm in unimpeded roots and 0–4 mm in impeded roots) while O2 demand for expansion compared the expanding terminal 10 mm of all roots. Using either criterion, impedance caused a 50–80 % increase in oxygen demand for growth.
Table 1.
Oxygen consumption per unit of elongation and volume expansion of unimpeded (0 kPa) and impeded (15 kPa) roots, using data from root growth and oxygen consumption experiments
| Root characteristic |
0 kPa |
15 kPa |
% increase |
|---|---|---|---|
| Extension rate (mm h−1) | 1·1 | 0·3 | |
| O2 consumption of growing tissues* (µmol h−1) | 200 | 100 | |
| O2 consumption per unit extension (µmol mm−1) | 180 | 330 | 80 |
| Rate of volume growth (mm3 h−1) | 0·93 | 0·83 | |
| O2 consumption of growing tissues† (µmol h−1) | 200 | 270 | |
| O2 consumption per unit volume growth (µmol mm−3) | 215 | 325 | 50 |
0–10 mm in unimpeded roots (0 kPa); 0–4 mm in impeded roots (15 kPa).
0–10 mm zone in both treatments.
DISCUSSION
Dynamics of root growth during exposure to impedance and hypoxia
Elongation of primary lupin roots was slowed by approx. 75 % when a mild external pressure was used to generate mechanical impedance. This level of impedance of root growth is not unusual in field conditions, where soil strength slows root growth up to 5-fold (Bennie, 1991) and even more stringent conditions have been applied in laboratory studies of mechanical impedance (Materechera et al., 1991). The experiment reported here demonstrates the dynamics of root response to mechanical impedance that are not evident from steady-state observations. For example, the slowing of elongation is not instantaneous in the externally pressurized matrix used in the experiment, probably in part because local displacement and compression of sand particles by root apices is necessary before external pressure is expressed as an impeding force on roots (Farrell and Greacen, 1966). The observation that 10 h elapsed before root elongation declined to its final rate could reflect a steady rise in external pressure (σN) perceived by roots as they penetrate a medium (Greacen and Oh, 1972). By contrast, when roots of barley made contact with ballotini beads (Goss and Scott Russell, 1980), growth slowed abruptly (tens of minutes). This essentially instantaneous inhibition of root elongation suggests that the manner in which mechanical resistance is imposed on roots influences the biological response. Solid, incompressible matrices (e.g. rock) or a change of phase from a fluid to a solid matrix (e.g. the Goss experiment cited) both contrast with these lupin experiments, where roots grew steadily through a granular matrix, as they commonly do in field conditions. The recovery phases after mechanical impedance was relieved reveals more about the internal root processes, with an initial rapid elongation followed by a slow phase that continued over several days. The initial surge is probably due to relaxed turgid cells expanding (Atwell and Newsome, 1990; Croser et al., 2000), with events such as the recovery of cell division underpinning the lag phase (see below).
Root dimensions are affected by mechanical impedance beyond the obvious inhibition of axial growth (Taylor and Ratliff, 1969; Bengough and Young, 1993). Experiments with lupin show that the commonly reported radial expansion of mechanically impeded roots is a highly controlled process rather than a generalized anisotropic growth response. The terminal 4 mm of lupin root axes was not thicker during impedance, a phenomenon that has not been commonly reported as part of the swelling response when roots are mechanically impeded (Wilson et al., 1977; Croser et al., 2000). Bengough et al. (1997) generalized that radial swelling encroached to within 2 mm of the apex of impeded roots. Even though impeded roots only elongated at 0·3 mm h−1 in a well-aerated matrix, growth was slowed by a further 50 % when O2 levels fell below 21 % O2 (full aeration). On the other hand, unimpeded roots grew optimally at moderate levels of hypoxia (10–20 % O2). Precisely why impeded roots were more sensitive to hypoxia than unimpeded roots is not evident from the present data. He et al. (1996) found that mechanical impedance and hypoxia synergistically enhance ethylene release, increasing the chance that ethylene-related effects influence roots in compacted soil. Radial swelling, a conspicuous response to impedance (Fig. 4), increased diffusive resistance to O2 flux (see analysis below) but this study does not determine what role ethylene might play in the swelling response. Because impeded roots swelled basal to the zone of elongation, radial swelling could not be invoked as a direct factor in the heightened sensitivity of elongation to hypoxia. A range of structural and metabolic changes could be induced in mechanically impeded roots that exacerbate O2 deficits. When the swollen mature axes of impeded roots became hypoxic, they would develop anoxic steles (Armstrong and Beckett, 1985; Thomson and Greenway, 1991), affecting metabolism throughout the root axis.
Differential growth in root tissues
When lupin roots were impeded, the basal boundary of the elongation zone shifted from approx. 10 mm to 4 mm from the apex (matching closely the observed sites of root hair initiation in both impeded and unimpeded roots) while the boundary of radial expansion moved from 4 mm (unimpeded roots) to 10 mm (impeded roots). A similar shortening of the elongation zone was observed in peas, where the basal boundary was 6 mm from the apex when roots were removed from impedance but moved to 12 mm over the following 2 d (Croser et al., 2000). Thus, the axial and radial growth vectors that describe cell expansion responded independently to external pressure. It remains to be seen whether this developmental uncoupling is observed in all species. It is conceivable that the extent to which root swelling encroaches into the apical zone could account for reported differences between species in tolerance to impedance reported by Materechera et al. (1991). This is significant because the calculation of root growth pressures relies on estimates of a cross-sectional area of the advancing root apex and should thus take into consideration whether the elongating cells also swell when impeded (see Abdalla et al., 1969).
The implications of altered growth distribution in impeded roots are complex because they simultaneously change the demand for internal resources (e.g. carbohydrates for cell wall synthesis) and the pathway of diffusion through metabolically active root apical tissues (e.g. O2, ethylene). Increments in tissue volume in impeded root axes are generally smaller than in unimpeded axes (Atwell, 1988; Table 1), resulting in a diminished demand for new cell constituents for growth and potentially smaller demand for O2 (Atwell, 1990; Bidel et al., 2000). However, the doubling of root diameter through mechanical impedance also restricts the delivery of O2 from the soil atmosphere to cells deep within root axes by increasing the length of the diffusion pathway. Estimates of resistance to O2 flux into unimpeded and impeded roots can be made by treating the root as defined by the epidermis as one cylinder and surface of the stele as a second concentric cylinder, these cylinders having radii of ro and ri, respectively (see Armstrong et al., 1994). Assumptions made in these calculations are referred to in the text below. In the roots shown in Fig. 4 (ri ≈0·01 cm), ro increased from ≈0·038 to ≈0·078 cm as roots swelled, increasing the resistance to O2 flux through the cortex from 1·04 × 102 s cm−3 to 1·59 × 102 s cm−3. Thus a doubling of root diameter caused a modest 50 % increase in resistance to O2 flux. This is relevant to the interpretation of O2 demand by roots (see the next section).
The effective radial fractional porosities of roots from each treatment and tissues from individual roots are likely to vary significantly, with O2 entering meristematic cells as a solute in the liquid phase and mature segments as a gas diffusing through air-filled pore spaces (Bidel et al., 2000). Thus important assumptions can be made that fractional porosities and cell packing arrangements in the two treatments are similar, acknowledging that they could modify O2 diffusion rates if they differed significantly. It has been established that impeded roots swell through radial and tangential cortical cell expansion while the number of cortical cell files increases by only 0–30 % (Wilson et al., 1977; Atwell, 1988; Croser et al., 1999; this study). Thus, while the contribution of vacuoles to the radial pathway increases, there was little addition of new cell walls that offer higher impedance to O2 diffusion (Armstrong et al., 1994).
Does hypoxia limit growth?
The interaction between O2 supply to roots, particularly apices, and mechanical impedance was the subject of several early studies (Gill and Miller, 1956; Eavis, 1969), driven by the rationale that compacted soils are likely to impede both root extension and diffusion of an adequate supply of O2 to root surfaces. However, other factors that can exacerbate the supply of O2 to cells deep within root tissues are the depth of root tissue that O2 must penetrate (hence radial root dimensions) and, potentially, the effect of changes in root anatomy (e.g. cell packing arrangements, porosities) on diffusivity of O2 through bulk tissues. In the experimental system reported here, local rates of root elongation were observed in a uniformly impeded matrix, then roots were rapidly removed to assess O2 uptake independently of the growth medium. Interpretation of these data is complex: the rate of root expansion as well as the morphology of impeded roots affects O2 demand through energy requirements (Bidel et al., 2000) and diffusive limitations (Armstrong et al., 1994), respectively. Removing roots from impeded soil had the advantage that intrinsic properties of O2 uptake into local root zones (e.g. critical O2 pressure of root apices) could be assessed independently of the permeability of the soil matrix, while it is conceded that the pathway for O2 movement was shorter via exposed cut surfaces.
The kinetics of O2 uptake show that the terminal apices of all roots were O2-limited, even after excision. Such an O2 limitation has been observed in other species (Berry and Norris, 1949; Atwell et al., 1985; Armstrong et al., 1994); paradoxically, this suggests that apical cell proliferation always occurs in hypoxic conditions. It also precludes O2 as the sole factor in the lower cell flux rates that are characteristic of impeded roots.
There were fundamental differences in the expanding tissues zones that lie basal to the zone of cell division, with contrasting O2 requirements under impedance (Fig. 6) and differential tissue responses to hypoxia (Fig. 8). All the elongating cells in impeded roots were located in the zone of rapid O2 consumption and high COPr, unlike unimpeded roots where the basal portion of the elongating zone consumed O2 more slowly and had a low COPr. It is postulated that this is linked to the high COPe measured in impeded roots. That impeded roots consumed O2 faster in the expanding zone as a whole (270 cf. 200 µmol O2 h−1) and particularly per unit volume growth (325 cf. 215 µmol O2 mm−3) emphasizes the vulnerability of impeded roots to hypoxia (high COPe). The sensitivity of impeded roots to hypoxia is illustrated by their slow extension rates: they consumed 80 % more O2 per unit of elongation growth than unimpeded roots. Atwell (1990) observed a doubling of demand for O2 per unit of elongation in wheat under field conditions.
The problem of O2 supply is further exacerbated in the field, where hypoxia can occur because O2 diffusion through compacted bulk soil limits the diffusive flow into roots (Eavis, 1972). While the proportion of root surface that is occluded by soil particles when the system is under pressure is not necessarily greater than in roots from friable soil (Barley and Greacen, 1967), any localized soil compression around impeded root axes potentially restricts O2 flow into roots (Armstrong and Beckett, 1985) and inhibits growth. Thus, hypoxia could conceivably affect metabolism in most of the growing zone of impeded roots in situ. Contrasting with the compressed zone of elongation in impeded root axes, unimpeded roots elongated up to 10 mm from the apex and would be much less susceptible to hypoxic conditions in friable soil. These two factors (restricted O2 flow through soil and high COPr values in elongating tissues) support the a priori case that impeded root axes are susceptible to hypoxia.
While a case is made for O2 affecting elongation of impeded roots, carbohydrate import has been cited as a potential limitation on root metabolism. However, earlier reports have provided no evidence that carbohydrate import limits growth in apices of impeded roots (Atwell and Newsome, 1990; Masle et al., 1990). Indeed, the acute limitations that apical root tissues impose on O2 diffusion in Prunus (Bidel et al., 2000) and lupin suggest that carbohydrates are probably rarely limiting, in spite of the high marginal demand for carbohydrates as roots penetrate hard soils (Atwell, 1990). Under most conditions it remains likely that the physical impedance that soils generate directly restricts root elongation through opposing turgor-driven expansion (Atwell and Newsome, 1990; Bengough et al., 1997) and restricting O2 diffusion. To the extent that root apices are dense and rapidly respiring, these are the most vulnerable sites for O2 deficits, whereas mature, vacuolated root tissues have more modest O2 demand (Berry and Norris, 1949) and experience less acute deficits of O2. All but the most basal cells of the elongating zone of impeded roots are hypoxic, even when roots are in an aerated medium (Fig. 8).
Control and dynamics of cell expansion
Cell formation in impeded root apices changed in a complex manner; epidermal cells were almost unaffected by impedance in the earliest stages of root expansion (0–4 mm from the apex) but older cells did not continue to elongate beyond about 40 µm in length. The wall loosening events described by Cleland (1977), Passioura and Fry (1992) and Frensch and Hsiao (1995) that sustain cell expansion in turgid cells are clearly reversed or opposed in the final phase of cell extension in impeded roots. The differential radial and axial growth observed along the axis of mechanically impeded lupin roots can be reconciled with the trivial variance in turgor pressure in these tissues (Atwell and Newsome, 1990; Croser et al., 2000) through fine control of cell wall loosening. In cases where turgor pressure has been shown expressly to respond to mechanical impedance (Clark et al., 1996), wall properties presumably play a role alongside turgor pressure in generating axial force on the soil. The data from lupin and a previous study with peas (Croser et al., 2000) confirm that local velocities of epidermal cell elongation (cf. overall axis elongation rates) vary with impedance, making a more compelling case for positional control of cell wall properties in axial and radial walls. The possibility that either wall extensibility or yield threshold turgor are affected by mechanical impedance, thereby changing irreversible expansion rate, are not separated in these experiments. Challenging roots with osmotic drought (Pritchard et al., 1991; Frensch and Hsiao, 1995) induced measurable changes within minutes in wall properties but equivalent experiments in impeded roots are lacking.
Cell flux through individual epidermal cell files was suppressed by 35 % when lupin roots were exposed to mechanical impedance, from 12 to 7·5 cells file−1 h−1. A number of authors from Barley (1965) to Croser et al. (1999) reported up to a 50 % decrease in cell flux when roots of cereals and pea were impeded, with smaller effects of impedance on the number of cell files (Fig. 4; Atwell, 1988; Croser et al., 1999). Thus lupin is typical of species in which cells of impeded roots exit their meristems more slowly, with implications for recovery when plants grow into loose soil. This is not easily reconciled with the fact that impedance did not affect growth in the terminal 3 mm of root axes (where the zone of cell division is located). The inhibition of cell fluxes by steady-state mechanical impedance illustrates that there are either subtle, direct effects of mechanical forces on meristems or that signals were acropetally transduced to apices from basal tissues (>3 mm from the apex). The 10–80 h recoveries in root growth that follow instantaneous release of mechanical impedance in the present and previous studies (Goss and Scott Russell, 1980; Croser et al., 1999) suggest that cell production was repressed through the action of metabolic events (e.g. cytological) rather than direct and immediate effects mediated by external pressure.
Supplementary Material
Acknowledgments
We thank Dr Hank Greenway for support throughout all phases of this study and Jane Speijers for advice on statistics. Judy Davis configured the images that make up Fig. 4.
LITERATURE CITED
- Abdalla AM, Hettiaratchi DRP, Reece AR. 1969. The mechanics of root growth in granular media. Journal of Agricultural Engineering Research 14: 236–248. [Google Scholar]
- Armstrong W, Beckett PM. 1985. Root aeration in unsaturated soil: a multi-shelled model of oxygen distribution and diffusion with and without sectoral blocking of the diffusion path. New Phytologist 100: 293–311. [Google Scholar]
- Armstrong W, Strange ME, Cringle S, Beckett PM. 1994. Microelectrode and modelling study of oxygen distribution in roots. Annals of Botany 74: 287–299. [Google Scholar]
- Atwell BJ. 1988. Physiological responses of lupin roots to soil compaction. Plant and Soil 111: 277–281. [Google Scholar]
- Atwell BJ. 1990. The effect of soil compaction on wheat during early tillering. III. Fate of carbon transported to the roots. New Phytologist 115: 43–49. [Google Scholar]
- Atwell BJ, Newsome JC. 1990. Turgor pressure in mechanically impeded lupin roots. Australian Journal of Plant Physiology 17: 49–56. [Google Scholar]
- Atwell BJ, Thomson CJ, Greenway H, Ward G, Waters I. 1985. A study of the impaired growth of roots of Zea mays seedlings at low oxygen concentrations. Plant, Cell and Environment 8: 179–188. [Google Scholar]
- Barley KP. 1965. The effect of localised pressure on the growth of the maize radicle. Australian Journal of Biological Sciences 18: 499–503. [Google Scholar]
- Barley KP, Greacen EL. 1967. Mechanical resistance as a soil factor influencing the growth of roots and underground shoots. Advances in Agronomy 19: 1–43. [Google Scholar]
- Bengough AG, Young IM. 1993. Root elongation of seedling peas through layered soil of different penetration resistances. Plant and Soil 149: 129–139. [Google Scholar]
- Bengough AG, Croser C, Pritchard J. 1997. A biophysical analysis of root growth under mechanical stress. Plant and Soil 189: 155–164. [Google Scholar]
- Bennie ATP. 1991. Growth and mechanical impedance. In: Waisel Y, Eshel A, Kafkafi U, eds. Plant roots. The hidden half. New York: Marcell Dekker, 393–414. [Google Scholar]
- Berry LJ, Norris WE. 1949. Studies of onion root respiration. I. Velocity of oxygen consumption in different segments of root at different temperatures as a function of partial pressure of oxygen. Biochimica et Biophysica Acta 3: 593–606. [Google Scholar]
- Bidel LPR, Renault P, Pagès L, Rivière LM. 2000. Mapping meristem respiration of Prunus persica (L.) Batsch seedlings: potential respiration of the meristems, O2 diffusional constraints and combined effects on root growth. Journal of Experimental Botany 51: 755–768. [PubMed] [Google Scholar]
- Clark LJ, Whalley WR, Dexter AR, Barraclough PB, Leigh RA. 1996. Complete mechanical impedance increases the turgor of cells in the apex of pea roots. Plant, Cell and Environment 19: 1099–1102. [Google Scholar]
- Clark LJ, Whalley WR, Dexter AR, Barraclough PB. 2003. How do roots penetrate strong soil? Plant and Soil 255: 93–104. [Google Scholar]
- Cleland RE. 1977. The control of cell enlargement. Symposium for Experimental Biology, Symposium XXXI, 101–117. [PubMed] [Google Scholar]
- Cockroft B, Barley KP, Greacen EL. 1969. The penetration of clays by fine probes and root tips. Australian Journal of Soil Research 7: 333–348. [Google Scholar]
- Croser C, Bengough AG, Pritchard, J. 1999. The effect of mechanical impedance on root growth in pea (Pisum sativum). I. Rates of cell flux, mitosis and strain during recovery. Physiologia Plantarum 107: 277–286. [Google Scholar]
- Croser C, Bengough AG, Pritchard J. 2000. The effect of mechanical impedance on root growth in pea (Pisum sativum). II. Cell expansion and wall rheology during recovery. Physiologia Plantarum 109: 150–159. [Google Scholar]
- Eavis BW. 1969. Mechanical impedance and root growth. In: Gibb JAC, ed. Proceedings, Agricultural Engineering Symposium, September 1967. Paper No. 4/F/39. London: Business Books. [Google Scholar]
- Eavis BW. 1972. Soil physical conditions affecting seedling root growth. I. Mechanical impedance, aeration and moisture availability as influenced by bulk density and moisture levels in a sandy loam soil. Plant and Soil 36: 613–622. [Google Scholar]
- Farrell DA, Greacen EL. 1966. Resistance to penetration of fine probes in compressible soil. Australian Journal of Soil Research 4: 1–17. [Google Scholar]
- Frensch J, Hsiao TC. 1995. Rapid response of the yield threshold and turgor regulation during adjustment of root growth to water stress in Zea mays Plant Physiology 108: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genstat 2002.Reference manual for Genstat Release 6·1. Lawes Agricultural Trust (Rothamsted Experimental Station). Oxford, UK: VSN International. [Google Scholar]
- Gill WR, Miller RD. 1956. A method for study of the influence of mechanical impedance and aeration on the growth of seedling roots. Proceedings of the Soil Science Society of America 20: 154–157. [Google Scholar]
- Goss MJ, Scott Russell RS. 1980. Effects of mechanical impedance on root growth in barley (Hordeum vulgare L.). III. Observations on the mechanism of response. Journal of Experimental Botany 31: 577–588. [Google Scholar]
- Greacen EL, Oh JS. 1972. Physics of root growth. Nature 234: 24–25. [DOI] [PubMed] [Google Scholar]
- He C-J, Finlayson SA, Drew MC, Jordan WR, Morgan PW. 1996. Ethylene biosynthesis during aerenchyma formation in roots of maize subjected to mechanical impedance and hypoxia. Plant Physiology 112: 1679–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masle J, Farquhar GD, Gifford RM. 1990. Growth and carbon economy of wheat seedlings as affected by soil resistance to penetration and ambient partial pressure of carbon dioxide. Australian Journal of Plant Physiology 17: 465–487. [Google Scholar]
- Materechera SA, Dexter AR, Alston AM. 1991. Penetration of very strong soils by seedling roots of different plant species. Plant and Soil 135: 31–41. [Google Scholar]
- Moss GI, Hall KC, Jackson MB. 1988. Ethylene and the response of roots of maize (Zea mays L.) to physical impedance. New Phytologist 109: 303–311. [Google Scholar]
- Passioura JB, Fry SC. 1992. Turgor and cell expansion: beyond the Lockhart equation. Australian Journal of Plant Physiology 19: 565–576. [Google Scholar]
- Pfeffer W. 1893. Druck und arbeitsleistung durch wachsende Pflanzen. Abhandlungen der Sachsischen Akademie der Wissenschaften Leipzig, Mathematische-Naturwissenschaftliche Klasse 33: 235–474. [Google Scholar]
- Pritchard J, Jones RGW, Tomos AD. 1991. Turgor, growth and rheological gradients of wheat roots following osmotic stress. Journal of Experimental Botany 42: 1043–1049. [Google Scholar]
- Richards BG, Greacen EL. 1986. Mechanical stresses on an expanding cylindrical root analogue in granular media. Australian Journal of Soil Research 24: 393–404. [Google Scholar]
- Sarquis JL, Jordan WR, Morgan PW. 1991. Ethylene evolution from maize (Zea mays L.) seedling roots and shoots in response to mechanical impedance. Plant Physiology 96: 1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BIH, Gulline H, Pallaghy CK. 1968. The electrochemical state of cells of broad bean roots. I. Investigations of elongating roots of young seedlings. Australian Journal of Biological Sciences 21: 185–200. [Google Scholar]
- Silk WK, Erickson RO. 1979. Kinematics of plant growth. Journal of Theoretical Biology 76: 481–501. [DOI] [PubMed] [Google Scholar]
- Taylor HM, Ratliff LF. 1969. Root growth pressures of cotton, peas and peanuts. Agronomy Journal 61: 398–402. [Google Scholar]
- Thomson CJ, Greenway H. 1991. Metabolic evidence for stelar anoxia in maize roots exposed to low O2 concentrations. Plant Physiology 96: 1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weele CM, Jiang HS, Palaniappan KK, Ivanov VB, Palaniappan K, Baskin TI. 2003. A new algorithm for computational image analysis of deformable motion at high spatial and temporal resolution applied to root growth. Roughly uniform elongation in the meristem and also, after an abrupt acceleration, in the elongation zone. Plant Physiology 132: 1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley GM, Utomo WH, Dexter AR. 1981. A comparison of penetrometer pressures and the pressures exerted by roots. Plant and Soil 61: 351–364. [Google Scholar]
- Wilson AJ, Robards AW, Goss MJ. 1977. Effects of mechanical impedance on root growth in barley, Hordeum vulgare L. II. Effects on cell development in seminal roots. Journal of Experimental Botany 28: 1216–1227. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.