Abstract
• Background and Aims Shade or inadequate water can inhibit photosynthesis and limit the development of maize (Zea mays) ovaries around the time of pollination, potentially reducing the number of kernels at harvest. This study investigated whether the decreased photosynthesis diminished only the sugar supply or also altered the transport path to the ovaries.
• Methods Photosynthesis and water potentials (Ψw) were measured in the leaves while dry matter delivery was monitored in the ovaries. Ovary glucose, starch and acid invertase activities were measured in situ. Stems were fed xylem-mobile safranin or phloem-mobile carboxyfluorescein (CF), and the dye transport to the ovaries was determined.
• Key Results Under normal conditions, the ovaries gained in dry mass, and starch accumulated in the pedicel and ovary wall. Glucose accumulated in the pedicel, apparently in the apoplast where insoluble (cell-wall-bound) acid invertase acted on the arriving sucrose. A glucose gradient developed from pedicel to nucellus. Safranin moved in the xylem and did not reach the ovary, but CF moved in the phloem and arrived at the ovary. CF also spread into the pedicel but unlike glucose it did not enter the nucellus. Low Ψw or shade decreased leaf photosynthesis, ovary dry mass accumulation, invertase activities, pedicel glucose, starch accumulation and CF delivery. Removal of these treatments reversed the effects.
• Conclusions The success of CF in tracing the general path and rate of carbohydrate transport gave visual evidence that phloem transport to the ovary decreased at low Ψw or in the shade but otherwise remained functional. The decreases indicated that losses in carbohydrate delivery are central features of failed ovary development under these conditions. The selectivity of transport into the nucellus resembled the situation later when embryo and endosperm are present and selective uptake occurs from the apoplast.
Keywords: Zea mays, carboxyfluorescein, shade, water deficiency, water potential, sucrose, glucose, invertase, pollination, ovary development, pedicel, starch, apoplast
INTRODUCTION
Plant reproduction depends on a supply of photosynthetic products. An inadequate supply can block the development of reproductive structures and have irreversible effects, i.e. cause the structures to abort when they are young. Later, an inadequate supply causes less abortion but results in smaller reproductive structures. For example, in maize, leaf water potentials (leaf Ψw) and photosynthesis decreased when water was inadequate, and the ovaries aborted early in ovary/embryo development (Westgate and Boyer, 1985, 1986). During a water shortage 2 weeks later, the kernels persisted and did not abort but their growth slowed, and final kernel size decreased (McPherson and Boyer, 1977; Jurgens et al., 1978). Many crop species show this sequence of reproductive susceptibility to low water supplies (Salter and Goode, 1967; Saini and Westgate, 2000).
In some respects the sequence of susceptibility resembles the effects resulting from insufficient light. Schussler and Westgate (1991a, b) and Setter et al. (2001) compared ovary abortion in shade to that occurring at low Ψw when photosynthesis was inhibited to the same degree. Abortion occurred in both treatments but was greater at low Ψw than in the shade, suggesting that carbohydrate supply was important, but additional factors may have operated at low Ψw. In an attempt to identify the factors involved, Boyle et al. (1991a, b) and Zinselmeier et al. (1995a, 1999) infused sucrose into plant stems to replace the photosynthetic products that otherwise would have been produced during ovary abortion at low Ψw. The infusions prevented or reversed most abortion, but partial abortion remained. Based on the depletion of downstream metabolite pools, Zinselmeier et al. (1999) concluded that losses in invertase activity limited sucrose processing by the developing ovaries. Acid invertase activity was inhibited at low Ψw (Zinselmeier et al., 1995b, 1999) and only partly recovered with sucrose feeding (Zinselmeier et al., 1999). Zinselmeier et al. (1999) attributed the partial recovery to either (1) an abnormal distribution of sugars in the ovaries that prevented some sucrose from reaching the invertase or (2) continued sugar starvation of the ovaries because of the low invertase activity. McLaughlin and Boyer (2004a, b) tested these concepts and observed a normal distribution of glucose, which is a product of invertase activity. This suggested that sucrose had reached the invertase normally. However, gene expression was down-regulated for invertase and up-regulated for certain senescence genes. A few of these genes were sugar-responsive. McLaughlin and Boyer (2004b) concluded that although low Ψw actively changed the expression of many genes, the sugar-responsive genes were likely to regulate abortion.
Working with later stages of reproduction, Hanft and Jones (1986) suggested that kernels induced to abort at the tip of the ear not only had reduced capacity to synthesize starch in the endosperm but also had reduced invertase activity and ability to unload sucrose from the phloem termini in the pedicel. Reed and Singletary (1989) reported that the basal endosperm cells of these kernels had decreased permeability to reducing sugars. When water was withheld from plants at early stages of ovary development, Schussler and Westgate (1991b) found decreased rates of sucrose uptake.
The explanation for these phenomena lies in the delivery of carbohydrates to the developing ovaries by the phloem and post-phloem transport system. Typically, the pathways for carbohydrate transport and phloem unloading are studied after fertilization, when embryo and endosperm are present (Bradford, 1994; Patrick and Offler, 1995, 1996; Fisher and Oparka, 1996; Patrick, 1997). The phloem-mobile dye carboxyfluorescein (CF) is often used to trace carbohydrate movement (Patrick, 1997), and the dye is supplied in the form of non-polar carboxyfluorescein diacetate that enters the phloem (Goodall and Johnson, 1982) and loses its acetate groups because of esterase activity inside the cells (Rotmann and Papermaster, 1966). The remaining CF is hydrophilic and effectively trapped in the phloem.
In contrast to the situation when embryos are present, there are few studies that explore unloading before fertilization, and it is at this time that reproductive organs are particularly susceptible to abortion (Westgate and Boyer, 1986; Boyle et al., 1991b; Zinselmeier et al., 1995b, 1999; McLaughlin and Boyer, 2004b). Consequently, the following experiments were undertaken with CF to explore carbohydrate transport to maize ovaries prior to fertilization, while low Ψw or shade inhibited photosynthesis in the parent plant.
MATERIALS AND METHODS
Plant material and growth conditions
Maize (Zea mays L. cv. DE2×H99) plants were grown in 22-L pots containing 11 kg of Evesboro loamy sand, loamy substratum (coated, mesic, Typic Quartzipsamments) amended by mixing peat : sand : soil in volumes of 1 : 1 : 1, and dolomitic limestone (275 g per pot) to adjust the soil pH to 6·9. Two seeds were planted in each pot, and the soil mix was saturated with nutrient solution (Hoagland and Arnon, 1950) and allowed to drain according to the method of Zinselmeier et al. (1999). The pots were placed in a controlled environment chamber (Environmental Growth Chambers, Chagrin Falls, OH, USA) with day/night temperatures and relative humidity of 30 °C/20 °C ± 1 °C and 40 %/95 ± 5 %, respectively. Cool-white fluorescent lamps provided a 14-h photoperiod with PPFD of 700 mmol m−2 s−1 throughout the day at the top of the canopy. After 2·5 weeks, seedlings were thinned to one per pot and supplied with the same nutrient solution until drainage. Nutrients were supplied twice a week. Supplemental KNO3 (12 mm) was supplied during rapid stem elongation (21–50 d after planting). All nutrient additions were terminated at silking, but water was supplied as required (approximately 1·5 L d−1). Planting density was approximately six plants per square metre, which is equivalent to field densities for this genotype. Individual plants were hand-pollinated as described in Zinselmeier et al. (1999). Under these conditions, the plants normally produce yields of about 7·5 tons of grain per hectare of controlled environment area.
Low Ψw treatments
The plants were supplied with adequate water except for a period of 6 d during flowering when water was withheld temporarily from some of them, starting when silks first appeared 5 d before pollination (designated day −5) according to Zinselmeier et al. (1999). Withholding water caused Ψw to decrease in the soil and plants. On the fifth day after silking, the plants were pollinated by hand (designated day 0). Water was re-supplied on the sixth day (day 1). The unshaded third leaf from the top of the plant was sampled 3–4 h into the photoperiod by removing a disc (4·2 cm2) and determining Ψw by isopiestic thermocouple psychrometry according to the method of Boyer (1995).
Low-irradiance treatments
Plants were maintained at the growth irradiances except for a few days during flowering when some were partially shaded to produce photosynthesis rates resembling those in plants subjected to low Ψw. Two days before pollination (day −2), irradiance was reduced to 350 µmol m−2 s−1. On day −1 and day 0, irradiance was further adjusted to 50 µmol m−2 s−1. On day 1, irradiance was returned to 350 µmol m−2 s−1 and finally to 700 µmol m−2 s−1 on day 2. During this sequence, there was a tendency for shade to cause lower air temperatures in the environment chamber, so care was taken to maintain air temperature at the usual 30 °C during each day. The control treatment was maintained continuously at 700 µmol m−2 s−1 and 30 °C during the day.
Stem infusions
In order to image phloem-mediated transport to the maize ovaries, carboxyfluorescein diacetate (CFDA, Molecular Probes Inc., Eugene, OR, USA) was infused into the main stem of maize plants. Using the method of Zinselmeier et al. (1999), the infusion was made at the ear internode on days −5, −2, 0 and +2 on separate plants each day. A cork borer was used to make a well in the stem. It was immediately filled with the solution to be delivered mostly by xylem to the leaves. After sealing the well with a serum stopper, a reservoir was attached and 20 mL of the dye solution was fed (Boyle et al., 1991a, b). After delivery to the leaves, the dye was loaded into the phloem where endogenous esterase activity cleaved off the diacetate moiety to leave the fluorescent CF molecule trapped in the phloem, where it could be transported.
Only one infusion was made in each plant on each day, in contrast to the repeated infusions in each plant used by Zinselmeier et al. (1999). Five milligrams of CFDA was dissolved in 20 mL of 50 mm KOH and the pH was adjusted to 6·3 with 1 m HCl. The location of the CF was determined after 24 h, and twice 4 and 8 h after feeding. Safranin O (Sigma Chemical Co., St Louis, MO, USA) was infused similarly [0·5 % safranin (w/w) in 90 mL of H2O] but only on day 0. The location of the fed safranin was determined after 24 h, or occasionally 42 h and twice 7 d after feeding. The safranin served as a control to test the specificity of CF transport.
Measurements
Net photosynthesis was measured on days −5, −2, 0 and +2 on the same leaves sampled for Ψw, using a LI-COR LI-6400 photosynthesis meter (Li-Cor Inc., Lincoln, NB, USA). Pots were weighed daily to monitor water use.
The whole ear was collected usually after 24 h for CFDA or safranin but occasionally after as much as 7 d after the infusion of safranin. Ten kernels were detached from the middle of the ear and ground in liquid nitrogen with a mortar and pestle. The ground powder was placed in 10 mm phosphate buffer (pH 6·3), vortexed, centrifuged at 10 000 g, and the supernatant was measured immediately for fluorescence in a spectrofluorophotometer (RF-1501, Shimadzu Scientific Instruments, Kyoto, Japan) with excitation at 492 nm and emission at 515 nm. Another set of ten kernels was detached, dried in the oven at 70 °C for 3 d and weighed.
Microscopy
For in situ CF localization, ovaries were removed from the middle of the ear 24 h after each CFDA infusion. The samples were frozen in liquid nitrogen to prevent migration of the fluorescent compounds. Samples stored at −80 °C were embedded in OCT compound (Ted Pella, Redding, CA, USA) and sliced in a cryotome at −20 °C to a thickness of 20 µm. The slices were kept at −20 °C while being mounted on a slide and lyophilized, then were viewed at room temperature with a laser scanning confocal microscope (LSM 510, Carl Zeiss, Germany).
For in situ starch localization, fresh ovaries were detached and sliced in the mid-plane parallel to the long axis of the ear. The ovary halves were stained with I2-KI solution [0·20 % (w/w) I2 and 0·53 % (w/w) KI], briefly rinsed with deionized water and viewed under a dissecting microscope.
Acid invertases were localized in situ in fresh ovaries in longitudinal hand sections (about 0·1 mm thick) maintained in phosphate buffer (0·38 m, pH 5·1) according to the method of McLaughlin and Boyer (2004a). Sections were viewed with a dissecting microscope.
Endogenous glucose was localized in situ from ovaries and young kernel tissues harvested in liquid nitrogen and stored at −80 °C, lyophilized, then observed using the Resorufin method of McLaughlin and Boyer (2004a). An epifluorescence microscope (Olympus AX70, Japan) with an attached CCD camera (Model 2.1.0, Diagnostic Instruments, Inc., Sterling Heights, MI, USA) was used to quantify the images using Image-Pro Plus software (Media Cybernetics, North Reading, MA, USA). Greyscale images were converted to pseudocolour with red equivalent to high concentrations and blue equivalent to low concentrations.
Statistical analysis
The experimental design was a generalized randomized complete block in which each chamber was considered a block with two replications of each treatment included in each block and two blocks in each of three experiments. For the statistical analyses the data were combined and analysed as one experiment. Differences in traits measured between treatments were established with ANOVA. Data shown are the means ± standard error of 3–8 replicate plants in each treatment.
RESULTS
Varying the sugar stream to the ovaries
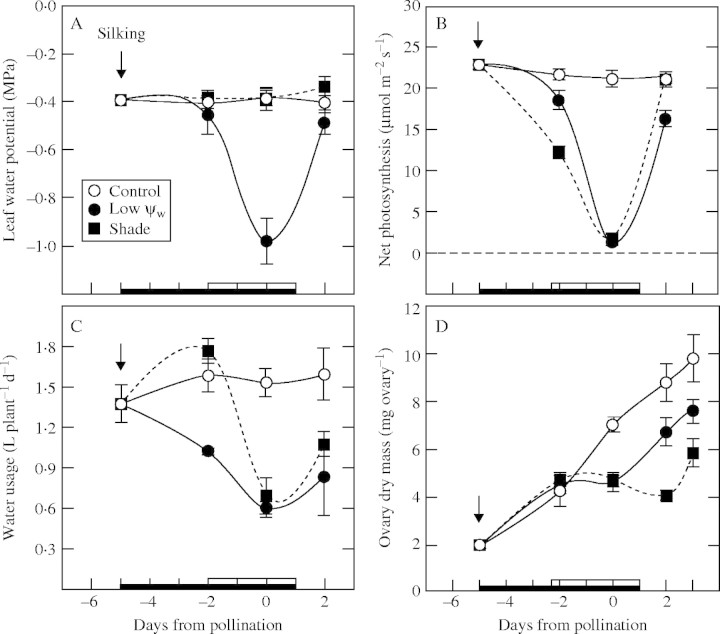
Leaf Ψw remained high at about −0·4 MPa in control plants supplied with adequate water (Fig. 1A). In plants from which water was withheld, leaf Ψw decreased noticeably by day −2 and reached −1·0 MPa on day 0. All plants were hand-pollinated on day 0, at the most severe Ψw. Westgate and Boyer (1986) showed that pollen tubes grew and fertilization occurred under these conditions. When abortion took place, an undeveloped embryo could be seen inside the ovary a few days later. Accordingly in the present study, 1 d was allowed for fertilization and the plants were rewatered on day 1. Leaf Ψw recovered to −0·5 MPa by day 2. In the shade, the plants were supplied with adequate water and Ψw remained as high as in the controls (Fig. 1A).
Fig. 1.
(A) Leaf water potential (Ψw), (B) net photosynthesis, (C) water use, and (D) ovary dry mass in maize deprived of water or shaded around the time of pollination. Open circles: control at high Ψw; closed circles: low Ψw; closed squares: shade. The black bar on the abscissa indicates when water was withheld from the soil and the white bar indicates the time of shading. The arrow marks the beginning of silking. Plants were hand-pollinated on day 0. Data are means ± s.e. of 4–6 plants.
These treatments had a large effect on net photosynthesis in the leaves. In both low Ψw and shade treatments, the rate decreased to 5 and 9 % of the control rate, respectively, by day 0 (Fig. 1B). The decrease occurred at approximately the same time in both treatments. After rewatering or removing the shade, net photosynthesis recovered to 76 % of the control level in plants treated with low Ψw, and recovered completely in the shaded plants (day 2, Fig. 1B).
Water use by the plants generally followed the pattern of changes seen in Ψw and net photosynthesis (Fig. 1C); however, in the shaded plants, use appeared to increase slightly on day −2 before it decreased to about the level seen at low Ψw on day 0. On day 0, water use at low Ψw or in the shade was 43 and 50 % of the control rate, respectively. Upon rewatering or removal of shade, use returned to 59 and 79 %, respectively, of the initial rate in the plants treated with low Ψw or shade, suggesting that stomata had not fully recovered in either treatment despite the recovery of Ψw and irradiance. If the measurements were repeated after another 2 d, use recovered almost completely (data not shown). In controls, water use remained fairly stable.
From day −5 to day −2, the ovaries gained 3 mg in dry mass (Fig. 1D) or about 1 mg per day. From day −2 to day 0, the rate was somewhat higher than 1 mg d−1, but it subsequently returned to this rate on later days. By contrast, there was no gain in dry mass in the shade or low Ψw treatments after day −2, and there was evidence that dry mass was being lost in the shade treatment (Fig. 1D). Although the inhibition lingered for 1 d after the shade was removed in the shade treatment, dry matter accumulation had fully resumed in both treatments after day 2 (slope of the line for ovary dry mass returned to about 1 mg d−1).
Ovary starch
Maize ovaries contained a spherical nucellus sitting in a cup-shaped structure at or near the terminus of the phloem (Fig. 2). The nucellus contained the embryo sac, which was not visible in the plane of section. There was a cylinder of vascular tissue leading to the cup, appearing here as two strands because of the median, axial section through the cylinder.
Fig. 2.
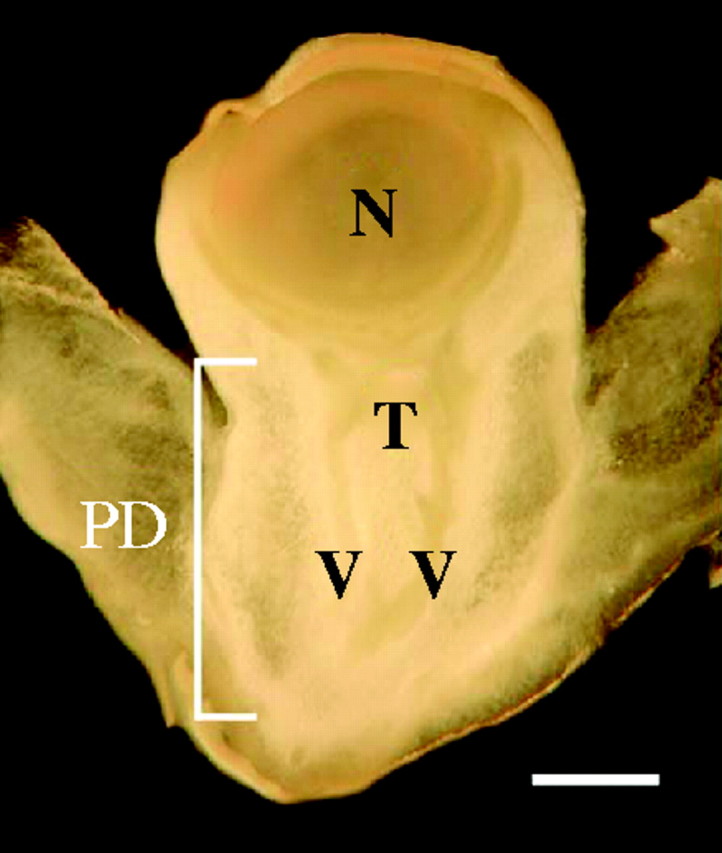
Structure of maize ovary on the day of pollination. Phloem terminates in or immediately below cup-shaped tissue below the nucellus. Stigma and style have been removed. N, nucellus; T, phloem termini; V, vascular structures; PD, pedicel. Scale bar = 1 mm.
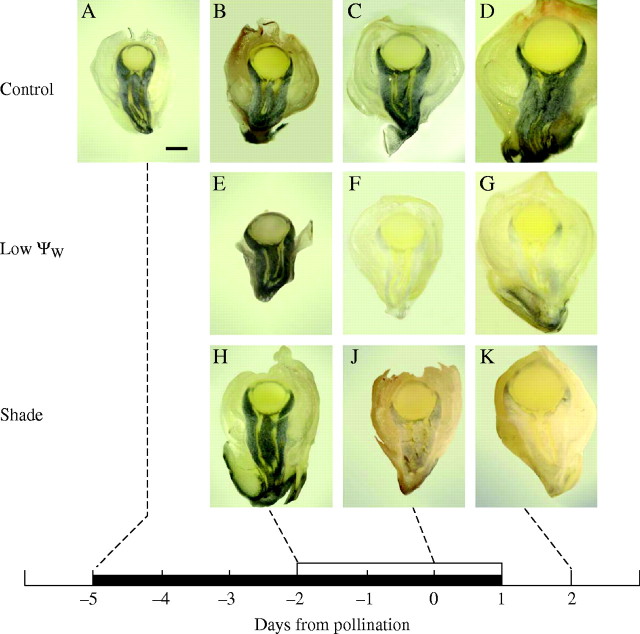
Starch increased in the tissue below the nucellus as the ovaries became larger from day −5 to day 2 (Fig. 3A–D). Shade or low Ψw nearly eliminated the starch by day 0 (Fig. 3F, J), but it began to reappear after rewatering (Fig. 3G). In shaded plants the reappearance of starch was delayed until after day 2 when the shade was removed (Fig. 3K). In a study at low Ψw, Zinselmeier et al. (1999) found similar results using a chemical assay of ovary starch content.
Fig. 3.
Starch in maize ovaries around the time of pollination. Control: (A–D) display starch (black) as ovaries develop. Starch was located around the vascular tissue in the pedicel and as part of the cup below the nucellus. No starch was detected in the nucellus. Low Ψw: starch was present in (E) but was depleted at low Ψw (F), and began to reappear (G) after rewatering. Shade: starch was present in (H) but depleted in shade (J) and remained depleted for 1 d after shade was removed (K). The black bar on the abscissa indicates when water was withheld from the soil and the white bar indicates the time of shading. Scale bar = 1 mm.
Tracing translocation with fluorescent dye
Because the shade and low Ψw treatments inhibited dry mass translocation to the ovaries and their starch nearly disappeared, the translocation of CF was measured to determine whether phloem retained its ability to transport solute. The specificity of CF for phloem was tested by infusing the main stem with the dye and comparing its location in the ear shoot with that of safranin, which stained xylem in maize (Tang and Boyer, 2002). After dye absorption for 1–3 h, CF and safranin could be detected in the nodal plexi of the ear shoot just below the ear 24 h later (Fig. 4). In a single plexus, CF was in the phloem alongside safranin in the xylem (Fig. 4A, B). Both dyes were confined to their respective vascular tissues and none could be detected in the surrounding tissues, which appeared black in the laser confocal microscope.
Fig. 4.
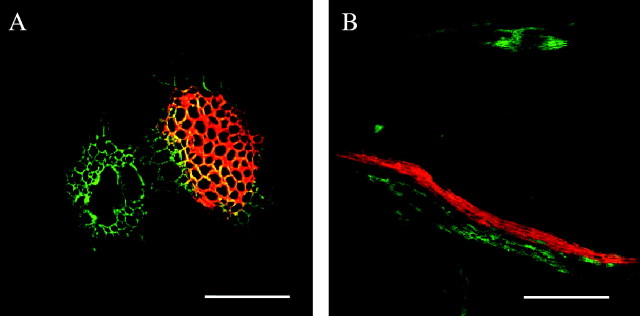
(A) Localization of carboxyfluorescein (green) and safranin (red) in horizontally cut node (cross-section) below ear on maize ear shoot. (B) Localization of carboxyfluorescein (green) and safranin (red) in vertically cut node (axial-section) below ear of maize ear shoot. Scale bars: (A) 100 mm, (B) 1 mm.
The dye was apparent in the ovaries by 24 h after dye infusion, with substantial CF in the phloem and surrounding pedicel tissue at the ovary base (Fig. 5A–D). There was no CF in the nucellus. The pattern was similar as development proceeded but the phloem became more visible at later stages, especially after pollination (Fig. 5D), as though the CF was increasingly restricted to the phloem. By contrast, safranin infused into the stem could not be detected in the ovaries. Safranin was absent even when the plants were fed for a short time with safranin and chased for 7 d with water (data not shown). By contrast, CF could be detected in as little as 4 h (data not shown).
Fig. 5.
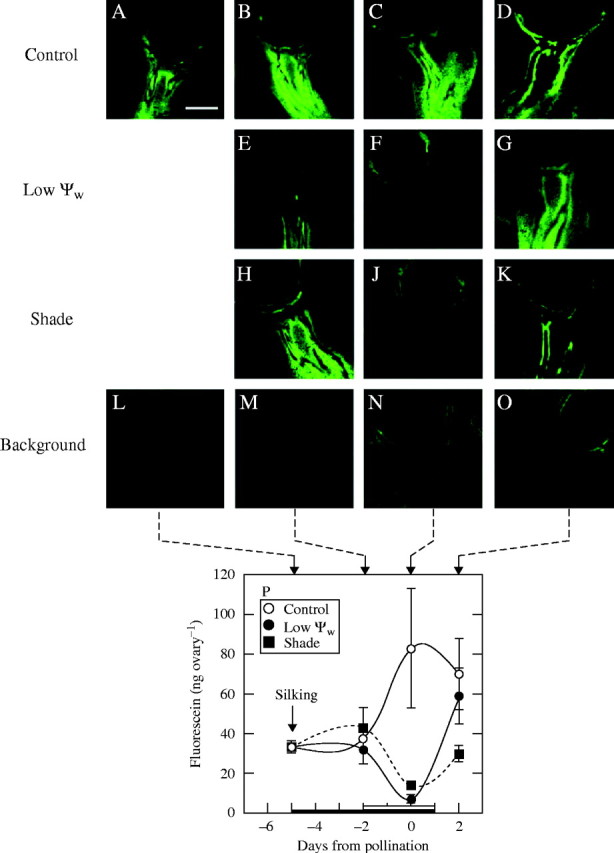
Localization and content of carboxyfluorescein (green) in maize ovaries 24 h after stem infusion around the time of pollination. Control: (A–D) as ovaries developed, carboxyfluorescein was increasingly evident (green) in and around the vascular tissues in the pedicel. Low Ψw: carboxyfluorescein was nearly absent (E, F) but reappeared 1 d after rewatering (G). Shade: (H) carboxyfluorescein was less abundant than in controls and disappeared (J). It began to reappear 1 d after shade was removed (K). Shade was imposed to match photosynthesis at low Ψw (see Fig. 1). Background: (L–O) autofluorescence in the absence of carboxyfluorescein. Scale bar = 1 mm. (P) Spectofluorophotometer measurement of fluorescence from carboxyfluorescein in extracts from ovaries in images. Open circles: control at high Ψw; closed circles: low Ψw; closed squares: shade. The black bar on the abscissa indicates when water was withheld from the soil and the white bar indicates the time of shading. Data are means ± s.e. of 3–5 plants.
In the low Ψw treatment, CF was faint on day −2 and scarcely detectable on day 0 (Fig. 5E, F). After rewatering on day 1, CF began to return to the phloem and surrounding tissues 1 d later (day 2, Fig. 5G). In the shade treatment, CF was present on day −2 but was nearly undetectable on day 0 (Fig. 5H, J). After removing the shade on day 1, CF was barely visible on day 2 and was confined to the phloem (Fig. 5K). There was hardly any autofluorescence in the maize ovaries (Fig. 5L–O).
The ovary content of CF was quantified by extracting the dye from the homogenized ovaries and determining the amount of fluorescence in a spectrofluorophotometer (Fig. 5P). On day 0, CF was at 8 and 16 % of controls in the low Ψw and shade treatments, respectively. After the treatments were removed on day 1, a recovery was detected on day 2 but less after shade than after low Ψw. These results correlate with the CF in the images (Fig. 5A–K), dry mass in the ovaries (Fig. 1D) and net photosynthesis in the parent plants (Fig. 1B). In the spectrofluorophotometer, the background autofluorescence was 2–4 ng per ovary, which was neglected (data not shown).
Localizing glucose
After unloading from the phloem, sucrose is hydrolysed by invertase, one of the products being glucose. The location of glucose thus establishes the site of hydrolysis, which was mostly in the upper pedicel tissues (Fig. 6A, B). At low Ψw on day 0, glucose was nearly depleted in the pedicel and only a slight amount could be seen near the phloem termini (Fig. 6C, D). In the shade, there was less glucose than in controls and it was most concentrated around the phloem termini (Fig. 6E, F). By quantifying the fluorescent dye (Resorufin) in the spectrofluorophotometer and relating the result to a standard curve of glucose concentrations as in McLaughlin and Boyer (2004a), glucose was detected at only about 26 % of the control concentration in the ovaries at low Ψw (Fig. 6G). In the shade, glucose was at 47 % of the controls.
Fig. 6.
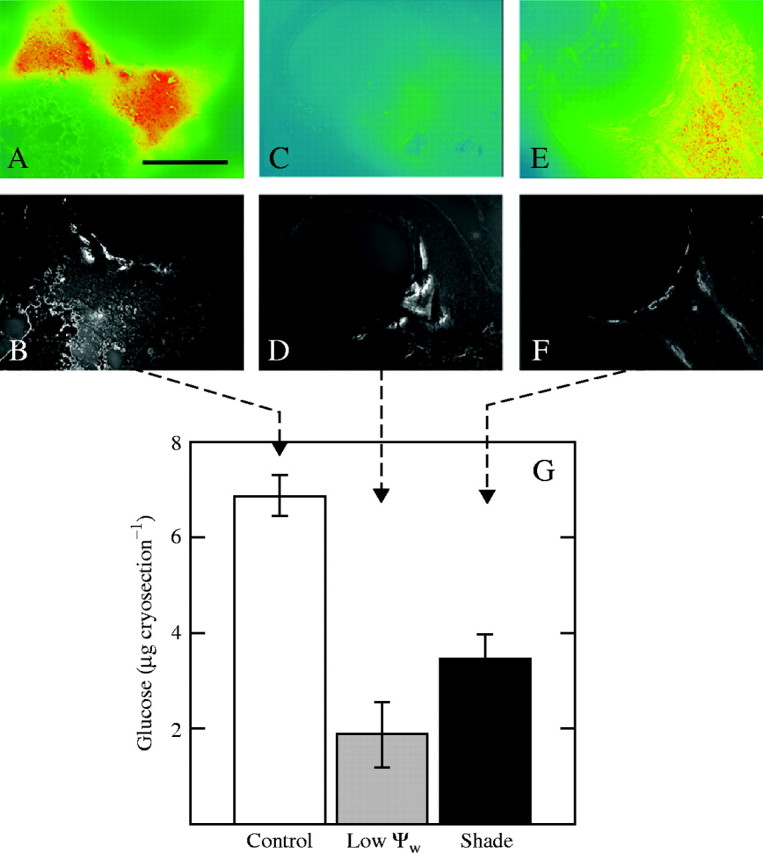
In vivo localization of glucose in maize ovaries on the day of pollination (day 0). Epifluorescence micrographs of glucose are paired with respective UV autofluorescence (below) showing ovary anatomy. High, moderate and low concentrations of glucose are shown as red, yellow and blue, respectively. Control: (A, B), glucose (A) was concentrated in the upper pedicel but was low in the nucellus shown in (B). Low Ψw: (C, D) glucose concentration was low in (C) compared with control in (A). Shade: (E, F) glucose concentration was low in (E) compared with control in (A). Scale bar = 1 mm. (G) Spectrofluorophotometer quantification of glucose concentrations in extracts from cryosections like those in (A–F). Data are means ± s.e. of three plants.
Localizing acid invertases
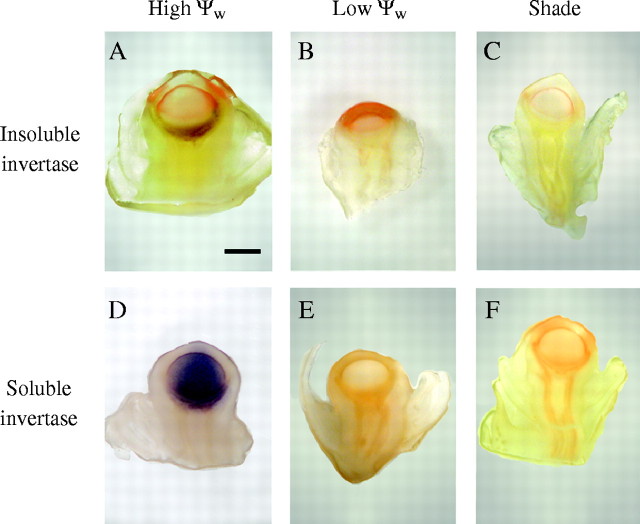
In maize, acid invertases are thought to mediate the hydrolysis of sucrose arriving from the phloem (Shannon, 1968, 1972; Shannon and Dougherty, 1972; Felker and Shannon, 1980; ap Rees, 1984; Doehlert and Felker, 1987; Griffith et al., 1987; Porter et al., 1987; Miller and Chourey, 1992; Thomas et al., 1993; Xu et al., 1995; Cheng et al., 1996). The low glucose concentrations at low Ψw and in the shade suggest that there could have been effects on invertase activities. Figure 7A shows an image of insoluble acid invertase obtained with living, extensively washed tissue. Because the tissue was washed and assay components were supplied from outside, the insoluble activity was outside the plasma membranes and bound to the cell walls (apoplast). There was a significant amount of activity in control maize ovaries on the day of pollination, below the nucellus in the cup region, as also reported by McLaughlin and Boyer (2004a). This form of activity was undetected in ovaries at low Ψw or in the shade on the same day (Fig. 7B, C).
Fig. 7.
Insoluble (A–C) and soluble (D–F) acid invertase activities in maize ovaries on the day of pollination (day 0). (A) Insoluble activity in vivo was localized in upper pedicel tissue. (B, C) Insoluble activity was not detected at low Ψw (B) or in shade (C) but was present if the assay was extended to 24 h. This indicated that insoluble activity was present but very low at low Ψw or in shade. (D) Soluble activity in situ was localized in the nucellus. Although there is some overlap of upper pedicel tissues and nucellus tissue in this section, other sections showed the soluble activity to be restricted to the nucellus (McLaughlin and Boyer, 2004a). (E, F) No activity was detected for soluble invertase at low Ψw (E) or in shade (F). Scale bar = 1 mm. Images are representative of eight control plants, eight plants at low Ψw and three plants in shade.
By contrast, soluble acid invertase was restricted to the nucellus (Fig. 7D), in agreement with the findings of McLaughlin and Boyer (2004a, b). In comparison with the high activity in the nucellus of the control, no soluble activity was detected in the nucellus at low Ψw or in shade on the day of pollination (Fig. 7E, F). In two studies of maize abortion at low Ψw, Zinselmeier et al. (1999) and Andersen et al. (2002) found similar results using chemical assays of invertase activities in vitro.
Note that the sections for the assays of soluble invertase had been frozen and thawed, but the soluble invertase remained trapped inside the cell walls because of its large size. Rupturing the plasma membranes by freezing and thawing allowed the small assay solutes to reach the invertase inside, but small cytoplasmic solutes leaked out and removed most of the insoluble invertase that had been bound to the cell walls (apoplast) by salt bridging. This insoluble activity was lost during subsequent washing in this assay (McLaughlin and Boyer, 2004a).
DISCUSSION
Transport of dry mass
Maize ovaries proved to be important sinks for carbon around the time of pollination. Considering only the persistent dry mass in excess of the losses in respiration, ovary dry mass was about 7 mg and increased by about 1–1·5 mg d−1. There were about 500 ovaries per ear thus accumulating a total of 0·5–0·8 g of dry mass, which was about 10–15 % of the 5 g gained each day by the whole plant under field conditions (Jurgens et al., 1978). Therefore, the arriving ovary dry mass, although a small part of the total output by the parent plant, made a major contribution to ovary development.
When net photosynthesis was inhibited by shading or by low Ψw, the dry matter stream decreased within 1 d and ovary dry mass stopped increasing or began to decrease, indicating not only that dry matter was not arriving but that it was being consumed in the ovaries. Starch normally accounts for about 0·4 mg of the ovary dry mass (Zinselmeier et al., 1999; Andersen et al., 2002) and it nearly disappeared with shade or low Ψw, giving visual confirmation of the consumed dry mass. The treatments thus deprived the ovaries of photosynthetic products from the parent.
However, the translocation system remained intact and dry mass began to move into the ovaries when the treatments were removed. This resumption of dry matter delivery contrasts with the observations of Zinselmeier et al. (1999), in which it did not occur because the ovaries were aborting. In the present work, abortion was not assessed but Ψw and water use were not as low as in the study of Zinselmeier et al. (1999), suggesting that abortion may not have been as extensive. This would allow dry matter delivery to resume after the shade was removed or after the plants were rewatered.
Assuming the arriving dry mass was mostly sucrose, the ovaries hydrolysed at least 1 mg d−1 of sucrose around the day of pollination under normal conditions. This hydrolysis caused glucose to accumulate in the upper pedicel tissues beneath the nucellus and, according to McLaughlin and Boyer (2004a), this pool accounted for about 0·2 mg of glucose each day, or about 40 % of the arriving sucrose (0·2 mg of glucose + 0·2 mg of fructose for each milligram of arriving sucrose). The other 60 % was devoted to starch synthesis and biosynthetic activity in the enlarging ovary tissues. As shown here and also by McLaughlin and Boyer (2004a), insoluble acid invertase was present in the apoplast where it was bound to the cell walls in vivo. Its activity was capable of hydrolysing 0·86 mg d−1 of sucrose (Zinselmeier et al., 1999), or nearly all of the incoming sucrose. It is thus reasonable to assume that most of the incoming dry mass was hydrolysed by invertase in the apoplast before entering ovary metabolism.
The invertases and sucrose synthases are the only known enzymes for sucrose breakdown in plants (Sturm and Tang, 1999). Although invertases appeared to dominate sucrose hydrolysis in maize ovaries (McLaughlin and Boyer, 2004a), Wittich and Vreugdenhil (1998) reported small amounts of sucrose synthase activity in the ovary tissues. UDPglucose, one of the products of the reaction mediated by sucrose synthase, was found in nanomolar quantities in the ovaries by Zinselmeier et al. (1999). Consequently, it seems possible that some sucrose was also broken down by sucrose synthase.
Transport of carboxyfluorescein
In general, these results indicate that effects of low Ψw and shade are similar for carbohydrate transport. Moreover, for both treatments, CF mimicked the carbohydrate transport and gave visual confirmation of the diminished delivery. Comparing the rates of CF arrival in Fig. 5P with the rates of dry mass accumulation calculated from the slopes of the data in Fig. 1D, Fig. 8A shows the two rates increased together and reached a maximum on the day of pollination in the control ovaries exposed to favourable conditions. When the plants were subjected to low Ψw or shade, dry mass delivery became slower than in the controls, and CF arrival was diminished, reaching a minimum on the day of pollination (Fig. 8B, C). These results are consistent with the concept that varying rates of photosynthesis varied the rates of bulk flow of sucrose in the phloem, and that CF was moving in that flow.
Fig. 8.
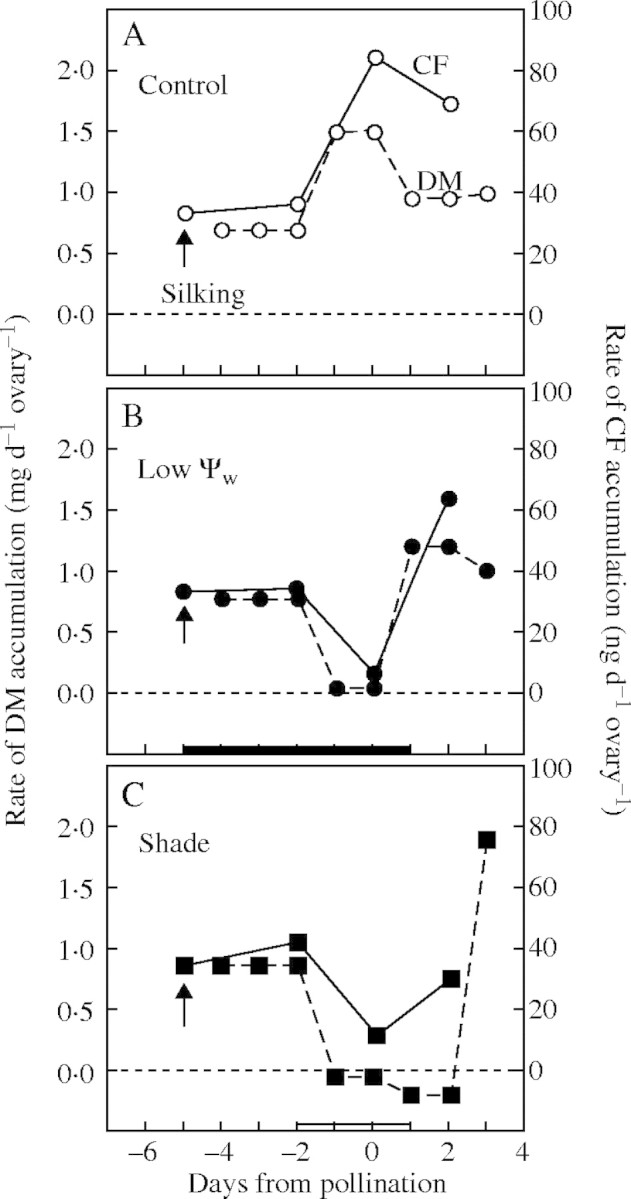
Comparison of mean rates of dry mass (DM) and carboxyfluorescein (CF) accumulation per day during the experiments. (A) Control, (B) low Ψw, and (C) shade. Rates of DM accumulation were calculated from the slope of the means shown in Fig. 1D. Rates of CF accumulation are means from Fig. 5P. The black bar on the abscissa indicates when water was withheld from the soil and the white bar indicates the time of shading.
After delivery to the phloem termini, CF spread through the surrounding tissues in the upper pedicel, where its localization was similar to that for glucose and starch. CF is generally considered to be unable to cross the plasma membrane, and is trapped inside those cells connected to the phloem by plasmodesmata (Patrick, 1997). However, because glucose and starch were outside the phloem and post-phloem path, CF also may have moved out of the post-phloem symplast and into the apoplast. Unequivocal evidence for movement into the apoplast remains to be determined, however.
Gradients in glucose
Glucose accumulated in the pedicel, giving a high glucose concentration that resulted in a substantial gradient towards the nucellus, where the concentration of glucose was very low. This gradient favoured glucose movement into the nucellus. The embryo sac was located in the nucellus and contained starch, as shown by Diboll and Larson (1965), which confirmed that glucose entered the nucellus. When low Ψw or shade decreased glucose in the pedicel, the treatments also decreased the gradient and thus a potential factor moving glucose into the nucellus.
CF mimicked these effects on the glucose gradient. The delivery of CF to the pedicel created a steep CF gradient favouring movement into the nucellus. Low Ψw or shade diminished the CF gradient. But in contrast to glucose, no CF moved into the nucellus. This coincidence of gradients but lack of entry into the nucellus indicates that the path of sugar movement was highly selective for the solute. Fisher and Oparka (1996) highlighted the differences in the molecular structure of sucrose and CF, and noted that membrane transport and cellular movement are unlikely to be the same for the two molecules. In the present experiments, their similar locations in the pedicel but differential uptake by the nucellus suggest that the molecular differences were recognized by the post-phloem cells or by the nucellus.
This selective barrier for ovary solutes seems to rule out the conclusion of Andersen et al. (2002) that the nucellus might be part of a post-phloem symplast path. Instead, it appears that there is a barrier to movement between the post-phloem symplast and the nucellus. The barrier controlled what reached the embryo sac, thus resembling the selective role of the transfer cells at the base of the kernel later in reproductive development when endosperm and embryo are present (Patrick and Offler, 1995).
Other barriers may also exist because xylem-mobile safranin failed to enter the ovary although the xylem passes around the nucellus and into the stylar tissue (Kiesselbach, 1949). Safranin binds to lignin in the xylem walls and tends to be removed from the transpiration stream (Tang and Boyer, 2002). Because water transport to the ovary is likely to be slow, the safranin may have been bound to the xylem walls before it reached the ovary xylem, in effect removing the safranin from the xylem stream. If the dye had no binding affinity for the walls, it might have moved more readily into the ovary where other filters would be important.
Low Ψw and shade effects on sugar signalling
McLaughlin and Boyer (2004b) reported the mRNA abundance for essentially all of the expressed genes for sucrose processing in maize ovaries. All were down-regulated at low Ψw but two invertase genes (Incw2 and Ivr2) were sugar-responsive, i.e. sucrose fed to the stems caused their mRNA to return toward control levels. In the present study, the response of glucose, starch and CF to shade resembled that for low Ψw, which suggests that sugar signals may occur in the shade as well. In both treatments, photosynthesis was inhibited, dry mass and CF delivery diminished, and glucose and starch were depleted 2 d later. Therefore, the sequence for sugar signalling may involve less sucrose arriving at the ovaries initially (day −2), triggering the breakdown of ovary starch and release of glucose. With only 0·4 mg of starch in the ovaries, the breakdown of starch would meet the demand for glucose (about 1 mg d−1) for only a short time. Glucose would be depleted by day 0, as observed. The sequence of sugar signals for changes in ovary gene expression thus may involve sucrose first and glucose later both during shade and low Ψw.
Koch (1996) and Sheen et al. (1999) describe sugar signalling for many genes, some of which are involved in sucrose processing. However, signalling from low sugar delivery seemed questionable based on the results of Schussler and Westgate (1991b), Zinselmeier et al. (1995b) and Andersen et al. (2002), who reported little change or increased concentrations of sugars at low Ψw. These studies expressed the data on a dry mass basis, and because the dry mass was 50–70 % sugars and starch (Zinselmeier et al., 1999; Andersen et al., 2002), concentrations depended on whether sugars or dry mass reacted more to the treatments. By contrast, sugar availability was directly altered by feeding sugars to the stems of plants in the work of Zinselmeier et al. (1999) and McLaughlin and Boyer (2004b). Sugar constituents were reported as a time course per ovary such that availability could be considered to meet demand if the sugars increased or remained stable through time (intake exceeded or equalled use). Demand was unmet if sugars declined (intake less than use). By this test, Zinselmeier et al. (1999) concluded that sucrose demands were unmet in the ovaries at low Ψw unless sugars were fed. Zinselmeier et al. (1995b) and Setter et al. (2001) reported sugar availability using both approaches and showed that flux differences detectable on an ovary basis became less apparent when expressed as concentrations on a dry mass basis. Therefore, the basis for expressing sugar concentration must be carefully considered when testing for possible signals for gene expression.
CONCLUSIONS
In maize, CF visually identified the transport path, relative rate of carbohydrate delivery and most of the gradient effects accompanying sugar movement into the ovary when photosynthesis was inhibited. The CF thus traced most of the carbohydrate movement. The CF confirmed the losses in carbohydrate transport to the ovary at low Ψw and in the shade, but phloem transport remained functional and recovered when the treatments were removed. The similarity in carbohydrate movement under the two treatments suggests that decreased carbohydrate delivery is a central feature of reproductive abortion whenever low rates of photosynthesis occur at critical times.
It is well known that phloem unloads to a post-phloem symplast and thence to the apoplast before entering the embryo and endosperm in reproductive sinks (Patrick, 1997). The present work indicates that a similar situation exists for glucose in the ovaries before an embryo and endosperm are present. For CF, however, the path ends in the symplast or possibly the apoplast. CF may be unable to exit the post-phloem cells or may be prevented from entering the nucellus but, in either scenario, the selectivity protected the embryo sac from CF delivered by the phloem.
Acknowledgments
We thank Jim A. Hawk and Teclemariam Weldekidan for making the crosses and supplying the seed for the hybrid used in this study, and An-Ching Tang and Ted Proseus for scientific and technical advice. This work was supported by the Academy of Finland (grant no. 52838) and the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2002-35100-12019.
Footnotes
Present address: Department of Applied Biology, Section of Crop Husbandry, PO Box 27, 00014 University of Helsinki, Finland.
LITERATURE CITED
- Andersen MN, Asch F, Wu Y, Jensen CR, Næsted H, Mogensen VO, Koch KE. 2002. Soluble invertase expression is an early target of drought stress during the critical, abortion sensitive phase of young ovary development in maize. Plant Physiology 130: 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS. 1995.Measuring the water status of plants and soils. San Diego: Academic Press. [Google Scholar]
- Boyle MG, Boyer JS, Morgan PW. 1991. Stem infusion of maize plants. Crop Science 31: 1241–1245. [Google Scholar]
- Boyle MG, Boyer JS, Morgan PW. 1991. Stem infusion of liquid culture medium prevents reproductive failure of maize at low water potential. Crop Science 3: 1246–1252. [Google Scholar]
- Bradford KJ. 1994. Water stress and the water relations of seed development: a critical review. Crop Science 34: 1–11. [Google Scholar]
- Cheng W-H, Taliercio EW, Chourey PS. 1996. The Miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. The Plant Cell 8: 971–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diboll AG, Larson DA. 1966. An electron microscopic study of the mature megagametophyte in Zea mays. American Journal of Botany 53: 391–402. [PubMed] [Google Scholar]
- Doehlert DC, Felker FC. 1987. Characterization and distribution of invertase activity in developing maize (Zea mays) kernels. Physiologia Plantarum 70: 51–57. [Google Scholar]
- Felker FC, Shannon JC. 1980. Movement of 14C-labeled assimilates into kernels of Zea mays L. III. An anatomical examination and microautoradiographic study of assimilate transfer. Plant Physiology 65: 864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DB, Oparka KJ. 1996. Post-phloem transport: principles and problems. Journal of Experimental Botany 47: 1141–1154. [DOI] [PubMed] [Google Scholar]
- Goodall H, Johnson MH. 1982. Use of carboxyfluorescein diacetate to study formation of permeable channels between mouse blastomeres. Nature 295: 524–526. [DOI] [PubMed] [Google Scholar]
- Griffith SM, Jones RJ, Brenner ML. 1987.In vitro sugar transport in Zea mays L. kernels. I. Characteristics of sugar absorption and metabolism by developing maize endosperm. Plant Physiology 84: 467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanft JM, Jones RJ. 1986. Kernel abortion in maize. II. Distribution of 14C among kernel carbohydrates. Plant Physiology 81: 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. 1950. The water culture method for growing plants without soil. California Agricultural Experimental Station Circular 347: 1–32. [Google Scholar]
- Jurgens SK, Johnson RR, Boyer JS. 1978. Dry matter production and translocation in maize subjected to drought during grain fill. Agronomy Journal 70: 678–682. [Google Scholar]
- Kiesselbach TA. 1949.The structure and reproduction of corn. Lincoln: University of Nebraska Press. [Google Scholar]
- Koch KE. 1996. Carbohydrate-modulated gene expression in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 509–540. [DOI] [PubMed] [Google Scholar]
- McLaughlin JE, Boyer JS. 2004. Glucose localization in maize ovaries when kernel number decreases at low water potential and sucrose is fed to the stems. Annals of Botany 94: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JE, Boyer JS. 2004. Sugar-responsive gene expression, invertase activity, and senescence in aborting maize ovaries at low water potentials. Annals of Botany 94: 675–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson HG, Boyer JS. 1977. Regulation of grain yield by photosynthesis in maize subjected to a water deficiency. Agronomy Journal 69: 714–718. [Google Scholar]
- Miller ME, Chourey PS. 1992. The maize invertase-deficient miniature-1 seed mutation is associated with aberrant pedicel and endosperm development. The Plant Cell 4: 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick JW. 1997. Phloem unloading: sieve element unloading and post-sieve element transport. Annual Review of Plant Physiology and Plant Molecular Biology 48: 191–222. [DOI] [PubMed] [Google Scholar]
- Patrick JW, Offler CE. 1995. Post-sieve element transport of sucrose in developing seeds. Australian Journal of Plant Physiology 22: 681–702. [Google Scholar]
- Patrick JW, Offler CE. 1996. Post-sieve element transport of photoassimilates in sink regions. Journal of Experimental Botany 47: 1165–1177. [DOI] [PubMed] [Google Scholar]
- Porter GA, Knievel DP, Shannon JC. 1987. Assimilate unloading from maize (Zea mays L.) pedicel tissues. Plant Physiology 83: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AJ, Singletary GW. 1989. Roles of carbohydrate supply and phytohormones in maize kernel abortion. Plant Physiology 91: 986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ap Rees T. 1984. Sucrose metabolism. In: Lewis DH, ed. Storage carbohydrates in vascular plants. London: Cambridge University Press, 53–73. [Google Scholar]
- Rotmann B, Papermaster BW. 1966. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proceedings of the National Academy of Sciences of the USA 55: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini HS, Westgate ME. 2000. Reproductive development in grain crops during drought. Advances in Agronomy 68: 59–96. [Google Scholar]
- Salter PJ, Goode JE. 1967.Crop responses to water at different stages of growth. Farnham Royal, Bucks., UK: Commonwealth Agricultural Bureau. [Google Scholar]
- Schussler JR, Westgate ME. 1991. Maize kernel set at low water potential. 1. Sensitivity to reduced assimilates during early kernel growth. Crop Science 31: 1189–1195. [Google Scholar]
- Schussler JR, Westgate ME. 1991. Maize kernel set at low water potential. II. Sensitivity to reduced assimilates at pollination. Crop Science 31: 1196–1203. [Google Scholar]
- Setter TL, Flannigan BA, Melkonian J. 2001. Loss of kernel set due to water deficit and shade in maize: carbohydrate supplies, abscisic acid, and cytokinins. Crop Science 41: 1530–1540. [Google Scholar]
- Shannon JC. 1968. Carbon-14 distribution in carbohydrates of immature Zea mays kernels following 14CO2 treatment of intact plants. Plant Physiology 43: 1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon JC. 1972. Movement of 14C-labeled assimilates into kernels of Zea mays L. I. Pattern and rate of sugar movement. Plant Physiology 49: 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon JC, Dougherty CT. 1972. Movement of 14C-labeled assimilates into kernels of Zea mays L. II. Invertase activity of the pedicel and placento-chalaza tissues. Plant Physiology 49: 203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J, Zhou L, Jang JC. 1999. Sugars as signaling molecules. Current Opinion in Plant Biology 2: 410–418. [DOI] [PubMed] [Google Scholar]
- Sturm A, Tang G-Q. 1999. The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends in Plant Science 4: 401–407. [DOI] [PubMed] [Google Scholar]
- Tang A-C, Boyer JS. 2002. Growth-induced water potentials and the growth of maize leaves. Journal of Experimental Botany 53: 489–503. [DOI] [PubMed] [Google Scholar]
- Thomas PA, Felker FC, Shannon JC, Crawford CG. 1993. Use of tassel-seed (Ts-5) maize for assimilate transport studies using intact or detached tassel branches. Crop Science 33: 325–328. [Google Scholar]
- Westgate ME, Boyer JS. 1985. Carbohydrate reserves and reproductive development at low water potentials in maize. Crop Science 25: 762–769. [Google Scholar]
- Westgate ME, Boyer JS. 1986. Reproduction at low silk and pollen water potentials in maize. Crop Science 26: 951–956. [Google Scholar]
- Wittich KE, Vreugdenhil D. 1998. Localization of sucrose synthase activity in developing maize kernels by in situ enzyme histochemistry. Journal of Experimental Botany 49: 1163–1171. [Google Scholar]
- Xu J, Pemberton GH, Almira EC, McCarty DR, Koch KE. 1995. The Ivr1 gene for invertase in Zea mays L. Plant Physiology 108: 1293–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinselmeier C, Jeong B-R, Boyer JS. 1999. Starch and the control of kernel number in maize at low water potentials. Plant Physiology 121: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinselmeier C, Lauer MJ, Boyer JS. 1995. Reversing drought-induced losses in grain yield: sucrose maintains embryo growth in maize. Crop Science 35: 1390–1400. [Google Scholar]
- Zinselmeier C, Schussler JR, Westgate ME, Jones RJ. 1995. Low water potential disrupts carbohydrate metabolism in maize ovaries. Plant Physiology 107: 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]