Abstract
• Background and Aims Morphological descriptions of the extrafloral nectaries (EFNs) of certain plant species are common in the literature, but they rarely relate morphology with histology, gland distribution and secretory attributes. In this study a morphological/secretory characterization of EFNs occurring on several plant species in a tropical coastal community is made and the implications of gland attributes discussed from a functional perspective.
• Methods The morphology and nectar secretion of the EFNs of 20 plant species are characterized through scanning electron microscopy, histochemical detection of reducing sugars (Fehling's reagent) and nectar volume/concentration estimates.
• Key Results Sixty-five per cent of plant species in coastal communities had EFNs on vegetative structures and 35 % of species had glands on reproductive and vegetative organs. The Fabaceae is the plant family with the most species with EFNs and most diversity of gland morphologies. Four types of vascularized nectaries and four of glandular trichomes are described; sugar-secreting trichomes are characterized using Fehling's technique, and the first descriptions of unicellular and peltate trichomes functioning as EFNs are provided. Glands of ten plant species and six genera are described for the first time. Four plant species possess more than one morphological type of EFN. Eleven species have EFNs in more than one location or organ. More complex glands secrete more nectar, but are functionally homologous to the aggregations of numerous secretory trichomes on specific and valuable plant organs.
• Conclusion Important diversity of EFN morphology was foundin the coastal plant community studied. Both vascularized and non-vascularized EFNs are observed in plants and, for the latter, previously non-existent morpho-secretory characterizations are provided with a methodological approach to study them. It is recommended that studies relating EFN attributes (i.e. morphology, distribution) with their differential visitation by insects (i.e. ants) and the cost of maintenance to the plants are carried out to understand the evolution of these glands.
Keywords: Extrafloral nectary morphology, secretory rates, nectary position, histology, taxonomy, ant-plant interactions, coastal plant communities
INTRODUCTION
Extrafloral nectaries (EFNs) are nectar-secreting vascularized or non-vascularized structures not directly involved with pollination (Elias, 1983; Koptur, 1992a), which are especially common on leaves, petioles, young stems, stipules and reproductive structures (e.g. buds, calyx, inflorescence axis, flower peduncles, fruit) (Rico-Gray, 1989, 1993; Rico-Gray et al., 2004). Extrafloral nectar contains large proportions of sugars (15–75 % per weight), lower but significant amounts of amino acids, and small amounts of other organic compounds (Elias, 1983; Beattie, 1985; Lanza, 1988; Galetto and Bernardello, 1992; Koptur, 1992b). These glands are structurally diverse and occur on a wide variety of plant taxa (both angiosperms and ferns) (Bentley, 1977a, b; Elias, 1983; Oliveira and Leitão-Filho, 1987; Koptur et al., 1998).
Given the taxonomic value of EFNs (Bhattacharya and Maheshwari, 1970; Lersten and Brubaker, 1987), pure morphological descriptions of these nectar-secreting glands is an ancient study topic. However, information on the secretory rates and gland morphology of the EFNs of different plant species have currently an aggregated value: these features influence plant attractiveness to nectar-foraging insect visitors (Apple and Feener, 2001; Hossaert-Mckey et al., 2001) thus affecting the outcome of the interaction among plants, their mutualistic insect visitors (i.e. ants) and herbivores (Rudgers and Gardener, 2004; Wäckers and Bonifay, 2004). In a field survey of EFN-bearing plants in coastal vegetation of Veracruz, Mexico, Díaz-Castelazo et al. (2004) found 41 species of EFN-bearing plants, which were more abundant during the rainy season and were constantly visited (day and night) by a variable ant assemblage. A high proportion of the community of plants bearing EFNs (66 % of the species) was associated with ants, and an even higher proportion of the ant species of the studied communities (84 % of the species found at honey baits) was associated with EFN-bearing plants. That study provided evidence of a strong seasonal variation in the number of ant and plant species associated through EFNs and some plant species were much more visited by ants or had a more species-rich-associated ant fauna) than others (see also Cuautle et al., 2005). These findings led to the question: are there different morphological or secretory attributes of the EFNs of different plant species that could shed some light on their differential associations with ants (‘attractiveness’)? Morpho-secretory data of the EFNs in most of the plant species surveyed at the site (Díaz-Castelazo et al., 2004) is practically non-existent, although morphological information on the glands of certain genera is available (for previous generic- or species-level records, see Table 1).
Table 1.
Morphology and distribution (taxonomical and on plant tissues) of EFNs in 20 plant species studied on coastal communities of Veracruz, Mexico
| Family/species |
Morphotype of EFN (vascularized) |
Morphotype of EFN (non-vascularized) |
Site (location) |
Distribution |
||||
|---|---|---|---|---|---|---|---|---|
| Apocynaceae | ||||||||
| *,†,‡Prestonia mexicana | Peltate trichomes | Blade/shoots | Scattered | |||||
| Bignoniaceae | ||||||||
| ‡Amphilophium paniculatum7,D | Scale-like trichomes | Blade/calyx/fruit | Uniform | |||||
| *,†,‡Mansoa hymenaea | Scale-like trichomes | Stem nodes | Aggregated | |||||
| ‡Tabebuia rosea6,A,D | Scale-like trichomes | Blade/stem | Uniform | |||||
| Boraginaceae | ||||||||
| *,†,‡Cordia spinescens | Peltate trichomes | Blade (abx) | Uniform | |||||
| Cactaceae | ||||||||
| Opuntia stricta7,A,D | Transformed nectaries: beneath thorns | Areoles | Single | |||||
| Combretaceae | ||||||||
| Conocarpus erectus4,A,D | Hollow nectaries | Petiole | Paired | |||||
| Compositae | ||||||||
| *,†,‡Bidens pilosa | Capitated trichomes | Nodes/bracts/phyllaries | Scattered | |||||
| Convolvulaceae | ||||||||
| Ipomoea pes-caprae3,7,A,D | Hollow nectaries (A) | Scale-like trichomes (B) | Petiole (A) | Paired (A) | ||||
| Petiole/stem (B) | Scattered (B) | |||||||
| Fabaceae (Caesalpinioideae) | ||||||||
| *,‡Caesalpinia cristaB,E,D | Unicellular trichomes (secretory basal cell) | Stipules/bracts | Uniform | |||||
| ‡Chamaecrista chamaecristoides7,B,G,D | Elevated nectaries | Rachis (int) | Single | |||||
| Senna occidentalis8,B,G,D | Elevated nectaries | Rachis (int) | Single | |||||
| Fabaceae (Mimosoideae) | ||||||||
| Acacia cornigera5,7,A,B,G | Elevated nectaries | Rachis (int) | Single | |||||
| Fabaceae (Papilionoideae) | ||||||||
| *,†,‡Calopogonium caeruleum | Transformed nectaries: invaginations on meristems (B) | Capitated trichomes (A) | Stipels (A) Meristems (B) (apx, axl) | Scattered (A) Scattered (B) | ||||
| Canavalia rosea7,A,B | Transformed nectaries: invaginations on inflorescence ‘cushions’ (A) | Inflorescence nodes (A) Leaf axil (B) | Scattered (A) Scattered (B) | |||||
| on leaf axil meristem (B) | ||||||||
| ‡Crotalaria incana2,B | Transformed nectaries: scars of fallen stipules (A) scars of aborted buds or fallen flowers (B) | Unicellular trichomes (secretory basal cell) (C) | Stipule (A) Inflorescence stem (B) Buds (C) | Single (A) Scattered (B) Scattered (C) | ||||
| Macroptilium atropurpureum7,F | Transformed nectaries: invaginations of aborted buds (B) | Capitated trichomes (A) on meristems (C) | Calyx/stipules (A) Inflorescence nodes (B) Meristems (C) (apx) | Scattered (A) Scattered (B) Scattered (C) | ||||
| Meliaceae | ||||||||
| ‡Cederlla odorata6 | Flattened nectaries | Stem/branches | Scattered | |||||
| Turneraceae | ||||||||
| Turnera ulmifolia1,7,A,D | Elevated nectaries | Petiole | Paired | |||||
| Verbenaceae | ||||||||
| *,‡Callicarpa acuminataA,C,D | Scale-like trichomes | Blade (abx) | Uniform | |||||
First report of EFNs on the plant species;
first report of EFNs on the plant genus;
first morphological description of EFNs provided for the species.
Superscript numbers indicate references from previous reports for species:
Superscript letters indicate reports for genus:
Pascal et al. (2000). Similar letters in parentheses within a plant species relate EFN morphotype, site and distribution. abx = abaxial; apx = apex; axl = axile; int = interjugal.
Although the list of species with EFNs in the literature is increasing, some records—especially those coming exclusively from field surveys where nectar was not explicitly obtained—are considered doubtful by some authors (Lerstern and Brubaker, 1987; Mckey, 1989). This is common since EF nectar is often secreted at low rates or is viscous and measures are not easily obtained. Furthermore, to characterize formless, minute (i.e. secretory trichomes) or very simple (i.e. modified stomata) nectar-secreting structures is not possible without advanced histological techniques. In this paper, a novel use of the well-known Fehling's reaction for reducing sugars to detect nectar on simple or even singled-celled EFNs is briefly described.
The aim of this work was to characterize the morphology and secretory activity of the EFNs present on selected plant species at the study site. The intention was to provide an overview of the morphological and secretory variation in the EFNs and to arrange them in the structural–topographical classification originally proposed by Zimmerman (1932) (Elias, 1983), with contributions of original observations made during this study.
MATERIALS AND METHODS
Study site
The study was carried out at Centro de Investigaciones Costeras La Mancha (CICOLMA), along the coast of the state of Veracruz, México (19°36′N, 96°22′W; elevation <100 m; approx. 70 ha of it protected). The climate is warm sub-humid with three distinct seasons occurring at the site: a rainy season between June and September, dry cold fronts from October to January (winter), and a dry season the rest of the year. Total annual precipitation is 1500 mm, mean annual temperature is 24–26 °C and minimum annual temperature is 15 °C (Moreno-Casasola et al., 1982; Rico-Gray, 1993). The major vegetation types selected for the present study at this site are: pioneer dune vegetation, dune scrub, tropical deciduous forest-growing either on young or old soil-, fresh water marsh (and its ecotone with flooded tropical evergreen forest) and mangrove forest (Rico-Gray, 1993; Castillo-Campos and Medina, 2000). There is also tropical dry lowland forest, grasslands and crop-fields which were did not surveyed. Approximately 290 species of flowering plants are known to occur in the reserve area (Rico-Gray, 1993), although more recent censuses estimate 139 species just of trees and bushes (Castillo-Campos and Medina, 2000).
Field work
Transects representative of the six vegetation types mentioned above were selected. These transects had been surveyed previously for the abundance of EFN-bearing plants and the frequency of ants visiting the EFNs of each plant (Díaz-Castelazo et al., 2004). The protocol for locating EFN-bearing plants was based on the following: available taxonomic lists (Elias, 1983; Oliveira and Oliveira-Filho, 1991; Koptur, 1992a), previous reports for the area (Rico-Gray, 1993), careful examination of the plants where stereotyped nectar-feeding behaviour of ants (Rico-Gray, 1993) or the presence of sooty moulds on or around the glands was observed (Pemberton, 1990; Koptur, 1992b), and detection of sugars with glucose test strips (Clinistix brand) applied to the secretion (when no previous taxonomic reports existed for EFN-bearing plant genera or species).
With EFN abundance estimates and ant censuses provided by Díaz-Castelazo et al. (2004) the 20 most abundant and most frequently ant-visited plant species of the communities studied were selected to characterize the morphology of their EFNs and the volume and concentration of the secreted nectar. Although the selected species constitute half of the EFN-bearing plant species in the selected communities, their overall abundance is about 61 % of the abundance of plants with EFNs present at the study site (Díaz-Castelazo et al., 2004). Furthermore, the frequency of visits of ants (occurrence of an ant species in a plant with EFNs in each census) in the 20 selected plants constitutes 88 % of their occurrence in all the EFN-bearing plants at the study site; thus this sample was considered to be truly representative of the plant community bearing EFNs in coastal Veracruz. In the present study, EFNs and their secretions could be obtained from plants located in different vegetation types (transects) within the coastal community studied; however, this was considered not to affect the sample for morpho-secretory characterization, since the abundance of EFN-bearing plants and ant visitation (an indirect measure of gland activity), as reported by Díaz-Castelazo et al. (2004) was not different among transects. That study also showed that seasonality is the most important source of variation in gland activity (abundance of EFN-bearing plants and ant visitation); thus, for the present study, the samples of nectar and glands were collected during the season of the year with the highest activity of EFNs—the rainy season. The plant species selected were: Prestonia mexicana (Apocynaceae), Bidens pilosa (Asteraceae), Amphilophium paniculatum, Mansoa hymenaea, Tabebuia rosea (Bignoniaceae), Cordia spinescens (Boraginaceae), Opuntia stricta (Cactaceae), Conocarpus erectus (Combretaceae), Ipomoea pes-caprae (Convolvulaceae), Acacia cornigera, Caesalpinia crista, Calopogonium caeruleum, Canavalia rosea, Chamaecrista chamaecristoides, Crotalaria incana, Macroptilium atropurpureum and Senna occidentalis (Fabaceae), Cedrella odorata (Meliaceae), Turnera ulmifolia (Turneraceae) and Callicarpa acuminata (Verbenaceae). For all the species, EFNs, whether located on vegetative or reproductive organs, are ‘extranuptial’ in function.
Extrafloral nectar was collected from a set of glands (the ‘sets’ are described below) from five to ten plant individuals of each species, after 12 h of accumulation (overnight) using standard-size paper bags and tanglefoot (The Tanglefoot Co.) to exclude insects. Among plant species, equivalent surfaces or sets of EFNs (i.e. two terminal branches per individual with a similar number of leaves, nodes or reproductive structures) were bagged to reduce heterogeneity of size or distribution of the glands. Extrafloral nectar was cumulatively obtained from these sets of glands within a plant individual (one measure per individual). Nectar volume was estimated using 1- or 5-µL disposable microcaps and nectar concentration was measured with 0–32 %, 28–62 % and 58–92 % Bausch and Lomb sugar hand-held refractometers. When nectar was evident but too viscous too permit measurements, a known amount of distilled water was applied to the set of previously bagged nectaries, the solution was collected and the proportional volume (V) and sugar concentration (C) was calculated [C1 = (V2C2)/V1]. Although nectar data for each species are reported here, variation among individuals is also specified [mean and standard deviation (SD)]; when a plant species has more than one morphological type of EFN, location of the glands is specified. After the nectar had been measured, the plant tissues where EFNs are located were collected from the selected plant species and fixed with glutaraldehyde.
Laboratory procedures
The material was dehydrated with a graded ethanol series and the tissue samples were infiltrated and embedded in polyethyleneglycol (1500 mol). Using a Leika 820 rotatory microtome, 30-µm sections for each plant were obtained.
For scanning electron microscopy (SEM), dehydrated sections were critical-point dried using CO2, sputter-coated with gold–palladium and examined using a JEOL scanning electron microscope at an accelerating voltage of 15–20 kV. Although presence of EFNs was confirmed for all the plant species selected, morphological characterization of the glands was not possible for all of them solely through SEM, since microscopic non-vascular trichomes were responsible for nectar secretion and, in some cases several types of trichomes were present on the plant structure studied. Thus, histochemical detection of sugars was necessary for the leaf stipules and bracts of Caesalpinia crista, nodes, leaf axils and bracts of Bidens pilosa, and flower buds and bracts of Crotalaria incana. For these species, the 30-µm sections previously obtained were placed in 5 × 30-mm test glass tubes, covered with a 50 % Fehling's reagent solution, heated for 1 min (just to boiling point) and placed on microscope slides with distilled water. Fehling's reaction with reducing sugars produces cupric oxide deposits that ‘stain’ the tissues or cells where nectar is produced/accumulated bright red.
Micrometry and photomicrography of treated nectary tissues was accomplished using a Nikon Eclipse E600 microscope. For nectary characterization the structural–topographical classification originally proposed by Zimmerman (1932) was used (Elias, 1983), nectary descriptions were from Mckey (1989), Vögel (1998) and Drewes (1998), and the authors' own descriptions of nectar-secreting trichomes, some of which were based in the morphological descriptions provided by Corsi and Bottega (1999) and Lopes et al. (2002) were used for certain secretory trichomes.
RESULTS
Location, distribution (taxonomical and on the plant surface) and morphological characterization of the EFNs of selected plant species is shown on Table 1. For 13 of the species studied (65 %), the EFNs were associated with vegetative tissues (leaves, stems, meristems, etc.). Roughly one-third (35 %) of the species (seven of 20) had EFNs associated with both vegetative and reproductive structures (buds, bracts, inflorescence stems, etc.); no plants with EFNs associated exclusively with reproductive structures were found for the selected species. Ten species distributed among five plant families presented EFNs in more than one location on the plant body. EFNs were observed on leaf blades of five of the 20 species, (25 %) on young stems (25 %), growing meristems (20 %), stipules (20 %), calyx/fruits/bracts (20 %), inflorescence stems (15 %), leaf petioles (15 %) and leaf rachises (15 %).
Both vascularized and non-vascularized nectaries were found. Among the first, elevated, flattened, hollow, pit (sensu Zimmerman, 1932; Elias, 1983) could be recognized and what are described as transformed nectaries that have common morphological attributes (i.e. abscission scars; sensu Blüthgen and Reifenrath, 2003). Among the non-vascularized EFNs, scale-like nectaries (sensu Zimmerman, 1932; Elias, 1983), capitate, peltate and unicellular secretory trichomes were found. Five plant species, four belonging to the Papilionoid legume subfamily, displayed more than one morphological type of EFN. The first records of EFN for five plant genera distributed among four families are presented here, and EFNs are reported for the first time for seven plant species (see details in Table 1). For 11 of these, the first morphological or topographical descriptions available in the literature are provided. Detailed information on the taxonomic distribution, nectary location, morphology and record or descriptions from the literature is also presented in Table 1. Volumes and sugar concentrations of nectar are variable among morphologies (Table 2) and plant species. Species with a small number of replicates occur because not enough nectar was accumulated in all the excluded plant individuals.
Table 2.
Mean (± s.d.) nectar secretion and sugar concentration for plant species grouped according to EFN morphology
| Nectary ‘morphotype’ |
Mean nectar volume (µL) |
Mean sugar concentration (%) |
|---|---|---|
| Unicellular | 1·45 ± 0·95 | 31·77 ± 0·33 |
| Capitate | 1·9 ± 0·42 | 9·95 ± 9·12 |
| Peltate | 0·66 ± 0·01 | 10·52 ± 4·62 |
| Scale-like | 0·42 ± 0·55 | 39·62 ± 38·56 |
| Flattened | 0·68* | 8·18* |
| Hollow | 2·05 ± 2·05 | 8·70 ± 3·25 |
| Transformed | 1·79 ± 1·29 | 17·94 ± 15·74 |
| Elevated | 2·8 ± 0·95 | 34·72 ± 30·55 |
Only one measure of nectar.
Species descriptions
Unicellular trichomes
Trichomes of this kind are long, slender, singled-celled epidermal emergences. Sections of these hairs revealed that they were hollow inside, and when they were submitted to sugar-detecting histochemical techniques their single- or two-celled base beneath the epidermis revealed accumulation of nectar. These trichomes were neither previously reported as nectar-secreting structures, nor as nectar ‘ducts’. The volume and concentration of nectar for the group of species with unicellular trichomes is shown in Table 2. Their features are described in two leguminous species:
1.Caesalpinia crista (Caesalpinaceae). The apical portion of the foliaceous stipules (the continuation of the mid-vein) ends in a truncate spine; the whole spine is covered with numerous simple, non-capitate trichomes (270 µm long) with a single basal cell (16 µm in diameter) which is believed to be responsible for nectar secretion (Fig. 1A–C; see the red coloration that indicates the presence of reducing sugars inside the trichomes and in their basal cells). When the plant is flowering, the spike secretes copious nectar from the same kind of trichomes located on the floral bracts, mainly on the abaxial surface, and on the surface of the flower buds. Although there was no evidence of pores or cracks on the trichome surface through which nectar is secreted, it is possible that secretions rise through the trichome's internal ‘channel’ to the top and are subsequently released, either by a permeable cuticle or when the species tip of the trichome breaks.
Fig. 1.
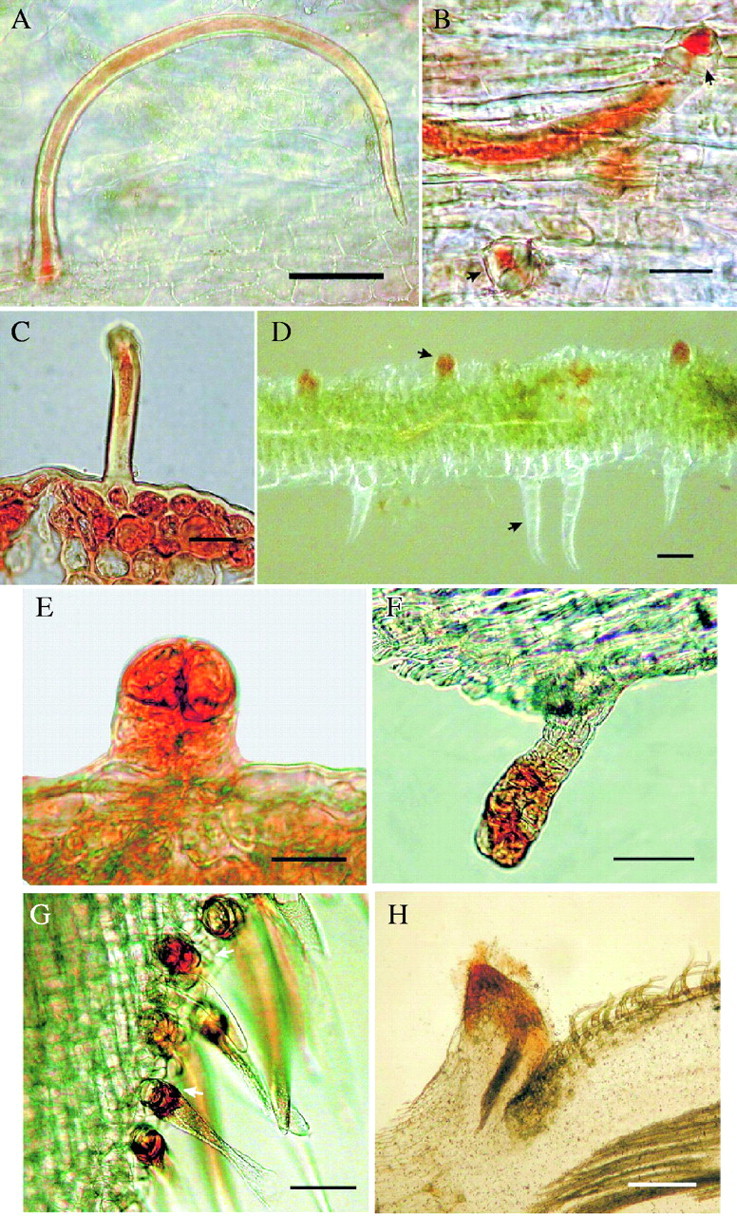
Unicellular trichomes, capitated trichomes and transformed nectaries showing the distinctive red-coloured indicator of reducing sugars. (A) Unicellular trichomes of Caesalpinia crista showing hollow space and sugar content. Scale bar = 50 µm. (B) Detail of the secretory basal nectar-secreting cell of the same species. Scale bar = 30 µm. (C) Transverse section of the apical spine of stipules of the same species showing the contents of the hollow unicellular trichome and the cells beneath. Scale bar = 30 µm. (D) Longitudinal section of the nodal stem area of Bidens pilosa showing differences between capitated secretory trichomes (red) and non-glandular trichomes (colourless). Scale bar = 50 µm. (E) Detail of a clavate secretory trichome of the same species. Scale bar = 30 µm. (F) Transverse section of the stem, near the flower phyllaries of the same species showing the more mature capitated trichome and its nectar-secreting head. Scale bar = 50 µm. (G) Section of the calyx of Crotalaria incana showing the unicellular trichomes with the sugar-secreting basal cells in red. Scale bar = 50 µm. (H) Transverse section of young stem of the same species showing the transformed nectary, stained in red, formed by the scar of a fallen stipule. Scale bar = 0.1 mm.
Nectar secretion: the nectar volume from the trichomes on the spine of the foliaceous stipule in Caesalpinia crista ranged from 0·21 to 5 µL (mean ± SD = 1·56 ± 1·51, n = 8) and its concentration varied between 20 % and 62 % (35·75 ± 13·69 %, n = 8); nectar volume from the trichomes covering the bracts of the inflorescence varied between 0·12 and 1 µL (0·54 ± 0·44, n = 3) and its concentration varied between 32 % and 44 % (38·66 ± 6·11 %, n = 3).
2.Crotalaria incana (Fabaceae). This leguminous shrub has unicellular trichomes scattered on the surface of flower buds, on the calyces of flowers, and developing fruits. The hairs consist of a somewhat long (240 µm long), helicoidal and collapsed distal cell ornamented with a tuberculate surface, supported by one basal nectar-secreting cell (36 µm in diameter), whose secretions are shed outside the trichomes through the collapsed (opened at the middle) distal cells (Fig. 1G; see the red-coloured cell indicative of the presence of reducing sugars, supporting the helicoidal trichome).
Nectar secretion: Only one measure of nectar secreted from the unicellular trichomes of the calyx was obtained: Nectar volume was 0·78 µL and its concentration was 32 %. More data of nectar from other types of EFNs in Crotalaria incana are presented later in this paper.
Capitate trichomes
These are clavate (club-shaped) secretory hairs, consisting of an elongated stalk (one to few-celled) and a secretory head (often multicellular). The cells of the head release their sugar-rich secretions directly onto the plant surface. This type of trichome is often considered nectar-secreting and is sometimes described as a glandular hair (Metcalfe and Chalk, 1972). The volume and concentration of nectar for the group of species having capitate trichomes is shown in Table 2. Capitate trichomes occur on:
1.Bidens pilosa (Asteraceae). The ‘shaggy’ stems of this plant have minute capitate secretory trichomes, most densely borne on the nodes and leaf axils (secretory head, 30 µm in diameter; total trichome length, 100 µm). These clavate glands were detected mainly with histochemical techniques, since they were ‘hidden’ by the conspicous non-secretory hairs that cover the whole plant. It can be seen that capitated nectar-secreting trichomes are coloured red by Fehling's technique (indicating presence of reducing sugars), while the uniseriated non-secretory hairs are not (Fig. 1D; detail of a capitated trichome on Fig. 1E). Nectaries of this type were scattered on the bracts and phyllaries of flower heads and have a more elongated aspect (Fig. 1F). Despite the well-known occurrence of capitate secretory hairs in Asteraceae (Metcalfe and Chalk, 1972), no record of nectar-secreting capitate hairs was available for this family.
Nectar secretion: Only one individual (of six) secreted enough nectar from the morphologically similar clavate trichomes distributed on nodes, leaf axils and flower phyllaries. Nectar volume was 1·6 µL and its concentration was of 3·5 %.
2.Macroptilium atropurpureum (Fabaceae). This herbaceous vine bears EFNs in widely varying locations. Minute capitate secretory trichomes (head 17 µm wide; total trichome length 37 µm) (see Fig. 2A) are very common and densely distributed on leaf stipels and calyces of flowers (Fig. 2B), but they can also occur on the surfaces of buds, fruit and leaflets, as reported for M. erythroloma (Drewes, 1998). These secretory hairs are dispersed among the non-secretory simple trichomes that provide pubescence on the plant.
Fig. 2.
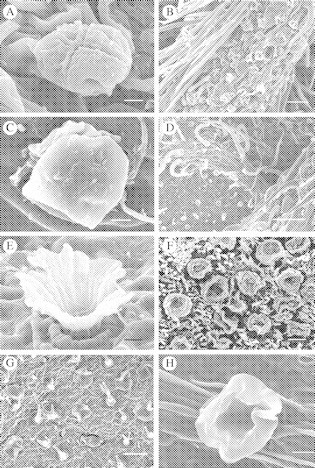
(A) Capitated secretory trichome of the calyx of Macroptilium atropurpureum. Scale bar = 5 µm. (B) Aggregation of capitated trichomes on the abaxial side of the stipule in the same species. Scale bar = 50 µm. (C) Peltate secretory trichome or papillae on the adaxial surface of a young leaf in Prestonia mexicana. Scale bar = 5 µm. (D) Peltate secretory trichomes on the abaxial leaf surface of Cordia spinescens showing density of non-secretory hairs. Scale bar = 200 µm. (E) Scale-like secretory trichome on the underside of the leaf. Scale bar = 5 µm. (F) Aggregation of scale-like secretory trichomes at the nodal position in Mansoa hymenaea. Scale bar = 100 µm. (G) Scale-like secretory trichome on the underside of the leaf of Amphilophium paniculatum showing distribution of nectaries. Scale bar = 100 µm. (H) Cupular scale-like trichome of the leaf underside of Callicarpa acuminata. Scale bar = 10 µm.
Nectar secretion: the morphologically similar clavate glandular trichomes located on the calyx, fruit and stipels of this plant secreted a nectar volume between 0·2 and 2·4 µL (mean = 2·19, n = 2) while nectar concentration varied between 20 % and 22·5 % (16·4 %, n = 2). However, other morphological types of EFNs present on Macroptilium atropurpureum secreted similar amounts of nectar and will be described further.
Peltate trichomes
These are papillose non-vascularized hairs consisting of a basal cell, a short (often unicellular) stalk and a spherical/umbelliform secretory head covered by a cuticle. SEM observation of the secretory head did not reveal pores or slits through which nectar could be released. Morphological evidence and literature (Corsi and Bottega, 1999) suggests that content release occurs when the cuticle breaks (due to nectar accumulation). Peltate hairs have been long recorded as secretory trichomes, but no detailed reports of nectar secretion from these structures exist. The volume and concentration of nectar for the group of species with peltate trichomes is shown in Table 2. Peltate trichomes occurred on:
1.Prestonia mexicana (Apocynaceae). This woody vine has small (25 µm of diameter) spherical papillose hairs (Fig. 2C) scattered and disperse on the adaxial surface of leaves. However, these glands secrete nectar more actively on young developing leaves and shoots (apical meristems) although no evidence for cuticle rupture was found.
Nectar secretion: Nectar volume measured on emerging leaf shoots varied between 0·72 and 1 µL (mean = 0·86, n = 2) and its concentration varied between 17 % and 22 % (19·5 %, n = 2).
2.Cordia spinescens (Boraginaceae) has similar peltate (32 µm in diameter) secretory trichomes associated with leaves, dispersed among the non-secretory hollow stout hairs of the pubescent leaf. In contrast to Prestonia mexicana, EFNs on Cordia spinescens are more numerous (Fig. 2D), active and more densely distributed, particularly on the abaxial surface of leaves, although they are present on the adaxial surface as well.
Nectar secretion: the volume of nectar secreted by the peltate trichomes on the leaf blade varied between 0·2 and 1·8 µL (mean ± SD = 0·65 ± 0·34, n = 4), while nectar concentration varied between 4·5 % and 10·5 % (7·25 ± 2·47 %, n = 4).
Scale-like nectaries
Originally described by Zimmerman (1932), these glands are squamiform elongations of the epidermis. They consist of a non-vascularized cuboidal head, either scuamiform or cup-shaped, and a short pedicel. This trichome morphology is characteristic of non-vascularized EFNs and, at least in Bignoniaceae, the clustering of many small, scale-like EFNs at different sites is considered an advanced strategy for ant attraction (Elias and Gelband, 1976). The volume and concentration of nectar for the group of species with scale-like EFNs is shown in Table 2.
1.Mansoa hymenaea (Bignoniaceae). This plant has a cluster of scale-like nectaries (161 µm in diameter each) (Fig. 2F), in the form of dishes with a low rim, in the nodal position on the stems. Due to their small size, each gland secretes modest amounts of nectar, but with a high sugar concentration, and is readily collected by ants.
Nectar secretion: the volume of nectar secreted by the aggregation of EFNs on the nodes varied between 0·1 and 0·33 µL (mean ± SD = 0·18 ± 0·11, n = 4); while a high nectar concentration ranged from 71 % to 78 % (mean 74·5 %, n = 2).
2.Amphilophium paniculatum (Bignoniaceae). This plant has scale-like nectaries with a more flattened aspect, due to the lower rims of the scales compared with those in Mansoa hymenaea. Glands are 82 µm in diameter and are evenly distributed on both adaxial and abaxial leaf surfaces (Fig. 2G). The plant has also more evident scale-like nectaries in the calyx of flowers and buds and along the dehiscence line of fruits.
Nectar secretion: the nectaries distributed on leaves secreted a nectar volume between 0·1 and 1 µL (mean = 0·55, n = 2) while its concentration ranged from 1·2 % to 10 % (mean = 5·6 %, n = 2); the larger but morphologically similar scale-like glandular trichomes on the calyx of flower buds and fruit surface secreted a nectar volume between 0·1 and 1 µL (mean = 0·55, n = 2), while nectar concentration varied between 24 % and 27 % (25·5 %, n = 2).
3.Tabaebuia rosea (Bignoniaceae). This plant has also scale-like glandular trichomes (50 µm in diameter) with a secretory centre and a more elevated loose cupular rim on both leaf surfaces and young stems. The stalk of the gland is partially embedded in the tissue of the leaf or stem (Fig. 2E). A very modest amount of viscous nectar is secreted from these glands.
Nectar secretion: the scale-like EFNs on the leaves of two plant individuals secreted a nectar volume between 0·1 and 0·2 µL (mean = 0·15, n = 2). However, measures of nectar concentration were obtained for only one plant (71 %).
4.Callicarpa acuminata (Verbenaceae). The minute (36 µm diameter) nectaries of this plant are evidently cup-shaped secretory trichomes (Fig. 2H) distributed among the non-glandular dendroid trichomes that gives the dense pubescence on the abaxial leaf surface.
Nectar secretion: Only one measure of nectar secreted from the scale-like trichomes on the abaxial surface of leaves was obtained. Nectar volume was 0·1 µL and its concentration was 0·83 %.
Flattened nectaries
These nectaries are closely pressed against the fundamental tissue of other organs (Zimmerman, 1932), so that the glandular surface is scarcely above or just beneath the surface level of the surrounding mesophyll tissue. These nectaries are vascularized and nectar-secreting tissue is represented by a palisade parenchyma (Blüthgen and Reifenrath, 2003). The volume and concentration of nectar for the group of species with flattened EFNs is shown in Table 2.
1.Cedrella odorata (Meliaceae). This plant has numerous dark green nectar-secreting spots scattered among the light green tissue of young stems and branches. These secretory structures are somewhat oval (0·5 mm long × 0·2 mm wide), parenchymatose and closely pressed against the fundamental tissue of stems and branches (Fig. 3G).
Fig. 3.
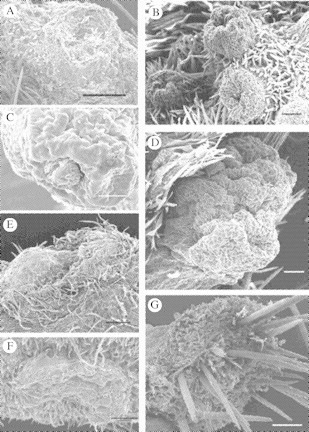
(A) Transformed nectary of Macroptilium atropurpureum showing invagination at infloresence stem. Scale bar = 200 µm. (B) Elevations and their respective central depressions of transformed nectaries of Calopogonium caeruleum showing their distribution on an apical meristem. Scale bar = 100 µm. (C) The transformed nectaries associated with the inflorescence ‘nodes’ of Canavalia rosea showing one of the openings or scars through which nectar is secreted. Scale bar = 100 µm. (D) Transformed nectary of the shoot or meristem on leaf axils of the same species, notice that each invagination is nectar-secreting. Scale bar = 100 µm. (E) Scar left by a flower along the stem of the plant, showing the ‘volcano-like’ dome structure. Scale bar = 200 µm. (F) Flattened nectary along the stem or branches of Cedrella odorata showing the compressed mesophyll tissue. Scale bar = 100 µm. (G) Areole of Opuntia stricta with detail of thorns and non-secretory trichomes. Scale bar = 100 µm.
Nectar secretion: Only one individual secreted enough nectar to be measured. Despite the glands being numerous on the young stems and branches, their minute size and inconspicuousness made nectar estimation very difficult. Nectar volume was 0·68 µL, while nectar concentration was 8·18 %.
Hollow nectaries
The are highly vascularized nectaries are deep cavities in other plant tissues or organs with a narrow channel, slit or pore extending to the surface. Very often the cavities are lined with secretory trichomes, especially scale-like nectaries (Elias, 1983).
1.Ipomoea pes-caprae (Convolvulaceae). This plant has a pair of hollow nectaries on both sides of the leaf petiole. Each nectary has a narrow channel that ends in a slit on the surface of the petiole (300 µm in diameter). Few scale-like secretory trichomes (44 µm wide) are scattered around the pore of each hollow nectary. Nectary-surrounding secretory trichomes have been reported previously for this and other Ipomoea spp. (Keeler and Kaul, 1979). However, the hollow nectary that was observed (Fig. 4B) is more similar to the so-called ‘crypt’ nectary that Keeler and Kaul (1979) describe, than the ‘basin’ nectaries they report for Ipomoea pes-caprae.
Fig. 4.
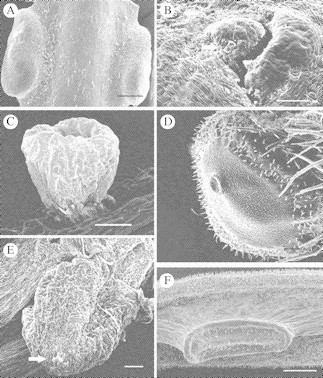
(A) Paired hollow nectarial glands of Conocarpus erectus showing petiolar position. Scale bar = 500 µm. (B) Detail of the surface slit (pore) of one of the hollow petiolar nectaries of Ipomoea pes-caprae; few scale-like trichomes surround the cavity. Scale bar = 500 µm. (C) Cup-shaped elevated nectary of Chamaecrista chamaecristoides with a stalked on the petiole or rachis of the compound leaf. Scale bar = 200 µm. (D) One of the paired petiolar elevated nectaries of Turnera ulmifolia showing central swelling due to nectar accumulation beneath the cuticle. Scale bar = 100 µm. (E) Single elevated nectary of Senna occidentalis with a stalk on the petiole or rachis showing apical swelling due to nectar accumulation under the cuticle. Scale bar = 200 µm. (F) Lateral view of a nectary of Acacia cornigera showing its elevation. Scale bar = 500 µm.
Nectar secretion: Nectar was obtained from the hollow petiolar nectaries of this species for only two individuals. Nectar volume was 0·6 µL in both cases and nectar concentration measured 8 % and 14 % (mean = 11 %, n = 2). Since the scale-like trichomes on this plant are few, scattered, and very close to the hollow nectaries on the petiole, it was not possible to separate its minute secretions from those of the vascularized nectaries, thus no measures of this nectar are available.
2.Conocarpus erectus (Combretaceae). This plant has a pair of elongated (1·66 mm long × 0·64 mm wide), swollen, hollow nectaries on both sides of the leaf petiole (see Fig. 4A), with a narrow channel extending to the surface and ending in a pore.
Nectar secretion: Paired petiolar EFNs of this plant secreted a nectar volume between 1 and 7 µL (mean ± SD = 3·5 ± 2·45, n = 5), while nectar concentration ranged from 2 % to 10 % (6·4 % ± 2·97 %, n = 5).
Transformed nectaries
In this newly described morphological category of EFNs (not used before as a categorization) such glands are included that are originated by the transformation of an organ, including abscission or abortion of an organ (where swelling of the surrounding tissue often occurs) and development of reproductive or vegetative meristems. Although transformed nectaries are vascularized and morphologically similar to elevated nectaries, they have structural peculiarities and distinct secretory activities. Blüthgen and Reifenrath (2003) refer to the plant tissues that secrete nectar ‘incidentally’ after organ abscission as ‘functional’ nectaries. It is believed that these glands are morphologically and ecologically intriguing, and many other authors have referred to them to some extent (Zimmerman, 1932; Elias, 1983; Kuo and Pate, 1985; Mckey, 1989; Koptur, 1992a; Drewes, 1998; Vögel, 1998). Nectar volume and concentration from the species with transformed EFNs is shown in Table 2.
1.Crotalaria incana (Fabaceae). This plant has two types of transformed EFNs and one morphological nectar-secreting structure. A pair of prophylls (or stipules) is located at the bifurcation of the stem, where young branches emerge; while the branch continues to grow the prophylls fall off, leaving elevated scars (200 µm in diameter) which secrete rich and abundant nectar (Fig. 1H; the red-coloured tissue is the elevated nectar-secreting scar, shows the presence of reducing sugars through Fehling's technique). EFNs are also formed when flower buds are aborted or when flowers are shed (both before and after anthesis), leaving a swollen scar (510 µm in diameter) with a central depression (Fig. 3E). Vögel (1998) states that the sugary sap is released through spontaneous disruption of phloem strands.
Nectar secretion: The vascularized secretory scars of prophylls and flowers which have fallen off secrete between 0·15 and 0·18 µL of nectar (mean = 0·16, n = 2) while nectar concentration varied between 55·3 % and 69 % (62·17 %, n = 2).
2.Calopogonium caeruleum (Fabaceae). This climbing trifoliate vine has recognizable transformed EFNs besides its stipelar capitate trichomes. Transformed nectaries are associated with the developing apical meristems of the twining stems. They consist of several well-defined cup-shaped elevations with a central depression (Fig. 3B) that presumably corresponds to the abscission ‘scar’ of an aborted bud primordium or bract, thus homologous to those described for Macroptilium (Drewes, 1991). The hollow depression fills with nectar that is readily foraged by ants.
Nectar secretion: The EFN associated with developing meristems secreted between 1 and 1·8 µL (mean ± SD = 1·4 ± 0·4, n = 3), while nectar concentration varied between 0·73 % and 14 % (9·58 ± 7·66 %, n = 3).
3.Macroptilium atropurpureum (Fabaceae). This plant has conspicuous secretory invaginations formed after abortion of flower buds located in the swollen nodes of the inflorescence rachis. As described by Drewes (1998) in M. erythroloma, when the perianth of a bud falls off, the pedicel base remains attached to the tissue underneath the future nectary (at the node of the inflorescence) and later disintegrates, leaving a cavity (Fig. 3A) where large amounts of nectar accumulate via phloem discharge.
Nectar secretion: The vascularized nectar-secreting invagination (scar) on the inflorescence stem secreted a nectar volume between 2 and 0·9 µL (mean = 1·34, n = 2), while nectar concentration ranged from 2 % to 8 % (mean = 21·25 %, n = 2).
4.Canavalia rosea (Fabaceae). This plant has two types of transformed EFNs. The nodes of the inflorescence stem have ‘cushion-like’ reduced side-branches. These swollen mounds of tissue support the flower buds, flowers and fruits (Mckey, 1989). When buds are aborted the pedicel base leaves a depression or scar (108 µm in diameter) and the tissue around it becomes tumescent and glandular (Fig. 3C), depositing its sugary content in the scar. Each cushion may have several scars that secrete nectar through the ‘lifetime’ of the inflorescence. The other type of EFN in this species is found on the developing shoots growing along the stem in the axils of leaves. The aspect of these glands (Fig. 3D), is similar to the cushions found on the inflorescence axis (Fig. 3C) with small invaginations (58 µm in diameter) where secretions accumulate, and tumescent surrounding tissue. Lerstern and Brubaker (1987) mention the occurrence of this leaf axil nectary on Canavalia gladiata (from a description of 1907) as a large green cushion or bolster in each leaf axil with up to ten dot-like surface depressions. It was observed that these nectaries are active mainly during new leaf formation and are not necessarily synchronized with inflorescence production.
Nectar secretion: Vascularized EFNs located on the modified side branches of the inflorescence stem secreted a nectar volume between 1 and 2 µL (mean = 1·5, n = 2), while nectar concentration ranged from 12 % to 20 % (mean = 16 %, n = 2). Nectaries from the axillary shoots secreted a nectar volume between 0·7 and 1 µL (mean = 0·85, n = 2), while nectar concentration varied between 2 % and 17 % (mean = 9·5 %, n = 2).
5.Opuntia stricta (Cactaceae). This cactus has EFNs on the areoles of the developing stem tissue of young cladodes (Fig. 3F) and in the floral cup surrounding the ovary in flowers and developing fruits. Each areole (2·5 mm in diameter) has one flat-topped gland raised on a base densely clothed with soft non-secretory trichomes. The long and numerous trichomes underneath and the thorns covering the epidermis on top of the gland ‘hide’ the EFN, but the whole flossy coating is moistened when nectar is secreted, attracting a number of foraging ants. Lloyd and Ridgway (1912) stated that nectar secretion in Opuntia occurs after the outer wall of the epidermis is released from the epidermal cells beneath, forming a chamber where nectar accumulates and is released after membrane (epidermis wall) rupture.
Nectar secretion: EFNs on the areoles associated mainly with developing tissue (i.e. young cladodes, shoots and flower cups) secreted a nectar volume between 1 and 7 µL (3·67 ± 3·06, n = 3), while nectar concentration ranged from 2 % to 4 % (2·67 ± 1·15 %, n = 3).
Elevated nectaries
These are vascularized, well-defined glands distinctly raised above the ground mesophyll tissue. These nectaries, often subsessile, consist of palisade secretory parenchyma, covered by a thick cuticle, which breaks down to release nectar. The volume and concentration of nectar of all species in the present study with elevated nectaries is shown in Table 2.
1.Chamaecrista chamaecristoides (Caesalpiniaceae). This plant has a small (510 µm in diameter) cup-shaped, elevated EFN at the inter-jugal position of the leaf rachis (Fig. 4C). This vascularized structure has secretory cells on the surfaces of a central colum from which the nectar arises (Pascal et al., 2000). The minute cupular EFNs of this plant are active mainly during the wet season.
Nectar secretion: the cup-shaped EFNs on petioles of the associated compound leaf petioles secreted a nectar volume between 0·9 and 2 µL (mean = 1·45, n = 2), while nectar concentration ranged from 2 % to 8 % (mean = 5 %, n = 2).
2.Senna occidentalis (Caesalpiniaceae). This small shrub has distinctively coloured large elevated nectary glands (>1 mm in diameter) (see Fig. 4E) on the base of the rachis (or petiole; sensu Fleet and Young, 2000), in the axil of the compound leaf. The top of the ‘mountain-like’ gland has secretory tissue covered by a cuticle, which breaks to release nectar.
Nectar secretion: the elevated EFNs on the leaf axils or petiole of the compound leaf secreted a nectar volume between 1 and 11 µL (mean ± SD = 4·57 ± 4·5, n = 4), while nectar concentration ranged from 19·85 % to 42 % (28·84 ± 10·29 %, n = 4).
3.Turnera ulmifolia (Turneraceae). This plant has a pair of well-developed (>1 mm in diameter) cupular elevated nectaries on both sides of the leaf petiole (Elias et al., 1975). The nectaries are subsessile and have a thick cuticle covering the secretory tissue (Fig. 4D); nectar accumulates between the intact cuticle and the epidermis and nectar is released through the thinner part of the cuticle that coincides in a thin invagination or pore (González, 1996).
Nectar secretion: The paired elevated EFNs on the petioles secreted a nectar volume between 0·27 and 6 µL (3·06 ± 2·62, n = 5), while nectar concentration ranged from 16 % to 44·65 % (27·53±11·28 %, n = 5).
4.Acacia cornigera (Mimosaceae). This plant has an elongated (1·6 mm long × 0·52 mm wide) vascularized nectary, elevated from the surrounding tissue but with no stalk (Fig. 4F). Each nectary is located on the rachis of the compound leaf at the inter-jugal position, and more than one gland can be found on the same rachis.
Nectar secretion: The pit nectary on the leaf rachis of this plant secreted a nectar volume between 2·5 and 3·5 µL (mean = 3, n = 2), while nectar concentration ranged from 75 % to 80 % (mean = 77·5 %, n = 2).
DISCUSSION
Characterization of non-vascularized EFNs
Detection of non-vascularized EFNs on a plant is easy when secretions accumulate: nectar can be collected if sufficient amounts are available or when they are modest, secretions can be absorbed with filter paper (which can be weighed to estimate the amount or analysed with chromatography), tested with glucose stripes or detected by persistent ant foraging. Morphological characterization of this kind of nectaries is, however, not so simple. Scanning microscopy is not enough when other secretory structures exist on the plant organ under study. Histochemical techniques are the solution but many of them are highly time- and effort-demanding.
In this study the reducing-sugar test with Fehling's reagent was used to detect plant tissues with sugars such as glucose or fructose. This technique is sensitive (1 mg of reducing sugar is enough to bring a positive result; Lancashire, 2003); compared with the Benedict and Barfoed methods for detecting reducing sugars (which can give positive but incorrect results according to Schreck and Loffredo, 1994), no ‘false positives’ are reported. It is the most practical method for detecting sugars that contain an aldehyde group (although it can be used for some aliphatic aldehydes as well) and it had been used to determine if plant secretions are extrafloral nectar or not (Bowden, 1971; Paiva et al., 2001). However, very few studies use this technique histologically to determine in which tissues or cells extrafloral nectar is secreted or accumulated (Rocha et al., 2002). For these purposes, Fehling's technique is highly recommend, since it is simple and useful for detecting reducing sugars in few-celled plant tissues or unicellular structures such as non-vascularized EFNs or nectar-secreting trichomes.
EFN morphology and taxonomic distribution
It was found that a great number of EFN-bearing plants belong to the Fabaceae, and present a high diversity in gland morphology and location. In subfamily Caesalpinoideae unicellular trichomes were observed on the stipular spine and on inflorescence bracts of Caesalpinia crista. Although capitated secretory trichomes had been described for the leaf rachis of other caesalpinoids (nectar-secreting in the Dimorphandra group; Pascal et al., 2000) and on leaves (non-nectariferous in the genus Caesalpinia; Lersten and Curtis, 1994), unicellular trichomes on caesalpinoids had not been considered before as EFNs. The simplicity of this trichome, with its abundance in plant organs, its high secretory ability and, presumably, the low structural costs they imply for a leguminous plant, make this structures worth-studying. Nectar secretion in this trichomes is clearly epidermal, but we are not certain if the basal cell(s) below each trichome is secretory or reservory. Evidently, anatomical studies of the trichomes (and their basal cells) of Caesalpinia are needed. In contrast, the vascularized elevated nectaries at the base of the leaf rachis had been reported before for caesalpinoids such as Senna and Chamaecrista and are well known, morphologically and anatomically (Elias, 1983; Mckey, 1989; Pascal et al., 2000). For Acacia cornigera—the only mimosoid studied in this paper—not only are EFNs frequently reported (Janzen, 1966) but the prescence of these glands at the base of the leaf rachises has been recorded for 44 of the 60 genus of the subfamily (Mckey, 1989; Pascal et al., 2000), for which morphological descriptions are common.
Continuing with the legumes, in the subtribe Phaseoleae of the subfamily Papilionoideae three contrasting EFN morphologies were observed: unicellular trichomes on the calyx of flowers and buds of Crotalaria incana; capitate trichomes on the stipels and calyx of Macroptilium atropurpureum; and transformed EFNs for Canavalia rosea, Crotalaria incana, Calopogonium caeruleum and Macroptilium atropurpureum, whose developmental roots reside in floral structures (Elias, 1983; Mckey, 1989). Capitated trichomes had been described for several genera within subtribe Phaseoleae (Papilionoideae), as aggregations of separate trichomes on leaf stipules or stipels (reviewed by Lersten and Brubaker, 1987). This trichome, also found in Macroptilium atropurpureum, is similar in distribution and density to those described by Drewes (1998) for M. erythroloma. However, it is considered that the presence of this type of EFN in the papilionoid species in their study site could be underestimated, since stipules or stipels were inconspicuous on individuals of some species. Similarly, flowers were not evident on some papilonoid species such as Calopogonium caeruleum and, given the fact that capitated trichomes were found on the calyxes and peduncles of M. atropurpureum, further revision of reproductive structures of Papilonoids to detect this kind of trichomes is suggested.
The transformed EFNs that are described within Fabaceae are interesting and efficient secreting structures that use the same vascular tissue that runs towards the inflorescences (Vögel, 1998); once some flowers have fallen the scars become tumescent and the vascular bundels secrete abundant extrafloral nectar that is readily harvested by ants (thus, the function of these nectaries is assumed to be the protection of remaining flowers and developing fruits). If, as suggested by Pascal et al. (2000), the simpler glands are plesiomorphic and the more complex are derived, the present observations with the leguminous species studied support the hypothesis: the structurally most simple EFNs (so ‘simple’ that were not previously considered as nectaries) were found in the genus Caesalpinia, belonging to the most primitive legume subfamily, while more complex ones (transformed EFNs) were found in Papilionoideae.
The three Bignoniaceous species whose EFNs are described posses scale-like nectaries either scattered on the vegetative tissues or aggregated on reproductive structures (mainly calyx). Scale-like EFNs, described as scuamiform secretory trichomes have been extensively studied in Bignoniaceae (Elias and Gelband, 1973; Elias, 1983; Rivera, 2000) where its aggregation on certain plant organs is considered an advanced form of ant attraction (Elias, 1983).
EFNs on reproductive structures
Although descriptions of EFNs exist for a variety of plant species, few studies describe EFNs on different locations on the same species (Kuo and Pate, 1985; Drewes, 1998). Here, morphological and secretory descriptions of the glands occurring on vegetative and reproductive structures within a plant species are provided for several species. The EFNs found to be associated with plant reproductive structures are simple in structure but diverse in morphological types. In some cases, gland morphology is exactly the same of nectaries found on vegetative structures (i.e. Bignoniaceae), but in others, strikingly different gland morphologies are found (i.e. Papilionoidea, Fabaceae) among plant organs. Presumably for the later, contrasting secretory patterns of EF nectar exist. Wäckers and Bonifay (2004) found that nectar secretion patterns differ among the EFNs occurring in reproductive and vegetative organs within the same plant species, following the theory of optimal defence (ant-derived defence against herbivores).
EFNs (not nuptial in function) associated with reproductive structures are especially interesting both morphologically and functionally. Commonly they are epidermal glands (Vögel, 1998), but some cases reported here are exceptions since they are not epithelial. Vögel (1998) made original histological observations of abscission scars turning into EFNs in family Lamiaceae and reports the same structures for some genera within the Phaseoleae (Fabacea, Papilionoideae). He describes how after abscission of a bract or flower peduncle, phloem tissue is increased through multiplication of parenchymatic cells. This phloematic tissue produces nectar that is liberated after penetrating the intercellular spaces of the distal non-glandular tissue, similar to bleeding. The authors found this specific case of nectaries for the Papilionoideaceus species Canavalia rosea, Crotalaria incana, Calopogonium caeruleum and Macroptilium atropurpureum. These are the first descriptions of what the authors called transformed EFNs on these species, although there are generic descriptions for most of them (Kuo and Pate, 1985; Lerstern and Brubaker, 1987; Mckey, 1989; Drewes, 1998).
Correlations among EFN attributes
EFN morphology and nectar secretion are highly correlated items. Gland morphology (whether phylogenetically determined or not) influences the volume of secretory tissue and vascular supply that highly determine secretion rates. Rudgers (2004) found that the size of EFNs on wild cotton (nectary length and width) was positively correlated with nectar volume. Similarly, in this study it was observed that the larger glands, with more complex structure and vascular supply (elevated EFNs, hollow EFNs) secrete more nectar. From a functional perspective, however, large numbers of small secretory units (i.e. trichomes) with lower structural costs can be as cost effective as large glands with morphological complexity. Example of this in the present study are the transformed EFNs which originate from abscission scars or the numerous capitated trichomes circumscribed to specific plant parts which, in comparison with the larger and more complex elevated and hollow EFNs, secrete almost equiparable amounts of nectar. Drewes (1998) and Bhattacharya and Maheshwari (1970) consider that for papilionoid legumes, groups of numerous nectariferous trichomes offer larger amounts of nectar, and are in that sense, similar to larger vascularized glands.
EFN attributes show important phenotypic plasticity. Substantial evidence suggests that foliar EFNs increase their secretions (Agrawal and Rutter, 1998; Heil et al., 2000; Wäckers et al., 2001), the concentration of sugar or amino acids of extrafloral nectar (Koptur, 1989; Ness, 2003; Rudgers and Gardener, 2004) and even their overall number on a plant (Mondor and Addicott, 2003) in response to herbivory or artificial damage (induction). Although natural variation in attributes of EFNs has not been measured within plant species, from the present observations in the coastal vegetation in Mexico, it is suspected that EFN are quite plastic in morphology and secretory attributes, specially for those glands whose developmental roots lie in other structures (i.e. transformed nectaries). At least it is known, from a community perspective, that gland activity (i.e. availability of EFNs and visitation by ants) in the study site is clearly influenced by seasonality (Rico-Gray, 1993; Díaz-Castelazo et al., 2004; Rico-Gray et al., 2004).
EFN attributes and their potential for selection
Although the genetic bases for EFN attributes are, as suggested by Mitchell (2004), almost unknown, some information on the heritability of EFNs is available: Rudgers (2004) found that, for wild cotton, the proportion of leaves with EFNs and the size of the glands had genetic variation and significant heritabilities. Rhyne (1969) found that the absence of EFNs is inherited as a single-locus trait in some crops (cited by Mitchell, 2004). The fact that, at least for some plant species, the presence and morphology of EFNs is an inheritable trait has important evolutionary implications since it can be subject to natural selection. It is suggested that morphological studies on EFNs focus also on their natural variation within plant species and on the degree of hereditability of morphological and secretory attributes. In this context, the insect community associated with EFNs can be the selective forces acting on gland and nectar traits. Rudgers (2004) found that visits of ants to naturally variable (in morphology and occurrence) EFNs affect plant fitness correlates, suggesting that the associated ant community (which defends the plant against herbivores) is influencing the evolution of EFN traits in wild cotton. In this context it cannot be overlooked that the morphological and secretory features of the EFNs of the plants in the present study influence attractiveness to ants and that, presumably this can affect the evolution of EFN traits. Although the species composition of the ant fauna associated with EFN-bearing plants in the study site is known (Díaz-Castelazo et al., 2004), there is still a need to find out if EFN attributes such as gland morphology influence ant preferences and the structure of the associated ant community. While there is increasing understanding of the evolutionary significance of EFNs in tropical communities, ecological studies of ant–plant mutualisms have until recently only considered the distribution of these structures among plant organs (Hossaert-Mckey et al., 2001; Wäckers and Bonifay, 2004) neglecting their morphology.
Acknowledgments
We thank Edgar Priego, Mariana Cuautle, Betzabé Salazar and Rosa Linda Robles for their help during field work. Plants were identified by Carlos Durán. We appreciate the help of Tiburcio Láes during scanning electron microscopy, of Victor Vázques for suggestions to histochemical techniques and the personnel of the plant anatomy laboratory of the Departamento de Productos Forestales of the Instituto de Ecología, A.C. We appreciate protocol suggestions by Suzanne Koptur and Paulo S. Oliveira. We thank Nels R. Lersten, Suzanne Koptur, Heather Gamper and Klaus Mehltreter for making literature available. This research was supported by Instituto de Ecología, A.C. (902-16 to V.R.-G.), CONACYT (118946 to C.D.-C.).
LITERATURE CITED
- Agrawal AA, Rutter MT. 1998. Dynamic anti-herbivore defense in ant-plants: the role of induced responses. Oikas 83: 227–236. [Google Scholar]
- Apple JL, Feener Jr DH. 2001. Ant visitation of extrafloral nectaries of Passiflora: the effects of nectary attributes and ant behavior on patterns in facultative ant-plant mutualisms. Oecologia 127: 409–416. [DOI] [PubMed] [Google Scholar]
- Baker HG, Opler PA, Baker I. 1978. A comparison of the amino acid complements of floral and extrafloral nectars. Botanical Gazette 139: 322–332. [Google Scholar]
- Beattie AJ. 1985.The evolutionary ecology of ant–plant mutualisms. Cambridge: Cambridge University Press. [Google Scholar]
- Bentley BL. 1977. The protective function of ants visiting the extrafloral nectaries of Bixa orellana (Bixaceae). Journal of Ecology 65: 27–38. [Google Scholar]
- Bentley BL. 1977. Extraflora nectaries and protection by pugnacious bodyguards. Annual Review of Ecology and Systematics 8: 407–427. [Google Scholar]
- Bhattacharya B, Maheshwari JK. 1970. Studies on extrafloral nectaries of the Leguminales. I. Papilionaceae, with a discussion on the systematics of the Leguminales. Proceedings of the Indian Academy of Sciences 37B: 11–30. [Google Scholar]
- Blüthgen N, Reifenrath K. 2003. Extrafloral nectaries in an Australian rainforest: structure and distribution. Australian Journal of Botany 51: 515–527. [Google Scholar]
- Boughton VH. 1985. Extrafloral nectaries of some Australian bipinnate acacias. Australian Journal of Botany 33: 175–184. [Google Scholar]
- Bowden BN. 1971 The leaf nectaries of Andropogon (Poaceae). Botanical Journal of the Linnean Society 64: 77–81. [Google Scholar]
- Castillo-Campos G, Medina MEA. 2000.Árboles y arbustos de las selvas y matorrales de la reserva natural de La Mancha, Veracruz, México: manual para la identificación de las especies. Xalapa, Veracruz, México: Instituto de Ecología, A.C. [Google Scholar]
- Cogni R, Freitas AVL, Oliveira PS. 2003. Interhabitat differences in ant activity on plant foliage: ants at extrafloral nectaries of Hibiscus pernambucensis in sandy and mangrove forests. Entomologia Experimentalis et Applicata 107: 125–131. [Google Scholar]
- Corsi G, Bottega S. 1999. Glandular hairs of Salvia officinalis: new data on morphology, localization and histochemistry in relation to function. Annals of Botany 84: 657–664. [Google Scholar]
- Cuautle M, Rico-Gray V, Díaz-Castelazo C. 2005. Dispersion in Turnera ulmifolia, an extrafloral nectaried-plant with elaiosome bearing seeds. Biological Journal of the Linnean Society (in press). [Google Scholar]
- Díaz-Castelazo C, Rico-Gray V, Oliveira PS, Cuautle M. 2004. Extrafloral nectary-mediated ant–plant interactions in the coastal vegetation of Veracruz, Mexico: richness, occurrence, seasonality and ant foraging patterns. Ecoscience 11: 472–481. [Google Scholar]
- Drewes SI. 1991. Typology of the synflorescence, development of extrafloral nectaries and growth forms in Macroptilium (Benth.) Urban (Leguminosae-Phaseoleae). Beitraege zur Biologie der Pflanzen 66: 407–420. [Google Scholar]
- Drewes SI. 1998. Nectarios en Macroptilium erythroloma (Fabaceae). Annales del Instituto de Biología de la Universidad Nacional Autónoma de México, Serie Botánica 69: 23–35. [Google Scholar]
- Elias TS. 1983. Extrafloral nectaries: their structure and distribution. In: Bentley B, Elias T, eds. The biology of nectaries. New York, NY: Columbia University Press, 174–203. [Google Scholar]
- Elias TS, Gelband H. 1976. Morphology and anatomy of floral and extrafloral nectaries in Campsis (Bignoniaceae). American Journal of Botany 63: 1349–1353. [Google Scholar]
- Elias TS, Rozich WR, Newcombe L. 1975. The foliar and floral nectaries of Turnera ulmifolia L. American Journal of Botany 62: 570–576. [Google Scholar]
- Fleet RR, Young BL. 2000. Facultative mutualism between imported fire ants (Solenopsis invicta) and a legume (Senna occidentalis) The Southwestern Naturalist 45: 289–298. [Google Scholar]
- Galetto L, Bernardello LM. 1992. Extrafloral nectaries that attract ants in Bromeliaceae: structure and nectar composition. Canadian Journal of Botany 70: 1101–1105. [Google Scholar]
- González AM. 1996. Nectarios extraflorales en Turnera, series Canaligerae y Leiocarpae Bonplandia 9: 129–143. [Google Scholar]
- Heil M, Fiala B, Baumann B, Linsenmair KE. 2000. Temporal, spatial and biotic variations in extrafloral nectar secretion by Macaranga tanarius Functional Ecology 14: 749–757. [Google Scholar]
- Herl M, Frab E, Bavinann B, Linsenmar RE. 2000. Temp al, spatial apd bretie varrations in extrattional nector extran Macaraing a tenarios Furtional Excology 14: 744–757. [Google Scholar]
- Hossaert-Mckey M, Orivel J, Labeyrie E, Pascal L, Delabie JHC, Dejean A. 2001. Differential associations with ants of three co-ocurring extrafloral nectary-bearing plants. Écoscience 8: 325–333. [Google Scholar]
- Janzen DH. 1966. Co-evolution of mutualism between ants and acacias in Central America. Evolution 20: 249–275. [DOI] [PubMed] [Google Scholar]
- Keeler KH, Kaul RB. 1979. Morphology and distribution of petiolar nectaries in Ipomoea (Convolvulaceae). American Journal of Botany 66: 946–952. [Google Scholar]
- Koptur S. 1989. Is extrafloral nectar production an inducible defense? In: Bock J, Linhart Y, eds. Evolutionary ecology of plants. Boulder, CO: Westview Press. [Google Scholar]
- Koptur S. 1992. Extrafloral nectary-mediated interactions between insects and plants. In: Bernays E, ed. Insect–plant interactions. Boca Ratón, FL: CRC Press, 81–129. [Google Scholar]
- Koptur S. 1992. Plants with extrafloral nectaries and ants in Everglades habitats. Florida Entomologist 75: 39–50. [Google Scholar]
- Koptur S, Rico-Gray V, Palacios-Rios M. 1998. Ant protection of the nectaried fern Polypodium plebeium in central México. American Journal of Botany 85: 736–739. [PubMed] [Google Scholar]
- Kuo J, Pate JS. 1985. The extrafloral nectaries of cowpea (Vigna unguiculata (L.) Walp). I. Morphology, anatomy and fine structure. Planta 166: 15–27. [DOI] [PubMed] [Google Scholar]
- Lancashire RJ. 2003.http://wwwchem.uwimona.edu.jm/courses/Fehling.html. The Department of Chemistry, University of the West Indies, Jamaica. [Google Scholar]
- Lanza J. 1988. Ant preferences for Passiflora nectar mimics that contain amino acids. Biotropica 20: 341–344. [Google Scholar]
- Lersten NR, Brubaker CL. 1987. Extrafloral nectaries in Leguminosae: review and original observations in Erythrina and Mucuna (Papilionoideae; Phaseoleae). Bulletin of the Torrey Botanical Club 114: 437–447. [Google Scholar]
- Lersten NR, Curtis JD. 1994. Leaf anatomy in Caesalpinia and Hoffmannseggia (Leguminosae, Caesalpinioideae) with emphasis on secretory structures. Plant Systematics and Evolution 192: 231–255. [Google Scholar]
- Lloyd FE, Ridgway CS. 1912. The behavior of the nectar gland in the Cacti, with a note on the development of the trichomes and areolar cork. The Plant World 15: 145–156. [Google Scholar]
- Lopes AV, Vögel S, Machado IC. 2002. Secretory trichomes, a substitutive floral nectar source in Lundia A.DC. (Bignoniaceae), a genus lacking a functional disc. Annals of Botany 90: 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckey D. 1989. Interactions between ants and leguminous plants. In: Stirton CH, Zarucchi JL, eds. Advances in legume biology Monographs of Systematic Botany of the Missouri Botanical Garden 29: 673–718. [Google Scholar]
- Metcalfe CR, Chalk L. 1972.Anatomy of the dicotyledons: leaves, stem and wood in relation to taxonomy, with notes of economic uses. Oxford: Oxford University Press. [Google Scholar]
- Mitchell RJ. 2004. Heritability of nectar traits: why do we know so little? Ecology 85: 1527–1533. [Google Scholar]
- Mondor EB, Addicott JF. 2003. Conspicuous extra-floral nectaries are inducible in Vicia faba. Ecology Letters 6: 495–497. [Google Scholar]
- Moreno-Casasola P, Maarel E, Castillo S, Huesca ML, Pisanty I. 1982. Ecología de la vegetación de dunas costeras: estructura y composición en el Morro de La Mancha, Veracruz. I. Biótica 7: 491–526. [Google Scholar]
- Ness JH. 2003.Catalpa bignonioides alters extrafloral nectar production after herbivory and attracts ant bodyguards. Oecologia 134: 210–218. [DOI] [PubMed] [Google Scholar]
- Oliveira PS, Leitão-Filho HF. 1987. Extrafloral nectaries: their taxonomic distribution and abundance in the woody flora of cerrado vegetation in southeast Brazil. Biotropica 19: 140–148. [Google Scholar]
- Oliveira PS, Oliveira-Filho AT. 1991. Distribution of extrafloral nectaries in the woody flora of tropical communities in western Brazil. In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW, eds. Plant–animal interactions. New York, NY: John Wiley and Sons, 163–175. [Google Scholar]
- Paiva ÉAS. 2001. Occurrence and structure of extrafloral nectaries in Pterodon pubescens Benth. and Pterodon polygalaeflorus Benth. Pesquisa Agropecuaria Brasileira 36: 219–224. [Google Scholar]
- Pascal LM, Motte-Florac EF, Mckey DB. 2000. Secretory structures on the leaf rachis of Caesalpinieae and Mimosoideae (Leguminosae): implications for the evolution of nectary glands. American Journal of Botany 87: 327–338. [PubMed] [Google Scholar]
- Pemberton RW. 1990. The occurrence of extrafloral nectaries in Korean plants. Korean Journal of Ecology 13: 251–266. [Google Scholar]
- Rhyne CL. 1969. Inheritance of extrafloral nectaries in cotton. Advancing Frontiers of Plant Sciences 13: 121–135. [Google Scholar]
- Rico-Gray V. 1989. The importance of floral and circum-floral nectar to ants inhabiting dry tropical lowlands. Biological Journal of the Linnean Society 38: 173–181. [Google Scholar]
- Rico-Gray V. 1993. Use of plant-derived food resources by ants in the dry tropical lowlands of coastal Veracruz, Mexico. Biotropica 25: 301–315. [Google Scholar]
- Rico-Gray V, Oliveira PS, Parra-Tabla V, Cuautle M, Díaz-Castelazo C. 2004. Ant–plant interactions: their seasonal variation and effects on plant fitness. In: Martínez ML, Psuty N, eds. Coastal dunes, ecology and conservation Ecological Studies 171: 221–239. Berlin: Springer-Verlag. [Google Scholar]
- Rivera G. 2000. Nectarios extranupciales florales en especies de Bignoniaceae de Argentina. Darwiniana 38: 1–10. [Google Scholar]
- Rocha JF. 2002. Estructuras secretoras em folhas de Hibiscus tiliaceus L. e Hibiscus pernambucensis Arrupa. Revista Universidade Rural, Série Ciências da Vida 22: 43–55. [Google Scholar]
- Rudgers JA. 2004. Enemies of herbivores can shape plant traits: selection in a facultative ant-plant mutualism. Ecology 85: 192–205. [Google Scholar]
- Rudgers JA, Gardener MC. 2004. Extrafloral nectar as a resource mediating multispecies interactions. Ecology 85: 1495–1502. [Google Scholar]
- Schreck JO, Loffredo WM. 1994. Qualitative testing in carbohydrates. In: Neidig HA, ed. Modular laboratory program in chemistry. Railroad, Palmira, PA: Chemical Educational Resources. [Google Scholar]
- Schupp EW, Feener DH. 1991. Phylogeny, lifeform and habitat dependence of ant-defended plants in a Panamanian forest. In: Huxley CR, ed. Ant–plant interactions. Oxford: Oxford University Press, 175–197. [Google Scholar]
- Vögel S. 1998. Remarkable nectaries: structure, ecology, organophyletic perspectives. IV. Miscellaneous cases. Flora 193: 225–248. [Google Scholar]
- Wäckers FL, Bonifay C. 2004. How to be sweet? Extrafloral nectar allocation by Gossypium hirsutum fits optimal defense theory predictions. Ecology 85: 1512–1518. [Google Scholar]
- Wäckers FL, Zuber D, Wunderlin R, Keller F. 2001. The effect of herbivory on temporal and spatial dynamics of extrafloral nectar production in cotton and castor. Annals of Botany 87: 365–370. [Google Scholar]
- Zimmerman JG. 1932. Über die extrafloralen Nektarien der Angiospermen. Beihefte zum Botanischen Zentralblatt 49: 99–196. [Google Scholar]


