Abstract
• Background and Aims Following a period of burial, more Actinotus leucocephalus (Apiaceae) and Tersonia cyathiflora (Gyrostemonaceae) seeds germinate in smoke water. The main aim of this study was to determine whether these fire-ephemeral seeds exhibit annual dormancy cycling during burial. This study also aimed to determine the effect of dormancy alleviation on the range of light and temperature conditions at which seeds germinate, and the possible factors driving changes in seed dormancy during burial.
• Methods Seeds were collected in summer, buried in soil in mesh bags in autumn and exhumed every 6 months for 24 months. Germination of exhumed and laboratory-stored (15 °C) seeds was assessed at 20 °C in water or smoke water. Germination response to light or dark conditions, incubation temperature (10, 15, 20, 25 and 30 °C), nitrate and gibberellic acid were also examined following burial or laboratory storage for 24 months. In the laboratory seeds were also stored at various temperatures (5, 15, 37 and 20/50 °C) for 1, 2 and 3 months followed by germination testing in water or smoke water.
• Key Results The two species exhibited dormancy cycling during soil burial, producing low levels of germination in response to smoke water when exhumed in spring and high levels of germination in autumn. In autumn, seeds germinated in both light and dark and at a broader range of temperatures than did laboratory-stored seeds, and some Actinotus leucocephalus seeds also germinated in water alone. Dormancy release of Actinotus leucocephalus was slow during dry storage at 15 °C and more rapid at higher temperatures (37 and 20/50 °C); weekly wet/dry cycles further accelerated the rate of dormancy release. Cold stratification (5 °C) induced secondary dormancy. By contrast, no Tersonia cyathiflora seeds germinated following any of the laboratory storage treatments.
• Conclusions Temperature and moisture influence dormancy cycling in Actinotus leucocephalus seeds. These factors alone did not simulate dormancy cycling of Tersonia cyathiflora seeds under the conditions tested.
Keywords: Dormancy cycling, fire ephemeral, germination stimulants, scarification, smoke water, soil burial, storage temperature, Actinotus leucocephalus, Tersonia cyathiflora
INTRODUCTION
Fire ephemerals are species that primarily germinate after fire and normally live for 3 months to 4 years (Bell et al., 1984). It is widely inferred that their seeds persist in the soil for many years between fires, although these species may occasionally germinate in areas of soil disturbance enriched with nutrients (Bell et al., 1984; Pate and Dixon, 1996). A number of Australian fire-ephemeral species possess non-deep physiological dormancy and consequently germination of freshly collected seeds can be difficult, even in response to fire-related cues such as heat and smoke (Baker et al., 2005a). However, in many fire-following species, germination in response to smoke is enhanced by a period of burial (Roche et al., 1997; Keeley and Fotheringham, 1998; Tieu et al., 2001; Baker et al., 2005b).
Different explanations have been proposed for enhanced germination in response to smoke following burial. Increased permeability of the seed coat or breakdown of seed covering structures may allow the entry and movement of chemicals in smoke (e.g. butenolides) to sites in seeds where they stimulate germination (de Lange and Boucher, 1993; Tieu and Egerton-Warburton, 2000; Flematti et al., 2004). Alternatively, it may be the temperature and moisture conditions in the soil that enhance the response of seeds to smoke following a period of burial (Keeley and Fotheringham, 1998). Buried seeds with non-deep physiological dormancy characteristically cycle seasonally in their degree of dormancy (Baskin and Baskin, 2004). As dormancy is alleviated, the range of conditions such as temperature and light, or responsiveness to germination stimulants suitable for germination widen (Vegis, 1964; Derkx and Karssen, 1993). Likewise, as dormancy is re-imposed there is a narrowing of the window of conditions at which seeds can germinate. Although fire ephemerals produce higher germination in response to smoke cues after a period of burial (Keeley and Fotheringham, 1998; Baker et al., 2005b), it has not been established whether these species cycle seasonally in their response to smoke water.
At present, temperature is considered to be the main factor that regulates dormancy levels in seeds with physiological dormancy (Vleeshouwers et al., 1995), and there is debate as to whether seed moisture content also plays a role. Bouwmeester and Karssen (1992, 1993) concluded that soil moisture had no effect on dormancy cycling in Sisymbrium officinale (Brassicaceae) or Polygonum persicaria (Polygonaceae) seeds. However, small increases in seed moisture or short periods of imbibition have been shown to accelerate dormancy release during after-ripening of Oryza sativa and Lolium rigidum (Poaceae; Leopold et al., 1988; Steadman et al., 2003; Gallagher et al., 2004). For species that exhibit dormancy loss at low temperatures, seeds must be imbibed for dormancy alleviation to occur, as in Polygonum aviculare (Polygonaceae; Kruk and Benech-Arnold, 1998).
Actinotus leucocephalus (Apiaceae) and Tersonia cyathiflora (Gyrostemonaceae) are both Australian fire ephemerals with soil-stored seeds (Bell et al., 1984). Both occur within the Mediterranean climate region of south-western Australia and under natural conditions primarily germinate in the first wet season after fire. Actinotus leucocephalus seeds have morphophysiological dormancy whereas Tersonia cyathiflora seeds have physiological dormancy only (Baker et al., 2005a). Germination of both species in response to smoke water is enhanced when seeds are buried in autumn and exhumed 12 months later (Baker et al., 2005b). This paper has two main aims: (1) to determine whether germination in response to smoke water increases with duration of seed burial or cycles annually in these species, and whether seeds can persist in the soil for at least 24 months; and (2) to examine whether the breadth of conditions suitable for germination, such as incubation temperatures and light regimes, are influenced by a period of burial. We investigate whether enhanced germination response to smoke water following exhumation in autumn is a result of summer burial only or whether winter followed by summer burial is required. This research also aims to determine what environmental components of burial influence the changes in dormancy in these species, such as temperature, seed moisture and physical weathering. These investigations will provide a better understanding of the germination ecophysiology of these two Australian fire ephemerals.
MATERIALS AND METHODS
Seed source
Freshly matured Actinotus leucocephalus seeds (technically mericarps but herein referred to as seeds) were collected in December, 2001 from plants in a Banksia woodland site (40 km north of Gin Gin, Western Australia, 31°01.231′S, 115°43.297′E) burnt the previous summer. Tersonia cyathiflora seeds were collected from plants at Beekeepers Reserve (north of Eneabba, Western Australia, 29°32.315′S, 115°03.415′E) in January, 2002 from a site burnt during a wildfire in January, 2000. Seeds were air-dried and stored in sealed containers at 15 °C prior to use. To determine the moisture content of seeds (percentage fresh weight) during storage, four replicates of 50 seeds were weighed and then placed at 103 °C for 17 h before reweighing (International Seed Testing Association, 1999).
Experiment 1: seed burial and retrieval over 24 months
The purpose of the first experiment was to determine whether seed germination was influenced by a period of burial and whether buried seeds could persist in the soil without germinating for at least 24 months. For each species, 130 seeds were placed in nylon bags with an approximately equal volume of white sand to seeds. The nylon material allowed the transfer of water and solutes between the contents of the bags and the soil environment. Seeds were buried in March, 2002 (autumn) in an area of Banksia woodland on Spearwood dunes (31°56.98′S, 115°47.83′E) at The University of Western Australia Shenton Park Field Station (Perth), part of which was burnt in January, 2002. Randomly distributed plots were established within burnt (four plots) and unburnt (four plots) areas with similar aspect (westerly) and slope (slight). Ten bags of each species were buried 2 cm below the soil surface in seedling trays at each of the eight plots. The trays enabled easy removal of bags at each exhumation date without disturbing other buried seeds. Seeds were exhumed during daylight hours in October (spring), 2002 and 2003 and March (autumn), 2003 and 2004.
After seeds were exhumed, damaged and flat seeds were counted and discarded. The remaining seeds were assigned to one of four treatments: water, smoke water, heat followed by incubation in water or heat followed by incubation in smoke water. All treatments consisted of four replicates of 25 seeds, and each of the four plots in the burnt and unburnt sites formed the replicates. For the heat treatment, seeds were wrapped in aluminium foil and placed in a 70 °C oven for 1 h and then transferred to 20 °C for 24 h before further treatment. To surface-sterilize seeds, and hence to minimize fungal contamination, all seeds were shaken for 30s in 2 % (v/v) NaOCl containing a non-ionic surfactant (one drop per 125mL of Plus 50, Ciba-Geigy, Sydney) and placed under vacuum (5 min on, 5 min off, 5 min on) to remove air bubbles and to ensure contact of the solution with the seed surface. Seeds were transferred to a laminar flow cabinet, rinsed three times with sterile distilled water, soaked for 10 min and rinsed again. Prior to incubation in smoke water, seeds were air-dried in the laminar flow cabinet for 8–12 h to ensure that smoke water was imbibed.
Seeds were placed in sterile plastic Petri dishes (90 mm in diameter) on two pieces of Whatman No. 1 filter paper. Three pieces of sponge each of 4 cm2 were placed under the filter papers to absorb excess moisture. Petri dishes were moistened with either 10mL of sterile distilled water or a 1:10 dilution of smoke water (Seed Starter, Kings Park and Botanic Garden, Perth), both containing 0·15 % (v/v) Previcur fungicide (Aventis, Melbourne, active ingredient 600 gL−1 propamocarb), and sealed with Parafilm. Smoke water aliquots were frozen and only thawed when required, to minimize any possible changes over time. After thawing, the smoke water was filtered (0·2 µm) and diluted. Seeds were incubated at 20 °C, and exposed to 12 h of light daily from cool white fluorescent tubes (50 µmolm−2s−1 photosynthetically active radiation). For all germination experiments, seeds were monitored every 7 d for 5 weeks and removed as they germinated (radicle emerged >2 mm). Seeds not germinating within 5 weeks were dissected and the number of filled seeds was noted. Water, smoke water, heat or heat and smoke water treatments were also undertaken on laboratory-stored seeds (15 °C) at the time seeds were buried and to coincide with each seed exhumation.
Breadth of conditions suitable for germination
Assignment of seeds from the burnt and unburnt sites to the different treatments was based on seed availability. After 24 months of burial (March, 2004), surface-sterilized seeds exhumed from the unburnt site were incubated at a range of constant temperatures (10, 15, 20, 25 and 30 °C) in water or smoke water. At all temperatures, seeds were exposed to 12 h of light daily. Seeds exhumed from the unburnt site were also placed in water or smoke water, wrapped in aluminium foil to exclude light and incubated at 20 °C. Dark-incubated seeds were only monitored at the end of the 5-week period to avoid exposing seeds to light during the trial.
Seeds exhumed from the burnt site in March, 2004 were surface sterilized and incubated at 20 °C in 10 mL of one of the following solutions: 30μm gibberellic acid (GA3), 10 mm KNO3, smoke water plus 30μm GA3, and 10 mm KNO3 plus 30μm GA3. The GA3 (Sigma-Aldrich) was dissolved in 4mLL−1 ethanol, and all solutions contained 0·15 % (v/v) Previcur. Additional seeds were soaked in H2SO4 for 1 min, rinsed five times in sterile distilled water in the laminar flow cabinet and incubated in water or smoke water. Seeds treated with H2SO4 were not surface sterilized. All treatments were undertaken concurrently on seeds stored in the laboratory at 15 °C.
Influence of burial on seed morphology
To determine whether burial altered the surface of seeds, seeds stored in the laboratory or buried in the unburnt site for 24 months (exhumed March, 2004) were examined and imaged using an Electroscan E3 environmental scanning electron microscope (ESEM). The influence of surface sterilization on buried and laboratory-stored seeds was also examined because this was a standard procedure before germination tests. The impact of soaking buried T. cyathiflora seeds in sulfuric acid for 1 min was investigated to tie in with pertinent impacts on germination. Images of A. leucocephalus seeds were captured with the Electroscan E3 ESEM operating at 30 kV, using both backscatter and secondary electron detectors. To detect finer surface detail, all T. cyathiflora images were captured at 15 kV using a secondary electron detector only (because secondary electrons originate from surface features).
Experiment 2: seed burial and retrieval over summer
Laboratory-stored seeds were buried in the unburnt site on 5 November, 2003 (late spring) to determine whether enhanced germination in autumn (expt 1) arose from winter followed by summer burial or from summer burial only. Nylon mesh bags were prepared as before (but this time with 150 Actinotus leucocephalus and 115 Tersonia cyathiflora seeds per bag). An equal number of seeds were also placed in sealed foil packets to separate the effects of temperature (foil packets) from combined temperature and moisture shifts (nylon mesh bags). The moisture content of seeds at the start of the experiment was determined using three replicates of 50 seeds of each species as outlined in expt 1. For each species, three nylon and two foil bags were buried in seedling trays adjacent to each of the four unburnt plots. Dataloggers (T-Tec 501-1, Temperature Technology, Henley Beach, South Australia) were buried (2 cm deep) in the seedling trays at two of the four unburnt plots, and the temperature was recorded at hourly intervals for 25d from the date of seed burial. Seeds were exhumed in March, 2004 and the germination of firm and undamaged seeds was tested in water or smoke water, with and without heat treatment of 70 °C for 1 h, as for seeds buried in March, 2002. In addition, non-surface-sterilized seeds from the foil packets and nylon bags were soaked in H2SO4 for 1 min, rinsed with distilled water five times and then germination tested in water or smoke water. All germination results were adjusted according to the number of filled seeds determined at the end of the trial using a cut test. However, germination results for the H2SO4 treatments were adjusted for the proportion of seeds filled according to the other treatments, as this treatment may have damaged seeds.
A tetrazolium chloride test was used to estimate the viability of seeds of each species prior to and following burial in nylon mesh and foil bags as it was suspected that seeds may have died in the foil bags but appeared viable because they were filled. For each of the four sites, three replicates of 25 intact and firm seeds were imbibed in distilled water for 24 h at 25 °C. A small portion of the T. cyathiflora seed coat and of the A. leucocephalus pericarp and seed coat were then removed from each seed to facilitate uptake of the stain. Seeds were placed in a 1 % solution of 2,3,5-triphenyl tetrazolium chloride for 24 h at 30 °C in the dark (International Seed Testing Association, 1999). The embryo in each seed was then examined microscopically and those that appeared healthy (firm and without necrotic tissue) and were stained red were scored as viable.
Experiment 3: temperature simulation of seasons under controlled conditions
Laboratory-based investigations were undertaken to determine whether temperature was the primary factor that drove dormancy cycling in the soil and whether seed moisture content also played a role. In August, 2003 laboratory-stored seeds were placed in paper bags and stored at either 20/50 °C (alternating regime with 12 h at each temperature) or 37 °C (constant) to simulate summer conditions in the soil. Seeds were removed after 1, 2 and 3 months of storage, surface sterilized and germination tested in water or smoke water at 20 °C with 12 h of light daily. To examine the effect of cold stratification (winter conditions), surface-sterilized seeds were incubated at 5 °C in Petri dishes moistened with 10mL of water and wrapped in aluminium foil to exclude light. After 1, 2 and 3 months, seeds of each species were transferred to fresh Petri dishes containing either water or smoke water, and incubated at 20 °C with 12 h of light daily for a further 5 weeks. In an additional treatment, seeds were cold stratified for 2 months, air-dried for 24 h at 20 °C and placed in paper envelopes at 20/50 °C for 1, 2 and 3 months (and 6 months for A. leucocephalus only). Seeds were again surface sterilized and incubated in either water or smoke water at 20 °C as above.
Statistical analysis
One- or two-way ANOVAs were performed to determine whether treatments influenced germination, whether the proportion of filled seeds remained unchanged over time during burial in each of the burnt and unburnt sites, and whether burial over summer in nylon and foil influenced seed viability across the four unburnt sites. When germination was absent from all four replicates, the treatment was excluded from analysis to satisfy the ANOVA assumption of equal variances. Prior to analysis, data were arcsine square-root transformed. Fisher's protected LSD test was used as a post-hoc test. Treatments were regarded as significantly different at P < 0·05. Statistical analyses were performed in Genstat version 6 (VSN International, Oxford, UK).
A linear regression was applied to the germination of laboratory-stored A. leucocephalus seeds in response to smoke water, heat and heat plus smoke water to determine whether there were any changes in germination response to each of these treatments over time. Germination of seeds following different durations of a range of laboratory temperature and moisture regimes was also tested against linear regressions to examine if these regimes altered germination over time. All regression analyses were performed in SigmaPlot version 8 (Systat Software, Richmond, CA, USA).
RESULTS
Experiment 1: seed burial and retrieval over 24 months
The number of Actinotus leucocephalus and Tersonia cyathiflora seeds that germinated following exhumation from burial cycled annually (Fig. 1). Prior to burial, no T. cyathiflora seeds germinated at 20 °C, even in response to smoke water (Fig. 1E–H). By contrast, some A. leucocephalus seeds germinated when treated with smoke water (35 %) or heat (29 %) or both (73 %) before burial (Fig. 1B–D). After 6 months of winter burial, germination in response to these treatments was very low (<5 %) in A. leucocephalus and low (0–15 %) in T. cyathiflora (Fig. 1). Following subsequent summer burial, all A. leucocephalus seeds and approximately 60 % of T. cyathiflora seeds germinated in smoke water (Fig. 1B and F). Seeds of A. leucocephalus exhumed from the unburnt site after summer also germinated in water (84 %, Fig. 1A) but smoke water was an absolute requirement for germination of T. cyathiflora (Fig. 1E–H). For both species, lower germination after winter burial and higher germination after summer burial was repeated in the subsequent year. Actinotus leucocephalus seeds exhumed in autumn produced maximum germination (100 %) in smoke water after 12 months of burial whereas germination of T. cyathiflora seeds buried in the unburnt site was enhanced by a longer duration of burial, with higher germination in smoke water after 24 months of burial than after 12 months (79 % vs. 65 %; F1,6 = 8·60, P = 0·026).
Fig. 1.
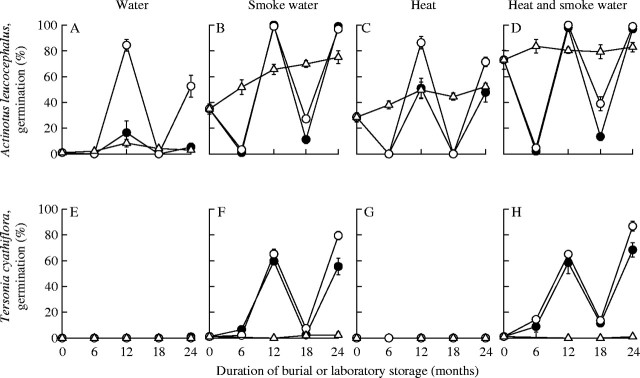
Germination (mean ± s.e.) of (A–D) Actinotus leucocephalus and (E–H) Tersonia cyathiflora seeds following 6, 12, 18 and 24 months of burial in nylon mesh bags in recently burnt (solid circles) or unburnt soil (open circles), or laboratory storage at 15 °C (open triangles). Germination was assessed by incubating seeds at 20 °C in water (A,C,E,G) or smoke water (B,D,F,H) for 35 days without preheating (A,B,E,F) or following pretreatment at 70 °C for 1 h (C,D,G,H).
Actinotus leucocephalus seeds stored in the laboratory at 15 °C (5·7 ± 0·3 % moisture content) exhibited an increase in germination response to smoke water (35–75 %) and a 70 °C heat treatment (29–52 %) applied separately (Fig. 1B and C). Germination in response to smoke water increased linearly over time (slope = 1·97, R2 = 0·82, F1,18 = 82·4, P < 0·0001) and at a slower rate in response to heat (slope = 0·40, R2 = 0·49, F1,18 = 17·4, P = 0·0006). By contrast, over the same time period, A. leucocephalus germination in water remained below 10 % (Fig. 1A). The combined heat and smoke water treatment produced approximately 80 % germination at the start of the trial and did not increase further over the 24-month storage period (F1,18 = 2·2, P = 0·15, Fig. 1D). Tersonia cyathiflora seeds stored in the laboratory at 15 °C (5·0 ± 0·1 % moisture content) did not germinate at 20 °C, irrespective of germination treatment (Fig. 1E–H).
During 24 months of burial the proportion of filled seeds declined in both species, particularly during the first winter of burial (Fig. 2). Despite seed fill declining over time, over 65 % of A. leucocephalus (Fig. 2A) and over 75 % of T. cyathiflora seeds (Fig. 2B) remained filled after 24 months of burial.
Fig. 2.
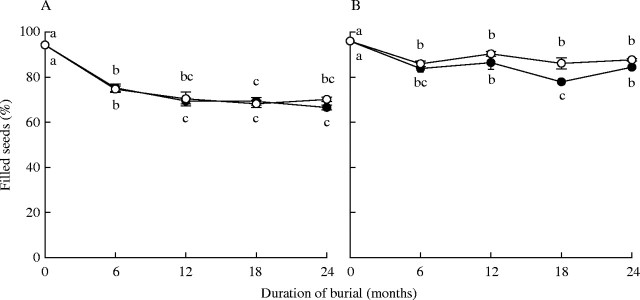
Number of (A) Actinotus leucocephalus and (B) Tersonia cyathiflora seeds filled (mean ± s.e.) after burial in nylon mesh bags for 6, 12, 18 and 24 months in unburnt (open circles) and recently burnt (solid circles) soil. Different lower-case letters indicate significant differences in the proportion of filled seeds over time. Letters above and below the symbols refer to seeds buried in the unburnt and burnt sites, respectively.
When seeds were dissected to determine the proportion that were filled, it was observed that embryo growth in A. leucocephalus, which has underdeveloped embryos at seed dispersal, had not visibly commenced during winter or summer burial.
Breadth of conditions suitable for germination
For both species, seeds exhumed from burial in autumn germinated at a broader range of temperatures than laboratory-stored seeds (Fig. 3). Germination of A. leucocephalus was >85 % following soil burial and incubation in smoke water at 15–25 °C (Fig. 3A). Buried seeds incubated in water alone produced maximum germination at a similar temperature range (15–25 °C) but germination levels were lower (50–75 %) than for seeds incubated in smoke water (87–99 %; F1,18 = 65·39, P < 0·001). Laboratory-stored seeds incubated in smoke water produced similar optimal levels of germination as buried seeds incubated in water, but the optimum incubation temperature range was narrower (15–20 °C). Germination of laboratory-stored seeds in water did not exceed 3 % across the range of temperatures tested. Tersonia cyathiflora seeds only germinated following burial and incubation in smoke water (Fig. 3B). High numbers of T. cyathiflora seeds germinated (70–80 %) across a broad range of temperatures (15–30 °C) following exhumation from the unburnt site in autumn and incubation in smoke water.
Fig. 3.
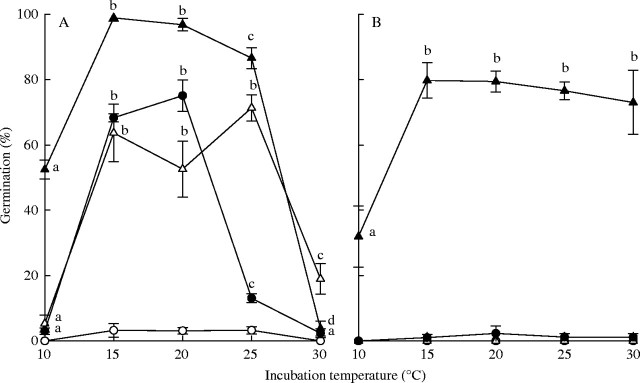
Germination (mean ± s.e.) of (A) Actinotus leucocephalus and (B) Tersonia cyathiflora seeds at a range of incubation temperatures (10, 15, 20, 25, 30 °C) following 24 months of burial in nylon mesh bags in unburnt soil (triangles) or laboratory storage (circles) at 15 °C. Seeds were incubated in water (open symbols) or smoke water (solid symbols) for 35 days. Lower-case letters indicate significant differences in germination across incubation temperatures for each treatment.
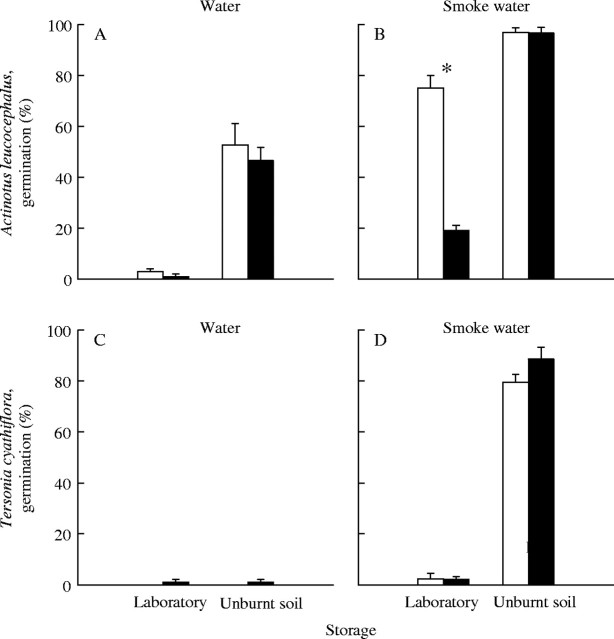
Following 24 months of laboratory storage, A. leucocephalus seeds produced higher germination in smoke water under an alternating light/dark regime (75 %) than under constant darkness (19 %; Fig. 4B). Conversely, the germination of A. leucocephalus seeds buried in the soil for the same duration of time was not promoted by light (Fig. 4A and B). Germination of T. cyathiflora seeds was unaffected by light environment irrespective of laboratory or soil storage (Fig. 4C and D).
Fig. 4.
Germination (mean ± s.e.) of (A,B) Actinotus leucocephalus and (C,D) Tersonia cyathiflora seeds under an alternating light/dark regime (12 h light/12 h dark, open bars) or continuous darkness (filled bars) for 35 days. Seeds were incubated in water (A,C) or smoke water (B,D) at 20 °C. Prior to testing, seeds had been stored in the laboratory (15 °C) or buried in nylon mesh bags in unburnt soil for 24 months. Asterisks (*) indicate significant differences in germination between light regimes for each treatment.
Following treatment with a range of chemicals, A. leucocephalus seeds exhumed from the soil in autumn or stored in the laboratory produced maximum germination in response to smoke water (Fig. 5A). Gibberellic acid, nitrate and sulfuric acid also stimulated germination of laboratory-stored A. leucocephalus seeds, but of these chemicals, only nitrate stimulated the germination of soil-stored seeds. Smoke water was the only chemical treatment that produced higher germination of A. leucocephalus seeds after exhumation from burial in autumn than after laboratory storage.
Fig. 5.
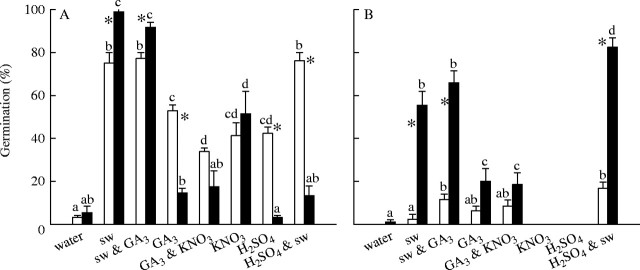
Germination (mean ± s.e.) of (A) Actinotus leucocephalus and (B) Tersonia cyathiflora seeds following 24 months of burial in nylon mesh bags in burnt bushland (filled bars) or laboratory storage (15 °C; open bars) when incubated at 20 °C for 35 days in water, smoke water (sw), sw plus 30 μm GA3, 30 μm GA3, 30 μm GA3 plus 10 mm KNO3, or 10 mM KNO3. Additional seeds were soaked in H2SO4 for 1 min prior to incubation in water or smoke water. Lower-case letters indicate differences in germination across treatments for each storage regime (laboratory or burial). Asterisks (*) indicate significant differences in germination between the storage regimes for each treatment.
Likewise, following a range of chemical treatments, germination of T. cyathiflora seeds was only higher following soil storage than laboratory storage in the presence of smoke water (Fig. 5B). Gibberellic acid induced low levels of germination in both laboratory- and soil-stored seeds but neither nitrate nor sulfuric acid alone induced any germination. However, pretreating seeds with sulfuric acid enhanced germination response to smoke water in both laboratory- and soil-stored seeds.
Influence of burial on seed morphology
A period of burial resulted in morphological changes to the outer layers of A. leucocephalus (Fig. 6) and T. cyathiflora seeds (Fig. 7). Most notably, in A. leucocephalus, hairs remained intact in laboratory-stored seeds (Fig. 6A and B) but were mostly lost during burial (Fig. 6C and D). In addition to the removal of hairs, a period of burial resulted in the lifting of a membrane over the base of the hairs (Fig. 6D). When seeds were surface sterilized prior to germination testing, laboratory-stored seeds were unaltered but the outer layer of the pericarp became completely detached from buried seeds (irrespective of whether they were exhumed in autumn or spring), exposing an underlying pitted layer of the pericarp (Fig. 6E and F). Laboratory-stored T. cyathiflora seeds had a fine surface texture (Fig. 7A and B) that was weathered by burial (Fig. 7C and D). The sulfuric acid treatment, which was associated with an increase in germination response to smoke water in both laboratory- and soil-stored T. cyathiflora seeds (Fig. 5B), fractured the seedcoat of both laboratory- and soil-stored seeds (Fig. 7E and F), exposing the outer layer of the endosperm.
Fig. 6.
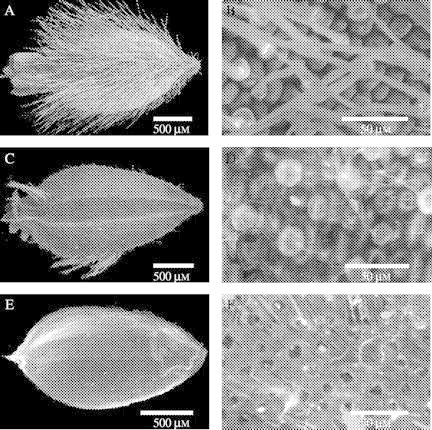
ESEM images of the surface of Actinotus leucocephalus seeds following laboratory storage (A,B), burial in the unburnt site for 24 months (C,D) and surface sterilization following burial in the unburnt site for 18 months (E,F). For each pair of images the whole dispersal unit is shown (A,C,E) and then the mid-section of the seeds is captured at higher magnification (images B, D and F, respectively).
Fig. 7.
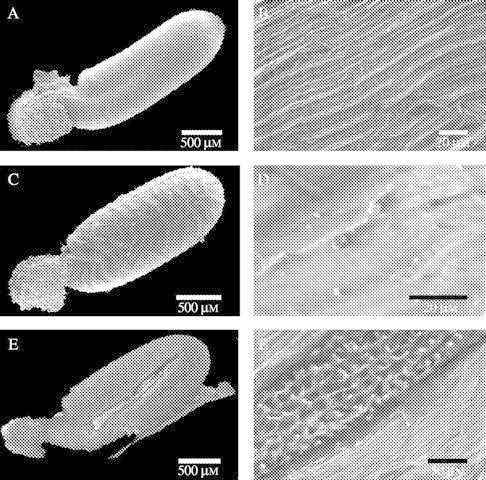
ESEM images of the surface of Tersonia cyathiflora seeds following laboratory storage (A,B), burial in the unburnt site for 24 months (C,D) and burial in the unburnt site for 24 months and soaking in H2SO4 for 1 min (E,F). For each pair of images the whole dispersal unit is shown (A,C,E) and then the mid-section of the seeds is captured at higher magnification (B, D and F, respectively).
Experiment 2: seed burial and retrieval over summer
Germination was generally higher following burial over summer in nylon mesh bags than in foil packets (Fig. 8). Germination of A. leucocephalus seeds exhumed in autumn following burial in nylon mesh bags for 4 months over summer in the unburnt site (Fig. 8A) was similar to that of seeds buried for 24 months at the same site (Fig. 1A–D). Sulfuric acid suppressed the smoke water response of A. leucocephalus seeds buried in the burnt site for 24 months (Fig. 5A), but did not suppress the germination response to smoke water of seeds buried in nylon mesh bags over summer only (Fig. 8A).
Fig. 8.
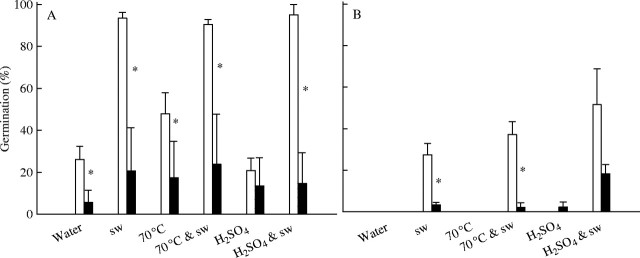
Germination (mean ± s.e.) of (A) Actinotus leucocephalus and (B) Tersonia cyathiflora seeds following burial in an unburnt area for 4 months over summer in nylon mesh bags (open bars) or sealed foil packets (filled bars). Seeds were incubated at 20 °C for 35 days in water or smoke water (sw), with or without a 1 h 70 °C heat or 1 min H2SO4 pretreatment. Asterisks (*) indicate significant differences between germination of seeds stored in nylon mesh bags vs. foil packets for each treatment.
The moisture content of A. leucocephalus seeds when sealed in the foil packets was 6·3 ± 0·1 %. Following summer burial in the foil packets, 91 ± 5·8 % of filled A. leucocephalus seeds from the most shaded plot (plot 3) were viable, but viability was <2 % at the other three plots (plots 1, 2 and 4). By contrast, viability of A. leucocephalus seeds buried over summer in nylon mesh bags exceeded 93 % at all four unburnt plots. The average soil temperatures at plots 1 and 3 during the first 25 d of burial, 2 cm beneath the soil surface, were 28·1 and 22·0 °C, respectively. At plot 1, temperatures ranged from 12·5 to 64·3 °C, and exceeded 50 °C on 18 of the 25 d. At plot 3, temperature fluctuations were lower with minimum and maximum temperatures of 14·2 and 33·3 °C.
The moisture content of T. cyathiflora seeds sealed in foil packets was 5·9 ± 0·1 %. After summer burial, T. cyathiflora seed viability did not differ significantly between nylon mesh bags and foil packets (F1,3 = 0·00, P = 0·969), and was higher in seeds exhumed from plot 3 (85 %) than from the three other plots (52–68 %; F3,16 = 16·06, P < 0·001).
Germination of T. cyathiflora seeds in smoke water, as a proportion of filled seeds, was higher after burial for 24 months in nylon mesh bags than after a single summer (Figs 1F and 8B). For seeds buried over summer only, germination response to smoke water was higher after burial in nylon mesh bags than in foil packets (Fig. 8B). Following sulfuric acid treatment, the germination response to smoke water of seeds buried in foil packets was enhanced (F3,12 = 6·56, P = 0·007), whereas the germination of seeds buried in nylon mesh was unchanged (F2,9 = 0·39, P = 0·685).
Experiment 3: temperature simulation of seasons under controlled conditions
At the commencement of expt 3, A. leucocephalus seeds were already partially after-ripened following storage at 15 °C for 20 months, producing 60 % germination in smoke water (Fig. 9A–D). Storage at alternating 20/50 °C temperatures for 3 months led to no detectable change in germination in smoke water (Fig. 9A; F1,14 = 0·4, P = 0·52). However, after 6 months of storage at 20/50 °C, germination increased to 81 ± 6 % (F1,6 = 10·08, P = 0·019). During 3 months of dry storage at 37 °C, A. leucocephalus germination in smoke water increased linearly to 83 % (Fig. 9B; F1,14 = 13·6, P = 0·0024). Subjecting seeds to weekly wet/dry cycles increased germination in smoke water to 90 % after 1 month of after-ripening at 37 °C (Fig. 9C).
Fig. 9.
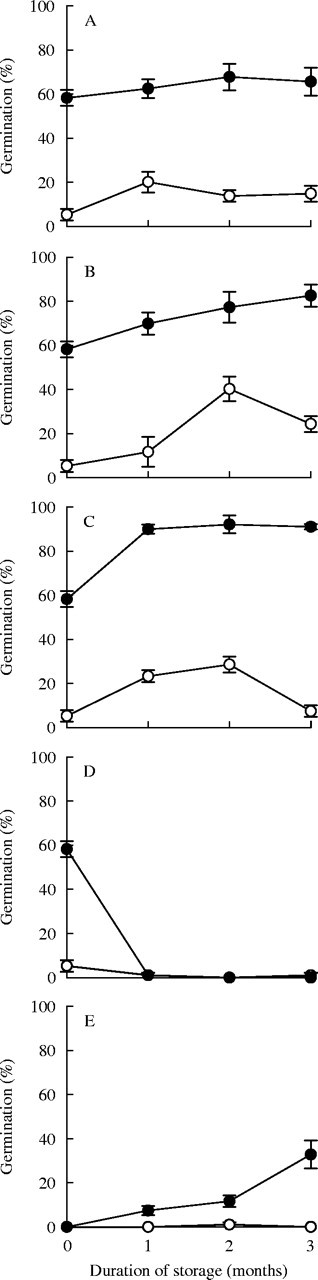
Germination (mean ± s.e.) of Actinotus leucocephalus in water (open circles) and smoke water (solid circles) at 20 °C following laboratory storage of seeds for 1, 2 and 3 months of air-dry storage at 20/50 °C (A), air-dry storage at 37 °C (B), a weekly regime of air-dry storage at 37 °C for 5 days, imbibition of seeds on wet absorbent sponge for 1 day at 20 °C followed by 1 day of air-drying at 20 °C (C), 5 °C stratification (D), or air-dry storage at 20/50 °C after 2 months of 5 °C stratification (E).
Conversely, cold stratification (5 °C) induced dormancy, suppressing subsequent germination of A. leucocephalus. Following 1 month of stratification, no A. leucocephalus seeds germinated in smoke water at 20 °C and germination remained negligible (≤1 %) following 2 and 3 months of stratification (Fig. 9D). Transferring seeds that were cold stratified for 2 months to warm dry storage (20/50 °C) resulted in a gradual increase in germination response to smoke with duration of storage (Fig. 9E).
In contrast to A. leucocephalus, germination of T. cyathiflora seeds was negligible (<1 %) following all laboratory temperature/moisture regimes tested when they were subsequently imbibed in water or smoke water at 20 °C.
DISCUSSION
Both Actinotus leucocephalus and Tersonia cyathiflora seeds exhibited annual dormancy cycling, with a widening of conditions suitable for germination occurring after summer burial and a narrowing after winter burial. According to the dormancy types of Baskin and Baskin (2004), T. cyathiflora possessed non-deep physiological dormancy and A. leucocephalus had morphophysiological dormancy (Baker et al., 2005a). Dormancy release in A. leucocephalus occurred following warm stratification, and observations indicated that embryo growth only proceeded after physiological dormancy was overcome, suggesting that A. leucocephalus seeds had non-deep, simple morphophysiological dormancy (Baskin and Baskin, 2004). This kind of dormancy was first described for another Apiaceae species, Chaerophyllum tainturieri (Baskin and Baskin, 1990). The germination phenology of seeds with non-deep morphophysiological dormancy is generally similar to winter annuals with non-deep physiological dormancy in terms of dormancy release occurring during summer and dormancy induction in winter, with the primary difference being the presence of underdeveloped embryos in the former and fully developed embryos in the latter (J. M. Baskin and Baskin, 1994).
Actinotus leucocephalus and T. cyathiflora are fire ephemerals and thus primarily germinate after fire (Bell et al., 1984). In south-western Australia most fires occur in summer and autumn (Lindesay, 2003). Seeds of both species germinated to higher levels in response to smoke water when exhumed in autumn than in spring. Smoke is an important component of fire and a germination stimulant in smoke, a butenolide (Flematti et al., 2004), becomes available to seeds when smoke deposits on the soil surface are leached through the soil profile with the onset of rain in autumn. Germination stimulation by smoke water ensures that these seedlings benefit from the post-fire environment when there is a flush of nutrient availability and reduced competition from other plants (Bell et al., 1984).
In addition to enhanced response to a germination stimulant, in the form of smoke water, seeds exhumed from burial in autumn could germinate at a broader range of temperatures than seeds stored dry in the laboratory at 15 °C. Buried A. leucocephalus seeds incubated in either water or smoke water produced >50 % germination across a wider range of temperatures than laboratory-stored seeds of the same age. A similar widening of temperatures appropriate for germination occurs in many other species as dormancy is released (Vegis, 1964; Bouwmeester and Karssen, 1992; Schütz et al., 2002). Furthermore, when dormancy was alleviated, the requirement for specific light conditions was overcome, as has been found in some other species (Vegis, 1964; C. C. Baskin and Baskin, 1994). Laboratory-stored A. leucocephalus seeds exhibited a strong light-stimulated germination response whereas dormancy release associated with soil burial removed any influence of light environment on germination. As seeds were retrieved during hot, dry environmental conditions and seeds must be hydrated to respond to a red-light stimulus (Bartley and Frankland, 1984), exposure of seeds to light during exhumation is unlikely to have influenced the germination of seeds subsequently incubated in the dark. Another winter annual, Corydalis flavula, germinates in both light and darkness at autumn temperatures and consequently lacks the potential to form large persistent seedbanks (J. M. Baskin and Baskin, 1994). The additional requirement for a smoke stimulant enables A. leucocephalus and T. cyathiflora to form persistent seedbanks.
Previously it has been inferred that seeds of fire ephemerals persist in the soil seedbank for long periods between fires (Bell et al., 1984; Pate and Dixon, 1996). Here, after 24 months of burial in Perth bushland, over 65 % of A. leucocephalus seeds and over 75 % of T. cyathiflora seeds persisted as filled seeds without germinating. Seed persistence is particularly important for the continuation of species that require fire to germinate and have a life span shorter than the average fire interval (Holmes and Newton, 2004). On the Northern Sandplain in south-western Australia, where both these species occur, estimates of natural fire intervals vary (as opposed to human-managed fire frequency). van der Moezel et al. (1987) suggest that fires occur naturally every 8–15 years, whereas Bell et al. (1984) estimate longer intervals of 25–50 years. Both estimates are substantially longer than the life spans (<1–4 years) of A. leucocephalus and T. cyathiflora plants. Actinotus leucocephalus may be able to germinate in the inter-fire period because autumn-exhumed seeds germinated in response to water; however, T. cyathiflora required a smoke cue to germinate, suggesting that germination may be restricted to the post-fire environment. Interestingly, the conditions in the unburnt soil compared with the burnt soil were more conducive to dormancy release in A. leucocephalus, allowing germination even in the absence of smoke water. This may be an ecological adaptation to ensure some germination and hence persistence of this species at a site in the absence of fire, especially as Pate and Hopper (1993) suggest that the seed longevity of monocarpic fire ephemerals, such as A. leucocephalus, is shorter than that of polycarpic fire ephemerals, such as T. cyathiflora.
Buried seeds of both these fire ephemerals varied seasonally in their response to germination stimulants, such as smoke water. Actinotus leucocephalus seeds buried in nylon mesh only over summer germinated to similar levels in autumn as seeds buried for 24 months, suggesting that summer conditions only are required to alleviate dormancy. Accordingly, after-ripening of dry seeds at moderate temperatures (15, 37 and 20/50 °C) enabled dormancy release of A. leucocephalus seeds, as in other Mediterranean climate species (Schütz et al., 2002; Steadman et al., 2003). Furthermore, seeds induced into secondary dormancy by cold stratification were also released from dormancy by warm, dry after-ripening. Actinotus leucocephalus occurs in south-western Australia, which has a Mediterranean climate, with hot, dry summers and cool, wet winters. Dormancy alleviation over summer and germination in autumn increases the likelihood that seeds will germinate when there is sufficient moisture available for seedling survival. Dormancy release of A. leucocephalus seeds occurred more rapidly under warm conditions when seeds were wetted and dried than when stored dry. Wetting and drying is usually associated with accelerating germination rates but has also alleviated dormancy (Lush et al., 1981; Gallagher et al., 2004). The acceleration of dormancy release in A. leucocephalus seeds by wetting and drying has practical applications for the ex situ germination of this species. Ecologically, the transition from the dry to the wet season is characterized by sporadic rain events during otherwise warm, dry periods. Thus, accelerated dormancy release associated with wetting and drying would increase the likelihood that dormancy has been alleviated by the onset of the wet season. Few A. leucocephalus seeds germinated at high temperatures (30 °C), indicating that germination would be unlikely in most sites following short periods of unseasonal summer rain.
In contrast to A. leucocephalus, laboratory storage at moderate temperatures did not simulate burial-induced dormancy release in T. cyathiflora seeds. This was unexpected because after-ripening occurs in many other seeds with non-deep physiological dormancy (Baskin and Baskin, 2004). Fewer T. cyathiflora seeds germinated in smoke water after summer burial only than after 24 months of burial, suggesting that something other than, or in addition to, warm conditions are involved in dormancy release in this species. In addition to temperature, T. cyathiflora seeds may require some form of seed-coat weathering because sulfuric acid treatment enhanced germination in response to smoke water. Scarification alone does not stimulate germination of T. cyathiflora seeds in water in contrast to many other species with non-deep physiological dormancy (Baskin and Baskin, 2004). Scarification may alter germination response to smoke water by altering embryo-covering layers other than, or in addition to, the seed coat. Many water-permeable seeds may be impermeable to certain solutes owing to the presence of a membrane in the inner layer of the seed coat adjacent to the endosperm (Taylor et al., 1997). An increase in permeability of the embryo-covering layers may be required for movement of the smoke chemical(s) to a site(s) that stimulates germination (Tieu and Egerton-Warburton, 2000). However, Baskin and Baskin (2004) argue that there is little evidence for the modification of embryo-covering layers being a natural germination requirement in water-permeable seeds. Indeed, the seed coats of both species exhibited alterations in morphology following burial, but changes in A. leucocephalus dormancy status were attributable to storage temperatures that did not alter the seed coat. In addition, the presence of dormancy cycling, wherein dormancy is alternately alleviated and re-imposed, suggests that weathering alone, which is a non-reversible process, is not wholly responsible for changes in germination response to smoke water. Thus, further work is required to determine conclusively whether breakdown of the embryo-covering layers plays a key role in enabling germination in T. cyathiflora under natural conditions and whether there are any other factors involved.
These fire ephemerals have similar ecological niches, primarily germinating after fire, having a short life span and persisting in the soil seedbank until a subsequent fire. Both species exhibited dormancy cycling, with dormancy being alleviated by autumn and re-imposed by spring, which would ensure that seeds germinate when moisture is most likely to be available for seedling establishment. Despite the similarities in the ecophysiology of the two species, some differences emerged. Smoke water was the only environmental stimulant tested that enabled T. cyathiflora to germinate when exhumed in autumn. By contrast, some A. leucocephalus seeds could germinate in water alone, suggesting that T. cyathiflora has a greater requirement for fire to germinate than A. leucocephalus. Temperatures alone drove dormancy cycling in A. leucocephalus but none of the temperature regimes enabled any T. cyathiflora seeds to germinate, even in smoke water, suggesting that other factors are required before germination in this species can proceed.
Supplementary Material
Acknowledgments
This research forms part of a postgraduate project undertaken with financial assistance from the Robert and Maude Gledden scholarship to K.S.B. Seed collection was undertaken with permission from the Western Australian Department of Conservation and Land Management (Licence number SW007276). Thanks go to John Murphy and Sharon Platten at the Centre for Microscopy and Microanalysis at The University of Western Australia for assistance with microscopy.
LITERATURE CITED
- Baker KS, Steadman KJ, Plummer JA, Dixon KW. 2005. Seed dormancy and germination responses of nine Australian fire ephemerals. Plant and Soil (in press). [Google Scholar]
- Baker KS, Steadman KJ, Plummer JA, Merritt DJ, Dixon KW. 2005. Dormancy release in Australian fire ephemeral seeds during burial increases germination response to smoke water or heat. Seed Science Research (in press). [Google Scholar]
- Bartley MR, Frankland B. 1984. Phytochrome intermediates and action spectra for light perception by dry seeds. Plant Physiology 74: 601–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin CC, Baskin JM. 1994. Germination requirements of Oenothera biennis seeds during burial under natural seasonal temperature cycles. Canadian Journal of Botany 72: 779–782. [Google Scholar]
- Baskin JM, Baskin CC. 1990. Germination ecophysiology of seeds of the winter annual Chaerophyllum tainturieri: a new type of morphophysiological dormancy. Journal of Ecology 78: 993–1004. [Google Scholar]
- Baskin JM, Baskin CC. 1994. Nondeep simple morphophysiological dormancy in seeds of the mesic woodland winter annual Corydalis flavula (Fumariaceae). Bulletin of the Torrey Botanical Club 121: 40–46. [Google Scholar]
- Baskin JM, Baskin CC. 2004. A classification system for seed dormancy. Seed Science Research 14: 1–16. [Google Scholar]
- Bell DT, Hopkins AJM, Pate JS. 1984. Fire in the kwongan. In: Pate JS, Beard JS, eds. Kwongan plant life of the Sandplain. Perth: University of Western Australia Press, 178–204. [Google Scholar]
- Bouwmeester HJ, Karssen CM. 1992. The dual role of temperature in the regulation of the seasonal changes in dormancy and germination of seeds of Polygonum persicaria L. Oecologia 90: 88–94. [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Karssen CM. 1993. Annual changes in dormancy and germination in seeds of Sisymbrium officinale (L.) Scop. New Phytologist 124: 179–191. [Google Scholar]
- Derkx MPM, Karssen CM. 1993. Changing sensitivity to light and nitrate but not to gibberellins regulates seasonal dormancy patterns in Sisymbrium officinale seeds. Plant, Cell and Environment 16: 469–479. [Google Scholar]
- Flematti GR, Ghisalberti EL, Dixon KW, Trengove RD. 2004. A compound from smoke that promotes seed germination. Science 305: 977. [DOI] [PubMed] [Google Scholar]
- Gallagher RS, Steadman KJ, Crawford AD. 2004. Alleviation of dormancy in annual ryegrass (Lolium rigidum) seeds by hydration and after-ripening. Weed Science 52: 968–975. [Google Scholar]
- Holmes PM, Newton RJ. 2004. Patterns of seed persistence in South African fynbos. Plant Ecology 172: 143–158. [Google Scholar]
- International Seed Testing Association. 1999. International rules for seed testing. Seed Science and Technology 27: supplement. [Google Scholar]
- Keeley JE, Fotheringham CJ. 1998. Smoke-induced seed germination in California chaparral. Ecology 79: 2320–2336. [Google Scholar]
- Kruk BC, Benech-Arnold RL. 1998. Functional and quantitative analysis of seed thermal responses in prostrate knotweed (Polygonum aviculare) and common purslane (Portulaca oleracea). Weed Science 46: 83–90. [Google Scholar]
- de Lange JH, Boucher C. 1993. Autecological studies on Audouinia capitata (Bruniaceae). 8. Role of fire in regeneration. South African Journal of Botany 59: 188–202. [Google Scholar]
- Leopold AC, Glenister R, Cohn MA. 1988. Relationship between water content and afterripening in red rice. Physiologia Plantarum 74: 659–662. [Google Scholar]
- Lindesay JA. 2003. Fire and climate in Australia. In: Cary G, Lindenmayer D, Dovers S, eds. Australia burning: fire ecology, policy and management issues. Melbourne: CSIRO, 32–40. [Google Scholar]
- Lush WM, Groves RH, Kaye PE. 1981. Presowing hydration–dehydration treatments in relation to seed germination and early seedling growth of wheat and ryegrass. Australian Journal of Plant Physiology 8: 409–425. [Google Scholar]
- van der Moezel PG, Loneragan WA, Bell DT. 1987. Northern sandplain kwongan: regeneration following fire, juvenile period and flowering phenology. Journal of the Royal Society of Western Australia 69: 123–132. [Google Scholar]
- Pate JS, Dixon KW. 1996. Convergence and divergence in the southwestern Australian flora in adaptations of roots to limited availability of water and nutrients, fire and heat stress. In: Hopper SD, Chappill JA, Harvey MS, George AS, eds. Gondwanan heritage: past, present and future of the Western Australian biota. Sydney: Surrey Beatty and Sons, 249–258. [Google Scholar]
- Pate JS, Hopper SD. 1993. Rare and common plants in ecosystems, with special reference to the south-west Australian flora. In: Schulze ED, Mooney HA, eds. Biodiversity and ecosystem function. Berlin: Springer-Verlag, 293–325. [Google Scholar]
- Roche S, Dixon KW, Pate JS. 1997. Seed ageing and smoke: partners cues in the amelioration of seed dormancy in selected Australian native species. Australian Journal of Botany 45: 783–815. [Google Scholar]
- Schütz W, Milberg P, Lamont BB. 2002. Seed dormancy, after-ripening and light requirements of four annual Asteraceae in South-western Australia. Annals of Botany 90: 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steadman KJ, Crawford AD, Gallagher RS. 2003. Dormancy release in Lolium rigidum seeds is a function of thermal after-ripening time and seed water content. Functional Plant Biology 30: 345–352. [DOI] [PubMed] [Google Scholar]
- Taylor AG, Beresniewicz MM, Goffinet MC. 1997. Semipermeable layer in seeds. In: Ellis RH, Black M, Murdoch AJ, Hong TD, eds. Basic and applied aspects of seed biology. Dordrecht: Kluwer, 429–436. [Google Scholar]
- Tieu A, Egerton-Warburton LM. 2000. Contrasting seed morphology dynamics in relation to the alleviation of dormancy with soil storage. Canadian Journal of Botany 78: 1187–1198. [Google Scholar]
- Tieu A, Dixon KW, Meney KA, Sivasithamparam K. 2001. Interaction of soil burial and smoke on germination patterns in seeds of selected Australian native plants. Seed Science Research 11: 69–76. [Google Scholar]
- Vegis A. 1964. Dormancy in higher plants. Annual Review of Plant Physiology 15: 185–224. [Google Scholar]
- Vleeshouwers LM, Bouwmeester HJ, Karssen CM. 1995. Redefining seed dormancy: an attempt to integrate physiology and ecology. Journal of Ecology 83, 1031–1037. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.