Abstract
• Background and Aims South African soils are not only low in phosphorus (P) but most nitrogen (N) is in organic form, and soil amino acid concentrations can reach 2·6 g kg−1 soil. The Proteaceae (a main component of the South African Fynbos vegetation) and some Fabaceae produce cluster roots in response to low soil phosphorus. The ability of these roots to acquire the amino acid glycine (Gly) was assessed.
• Methods Uptake of organic N as 13C–15N-Gly was determined in cluster roots and non-cluster roots of Leucadendron laureolum (Proteaceae) and Lupinus albus (Fabaceae) in hydroponic culture, taking account of respiratory loss of 13CO2.
• Key Results Both plant species acquired doubly labelled (intact) Gly, and respiratory losses of 13CO2 were small. Lupin (but not leucadendron) acquired more intact Gly when cluster roots were supplied with 13C–15N-Gly than when non-cluster roots were supplied. After treatment with labelled Gly (13C : 15N ratio = 1), lupin cluster roots had a 13C : 15N ratio of about 0·85 compared with 0·59 in labelled non-cluster roots. Rates of uptake of label from Gly did not differ between cluster and non-cluster roots of either species. The ratio of C : N and 13C : 15N in the plant increased in the order: labelled roots < rest of the root < shoot in both species, owing to an increasing proportion of 13C translocation.
• Conclusions Cluster roots of lupin specifically acquired more intact Gly than non-cluster roots, whereas Gly uptake by the cluster and non-cluster roots of leucadendron was comparable. The uptake capacities of cluster roots are discussed in relation to spatial and morphological characteristics in the natural environment.
Keywords: Amino acid, cluster roots, 13C–15N-Gly, Leucadendron laureolum, Lupinus albus, Fynbos, Proteaceae, organic nitrogen
INTRODUCTION
The Fynbos (‘fine bush’) biome is part of the Cape Floristic Region (CFR) in the south-west of South Africa and is one of the world's biodiversity hotspots. Fynbos is characterized by sclerophyllous plants and the presence of three plant families: Proteaceae, Restionaceae (largely non-mycorrhizal) and Ericaceae (ericoid mycorrhizal). These and other families grow on ancient, highly weathered, low-nutrient, mostly acid soils where nitrogen (N) and phosphorus (P) are limiting (Richards et al., 1997). It is possible that the age and/or the related depletion of the soils of the CFR are factors responsible for the high plant diversity and that different nutrient acquisition strategies (e.g. mycorrhiza, carnivory, cluster roots) facilitate survival of species found in the Fynbos. Families that do not have mycorrhiza are usually those with cluster roots or analogous structures while some species have both (Allsopp and Stock, 1993), or have the tripartite relationship of N2-fixing bacteria, cluster roots and mycorrhiza (e.g. Aspalathus linearis, rooibos tea).
Soil P in the Fynbos is low, varying between 3 and 20 mg kg−1 soil. There is a wealth of evidence that mycorrhizal symbiosis (Tarafdar and Marschner, 1994; Joner and Jakobsen, 1995) as well as formation of cluster roots (Gilbert et al., 2000; Neumann et al., 2000) are adaptations to low P and that their formation is, in fact, driven by P supply. Cluster roots are able to enhance plant P uptake from poorly available sources through the production of exudates. These exudates solubilize P bound to metal ions via ligand exchange (Gerke, 1992). How the plants adapt to low levels of available mineral N in the Fynbos is less clear. The levels of inorganic N are low (3–6 mg kg−1 soil depending on soil age) owing to low rates of N mineralization. However, the amount of organic N can be up to 100 times that of inorganic N (Stock et al., 1995). In nine South African high-veld soils, amino acids constituted between 11 and 47 per cent (550–2600 mg amino acid kg−1 soil) of total soil-N, with neutral amino acids [glycine (Gly), alanine, serine, threonine and lysine: 30–350 mg kg−1 soil] and the two acidic amino acids, glutamic and aspartic acid (50–450 mg kg−1 soil), being the most common (Botha et al., 1989; Brodowski et al., 2004). It is known that mycorrhiza may contribute significantly to organic N uptake (ectomycorrhiza, Lipson et al., 1999b; arbuscular mycorrhiza, Hawkins et al., 2001; ericoid mycorrhiza, Read, 1996). However, the Proteaceae are largely non-mycorrhizal and yet form a dominant part of the vegetation (about 23 %), existing as fairly large to tree-like shrubs. Although inorganic N uptake rates of the Proteaceae have been shown to be quite low, at 3–4 µmol 15N g−1 dry mass d−1 (Stock and Lewis, 1984), these plants have a moderate growth rate, a very high photosynthetic capacity and produce large woody flowering stems each season, representing a large investment of C and N.
Non-mycorrhizal plants are not necessarily dependent on inorganic forms of N. It has been shown that a wide range of growth forms (trees, shrubs and grasses) from boreal forests take up organic N irrespective of mycorrhizal status (Näsholm et al., 1998). The non-mycorrhizal arctic sedge Eriophorum vaginatum can obtain up to 60 % of its N budget in organic form (Chapin et al., 1993) and members of the Cyperaceae can utilize soil amino acids (Raab et al., 1999). Most of the work demonstrating the ability of plants to take up organic N in the absence of mycorrhiza comes from arctic sedge (e.g. Kielland, 1994) and boreal forest (Näsholm et al., 1998) species, in environments where low temperatures inhibit microbial degradation of organic matter. However, species from a wide range of Australian environments, not necessarily limited by low mineralization rates, take up Gly, a simple amino acid (Schmidt and Stewart, 1999). Although many families within the Fynbos do form mycorrhizal associations, the Cyperaceae, Restionaceae and Proteaceae are mostly non-mycorrhizal (Allsopp and Stock, 1993). These families may not have mycorrhiza, but they do have cluster roots (in the Proteaceae) or, possibly, analogous structures such as dauciform roots, as has been shown for Australian Cyperaceae (Shane et al., 2005).
Cluster roots (Lamont, 1982) have been known in the Proteaceae since the beginning of the 19th Century and were initially named ‘proteoid roots’ by Purnell (1960). Cluster roots or analogous structures have been found also in other families: Betulaceae, Casuarinaceae, Cucurbitaceae, Cyperaceae, Eleagnaceae, Fabaceae, Moraceae and Myricaceae (cited in Lambers et al., 2003). A cluster root is composed of longitudinal rows of tightly packed, short-lived and determinate rootlets (Purnell, 1960; Watt and Evans, 1999), which give a cluster its distinctive bottlebrush appearance. The rootlets originate opposite each protoxylem pole within the cluster root axis (Purnell, 1960; Johnson et al., 1996; Skene, 2001) and occur in soil patches, corresponding to localized availability of nutrients. Important areas of nutrient research include the contribution of cluster roots to mobilization and uptake of inorganic P and N (Lamont, 1981; Lamont et al., 1984; Keerthisinghe et al., 1998; Neumann et al., 2000), Fe (Arahou and Diem, 1997) and, recently, organic N (Schmidt et al., 2003).
Purnell (1960) gave the first hint that cluster roots may be involved in the uptake of organic nutrients when she described the proliferation of cluster roots in a layer of blood and bone, and cluster roots are found wherever an organic layer occurs, normally close to the soil surface. More specifically, Schmidt and Stewart (1999) found that proteoid roots of Hakea (Proteaceae) took up  and Gly in equal amounts, whereas non-cluster roots took up
and Gly in equal amounts, whereas non-cluster roots took up 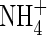 in greater amounts than Gly. Field data of Spriggs et al. (2003) showed that the natural abundance of 15N in selected South African Proteaceae leaves followed closely that of the soil, the content of which is mostly organic N. Although there are several factors that must be considered in interpreting natural abundance of 15N in plants, it was speculated that local Proteaceae may be acquiring significant proportions of their N supply from organic soil N to meet their growth needs. The present study investigates whether cluster and non-cluster roots of a member of the Fynbos Proteaceae (Leucadendron laureolum) and Lupinus albus (Fabaceae, white lupin, a well-known plant used in cluster root research) are able to take up doubly labelled Gly (13C2–15N-Gly) as an intact molecule.
in greater amounts than Gly. Field data of Spriggs et al. (2003) showed that the natural abundance of 15N in selected South African Proteaceae leaves followed closely that of the soil, the content of which is mostly organic N. Although there are several factors that must be considered in interpreting natural abundance of 15N in plants, it was speculated that local Proteaceae may be acquiring significant proportions of their N supply from organic soil N to meet their growth needs. The present study investigates whether cluster and non-cluster roots of a member of the Fynbos Proteaceae (Leucadendron laureolum) and Lupinus albus (Fabaceae, white lupin, a well-known plant used in cluster root research) are able to take up doubly labelled Gly (13C2–15N-Gly) as an intact molecule.
MATERIALS AND METHODS
Plant culture
Seeds of Lupinus albus L. (white lupin) were sterilized in 10 per cent H2O2 for 10 min, pre-germinated in saturated, aerated CaSO4 solution overnight and germinated in sand. After 1 week, Long Ashton nutrient solution (Hewitt, 1966) was supplied every second day. Twenty-one-day-old plants were transferred to a hydroponic solution of 10 per cent Long Ashton minus P (pH 5·5, corrected daily). Conditions in the growth room were a 14 : 10 h day–night regime at 25 °C, ambient relative humidity and a light intensity of 300–400μmolm−2 s−1. After 3 weeks, when the plants were showing signs of P deficiency, 2 % P was added to the nutrient solution. Two-year-old female Leucadendron laureolum (Lam.) Fourc. plants (golden conebush, here referred to simply as leucadendron) were obtained from the Kirstenbosch National Botanical Garden, Cape Town. Plant roots were washed free of soil and grown in deionized H2O for 2 weeks under the same conditions as for white lupin to acclimatize the roots to a hydroponic environment and deplete stored P. Plants were then placed in 10 % Long Ashton solution plus 2 % P (pH 5·5, corrected daily). As the Gly-feeding period for L. laureolum was 16 h, the day–night regime was changed to 18 : 6 h 1 week before commencement of labelling.
13C2–15N-Gly labelling
Doubly labelled Gly (13C2–15N-Gly, labelled on C2 only, 99 atom per cent 15N, 99 atom per cent 13C, Sigma) with a 13C : 15N ratio of 1 : 1 was supplied to cluster and non-cluster roots of lupin and leucadendron to determine the proportion of the amino acid taken up in the intact form. Only mature, metabolically active cluster roots were used for labelling experiments: maturity was tested by dipping cluster roots into a pH indicator [bromocresol purple (0·04 % w/v], where roots that coloured yellow (pH 4·5; active H+-ATPase and organic acid exudation) were considered active and ‘mature’, whereas roots that coloured red (pH 6) or purple (pH 7) were either ‘juvenile’ or ‘senescent’, respectively. Typically, under the growth conditions described, ‘mature’ cluster roots were 10–12d old. Before the uptake experiment, three leaves each from three plants were taken in order to determine the 15N and 13C natural abundance in the lupin and leucadendron prior to labelling. Plants were inserted into 5-L hydroponic containers containing two 50-mL cuvettes to accommodate a proportion of the cluster or non-cluster roots (typically a few grams fresh mass) and the whole system placed in a respiration chamber (Fig. 1). Most of the cluster roots of a plant could be accommodated in the cuvette whereas the non-cluster roots in the labelling cuvette represented only a small proportion of the total root mass. Initially, cuvettes contained N-free nutrient solution plus unlabelled 2 mm Gly. Nutrient solutions in the 5-L container and the cuvettes were as described for plant culture. The plants were left for 1 h to acclimatize and to alleviate any stress resulting from manipulation into the experimental chamber. At the start of the Gly labelling experiment, the solution in the two cuvettes was replaced with 40 mL each of fresh N-free nutrient solution plus 2 mm doubly labelled Gly so that, in total, 160mmol Gly was supplied to each replicate. The concentration of Gly used was within ecological levels, which can range between 5 and 5 mm (Monreal and McGill, 1985; Schobert and Komor, 1987). A concentration of 2 mm was chosen in order to give sufficient plant enrichment with isotopes (about 2 %). Once the cluster roots had been placed in the containers, the container tops were carefully screwed closed and sealed as described below. Lupin plants were labelled for 4 h and leucadendron plants for 16 h, using the low mineral-N uptake rates for Fynbos Proteaceae species as a guideline. At harvest, the roots from the cuvettes (cluster or non-cluster roots) were washed three times for 15s with nutrient solution containing 2 mm unlabelled Gly to flush out labelled Gly in extracellular spaces and the apparent free space (Hawkins et al., 2001). The clusters were then washed three times in distilled water for 15s. The harvested plants were separated into shoot, labelled cluster or non-cluster roots (those roots in cuvettes), and the rest of the (unlabelled) root, termed bulk root. Leaf material harvested before labelling was termed unlabelled shoot. Plant material was dried at 60 °C for 72 h, coarsely milled and then ground by hand with a mortar and pestle in liquid N2. Five white lupin and four leucadendron plants were used per treatment.
Fig. 1.
Respiration chamber used for 15N–13C-Gly labelling of cluster and non-cluster roots of Lupinus albus (white lupin) and Leucadendron laureolum (leucadendron) and determination of respiration rates and 13C loss as 13CO2 during the labelling period. An example of labelling cluster roots of lupin is shown. C = control beaker.
Gly uptake
Glycine uptake was calculated from 13C : 15N ratios of the roots that had been supplied with label. Total uptake is given per gram of labelled root, instead of per plant because ratios of 13C : 15N in tissues other than the labelled root may not necessarily reflect intact Gly, but rather labelled C or N from 13C–15N-Gly after undergoing several reactions. Translocation rates into the rest of the root and shoot were calculated using tissue mass of root and shoot, respectively.
Respiratory 13C loss and respiration rates
The proportion of 13C lost by decarboxylation of Gly during respiration was determined from the amount of respired 13CO2 that had been trapped by NaOH (Fig. 1). Plants were placed in a respiration chamber during 13C2–15N-Gly labelling (Fig. 1). The hypocotyls of plants were fixed into the lid of 5-L containers using closed cell Neoprene rubber, and sealed with paraffin wax and petroleum jelly. Air inlet and air outlet tubing for shoot and root were sealed to the chamber with silicone glue. Two fans at the bottom and top of the chamber maintained air mixing. Total shoot and root net respiration rates were determined by bubbling air from shoot and root compartments into 0·3 m NaOH trap solutions, which were later titrated against 0·3 m HCl. A control beaker with the same amount of NaOH was placed next to test traps during labelling to correct for the amount of CO2 in the atmosphere. Before titration, the carbonate from Na2CO3 formed was precipitated with 1m BaCl2 so that the residual NaOH could be determined. Phenolphthalein (1 %) was used to indicate neutrality at the titration end-point. The shoot or root respiration of 13CO2 was calculated from the difference between 13C levels in the control and root or shoot NaOH traps. Because it was possible that 13CO2 respired by the roots could be re-fixed during dark fixation as 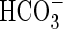 by root PEPCase and carbonic anhydrase (Cramer, 2002), the air flow in the root solution was kept high so that most of the CO2 would be quickly removed from solution and driven into the NaOH traps.
by root PEPCase and carbonic anhydrase (Cramer, 2002), the air flow in the root solution was kept high so that most of the CO2 would be quickly removed from solution and driven into the NaOH traps.
Mass spectrometry of 15N and 13C
Ground shoot and root material (0·1 mg) and air-dried, powdered NaOH (0·07–0·1 mg, used as traps for 13CO2 respired) were weighed into tin caps for Dumas combustion prior to analysis on a mass spectrometer (Finnigan MAT 252, Bremen, Germany). Total amounts of Gly or the mineralization product taken up were calculated from the sums of total 15N found in roots and shoots (leaves only for leucadendron because the stems are woody) and the 13C : 15N ratios of the respective tissues. Gly uptake rates were expressed as mmol 13C or 15N g−1 dry mass h−1 calculated from the 15N and 13C atom per cent excess values corrected against a known leaf standard. The natural abundance obtained from the leaf samples before the uptake experiment was subtracted from the final atom per cent excess value for each plant.
Nitrogen determinations
Total soil N, nitrate-N (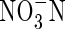 ), ammonium-N (
), ammonium-N ( -N) and organic N of the 1-mm sieved soil fraction (0–10 cm) from four field sites in the CFR were determined. Total soil N was determined by digestion with an FP-528 Nitrogen Analyzer (Leco Corp., St Joseph, USA);
-N) and organic N of the 1-mm sieved soil fraction (0–10 cm) from four field sites in the CFR were determined. Total soil N was determined by digestion with an FP-528 Nitrogen Analyzer (Leco Corp., St Joseph, USA);  and
and 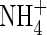 -N were determined using a Technicon Auto Analyzer II (Technicon Corp., Tarrytown, USA) after extraction with 1m KCl. Ammonium concentrations in the labelled Gly solution before and after the feeding period were determined using the salicylate method (Nelson, 1983).
-N were determined using a Technicon Auto Analyzer II (Technicon Corp., Tarrytown, USA) after extraction with 1m KCl. Ammonium concentrations in the labelled Gly solution before and after the feeding period were determined using the salicylate method (Nelson, 1983).
Statistical analysis
Data were analysed using Student's t-tests for equal variances at the P < 0·05 level (Statistica 6·01, Statsoft). Percentage data were normalized using arcsine square root transformation.
RESULTS
Soil nitrogen
At four sites distributed over the CFR, 99 % of the soil N was organic (2·3 ± 0·36–4·8 ± 0·12 mg N g−1 soil) with inorganic N occurring mostly as  (12·6 ± 0·71–18·6 ± 3·64 mg N g−1 soil) and very little as
(12·6 ± 0·71–18·6 ± 3·64 mg N g−1 soil) and very little as  (1·62 ± 0·28–2·46 ± 0·14 mg N g−1 soil).
(1·62 ± 0·28–2·46 ± 0·14 mg N g−1 soil).
Uptake of 13C2–15N-Gly
Both lupin and leucadendron took up N derived from doubly-labelled Gly (Fig. 2). Total 15N uptake was similar in cluster and non-cluster roots of both lupin and leucadendron (Fig. 2), but lupin acquired more 15N as intact Gly when cluster roots were supplied with 13C–15N-Gly than when non-cluster roots were supplied (Fig. 2). This was not the case for leucadendron, for which there was no difference between the amount of intact Gly taken up by cluster and non-cluster roots (Fig. 2). Plant 15N uptake that could not be accounted for by Gly uptake may have been due to  uptake [as
uptake [as  was found in the labelled solution after the labelling period (48 ± 5 mm for lupin and 124 ± 11 mm for leucadendron)], some other mineralization product of Gly or Gly metabolism in the plant (Fig. 2). In the case of leucadendron, very little of the 15N uptake could be attributed to Gly uptake (Fig. 2).
was found in the labelled solution after the labelling period (48 ± 5 mm for lupin and 124 ± 11 mm for leucadendron)], some other mineralization product of Gly or Gly metabolism in the plant (Fig. 2). In the case of leucadendron, very little of the 15N uptake could be attributed to Gly uptake (Fig. 2).
Fig. 2.
15N uptake from 15N–13C-Gly by cluster or non-cluster roots of Lupinus albus (white lupin) and Leucadendron laureolum (leucadendron). Values are means of four or five replicates ± s.e.
The ratio 13C : 15N in cluster or non-cluster roots after the labelling period indicated the proportion of Gly that had been taken up in intact form. Because 13C : 15N in the Gly supplied was 1·0, a value of around 1·0 would indicate that Gly had been taken up as the intact molecule. The ratio varied between 0·85 and 0·19 depending on species and type of root (Table 1). After supplying 13C–15N-Gly to selected lupin cluster or non-cluster roots in a cuvette, the cluster roots had a 13C : 15N ratio of about 0·85 compared with 0·59 in non-cluster roots (Table 1, P = 0·030, Student's t-test). There was no significant difference between the ratio for cluster (0·19) and non-cluster (0·21) roots of leucadendron (Table 1, P = 0·561).
Table 1.
13C : 15N ratios, C : N ratios, 15N- and 13C-uptake rates of cluster (cl) or non-cluster roots (ncl), and translocation rates of Lupinus albus (white lupin) and Leucadendron laureolum (leucadendron) after labelling with 2 mm 13C–15N-Gly for 4 h (lupin) or 16 h (leucadendron)
|
13C uptake rate* (µmol g−1 dm h−1) |
15N uptake rate* (µmol g−1 dm h−1) |
13C : 15N ratio |
C : N ratio |
|||||
|---|---|---|---|---|---|---|---|---|
| Lupinus albus | ||||||||
| Shoot before labelling | – | – | – | 27·7 ± 2·3 | ||||
| Labelled cluster root (cl) | 13·36 ± 6·46a | 8·09 ± 0·67a | 0·85 ± 0·09a | 21·9 ± 1·1a | ||||
| Labelled non-cluster root (ncl) | 2·76 ± 0·61a | 6·49 ± 0·73a | 0·59 ± 0·04b | 28·5 ± 3·5b | ||||
| Bulk root (cl) | 1·41 ± 0·41a | 0·16 ± 0·07a | 40·98 ± 14·81a | 34·8 ± 0·7a | ||||
| Bulk root (ncl) | 0·58 ± 0·66b | 0·07 ± 0·01a | 45·22 ± 7·72a | 35·4 ± 1·6a | ||||
| Shoot (cl) | 0·56 ± 0·18a | 0·08 ± 0·01a | 41·45 ± 8·72a | 40·4 ± 3·1a | ||||
| Shoot (ncl) | 0·27 ± 0·002a | 0·03 ± 0·01b | 80·77 ± 11·80b | 47·9 ± 3·6b | ||||
| Leucadendron laureolum | ||||||||
| Shoot before labelling | – | – | – | 89·1 ± 5·6 | ||||
| Labelled cluster root (cl) | 2·42 ± 0·08a | 2·24 ± 0·17a | 0·19 ± 0·03a | 90·5 ± 3·1a | ||||
| Labelled non-cluster root (ncl) | 1·28 ± 0·42a | 2·12 ± 0·44a | 0·21 ± 0·01a | 83·6 ± 7·8a | ||||
| Bulk root (cl) | 0·18 ± 0·01a | 0·05 ± 0·01a | 6·13 ± 1·11a | 92·3 ± 5·0a | ||||
| Bulk root (ncl) | 0·18 ± 0·03a | 0·10 ± 0·01b | 3·08 ± 0·11a | 89·4 ± 7·9a | ||||
| Shoot (cl) | 0·08 ± 0·06a | 0·01 ± 0·003a | 63·66 ± 13·67a | 95·4 ± 6·5a | ||||
| Shoot (ncl) | 0·06 ± 0·02a | 0·01 ± 0·001a | 45·90 ± 18·80a | 99·0 ± 8·2a | ||||
Translocation rate for bulk root and shoot.
Values are means of 4–5 replicates ± s.e. Different letters indicate significant differences (at P < 0·05 after Student's t-tests) between separate plant parts when cluster roots or non-cluster roots were labelled. dm = dry mass.
The uptake rates of 15N or 13C by labelled roots on a mmol g−1 dry root mass h−1 basis did not vary significantly between cluster and non-cluster roots of either species tested (Table 1). There was a significantly higher rate of uptake of 13C into the bulk of the root (P = 0·001), and 15N into the shoot (P = 0·008), in cluster roots compared with non-cluster roots of lupin (P = 0·001), while uptake rates from the labelled roots to the rest of the root or shoot (or translocation rates) were very low in both treatments (Table 1).
Translocation of assimilate
Most of the 15N and 13C taken up was found in the labelled roots (cluster or non-cluster) with relatively less in the rest of the root (`bulk root') and still less in the shoot, regardless of species (Table 1 and Fig. 3). In lupin, a similar amount of 15N label was translocated (about 15 %) regardless of whether cluster or non-cluster roots were labelled, whereas more 13C was translocated to the bulk root and shoot in non-cluster roots than in cluster roots (35 % vs. 45 %, Fig. 3). A general increase in the translocation of 13C label from labelled root to bulk root to shoot was observed, for both lupin treatments, from increasing C : N ratios, although this was more obvious for plants in which the non-cluster roots had been labelled (Table 1). In leucadendron, a similar trend was apparent, except that there was also slightly more label translocated into non-cluster roots (Fig. 3). As was found for lupins, a general increase in the translocation of 13C label from labelled root to bulk root to shoot was observed, for both leucadendron treatments, from increasing C : N ratios and this was again more obvious for plants in which the non-cluster roots had been labelled (Table 1).
Fig. 3.

Proportion of (A) total 15N and (B) total 13C label found in labelled cluster or non-cluster roots, the rest of the root, and shoots after feeding 2 mm 15N–13C-Gly to Lupinus albus (Lupin) and Leucadendron laureolum (Leuc). Values are means of four or five replicates ± s.e.
Respiratory 13C loss and respiration rates
The small amounts of 13C lost as respiratory CO2 (Table 2) were added to the amounts of 13C uptake, increasing 13C : 15N ratios. There was a significantly greater loss of respiratory 13C from roots compared with shoots of both lupins (P = 0·003) and leucadendron (P = 0·006, Table 2). Leucadendron had a greater respiratory loss of 13C than lupin, largely owing to a larger biomass given that the actual respiration rates were lower (Table 2). Root respiration was greater than shoot respiration in lupin but the opposite was found for leucadendron (Table 2), perhaps because of the larger allocation of biomass to the leucadendron shoot than root (Table 3).
Table 2.
Total respiratory 13C loss and respiration rates from shoot and root during labelling of Lupinus albus (white lupin) and Leucadendron laureolum (leucadendron)
|
Lupinus albus |
Leucadendron laureolum |
|||
|---|---|---|---|---|
| Total respiratory 13C loss (pmol 13C) | ||||
| Shoot | 6·16a ± 0·96 | 34·89a ± 4·81 | ||
| Root | 41·80b ± 7·29 | 109·24b ± 17·44 | ||
| Respiration (mmol CO2 g−1 h−1) | ||||
| Shoot | 0·04a ± 0·01 | 0·15a ± 0·002 | ||
| Root | 0·20b ± 0·03 | 0·03b ± 0·004 | ||
Values are means of four replicates ± s.e. Letters indicate significant differences at P < 0·05.
Table 3.
Shoot, root dry mass and shoot : root ratio of Lupinus albus and Leucadendron laureolum plants used for 13C–15N-Gly labelling. Only total leaf mass is given for Leucadendron laureolum as the stems are woody
| Shoot or leaves* (g) |
Root (g) |
Shoot : root ratio |
|
|---|---|---|---|
| Lupinus albus | 1·87 ± 0·17 | 1·07 ± 0·11 | 1·76 ± 0·06 |
| Leucadendron laureolum* | 8·35 ± 1·12 | 4·21 ± 1·01 | 2·17 ± 0·20 |
Values are means of 6–8 replicates ± s.e.
DISCUSSION
This study demonstrates and quantifies the uptake of intact amino acid by a non-mycorrhizal member of the Fynbos: both cluster and non-cluster roots of Leucadendron laureolum (Proteaceae) took up Gly in an intact form. Uptake rates of Gly by leucadendron were similar to those found for inorganic N uptake by Proteaceae (Stock and Lewis, 1984). As expected, the crop plant white lupin (Fabaceae) took up more intact Gly and at a higher rate than leucadendron.
Unlike those of lupin, cluster roots of the leucadendron did not have a higher capacity for Gly uptake compared with non-cluster roots, as also found for Hakea actities (Schmidt and Stewart, 1999). However, the relative importance of cluster compared with non-cluster roots of Proteaceae for amino acid uptake should be considered together with spatial and morphological aspects of cluster roots in soil: unlike non-cluster roots, cluster roots do occur predominantly in the litter layer (Purnell, 1960; Lamont et al., 1984) or wherever organic matter accumulates (Lamont, 1993), developing specifically in response to poorly soluble forms of N and P. In addition, diffusion is limited in soil compared with hydroponic solution and N uptake by cluster roots may be more efficient compared with non-cluster roots as cluster roots comprise tightly packed rootlets with many root hairs. A Gly transporter has been identified on Hakea cluster roots (Schmidt et al., 2003). Possibly, the concentration of Gly transporters is higher on cluster than on non-cluster roots.
The generally lower uptake of intact Gly by leucadendron compared with lupin can be explained by a lower rate of uptake. Members of the Proteaceae are adapted to a low-nutrient environment and Proteaceae species may not be able to exploit high concentrations of available N (Lewis and Stock, 1978). Thus, the limit to amino acid uptake may not be at the level of the cluster root but at the level of the whole plant. Plants growing in low-nutrient environments usually have low uptake capacities (low Km and Imax) (Neumann and Römheld, 1999), and even white lupin plants grown in a P-deficient environment have a lower Km than P-sufficient plants (Neumann and Römheld, 1999). However, in a P-deficient environment cluster roots have a higher Vmax than non-cluster roots, suggesting a higher concentration of transporters in cluster roots (Schachtman et al., 1998); this means that, as nutrients become more limiting, P uptake by cluster roots becomes more efficient. If this is also true for amino acid uptake by cluster roots of Proteaceae, then it is likely that the uptake of amino acids is not limited by the cluster roots but by the internal regulation of the whole plant.
The 15N uptake that could not be accounted for by 15N–13C-Gly uptake may be attributable to 15 uptake after bacterial mineralization in the labelled solution or to an alternative product after 15N–13C-Gly metabolism in the plant. Some ammonium was present in the labelled solution after the feeding period and this may have resulted from plant mineralization of Gly after uptake and subsequent efflux or bacterial mineralization of Gly under the non-sterile conditions.
uptake after bacterial mineralization in the labelled solution or to an alternative product after 15N–13C-Gly metabolism in the plant. Some ammonium was present in the labelled solution after the feeding period and this may have resulted from plant mineralization of Gly after uptake and subsequent efflux or bacterial mineralization of Gly under the non-sterile conditions.
Although use of hydroponics facilitated easy and quantitative determination of Gly uptake by roots, the results obtained cannot be directly extrapolated to soil. For instance, in soil, competition between plant roots and micro-organisms for amino acids is intense (Jones and Hodge, 1999; Owen and Jones, 2001) and microbes are thought to out-compete plant roots for nutrients as they have high substrate affinities, rapid growth rates and high surface area to volume ratios (Jackson et al., 1989). However, these studies did not take the spatio-temporal aspects of root growth into account (Hodge et al., 2000). Additionally, Gly and Ser are poor substrates for microbial growth compared with Glu (and other amino acids) (Lipson et al., 1999a) and plants capture amino acids at relatively higher rates than micro-organisms when soil solution concentrations of amino acids are high (Jones et al., 2005). In plant–microbe competition studies, Gly uptake by the non-mycorrhizal, alpine sedge Kobresia myosuroides was 3·25 times higher than that of microbes and the sedge had a higher uptake of Gly than of Glu (Lipson et al., 1999a). Ten other arctic plants also had a preference for Gly over Glu (Kielland, 1994). This suggests that microbes and plants have different affinities for the two most predominant amino acids present in the soil solution (Gly and Glu) and that the poor growth of microbes on Gly may present plants with a niche for competitive Gly uptake in soils. Therefore, uptake of Gly by both leucadendron and lupin in hydroponics is a reasonable initial assessment of their capacity for uptake in soil. Although this is to be confirmed by studies in soil pot experiments and the field, it can be speculated that use of soil amino acids, including those from microbial turnover (Bardgett et al., 2003), makes ecological sense for plants in the Fynbos environment, where soil N is largely organic N. Even in agricultural environments, where mineral N is not normally limiting, the amino acid content can be between 5 μm and 5 mm (Monreal and McGill, 1985; Schobert and Komor, 1987) and could contribute to N nutrition of crops such as lupin, as has been shown for wheat and carrot (Hawkins et al., 2001).
The form in which the assimilated Gly was translocated is not known. However, there is an indication that it was predominantly as organic acids on the basis of the increasing 13C : 15N and C : N ratios as the labels were translocated from the labelled roots (cluster or non-cluster) to the rest of the root and the shoot, especially for leucadendron. Generally, more 13C (and 15N in leucadendron) was retained in cluster roots than in non-cluster roots. An explanation for this may be that cluster roots are more C- and N-limited compared with non-cluster roots owing to high rates of respiration and organic acid synthesis.
Future research on the non-mycorrhizal members of the Fynbos (Proteaceae, Restionaceae, Cyperaceae) as well as mycorrhizal members should determine the extent to which inorganic and organic N are acquired in N- and P-poor soil, to what extent microbial competition occurs in these soils, and whether low mineralization rates in the Fynbos (Stock et al., 1988) necessarily mean high rates of organic N uptake in this system. In addition, the affinity of inorganic and organic N transporters of Fynbos plants is unknown, as is the internal regulation of that uptake by growth rates.
Acknowledgments
We thank the National Research Foundation (NRF) of South African for financial support during this study.
LITERATURE CITED
- Allsopp N, Stock WD. 1993. Mycorrhizal status of plants growing in the Cape Floristic Region, South Africa. Bothalia 23: 91–104. [Google Scholar]
- Arahou M, Diem HG. 1997. Iron deficiency induces cluster (proteoid) root formation in Casuarina glauca Plant and Soil 196: 71–79. [Google Scholar]
- Bardgett RD, Streeter TC, Bol R. 2003. Soil microbes compete effectively with plants for organic-nitrogen inputs to temperate grasslands. Ecology 84: 1277–1287. [Google Scholar]
- Botha ADP, Philpott MF, van der Merwe MJ. 1989. Amino acids in eight South African topsoil samples. Suid Afrikaanse Tydskrif van Plant en Grond 6: 210–211. [Google Scholar]
- Brodowski S, Amelung W, Lobe I, Du Preez CC. 2004. Losses and biogeochemical cycling of soil organic nitrogen with prolonged arable cropping in the South African Highveld – evidence from D- and L-amino acids. Biogeochemistry 71: 17–42. [Google Scholar]
- Chapin FS, III, Moilanen L, Kielland K. 1993. Preferential use of organic nitrogen for growth by a non-mycorrhizal arctic sedge. Nature 361: 150–153. [Google Scholar]
- Cramer M. 2002. Inorganic carbon utilization by root systems. In: Waisel Y, Eshel A, Kafkafi U, eds. Plant roots: the hidden half. New York: Marcel Dekker Inc., 699–715. [Google Scholar]
- Gerke J. 1992. Phosphate aluminium and iron in the soil solution of three different soils in relation to varying concentrations of citric acid. Zeitschrift fuer Pflanzenernaehrung und Bodenkunde 155: 339–343. [Google Scholar]
- Gilbert GA, Knight JD, Vances CP, Allan DL. 2000. Proteoid root development of phosphorus deficient lupin is mimicked by auxin and phosphonate. Annals of Botany 85: 921–928. [Google Scholar]
- Hawkins H-J, Johansen A, George E. 2001. Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant and Soil 226: 275–285. [Google Scholar]
- Hewitt EJ. 1966.Sand and water culture methods used in the study of plant nutrition, 2nd revised edition. Commonwealth Bureau of Horticulture and Plantation Crops, East Malling, Technical Communication No. 22. Farnham Royal, UK: Commonwealth Agriculture Bureau, 431–432. [Google Scholar]
- Hodge A, Robinson DL, Fitter A. 2000. Are microorganisms more effective than plants at competing for nitrogen? Trends in Plant Science 5: 304–308. [DOI] [PubMed] [Google Scholar]
- Jackson LE, Schimel JP, Firestone MK. 1989. Short-term partitioning of ammonium and nitrate between plants and microbes in an annual grassland. Soil Biology and Biochemistry 21: 409–415. [Google Scholar]
- Johnson JF, Vance CP, Allan DL. 1996. Phosphorus deficiency in Lupinus albus: altered lateral root development and enhanced expression of phosphoenolpyruvate carboxylase. Plant Physiology 112: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joner E, Jakobsen I. 1995. Uptake of 32P from labelled organic matter by mycorrhizal and non-mycorrhizal subterranean clover (Trifolium subterraneum L.). Plant and Soil 172: 221–227. [Google Scholar]
- Jones DL, Hodge A. 1999. Biodegradation kinetics and sorption reactions of three differently charged amino acids in soil and their effect on plant organic nitrogen availability. Soil Biology and Biochemistry 31: 1331–1342. [Google Scholar]
- Jones DL, Shannon D, Junvee-Fortune T, Farrar JF. 2005. Plant capture of free amino acids is maximized under high soil amino acid concentrations. Soil Biology and Biochemistry 37: 179–181. [Google Scholar]
- Keerthisinghe G, Hocking PJ, Ryan PR, Delhaize E. 1998. Effect of phosphorus supply on the formation and function of proteoid roots of white lupin (Lupinus albus L.). Plant, Cell and Environment 21: 467–478. [Google Scholar]
- Kielland K. 1994. Amino acid absorption by arctic plants: implications for plant nutrition and nitrogen cycling. Ecology 75: 2373–2383. [Google Scholar]
- Lambers H, Cramer MD, Shane MW, Wouterlood M, Poot P, Veneklaas EJ. 2003. Introduction: structure and function of cluster roots and plant responses to phosphate deficiency. Plant and Soil 248: ix–xix. [Google Scholar]
- Lamont BB. 1981. Specialized roots of non-symbiotic origin. In: Specht RL, ed. Heathlands and related shrublands. B. Analytical studies. Amsterdam: Elsevier, 183–195. [Google Scholar]
- Lamont BB. 1982. Mechanisms for enhancing nutrient uptake in plants with particular reference to Mediterranean South Africa and Western Australia. Botanical Reviews 48: 597–689. [Google Scholar]
- Lamont BB. 1993. Why are hairy root clusters so abundant in the most nutrient-impoverished soils of Australia? Plant and Soil 155/156: 269–272. [Google Scholar]
- Lamont BB, Brown G, Mitchell DT. 1984. Structure, environmental effects on their formation, and function of proteoid roots in Leucadendron laureolum (Proteaceae). New Phytologist 97: 381–390. [Google Scholar]
- Lewis OAM, Stock WD. 1978. A preliminary study of the nitrogen nutritional status of members of the South African Proteaceae. Journal of South African Botany 44: 143–151. [Google Scholar]
- Lipson DA, Raab TK, Schmidt SK, Monson RK. 1999a. Variation in competitive abilities of plants and microbes for specific amino acids. Biology and Fertility of Soils 29: 257–261. [Google Scholar]
- Lipson DA, Schadt CW, Schmidt SK, Monson RK. 1999b. Ecotomycorrhizal transfer of amino acid-nitrogen to the alpine sedge Kobresia myosuroides New Phytologist 142: 163–167. [Google Scholar]
- Monreal CM, McGill WB. 1985. Centrifugal extraction and determination of free amino acids in soil solution by TLC using tritiated 1-fluoro-2,4-dinitrobenzene. Soil Biology and Biochemistry 17: 533–539. [Google Scholar]
- Näsholm TA, Ekblad A, Nordin R, Giesler M, Hogberg M, Hogberg P. 1998. Boreal forests plants take organic nitrogen. Nature 392: 914–916. [Google Scholar]
- Nelson DW. 1983. Determination of ammonium in KCl extracts by the salicylate method. Communications in Soil Science and Plant Analysis 14: 1051–1062. [Google Scholar]
- Neumann G, Römheld V. 1999. Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant and Soil 211: 121–130. [Google Scholar]
- Neumann G, Massonneau A, Langlade N, Dinkelaker B, Hengeler C, Römheld V, Martinoia E. 2000. Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Annals of Botany 85: 909–919. [Google Scholar]
- Owen AG, Jones DL. 2001. Competition for amino acids between wheat roots and rhizosphere microorganisms and the role of amino acids on plant N acquisition. Soil Biology and Biochemistry 33: 651–657. [Google Scholar]
- Purnell HM. 1960. Studies of the family Proteaceae. I. Anatomy and morphology of the roots of some Victorian species. Australian Journal of Botany 8: 38–50. [Google Scholar]
- Raab TK, Lipson DA, Monson RK. 1999. Soil amino acid utilization among species of the Cyperaceae: plant and soil processes. Ecology 80: 2408–2419. [Google Scholar]
- Read DJ. 1996. The structure and function of the ericoid mycorrhizal root. Annals of Botany 77: 365–374. [Google Scholar]
- Richards MB, Stock WD, Cowling RM. 1997. Soil nutrient dynamics and community boundaries in the Fynbos vegetation of South Africa. Plant Ecology 130: 143–153. [Google Scholar]
- Schachtman DP, Reid RJ, Ayling SM. 1998. Phosphorus uptake by plants: from soil to cell. Plant Physiology 116: 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Stewart GR. 1999. Glycine metabolism and its occurrence in Australian plant communites. Australian Journal of Plant Physiology 26: 253–264. [Google Scholar]
- Schmidt S, Mason M, Sangtiean T, Steward GR. 2003. Do cluster roots of Hakea actities (Proteaceae) acquire complex organic nitrogen? Plant and Soil 248: 157–165. [Google Scholar]
- Schobert C, Komor E. 1987. Amino acid uptake by Ricinus communis roots: characterization and physiological significance. Plant, Cell and Environment 10: 493–500. [Google Scholar]
- Shane MW, Dixon KW, Lambers H. 2005. The occurrence of dauciform roots amongst Western Australian reeds, rushes and sedges, and the impact of phosphorus supply on dauciform-root development in Schoenus unispiculatus (Cyperaceae). New Phytologist 165: 887–898. [DOI] [PubMed] [Google Scholar]
- Skene KR. 2001. Cluster roots: model experimental tools for key biological problems. Journal of Experimental Botany 52: 479–485. [DOI] [PubMed] [Google Scholar]
- Spriggs AC, Stock WD, Dakora FD. 2003. Influence of mycorrhizal associations on foliar δ15N values of legume and non-legume shrubs and trees in the Fynbos of South Africa: implications for estimating N2 fixation using the 15N natural abundance method. Plant and Soil 255: 495–502. [Google Scholar]
- Stock WD, Lewis OAM. 1984. Uptake and assimilation of nitrate and ammonium by an evergreen Fynbos shrub species Protea repens L. (Proteaceae). New Phytologist 97: 261–268. [Google Scholar]
- Stock WD, Lewis OAM, Allsopp N. 1988. Soil nitrogen mineralisation in a coastal Fynbos succession. Plant and Soil 106: 295–298. [Google Scholar]
- Stock WD, Wienand KT, Baker AC. 1995. Impacts of invading N2-fixing Acacia species on patterns of nutrient cycling in two Cape ecosystems – Evidence from soil incubation studies and 15N natural abundance values. Oecologia 101: 375–382. [DOI] [PubMed] [Google Scholar]
- Tarafdar JC, Marschner H. 1994. Efficiency of VAM hyphae in utilization of organic phosphorus by wheat plants. Journal of Soil Science and Plant Nutrition 40: 593–600. [Google Scholar]
- Watt M, Evans JR. 1999. Proteoid roots. Physiology and development. Plant Physiology 121: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]




